PURPOSE
The optimum number of treatment courses for younger patients with acute myeloid leukemia (AML) is uncertain. The United Kingdom National Cancer Research Institute AML17 trial randomly assigned patients who were not high risk to a total of three versus four courses.
PATIENTS AND METHODS
Patients received two induction courses based on daunorubicin and cytarabine (Ara-C), usually with gemtuzumab ozogamicin. Following remission, 1,017 patients were randomly assigned to a third course, MACE (amsacrine, Ara-C, and etoposide), plus a fourth course of MidAc (mitoxantrone and Ara-C) and following an amendment to one or two courses of high-dose Ara-C. Primary end points were cumulative incidence of relapse (CIR), relapse-free survival (RFS), and overall survival (OS). Outcomes were correlated with patient characteristics, mutations, cytogenetics, induction treatments, and measurable residual disease (MRD) postinduction.
RESULTS
In logrank analyses, CIR and RFS at 5 years were improved in recipients of four courses (50% v 58%: hazard ratio [HR] 0.81 [0.69-0.97], P = .02 and 43% v 36%: HR 0.83 [0.71-0.98], P = .03, respectively). While OS was not significantly better (63% v 57%: HR 0.84 [0.69-1.03], P = .09), the noninferiority of three courses to four courses was not established. The impact on relapse was only significant when the fourth course was Ara-C. In exploratory analyses, although MRD impacted survival, a fourth course had no effect in either MRD-positive or MRD-negative patients. A fourth course was beneficial in patients who lacked a mutation of FLT3 or NPM1, had < 3 mutations in other genes, or had a presenting WBC of < 10 × 109 L−1.
CONCLUSION
Although a fourth course of high-dose Ara-C reduced CIR and improved RFS, it did not result in a significant OS benefit. Subsets including those with favorable cytogenetics, those lacking a mutation of FLT3 or NPM1, or those with < 3 other mutations may derive survival benefit.
INTRODUCTION
A number of different induction schedules and combinations given to patients with acute myeloid leukemia (AML) will achieve morphological marrow blast clearance in 75%-85% of younger patients, arbitrarily defined as < 60 years of age.1-4 More intensive combinations often only require a single induction course.4 In remission, the risk of relapse is based on several factors including presenting WBC count, age, secondary disease, morphological response of the bone marrow if not in remission, cytogenetics, and mutation analysis.5,6 More recently, estimates of minimal residual disease (MRD) assessed after the first or second induction course have also been shown to be important for relapse risk,7-9 although further data are required to establish when the MRD status is predictive of what the optimal treatment should be. From the combined prognostic information, patients will be regarded as at high, intermediate, or low risk of relapse. Usually, the issue requiring clarification is whether a stem cell transplant should be recommended. Prospective studies have now demonstrated that for patients who have FLT3 mutation type 1 inhibitors, midostaurin10 but not lestaurtinib11 will reduce relapse risk and improve survival. Other recently approved drugs may deliver further reductions in relapse for particular subgroups.
CONTEXT
Key Objective
After two courses of induction treatment, patients who were in remission and not high risk were randomly assigned to one or two more courses of treatment.
Knowledge Generated
-
Patients given two courses of high-dose cytarabine (ie, a total of four courses) had a reduced cumulative incidence of relapse and an improved relapse-free survival but did not result in a significant benefit in overall survival.
The trial did not establish that three courses of chemotherapy were noninferior to the standard of care of four courses.
Although measurable residual disease (MRD) predicted the risk of relapse, overall administration of a fourth course had no survival benefit on either MRD-positive or MRD-negative patients.
Relevance
This study contributes information to the question of how much chemotherapy is required for younger patients with acute myeloid leukemia.
High-dose cytarabine (Ara-C) has been an established standard of care for consolidation following the landmark study of Mayer et al,12 who showed that a higher dose of 3 g/m2 was superior to 400 mg/m2 or 100 mg/m2 each for four courses after which four courses of maintenance were given.
United Kingdom trials have tried to establish the optimum number of consolidation courses since we explored giving two versus six courses in AML8.13 In order to explore the dose level of cytarabine and the total number of courses required, in our consecutive Medical Research Council (MRC) AML12 and 15 trials, we randomly assigned > 1,300 patients between four and five courses of total chemotherapy and established that adding a fifth course (Ara-C 1.5 g/m2) provided no additional benefit.14,15 We also demonstrated that patients randomly assigned to Ara-C 1.5 g/m2 for the third and fourth courses did not have a significantly inferior survival outcome to those receiving Ara-C 3 g/m2, either overall or in any risk subgroup.15 We now report the experience in the National Cancer Research Institute AML17 trial, which compared three courses versus four courses of total treatment for non–high-risk patients.
PATIENTS AND METHODS
The AML17 Trial
The United Kingdom MRC AML17 trial (ISRCTN55675535) tested a number of interventions for induction, which have already been reported.11,16-18 Briefly, patients of age from 18 years usually up to 60 years were initially randomly assigned to receive ADE (Ara-C, daunorubicin, and etoposide) or DA (daunorubicin and Ara-C) for the first two courses combined with gemtuzumab ozogamicin as a single dose of 3 mg/m2 or 6 mg/m2 in course 1, in which neither the addition of etoposide nor dosing of gemtuzumab ozogamicin at 6 mg/m2 improved the result.18 In a subsequent amendment, induction treatment was DA treatment where the daunorubicin dose in course 1 was either 60 mg/m2 or 90 mg/m2. In this comparison, no overall difference was found,4 but it later emerged that patients with a FLT3 mutation benefited from the dose of 90 mg/m2.19 Patients with high-risk myelodysplastic syndrome (defined as > 10% marrow blasts), with de novo or secondary AML, with any WHO performance score could be included, but the blast transformation of chronic myeloid leukemia and acute promyelocytic leukemia was excluded. After the first course of induction treatment, patients were designated as high-, intermediate-, or low-risk based on our validated weighted risk score,5,6 which is based on the presenting WBC count, age, cytogenetics, and secondary disease and is presented in detail in the Protocol (online only). High-risk patients were subjected to a separate random assignment of FLAG-Ida (fludarabine, Ara-C, granulocyte colony-stimulating factor, and idarubicin) versus DClo (daunorubicin and clofarabine) with the intention to proceed to transplantation, which has previously been reported,16 showing the superiority of FLAG-Ida in this setting. Intermediate-risk patients with a FLT3 mutation could enter a random assignment of the addition of the FLT3 inhibitor, lestaurtinib, or not, while other intermediate-risk patients without the FLT3 mutation could be randomly assigned to the addition of the mammalian target of Rapamicin inhibitor, everolimus, or not. The results of both interventions have previously been reported with neither addition showing overall benefit.11,17 After the two induction courses, all intermediate- and good-risk patients, whether receiving lestaurtinib or everolimus or not, were eligible to be randomly assigned to have one or two consolidation courses following the confirmation of remission (ie, three v four courses of treatment in total). This random assignment helped in recruiting patients from April 2009 to December 2014. Initially, until June 2010, the consolidation treatment random assignment for the third and fourth courses was between MACE (amsacrine, Ara-C, and etoposide) and MACE plus MidAc (mitoxantrone and Ara-C) (n = 120). In light of the results of the previous MRC AML15 trial, which compared MACE/MidAc with two courses of Ara-C,15 a subsequent protocol amendment changed the random assignment to one versus two courses of high-dose Ara-C (3 g/m2 twice a day days 1, 3, and 5) (n = 897). Random assignment took place after count recovery following the second induction course. The aim of the three versus four random assignment was to define if a fourth treatment course was necessary and whether the treatments involved were relevant. The trial flow diagram and details of drugs used are shown in Fig 1.
FIG 1.
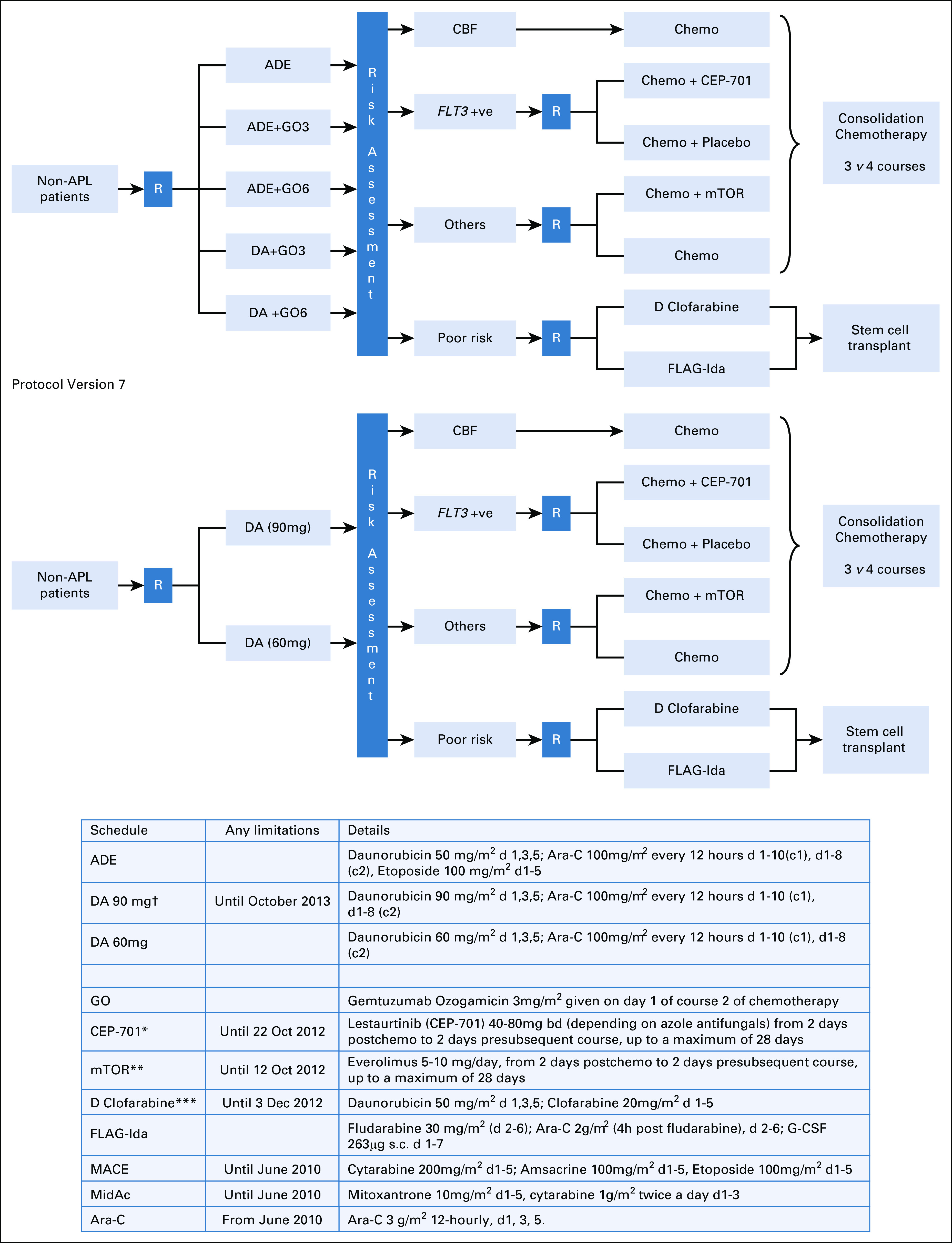
Protocol flow diagram. *Following closure of the CEP-701 randomly assigned, patients were guided by risk score to either poor risk or nonpoor risk options. **Following closure of the mTOR inhibition random assignment, patients in this group received DA 50mg alone. ***Following closure of the D Clofarabine arm, patients were recommended to receive FLAG-Ida (which was also the case if renal criteria were not met). †Following closure of the high-dose daunorubicin arm, patients were allocated DA60. ADE, Ara-C, daunorubicin, and etoposide; APL, acute promyelocytic leukemia; CBF, core binding factor; DA, daunorubicin and Ara-C; FLAG-Ida, fludarabine, Ara-C, granulocyte colony-stimulating factor, and idarubicin; GO, gemtuzumab ozogamicin; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C; mTor, mammalian target of Rapamicin.
Correlative Studies
Cytogenetic analyses were undertaken locally in laboratories that participate in the national quality assurance scheme, centrally reviewed, and classified according to our established criteria.20 Mutation analysis of the FLT3 and NPM1 status was performed in a single reference lab. Although not integral to therapeutic decisions in the trial, samples for MRD, which were not disclosed to investigators, were collected after each induction course and undertaken by flow cytometry in one of the two reference labs by methods previously described,8,21 and whole genome sequencing (Sanger sequencing) as described elsewhere22 of additional 82 genes was undertaken on 443 stored samples from participants in this random assignment at the Sanger Centre (Cambridge, United Kingdom). The FLT3 mutation status was provided during the trial to enable entry to the lestaurtinib random assignment.
Patients were randomly assigned in 110 centers in the United Kingdom, five in Denmark, and five in New Zealand. The trial was sponsored by Cardiff University and approved by the All Wales Research Ethics Committee on behalf of all UK investigators, by the Danish Medicines Agency for sites in Denmark, and by the New Zealand Medicines and Medical Devices Safety Agency for sites in New Zealand.
Written consent was obtained for each random assignment and for the storage of diagnostic samples. The trial was conducted in accordance with the Declaration of Helsinki.
Statistical Considerations and End Points
The primary outcome measure for this random assignment was overall survival (OS) at 5 years. It was anticipated that about 55% of patients who entered the whole AML17 trial would be available for the consolidation chemotherapy random assignment. The trial was anticipated to detect, with 90% power, a difference in survival from 55% to 65%, equivalent to a hazard ratio (HR) of 0.71; a critical number of 370 events was required to evaluate this difference. The primary question was whether three courses were noninferior to four courses, at a one-sided significance of 0.025; consequently, effect sizes are reported with 95% two-sided CIs throughout. Noninferiority would be concluded if the lower 95% CI bound was above 0.71. Toxicity (hematologic recovery times and nonhematologic toxicity) was scored using the National Cancer Institute Common Toxicity Criteria, Version 3, and resource use data (blood product support, days on antibiotics, and hospitalization) were collected. All end points were defined according to the revised International Working Group criteria,23 where OS and relapse-free survival (RFS) were measured from the point of random assignment.
The analyses are by intention to treat. Categorical end points (eg, OS) were compared using Mantel-Haenszel tests, giving Peto odds ratios (ORs) and CIs. Continuous/scale variables were analyzed by nonparametric (Wilcoxon rank-sum) tests. Time-to-event outcomes were analyzed using the logrank test, with Kaplan-Meier survival curves. Analyses adjusted for random assignment parameters are performed using the Cox regression and given in parallel to the assumption-free logrank approach. ORs/HRs < 1 indicate benefit for the extra course of chemotherapy. All survival percentages are at 5 years unless otherwise stated. The median follow-up at the time of final analysis was 55.1 months (range, 1.2-99.4 months).
In addition to overall analyses, exploratory analyses were performed stratified by the random assignment stratification parameters and other important variables, including correlations with MRD, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
RESULTS
Patient Characteristics
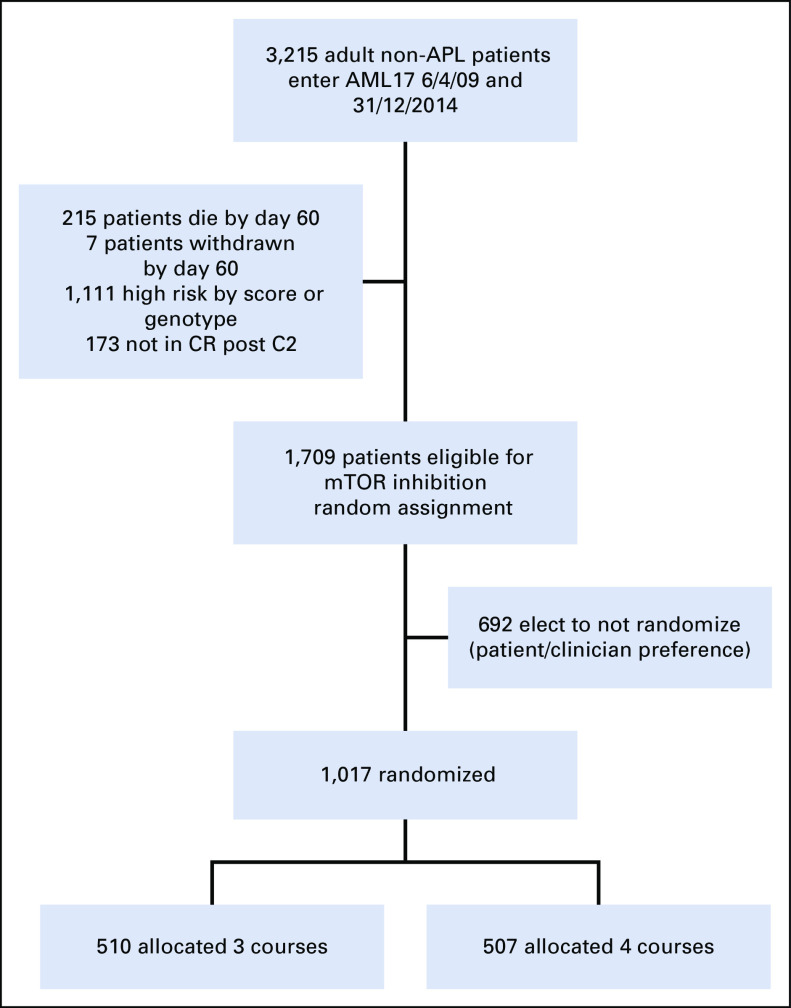
Between April 2009 and December 2014, a total of 1,709 patients, on recovery from induction course 2, were eligible for this random assignment of whom 1,017 (60%) were randomly assigned. The reasons for not being randomly assigned were only listed as patient or clinician preference. The interval between diagnosis and random assignment was 2.6 months (range, 1.4-5.2 months). Patients not entering the random assignment were generally similar but were less likely to be de novo AML, to have worse cytogenetics, and to have received DA60 in induction (Protocol); however, the OS at 5 years of those eligible who reached the median time of random assignment but did not enter the random assignment was 60%, which was the same for those who were randomly assigned (60%; P = .4). The characteristics and treatments of the randomly assigned patients are shown in Table 1. There were no differences between those randomly assigned with respect to age, sex, performance score, presenting WBC count, cytogenetic risk group, NPM1 status, induction treatments, and risk score or number given stem cell transplant overall or in CR1. There was a modest difference in 479 patients whose MRD status was known after course 1 or in 365 randomly assigned patients whose MRD status was known after course 2, with fewer patients allocated to three courses to be MRD-negative. As previously stated, the MRD status of patients was not made available to investigators. There was no difference in the frequency of mutations or in the numbers of patients with different numbers of mutations detected. The deployment of patients is shown in Fig 2.
TABLE 1.
Patient Characteristics

FIG 2.
CONSORT diagram. AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; CR, complete remission; mTor, mammalian target of Rapamicin.
Although the addition of a fourth course significantly reduced the cumulative incidence of relapse (CIR) and RFS, the OS difference at 5 years failed to reach significance.
CIR and RFS
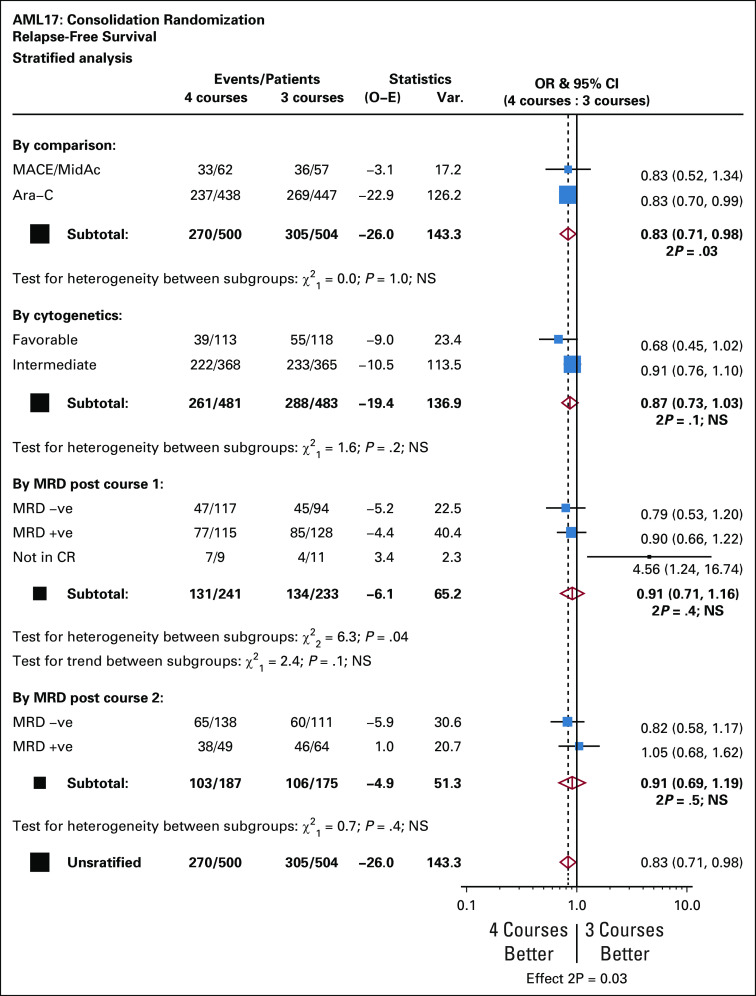
Overall, those allocated to the fourth course had a significantly lower CIR: 50% v 58% (raw HR 0.81 [0.69-0.97], P = .02; adjusted HR 0.82 [0.69-0.97], P = .02) (Data Supplement and Fig 3A). With respect to the treatment received, there was no significant interaction. While the effect on CIR was only significant with Ara-C as the fourth course (Figs 3B-3C), effect sizes are similar (0.82 [0.49-1.38] MACE/MidAc, v 0.81 [0.68-0.98] Ara-C). Similarly, while the effect appeared stronger in favorable-risk patients (0.67 [0.45-1.02]) than those with intermediate cytogenetics (0.90 [0.74-1.10]), there was no significant interaction (P = .2, Figs 3D-3E).
FIG 3.
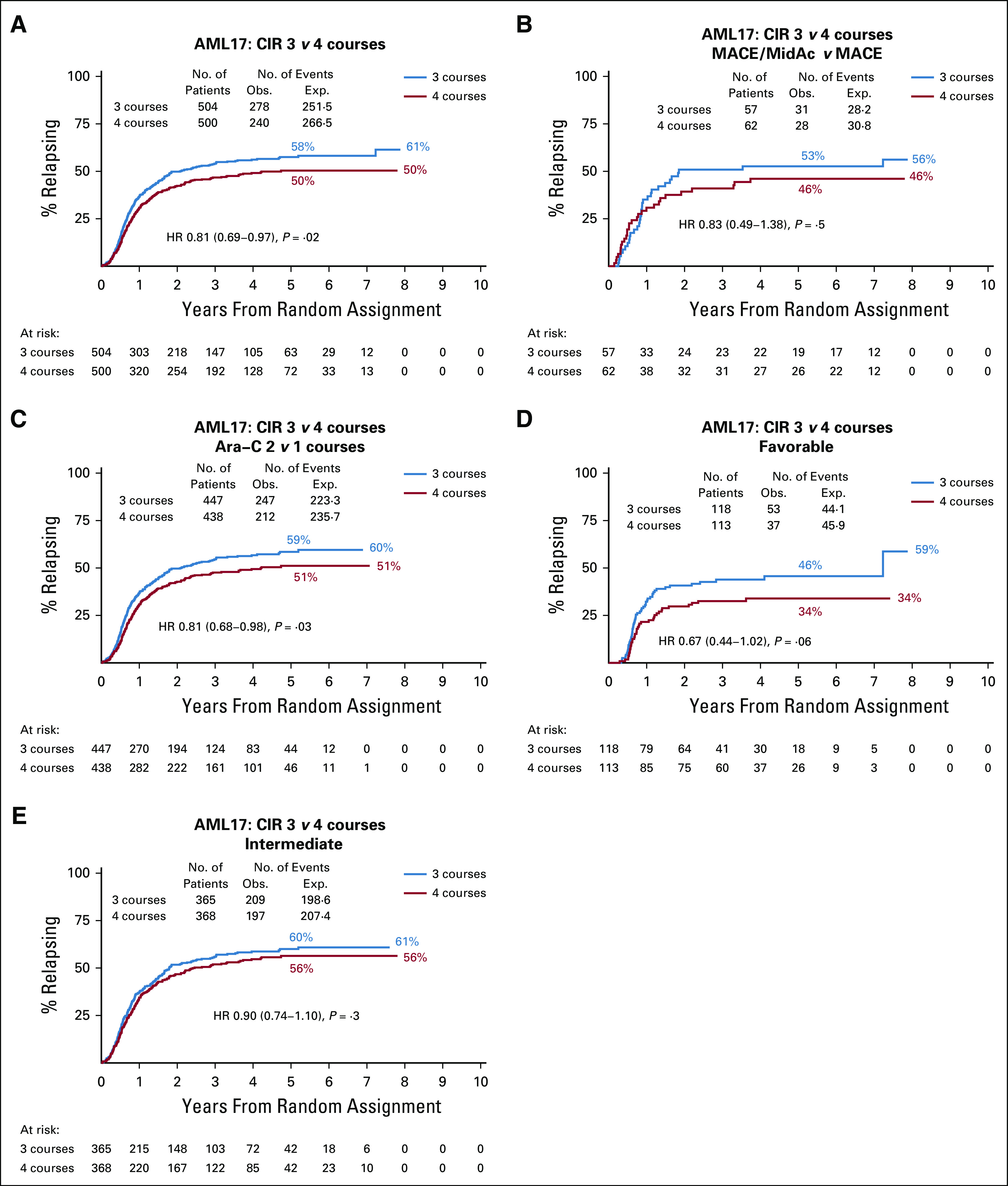
Cumulative incidence of relapse. AML, acute myeloid leukemia; CIR, cumulative incidence of relapse; HR, hazard ratio; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C.
Overall RFS differences, by treatment and risk groups, showed the same differences as CIR (Protocol), namely that there was benefit in a fourth course with respect to prevention of relapse.
Treatment Compliance
Of the 507 patients allocated to four courses, 74% received all intended courses, which resulted in a 67% survival, whereas for those allocated four but who only received three, survival was 54% (P = .002; HR, 0.58 [0.040-0.84]). The recipients of four courses required a median time of an extra 23 days of hospitalization, 9 days on antibiotics, 5.6 and 5.9 units of RBCs and platelets and 4 and 5 weeks for recovery of neutrophils and platelets, and had a 2% risk of death within 60 days.
OS
There was a nonsignificant difference in favor of four courses (63% v 56%) with respect to survival (Fig 4A). Considering the question of whether three courses were noninferior to four courses, the CI in both unadjusted and adjusted analyses crosses the threshold of 0.71, meaning that there is no evidence to conclude noninferiority at this threshold. In spite of the reduced CIR, there was no detectable survival difference in patients treated in the MACE/MidAc arm (Fig 4B), but there were nonsignificant differences in favor of four courses in patients treated in the Ara-C arms in both risk groups (Figs 4C-4E). If the 79 patients who received a transplant in CR1 are censored at transplant, the survival rates are 63% for three courses and 72% for four courses. The overall outcomes are summarized in the Protocol.
FIG 4.

Overall survival. AML, acute myeloid leukemia; HR, hazard ratio; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C.
Exploratory Subgroup Analyses
The outcome was not affected by any of the patients' characteristics or induction treatments (Protocol), although those presenting with a low WBC of < 10.0 × 109/L had a significant benefit from four courses. MRD information was obtained after course 1 in 456 patients and in 365 patients after course 2. The OS at 5 years in patients who were MRD-negative after the first or second induction courses (n = 464) at 73% was better than that in the MRD-positive patients (n = 357) at 50%. In patients who were assessed after course 1 of induction, the OS was not significantly different between the treatment arms, irrespective of the MRD status (Figs 5A-5B). In patients with MRD information after course 2, the OS was 69% if MRD-negative and 37% if MRD-positive, but again there was no significant difference in either groups if allocated to three or four courses (Figs 5C-5D).
FIG 5.

Effect of measurable residual disease (MRD). AML, acute myeloid leukemia; HR, hazard ratio.
Although there appears to be a significant advantage of four courses in patients who received daunorubicin 90 mg/m2 in induction (Protocol), the test for heterogeneity was not significant, suggesting that any apparent benefit is not conclusive.
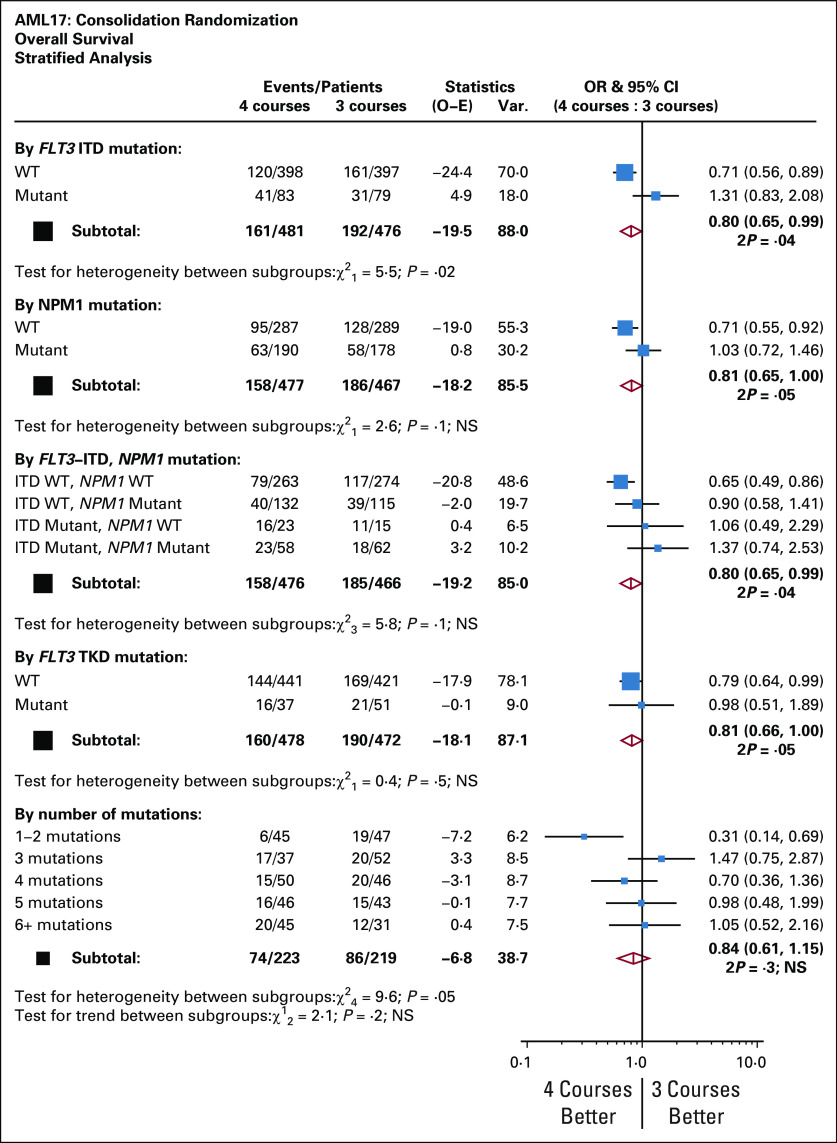
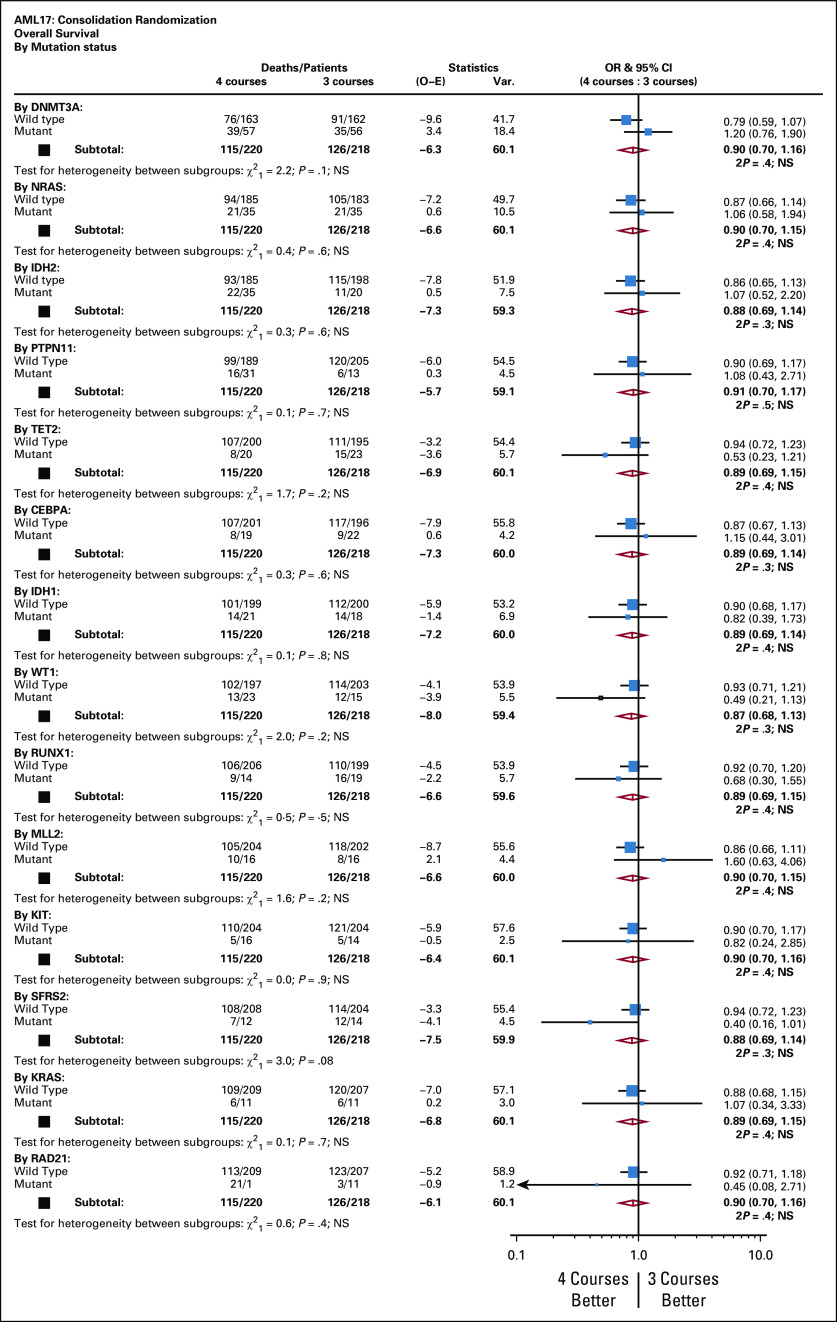
Four courses were significantly beneficial in patients without an FLT3 internal tandem duplication or tyrosine kinase domain or NPM1 mutation or in 92 of the 433 patients with < 3 mutations as detected using Sanger sequencing (Fig 6). The benefit appeared greatest in patients with FLT3/NMP1 wild type, although there was no significant interaction. For the purpose of assessing the prognostic value of the mutations detected using Sanger sequencing, only mutations that occurred in more than 20 of the 433 patients were considered, but no correlations were observed (Protocol).
FIG 6.
Analysis stratified by mutation status. AML, acute myeloid leukemia; ITD, internal tandem duplication; OR, odds ratio; TKD, tyrosine kinase domain; WT, wild type.
DISCUSSION
The recent approvals of new drugs for AML may move the treatment algorithm in the relevant subgroups. However, it is yet to be established for some of these new drugs whether combination with standard chemotherapy may be their optimal use.24 It therefore remains important to define the optimal total treatment with chemotherapy that is required. There has been extensive effort to establish the best agent and doses for induction, but less attention to the dose and number of courses of postinduction treatment. Recruiting sufficiently large numbers to reliably answer questions at this stage of treatment is a logistical challenge. Definitive studies 25 years ago established high-dose Ara-C as the standard of care for up to four courses at a dose level of 3 g/m2. The MRC AML15 trial (ISRCTN17161961) established that our previous standard of care (MACE plus MidAc) was superior to high-dose Ara-C in adverse-risk patients.15 There was little survival difference between 3 g/m2 and 1.5 g/m2 Ara-C dose levels. The addition of a fifth course was tested in the MRC AML12 and 15 trials14,15 with no evidence of benefit for a fifth course. A number of collaborative group trials have assessed the number of courses without providing a universally accepted conclusion.25-27
In this trial, random assignment took place after the completion of two induction courses and 1,709 (53%) of the original trial entrants were eligible. In the intent-to-treat analysis of the 1,017 patients randomly assigned, it emerged that the addition of the fourth course significantly reduced the CIR and significantly improved the RFS, but the trend for better OS was not significant. This was the case in the 885 patients who received exclusively Ara-C. Because of carry over random assignment from our AML15 trial, 120 patients received MACE or MACE plus MidAc among whom there was a similar trend for reduction in CIR and improvement in RFS, but neither of which reached statistical significance. Although the numbers become too small for confidence, the benefit (CIR and RFS) of the fourth course is more obvious in the favorable-risk group. In terms of OS, based on the number of patients assessable, there is a strong trend for benefit in the recipients of Ara-C, but not for the recipients of MACE/MidAc.
Among the several subgroups examined where a significant difference was observed in conjunction with a test for heterogeneity, which reached significance, were patients who lacked a FLT3 or NPM1 mutation, patients with one or two mutations compared with > 2, and patients with presenting WBCs of < 10 × 109/L. Patients who were MRD-negative (at a level of 1 in 104) after course 1 or 2 had a significantly better survival than those who were positive at either time point, but the addition of a fourth course was not beneficial in either group. In general, patients with more favorable characteristics appear to benefit from a fourth course, but only when high dose Ara-C consolidation is used, whereas those with intermediate-risk characteristics do not, although these were only trends for benefit.
There was a price to pay for the fourth course with respect to days in hospital, days on antibiotics, and blood product support although there were no excessive deaths in remission. However, as a consequence of the greater number of relapses, more salvage transplants were required in patients receiving just three courses (Table 1). It could be speculated that the most useful interaction will eventually be the initial discrimination based on the MRD status after the first or second induction course, where there may be little benefit in a fourth course for those who are MRD-positive, but benefit for those who are negative, or vice versa.
Overall, this experience suggests that if Ara-C is the chosen consolidation treatment, a fourth course of overall treatment is probably beneficial. In a retrospective analysis of the induction in the AML15 trial,15 patients who received two courses of FLAG-Ida as induction without consolidation had a similar survival to control patients who received two induction courses of DA treatment followed by two courses of consolidation. These were nonrandomized data and are currently being tested in the ongoing AML19 trial (ISRCTN78449203).
ACKNOWLEDGMENT
We would like to thank Cancer Research UK for research support and the Cardiff University Haematology Clinical Trials Unit staff for supervision of the trial and the staff of the Sanger Centre, Cambridge, for undertaking sequencing under contract with the sponsor.
APPENDIX
The following investigators recruited patients: Aalborg Hospital: Maria Kallenbach; Aarhus University Hospital: Hans Beier Ommen, Ingolf Molle, Jan Maxwell Norgaard, Mette Holm; Arrowe Park Hospital: Ranjit Dasgupta; Auckland City Hospital: Leanne Berkhan, Lucy Pemberton, Nigel Patton, Richard Doocey, Sophie Lee, Timothy Hawkins; Ayr Hospital: Fiona Cutler, Paul Eynaud; Barnet General Hospital: Andres Virchis, Sylvia Berney; Barts and the London NHS Trust: Jamie Cavenagh, Matthew Smith, Simon Hallam, Heather Oakervee; Basingstoke and North Hampshire Foundation NHS Trust: Ashok Roy, Sylwia Simpson; Beatson West of Scotland Cancer Center: Anne Parker, Mark Drummond, Mike Leach, Pam McKay, Richard Soutar; Belfast City Hospital: Claire Arnold, Damian Finnegan, Mary Frances McMullin, Robert Cuthbert, Victoria Pechey; Birmingham Heartlands Hospital: Donald Milligan, Guy Pratt, Manos Nikolousis, Matthew Lumley, Neil Smith, Richard Lovell, Shankara Paneesha; Blackpool Victoria Hospital NHS Foundation Trust: Paul Cahalin; Borders General Hospital: John Tucker; Bradford Royal infirmary: Adrian Wiliams, Anita Hill, Lisa Newton, Nitin Sood, Sam Ackroyd; Bristol Haematology and Oncology Center: David Marks, Graham Standen, Jenny Bird, Priyanka Mehta, Roger Evely; Cambridge University Hospitals NHS Foundation Trust: Charles Crawley, George Follows, Jenny Craig, Pramila Krishnamurthy; Cheltenham General Hospital: Adam Rye, Richard Lush; Chesterfield Royal Hospital: Andrew Fletcher, Mark Wodzinski, Peter Toth, Robert Cutting; Christchurch Hospital: Andrew Butler, Mark Smith, Peter Ganly, Ruth Spearing, Steve Gibbons; Christie Hospital NHS Trust: Mike Dennis; Countess of Chester hospital: Salah Tuegar; Crosshouse Hospital: Julie Gillies, Mark McColl, Peter Maclean; Darent Valley Hospital: Anil Kamat, Raphael Ezekwesili; Derby Hospitals NHS Foundation Trust: Christopher Millar, David Allotey, Juanah Addada; Derriford Hospital: Hannah Hunter, Patrick Medd, Simon Rule, Tim Nokes, Wayne Thomas; Doncaster Royal Infirmary: Joe Joseph, Robert Cutting, Stuti Kaul, Youssef Sorour; Dorset County Hospital NHS Foundation Trust: Akeel Moosa; Dunedin Hospital: Annette Neylon, Hilda Mangos; East Kent Hospitals University NHS Foundation Trust: Jindriska Lindsay, Kamiran Saied, Christopher Pocock, Vijay Ratnayake; East Sussex Hospitals NHS Trust: Judy Beard, Satyajit Sahu; Epsom and St Helier University NHS Trust: Jane Mercieca; Falkirk and District Royal Infirmary: Christopher Brammer, Marie Hughes, Roderick Neilson; Glan Clwyd Hospital: Christine Hoyle, Earnest Heartin, Margaret Goodrick; Gloucestershire Royal Hospital: Adam Rye, Eve Blundell, Rebecca Frewin, Sally Chown; Great Western Hospital: Alex Sternberg, Atherton Gray, Norbert Blessing; Guys and St Thomas' Foundation Trust: Kavita Raj, Robert Carr; Hammersmith Hospital: Jiri Pavlu; Heatherwood and Wexham Park NHS foundation Trust: Mark Offer, Nicola Bienz, Nicola Philpott, Simon Moule; Hereford County Hospital: Lisa Robinson, Sara Willoughby; Herlev Hospital: Morten Krogh Jensen, Peter Moller, Ulrik Overgaard; Hillingdon Hospital: Riaz Janmohamed, Richard Kaczmarski; Hull Royal Infirmary: Christopher Carter, Sahra Ali; Ipswich Hospital NHS Trust: Andrew Hodson, Debo Ademokun; James Cook University Hospital: Angela Wood, Ray Dang; James Paget University Hospital: Cesar Gomez, Manzoor Mangi, Shala Sadullah; John Radcliffe Hospital: Paresh Vyas; Kettering General hospital: Isaac Wilson-Morkeh, Karyn Longmuir, Mark Kwan, Matthew Lyttelten; Leicester Royal Infirmary: Ann Hunter, Kaljit Bhuller, Murray Martin; Lincoln County Hospital: Kandeepan Saravanamuttu; Maidstone Hospital: Evangelia Dimitriadou, Richard Gale; Manchester Royal Infirmary: Eleni Tholouli, Guy Lucas, John Yin, Sarah Burns; Medway Maritime Hospital: Maadh Aldouri, Vivienne Andrews; Milton Keynes Hospital NHS Foundation Trust: Moez Dungarwalla; Monklands Hospital: Alaeddin Raafat, Charlotte Thomas, Jane Laird, John Murphy, Pamela Paterson; NHS Grampian: Dominic Culligan, Jane Tighe; New Cross Hospital: Abraham Jacob, Alan MacWhanell, Sunil Hada, Supratik Basu; Ninewells Hospital and Medical Center: Keith Gelly, Sudhir Tauro; Norfolk and Norwich University Hospital NHS Foundation Trust: Matthew Lawes; North Middlesex University Hospital: Neil Rabin; Northampton General Hospital: Angela Bowen, Sajan Mittal, Suchitra Krishnamurthy; Northwick Park Hospital: Nicki Panoskaltsis, Robert Ayto; Nottingham University Hospitals NHS Trust: Emma Dasgupta, Jenny Byrne, Nigel Russell, Simone Stokley; Odense University Hospital: Claus Marcher, Lone Friis, Poul Gram Hansen; Palmerston North Hospital: Bart Baker, Paul Harper; Peterborough District Hospital: Kanchan Rege, Muthuswamy Sivakumaran, Sateesh Nagumantry; Pinderfield General Hospital: David Wright, Kavita Patil, Mary Chapple, Paul Moreton; Poole General Hospital: Fergus Jack, Ram Jayaprakash; Princess Royal University Hospital: Bipin Vadhir; Queen Alexandra Hospital: Christopher Jones, Helen Dignum, Mary Ganczakowski, Robert Corser, Tanya Cranfield; Queen Elizabeth Hospital: Charles Craddock, Jim Murray; Queen Elizabeth Hospital Woolwich: Ana Duran; Queen Elizabeth Hospital, Norfolk: Jane Keidan, Lisa Cooke; Queens Hospital: Claire Hemmaway, Jane Stevens; Raigmore Hospital NHS Highland: Chris Lush, Joanne Craig, Peter Forsyth; Rigshospitalet: Carsten Niemann, Lars Kjeldsen, Ole Wei Bjerrum, Ove Juul Nielsen, Peter Kampmann; Rotherham General Hospital: Arun Alfred, Richard Went; Royal Berkshire Hospital: Henri Groch, Rebecca Sampson, Stuart Mucklow; Royal Bournemouth Hospital: Joseph Chacko, Rachel Hall; Royal Cornwall Hospital: Bryson Pottinger; Royal Devon and Exeter Hospital: Anthony Todd, Claudius Rudin, Loretta Ngu, Malcolm Hamilton; Royal Free Hospital: Archie Prentice, Panos Kottaridis; Royal Hallamshire Hospital: Chris Dalley, Harpreet Kaur, John Snowden, Sameer Tulpule; Royal Marsden Hospital: David Taussig, Mark Ethell; Royal Oldham: Allameddine Allameddine, David Osborne, Hayley Greenfield, Sumaya Elhanash, Vivek Sen; Royal Shrewsbury Hospital: Andreea Corcoz; Royal Surrey County Hospital: Elisabeth Grey-Davies, Johannes DeVos, Louise Hendry; Royal United Hospital: Charles Singer, Christopher Knechtli, Josephine Crowe; Russells Hall Hospital: Jeff Neilson, Savio Fernandes, Stephen Jenkins; Salford Royal Hospital: John Houghton, Rowena Thomas-Dewing, Simon Jowitt, Sonya Zaman; Salisbury Hospital NHS Foundation: Effie Grand, Jonathan Cullis, Louise Fraser, Tamara Everington; Sandwell Hospital: Farooq Wandoo, Yasmin Hasan; Singleton Hospital: Hamdi Sati, Saad Ismail; South Devon Healthcare NHS Foundation Trust: Deborah Turner, Nicholas Rymes, Patrick Roberts, Steve Smith; Southampton University Hospital NHS Trust: Deborah Richardson, Kim Orchard, Matthew Jenner; Southern General Hospital: Anne Morrison, Ian Macdonald; St Helens and Knowsley NHS Trust: Toby Nicholson; St James University Hospital: David Bowen; St Richards Hospital: Phillip Bevan, Sarah Janes; Stafford Hospital: Andrew Amos, Aurangzeb Razzak; Stoke Mandeville Hospital: Anne-Marie O'Hea, Jonathan Pattinson, Robin Aitchison; Sunderland Royal Hospital: Annette Nicolle, Scott Marshall, Yogesh Upadhye; Taunton and Somerset Foundation Trust: Sarah Allford, Simon Bolam; The Newcastle upon Tyne NHS Foundation Trust: Anne Lennard, Chris Williams, Gail Jones, Graham Jackson; University College London Hospitals: Andres Virchis, Asim Khwaja, Kwee Yong, Nishal Patel; University Hospital Aintree: Arpad Toth, Barbara Hammer, Barrie Woodcock, Lynny Yung, Walid Sadik; University Hospital Coventry and Warwickshire NHS Trust: Anand Lokare, Anton Borg, Beth Harrison, Mekkali Narayanan, Nicholas Jackson, Oliver Chapman, Sarah Nicolle, Shailesh Jobanputra, Syed Bokhari; University Hospital Lewisham: Naheed Mir; University Hospital of North Staffordshire NHS Trust: Deepak Chandra, Kamaraj Karunanithi, Neil Phillips, Richard Chasty, Srinivas Pillai; University Hospital of Wales: Caroline Alvares, Jonathan Kell, Steve Knapper; University Hospital of North Tees and Hartlepool: Philip Mounter, Philip Saunders, Zor Maung; University of Liverpool and Royal Liverpool University Hospital: Rahuman Salim, Richard Clark; Victoria Hospital NHS Fife: Kerri Davidson, Peter Williamson, Stephen Rogers; Waikato Hospital: Gillian Corbett, Humphrey Pullon, Shahid Islam; Wellington Hospital: Alwyn D'Souza, Huib Buyck, John Carter, Kentneth Romeril; Western General Hospital: PH Roddie, Peter Johnson; Wishaw General Hospital: Annielle Hung, Gila Helenglass; Worcestershire Royal Hospital: Elizabeth Maughan, Juliet Mills, Salim Shafeek; Worthing Hospital: Santosh Narat; York Hospital: Laura Munro, Lee Bond, Martin Howard; Ysbyty Gwynedd: David Edwards, James Seale.
FIG A1.

Patients' score derived from multiplying each relevant characteristic by the value derived from the Cox model: 0.01325*age (in years) + 0.16994*sex (1 = male, 0 = female) + 0.22131*diagnosis (1 = de novo, 2 secondary) + 0.65082*cytogenetics (1 = favorable, 2 = intermediate, 3 adverse) + 0.19529*status post C1 (1 = complete remission, 2 = partial remission, 3 = no response) + 0.00169* WBC (x109/l). Distribution of patients in MRC AML10,12 trials by index: Taking into account the apparent bimodality of the curve, patients with an index of 2 or below were deemed good risk, and the data were arbitrarily divided at the 75th centile between standard and poor risk. Survival index from complete remission in AML10,12 according to the risk groups was validated on data from MRC AML15 Trial.5
FIG A2.
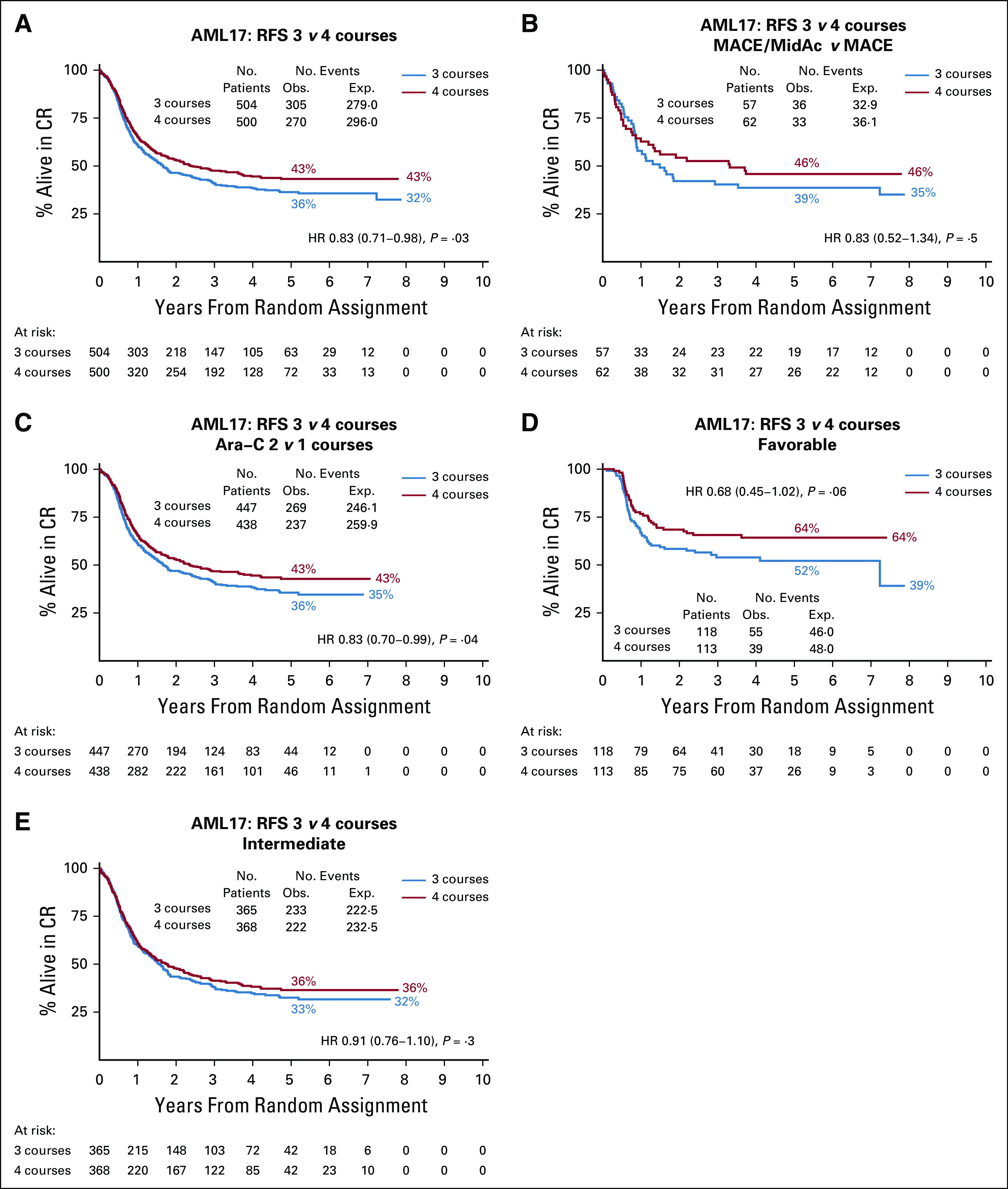
Relapse-free survival (RFS). AML, acute myeloid leukemia; CR, complete remission; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C.
FIG A3.
Stratified analysis of relapse-free survival. AML, acute myeloid leukemia; CR, complete remission; MACE, amsacrine, Ara-C, and etoposide; MidAc, mitoxantrone and Ara-C; MRD, measurable residual disease; OR, odds ratio.
FIG A4.
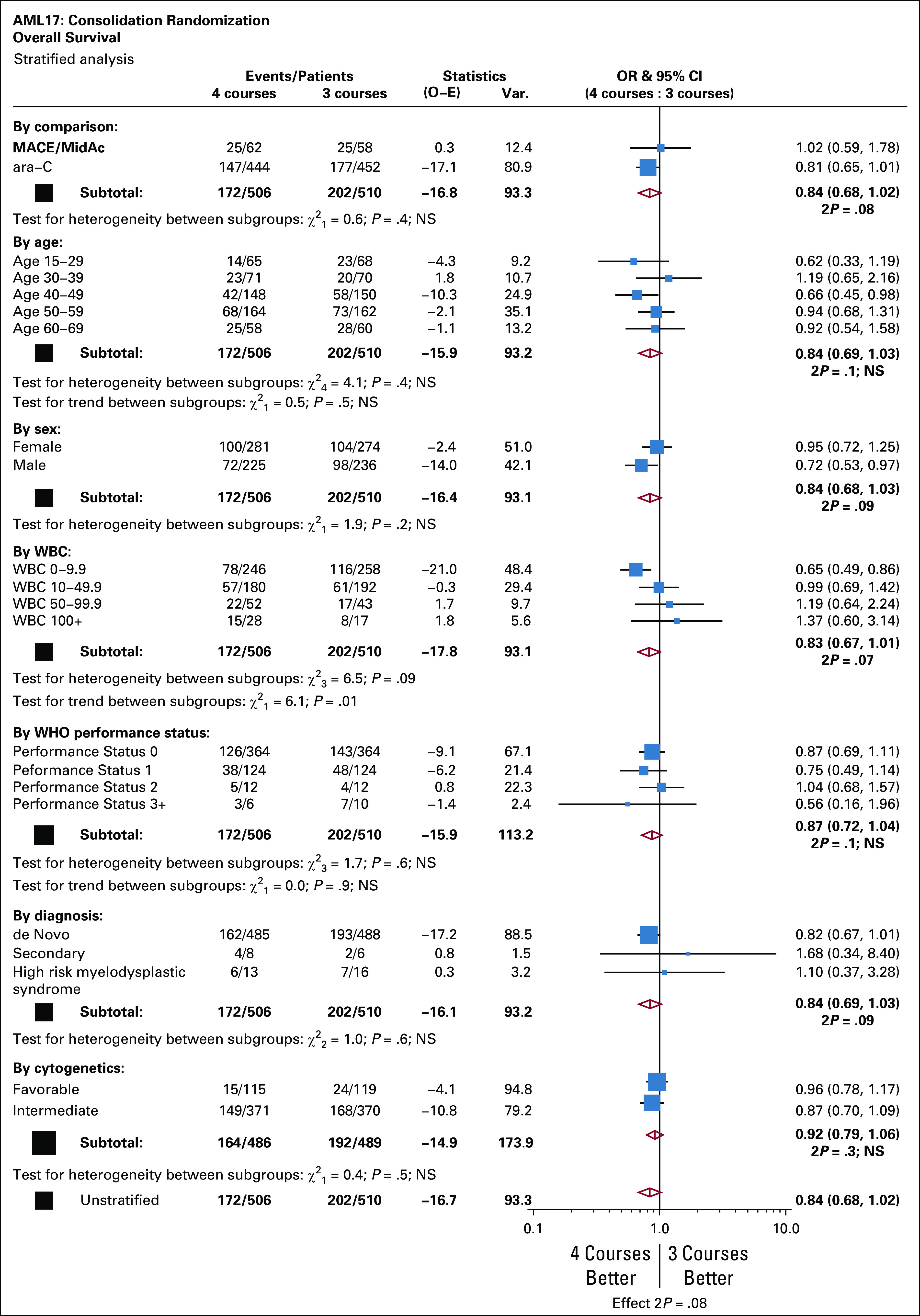
Stratified analysis of overall survival. AML, acute myeloid leukemia; CR, complete remission; MACE, amsacrine, Ara-C, and etoposide; MidAC, mitoxantrone and Ara-C; OR, odds ratio.
FIG A5.
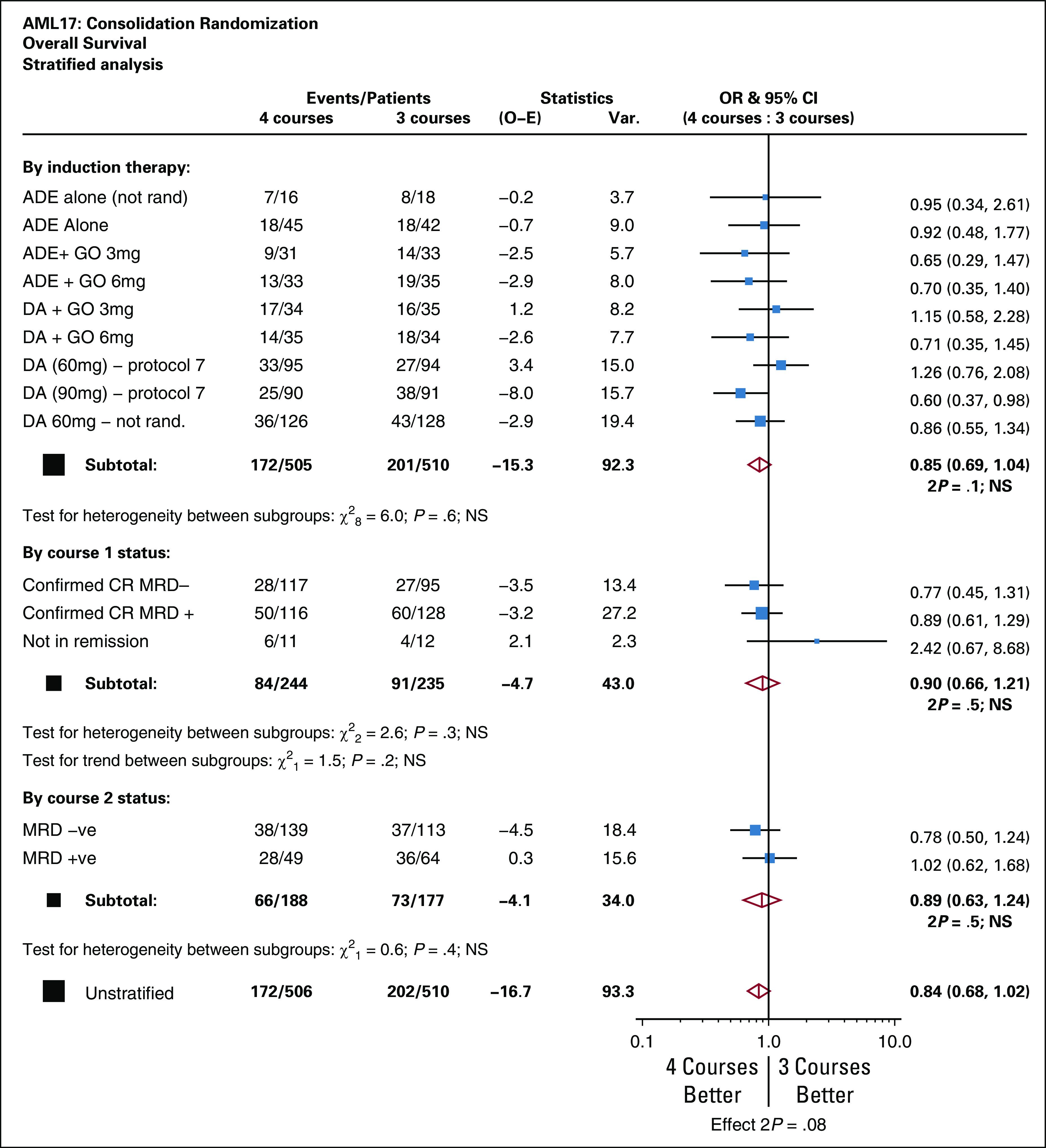
Analysis stratified by prior treatment and MRD status. ADE, Ara-C, daunorubicin, and etoposide; AML, acute myeloid leukemia; CR, complete remission; DA, daunorubicin and Ara-C; GO, gemtuzumab ozogamicin; MACE, amsacrine, Ara-C, and etoposide; MidAC, mitoxantrone and Ara-C; MRD, measurable residual disease; OR, odds ratio.
FIG A6.
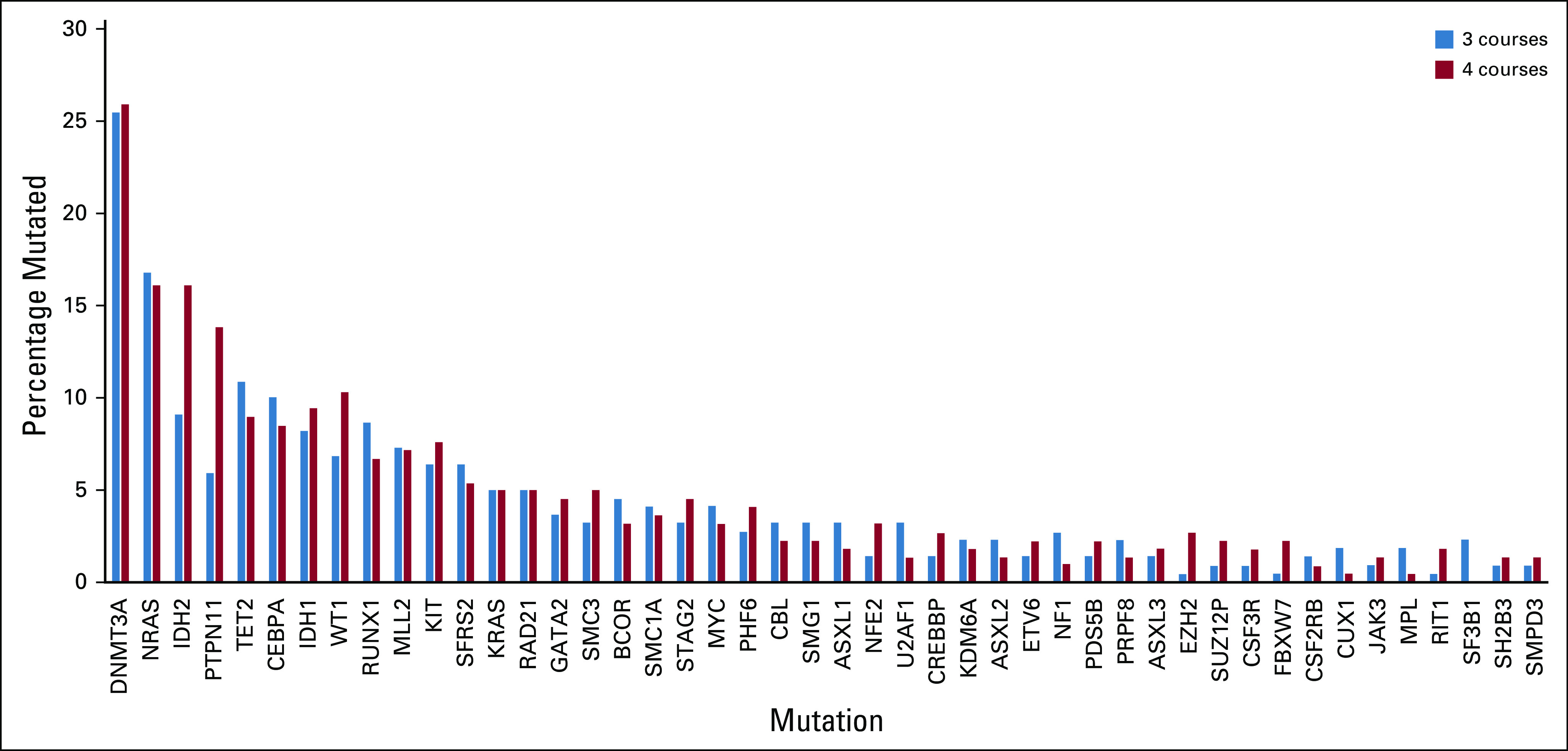
Sanger sequencing.
FIG A7.
Analysis stratified by Sanger sequencing. AML, acute myeloid leukemia; OR, odds ratio.
TABLE A1.
Patient Characteristics of Those Eligible by Entry to Random Assignment (n = 1017) or Not (n = 692)
TABLE A2.
Clinical Outcomes by Treatment Armback
See accompanying Oncology Grand Rounds on page 870
CLINICAL TRIAL INFORMATION
ISRCTN55675535 (AML17)
AUTHOR CONTRIBUTIONS
Conception and design: Alan K. Burnett, Nigel H. Russell, Robert K. Hills
Administrative support: Ian F. Thomas
Provision of study materials or patients: Nigel H. Russell, Stephen Knapper, Brian Huntly, Richard E. Clark, Lars Kjeldsen, Mary Frances McMullin, Mark Drummond, Jonathan Kell, Ruth Spearing
Collection and assembly of data: Robert K. Hills, Ian F. Thomas
Data analysis and interpretation: Alan K. Burnett, Nigel H. Russell, Robert K. Hills, Sylvie Freeman
Manuscript writing: Alan K. Burnett, Nigel H. Russell
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Defining the Optimal Total Number of Chemotherapy Courses in Younger Patients With Acute Myeloid Leukemia: A Comparison of Three Versus Four Courses
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nigel H. Russell
Honoraria: Jazz Pharmaceuticals, Pfizer, Astellas Pharma
Research Funding: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Stephen Knapper
Honoraria: Novartis, Jazz Pharmaceuticals
Consulting or Advisory Role: Novartis, Jazz Pharmaceuticals, Astellas Pharma, Pfizer, Eurocept
Research Funding: Novartis
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Sylvie Freeman
Speakers' Bureau: Jazz Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Vyas, P., Goardon, N., & Freeman, S. (2011). U.S. Patent Application 13/995,347. Title: Detection of Acute Myeloid Leukaemia. Granted 2018
Travel, Accommodations, Expenses: BD Biosciences
Brian Huntly
Honoraria: Novartis
Speakers' Bureau: Novartis, Pfizer
Richard E. Clark
Honoraria: Novartis, Pfizer
Consulting or Advisory Role: Novartis, Pfizer
Research Funding: Pfizer
Ian F.Thomas
Research Funding: Celgene, BCTI, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: CTI, Daiichi Sankyo, Pfizer
Stock and Other Ownership Interests: Novo Nordisk, Abbvie
Honoraria: Celgene, Daiichi Sankyo
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis
Lars Kjeldsen
Stock and Other Ownership Interests: Novo Nordisk, Abbvie
Honoraria: Celgene, Daiichi Sankyo
Research Funding: Novartis
Travel, Accommodations, Expenses: Novartis
Mary Frances McMullin
Consulting or Advisory Role: Novartis
Speakers' Bureau: Celgene, Jazz Pharmaceuticals
Mark Drummond
Honoraria: Novartis, Pfizer, Jazz Pharmaceuticals, Astellas Pharma
Speakers' Bureau: Novartis, Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Novartis, Celgene
Jonathan Kell
Honoraria: Novartis
Consulting or Advisory Role: Novartis
Ruth Spearing
Travel, Accommodations, Expenses: Roche New Zealand
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bishop JF Matthews JP Young GA, et al. : A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood 87:1710-1717, 1996 [PubMed] [Google Scholar]
- 2.Holowiecki J Grosicki S Giebel S, et al. : Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: A multicenter, randomized phase III study. J Clin Oncol 30:2441-2448, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Lee JH Joo YD Kim H, et al. : A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 118:3832-3841, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Burnett AK Russell NH Hills RK, et al. : A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: Results from the UK NCRI AML17 trial in 1206 patients. Blood 125:3878-3855, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett AK Hills RK Wheatley K, et al. : A sensitive risk score for directing treatment in younger patients with AML. Blood 108:10a, 2006. [Google Scholar]
- 6.Ling V Burnett AK Bradstock K, et al. : Utility of a clinical risk score to identify high-risk patients with de novo acute myeloid leukaemia in first remission after high-dose cytarabine (HiDAC) based induction chemotherapy. Br J Haematol 160:861-863, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Ravandi F, Walter RB, Freeman SD: Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv 2:1356-1366, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman SD Hills RK Virgo P, et al. : Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 36:1486-1497, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuurhuis GJ Heuser M Freeman S, et al. : Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 131:1275-1291, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone RM Mandrekar SJ Sanford BL, et al. : Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377:454-464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knapper S Russell N Gilkes A, et al. : A randomized assessment of adding the kinase inhibitor lestaurtinib to first-line chemotherapy for FLT3-mutated AML. Blood 129:1143-1154, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer RJ Davis RB Schiffer CA, et al. : Intensive post-remission therapy in adults with acute myeloid leukemia. N Engl J Med 331:896-903, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Rees JKH Gray R Swirsky D, et al. : The treatment of 1127 patients with acute myeloid leukaemia; principal results of the Medical Research Council’s 8th AML trial. Lancet 2:1236-1231, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Burnett AK Hills RK Milligan DW, et al. : Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: Results of the MRC AML12 trial. J Clin Oncol 28:586-595, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Burnett AK Russell NH Hills RK, et al. : Optimization of chemotherapy for younger patients with acute myeloid leukemia: Results of the Medical Research Council AML15 trial. J Clin Oncol 31:3360-3368, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Burnett AK Hills RK Nielsen OJ, et al. : A comparison of FLAG-Ida and daunorubicin combined with clofarabine in high-risk acute myeloid leukaemia: Data from the UK NCRI AML17 Trial. Leukemia 32:2693-2697, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett AK Das Gupta E Knapper S, et al. : Addition of the mammalian target of rapamycin inhibitor, everolimus, to consolidation therapy in acute myeloid leukemia: Experience from the UK NCRI AML17 trial. Haematologica 103:1654-1661, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnett A Cavenagh J Russell N, et al. : Defining the dose of emtuzumab oogamicin in combination with induction chemotherapy in acute myeloid leukemia: A comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML 17 trial. Haematologica 101:724-731, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnett AK Russell NH Hills RK, et al. : Higher daunorubicin exposure benefits FLT3 mutated acute myeloid leukemia. Blood 128:449-452, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Grimwade D Hills RK Moorman AV, et al. : Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354-365, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Freeman SD Virgo P Couzens S, et al. : Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 31:4123-4131, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Basheer F Giotopoulos G Meduri E, et al. : Contrasting requirements during disease evolution identify EZH2 as a therapeutic target in AML. J Exp Med 216: 966-981, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheson BD Bennett JM Kopecky KJ, et al. : Revised recommendation of the International Working Group for diagnosis standardisation, of response criteria treatment outcomes and reporting standards for therapeutic trials in acute myeloid leukaemia. J Clin Oncol 21:4642-4649, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Burnett AK, Stone RM: AML: New drugs but new challenges. Clin Lymphoma Myeloma Leuk 20:341-350, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Elonen E Almqvist A Hanninen A, et al. : Comparison between four and eight cycles of intensive chemotherapy in adult acute myeloid leukemia: A randomised trial of the Finnish Leukemia Group. Leukemia 12:1041-1048, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Preisler H Davis RB Krishner J, et al. : Comparison of three induction regimens and two postinduction strategies for the treatmen of acute nonlymphocytic leukemia: A Cancer and Leukemia Group B study. Blood 5:1441-1449, 1987 [PubMed] [Google Scholar]
- 27.Lowenberg B: Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 121:26-28, 2013 [DOI] [PubMed] [Google Scholar]