Abstract
The number of newly diagnosed prostate cancer cases varies across Asia, with higher mortality‐to‐incidence ratio reported in developing nations. Androgen deprivation therapy (ADT), alone or in combination, remains the mainstay of first‐line treatment for advanced prostate cancer. Key findings of extensive research and randomized controlled trials have shaped current clinical practice and influenced clinical guideline recommendations. We describe here the recent trend of ADT in newly diagnosed prostate cancer for Asia focusing on Japan (high‐income country) and Malaysia (middle‐income country) based on the Asian Prostate Cancer (A‐CaP) Study. The combination of radiotherapy and ADT or ADT alone was common in patients with intermediate‐to‐high risk localized and locally advanced disease. For metastatic prostate cancer, maximum androgen blockade (gonadotrophin‐releasing hormone [GnRH] agonist/antagonist plus antiandrogen) was prevalent among the Japanese patients while primary ADT alone with GnRH agonist/antagonist was widely practiced in the Malaysian cohort. Upfront combined therapy (ADT plus docetaxel or androgen receptor pathway inhibitor) has significantly improved the outcomes of patients with metastatic castration‐naïve prostate cancer. Its application, however, remains low in our cohorts due to patients’ financial capacity and national health insurance coverage. Early detection remains the cornerstone in prostate cancer control to improve treatment outcome and patient survival.
Keywords: abiraterone acetate, A‐CaP Study, adjuvant ADT, chemohormonal therapy, metastatic prostate cancer
The A‐ Asian Prostate Cancer Study revealed a high proportion of metastatic prostate cancer was reported in Asia, particularly in developing nations. Androgen deprivation therapy, alone or in combination, remains the mainstay of first‐line treatment for advanced prostate cancer.

Abbreviations
- A‐CaP
Asian Prostate Cancer
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ASEAN
Association of Southeast Asian Nations
- ASR
age‐standardized incidence and mortality rate
- CI
confidence interval
- EAU
European Association of Urology
- GLOBOCAN
Global Cancer Incidence, Mortality, and Prevalence
- GnRH
gonadotrophin‐releasing hormone
- HR
hazard ratio
- IQR
interquartile range
- J‐CaP
Japan Prostate Cancer Study Group
- J‐CAPRA
Japan Cancer of the Prostate Risk Assessment
- MAB
maximum androgen blockade
- M‐CaP
Malaysia Prostate Cancer Study Group
- mCNPC
metastatic castration‐naïve prostate cancer
- OS
overall survival
- PSA
prostate‐specific antigen
- RCT
randomized controlled trial
- RP
radical prostatectomy
- rPFS
radiographic progression‐free survival
- RT
radiotherapy
- STAMPEDE
systemic therapy in advancing or metastatic prostate cancer: evaluation of drug efficacy
1. INTRODUCTION
Prostate cancer is the second most common non‐cutaneous cancer in men worldwide. 1 The GLOBOCAN 2020 revealed significant differences in prostate cancer incidence and mortality estimates between Western and Asian countries. 1 In North America, prostate cancer is common, with estimated ASRs of 72.0 per 100 000 and 8.3 per 100 000, respectively. 1 Conversely, a low incidence estimate (ASR 13.6 per 100 000) is reported in Asia with a relatively high mortality rate (ASR 4.4 per 100 000). 1 These variations could be attributed to the number of advanced diseases at diagnosis, access to survival‐prolonging treatments, local screening programs, diagnostic practices, dietary intake, and population genetics.
Prostate cancer is a highly endocrine‐responsive disease. It relies profoundly on the androgen signalling pathway for its growth and survival. Treatment of prostate cancer depends on the disease risk and stage, life expectancy, as well as other competing mortality risks. Androgen deprivation therapy is an important component of prostate cancer treatment throughout the whole disease continuum including high‐risk, locally advanced non‐metastatic (M0) and metastatic (M1) diseases. 2 Testosterone suppression to castration level has formed the basis of ADT as a standard treatment for metastatic prostate cancer. Castration can be achieved by surgical orchidectomy or medical castration with pharmacological methods. Gonadotrophin‐releasing hormone agonists profoundly downregulate the hypothalamic‐pituitary‐testicular axis, either alone or in combination with antiandrogen, inducing reduction of testosterone production and hormone‐sensitive tumor regression. 3 , 4 , 5 In high‐risk, locally advanced prostate cancer, combination of RT with ADT increased the radiosensitivity and vulnerability of tumor cells to be damaged by radiation, improving patients’ OS, biochemical progression free survival and disease‐free survival. 6 , 7 , 8 , 9 , 10
The landscape of prostate cancer treatment has evolved rapidly in recent years particularly for advanced prostate cancer. Relugolix, the first oral GnRH antagonist showing non‐inferiority and superiority to leuprolide (GnRH agonist), 11 was recently approved for advanced prostate cancer by the Food and Drug Administration (FDA). Implementing recommendations of evidence‐based clinical guidelines and expert consensus 12 , 13 , 14 is a challenge we all face in routine clinical practice, especially in Asia due to limited resources and access to certain treatments. 15 Many patients cannot afford the high cost of novel AR‐targeting agents (eg, abiraterone acetate and enzalutamide) or other life‐prolonging treatments in low‐to‐middle income countries without adequate national or personal health insurance coverage. In this review, we focus on the recent trend of ADT for newly diagnosed prostate cancer patients in Asia, particularly in Japan and Malaysia, from 2016 to 2018.
2. RECENT TRENDS OF PROSTATE CANCER IN ASIA
In recent decades, prostate cancer incidence has been growing rapidly in Asia particularly in developed countries with the introduction of PSA testing 16 and increased aging populations. It is imperative to address the lack of high quality population‐based prostate cancer registries in low‐to‐middle income countries for better cancer control planning and reduced cancer burden in the community.
According to the GLOBOCAN 2020 report, prostate cancer was the fifth most commonly diagnosed cancer and the seventh leading cause of cancer mortality among Asian men. 1 The age‐standardized (world) incidence and mortality estimates vary across Asia (Figure 1A). High‐income Asian nations such as Japan, Singapore, and South Korea recorded lower mortality rates but higher incidence rates compared to other low‐to‐middle income Asian countries (Figure 1A).
FIGURE 1.
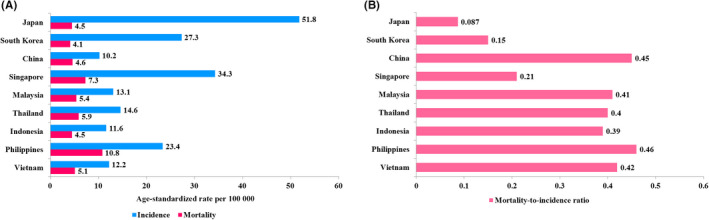
Estimated age‐standardized incidence and mortality rate (A) and mortality‐to‐incidence ratio of prostate cancer (B) in selected Asian countries based on GLOBOCAN 2020 data
The cancer mortality‐to‐incidence ratio serves as one of the key measurements to evaluate long‐term success of cancer surveillance and efficacy of cancer control programs, particularly cancer screening. 17 Japan recorded the lowest mortality‐to‐incidence ratio of 0.087, followed by South Korea (0.15) and Singapore (0.21) (Figure 1B). These findings were in line with other developed countries and regions, including North America (0.12), Australia (0.13), and the United Kingdom (0.16). 1 Conversely, developing Southeast Asian countries had a higher mortality‐to‐incidence ratio, with a range between 0.39 and 0.46 (Figure 1B), suggesting inequalities in cancer survival rates across high and low‐to‐middle income countries. 18
A longitudinal research initiative named the A‐CaP Study provides further insights into the recent trend of prostate cancer across several Asian countries including Japan, South Korea, China, Singapore, Malaysia, Thailand, Indonesia, Philippines, and Vietnam. 19 Of note, prostate cancer patients diagnosed between 2016 and 2018 were recruited into the A‐CaP Study with a minimum follow‐up period of 7 years. The A‐CaP Study revealed that approximately 25%‐54% of prostate cancer cases from developing Asian countries were diagnosed at M1 stage (Figure 2). Middle‐income Asian countries such as Indonesia and Malaysia had a higher percentage of M1 disease, accounting for 46.6% and 53.9% of all new prostate cancer cases, respectively, than those of high income Asian nations including Japan (10.2%), South Korea (4.5%), and Singapore (15.5%) (Figure 2). Comparing the proportion of advanced prostate cancer in Japan across years 2000‐2010, it is worth noting that metastatic prostate cancer was significantly reduced from 23% in 2000 20 to 12.1% in 2004 21 and 10.4% in 2010 22 amongst Japanese men, due to increased PSA exposure rates. Thus, early detection of prostate cancer could decrease the high mortality‐to‐incidence ratio observed in developing Asian countries (Figure 1B).
FIGURE 2.
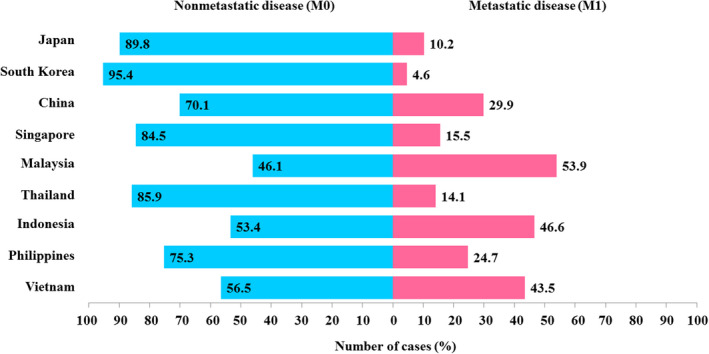
Trends of nonmetastatic (M0) and metastatic (M1) prostate cancer cases in countries included in the Asian Prostate Cancer (A‐CaP) Study
3. ANDROGEN DEPRIVATION THERAPY IN M0 PROSTATE CANCER PATIENTS
For M0 prostate cancer, ADT may be given as an adjunct to primary local therapy, namely RP and radical RT. Findings from previous studies provided strong evidence showing benefits of using ADT as neoadjuvant and adjuvant therapy in combination with RT for men with intermediate‐ and high‐risk diseases. In the Radiation Therapy Oncology Group (RTOG) 94‐08 trial, 10 low‐risk and intermediate‐risk prostate cancer patients were randomly assigned RT either alone or combined with short‐term ADT for 4 months. Results showed that 10‐year OS was increased from 57% to 62% (HR 1.17; 95% CI, 1.01‐1.35), disease‐specific mortality decreased from 8% to 4% (HR 1.87; 95% CI, 1.27‐2.74), and 10‐year biochemical failure reduced from 41% to 26% (HR 1.74; 95% CI, 1.48‐2.04) for combined therapy. These significant improvements were primarily observed in intermediate‐risk, but not low‐risk prostate cancer patients. 10
In addition, benefits of long‐term ADT (24‐36 months) with RT for high‐risk and locally advanced disease were well‐established in previous studies. 6 , 9 , 10 , 23 , 24 Adjuvant ADT was superior compared to neoadjuvant ADT in improving progression‐free survival, biochemical failure, distant metastasis, and metastasis‐free survival of prostate cancer patients treated with RT (P < .05). 25 The latest EAU 2020 guidelines recommended short‐term neoadjuvant plus concomitant ADT for 4‐6 months in patients with intermediate‐risk disease receiving RT, while patients with high‐risk localized and locally advanced disease should receive ADT for up to 2‐3 years. 26
In contrast to RT, neoadjuvant or adjuvant ADT is not considered as standard of care for patients undergoing RP. The survival advantages were restricted to adjuvant ADT in patients with nodal metastases (pN1) who underwent post‐RP and pelvic lymphadenectomy. The ECOG 3886 trial showed that OS (HR 1.84; 95% CI, 1.01‐3.35), prostate cancer‐specific survival (HR 4.09; 95% CI, 1.76‐9.49), and progression‐free survival (HR 3.42; 95% CI, 1.96‐5.98) were significantly improved in pN1 prostate cancer patients who received immediate ADT after RP and pelvic lymphadenectomy compared to those with deferred ADT. 27 , 28
There is inadequate evidence to support the use of ADT alone as primary treatment, in the absence of RP or RT, in patients with localized prostate cancer. For newly diagnosed, high‐risk or locally advanced prostate cancer, patients who underwent combined ADT and RT had better OS than those who received ADT at the 7‐year follow‐up point (74% vs 66%; P = .033). 29 Nevertheless, treatment with ADT alone may be an option for high‐risk prostate cancer patients with PSA greater than 50 ng/mL and/or PSA doubling time less than 12 months, who were deemed unsuitable for local curative treatment due to advanced local tumor, short life expectancy, and/or more severe comorbidities as described in the European Organisation for Research and Treatment of Cancer (EORTC) trial 30891. 30
3.1. Current ADT trend among M0 prostate cancer patients in Japan (high‐income country) and Malaysia (middle‐income country)
The A‐CaP Study investigated the clinical characteristics, treatment patterns, and disease outcomes of prostate cancer patients in nine Asian countries. 19 Here, we report the ADT trend among newly diagnosed M0 prostate cancer patients between 2016 and 2018 in the A‐CaP Study, particularly those from J‐CaP (Japan) and M‐CaP (Malaysia). A summary of clinical characteristics of both J‐CaP and M‐CaP cohorts was presented in Table 1. The M‐CaP M0 patients receiving ADT, in combination or alone, were younger than those in J‐CaP with a median age at diagnosis of 70 years (IQR 8) vs 75 years (IQR 10). Overall, 5048 (51%) patients had a Gleason score 8‐10. Majority of cases (71.2%) were diagnosed at high‐risk localized or locally advanced disease stage. A high burden of comorbidity was observed in the M‐CaP patients with 29.3% reported with at least three comorbidities. Most M‐CaP patients (75.7%) were categorized into the J‐CAPRA intermediate risk group 31 while 60% of J‐CaP patients were in the J‐CAPRA low risk group. The combination of RT and ADT (65.7%) was the most common treatment in the M‐CaP cohort, while ADT alone or in combination with RT accounted for 46.4% and 47% of the J‐CaP patients, respectively.
TABLE 1.
Clinical characteristics of newly diagnosed nonmetastatic (M0) castration‐naïve prostate cancer patients undergoing androgen deprivation therapy (ADT), in combination or alone
| Characteristic | Frequency distribution, n (%) | ||
|---|---|---|---|
|
Overall (N = 9914) |
J‐CaP (n = 9497) |
M‐CaP (n = 417) |
|
| Age at diagnosis (y) | |||
| ≤64 | 913 (9.2) | 843 (8.9) | 70 (16.8) |
| 65‐69 | 1696 (17.1) | 1573 (16.6) | 123 (29.5) |
| 70‐74 | 2252 (22.7) | 2128 (22.4) | 124 (29.7) |
| 75‐79 | 2667 (26.9) | 2584 (27.2) | 83 (19.9) |
| ≥80 | 2386 (24.1) | 2369 (24.9) | 17 (4.1) |
| PSA at diagnosis (ng/mL) | |||
| ≤10 | 4106 (41.4) | 4050 (42.6) | 56 (13.4) |
| 10.01‐20.00 | 2728 (27.5) | 2629 (27.7) | 99 (23.7) |
| 20.01‐50.00 | 1735 (17.5) | 1619 (17.0) | 116 (27.8) |
| >50 | 1345 (13.6) | 1199 (12.6) | 146 (35.0) |
| Gleason score | |||
| ≤6 | 880 (8.9) | 821 (8.6) | 59 (14.2) |
| 7 | 3979 (40.2) | 3821 (40.3) | 158 (38.0) |
| ≥8 | 5049 (51.0) | 4850 (51.1) | 199 (47.8) |
| Unknown | 6 | 5 | 1 |
| Disease stage a | |||
| Low‐risk localized | 366 (3.7) | 365 (3.9) | 1 (0.2) |
| Intermediate‐risk localized | 2459 (25.0) | 2431 (25.9) | 28 (6.7) |
| High‐risk localized | 3893 (39.6) | 3770 (40.1) | 123 (29.5) |
| Locally advanced | 3101 (31.6) | 2836 (30.2) | 265 (63.5) |
| Unknown | 95 | 95 | 0 |
| J‐CAPRA risk group | |||
| Low (0‐2) | 5740 (58.9) | 5659 (60.4) | 81 (21.4) |
| Intermediate (3‐7) | 3931 (40.3) | 3645 (38.9) | 286 (75.7) |
| High (≥8) | 78 (0.8) | 67 (0.7) | 11 (2.9) |
| Unknown | 165 | 126 | 39 |
| Type of ADT | |||
| Prostatectomy + ADT | 650 (6.6) | 627 (6.6) | 23 (5.5) |
| Radiotherapy + ADT | 4742 (47.8) | 4468 (47.0) | 274 (65.7) |
| ADT alone | 4522 (45.6) | 4402 (46.4) | 120 (28.8) |
| Comorbidity count | |||
| 0 | 3387 (34.2) | 3294 (34.7) | 93 (22.3) |
| 1 | 3280 (33.1) | 3184 (33.5) | 96 (23.0) |
| 2 | 2048 (20.7) | 1942 (20.4) | 106 (25.4) |
| ≥3 | 1199 (12.1) | 1077 (11.3) | 122 (29.3) |
Abbreviations: J‐CaP, Japan Prostate Cancer Study Group; J‐CAPRA, Japan Cancer of the Prostate Risk Assessment; M‐CaP, Malaysia Prostate Cancer Study Group; PSA, prostate‐specific antigen.
Disease stage was classified based on the European Association of Urology 2020 guidelines. Low‐risk localized prostate cancer is defined as PSA < 10 ng/mL and Gleason score < 7 (International Society of Urological Pathology [ISUP] grade 1) and cT1‐2a. Intermediate‐risk localized prostate cancer is defined as PSA 10‐20 ng/mL or Gleason score 7 (ISUP grade 2/3) or cT2b. High‐risk localized prostate cancer is defined as PSA > 20 ng/mL or Gleason score > 7 (ISUP grade 4/5) or cT2c. Locally advanced prostate cancer is defined as cT3‐4 or cN+ with any PSA and Gleason score (any ISUP grade).
Table 2 further illustrated the patients’ baseline characteristics based on various treatment groups. The J‐CaP patients receiving a combination of RP and ADT were relatively younger with median age at diagnosis of 69 years (IQR 8) than those treated with a combination of RT and ADT (age at diagnosis 73 years, IQR 8) or ADT alone (age at diagnosis 79 years, IQR 8). A similar trend was observed in the M‐CaP cohort. These findings reflect that it is not uncommon for M0 patients to be treated with ADT alone in Asia, particularly in Japan, due to the higher proportion of elderly prostate cancer patients. Combined RT and ADT was predominantly used in treating intermediate‐risk (92.9%), high‐risk localized (56.1%), and locally advanced prostate cancer (67.2%) in the M‐CaP cohort. Conversely, J‐CaP patients with high‐risk localized and locally advanced prostate cancer were either treated with ADT alone (43.7% vs 52.7%) or combined RT and ADT (48% vs. 40.8%). While half of the J‐CaP patients undergoing combined RP and ADT had high‐risk localized disease, majority of patients (82.6%) receiving combined RP and ADT were with locally advanced disease in the M‐CaP cohort.
TABLE 2.
Subgroup characterization of newly diagnosed nonmetastatic (M0) castration‐naïve prostate cancer patients stratified by types of androgen deprivation therapy (ADT)
| Characteristics | Frequency distribution, n (%) | |||||
|---|---|---|---|---|---|---|
| Prostatectomy + ADT | Radiotherapy + ADT | ADT alone | ||||
|
J‐CaP (n = 627) |
M‐CaP (n = 23) |
J‐CaP (n = 4468) |
M‐CaP (n = 274) |
J‐CaP (n = 4402) |
M‐CaP (n = 120) |
|
| Age at diagnosis (y) | ||||||
| ≤64 | 132 (21.1) | 7 (30.4) | 554 (12.4) | 48 (17.5) | 157 (3.6) | 15 (12.5) |
| 65‐69 | 207 (33.0) | 7 (30.4) | 982 (22.0) | 98 (35.8) | 384 (8.7) | 18 (15.0) |
| 70‐74 | 184 (29.3) | 7 (30.4) | 1231 (27.6) | 80 (29.2) | 713 (16.2) | 37 (30.8) |
| 75‐79 | 95 (15.2) | 2 (8.7) | 1274 (28.5) | 44 (16.1) | 1215 (27.6) | 37 (30.8) |
| ≥80 | 9 (1.4) | 0 (0.0) | 427 (9.6) | 4 (1.5) | 1933 (43.9) | 13 (10.8) |
| PSA at diagnosis (ng/mL) | ||||||
| ≤10 | 250 (39.9) | 4 (17.4) | 2261 (50.6) | 48 (17.5) | 1539 (35.0) | 4 (3.3) |
| 10.01‐20.00 | 180 (28.7) | 3 (13.0) | 1242 (27.8) | 82 (29.9) | 1207 (27.4) | 14 (11.7) |
| 20.01‐50.00 | 136 (21.7) | 11 (47.8) | 628 (14.1) | 75 (27.4) | 855 (19.4) | 30 (25.0) |
| >50 | 61 (9.7) | 5 (21.7) | 337 (7.5) | 69 (25.2) | 801 (18.2) | 72 (60.0) |
| Gleason score | ||||||
| ≤6 | 43 (6.9) | 2 (8.7) | 313 (7.0) | 46 (16.8) | 465 (10.6) | 11 (9.2) |
| 7 | 197 (31.5) | 8 (34.8) | 2041 (45.7) | 112 (40.9) | 1583 (36.0) | 38 (31.9) |
| ≥8 | 386 (61.7) | 13 (56.5) | 2114 (47.3) | 116 (42.3) | 2350 (53.4) | 70 (58.8) |
| Unknown | 1 | 0 | 0 | 0 | 4 | 1 |
| Disease stage a | ||||||
| Low‐risk localized | 18 (2.9) | 0 (0.0) | 131 (3.0) | 1 (0.4) | 216 (5.0) | 0 (0.0) |
| Intermediate‐risk localized | 104 (16.7) | 0 (0.0) | 1339 (30.2) | 26 (9.5) | 988 (22.7) | 2 (1.7) |
| High‐risk localized | 314 (50.6) | 4 (17.4) | 1810 (40.8) | 69 (25.2) | 1646 (37.9) | 50 (41.7) |
| Locally advanced | 185 (29.8) | 19 (82.6) | 1157 (26.1) | 178 (65.0) | 1494 (34.4) | 68 (56.7) |
| J‐CAPRA risk group | ||||||
| Low (0‐2) | 343 (55.4) | 2 (8.7) | 2978 (67.2) | 72 (27.1) | 2338 (54.1) | 7 (7.9) |
| Intermediate (3‐7) | 276 (44.6) | 21 (91.3) | 1442 (32.6) | 193 (72.6) | 1927 (44.6) | 72 (80.9) |
| High (≥8) | 0 (0.0) | 0 (0.0) | 10 (0.2) | 1 (0.4) | 57 (1.3) | 10 (11.2) |
| Unknown | 8 | 0 | 38 | 8 | 80 | 31 |
| Comorbidity count | ||||||
| 0 | 228 (36.4) | 9 (39.1) | 1569 (35.1) | 58 (21.2) | 1497 (34.0) | 26 (21.7) |
| 1 | 212 (33.8) | 3 (13.0) | 1528 (34.2) | 67 (24.5) | 1444 (32.8) | 26 (21.7) |
| 2 | 138 (22.0) | 6 (26.1) | 935 (20.9) | 70 (25.5) | 869 (19.7) | 30 (25.0) |
| ≥3 | 49 (7.8) | 5 (21.7) | 436 (9.8) | 79 (28.8) | 592 (13.4) | 38 (31.7) |
Abbreviations: J‐CaP, Japan Prostate Cancer Study Group; J‐CAPRA, Japan Cancer of the Prostate Risk Assessment; M‐CaP, Malaysia Prostate Cancer Study Group; PSA, prostate‐specific antigen.
Disease stage was classified based on European Association of Urology 2020 guidelines. Low‐risk localized prostate cancer is defined as PSA < 10 ng/mL and Gleason score < 7 (International Society of Urological Pathology [ISUP] grade 1) and cT1‐2a. Intermediate‐risk localized prostate cancer is defined as PSA 10‐20 ng/mL or Gleason score 7 (ISUP grade 2/3) or cT2b. High‐risk localized prostate cancer is defined as PSA > 20 ng/mL or Gleason score > 7 (ISUP grade 4/5) or cT2c. Locally advanced prostate cancer is defined as cT3‐4 or cN+ with any PSA and Gleason score (any ISUP grade).
4. ANDROGEN DEPRIVATION THERAPY IN M1 CASTRATION‐NAÏVE PROSTATE CANCER PATIENTS
Metastatic castration‐naïve prostate cancer (mCNPC) or M1 prostate cancer is commonly diagnosed in Asian men. 32 The diagnosis is established using clinical criteria (high PSA) and conventional imaging studies (computed tomography scan, MRI, and bone scan) routinely. Androgen deprivation therapy is the core treatment option of mCNPC. The paradigm of mCNPC treatment has been rapidly evolving since 2015. Combination therapies with survival advantages were introduced, including chemohormonal therapy (docetaxel plus ADT) in 2015, 33 , 34 abiraterone plus ADT in 2017, 35 , 36 enzalutamide plus ADT, 37 and apalutamide plus ADT 38 in 2019. Details of these key RCTs are outlined in Table 3.
TABLE 3.
Key randomized controlled trials (RCTs) of combination therapy in metastatic (M1) castration‐naïve prostate cancer patients
| RCT | Study arm | Primary endpoint | Median follow‐up (mo) | HR (95% CI) | AE grade 3‐5 (%) |
|---|---|---|---|---|---|
| Docetaxel | |||||
| CHAARTED 33 | ADT + Doc vs ADT alone | OS | 28.9 | 0.61 (0.51‐0.72) | 29.6 |
| STAMPEDE Arm C 34 | ADT + Doc vs ADT alone | OS | 43 | 0.76 (0.62‐0.92) | 52.0 vs 32.0 † |
| ADT + Doc + ZA vs ADT alone | OS | 43 | 0.79 (0.66‐0.96) | 52.0 vs 32.0 † | |
| Abiraterone | |||||
| LATITUDE 35 | ADT + Abi + Pred vs ADT + placebos | OS | 30.4 | 0.62 (0.51‐0.76) | 63.0 vs 48.0 |
| rPFS | 0.47 (0.39‐0.55) | ||||
| STAMPEDE Arm G 36 | ADT + Abi + Pred vs ADT alone | OS | 40 | 0.61 (0.49‐0.75) | 47.0 vs 33.0 † |
| Enzalutamide | |||||
| ENZAMET 37 | ADT + Enza vs ADT + NSAA | OS | 34 | 0.67 (0.52‐0.86) | 57.0 vs 43.0 |
| Apalutamide | |||||
| TITAN 38 | ADT + Apa vs ADT + placebo | OS | 22.7 | 0.67 (0.51‐0.89) | 42.2 vs 40.8 |
| rPFS | 0.48 (0.39‐0.60) |
Abbreviations: Abi, abiraterone acetate; ADT, androgen deprivation therapy; AE, adverse event; Apa, apalutamide; CHAARTED, chemohormonal therapy vs androgen ablation randomized trial for extensive disease in prostate cancer; CI, confidence interval; Doc, docetaxel; Enza, enzalutamide; ENZAMET, enzalutamide in first line androgen deprivation therapy for metastatic prostate cancer; HR, hazard ratio; LATITUDE, a study of abiraterone acetate plus low‐dose prednisone plus androgen deprivation therapy vs androgen deprivation therapy alone in newly diagnosed participants with high‐risk, metastatic hormone‐naïve prostate cancer; NSAA, nonsteroidal antiandrogen drug; OS, overall survival; Pred, prednisone; rPFS, radiographic progression‐free survival; STAMPEDE, systemic therapy in advancing or metastatic prostate cancer: evaluation of drug efficacy; TITAN, targeted investigational treatment analysis of novel antiandrogen; ZA, zoledronic acid.
Both metastatic and nonmetastatic prostate cancer patients were included in the AE assessment.
The chemohormonal therapy vs androgen ablation randomized trial for extensive disease in prostate cancer (CHAARTED) study 33 reported an OS benefit for those receiving docetaxel plus ADT (median OS, 57.6 months) compared to the ADT alone group (median OS, 44.0 months; HR 0.61; 95% CI, 0.51‐0.72). This finding was further supported by results of the STAMPEDE trial. 34 For abiraterone acetate (a cytochrome P450 17 inhibitor) plus ADT, the STAMPEDE trial revealed that a 39% relative improvement in OS (HR 0.61; 95% CI, 0.49‐0.75) was observed in the mCNPC group receiving this combination therapy. 36 The median rPFS was significantly prolonged among patients receiving abiraterone acetate and prednisone in addition to ADT, compared to those treated with ADT alone (33 vs 14.8 months; HR 0.47; 95% CI, 0.39‐0.55) in the abiraterone acetate plus low‐dose prednisone plus androgen deprivation therapy vs ADT alone in newly diagnosed participants with high‐risk, metastatic hormone‐naïve prostate cancer (LATITUDE) trial. 30
Enzalutamide, an AR antagonist, showed a survival advantage in the mCNPC setting. The enzalutamide in first line androgen deprivation therapy for metastatic prostate cancer (ENZAMET) trial revealed a 61% risk reduction in PSA progression (HR 0.39; 95% CI, 0.33‐0.47) and a significant improvement in OS (HR 0.67; 95% CI, 0.52‐0.86) for mCNPC patients treated with enzalutamide plus ADT compared with ADT plus placebo. 37 Similarly, apalutamide (another AR antagonist) has become a potential combination treatment for mCNPC. The targeted investigational treatment analysis of novel antiandrogen (TITAN) study showed that OS (HR 0.67; 95% CI, 0.51‐0.89) and rPFS (HR 0.48; 95% CI, 0.39‐0.60) benefits were observed in mCNPC patients treated with apalutamide plus ADT compared with ADT plus placebo. 38 Based on the level 1 evidence of survival benefits from these key RCTs, the EAU 2020 guidelines recommended use of ADT combined with chemotherapy (docetaxel) or abiraterone acetate plus prednisone or apalutamide or enzalutamide in patients whose first presentation is M1 disease and who are fit for the regimen, as standard of care. 39
In addition, another therapeutic area of interest is the use of RT in patients with oligometastatic CNPC. Evidence from STAMPEDE, 40 HORRAD (a randomized study about the effect on survival of hormonal therapy vs hormonal therapy plus local external radiation therapy in patients with primary diagnosed metastasized (M+) prostate cancer) 41 and STOPCAP (speeding up the evaluation of therapies for metastatic hormone‐sensitive prostate cancer) 42 trials showed OS benefit of RT to the primary tumor, in addition to standard of care therapies. Of note, definition of oligometastatic disease is still under debate. Rapid popularization of next generation imaging techniques, such as prostate‐specific membrane antigen scan, will confound the decision‐making process as current RCTs were mostly based on conventional imaging studies.
4.1. Current ADT trend among mCNPC patients in Japan (high‐income country) and Malaysia (middle‐income country)
Based on the A‐CaP Study, M1 prostate cancer accounts for approximately 50% of newly diagnosed prostate cancer in developing Asian countries (Figure 2). For instance, 53.9% of new prostate cancer cases were diagnosed at M1 stage in Malaysia, a developing country. Conversely, only 10.2% of newly diagnosed, M1 prostate cancer cases were recorded in Japan, a high‐income country. A summary of clinical characteristics and ADT treatment trends of both J‐CaP and M‐CaP cohorts was described in Table 4.
TABLE 4.
Baseline characteristics of newly diagnosed metastatic (M1) castration‐naïve prostate cancer patients undergoing primary androgen deprivation therapy (ADT)
| Characteristic | Frequency distribution, n (%) | ||
|---|---|---|---|
|
Overall (N = 3095) |
J‐CaP (n = 2203) |
M‐CaP (n = 892) |
|
| Age at diagnosis (y) | |||
| ≤64 | 455 (14.7) | 235 (10.7) | 220 (24.7) |
| 65‐69 | 600 (19.4) | 404 (18.3) | 196 (22.0) |
| 70‐74 | 636 (20.5) | 404 (18.3) | 232 (26.0) |
| 75‐79 | 633 (20.5) | 484 (22.0) | 149 (16.7) |
| ≥80 | 771 (24.9) | 676 (30.7) | 95 (10.7) |
| PSA at diagnosis (ng/mL) | |||
| ≤10 | 147 (4.8) | 121 (5.5) | 26 (3.0) |
| 10.01‐20.00 | 196 (6.4) | 155 (7.0) | 41 (4.7) |
| 20.01‐50.00 | 364 (11.8) | 253 (11.5) | 111 (12.7) |
| > 50 | 2373 (77.0) | 1674 (76.0) | 699 (79.7) |
| Unknown | 15 | 0 | 15 |
| Gleason score | |||
| ≤6 | 50 (1.7) | 19 (0.9) | 31 (3.8) |
| 7 | 378 (12.6) | 199 (9.1) | 179 (22.0) |
| ≥8 | 2581 (85.8) | 1977 (90.1) | 604 (74.2) |
| Unknown | 86 | 8 | 78 |
| Modified J‐CAPRA risk group | |||
| Low (0‐2) | 17 (0.6) | 0 (0.0) | 17 (2.0) |
| Intermediate (3‐5) | 477 (15.7) | 351 (16.0) | 126 (15.1) |
| High (≥6) | 2537 (83.7) | 1844 (84.0) | 693 (82.9) |
| Unknown | 64 | 8 | 56 |
| Primary ADT | |||
| GnRH agonists or antagonists | 755 (32.4) | 195 (13.6) | 560 (62.8) |
| Orchidectomy | 209 (9.0) | 55 (3.8) | 154 (17.3) |
| GnRH agonists or antagonists + AA (MAB) | 1119 (48.1) | 1068 (74.6) | 51 (5.7) |
| Orchidectomy + AA (MAB) | 106 (4.6) | 85 (5.9) | 21 (2.4) |
| Chemohormonal therapy | 48 (2.1) | 4 (0.3) | 44 (4.9) |
| AA monotherapy | 25 (1.1) | 14 (1.0) | 11 (1.2) |
| Abiraterone acetate and prednisone + ADT | 5 (0.2) | 0 (0.0) | 5 (0.6) |
| Others | 61 (2.6) | 15 (1.0) | 46 (5.2) |
| Comorbidity count | |||
| 0 | 1092 (35.3) | 867 (39.4) | 225 (25.2) |
| 1 | 872 (28.2) | 670 (30.4) | 202 (22.6) |
| 2 | 650 (21.0) | 431 (19.6) | 219 (24.6) |
| ≥3 | 481 (15.5) | 235 (10.7) | 246 (27.6) |
Abbreviations: AA, antiandrogen; GnRH, gonadotropin‐releasing hormone; J‐CaP, Japan Prostate Cancer Study Group; J‐CAPRA, Japan Cancer of the Prostate Risk Assessment; MAB, maximum androgen blockade; M‐CaP, Malaysia Prostate Cancer Study Group; PSA, prostate specific antigen.
The M1 patients were diagnosed at an older age in the J‐CaP cohort with a median age at diagnosis of 75 years (IQR 12) than those of the M‐CaP cohort (median age at diagnosis, 70 years; IQR 10). Overall, 2373 (77%) M1 patients had a PSA at diagnosis above 50 ng/mL and 85.8% of M1 cases were high grade tumor with Gleason score 8‐10. A total of 2537 (83.7%) M1 cases were stratified into the modified J‐CAPRA high risk group in parallel, based on PSA level, biopsy Gleason score, and clinical M stage. 43
For newly diagnosed M1 prostate cancer, MAB was the most common form of ADT in the J‐CaP cohort, achieved by either GnRH agonists/antagonists plus antiandrogen (74.6%) or orchidectomy plus antiandrogen (5.9%). The MAB therapy was consistently received by 70% of stage IV patients in 2000, 44 84.1% of M1 patients in 2004, 21 and 87.6% of M1 patients in 2010 22 among the Japanese population. On the contrary, 62.8% opted for GnRH agonists or antagonists, and 17.3% were treated with orchidectomy in the M‐CaP cohort. The proportion of patients receiving the latest mCNPC combination therapies was low in the present J‐CaP and M‐CaP cohorts; of which, chemohormonal therapy only accounted for 2% of mCNPC treatments and less than 1% of mCNPC patients were treated with abiraterone acetate and prednisone. Notably, clinical decision‐making of mCNPC management is influenced by clinical and environmental factors as well as financial capacity, albeit several combination therapies are available with survival advantages (Table 3).
In Japan, upfront administration of abiraterone, enzalutamide, and apalutamide was recently approved by the Japanese National Health Insurance. However, the cost of docetaxel in mCNPC is yet to be covered by the Japanese National Health Insurance. Thus, this could explain the low uptake of combination therapies amongst the J‐CaP mCNPC patients diagnosed between 2016 and 2018. Majority of Malaysian men with advanced prostate cancer prefer hormone therapy to chemotherapy. 15 However, novel AR‐targeting agents or other life‐prolonging treatments for mCNPC are expensive. For instance, the cost of abiraterone (~$2800/mo) is twice the country’s median monthly household income (~$1300), 45 which is affordable mostly by those with adequate personal health insurance coverage. Recent evidence from the ACTION (ASEAN Costs in Oncology) study showed the severity of financial catastrophe (out‐of‐pocket health costs 30% or more of annual household income) and economic hardship (inability to make necessary household payments) experienced by cancer patients from low‐ and middle‐income ASEAN countries. 46 In Malaysia, 48% were at risk of financial catastrophe and 45% encountered economic hardship within 12 months after a cancer diagnosis, resulting from medical costs for inpatient/outpatient care and purchasing medical supplies, drugs, and equipment. 46
5. CONCLUSION
The cancer mortality‐to‐incidence ratio of prostate cancer varies significantly across Asia. It is an alarming health issue with a high proportion of metastatic prostate cancer diagnosed particularly in low‐to‐middle income countries. Androgen deprivation therapy has been the most common treatment option in advanced prostate cancer over the past decades. It remains an important component in treating both localized and metastatic prostate cancer across Asia. Evidence‐based clinical practice guidelines are pivotal for prostate cancer management; however, the latest combination ADT therapies may place enormous strain on a country’s health‐care system and patients’ financial health. Early detection is an imperative, ongoing effort to improve prostate cancer outcome and survival as well as to reduce financial catastrophe and economic hardship in cancer patients.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We would like to thank the Director of Health Malaysia for permission to publish this paper and all urology associates for their assistance in data collection at all participating hospitals under the A‐CaP Study, particularly the J‐CaP and M‐CaP cohorts. The M‐CaP was funded by the Ministry of Health Malaysia (NMRR‐16‐1340‐31177 to RM) and University of Tokyo (to TAO). The J‐CAP Study Group was supported in part by Takeda Pharmaceutical Company Limited (to HA).
Lim J, Onozawa M, Saad M, et al. Recent trend of androgen deprivation therapy in newly diagnosed prostate cancer patients: Comparing between high‐ and middle‐income Asian countries. Cancer Sci. 2021;112:2071–2080. 10.1111/cas.14889
Contributor Information
Jasmine Lim, Email: jasmine.lim@um.edu.my.
Hideyuki Akaza, Email: akazah@siccn.org.
REFERENCES
- 1. GLOBOCAN . 2020: Estimated cancer incidence, mortality and prevalence worldwide in 2020. http://GLOBOCAN.iarcfr/Defaultaspx
- 2. Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293‐297. [DOI] [PubMed] [Google Scholar]
- 3. Ahmann FR, Citrin DL, deHaan HA, et al. Zoladex: a sustained‐release, monthly luteinizing hormone‐releasing hormone analogue for the treatment of advanced prostate cancer. J Clin Oncol. 1987;5:912‐917. [DOI] [PubMed] [Google Scholar]
- 4. Labrie F, Dupont A, Belanger A, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267‐275. [PubMed] [Google Scholar]
- 5. Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419‐424. [DOI] [PubMed] [Google Scholar]
- 6. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516‐2527. [DOI] [PubMed] [Google Scholar]
- 7. D'Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299:289‐295. [DOI] [PubMed] [Google Scholar]
- 8. Denham JW, Steigler A, Lamb DS, et al. Short‐term androgen deprivation and radiotherapy for locally advanced prostate cancer: results from the Trans‐Tasman Radiation Oncology Group 96.01 randomised controlled trial. Lancet Oncol. 2005;6:841‐850. [DOI] [PubMed] [Google Scholar]
- 9. Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma–long‐term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285‐1290. [DOI] [PubMed] [Google Scholar]
- 10. Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short‐term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107‐118. [DOI] [PubMed] [Google Scholar]
- 11. Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen‐deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187‐2196. [DOI] [PubMed] [Google Scholar]
- 12. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the advanced prostate cancer consensus conference 2019. Eur Urol. 2020;77:508‐547. [DOI] [PubMed] [Google Scholar]
- 13. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: the report of the advanced prostate cancer consensus conference APCCC 2017. Eur Urol. 2018;73:178‐211. [DOI] [PubMed] [Google Scholar]
- 14. Gillessen S, Omlin A, Attard G, et al. Management of patients with advanced prostate cancer: recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26:1589‐1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saad M, Alip A, Lim J, et al. Management of advanced prostate cancer in a middle‐income country: real‐world consideration of the advanced prostate cancer consensus conference 2017. BJU Int. 2019;124:373‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsing AW, Tsao L, Devesa SS. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60‐67. [DOI] [PubMed] [Google Scholar]
- 17. Choi E, Lee S, Nhung BC, et al. Cancer mortality‐to‐incidence ratio as an indicator of cancer management outcomes in organization for economic cooperation and development countries. Epidemiol Health. 2017;39:e2017006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farmer P, Frenk J, Knaul FM, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. 2010;376:1186‐1193. [DOI] [PubMed] [Google Scholar]
- 19. Akaza H, Hirao Y, Kim CS, et al. Asia prostate cancer study (A‐CaP Study) launch symposium. Prostate Int. 2016;4:88‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cancer Registration Committee of the Japanese Urological A . Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol. 2000;2005(12):46‐61. [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto H, Nakanishi H, Miki T, et al. Oncological outcomes of the prostate cancer patients registered in 2004: report from the Cancer Registration Committee of the JUA. Int J Urol. 2011;18:876‐881. [DOI] [PubMed] [Google Scholar]
- 22. Onozawa M, Hinotsu S, Tsukamoto T, et al. Recent trends in the initial therapy for newly diagnosed prostate cancer in Japan. Jpn J Clin Oncol. 2014;44:969‐981. [DOI] [PubMed] [Google Scholar]
- 23. Pilepich MV, Winter K, John MJ, et al. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243‐1252. [DOI] [PubMed] [Google Scholar]
- 24. Lawton CAF, Lin X, Hanks GE, et al. Duration of androgen deprivation in locally advanced prostate cancer: long‐term update of NRG oncology RTOG 9202. Int J Radiat Oncol Biol Phys. 2017;98:296‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spratt DE, Malone S, Roy S, et al. Prostate radiotherapy with adjuvant androgen deprivation therapy (ADT) improves metastasis‐free survival compared to neoadjuvant ADT: an individual patient meta‐analysis. J Clin Oncol. 2021;39:136‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mottet N, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer‐2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2020. [DOI] [PubMed] [Google Scholar]
- 27. Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node‐positive prostate cancer. N Engl J Med. 1999;341:1781‐1788. [DOI] [PubMed] [Google Scholar]
- 28. Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node‐positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7:472‐479. [DOI] [PubMed] [Google Scholar]
- 29. Warde P, Mason M, Ding K, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104‐2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Studer UE, Collette L, Whelan P, et al. Using PSA to guide timing of androgen deprivation in patients with T0–4 N0–2 M0 prostate cancer not suitable for local curative treatment (EORTC 30891). Eur Urol. 2008;53:941‐949. [DOI] [PubMed] [Google Scholar]
- 31. Cooperberg MR, Hinotsu S, Namiki M, et al. Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy. J Clin Oncol. 2009;27:4306‐4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu Y, Uemura H, Ye D, et al. Prostate cancer in Asia: design of a patient registry to inform real‐world treatments, outcomes, and quality of life. Prostate Int. 2019;7:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N Engl J Med. 2015;373:737‐746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first‐line long‐term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2017;377:352‐360. [DOI] [PubMed] [Google Scholar]
- 36. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first‐line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121‐131. [DOI] [PubMed] [Google Scholar]
- 38. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration‐sensitive prostate cancer. N Engl J Med. 2019;381:13‐24. [DOI] [PubMed] [Google Scholar]
- 39. Cornford P, van den Bergh RCN, Briers E, et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer. Part II‐2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263‐282. [DOI] [PubMed] [Google Scholar]
- 40. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353‐2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boeve LMS, Hulshof M, Vis AN, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410‐418. [DOI] [PubMed] [Google Scholar]
- 42. Burdett S, Boeve LM, Ingleby FC, et al. Prostate radiotherapy for metastatic hormone‐sensitive prostate cancer: a STOPCAP systematic review and meta‐analysis. Eur Urol. 2019;76:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim J, Hinotsu S, Onozawa M, et al. Modified J‐CAPRA scoring system in predicting treatment outcomes of metastatic prostate cancer patients undergoing androgen deprivation therapy. Cancer Med. 2020;9:9346‐9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hinotsu S, Akaza H, Usami M, et al. Current status of endocrine therapy for prostate cancer in Japan analysis of primary androgen deprivation therapy on the basis of data collected by J‐CaP. Jpn J Clin Oncol. 2007;37:775‐781. [DOI] [PubMed] [Google Scholar]
- 45. Key Statistics of Household Income & Expenditure 2016 . https://wwwdosmgovmy/v1/indexphp?r=column/cone&menu_id=UllqdFZoVFJhMi9zekpWKzFaSTdvUT09
- 46. The ACTION Study Group B‐PN , Yip CH, Peters SAE, et al. Policy and priorities for national cancer control planning in low‐ and middle‐income countries: Lessons from the Association of Southeast Asian Nations (ASEAN) Costs in Oncology prospective cohort study. Eur J Cancer. 2017;74:26‐37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


