Abstract
Background
Osteoarthritis (OA) of the knee is a common cause of chronic pain. Analgesics that are currently available have limited efficacy and may be poorly tolerated. Tricyclic antidepressants are used as analgesics for other chronic conditions, but they have not been evaluated as analgesics in OA.
Aim
To investigate the analgesic efficacy of nortriptyline in people with knee OA.
Design and setting
A two-arm, parallel-group, 1:1, double-blind, randomised, placebo-controlled trial in Christchurch, New Zealand.
Method
Participants were recruited from orthopaedic outpatient clinics, primary care, and through public advertising. Adults with knee OA and a pain score of ≥20 points on the 50-point Western Ontario and McMaster University Osteoarthritis Index (WOMAC) pain subscale were randomised to receive either nortriptyline or identical placebo for 14 weeks. The primary outcome was knee pain at 14 weeks measured using the WOMAC pain subscale. Secondary outcomes included: function; stiffness; non-steroidal anti-inflammatory drug, opioid, and/or paracetamol use; each participant’s global assessment; and adverse effects at 14 weeks.
Results
Of the 205 randomised participants, 201 (98.0%) completed follow-up at 14 weeks. The baseline-adjusted mean WOMAC pain subscale score at week 14 was 6.2 points lower (95% confidence interval = −0.26 to 12.6, P = 0.06) in the nortriptyline arm versus the placebo arm. Differences in secondary outcomes generally favoured the nortriptyline arm, but were small and unlikely to be clinically relevant. However, the following were all more commonly reported by participants taking nortriptyline than those taking a placebo: dry mouth (86.9% versus 51.0%, respectively, P<0.001), constipation (58.6% versus 30.4%, respectively, P<0.001), and sweating (31.3% versus 20.6%, respectively, P = 0.033).
Conclusion
This study suggests nortriptyline does not significantly reduce pain in people with knee OA. The adverse effect profile was as expected.
Keywords: analgesia, general practice, knee osteoarthritis, randomised controlled trial
INTRODUCTION
Osteoarthritis (OA) is the most common joint disease and a major cause of pain, disability, reduced quality of life, and large healthcare costs;1–3 in addition, the burden of disease is predicted to rise.4–5 Osteoarthritis management is focused on advice, exercise, weight loss (if obese), analgesia, and maintenance of function.6 The analgesics currently recommended for OA are not ideal: paracetamol is minimally effective7 and has been linked with increased risk of mortality, cardiovascular disease, gastrointestinal bleeding, and renal adverse events;8 although oral non-steroidal anti-inflammatory drugs (NSAIDs) are effective in reducing pain,9 they are associated with renal, gastrointestinal, and cardiovascular toxicity.10 Opioids may also be used, despite poor evidence of efficacy, a significant side-effects burden, and risk of dependency.11–13 Patients may be offered intra-articular corticosteroid injections, but their analgesic efficacy and safety are uncertain.14 In addition, knee OA may be treated with joint replacement, but access to this intervention is limited by resource constraints in many countries15 and by patient comorbidity. For younger people (aged <60 years), delayed joint replacement may be desirable due to an increased lifetime risk of prosthetic failure.16 Given all of these factors, there is a need for more-effective and better-tolerated pain management for people with OA.
Central sensitisation may play an important role in the chronic pain of OA,17–18 and some centrally acting agents have been shown to reduce OA pain.19–20 Tricyclic antidepressants (TCAs) are used in other chronic pain conditions,21–22 but their analgesic effect in OA has not previously been evaluated; the aim of this study was to assess the efficacy and safety of the TCA nortriptyline for analgesia in knee OA when used in addition to participants’ usual analgesia.
METHOD
Trial design
A two-arm, parallel-group, 1:1, randomised, double-blind, placebo-controlled trial was conducted to investigate whether nortriptyline (25 mg–100 mg per day) provides clinically significant pain relief in patients with OA of the knee. A full protocol has previously been published23 and results are presented in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines and extension for harms.24–25
How this fits in
| Patients with knee osteoarthritis (OA) frequently require analgesics, but the analgesics commonly used are not ideal as they are either insufficiently effective or have serious side-effects. The authors hypothesised that tricyclic antidepressants (TCAs), which are used as analgesics for other chronically painful conditions, may be helpful for patients with OA. In this randomised, double-blind, placebo-controlled trial, it was found that one TCA, nortriptyline, did not significantly reduce pain or improve physical function, stiffness, or participants’ global assessment of the impact of their OA; as such, nortriptyline is unlikely to be a useful treatment for patients with knee OA. |
Participant recruitment and eligibility
Potential participants were largely recruited from urban and suburban areas of Christchurch, New Zealand’s second largest city. Invitation letters to participate were sent to people with knee OA who had been declined specialist orthopaedic assessment for knee replacement (by a referral triaging orthopaedic surgeon, who read the referral letters and examined accompanying prereferral X-rays) and returned to their GP for ongoing care. These people were identified on lists held by the Canterbury District Health Board orthopaedic department. The authors did not attempt further contact for those who did not respond to initial invitation letters.
A range of local marketing initiatives were used. These included advertisements and articles in local newspapers and bulletins; posters in primary healthcare premises, libraries, and clubs; and stalls in shopping centres. Research nurses provided individuals who responded to these invitations with study information by post or over the telephone, and conducted in-person screening assessments. For inclusion in the study, participants had to meet all of the following criteria:
have primary knee OA defined according to the American College of Rheumatology clinical criteria for the classification of idiopathic OA of the knee;26
have knee pain scoring ≥20 points on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (range: 0–50 points);27 and
have been on a stable analgesic regime for, at least, the previous 2 months.
Exclusion criteria included:
previous joint replacement of the study knee;
intra-articular steroid injection in the previous 3 months;
secondary OA;
concurrent use of any antidepressant; and
any established contraindications to TCAs.
The full list of exclusion criteria can be found elsewhere.23
Randomisation and blinding
An unstratified, 1:1-allocation, computer-generated randomisation list with blocks of varying size (one to four) was prepared by the study statistician (https://cran.r-project.org/web/packages/blockrand/index.html). Participants, as well as all investigators and research staff who were enrolling participants, dispensing medication, and/or assessing outcomes, were blinded to the treatment allocation. To assess the effectiveness of blind allocation, participants and research nurses were asked which study arm they believed the participant had been allocated to at the final study visit.
Trial regimen and procedures
Treatment occurred over 14 weeks and comprised an 8-week dose-adjustment phase and a 6-week steady-dose phase. Participants were instructed to commence taking one capsule (containing either nortriptyline 25 mg or placebo) daily for 2 weeks, at which time they were contacted by a research nurse by telephone who advised them to increase or decrease their dose by one capsule per day according to the participant’s level of knee pain and adverse effects. Further dose adjustments occurred at 4 weeks, 6 weeks, and 8 weeks, up to a maximum dose of four capsules daily (100 mg nortriptyline daily in the treatment group). At 8 weeks, participants were instructed to maintain their current dose until week 14, when the final assessment was undertaken. Throughout the study, participants were free to use and adjust their usual analgesic medication as prescribed by their GP, but were requested not to use any other antidepressants or receive intraarticular steroid injections.
Outcome measures
Primary outcome
The primary outcome was self-reported pain in the affected knee at week 14 over the previous 48 hours, captured using the WOMAC 3.1 pain subscale.
Secondary outcomes
Secondary outcomes comprised:
physical function, measured using the WOMAC pain subscale;
stiffness, measured using the WOMAC pain subscale;
global assessment, measured using a visual analogue scale (VAS);
response to treatment, according to the Outcome Measures in Rheumatology and Osteoarthritis Research Society International (OMERACT–OARSI) responder criteria;28
quality of life, using the RAND 36-Item Health Survey 1.0 (RAND 36);29
the proportion of participants reporting adverse events and the severity of these events, measured using the Antidepressant Side-Effect Checklist at week 14; and
use of NSAIDs and other analgesics.
WOMAC and VAS scores were standardised to a range of 0 (best-possible outcome) to 100 (worst-possible outcome). RAND 36 physical and mental component summary scores were calculated according to simple item sums.30
Data collection
Information was collected at baseline by a research nurse in a face-to-face interview and included demographics, clinical characteristics, baseline measures of outcome variables (WOMAC 3.1 OA index, global assessment by VAS, and RAND 36), and analgesic use during the previous 2 weeks.
Interviews were conducted by a research nurse 14 weeks after commencing the study medication, and self-reported items were completed during these interviews. Interviews were conducted at the Department of General Practice, University of Otago, and lasted circa 20 min.
Adverse events were captured at weeks 2, 4, 6, 8, and 14 in free-text fields incorporated into each tool and coded by a research nurse and study investigator using the Common Terminology Criteria for Adverse Events, version 5.31 The occurrence and severity of expected adverse events resulting from antidepressant use at any time during the study were captured for all participants at week 14 using the Antidepressant Side-Effect Checklist.32 Unexpected adverse events were collected at any point of contact with the study team.
Analgesic use during the final 2 weeks of the study was recorded by participants in daily diaries. Daily doses were calculated for each analgesic, and pre-specified rules were used to derive equivalent doses of NSAIDs and opioids (see Supplementary Table S1).
Sample-size calculation
The study was powered to detect the minimum clinically important difference for reduction in pain at 14 weeks, measured using the WOMAC pain subscale; this was deemed to be 10% of the scale maximum,33 with 90% power at a two-sided significance level of 0.05. It was calculated that a sample size of 200 was needed, which conservatively assumed a pooled standard deviation of 20 points, no correlation between baseline and follow-up scores, and an attrition rate of up to 15%.34
Statistical analysis
The statistical analysis plan and analysis code were prepared by the study statistician and agreed on by the principal investigator following blinded review of the data but before the randomisation code was broken. Analysis was conducted using R (version 3.5.1). The primary analysis followed an intention-to-treat approach and missing data were imputed using multivariate normal multiple imputation.35 A P-value of <0.05 was considered to be statistically significant.
The mean absolute treatment effect in the primary outcome was estimated using linear regression modelling, with baseline WOMAC pain subscale scores included as a covariate. Similar analyses were conducted for the secondary outcomes (namely, physical function, stiffness, global assessment, and quality of life). Absolute and relative differences in the proportion of participants who responded to treatment, according to the OMERACT-OARSI responder criteria,28 were calculated with 95% confidence intervals (CIs) using generalised linear regression models.
Heterogeneity of treatment effects (subgroup) analysis followed the framework presented by Kent et al.36 Additional sensitivity analyses were performed adjusting for age, body mass index (BMI), sex, years with OA, use of assistive devices, presence of any chronic comorbidities, and mental health status.
Generalised mixed-effects hurdle models were used to estimate differences in:
the daily likelihood of a participant using an analgesic; and
the average number of ‘standardised’ analgesic tablets taken daily by those who took any.
A logistic model was used for the daily likelihood of a participant using an analgesic and a truncated generalised Poisson model was used for the average number of ‘standardised’ analgesic tablets taken daily by those who took any. Both models included time of observation, treatment group, and time by treatment as fixed effects, and subject as a random effect.
RESULTS
Participant recruitment and characteristics
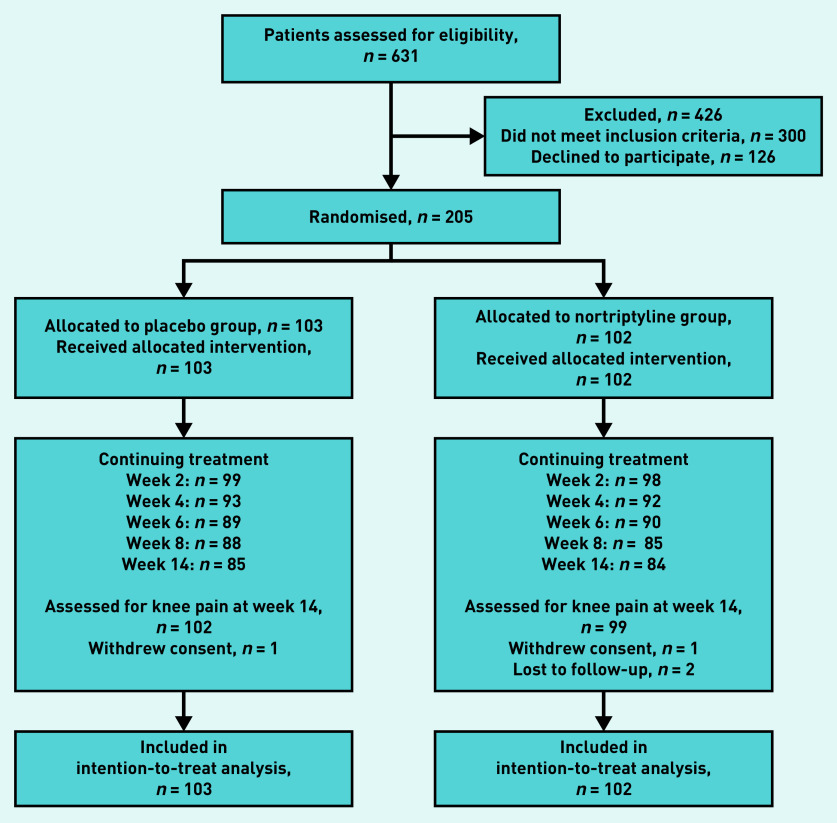
The participant recruitment process is outlined in Figure 1. A total of 205 participants were enrolled between November 2015 and October 2017, and the final follow-up interview was conducted in February 2018.
Figure 1.
Participant recruitment and randomisation. All participants were included at the final point of assessment irrespective of whether they had continued or ceased study medication.
Participants’ demographic and clinical characteristics are presented in Table 1; their baseline characteristics were generally similar in both the placebo and nortriptyline arms, but the nortriptyline arm included a greater proportion of individuals who were obese (73.5% versus 47.6%), and the placebo arm a greater proportion of individuals who were overweight (46.6% versus 19.6%). Baseline VAS data were not collected for the first 24 participants (12 participants in both study arms) due to an error in the preparation of their record sheets. Multiple imputation assuming a multivariate normal distribution was used to impute the missing data for the baseline VAS at the same time that missing data was imputed for the outcome variables. The baseline VAS is relatively highly correlated with the WOMAC pain subscale and physical function scores (Pearson’s r = 0.4), which are also included as auxiliary variables in the multiple imputation models. Outcome data were not collected for four participants: two withdrew from the study after randomisation and two were lost to follow-up.
Table 1.
Study participants’ characteristics at baseline in placebo (n = 103) and nortriptyline (n = 102) groups
| Characteristic | Placebo | Nortriptyline |
|---|---|---|
| Mean age, years (SD) | 64.6 (10.3) | 64.4 (7.9) |
|
| ||
| Female, n (%) | 43 (41.7) | 44 (43.1) |
|
| ||
| Ethnicity, n (%)a | ||
| European | 87 (84.5) | 96 (94.1) |
| Māori | 9 (8.7) | 12 (11.8) |
| Other | 11 (10.7) | 5 (4.9) |
|
| ||
| BMI (kg/m2), mean (SD) | 31.3 (6.2) | 33.2 (5.7) |
| Healthy: 18–<25, n (%) | 6 (5.8) | 7 (6.9) |
| Overweight: 25–<30, n (%) | 48 (46.6) | 20 (19.6) |
| Obese: ≥30, n (%) | 49 (47.6) | 75 (73.5) |
|
| ||
| Years with knee OA, mean (SD) | 6.6 (7.1) | 8.5 (7.9) |
|
| ||
| Use of assistive device, n (%) | 39 (37.9) | 41 (40.2) |
|
| ||
| Chronic conditions, n (%) | 60 (58.3) | 61 (59.8) |
Ethnicity was self-identified; participants could select >1 ethnicity, so totals may add to >100%. BMI = body mass index. OA = osteoarthritis. SD = standard deviation.
Efficacy
Primary outcome
On average, participants given nortriptyline had a WOMAC pain subscale score at week 14 that was 6.2 points lower (95% CI = −0.26 to 12.56, P = 0.060) than that of those who received a placebo. Results were, effectively, unchanged in sensitivity analyses when adjusting for baseline covariates or excluding participants with protocol violations (Table 2).
Table 2.
Primary and secondary continuous outcomesa in placebo (n = 103) and intervention (n = 102) groups
| Placebo, mean (SD) | Nortriptyline, mean (SD) | Baseline-adjusted difference at 14 weeks | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Baseline | 14 weeks | Change | Baseline | 14 weeks | Change | Difference | 95% CI | P-value | |
| Primary outcome | |||||||||
| Pain (WOMAC)b | 61.2 (12.5) | 42.5 (24.0) | −18.7 (25.8) | 60.2 (13.5) | 36.0 (23.2) | −24.3 (22.5) | −6.2 | −0.26 to 12.56 | 0.06 |
|
| |||||||||
| Secondary outcomes | |||||||||
| Function (WOMAC)b | 59.9 (14.8) | 41.9 (24.2) | −18.0 (23.2) | 62.8 (15.0) | 39.6 (24.4) | −23.2 (21.5) | −4.4 | −10.48 to 1.79 | 0.16 |
| Stiffness (WOMAC)b | 61.2 (20.1) | 47.2 (27.9) | −14.0 (29.5) | 65.2 (20.3) | 45.4 (27.4) | −19.9 (27.4) | −3.6 | −10.94 to 3.72 | 0.33 |
| Global assessment VASb | 72.6 (21.1) | 53.6 (28.6) | −19.0 (33.0) | 74.3 (20.2) | 49.3 (30.8) | −25.0 (33.5) | −4.7 | −12.91 to 3.46 | 0.26 |
|
| |||||||||
| Quality of lifec | |||||||||
| Physical function | 31.6 (9.9) | 31.0 (10.1) | −0.6 (8.8) | 29.9 (10.6) | 31.9 (11.5) | 2.0 (10.0) | 2.0 | −0.39 to 4.43 | 0.10 |
| Role limitations due to physical health | 39.7 (11.1) | 39.7 (12.1) | −0.1 (11.5) | 39.9 (11.0) | 41.7 (11.9) | 1.8 (11.9) | 2.0 | −0.95 to 4.88 | 0.19 |
| Bodily pain | 35.5 (7.5) | 38.6 (8.8) | 3.1 (9.4) | 35.7 (8.1) | 41.4 (10.0) | 5.8 (8.8) | 2.8 | 0.42 to 5.08 | 0.02 |
| General health | 46.1 (8.6) | 46.7 (10.1) | 0.6 (7.8) | 47.4 (8.4) | 47.4 (8.7) | 0.1 (7.3) | −0.1 | −2.15 to 1.88 | 0.90 |
| Energy and vitality | 47.1 (10.0) | 47.1 (10.8) | 0.0 (9.9) | 46.2 (8.8) | 46.8 (10.9) | 0.6 (8.5) | 0.3 | −2.15 to 2.72 | 0.82 |
| Social function | 44.8 (11.4) | 43.2 (13.5) | −1.6 (12.8) | 44.7 (11.5) | 45.0 (12.7) | 0.4 (13.2) | 1.9 | −1.40 to 5.14 | 0.26 |
| Role limitations due to emotional health | 47.8 (11.4) | 44.7 (12.9) | −3.1 (13.9) | 47.3 (11.8) | 46.0 (12.9) | −1.3 (11.8) | 1.6 | −1.63 to 4.79 | 0.33 |
| Emotional wellbeing | 52.1 (8.8) | 51.7 (10.0) | −0.4 (8.9) | 51.9 (9.1) | 51.4 (10.9) | −0.6 (9.7) | −0.2 | −2.66 to 2.21 | 0.86 |
All outcomes have been standardised to a range of 0–100.
0 = best-possible outcome.
100 = best-possible outcome. SD = standard deviation. VAS = visual analogue scale. WOMAC = McMaster Universities Osteoarthritis Index.
Secondary outcomes
Participants in the nortriptyline arm achieved a greater improvement in the bodily pain subscale of the RAND 36 (baseline-adjusted difference 2.8, 95% CI = 0.42 to 5.08, P = 0.02) than the group receiving placebo; however, no statistically significant differences were observed in the other items of the RAND 36, the WOMAC subscales for physical function or stiffness, the VAS, or the proportion of responders (Table 2). There was no evidence of heterogeneity in treatment effects according to the predicted WOMAC pain subscore at week 14 (P = 0.82) or the predicted responder status (P = 0.67); as such, further subgroup analyses were not undertaken.
The estimated proportion of individuals who achieved the OMERACT-OARSI responder criteria was 56.4% (95% CI = 46.8 to 66.0) in those treated with placebo and 68.5% (95% CI = 59.4 to 77.6) in those treated with nortriptyline. This represents an absolute difference of 12.1% (95% CI = −1.4 to 25.6, P = 0.08) and a relative improvement of 1.2 (95% CI = 0.98 to 1.51, P = 0.08) (data not shown).
Analgesic use
Participants in the nortriptyline group, in comparison with those receiving placebo, had a greater proportion of days when they did not take any NSAIDs (73.9% versus 69.1%, adjusted odds ratio [OR] 3.91, 95% CI = 2.49 to 6.16) or paracetamol (62.6% versus 57.6%, adjusted OR 1.55, 95% CI = 1.03 to 2.37). If they did take NSAIDs they took, on average, fewer tablets (adjusted rate ratio 0.85, 95% CI = 0.80 to 0.90) (Table 3).
Table 3.
| Analgesic | Placebo | Nortriptyline | Adjusted odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 14 weeks | Difference | Baseline | 14 weeks | Difference | ||
| Mean proportion of days when analgesic was not taken | |||||||
| Paracetamol, % | 52.5 | 57.6 | 4.3 | 53.0 | 62.6 | 9.1 | 1.55 (1.03 to 2.37) |
| NSAID, % | 66.6 | 69.1 | 4.1 | 61.6 | 73.9 | 11.5 | 3.91 (2.49 to 6.16) |
| Opioid, % | 81.5 | 82.8 | 0.6 | 76.9 | 80.8 | 4.6 | 1.60 (0.86 to 2.99) |
| Mean standard analgesic tablet count on days when analgesic was taken | Adjusted rate ratio (95% CI) | ||||||
| Paracetamol, n | 4.2 | 4.2 | 0.0 | 3.6 | 3.5 | −0.1 | 0.97 (0.92 to 1.03) |
| NSAID, n | 6.4 | 6.1 | −0.3 | 7.4 | 6.7 | −0.7 | 0.85 (0.80 to 0.90) |
| Opioid, n | 7.0 | 6.6 | −0.4 | 5.9 | 6.8 | 0.9 | 0.98 (0.90 to 1.07) |
One pill is defined as the equivalent of 500 mg paracetamol, 200 mg ibuprofen, or 15 mg codeine.
Of the 205 participants enrolled in the study (n = 103 placebo, n = 102 intervention), seven (n = 4 placebo, n = 3 intervention) did not complete a medication diary at week 14 and therefore could not be included in the analgesic analysis. Four withdrew or were lost to follow-up (n = 1 placebo, n = 3 intervention) and had no week 14 data, a further three (all placebo) completed the week 14 assessment (WOMAC questionnaire) but did not complete the medication diary. NSAID = non-steroidal anti-inflammatory drug.
Adverse events
Seven serious adverse events (SAEs) were reported: one — for a participant receiving nortriptyline — was life threatening, and in both study arms three participants required hospitalisation (three for those in the placebo group, three for those receiving nortriptyline). Two SAEs (the hospitalisations for myocardial infarction and atrial fibrillation) were considered to be related to the study medication (Table 4).
Table 4.
Adverse eventsa (serious adverse events, overall, and most common) in placebo (n = 102) and nortriptyline (n = 99) groupsb
| Event | Placebo, n (%) | Nortriptyline, n (%) | P-value |
|---|---|---|---|
| Serious adverse events | |||
| Overall | 3 (2.9) | 4 (4.0) | 0.72 |
| Life-threatening myocardial infarctionc | 0 (0.0) | 1 (1.0) | — |
| Hospitalisation for lower back pain | 1 (1.0) | 0 (0.0) | — |
| Hospitalisation for atrial fibrillationc | 0 (0.0) | 1 (1.0) | — |
| Hospitalisation for epistaxis | 0 (0.0) | 1 (1.0) | — |
| Hospitalisation for renal calculi | 1 (1.0) | 0 (0.0) | — |
| Hospitalisation for lung infection | 1 (1.0) | 0 (0.0) | — |
| Hospitalisation for hyperglycaemia | 0 (0.0) | 1 (1.0) | — |
|
| |||
| Adverse events (Antidepressant Side-Effect Checklist at week 14) | |||
| Any adverse events | 88 (86.3) | 97 (98.0) | 0.001 |
| Largest differences | |||
| Dry mouth | 52 (51.0) | 86 (86.9) | <0.001 |
| Constipation | 31 (30.4) | 58 (58.6) | <0.001 |
| Sweating | 21 (20.6) | 31 (31.3) | 0.033 |
| Sexual dysfunction | 9 (8.8) | 17 (17.2) | 0.084 |
| Headache | 27 (26.5) | 14 (14.1) | 0.009 |
| Diarrhoea | 21 (20.6) | 11 (11.1) | 0.060 |
Each event is only recorded once per patient.
Antidepressant Side-Effect Checklist data relates to those assessed for knee pain at week 14 only.
Considered to be related to the study medication.
For adverse events, dry mouth (86.9% versus 51.0%, P<0.001), constipation (58.6% versus 30.4%, P<0.001), and sweating (31.3% versus 20.6%, P = 0.033) were all more commonly reported by those taking nortriptyline, as was sexual dysfunction (17.2% versus 8.8%, P = 0.084), however, this was not statistically significant (Table 4).
Test of blinding
When participants were asked what treatment they thought they received, the proportion who correctly guessed was similar among those who guessed that they were taking nortriptyline (62.7%, 95% CI = 53.3 to 71.4) and those who guessed that they were taking placebo (70.3% [95% CI = 58.5 to 80.3]) (P = 0.06) (data not shown).
DISCUSSION
Summary
In people with knee OA, adding nortriptyline to their usual analgesic treatment did not meaningfully improve pain, function, stiffness, or each participant’s global assessment of OA. Participants randomised to nortriptyline were more likely to have days when they did not take paracetamol or NSAIDs and, when they did take NSAIDs, they took fewer doses; however, these differences in analgesic use were small and unlikely to be clinically important. Dry mouth, constipation, sweating, and sexual dysfunction were more likely in participants taking nortriptyline, though sexual dysfunction was not statistically significant. Serious adverse events occurred in four participants taking nortriptyline and three taking placebo.
Of the SAEs, two were judged to be related to study medication and one was life-threatening (all of these occurred in participants taking nortriptlyine).
Strengths and limitations
To the authors’ knowledge, this is the first published, double-blind randomised controlled trial of the analgesic effect of a TCA on OA. In addition, there are a number of strengths to the study design: primary and secondary outcome measures were recorded at 14 weeks, a follow-up period that is consistent with OMERACT-OARSI OA study recommendations;37 the primary outcome was measured using the WOMAC pain subscale, a valid, reliable, and responsive measure of pain and disability in OA;38–39 and the study was adequately powered to detect the minimum clinically important difference in pain. However, although this study was adequately powered to detect a clinically important effect of nortriptyline on pain, the sample size was too small to allow for subgroup analyses of participants who may have been predisposed to derive greater benefit from nortriptyline — for example, those with low mood or higher baseline levels of pain.
The pragmatic study design, in which study medication was taken in addition to usual treatment, is considered a strength: as a result of this, the effect of nortriptyline was tested in the context in which it would be used in clinical practice. However, as the design placed no limitation on participants’ use of other analgesics, it is possible that an analgesic effect of nortriptyline may have been masked that would have, otherwise, been apparent had the use of other analgesics been restricted.
Study participants were recruited from a number of sources. The majority were those with knee OA who had been declined assessment for knee replacement and returned to their GP’s care; others were recruited through a range of community-based advertising approaches. Participant demographics were broadly representative of patients with OA, though people of non-European ethnicity were under-represented. The recruitment of individuals referred for, and declined, specialist assessment may have meant that the range of disease severity in participants was greater than is typically encountered in primary care; it is important to be aware, therefore, that this may limit extrapolation of the findings to the full range of patients with knee OA that is encountered in primary care.
The study design also required participants’ study medication doses to be individually titrated according to their levels of pain and adverse effects believed by the participant to have been caused by study medication. This is important as nortriptyline metabolism and, hence, dosing have high inter-individual variability: the effective and tolerated daily dose ranges from <25 mg to >100 mg, so individualised dosing is essential.40–41 The choice of a dosing range from 25 mg to 100 mg daily could have been a limitation in that some participants may have received subtherapeutic dosing even at the maximum study dose, while others may have developed intolerable adverse effects at the minimum dose and ceased study medication as a result. However, the study dosing range was consistent with current clinical recommendations40 and was a pragmatic approach to permit flexible individualised dosing in the limited time available in the study. Furthermore, this process of dose adjustment closely resembles usual clinical practice when initiating a TCA.
The authors were able to demonstrate reasonably effective blinding of participants. This is of particular importance in trials of TCAs, as these medicines have a well-recognised set of adverse effects (in particular, dry mouth, constipation, and sedation) that may lead to unblinding, which could have compromised the internal validity of the study.
Participants’ baseline characteristics were generally similar in the placebo and nortriptyline arms. The nortriptyline arm, however, included a greater proportion of individuals who were obese. Individuals with a higher BMI report greater OA pain than those with lower BMI;42 therefore, the greater proportion of obese individuals in the nortriptyline arm may have biased results towards the null. However, mean BMI and the proportion of individuals with healthy BMI were similar in the two study arms, and the nortriptyline arm included a smaller proportion of those classified as overweight, so any effect on the findings is likely to have been small.
This may have introduced bias to the findings, although the mean BMI was similar in the two study arms, and adjusting for BMI and other key baseline variables did not substantially alter them.
Comparison with existing literature
To the authors’ knowledge, this is the first trial of a TCA for pain in OA; therefore, no directly comparable studies exist. The null finding is disappointing as achieving adequate, well-tolerated, and safe analgesia for patients with OA is challenging, and few trials of analgesic agents demonstrate an effect size equal to the minimum clinically important difference.11,19,33
The evidence of TCAs’ efficacy in other chronic pain conditions is mixed: TCAs are established as first-line agents in neuropathic pain43 and have been shown to be effective in chronic headache,22 post-herpetic neuralgia, and diabetic neuropathy.21 Their efficacy in fibromyalgia is less clear,44–45 and a recent RCT of amitriptyline in chronic back pain did not show a statistically significant improvement in pain.46 In OA, the relative contribution of central and peripheral sensitisation and nociceptive pain varies between individuals;47 this may mean that some people are more likely to benefit from centrally acting analgesics than others.
Implications for practice
Nortriptyline taken in addition to standard analgesia does not reduce pain in people with knee OA.
The degree of central sensitisation in an individual can be estimated using clinical scoring systems,48 and future work on centrally acting analgesics in OA could explore the potential for stratifying participants based on their degree of central sensitisation in order to determine whether centrally acting agents might usefully be targeted to those patients with higher levels of central sensitisation.
Acknowledgments
The authors would like to thank: the study participants; research nurses Gwyneth Steenson and Rae Noble-Adams; research administrator Alison Parsons; departmental colleagues Ruth Savage and Kim Pasley; the Canterbury District Health Board orthopaedics outpatient clinic administrative team; and Aarti Patel, the general manager of Canterbury Community Pharmacy Group. They would also like to thank the Health Research Council of New Zealand for funding the study.
Funding
This study was funded with a project grant from the Health Research Council of New Zealand (reference number: 14/152). The funding source had no role in the design, execution, analysis, interpretation of the data, or the decision to submit results of this study.
Ethical approval
This study was approved by the New Zealand Northern A Health and Disability Ethics Committee (reference number: 14/NTA/139).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Abbott JH, Usiskin IM, Wilson R, et al. The quality-of-life burden of knee osteoarthritis in New Zealand adults: a model-based evaluation. PLoS One. 2017;12(10):e0185676. doi: 10.1371/journal.pone.0185676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res. 2015;67(2):203–215. doi: 10.1002/acr.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jinks C, Ong BN, Richardson J. A mixed methods study to investigate needs assessment for knee pain and disability: population and individual perspectives. BMC Musculoskelet Disord. 2007;8:59. doi: 10.1186/1471-2474-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Access Economics The economic cost of arthritis in New Zealand in 2010. 2010 https://www.arthritis.org.nz/pdfs/economic-cost-of-arthritis-in-newzealand-final-print.pdf (accessed 21 May 2021). [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence Osteoarthritis: care and management. 2020. Clinical guideline [CG177] http://nice.org.uk/cg177 (accessed 21 May 2021).
- 7.Leopoldino AO, Machado GC, Ferreira PH, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev. 2019;2(2):CD013273. doi: 10.1002/14651858.CD013273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts E, Delgado Nunes V, Buckner S, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. 2016;75(3):552–559. doi: 10.1136/annrheumdis-2014-206914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 10.Coxib and Traditional NSAID Trialists’ (CNT) Collaboration Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Costa BR, Nüesch E, Kasteler R, et al. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2014;(9):CD003115. doi: 10.1002/14651858.CD003115.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth. 2018;120(6):1335–1344. doi: 10.1016/j.bja.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872–882. doi: 10.1001/jama.2018.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwynne-Jones D. Quantifying the demand for hip and knee replacement in Otago, New Zealand. N Z Med J. 2013;126(1377):7–17. [PubMed] [Google Scholar]
- 16.Bayliss LE, Culliford D, Monk AP, et al. The effect of patient age at intervention on risk of implant revision after total replacement of the hip or knee: a population-based cohort study. Lancet. 2017;389(10077):1424–1430. doi: 10.1016/S0140-6736(17)30059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mease PJ, Hanna S, Frakes EP, Altman RD. Pain mechanisms in osteoarthritis: understanding the role of central pain and current approaches to its treatment. J Rheumatol. 2011;38(8):1546–1551. doi: 10.3899/jrheum.100759. [DOI] [PubMed] [Google Scholar]
- 18.Neogi T, Frey-Law L, Scholz J, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: trait or state? Ann Rheum Dis. 2015;74(4):682–688. doi: 10.1136/annrheumdis-2013-204191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZY, Shi SY, Li SJ, et al. Efficacy and safety of duloxetine on osteoarthritis knee pain: a meta-analysis of randomized controlled trials. Pain Med. 2015;16(7):1373–1385. doi: 10.1111/pme.12800. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan M, Bentley S, Fan M-Y, Gardner G. A single-blind placebo run-in study of venlafaxine XR for activity-limiting osteoarthritis pain. Pain Med. 2009;10(5):806–812. doi: 10.1111/j.1526-4637.2009.00637.x. [DOI] [PubMed] [Google Scholar]
- 21.Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;(4):CD005454. doi: 10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222. doi: 10.1136/bmj.c5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson B, Williman JA, Stamp LK, et al. Nortriptyline in knee osteoarthritis (NortIKA Study): study protocol for a randomised controlled trial. Trials. 2015;16:448. doi: 10.1186/s13063-015-0961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JPA, Evans SJW, Gøtzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 26.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 28.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 30.Grassi M, Nucera A, European Community Respiratory Health Study Quality of Life Working Group Dimensionality and summary measures of the SF-36 v1.6: comparison of scale- and item-based approach across ECRHS II adults population. Value Health. 2010;13(4):469–478. doi: 10.1111/j.1524-4733.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 31.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE): Version 5.0. 2021. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (accessed 17 May 2021).
- 32.Uher R, Farmer A, Henigsberg N, et al. Adverse reactions to antidepressants. Br J Psychiatry. 2009;195(3):202–210. doi: 10.1192/bjp.bp.108.061960. [DOI] [PubMed] [Google Scholar]
- 33.Ehrich EW, Davies GM, Watson DJ, et al. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641. [PubMed] [Google Scholar]
- 34.Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage. 2004;28(1):59–71. doi: 10.1016/j.jpainsymman.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Honaker J, King G, Blackwell M. Amelia II: a program for missing data. Journal of Statistical Software. 2011;45(7):1–47. [Google Scholar]
- 36.Kent DM, Rothwell PM, Ioannidis JPA, et al. Assessing and reporting heterogeneity in treatment effects in clinical trials: a proposal. Trials. 2010;11:85. doi: 10.1186/1745-6215-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altman R, Brandt K, Hochberg M, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 38.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45(5):453–461. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 39.Rogers JC, Irrgang JJ. Measures of adult lower extremity function: The American Academy of Orthopedic Surgeons Lower Limb Questionnaire, The Activities of Daily Living Scale of the Knee Outcome Survey (ADLS), Foot Function Index (FFI), Functional Assessment System (FAS), Harris Hip Score (HHS), Index of Severity for Hip Osteoarthritis (ISH), Index of Severity for Knee Osteoarthritis (ISK), Knee Injury and Osteoarthritis Outcome Score (KOOS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC™) Arthritis Care Res. 2003;49(S5):S67–S84. [Google Scholar]
- 40.New Zealand Government New Zealand consumer medicine information Norpress: nortriptyline (as hydrochloride) 10mg & 25mg tablets. 2021 https://www.medsafe.govt.nz/Consumers/CMI/n/norpress.pdf (accessed 18 May 2021). [Google Scholar]
- 41.Wolf CR, Smith G, Smith RL. Science, medicine, and the future: pharmacogenetics. BMJ. 2000;320(7240):987–990. doi: 10.1136/bmj.320.7240.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss E. Knee osteoarthritis, body mass index and pain: data from the Osteoarthritis Initiative. Rheumatology (Oxford) 2014;53(11):2095–2099. doi: 10.1093/rheumatology/keu244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Häuser W, Bernardy K, Uçeyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA. 2009;301(2):198–209. doi: 10.1001/jama.2008.944. [DOI] [PubMed] [Google Scholar]
- 45.Häuser W, Walitt B, Fitzcharles M-A, Sommer C. Review of pharmacological therapies in fibromyalgia syndrome. Arthritis Res Ther. 2014;16(1):201. doi: 10.1186/ar4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Urquhart DM, Wluka AE, van Tulder M, et al. Efficacy of low-dose amitriptyline for chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2018;178(11):1474–1481. doi: 10.1001/jamainternmed.2018.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hochman JR, Gagliese L, Davis AM, Hawker GA. Neuropathic pain symptoms in a community knee OA cohort. Osteoarthritis Cartilage. 2011;19(6):647–654. doi: 10.1016/j.joca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Hochman JR, Davis AM, Elkayam J, et al. Neuropathic pain symptoms on the modified painDETECT correlate with signs of central sensitization in knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1236–1242. doi: 10.1016/j.joca.2013.06.023. [DOI] [PubMed] [Google Scholar]