Abstract
The use of patient‐derived xenografts (PDXs) has recently attracted attention as a drug discovery platform with a high predictive clinical efficacy and a preserved tumor heterogeneity. Given the racial differences in genetic variations, it would be desirable to establish a PDX library from Japanese cancer patients on a large scale. We thus tried to construct the Japanese PDX (J‐PDX) library with a detailed clinical information for further clinical utilization. Between August 2018 and May 2020, a total of 1126 cancer specimens from 1079 patients were obtained at the National Cancer Center Hospital and National Cancer Center Hospital East, Japan, and were immediately transplanted to immunodeficient mice at the National Cancer Center Research Institute. A total of 298 cross‐cancer PDXs were successfully established. The time to engraftment varied greatly by cancer subtypes, especially in the first passage. The engraftment rate was strongly affected by the clinical stage and survival time of the original patients. Approximately 1 year was needed from tumor collection to the time when coclinical trials were conducted to test the clinical utility. The 1‐year survival rates of the patients who were involved in establishing the PDX differed significantly, from 95.6% for colorectal cancer to 56.3% for lung cancer. The J‐PDX library consisting of a wide range of cancer subtypes has been successfully established as a platform for drug discovery and development in Japan. When conducting coclinical trials, it is necessary to consider the target cancer type, stage, and engraftment rate in light of this report.
Keywords: coclinical trial, J‐PDX, patient‐derived xenograft
We have successfully established a PDX library derived from Japanese cancer patients. In less than 2 years, we have registered more than 1000 specimens and established nearly 300 PDXs. We will further enrich the library and use it as a platform to accelerate drug discovery in Japan.
1. INTRODUCTION
In recent years, there has been much interest in the use of patient‐derived xenografts (PDXs), a cancer‐bearing mouse model generated by transplanting a patient's tumor directly into an immunodeficient mouse, as a platform for anticancer drug development. 1 , 2 Compared with traditional cell line and cell line xenograft models, a PDX is reported to better reflect the efficacy of treatment in the clinic. 3 It is expected to increase the probability of success, which is estimated to be around 5% in cancer drug development, reduce the cost of development by hundreds of millions of dollars, and shorten development times. 4 , 5 Large PDX libraries have already been created and widely used by EuroPDx, the National Cancer Institute Patient‐Derived Models Repository (NCI‐PDMR), and several commercial companies, mainly in Europe and the USA. 6 , 7 In Japan, however, there is no large‐scale PDX library, and considering racial differences in oncogenes, it is hoped that a library will be established as soon as possible. 8
There are several possible issues in using PDX models for drug development. In the preclinical phase, it is expected that a PDX model will be used to evaluate the efficacy of a drug in a “PDX basket trial” to confirm proof of concept and to select target cancer types and fractions. In the clinical development phase, pretreatment PDXs in parallel with clinical trials, called “coclinical trials,” can be used to search for biomarkers of drugs and identify factors associated with refractory response. Post‐treatment PDXs in the drug‐resistant phase can be used to explore the mechanism of resistance, provide a basis for the next phase of drug discovery and development, and enable the search for combination therapies. After regulatory approval, it is also expected that PDXs can be used to evaluate new indications. Coclinical trials are particularly important because of the potential to compare the effects of treatment in PDXs and original patients. They are expected to become increasingly important soon. In short, the use of PDX models has the potential to provide the ultimate in personalized medicine 1 , 6 ; if a PDX model can be pregenerated as a patient's avatar with the ability to predict the effects of a drug before it is administered to a patient, it could not only have a significant impact on patient treatment decisions but also provide an essential platform for drug discovery and development.
On the other hand, the major challenges in using PDX models and conducting coclinical trials are the low transplantation rate, the long transplantation time, and most importantly, the paucity of basic information. Previous reports have shown an engraftment rate of 20%‐50%, depending on the patient's cancer type, stage, amount of specimen transplanted, and type of immunodeficient mouse used. 9 It takes 6‐12 months after transplantation before a PDX can be evaluated for drug efficacy. 10 Several retrospective studies have been reported comparing anticancer drugs’ efficacy in established PDX models and in the original patients. 11 , 12 , 13 , 14 , 15 , 16 , 17 In addition, a high concordance rate of anticancer drug efficacy between PDXs and the corresponding clinical trials has been reported. 1 , 18 , 19 , 20 , 21 However, these reports are studies of specific drugs in specific tumors and are rarely systematic.
There is no doubt that the acquisition of preclinical proof of concept will play a role in the success or failure of anticancer drug development in the development of treatments. As the importance of coclinical trials with PDXs is expected to increase, there is an urgent need to obtain basic information for preparing coclinical trials on PDX engraftment rates, time to engraftment, and time to drug efficacy studies for different types of cancer.
We initiated the J‐PDX library project in 2018 to create a PDX library from Japanese cancer patients and to innovate in drug development. In this project, we recruited patients focusing on advanced‐ and recurrent‐stage cancers resistant to standard treatment and eligible for early clinical trials. In addition, unmet medical needs of pediatric cancers and rare cancers were also set as priority targets. Over the 21 months to May 30, 2020, we have enrolled 1126 cases and established nearly 300 PDXs. This paper reports on our experience in establishing cross‐cancer PDX libraries in the J‐PDX library project, cancer type–specific engraftment rates, and time to engraftment. Moreover, the probability of patient survival in conducting a coclinical trial is presented, and insights into planning coclinical trials are discussed.
2. METHODS
2.1. Patient selection and consent
Between August 22, 2018 and May 31, 2020, a total of 1126 specimens from 1079 patients were enrolled at the National Cancer Center Hospital and National Cancer Center Hospital East in Japan. The protocol was approved by the institutional review board (NCCRI: 2015‐123), and all patients gave written informed consent. All cancers, including rare and pediatric cancers, were included in this study. To prevent workers' exposure to infection, specimens from Hepatitis B virus–, Hepatitis C virus–, and human immunodeficiency virus–infected patients, and previously infected cases were excluded. The study was performed according to the precepts established by the Helsinki Declaration. The study design and its conduct complied with all applicable regulations, guidance, and local policies. Animal experiments were performed in compliance with the guidelines of the Institute for Laboratory Animal Research, National Cancer Center Research Institute (T17‐073 and T19‐008).
2.2. Sample collection
After confirming consent acquisition, surgical specimens were separated in the pathology department, and biopsy specimens were received in the collection department, including the endoscopy room. The following samples were used: 2 to 10–mm3 surgical specimens, 1‐2 punctures for needle biopsies, 1‐3 tissues for endoscopy, and more than 20 mL of pleural and ascitic fluid. Tissues were immediately soaked in storage solution (Theliokeep, Bio Verde Inc) after collection and stored at 4°C. The specimens were anonymized after receipt and transported to the National Cancer Center Research Institute.
2.3. PDX establishment
2.3.1. Sample processing and tumor implantation
All procedures complied with Standard Operating Procedures for the J‐PDX Library, and workers were provided with regular technical guidance. After receipt of the specimens in the laboratory, they were stored at 4°C until transplantation. Solid tumor samples were cut into 2 mm3 and implanted. Pleural and ascitic fluid samples were centrifuged by density gradient centrifugation (Oncoquick, Greiner Bio‐one) to collect tumor cells, suspended in Theliokeep, mixed with equal amounts of Matrigel (Matrigel Matrix Basement Membrane Growth Factor Reduced, Corning), and injected subcutaneously. In principle, the transplantation site was subcutaneous around the flank, and only breast cancer specimens were transplanted into the mammary gland in female 6‐week‐old NOG mice (NOD. Cg‐Prkdcscid Il2rgtm1Sug/ShiJic, In‐Vivo Science Inc). Mice were housed in sterile, filter‐capped, polycarbonate cages, maintained in a barrier facility on a 12‐hour light/dark cycle, and provided sterilized food and water. All invasive procedures were performed by intraperitoneal administration of three types of mixed anesthesia (medetomidine hydrochloride, Meiji Seika Pharma; midazolam, Maruishi Pharmaceutical Co.; betorfal tartrate, Meiji Seika Pharma) or inhalation of isoflurane (Zoetis Japan) to reduce the pain of the experimental animals.
2.3.2. Monitoring and passage
Mice were monitored weekly for tumor growth and body weight. When the tumor was palpable, tumor volume was calculated using the following formula: tumor volume (mm3) = (tumor length (mm) × [tumor width (mm)]2)/2. The tumor was then designated as Trans Generation 1 (TG1), and the tumor was passaged to mice for further generations (TG2, 3…). Tumor passage was conducted if the tumor volume reached 200 to 2000 mm3 or if the humanitarian endpoint was met. The following points were defined as humanitarian endpoints 22 , 23 : dehydration, serious emaciation, weight loss of 20% or more compared with the previous week, motility problems (inability to take food and water), unable to stand, persistently supine or prone, decreased spontaneous movement, signs of muscle atrophy, respiratory slowness, tachypnea, dyspnea, and effortless breathing, progressive drop in body temperature, paralytic gait, clonic spasms, tonic spasms, persistent hemorrhage from an opening, tumor volume exceeds 10% of the body weight, ulceration and necrosis of the tumor mass, or the tumor is extremely large and severely restricts normal behavior. Mice were euthanized by cervical dislocation under anesthesia, and the tumors were removed. Removed tumors were cut into 2‐mm squares and immediately passaged for a further generation, and the rest of the tumors were used as samples for reimplantation, samples for the preparation of pathological blocks, and fresh frozen samples.
2.3.3. Storage
The tumor samples for reimplantation were temporarily stored at −80°C in cell cryopreservation solution (STEM‐CELLBANKER DMSO Free GMP grade, ZENOAQ RESOURCE; LABO Banker 2, TOSC Japan Ltd.; or CELLBANKER 1 plus, ZENOAQ RESOURCE) using Bicell (BICELL, Nihon Freezer Co. Ltd.) and then stored at −150°C or liquid nitrogen within 72 hours. Fresh frozen samples were flash‐frozen immediately after collection with liquid nitrogen and stored at −80°C.
2.4. PDX tumor sample validation
2.4.1. Immunohistochemistry
Pathological tissues were routinely subjected to hematoxylin‐eosin staining, human CD45 (clone, D9M8I, Cell Signaling Technology) staining, human COX IV (clone, 3E11, Cell Signaling Technology), and rodent COX IV (clone D6I4K, Cell Signaling Technology) staining to confirm replacement by lymphoma outgrowth and murine tumors. If human CD45 was determined to be 3+ in any of the TG1‐3 samples, the PDX was determined to be a lymphoma outgrowth. The percentage of human tissue was confirmed by human COX IV staining, and if the percentage of human COX IV–positive cells was low, staining was performed with rodent COX IV to confirm the presence of murine tumor.
2.5. PDX database construction
2.5.1. Patient medical data
Patients' characteristics were collected, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, medical history, and family history. According to the Union for International Cancer Control classification for each tumor, tumor characteristics were noted, including histology and tumor‐node‐metastasis (TNM) stage. Biomarker data were collected from medical records, including gene analysis results of companion diagnostics, and clinical sequencing. Prior treatment characteristics, including surgery, surgical procedure, radiation dose, chemotherapy regimens, cycles of chemotherapy, best response, progression‐free survival, and overall survival (OS), were collected. OS was defined as the time from the day of study enrollment to the last day on which the patient was confirmed alive or dead from any cause.
2.5.2. PDX establishment data
All information on tumor volume and body weight changes related to PDX implantation, passage, and establishment, as well as biomarker analysis and drug administration results using PDX samples, were aggregated in a database. As an assessment of the time to the growth of PDX tumors, the time to reach a tumor volume of 200 mm3 (TTV200), defined as days from tumor implantation to the first day when the tumor volume exceeds 200 mm3, and the time to the passage (TTP), defined as days from tumor implantation to the passage, were evaluated. The time to establishment (TTE) was defined as the sum of the TTP for each passage.
2.6. Statistical analysis
The results are expressed as means ± SEM. OS differences were analyzed using the Kaplan‐Meier method, and the log‐rank test was used to compare survival. Logistic regression analysis was used to investigate the associations between passageable tumors and factors related to patient characteristics. Analyses were performed using STATA SE version 16.1 (Stata Corp) and Graphpad Prism version 8.4.3 (GraphPad Software). Probability values of <.05 indicated a significant difference.
3. RESULTS
3.1. Enrollment progress
From August 22, 2018 to May 31, 2020, 1126 specimens were received from 1079 patients, with three specimens from one patient and two specimens from 45 patients. Due to the SARS‐CoV‐2 pandemic's impact, enrollment was temporarily suspended from March 30, 2020 (Figure 1A).
FIGURE 1.
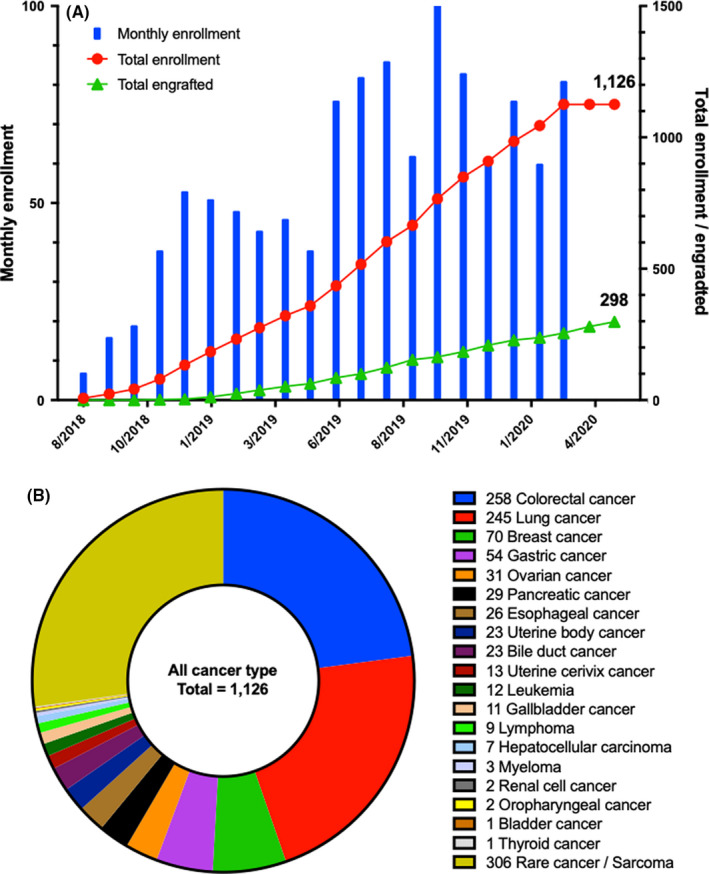
Enrollment progress and enrolled cancer types. A, Graph depicting the enrollment progress from August 22, 2018 to May 31, 2020. Blue bars indicate the number of enrollments per month, red lines indicate the total number of enrollments, and green lines indicate the total number of engrafted PDXs. As of May 31, 2020, there were a total of 1126 cases enrolled and 298 engrafted. B, The breakdown of enrolled cases by cancer type. A breakdown of rare cancers is shown in Figure S2
The CONSORT diagram is shown in Figure S1. Of the 1126 specimens enrolled, 16 specimens were unavailable due to inability to obtain specimens after consent. Ten hematologic tumor specimens are awaiting transplantation because the method of transplantation differs from that of solid tumors. As a result, 1100 transplanted specimens were determined to be “Totally Assessable Specimens”. A total of 476 specimens were defined as “Discontinued”: 290 specimens that failed to grow in TG1, 39 specimens that failed to grow in TG2, 10 specimens with a murine tumor, and 137 specimens with lymphoma outgrowth. As of May 31, 2020, 326 specimens were TG1 ongoing. Finally, a total of 298 specimens were judged to be “Passageable tumors”: 54 TG2 ongoing specimens, 43 TG3 ongoing specimens, and 201 specimens established up to TG3.
A wide range of major cancer types was enrolled, including 258 samples of colorectal cancer, 245 samples of lung cancer, 70 samples of breast cancer, and 54 samples of gastric cancer (Figure 1B). Moreover, rare cancers and sarcomas were also collected in 306 samples from 69 cancer types (Figure S2).
3.2. Patient characteristics
The patients' background characteristics are shown in Table 1. The median age was 61 years, with a range of 5‐91 years. Stage III, IV, and recurrent cases accounted for 60% of all cases, with a significantly higher frequency of Passageable tumors compared with Discontinued (P = .002). Overall, 91% of patients received some kind of prior treatment, with 79% of patients receiving surgery, 13% receiving radiation therapy, and 30% receiving chemotherapy. Prior chemotherapy history was significantly higher in Passageable tumors compared with Discontinued (35% vs 27%, P = .014), and immune checkpoint inhibitors were used in 12% of specimens. Thirty percent of the specimens were biopsies, and 70% were surgical specimens, of which 48% (374/783) were surgically resected specimens from stage III, stage IV, or recurrent cases. The biopsy and surgical specimens had similar establishment rates (biopsy: 99/343, 28.9% vs surgery: 199/783, 25.4%. P = .227). Of 306 rare cancer specimens, 211 (69%) were obtained from surgical specimens. The average time from specimen collection to transplantation was 1.25 day (range, 0‐11 days), with Passageable tumors being transplanted in a significantly shorter period of time than Discontinued (Passageable tumors: average 1.03 days vs Discontinued: 1.27 days, P = .005). The average time to transplantation for specimen type was 0.87 days (0‐11 days) for biopsy specimens vs 1.40 days (0‐10 days) for surgical specimens and significantly shorter for biopsy specimens (P <.001). The longer time to transplantation was due to the end‐of‐year holidays and consecutive holidays. Details on the distribution of stages and specimen types by carcinoma are shown in Table S1.
TABLE 1.
Patient characteristics
|
Total enrolled N = 1126 |
Passageable tumors N = 298 |
Discontinued N = 476 |
Not yet assessable N = 326 |
P‐value | |
|---|---|---|---|---|---|
| Age (median, range) | 61 (5‐91) | 61 (12‐88) | 61 (12‐91) | 61 (5‐87) | .406 |
| Sex (M/F, %) | 545/581 (48%/52%) | 152/146 (51%/49%) | 236/240 (50%/50%) | 139/187 (43%/57%) | .699 |
| Smoking history (Yes/No/NA, %) | 574/548/4 (51%/49%/0%) | 156/142/0 (52%/28%/0%) | 243/232/1 (51%/49%/0%) | 162/162/2 (50%/50%/0%) | .725 |
| Stage (I‐II/III, IV, recurrence/NE, %) | 355/667/104 (32%/59%/9%) | 64/218/16 (21%/73%/5%) | 143/285/48 (30%/60%/10%) | 147/152/27 (45%/47%/8%) | .002 |
| Prior‐treatment history (Yes/No, %) | 1023/103 (91%/9%) | 266/32 (89%/11%) | 426/50 (90%/10%) | 313/13 (96%/4%) | .918 |
| Prior‐surgery (Yes/No, %) | 892/232 (79%/21%) | 227/70 (76%/24%) | 372/103 (78%/22%) | 288/38 (88%/12%) | .541 |
| Prior‐radiotherapy (Yes/No, %) | 149/975 (13%/87%) | 45/252 (15%/85%) | 63/412 (13%/87%) | 37/289 (11%/89%) | .462 |
| Prior‐chemotherapy (Yes/No, %) | 339/787 (30%/70%) | 105/193 (35%/65%) | 128/348 (27%/73%) | 89/237 (27%/73%) | .014 |
| Prior‐chemotherapy line (1st line/2nd line/3rd line ‐, %) | 145/77/117 (43%/23%/32%) | 40/23/42 (38%/22%/40%) | 52/28/48 (41%/22%/38%) | 44/21/24 (48%/24%/28%) | |
| Prior‐cytotoxic chemotherapy (n, %) | 292 (86%) | 95 (90%) | 103 (80%) | 79 (89%) | |
| Prior‐tyrosine kinase inhibitor (n, %) | 84 (25%) | 23 (22%) | 43 (33%) | 15 (17%) | |
| Prior‐immune checkpoint inhibitor (n, %) | 42 (12%) | 14 (13%) | 18 (14%) | 9 (10%) | |
| Prior‐investigational new drug (n, %) | 22 (6%) | 7 (7%) | 8 (6%) | 7 (8%) | ‐ |
| Specimen type (Biopsy/Surgical) | 343/783 (30%/70%) | 99/199 (33%/67%) | 151/325 (32%/68%) | 67/259 (21%/79%) | .664 |
| Time to transplantation (Days, average, range) | 1.25 (0‐11) | 1.03 (0‐5) | 1.27 (0 ‐ 11) | 1.40 (0‐10) | .005 |
3.3. PDX establishment and engraftment rate
The specimens' establishment status is shown in Table 2, where the percentage of Passageable tumors of the Total Assessable Specimens was calculated as the engraftment rate. The engraftment rate was 27.1% overall, 36.4% in colorectal cancer, 22.7% in lung cancer, 22.9% in breast cancer, and 21.6% in rare cancer/sarcoma. As of May 31, 2020, there were 326 TG1 ongoing specimens, and the final engraftment rate is expected to be around 40%.
TABLE 2.
Engraftment results
| Total enrolled | Not assessable specimens | Total assessable specimens | Engraftment rate of assessable specimens | Passageable tumors | Discontinued |
Not yet assessable: TG1 ongoing |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TG2/3 ongoing | TG3 established | Failed to grow in TG1 | Failed to grow in TG2 | Murine tumor | Lymphoma | ||||||
| Colorectal cancer | 258 | 258 | 36.4% | 21 | 73 | 50 | 11 | 2 | 71 | 30 | |
| Lung cancer | 245 | 3 | 242 | 22.7% | 21 | 34 | 85 | 8 | 1 | 24 | 69 |
| Breast cancer | 70 | 70 | 22.9% | 8 | 8 | 28 | 2 | 3 | 21 | ||
| Gastric cancer | 54 | 1 | 53 | 17.0% | 5 | 4 | 11 | 12 | 21 | ||
| Ovarian cancer | 31 | 31 | 22.6% | 5 | 2 | 3 | 2 | 19 | |||
| Pancreatic cancer | 29 | 1 | 28 | 57.1% | 2 | 14 | 7 | 1 | 2 | 2 | |
| Esophageal cancer | 26 | 2 | 24 | 37.5% | 4 | 5 | 8 | 2 | 5 | ||
| Bile duct cancer | 23 | 2 | 21 | 33.3% | 1 | 6 | 2 | 4 | 1 | 7 | |
| Uterine body cancer | 23 | 23 | 30.4% | 7 | 1 | 1 | 14 | ||||
| Uterine cervix cancer | 13 | 13 | 30.8% | 2 | 2 | 1 | 1 | 1 | 6 | ||
| Leukemia | 12 | 7 | 5 | 0.0% | 4 | 1 | |||||
| Gallbladder cancer | 11 | 1 | 10 | 40.0% | 4 | 2 | 4 | ||||
| Lymphoma | 9 | 1 | 8 | 12.5% | 1 | 5 | 2 | ||||
| Hepatocellular carcinoma | 7 | 7 | 14.3% | 1 | 1 | 5 | |||||
| Myeloma | 3 | 3 | 0 | 0.0% | |||||||
| Oropharyngeal cancer | 2 | 2 | 50.0% | 1 | 1 | ||||||
| Renal cell cancer | 2 | 2 | 50.0% | 1 | 1 | ||||||
| Bladder cancer | 1 | 1 | 100.0% | 1 | |||||||
| Thyroid cancer | 1 | 1 | 0.0% | 1 | |||||||
| Rare cancer/Sarcoma | 306 | 5 | 301 | 21.6% | 20 | 45 | 84 | 11 | 5 | 16 | 120 |
| Total | 1126 | 26 | 1100 | 27.1% | 97 | 201 | 290 | 39 | 10 | 137 | 326 |
Bold indicate the important values.
4. Cases with multiple enrollments
Forty‐five cases had two specimens, and one case had three specimens enrolled in this study. Forty‐five cases with two specimens enrolled are summarized in Table S2. Fourteen cases had multiple specimens collected simultaneously (the same day or the next day), and 31 cases had specimens collected over time. Of the cases in which multiple specimens were collected simultaneously, four were from synchronous tumors and 11 were from different sites of the same tumor. Of the 31 cases collected over time, 17 were collected before and after chemotherapy for the same tumor, 11 were collected over time with no intermediate treatment, and three were collected from metachronous tumors. The patients who registered three specimens were combined resection of colorectal cancer and gastric cancer. They provided two specimens from colorectal cancer and one specimen from gastric cancer. Finally, 20 PDXs generated from 10 cases were able to be established in pairs over time.
5. Histopathological findings
Typical histopathological images for each carcinoma are shown in Figure S3. Overall, the histopathological structures of the PDX tumors were retained even after passaging up to TG3 compared with the original tumors.
Representative immunostaining images of lymphoma outgrowth and murine tumor are shown in Figure S4. Figure S4 A shows a tumor generated from a surgical specimen of colorectal cancer. In TG1, there were only a few CD45‐positive cells, but in TG2, all of the human cells were positive for CD45, and it was judged to be a lymphoma outgrowth. Figure S4B is a tumor generated from a biopsy specimen of pancreatic cancer, which showed almost no human COX IV–positive cells from TG1, and was judged to be a murine tumor.
6. Assessment of the time to the growth of PDX tumor
For the 201 specimens that could be established up to TG3, the TTV200 in each cancer type is shown in Figure 2A. Overall, the mean TTV200 in each passage was 72.9 days in TG1, 44.9 days in TG2, and 43.1 days in TG3. Figure S5 shows the tumor growth curves up to the passage at TG1, 2, and 3 for each cancer type. The TTP is shown in Figure 3B. The average TTP was 92.6, 64.3, and 61.3 days for TG1, TG2, and TG3, respectively, and 218.2 days for establishment.
FIGURE 2.
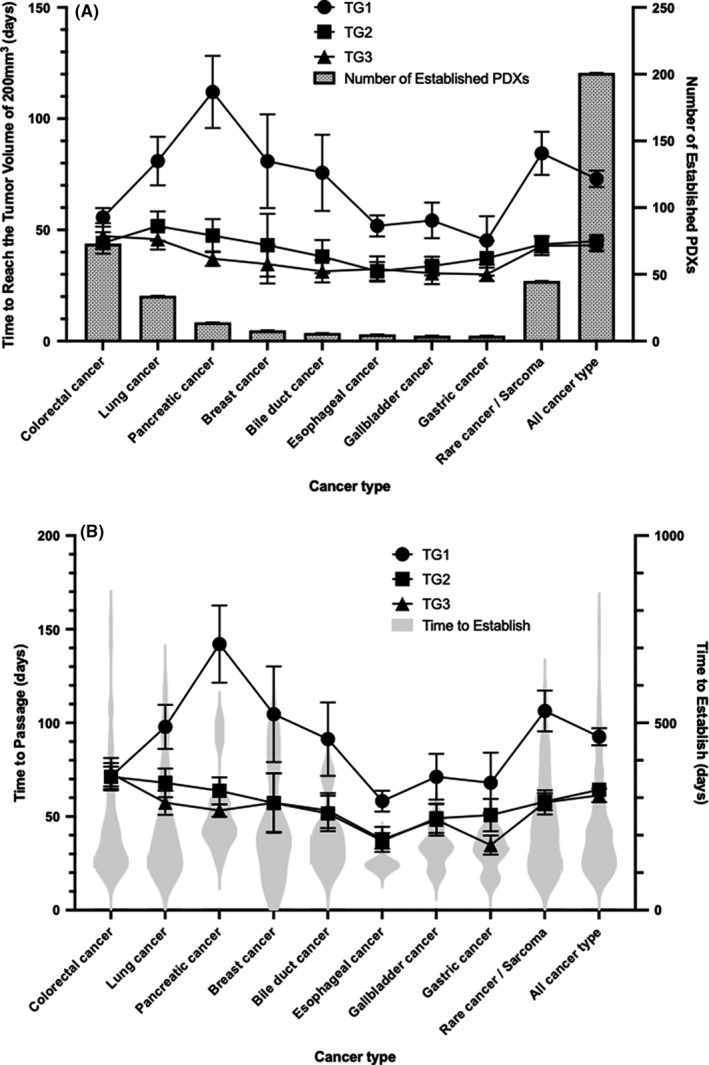
Time to reach tumor volume of 200 mm3 and time to passage. A, Time to reach tumor volume of 200 mm3 by cancer type. Only carcinomas that have been established in more than two PDXs are indicated. The line graph shows the number of days in each passage (TG1, 2, 3) and corresponds to the left y‐axis. The bars show the number of PDXs established for each carcinoma and correspond to the y‐axis on the right. B, Time to passage by cancer type. The line graph shows the number of days in each passage (TG1, 2, 3) and corresponds to the left y‐axis. The violin plot shows the time to establishment and corresponds to the right y‐axis
FIGURE 3.
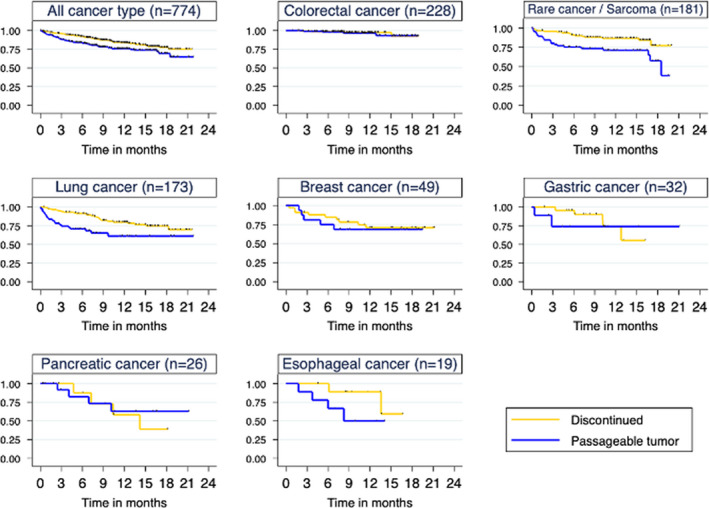
Comparison of survival of original patients with Discontinued and Passageable tumors. Discontinued is indicated by a yellow line and Passageable tumor by a blue line
7. Factors associated with PDX tumor growth
To explore factors affecting tumor growth in PDX, 774 specimens classified as Passageable tumor and Discontinued were examined in relation to their clinical background characteristics (Table 3): age, sex, specimen type (surgical vs biopsy), time to transplantation (with the median as the cutoff), stage (I, II vs III, IV, recurrence), PS (0 vs 1‐4), smoking history, cancer type (major cancer vs rare cancer/sarcoma), prior chemotherapy (naïve vs resistant), and OS (with the median as the cutoff). On univariate analysis, time to transplantation, stage, prior chemotherapy, and OS were found to be significant factors on univariate analysis, and the significance of stage and OS was confirmed on multivariate analysis (P = .046 and P = .001, respectively).
TABLE 3.
COX proportional hazards model for PDX engraftment
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Coefficients | 95% CI | P value | Coefficients | 95% CI | P value | |
| Sex (Female vs male) | 0.06 | −0.23‐0.35 | .699 | |||
| Age (75< vs ≥75) | −0.22 | −0.63‐0.19 | .299 | −0.29 | −0.89‐0.31 | .340 |
| Specimen (Surgical vs biopsy) | 0.07 | −0.24‐0.38 | .664 | −0.17 | −0.71‐0.36 | .528 |
| Time to transplantation (Short vs long) | −0.43 | −0.80‐−0.46 | .028 | −0.21 | −0.76‐0.35 | .463 |
| Stage (I, II vs III, IV, Rec) | 0.54 | 0.19‐0.88 | .002 | 0.54 | 0.01‐1.07 | .046 |
| PS (0 vs 1‐4) | 0.09 | −0.24‐0.42 | .587 | |||
| Smoking history (No vs yes) | 0.05 | −0.24‐0.34 | .725 | 0.09 | −0.35‐0.52 | .696 |
| Cancer type (Major cancer vs rare cancer) | −0.14 | −0.49‐0.20 | .414 | |||
| Prior‐chemotherapy (naïve vs resistant) | 0.39 | 0.08‐0.70 | .014 | 0.37 | −0.17‐0.91 | .181 |
| OS (Long vs short) | 4.19 | 3.63‐4.75 | .001 | 4.13 | 3.56‐4.70 | .001 |
Abbreviations: CI, confidence interval; OS, overall survival; PDX, patient‐derived xenograft; PS, performance status.
Bold italics indicate P value < .05.
8. Survival analysis of original patient and PDX engraftment status
To investigate the association between patient survival and PDX engraftment in detail, the differences in OS between Passageable tumors and Discontinued were examined using Kaplan‐Meier curves. In all, Passageable tumors had a shorter OS (P = .003), especially lung cancer and rare cancer/sarcoma (P = .004, respectively) (Figure 3). In addition, the data showed that the average time to establish was 218.2 days, and it was assumed that about 1 year would be required to start a drug efficacy study in the shortest time. For this reason, the survival rate of the original patients at 1 year was examined in Passageable tumors and Discontinued (Table S3). Overall, the 1‐year survival rate tended to be lower in Passageable tumors than in Discontinued (72.1% vs 81.9%), especially in rare, lung, and esophageal cancers.
In contrast, there was no difference in 1‐year survival rates for colorectal, breast, gastric, and pancreatic cancers. In stage IV, there was a trend toward lower 1‐year survival rates for Passageable tumors for lung and esophageal cancers, but it was similar to that of Discontinued in colorectal cancer. Among the rare cancers, rhabdomyosarcoma and leiomyosarcoma showed a high long‐term survival rate, whereas osteosarcoma, glioblastoma, and Ewing sarcoma showed a trend toward shorter survival.
9. DISCUSSION
Overall, as many as 1100 specimens from more than 50 cancer types were transplanted, and 298 PDXs were successfully generated. Approximately 60% of the enrolled patient population was in an advanced or recurrent stage, and more than 90% of patients had some prior treatment. In particular, 30% of the patients had a history of chemotherapy. The overall engraftment rate was 27.1%. Among carcinomas with more than 10 transplanted specimens, pancreatic cancer (57.1%), gallbladder cancer (40%), esophageal cancer (37.5%), and colorectal cancer (36.4%) had a high engraftment rate. There was a significant difference in the time to tumor growth of 200 mm3 in TG1 tumors by cancer type, although it was approximately 50 days in TG2 and TG3, regardless of the cancer type. Factors affecting the likelihood of tumor engraftment were identified as advanced or recurrent stage and short survival time after sample collection. A log‐rank test showed a shorter survival for Passageable tumors compared with Discontinued in all cancer types. There was a strong tendency for survival to be shorter for rare cancers and lung cancer, whereas there were no differences in survival for colorectal, breast, and pancreatic cancers. Similarly, there were no clear differences in 1‐year survival rates for colorectal, breast, gastric, and pancreatic cancers, regardless of successful engraftment.
This paper is the first report of large‐scale, systematic, and consecutive PDX establishment by a single institute in Japan. It was possible to enroll 1126 specimens in a short period of about 19 months, collecting a large number of specimens from a wide range of cancer types, and, of special note, 306 specimens of rare cancers were obtained. In addition, eight childhood cancers aged 0‐14 years and 108 cases of cancer in adolescents and young adults aged 15‐39 years were included. Patients with a history of chemotherapy were treated with various regimens, including cytotoxic anticancer agents, tyrosine kinase inhibitors, immune checkpoint inhibitors, and even investigational new drugs. Together with a large number of advanced‐stage or relapsed cases, this may have enriched the patient population for early clinical trials.
The overall engraftment rate was 27.1%, with rates of 36.4% for colorectal cancer, 22.7% for lung cancer, and 22.9% for breast cancer. In a previous large report by Izumochenko et al on PDX establishment using tumor specimens from 1163 cases, the overall engraftment rate was 49%, 10 with rates of 88% in melanoma, 85% in colorectal cancer, and 50% in lung cancer, much higher engraftment rates than in the present report. This may be partly due to the different types of tumors transplanted, the small amount of tumor samples used in the present study, and the fact that 30% of the specimens in the present report were under observation with TG1. In the present study, 80% of the specimens were transplanted by the next day, and 99% were transplanted within 4 days after collection, suggesting the advantage of being located close to the hospital and our laboratory. The failure rate of establishment was 43.3%, with 290 (290/1100, 26.4%) that failed to grow in TG1, 39 (39/810, 4.8%) that failed to grow in TG2, and 0 (0/771, 0%) that failed to grow in TG3, confirming the high probability of implantation after TG2 if TG1 was successfully grown.
The incidence of lymphoma outgrowth was 12.5%, with the highest incidence for colorectal cancer (27.5%), gastric cancer (21.1%), thymic carcinoma (16.7%), and lung cancer (9.9%). Reports on the incidence of lymphoma outgrowth varied widely, but they were generally comparable to previous reports. 19 , 24 , 25 , 26 , 27 , 28 Furthermore, some mice whose tumors were not grown in TG1 or TG2 showed dermatitis and rapid weight loss, and their autopsy findings showed the accumulation of human CD45‐positive cells in dermatitis sites and hepatosplenomegaly. These are thought to be part of the xenograft‐associated lymphoproliferative disorder (XALD) of mice caused by human lymphocytes in the transplanted tumor tissue. It has been reported that XALD has a significant effect on the PDX engraftment rate, and that pretreatment with rituximab is effective. 29 , 30 It is necessary to investigate the mechanism of XALD and how to prevent it in the future.
Different time to tumor growth has been reported in the past, but there has been no report of a single institute with multiple cancer types in a systematic manner, to the best of our knowledge. In the present study, the TTV200 and the TTP were evaluated as endpoints to assess differences among tumors. The median TTV200 was 57 days for TG1, 36 days for TG2, and 33 days for TG3. By cancer type, colorectal cancer showed a median TTV200 of approximately 40 days among passages, whereas pancreatic cancer showed a significant reduction in TTV200 to a median of 108.5 days in TG1, 39 days in TG2, and 32.5 days in TG3. Although the duration of TG1 tumor growth differed greatly depending on the carcinoma, TG2 and TG3 were found to be shortened to around 40 days. As tumor volume of 200 mm3 is one of the criteria for starting a drug efficacy study, this information may be important as preliminary information for planning a drug efficacy study.
An important challenge in PDX establishment is the inability to predict the growth of PDX tumors. 9 In fact, in the present study, a huge number of transplants (1100 transplants) have been performed, and 476 specimens have already failed as of May 31, 2020. This is a great disadvantage in terms of patient invasiveness, cost, and time. For this reason, the factors that affect the engraftment rate were examined. On multivariate analysis, stage and survival were the most influential factors, rather than specimen type, time to transplantation, and chemotherapy history. Furthermore, when engraftment rate and original patient survival time were examined by cancer type, especially for rare cancers and lung cancer, shorter survival times from sample collection were more likely to result in PDX engraftment success. Even when limiting to the stage III, IV, and recurrent cohorts, survival was shorter in Passageable tumors than in Discontinued, as has been previously reported in several cancer PDXs (P = .04, data not shown).
Coclinical trials are expected to be useful for selecting the right drugs and evaluating resistance mechanisms in individual patients by generating a PDX as a patient's avatar, and it can be considered the ultimate in personalized medicine (Figure S6). As we have shown in this study, the growth speed of PDX tumor increases with the passage, which may be due to the replacement of tumor stroma with mouse origin, the tumor's adaptation to the environment in the mice’s body, or selection of the tumor cells. Based on the average TTP in TG1 and TG2 (92.6 and 64.3 days, respectively) and the average TTV200 in TG3 (43.1 days), an average time of 200 days is required from transplantation to the start of the drug efficacy study. We think that about 1 year is necessary from transplantation to the end of the drug efficacy study. The present data shows that patients with colorectal, breast, gastric, and pancreatic cancers are more likely to be alive 1 year after PDX implantation (Figure 3, Table S2). Such types of cancer would be good candidates for coclinical trials. It is difficult to make a general statement about rare cancers, because the tumor doubling time varies greatly with each specific tumor type. Adenoid cystic carcinoma (ACC), for example, is a very slow‐growing tumor with a high 1‐year survival rate. However, ACC‐PDX tumors are very slow‐growing and require a longer time to initiate drug efficacy testing with a PDX. 31 Such slow‐growing tumors may not be suitable for coclinical trials because of their difficulty in responding to drugs and in evaluating efficacy. Lung cancer, for which anticancer agents have been progressing rapidly in recent years, has a low 1‐year survival rate overall. However, there is a high potential for long‐term survival with molecular‐targeted agents and immunotherapy. It has also been reported that the engraftment rates of lung cancer PDXs vary largely according to genetic mutation and histological subtype; thus, it is necessary to consider the feasibility of coclinical trials by looking at the survival rate according to individual molecular profiles. 13 , 17
We previously conducted a coclinical trial for patients with very poor prognostic histology in uterine cancer. As a high rate of postoperative recurrence was predicted in this population, we prepared for a coclinical trial by generating PDXs from surgical specimens in advance, and we successfully conducted a coclinical trial with multiple patients. We will conduct coclinical trials in collaboration with hospital physicians, basic researchers, and pharmaceutical company researchers with basic and clinical data such as histological type, molecular profile, therapeutic efficacy, and survival status in PDXs and the original patients. Moreover, we will use these data to validate the utility of PDXs and conduct drug discovery and development research using PDXs.
One of the issues we need to work on in the future is understanding the molecular profiles of original patient tumors and corresponding PDX tumors. It has been reported that different genetic mutations in lung cancer led to different PDX engraftment rates. 17 It has also been reported that copy number variations in PDX tumors increase with passaging, and that major genetic mutations in the original tumor are inherited. 32 , 33 The effects of these genetic changes associated with passaging in the original and PDX tumors on growth characteristics and drug sensitivity will need to be evaluated in the future.
A key challenge in using PDX for patient avatars and personalized medicine is that tumor characteristics are constantly changing in the patient's body. A PDX created from a treatment‐naïve tumor is expected to be different from the tumor status of a patient who has relapsed after chemotherapy. Conversely, PDXs generated from postchemotherapy tumors will be different from treatment‐naïve tumors. In addition, considering that it takes several months to a year to be able to evaluate the efficacy of PDXs and that the establishment rate is about 30%, there is a good chance that the patient's tumor characteristics will change while the PDXs are being established and that the PDXs cannot be established. In the future, it is necessary to study the possibility of improving the establishment rate and shortening the establishment period of PDXs, as well as to examine phenotypic changes with PDXs created over time in the same patient. In the J‐PDX, subcutaneous transplantation is used for all patients except breast cancer in order to standardize and unify the technique. It is known that the intratumor microenvironment and growth rate differ between orthotopic and xenotopic transplantation, and orthotopic transplantation is preferred to preserve tumor biology. Because of the difficulty of assessing tumor size in orthotopic transplantation, it is necessary to examine the differences in biology and drug response between orthotopic and xenotopic transplantation.
We hope to use the J‐PDX library to promote drug discovery and development research with pharmaceutical companies around the world, as well as biological research with academia and other researchers. However, standardization of evaluation methods is needed to predict the clinical effects in humans from PDX results. The number of PDX mice to be used, the method of drug administration, the timing of drug administration initiation, the number of passages to be used, the method of determining efficacy, and pharmacokinetics/pharmacodynamics considerations will all need to be developed with clinical efficacy as an endpoint. Our J‐PDX library has been constructed to focus on patients with advanced or recurrent stage and resistance to standard treatment. We have also compiled a database of clinical drug administration history and treatment response. This makes it a unique platform to contrast PDX results with those of the original patients.
We have successfully established a library of 300 Japanese cancer patient–derived PDXs as a platform for drug discovery and development in Japan. These data provide important information on survival rates of the original patients for planning coclinical trials. We will continue to actively promote coclinical trials to establish the use of PDXs and improve the probability of drug discovery and development (Figure S6).
DISCLOSURE
The authors declare no potential conflicts of interest.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Tab S1
Tab S2
Tab S3
ACKNOWLEDGEMENT
We are grateful to all the patients, oncologists, allied healthcare professionals, research concierges, and research assistants who participated in this study. We also thank Mr Hideaki Kakinuma from LSI Medience Corporation for maintaining PDX mice.
Yagishita S, Kato K, Takahashi M, et al. Characterization of the large‐scale Japanese patient‐derived xenograft (J‐PDX) library. Cancer Sci. 2021;112:2454–2466. 10.1111/cas.14899
Funding information
This work was partly supported by the Japan Agency for Medical Research and Development (AMED), Cyclic Innovation for Clinical Empowerment (CiCLE) under Grant Number 17pc0101011h0001, and National Cancer Center Research and Development Fund, Japan.
REFERENCES
- 1. Hidalgo M, Amant F, Biankin AV, et al. Patient‐derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998‐1013. 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aparicio S, Hidalgo M, Kung AL. Examining the utility of patient‐derived xenograft mouse models. Nat Rev Cancer. 2015;15(5):311‐316. 10.1038/nrc3944 [DOI] [PubMed] [Google Scholar]
- 3. Gao H, Korn JM, Ferretti S, et al. High‐throughput screening using patient‐derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21(11):1318‐1325. 10.1038/nm.3954 [DOI] [PubMed] [Google Scholar]
- 4. Paul SM, Mytelka DS, Dunwiddie CT, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9(3):203‐214. 10.1038/nrd3078 [DOI] [PubMed] [Google Scholar]
- 5. Mullard A. Parsing clinical success rates. Nat Rev Drug Discov. 2016;15(7):447. 10.1038/nrd.2016.136 [DOI] [PubMed] [Google Scholar]
- 6. Byrne AT, Alferez DG, Amant F, et al. Interrogating open issues in cancer precision medicine with patient‐derived xenografts. Nat Rev Cancer. 2017;17(4):254‐268. 10.1038/nrc.2016.140 [DOI] [PubMed] [Google Scholar]
- 7. Meehan TF, Conte N, Goldstein T, et al. PDX‐MI: minimal information for patient‐derived tumor xenograft models. Cancer Res. 2017;77(21):e62‐e66. 10.1158/0008-5472.CAN-17-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saito M, Shiraishi K, Kunitoh H, Takenoshita S, Yokota J, Kohno T. Gene aberrations for precision medicine against lung adenocarcinoma. Cancer Sci. 2016;107(6):713‐720. 10.1111/cas.12941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins AT, Lang SH. A systematic review of the validity of patient derived xenograft (PDX) models: the implications for translational research and personalised medicine. PeerJ. 2018;6:e5981. 10.7717/peerj.5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Izumchenko E, Paz K, Ciznadija D, et al. Patient‐derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28(10):2595‐2605. 10.1093/annonc/mdx416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient‐derived human breast cancer xenograft models. Cancer Res. 2013;73(15):4885‐4897. 10.1158/0008-5472.Can-12-4081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weroha SJ, Becker MA, Enderica‐Gonzalez S, et al. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014;20(5):1288‐1297. 10.1158/1078-0432.Ccr-13-2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart EL, Mascaux C, Pham NA, et al. Clinical utility of patient‐derived xenografts to determine biomarkers of prognosis and map resistance pathways in egfr‐mutant lung adenocarcinoma. J Clin Oncol. 2015;33(22):2472‐2480. 10.1200/jco.2014.60.1492 [DOI] [PubMed] [Google Scholar]
- 14. Marangoni E, Vincent‐Salomon A, Auger N, et al. A new model of patient tumor‐derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13(13):3989‐3998. 10.1158/1078-0432.Ccr-07-0078 [DOI] [PubMed] [Google Scholar]
- 15. Garrido‐Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma‐ related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res. 2011;17(17):5793‐5800. 10.1158/1078-0432.Ccr-11-0341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fichtner I, Slisow W, Gill J, et al. Anticancer drug response and expression of molecular markers in early‐passage xenotransplanted colon carcinomas. Eur J Cancer. 2004;40(2):298‐307. 10.1016/j.ejca.2003.10.011 [DOI] [PubMed] [Google Scholar]
- 17. Dong X, Guan J, English JC, et al. Patient‐derived first generation xenografts of non‐small cell lung cancers: promising tools for predicting drug responses for personalized chemotherapy. Clin Cancer Res. 2010;16(5):1442‐1451. 10.1158/1078-0432.Ccr-09-2878 [DOI] [PubMed] [Google Scholar]
- 18. Migliardi G, Sassi F, Torti D, et al. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient‐derived xenografts of RAS‐mutant colorectal carcinomas. Clin Cancer Res. 2012;18(9):2515‐2525. 10.1158/1078-0432.Ccr-11-2683 [DOI] [PubMed] [Google Scholar]
- 19. Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7(4):776‐790. 10.1016/j.molonc.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bertotti A, Migliardi G, Galimi F, et al. A molecularly annotated platform of patient‐derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab‐resistant colorectal cancer. Cancer Discov. 2011;1(6):508‐523. 10.1158/2159-8290.Cd-11-0109 [DOI] [PubMed] [Google Scholar]
- 21. Julien S, Merino‐Trigo A, Lacroix L, et al. Characterization of a large panel of patient‐derived tumor xenografts representing the clinical heterogeneity of human colorectal cancer. Clin Cancer Res. 2012;18(19):5314‐5328. 10.1158/1078-0432.Ccr-12-0372 [DOI] [PubMed] [Google Scholar]
- 22. Wallace J. Humane endpoints and cancer research. Ilar J. 2000;41(2):87‐93. 10.1093/ilar.41.2.87 [DOI] [PubMed] [Google Scholar]
- 23. Stokes WS. Humane endpoints for laboratory animals used in regulatory testing. Ilar J. 2002;43(Suppl):S31‐S38. [PubMed] [Google Scholar]
- 24. Wetterauer C, Vlajnic T, Schüler J, et al. Early development of human lymphomas in a prostate cancer xenograft program using triple knock‐out immunocompromised mice. Prostate. 2015;75(6):585‐592. 10.1002/pros.22939 [DOI] [PubMed] [Google Scholar]
- 25. John T, Kohler D, Pintilie M, et al. The ability to form primary tumor xenografts is predictive of increased risk of disease recurrence in early‐stage non‐small cell lung cancer. Clin Cancer Res. 2011;17(1):134‐141. 10.1158/1078-0432.Ccr-10-2224 [DOI] [PubMed] [Google Scholar]
- 26. Ilie M, Nunes M, Blot L, et al. Setting up a wide panel of patient‐derived tumor xenografts of non‐small cell lung cancer by improving the preanalytical steps. Cancer Med. 2015;4(2):201‐211. 10.1002/cam4.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bondarenko G, Ugolkov A, Rohan S, et al. Patient‐derived tumor xenografts are susceptible to formation of human lymphocytic tumors. Neoplasia. 2015;17(9):735‐741. 10.1016/j.neo.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mukohyama J, Iwakiri D, Zen Y, et al. Evaluation of the risk of lymphomagenesis in xenografts by the PCR‐based detection of EBV BamHI W region in patient cancer specimens. Oncotarget. 2016;7(31):50150‐50160. 10.18632/oncotarget.10322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler KA, Hou X, Becker MA, et al. Prevention of human lymphoproliferative tumor formation in ovarian cancer patient‐derived xenografts. Neoplasia. 2017;19(8):628‐636. 10.1016/j.neo.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Corso S, Cargnelutti M, Durando S, et al. Rituximab treatment prevents lymphoma onset in gastric cancer patient‐derived xenografts. Neoplasia. 2018;20(5):443‐455. 10.1016/j.neo.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morelli MP, Calvo E, Ordoñez E, et al. Prioritizing phase I treatment options through preclinical testing on personalized tumorgraft. J Clin Oncol. 2012;30(4):e45‐e48. 10.1200/jco.2011.36.9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ricci F, Bizzaro F, Cesca M, et al. Patient‐derived ovarian tumor xenografts recapitulate human clinicopathology and genetic alterations. Cancer Res. 2014;74(23):6980‐6990. 10.1158/0008-5472.CAN-14-0274 [DOI] [PubMed] [Google Scholar]
- 33. Ben‐David U, Ha G, Tseng YY, et al. Patient‐derived xenografts undergo mouse‐specific tumor evolution. Nat Genet. 2017;49(11):1567‐1575. 10.1038/ng.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Tab S1
Tab S2
Tab S3


