Abstract
Objectives
To evaluate the prevalence of infection prevention behaviors in Taiwan—wearing facemasks and alcohol-based hand hygiene (AHH)—and compare their practice rates during SARS and COVID-19.
Methods
We surveyed 2328 Taiwanese from July 29 to August 6, 2020, assessing demographics, information sources, and preventive behaviors during the 2003 SARS outbreaks, 2009 pandemic influenza H1N1, COVID-19, and with post-survey intentions. Characteristics associated with the practice of preventive behaviors in 2020 were identified through logistic regression.
Results
Preventive behaviors were conscientiously practiced by 70.2% of participants. Compared with 2003 SARS/2009 H1N1, the percentages of facemask use (66.6% vs 99.2% [indoors], P < 0.001) and on-person AHH (44.2% vs 65.4% [hand sanitizers], P < 0.001) significantly increasedduring 2020 COVID-19. Highest adherence to preventive behaviors in 2020 was among females (adjusted odds ratio [aOR], 1.72), those receiving government COVID-19 information (aOR, 1.52), participants recruited from primary-care clinics (aOR, 1.43), and those who practiced AHH during 2003 SARS/2009 H1N1 (aOR, 1.37).
Conclusions
Government leadership, healthcare providers risk communication, and public cooperation rapidly mitigated the spread of COVID-19 in Taiwan even before vaccination. Future global efforts must implement such population-based preventive behaviors at a level above the viral-transmission-threshold, particularly in areas with fast-spreading SARS-CoV-2 variants.
Keywords: SARS-CoV-2, COVID-19, Public health policies, Face mask, Alcohol-based hand hygiene, Threshold-based bundle strategy, Taiwan
Introduction
The COVID-19 pandemic has had an immense impact on global health. It is crucial to examine how certain countries have successfully mitigated this threat and contributed to controlling the spread of SARS-CoV-2 more efficiently on a global scale.
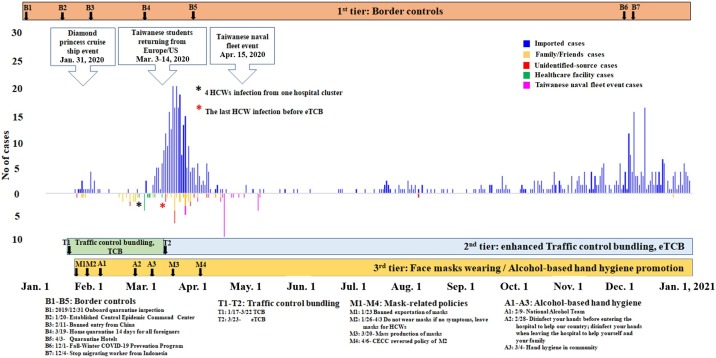
Taiwan has had close cultural and economic exchanges with China and was expected to be one of the most at-risk areas for large-scale outbreaks of COVID-19. From January 1 to February 29, 2020, Taiwan had 45 cases (including 25 indigenous cases) when several cities in China were facing lockdowns (Taiwan CDC, 2020a). However, in contrast to the predictions, Taiwan’s actual numbers had thus far been minimal and well-controlled (Wang and Ellis, 2020). Many scholars attribute Taiwan’s effective responses to their past experiences with the 2003 SARS outbreaks and the 2009 pandemic influenza H1N1, the use of big data to enhance surveillance and contact tracing, effective communication, early deployment of strict border controls (Chiu et al., 2020, Wang et al., 2020), and strengthening the health care system with the enhanced traffic control bundling (TCB) (Yen et al., 2021). This TCB, which was developed in Taiwan during 2003 SARS, had been proved to effectively curtail nosocomial transmission and help control the outbreaks. The TCB incorporates three components: (1) Triage before entering hospitals; (2) Separating zones of risks; (3) Checkpoint hand disinfection. However, there were 3 major leaks without quarantine (Figure 1 ) that could have resulted in community spread: (1) the Diamond Princess cruise ship docked at Keelung Port, where 2694 passengers went ashore on January 31 and visited many sites; (2) the early-March influx of Taiwanese students returning from Europe and the USA, where SARS-CoV-2-positive cases were on the rise (Murray, 2020); and (3) the debarking of 744 crew members from a Taiwanese naval fleet in mid-April with 36 SARS-CoV-2-positive cases who had visited many cities (Taiwan CDC, 2020b). Nonetheless, Taiwan had only 808 laboratory-confirmed SARS-CoV-2 cases, including 56 indigenous cases and 7 deaths, as of December 31, 2020 (National Center for High-performance Computing (NCHC, 2021).
Figure 1.
Countermeasures and an epidemic curve of the laboratory-confirmed SARS-CoV-2 cases in Taiwan from January 1 to December 31, 2020, based on the dates of illness onset for most cases.
The bottom parts involve the 4 major public health policies, which are shown as “B” for border control, “T” for policies related to traffic control bundle or enhanced TCB (TCB or eTCB), “M” for mask-related policies, and “A” for alcohol-based hand hygiene. All the numbers are based on the order of calendar dates from when the policy started to be implemented (Taiwan Ministry of Health and Welfare, 2020). The top 3 arrow-shaped text boxes represent the 3 major leaks (see details in the Introduction section). The overall public health prevention measures involve 3 tiers: 1st tier with border control, 2nd tier with eTCB, and 3rd tier with public cooperation in terms of wearing masks and exercising alcohol-based hand hygiene. Border control involves all travelers entering Taiwan requiring 14 days for quarantine plus an additional 7 days of self-health-management (effective March 19, 2020). The surge of medical demand for surgical masks and the prohibition on exports of surgical masks after the lockdown in Wuhan, China, on January 23, 2020, provoked social panic, resulting in Taiwanese citizens rushing to stand in long lines to buy facemasks until a massive increase in facemask production was achieved.
We used the dates of illness onset for most cases (529/808, 65.5%) with clear information. However, 279 cases (279/808, 34.5%) without clear onset dates of illness, including 258 asymptomatically infected SARS-CoV-2 cases (258/ 279, 92.5% [identified by antibody test]) were plotted by laboratory-confirmation dates because onset dates of illness were not available for asymptomatic SARS-CoV-2 cases and a small proportion of the cases were identified either from contact tracing with time delays or from retrospective laboratory tests of SARS-CoV-2 for the reported human influenza severe cases with influenza-negative laboratory results, based on the recommendations from the Taiwan COVID-19 advisory group meeting. All these data were released by Taiwan CDC and were accessible on January 16, 2021 (National Center for High-performance Computing (NCHC, 2021).
During the 2003 SARS outbreaks, Taiwan had suffered from heavy loss of healthcare workers (HCWs) (Figure 4) (Lee and Hsueh, 2020, Yen et al., 2021). The specific aims of this study were: (1) to identify the preventive behaviors practiced during 2003 SARS or 2009 H1N1, (2) to investigate the role of wearing facemasks and practicing alcohol-based hand hygiene (AHH) as part of the Taiwanese preventive behaviors in COVID-19, and (3) to search for characteristics associated with persistently practicing these preventive behaviors from the 2003 SARS/2009 H1N1 era to COVID-19 in 2020. We hope our findings can help in combating COVID-19 more effectively.
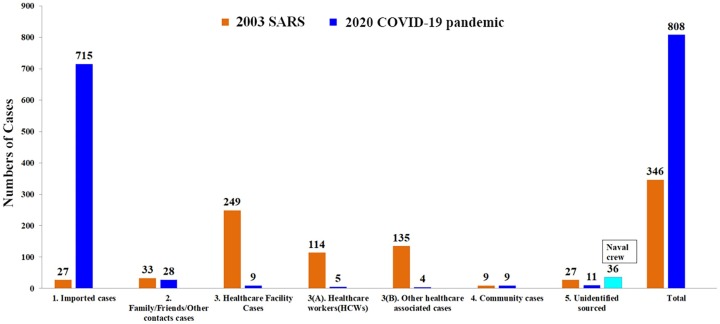
Figure 4.
The number of cases originating in the 5 major sources of the risk groups during the 2003 SARS outbreak and COVID-19.
The 5 risk groups in Taiwan during the 2003 SARS outbreak (shown in brown) versus COVID-19 (shown in blue) were: (1) imported cases; (2) family, friends, and other contact cases infected directly from the imported cases; (3) healthcare facility-associated cases, including 3(A) healthcare workers (HCWs) and 3(B) others; (4) community cases (i.e., cases in which the sources of the SARS-CoV-2 infections were schools, stores, apartments, commercial buildings, and transportation); and (5) unidentified-source cases (i.e., cases without clear sources of infection, even after thorough contact tracing and epidemiological investigations by public health professionals at local departments of health with joint efforts of local Taiwan CDC branches). All these data were released from Taiwan CDC and were accessible on January 16, 2021 (National Center for High-performance Computing (NCHC, 2021).
Methods
Study design and setting
To understand the prevention behaviors practiced in Taiwan during COVID-19 compared with those practiced during the 2 specific past outbreaks of 2003 SARS and 2009 H1N1, we conducted a retrospective survey to determine the significant characteristics underlying Taiwanese citizens’ participation in effectively containing the COVID-19 epidemic. All laboratory-confirmed SARS-CoV-2 cases documented in Taiwan’s Central Epidemic Command Center (CECC) were used to evaluate the effectiveness of the overall prevention and control measures.
Taiwan’s two major strategies involving citizens in mitigating COVID-19
Community alcohol-based hand hygiene (AHH) initiative
AHH became a standard nosocomial infection control policy in the aftermath of SARS. This policy extended beyond hospitals into broader communities during 2009 H1N1 emphasizing the use of alcohol-based hand sanitizers (ABHS) to prevent disease transmission.
During COVID-19, the Taiwanese government encouraged citizens to participate in the national campaign of disinfecting hands before entering and leaving hospitals to prevent infections (Taiwan CDC, 2020c).
Community facemask initiative
The practice of wearing facemasks in response to an emerging respiratory disease started from countries affected by the 2003 SARS outbreaks and it has become a personal protection behavior since then (Chen et al., 2004, Feng et al., 2020). As SARS-CoV-2 proved to be transmissible via droplets even before symptoms appear (To et al., 2020), government guidelines reinforced that citizens should wear facemasks in 8 types of public venues (Footnotes of Table 1 ) and in areas where appropriate physical distancing is not possible (Taiwan CDC, 2020d).
Table 1.
Characteristics of participants with good and poor preventive behaviors against SARS-CoV-2 infection.
| Characteristics | No. (%) of participants* |
||||
|---|---|---|---|---|---|
| Total, N = 2328 | Individuals with poor preventive behaviors against SARS-CoV-2 infection (PPBG), n = 694 | Individuals with good preventive behaviors against SARS-CoV-2 infection (GPBG), n = 1634 | P value | ||
| Demographics | |||||
| Age, years (mean ± SD) | 48.9 ± 12.1 | 49.0 ± 12.6 | 48.8 ± 11.9 | 0.748 | |
| 18–49 | 1142 (49.1) | 334 (48.1) | 808 (49.5) | 0.559 | |
| ≥50 | 1186 (51.0) | 360 (51.9) | 826 (50.6) | ||
| Gender | |||||
| Female | 1403 (60.3) | 357 (51.4) | 1046 (64.0) | <0.001 | |
| Male | 925 (39.7) | 337 (48.6) | 588 (36.0) | ||
| Recruitment methods | |||||
| Web system | 1583 (68.0) | 496 (71.5) | 1087 (66.5) | 0.064 | |
| Primary-care clinics | 260 (11.2) | 68 (9.80) | 192 (11.8) | ||
| Others | 485 (20.8) | 130 (18.7) | 355 (21.7) | ||
| Sources of COVID-19 prevention information | |||||
| Taiwan Central Epidemic Command Center | 1917 (82.4) | 536 (77.2) | 1381 (84.5) | <0.001 | |
| Internet news | 1522 (65.4) | 446 (64.3) | 1076 (65.9) | 0.462 | |
| Television | 1479 (63.5) | 445 (64.1) | 1034 (63.3) | 0.700 | |
| Social media | 1420 (61.0) | 424 (61.1) | 996 (61.0) | 0.949 | |
| Online information | 671 (28.8) | 182 (26.2) | 489 (29.9) | 0.071 | |
| Newspapers | 312 (13.4) | 114 (16.4) | 198 (12.1) | 0.005 | |
| Relatives/friends | 192 (8.3) | 64 (9.2) | 128 (7.8) | 0.265 | |
| Past preventive behaviors | |||||
| Wearing mask | 1551 (66.6) | 453 (65.3) | 1098 (67.2) | 0.368 | |
| Regularly disinfecting hands with ABHS | 1029 (44.2) | 273 (39.3) | 756 (46.3) | 0.002 | |
| Present preventive behaviors | |||||
| Wearing mask indoors or while taking mass transportation | 2309 (99.2) | 679 (97.8) | 1630 (99.8) | <0.001 | |
| Wearing mask outdoors | 1884 (80.9) | 639 (92.1) | 1245 (76.2) | <0.001 | |
| Disinfecting hands with ABHS while entering a building | 2290 (98.4) | 658 (94.8) | 1632 (99.9) | <0.001 | |
| Carrying ABHS | 1523 (65.4) | 66 (9.5) | 1457 (89.2) | <0.001 | |
| Increasing frequency of washing hands with water and soap in public areas | 2172 (93.3) | 558 (80.4) | 1614 (98.8) | <0.001 | |
| Future preventive behaviors | |||||
| Maintaining the COVID-19 preventive behaviors when no indigenous SARS-CoV-2 cases are laboratory-confirmed | 1933 (83.0) | 557 (80.3) | 1376 (84.2) | 0.020 | |
| Maintaining the COVID-19 preventive behaviors when sporadic cases of indigenous SARS-CoV-2 are laboratory-confirmed | 2183 (93.8) | 633 (91.2) | 1550 (94.9) | 0.001 | |
| Agreeing with and following the COVID-19 new lifestyle if global COVID-19 pandemic would last for 1-2 years | 2259 (97.0) | 664 (95.7) | 1595 (97.6) | 0.012 | |
*Unless stated otherwise.
ABHS, alcohol-based hand sanitizer; GPBG, good prevention behavior group; PPBG, poor prevention behavior group; SD, standard deviation.
*See the details in the Methods section.
The “good preventive behavior group” in 2020 (2020-GPBG) was defined as participants who had ≥4 points (taking at least 4 correct prevention measures). In contrast, the “poor preventive behavior group” (2020-PPBG) was defined as those who had ≤3 points.
The government guideline is to urge citizens to wear facemasks in 8 types of public venues, including healthcare facilities, markets and shopping centers, schools/educational centers, sporting events and exhibition venues, religious places, entertainment sites, public transportation, any type of gathering, and areas where appropriate physical distancing (1.5-m separation indoors, and 1-m separation outdoors) is not possible (Taiwan Centers for Disease Control (Taiwan CDC, 2020d).
Survey of preventive behaviors practiced by Taiwanese citizens
Study population
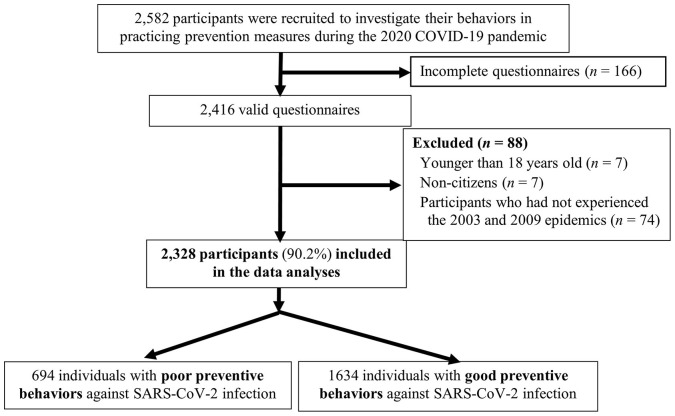
To explore Taiwanese citizens’ (including patients) preventive behaviors regarding wearing facemasks and practicing AHH in COVID-19, we administered an anonymous questionnaire to Taiwanese residents from July 29 to August 6, 2020, through several channels. Among the 2416 questionnaires returned with complete answers for each question (Figure 2 ), 1646 (68.1%), 265 (11.0%), and 505 (20.9%) were collected from the web system, local primary-care clinics, and other channels (such as e-mail, Facebook, and LINE groups), respectively.
Figure 2.
A flow chart of participant enrollment for investigating their practice of prevention behaviors during COVID-19 (2020).
Finally, we excluded 7 persons younger than 18 years old, 7 non-citizens, and 74 participants who had not experienced the 2003 and 2009 epidemics (due to using McNemar’s tests for the paired data of those who experienced both epidemics—of 2003/2009 and 2020). The remaining 2328 individuals were included in the data analysis.
Data collections and variables
The questionnaire involved 3 parts: (1) demographic characteristics (age, gender, and location of residence in Taiwan); (2) sources of information (government agencies (such as the Taiwan CECC), television, newspaper, internet news, social media, online information, and relatives/friends); and (3) preventive behaviors during the 3 periods, that are: (1) the past (i.e., during the 2003 SARS outbreaks or the 2009 H1N1 pandemic influenza), (2) present period in 2020 (from the beginning of the COVID-19 lockdown in Wuhan in 2020 and the subsequent period of intense transmission of SARS-CoV-2 in Taiwan until June 7, 2020), and (3) the future, meaning, after the easing of COVID-19 restrictions in Taiwan (i.e., after this survey) (see Supplementary 1: questionnaire). To fully understand the questions in the questionnaire, we held a small focus group involving 35 participants in a pilot study. After collecting this feedback, several questions were revised before administering the final questionnaire.
Behavior scoring, outcomes, and statistical analysis
To ascertain the characteristics associated with the persistent adoption of preventive measures during the 2003 SARS/2009 H1N1 outbreaks and during COVID-19, we developed a scoring system as follows: a participant practicing any one of the correct preventive behaviors scored “1 point” whereas a participant neglecting to practice any one of the correct preventive behaviors scored “0 points”. Therefore, the highest score possible for the 2020 preventive behaviors was 5 points. Wearing masks outdoors scored 0 points in accordance with government rules in 2020 (Taiwan CDC, 2020e); however, wearing masks during mass gatherings outdoors scored 1 point. Because many cautious Taiwanese citizens might have worn facemasks outdoors at all times, they could potentially have scored 4 points. Therefore, the “good preventive behavior group” in 2020 (2020-GPBG) was defined as participants who had ≥4 points (i.e., taking at least 4 correct prevention measures). In contrast, the “poor preventive behavior group” (2020-PPBG) was defined as those who earned ≤3 points.
Both univariable and multivariable analyses were performed to compare the 2020-GPBG and 2020-PPBG. In univariable analysis, means of age in these 2 groups were compared using the student’s t-test. The association of categorical variables between the 2 prevention groups was examined using either the chi-squared test or Fisher’s exact test. In addition, McNemar’s test for paired data was used to test significant differences in the practice of preventive behaviors between the 2003 SARS/2009 H1N1 and COVID-19 in 2020 (i.e., paired 2003/2009 and 2020 data of the same person for each individual belonging to the 2020-GPBG vs the 2020-PPBG). In testing the preventive behaviors in 2003/2009 versus 2020, a P-value <0.05 was considered statistically significant.
To assess the associations of selected characteristics with the 2020-GPBG (preventive behavior scores ≥4), univariable and multivariable logistic regression analyses were performed. All variables found to be significant (P < 0.10) through univariable analysis were included in multivariable analysis using the SAS program. Subsequently, multivariable analyses were conducted to identify the factors associated with the 2020-GPBG. A logistic regression model with a stepwise selection of variables was used. The variables included in the regression model were age, gender, recruitment methods, sources of COVID-19-prevention information, wearing of masks during the 2003 SARS/2009 H1N1 in Taiwan, and regular disinfecting of hands with ABHS during 2003 SARS/2009 H1N1 in Taiwan. The best model was selected from the candidate models through a stepwise search. The odds ratios (ORs) were calculated from the coefficients of the regression models, and P-values of <0.05 were regarded as statistically significant. The crude ORs and adjusted ORs (aORs) with 95% confidence intervals (CIs) were reported to show the strength and direction of these associations.
We used the ‘stats’ package in the R studio version 4.0.2 (2020-06-22) (R Core Team, 2018) to do the statistical tests, including chi-squared tests, McNemar tests, and fitting Generalized Linear Models. All the multivariable data analyses were examined as 3 independent analyses. The logistic regression results from the SAS program version 9.4 were double-checked by 2 other persons using the R version 4.0.2 and SPSS programs version 22 to enhance internal validity.
Evaluation of prevention and control measures
Physicians in Taiwan are required to report any suspected case of COVID-19 to the CECC within 24 h (Taiwan CDC, 2020f). Laboratory-confirmed cases in Taiwan are defined as SARS-CoV-2-positive results mainly by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) (Wölfel et al., 2020) and partially by serological tests that quantitatively detect SARS-CoV-2 antibodies in human serum, using commercial kits from Elecsys (Roche Labs, Basel, Switzerland) (Long et al., 2020) for identifying asymptomatic cases or late reported cases.
We calculated the overall monthly incidence rates in 2020 and 2003 and also evaluated them using the Wilcoxon rank-sum test. Additionally, the total numbers of cases in the 5 risk groups (see the Legends and footnotes of the Figure 4) in 2020 and 2003 were further compared using chi-square tests (Taiwan CDC, 2020f; Taiwan CDC, 2013) (Figure 4).
Results
Participants in the survey of preventive behaviors
This study recruited 2582 participants to investigate their practice of preventive behaviors during COVID-19 in 2020. After excluding 7 persons younger than 18 years old, 7 non-citizens, and 74 participants who reported not having engaged in protective practices such as mask wearing during both the 2003 SARS and 2009 H1N1 from the 2416 participants with completed questionnaire, the remaining 2328 individuals were included in the data analysis (Figure 2). Their demographic information was as follows: the overall mean and standard deviation (mean ± standard deviation) of age was 48.9 ± 12.1 years (68.6% were older adults aged 40–64 years and only 4.8% were younger adults aged <25 years); 60.3% of participants were female; and 60.6%, 16.8%, 20.5%, and 2.1% were living in northern, central, southern, and eastern Taiwan, respectively (Table 1).
Comparison of Taiwanese participants’ 2 important preventive behaviors against infection during the 2003 SARS/2009 H1N1 epidemic versus COVID-19 in 2020
Wearing facemasks in 2003/2009 versus 2020
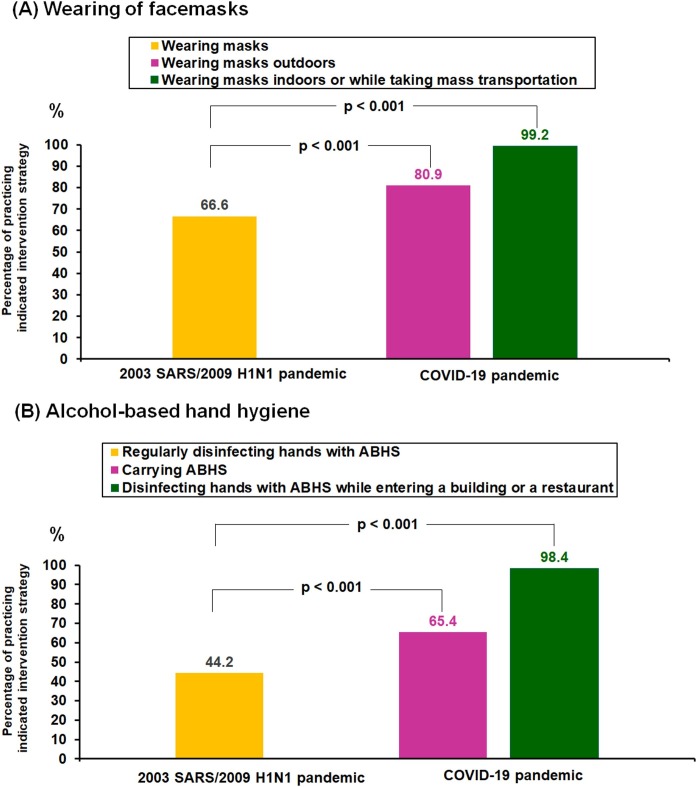
The proportion of participants wearing facemasks significantly increased from 66.6% during the 2003 SARS/2009 H1N1 pandemic to 99.2% (P < 2.2e-16) and 80.9% (P < 2.2e-16) in indoor and outdoor environments, respectively, during COVID-19 (Figure 3A ).
Figure 3.
Percentages of Taiwanese citizens practicing (A) wearing of facemasks, and (B) alcohol-based hand hygiene during the 2003 SARS/2009 H1N1 pandemics versus during the COVID-19 pandemic in 2020.
The proportion of participants wearing facemasks and the proportion of those disinfecting hands with alcohol-based hand sanitizer (ABHS) significantly increased from the 2003 SARS/2009 H1N1 pandemics to the COVID-19 pandemic from January 23 to June 7, 2020, in Taiwan.
Practicing hand hygiene in 2003/2009 versus 2020
The proportion of participants disinfecting their hands with ABHS significantly increased from 44.2% during the 2003 SARS/2009 H1N1 pandemic to 98.4% disinfecting hands with ABHS on entering a commercial building or restaurant (P < 2.2e-16) and 65.4% carrying ABHS (P = 2.695e-05) during COVID-19 (Figure 3B).
Characteristics of the participants with good versus poor preventive behaviors against COVID-19
A total of 70.2% of the Taiwanese participants belonged to the “good preventive behavior group in 2020” (2020-GPBG) (Table 1). Univariable analysis showed that those with good preventive behaviors during COVID-19 were significantly more likely to be: (1) female; (2) those who received COVID-19-prevention information from the Taiwan CECC; and (3) those who disinfected their hands with ABHS during the 2003 SARS outbreaks/2009 H1N1 pandemic (P < 0.01) (Table 2 ). Moreover, it was found that these participants were more likely to maintain such preventive behaviors even if there were only sporadic or zero indigenous COVID-19 cases. However, those participants who had received COVID-19 prevention information from newspapers (rather than directly from the official CECC) were less likely to practice good preventive behavior against COVID-19.
Table 2.
Crude and adjusted odds ratios (ORs) for the characteristics associated with the good preventive behaviors group against SARS-CoV-2.
| Variables | Number of participants (n = 2,328) |
No. (%) of individuals in good preventive behaviors group (GPBG) against SARS-CoV-2 |
Crude ORs a (95% CI) |
Adjusted ORs b (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) 18–49 | 1,142 | 808 (70.8) | 1 | |
| ≥50 | 1,186 | 826 (69.6) | 0.95 (0.79 to 1.13) | |
| Gender Male | 925 | 588 (63.6) | 1 | 1 |
| Female | 1,403 | 1,046 (74.6) | 1.68 (1.40 to 2.01)*** | 1.72 (1.42 to 2.07)*** |
| Recruitment methods | ||||
| Web system | 1,583 | 1,087 (68.7) | 1 | 1 |
| Primary-care clinics | 260 | 192 (73.8) | 1.29 (0.96 to 1.73) | 1.43 (1.06 to 1.95)* |
| Others | 485 | 355 (73.2) | 1.25 (0.99 to 1.56) | 1.18 (0.93 to 1.48) |
| Sources of COVID-19 prevention information | ||||
| Taiwan Central Epidemic Command Center (CECC) | 1,917 | 1,381 (72.0) | 1.61 (1.29 to 2.01)*** | 1.52 (1.21 to 1.91)*** |
| Online information | 671 | 489 (72.9) | 1.20 (0.98 to 1.47) | 1.22 (0.99 to 1.49) |
| Internet news | 1,522 | 1,076 (70.7) | 1.07 (0.89 to 1.29) | |
| Social media | 1,420 | 996 (70.1) | 0.99 (0.83 to 1.19) | |
| Television | 1,479 | 1,034 (69.9) | 0.96 (0.80 to 1.16) | |
| Relatives/friends | 192 | 128 (66.7) | 0.84 (0.61 to 1.15) | |
| Past preventive behaviors | ||||
| Regularly disinfecting hands with ABHS | 1,029 | 756 (73.5) | 1.33 (1.11 to 1.59)** | 1.37 (1.14 to 1.65)*** |
| Wearing mask | 1,551 | 1,098 (70.8) | 1.09 (0.90 to 1.31) |
CI, confidence interval; ABHS, alcohol-based hand sanitizer. P values: *<.05; **<.01; ***<.001.
Sources of COVID-19 prevention information: Each participant can select up to 4 of 8 choices. In data analysis, each choice was analyzed with or without using that specific information channel.
Models:
Logit(P) =0 +, where P was the probability of belonging to “good-preventive behaviors” group against SARS-CoV-2 and k was the total number of explanatory variables. There were 2 models with detailed descriptions as follows.
a. Crude Model was to explore how a single factor (k = 1) affects the outcome measure (GPBG) expressing as “Crude OR” without adjusting potential covariates.
b. Final Model# with the same outcome measure as the Crude Model but involved 5 predictor variables (k = 5), including: (1) gender, (2) recruitment methods, (3-4) 2 sources of COVID-19 prevention information variables (Taiwan CECC, online information), and (5) past preventive behavior (regularly disinfecting hands with ABHS) expressing as “Adjusted OR” with adjusting important covariates.
Logit(P) =0 + [gender] + [recruitment methods] + [Taiwan CECC] + on-line information] + [past preventive behavior (regularly disinfecting hands with ABHS)].
#The variable of “living areas in Taiwan” was not accommodated in the model because there were zero SARS-CoV-2 cases in eastern Taiwan, and the sample size in this study was also quite small.
Characteristics associated with good preventive behaviors against COVID-19
Multivariable logistic regression was used to identify the independent characteristics associated with good preventive behaviors against COVID-19 in Taiwanese participants. After controlling for demographic variables, recruitment methods, and practice of preventive behaviors during the 2003 SARS/2009 H1N1 outbreaks, the results from the SAS program revealed 4 variables were significantly associated with good preventive behaviors against COVID-19 in 2020 (aOR >1) (Table 2): (1) females (aOR 1.72, 95% CI 1.42–2.07, P < 0.001); (2) those who received COVID-19-prevention information from the official CECC channel (aOR 1.52 (1.21–1.91), P < 0.001); (3) participants recruited from primary-care clinics (aOR 1.43 (1.06–1.95), P < 0.05); and (4) those who often disinfected their hands with ABHS during the 2003 SARS outbreaks/2009 H1N1 pandemic (aOR 1.37 (1.14–1.65), P < 0.001). The aOR values from R and SPSS also showed similar results (Supplementary Table 1).
Evaluation for infection control in healthcare facilities and overall incidence and case fatality rate
The percentage of healthcare-associated infections and healthcare worker (HCW)-related infections during COVID-19 were significantly lower than that during the 2003 SARS outbreaks (healthcare-associated infections: 1.1% (9/808) vs 72.0% (249/346), P < 2.2e-16; HCWs: 0.6% (5/808) vs 32.9% (114/346), P < 2.2e-16) (Figure 4). The percentages of cases of community and unidentified sources in 2020 were also significantly lower compared with 2003 (community cases: 1.1% (9/808) vs 2.6% (9/346), P = 0.061737; unidentified sources: 1.4% (11/808) vs 7.8% (27/346), P = 1.9221e-08) (Figure 4). As of December 31, 2020, the monthly incidence rate of laboratory-confirmed SARS-CoV-2 cases in Taiwan (number of cases for total 12 months per 1 million of the population) in 2020 was lower than that of SARS-CoV-1 in Taiwan in 2003 [0.2 ± 0.4 (57/23,574,334/12) vs 1.2 ± 2.7 (330/22,604,550/12), P > 0.05]. The case fatality rate in Taiwan also had a statistically significant decrease in 2020 compared with 2003 (0.9% (7/808) vs 21.1% (73/346), P < 2.2e-16).
Discussion
COVID-19 has caused major global disruptions. SARS-CoV-2 is highly transmissible and requires public participation in prevention and control measures (Lee and Hsueh, 2020, Lai et al., 2020). Our study has 3 unique findings with global applications. First, the percentages of participants wearing facemasks while also practicing AHH showed statistically significant increases from the 2003 SARS outbreaks to COVID-19 in 2020 (P < 0.001), suggesting the importance of combining both prevention measures to halt the local transmission of SARS-CoV-2. Second, risk communication is important because participants in the 2020-GPBG acquired most information on COVID-19 from the government CECC, had their questionnaires collected from primary-care clinics, and practiced AHH during the 2003 SARS outbreaks, leading to Taiwanese citizens successfully following prevention strategies in 2020. Third, a nationwide, integrated prevention strategy is vital to the reduction of SARS-CoV-2 infections. Taiwan’s integrated approach involved government–public cooperation not just in healthcare facilities but also in non-healthcare settings. SARS-CoV-2 infections in healthcare facilities and among HCWs were both significantly lower in comparison with the SARS-CoV-1 outbreaks. We believe that a reported 99.2% practice rate of wearing facemasks indoors and a 98.4% practice rate of AHH upon entering buildings/restaurants contributed to Taiwan not having to implement a nationwide lockdown.
This study is the first to address the significant role of the willingness of citizens to practice AHH and wear facemasks in curbing the spread of SARS-CoV-2. Our multivariable analyses showed that past AHH practices and visits to healthcare facilities boosted the practice of good preventive behaviors in 2020. AHH has been applied in healthcare settings since 2005, when the World Health Organization (WHO) started paying more attention to hand hygiene (Pittet and Donaldson, 2005). From the 2009 H1N1 influenza pandemic to the COVID-19 pandemic in 2020, a series of public health policies have resulted in the availability of alcohol-based disinfectant dispensers at the entrances of hospitals and major buildings, and the 2020 public health initiatives further consolidated AHH as a cultural norm in Taiwan. Fomite transmission had been mostly ignored in emerging infectious diseases until COVID-19 (Santarpia et al., 2020). In addition to hand hygiene, wearing facemasks is also helpful in curbing fomite transmission through source control of droplet transmission (Lai et al., 2012, Chin et al., 2020). Our findings show that a high percentage of AHH compliance, together with wearing facemasks, has proven effective in reducing community spread. Future projection on the compliance of prevention behaviors (Supplementary Table 2) also showed that the crude odds ratios increased from Scenario 1 (zero indigenous COVID-19 cases) to Scenario 2 (sporadic COVID-19 cases) and to Scenario 3 (the COVID-19 pandemic lasts for 1 to 2 years). However, wearing facemasks has been a controversial issue in Western countries, where many citizens believe that only infected persons have to wear facemasks or that mask mandates threaten personal liberty (Olivera-La Rosa et al., 2020). Mask-wearing compliance in these Western countries thus is not sufficiently high to effectively curb viral transmission (Chen and Fang, 2021). Although comprehensive data is difficult to obtain, progressively more and more evidence has shown that wearing facemasks provides community-wide benefits (Lyu and Wehby, 2020, Chu et al., 2020, Cowling et al., 2020). Among studies where the benefits of wearing facemask are still controversial, most had ignored the complementary efficacy of wearing facemasks and practicing AHH and overlooked the significance of fomite transmission either when the percentage of people wearing facemasks was inadequate or when water and soap for hand hygiene were inaccessible (Pittet et al., 2000, Bundgaard et al., 2020).
Effective governance is crucially important to address the initial COVID-19 challenges. Two widely used approaches are top-down governance, such as fast lockdown in authoritarian countries, and bottom-up governance, like that in democratic countries, where wearing facemasks and AHH may not be mandatory. Public mobilization, such as the availability of the polio vaccine in the U.S. in 1955 due almost entirely to the efforts of the March of Dimes (Wilson, 2005) and the national hand-hygiene campaign in Germany (Reichardt et al., 2013), have successfully achieved their intended goals. Since the Taiwanese government wants their citizens to have a high percentage of protection behavior compliance, after understanding the dynamics of the COVID-19 pandemic, the CECC had almost daily press briefings plus social media risk communication covering the most updated epidemiological data in Taiwan and global trends plus the Chinese translation of the WHO guidelines and the CECC guidelines. We found that participants who maintained good preventive behaviors in 2020 obtained risk communication from the government CECC and public initiatives and also acquired the appropriate prevention guidance from health professionals. Recent models from Taiwan indicate that facemasks, when worn in over 75% of the population, coupled with quarantine requirement for all travelers entering Taiwan (effective March 19, 2020), leads to SARS-CoV-2 transmission becoming unsustainable (Chen and Fang, 2021). Importantly, policies that emphasize combined strategies, including early border controls, quarantine policies, innovative health information technology integrated with national healthcare insurance, and contact tracing, have helped effectively interrupt viral transmission (Chiu et al., 2020, Cheng et al., 2020). The 70.2% of participants closely adhering to Taiwan's recommended preventive behavior practices exceeded the estimated herd immunity threshold of 60%–67% (Buss et al., 2021) prior to COVID-19 vaccination (starting March 22, 2021). Based on our data, public health agencies worldwide could contain highly contagious emerging viruses with sufficient coverage of population-based non-biological preventive measures before the establishment of adequate herd immunity. Our population-based countermeasures demonstrated why countries that reported low adherence to comprehensive personal protection measures have had multiple waves of COVID-19 outbreaks (Machida et al., 2020, Machida et al., 2021). Taiwan’s success was further supported by the absence of domestic SARS-CoV-2-positive cases for 253 consecutive days (from April 13 to December 21, 2020). New Zealand quickly responded to its first COVID-19 wave in 2020 by rapidly implementing strict border closures, national lockdown, and surveillance enhancements (Jefferies et al., 2020, Summers et al., 2020). However, subsequent transmission from potentially unnoticed cases occurred, which could have been avoided by wearing masks and practicing AHH. It is time for public health agencies worldwide to make rational recommendations on the appropriate use of facemasks to complement other preventive measures (Feng et al., 2020).
From September to November 2020, there were near synchronous resurgences of SARS-CoV-2 cases across the US, Europe, and Asia. Undoubtedly, the resumption of daily activities and gatherings with asymptomatic or pre-symptomatic people accounted for as much as 40% to 60% of total cases (He et al., 2020). Similarly, the third COVID-19 wave in Europe, which started in March 2021, has demonstrated difficulties in fighting against more contagious SARS-CoV-2 variants without timely adequate population-based preventive measures (Kluge, 2021, Khan, 2021). These unexpected surges might occur from a single episode of cluster of cases and then have initiated a series of transmission chains. Without successful control mechanism, these cluster cases can quickly increase the effective reproductive number above the community threshold, which would threaten the healthcare systems (Yen et al., 2021), creating a vicious cycle of community-hospital-community infections, leading to broader, longer, and more severe waves, or even a large-scale epidemic (Yen et al., 2020). On the other hand, Taiwan’s public initiatives against COVID-19 focused on high participation in the 2 population-based preventive behaviors to interrupt the viral transmission (i.e., population-blockade) quite early in the Susceptible-Exposed-Infectious-Removed (SEIR) dynamic model (Faranda and Alberti, 2020). Our study indicates that the policies governing universal mask usage must target a high population coverage, be coupled with AHH, and these efforts effectively foster government–civilian cooperation to improve public compliance. Indeed, such a total coverage rate must be even higher in areas dominated by fast-spreading SARS-CoV-2 variants that could reduce the neutralization capacity of the vaccine (Lazarevic et al., 2021, Lauring and Hodcroft, 2021, Rowan and Moral, 2021, Gondim, 2021, Weisblum et al., 2020). Therefore, we propose a “threshold-based bundle strategy” combining facemask-wearing or physical distancing (physical intervention), ABHS or other hand sanitation methods (chemical interruption), and population immunity from vaccine plus natural infection (biological interference). The total percentage of participating in these three (physical + chemical + biological) preventive strategies should be greater than the required threshold for continuous viral transmission in a community in order to effectively curb the spread of COVID-19 pandemic and/or future respiratory transmitted emerging infectious diseases. Importantly, the demand for compliance with such population protective behaviors cannot be relaxed, if total percentage of all population-based interventions against SARS-CoV-2 has not been reached (Huang et al., 2021, Tirachini and Cats, 2020). In other words, citizens’ preventive behavior is the most cost-effective way to combat viral spread before the final defending capability (i.e., sufficiently high total intervention percentage) has been reached globally.
This study has 4 major limitations. First, this was a retrospective survey, and several explanatory variables leading to preventive behaviors were not collected, as they were considered to be barriers to recruitment of participants. Second, our study population was recruited via the internet system, primary-care clinics or social media, which may have resulted in social desirability bias, recall bias, and/or sampling bias. Nonetheless, our findings are supported by another recent study showing that over 70% of the targeted general population in Taiwan wear facemasks (Chen et al., 2020). Third, this study cannot differentiate between the combined effect of wearing facemasks and hand-hygiene disinfection and the isolated effect of each practice, and we also cannot exclude the effect of other preventive/control measures (Greenhalgh et al., 2020, Godlee, 2020). Fourth, our seroepidemiological data on the infection rate of SARS-CoV-2 among HCWs, which was used to evaluate the infection control at healthcare facilities, was not nationwide (Tseng et al., 2021, Pan et al., 2021, Ho et al., 2020, Everington, 2020).
In conclusion, our results confirm that Taiwan’s integrated intervention strategies, including wearing facemasks, practicing AHH, and implementing enhanced traffic control bundles in healthcare facilities, have increased the effectiveness of the preventive measures during the COVID-19 pandemic responses. Furthermore, taken together, early border controls, government leadership, and widespread risk communication emphasizing the importance of preventive behaviors have successfully enabled Taiwan to avoid lockdowns. These comprehensive preventive responses to COVID-19 in 2020, like immune-boosting responses, are more efficient and effective than those implemented during 2003 SARS. Given the likelihood of additional waves plus the dominance of fast-spreading SARS-CoV-2 variants in many countries, we recommend that our strategies could be adopted internationally and modified to fit the social/cultural context of other countries to effectively control the COVID-19 pandemic before mass vaccination worldwide is finally achieved.
Ethical approval
This study was approved by the Institutional Review Board of Taipei City Hospital (TCH) (no. TCHIRB-10907007-E) on July 26, 2020. Questionnaires and data were fully anonymized to protect participants’ privacy, and only aggregated data were used for further analyses and statistical tests.
Availability of data and materials
All the data for this study will be available upon request to the corresponding authors.
Funding source
This study was supported by grants from the Department of Health, Taipei City Government (Taipei City Grant No. 10901-62-007), Department of Emergency Medicine, NTU Hospital Research Support Grant, Taiwan CDC (Grant No. DOH92-DC-SA), National Science Council (reorganized as Ministry of Science and Technology since 2014) in Taiwan (Grant No. 92-2751-B-002-020-Y), and National Health Research Institutes (Grant Numbers: MR-109-GP-14, MR-110-CO-18, NHRI-110A1-MRCO-0121210).
Conflict of interest
None declared.
Author contributions
Concept and design of the study: M.Y.Y., C.C.K., and Y.F.Y. Acquisition of the data: T.Y.C., K.H.H., T.I.L., T.H.T., M.Y.Y., and C.C.K. Data analysis and interpretation of the data: Y.F.Y., T.I.L., K.H.H., L.Y.H., T.H.T., C.C.K., M.Y.Y., S.Y.C., and T.C.C. Data validation and visualization: K.H.H., T.I.L., L.Y.H., T.H.T., C.C.K., Y.F.Y., T.C.C., S.Y.C., and M.Y.Y. Drafting the manuscript: M.Y.Y., C.C.K., and Y.F.Y. Review, critical revision of the manuscript, and editing: C.C.K., M.Y.Y., S.Y.C., T.C.C., L.Y.H., Y.F.Y., P.R.H., T.I.L., K.H.H., and T.H.T. All authors approved the final version of the manuscript.
Acknowledgments
We would like to sincerely thank medical and public health professionals in Taiwan, Centers for Disease Control (Taiwan CDC), local Departments of Health, Central Epidemic Command Center (CECC),and National Health Research Institute (NHRI) in Taiwan, for their assistance in epidemiological investigation, data collection of laboratory-confirmed SARS-CoV-2 cases, and prevention/control efforts in Taiwan. The administrative support from Taipei City Hospital and local clinics is unforgettable. The discussion inputs from the following scholars are highly appreciated: Dr. Chi-Tai Fang, Ms. Yi-Hsuan Chen, Dr. Hsien-Ho Lin, Dr. Hsiu-Hsi Chen, and Dr. Wen-Chung Lee at the Institute of Epidemiology and Preventive Medicine (IEPM), College of Public Health, National Taiwan University (NTU-CPH), and Dr. Pau-Chung Chen at the Institute of Environmental and Occupational Health Sciences, NTU-CPH, Taipei, Taiwan, Dr. Jonathan Schwartz at the Department of Political Science, State University of New York, New Paltz, NY, USA., Dr. Barry T. Rouse at the University of Tennessee, USA (on epidemiology and public health policies), Dr. Li-Yu Daisy Liu at the NTU Department of Agronomy, Dr. Wan-Yu Lin, and Dr. Jui-Hsiang Lin at the NTU-IEPM (on biostatistical data analyses and interpretation of the results). We also would like to express our sincere gratitude to the English editors Mr. Marc Anthony, Ms. Anita Zhang, Dr. Neal Lin, Mr. James Steed, Mr. Nicholas T. Minahan, Mr. Iddrisu Bukari, Dr. Jonathan Kao, Mr. Pei-En Huang, Mr. Russ Shean, Mr. Rodger Tsai, Mr. Brad J. Armstrong and Ms. Amy H. Kao for editing/proofing of the manuscript and translating the first draft of the questionnaire.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi: 10.1016/j.ijid.2021.06.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bundgaard H., Bundgaard J.S., Raaschou-Pedersen D.E.T., Mariager A.F., Schytte N., von Buchwald C., et al. Face masks for the prevention of COVID-19 — rationale and design of the randomised controlled trial DANMASK-19. Dan Med J. 2020;67(9) [PubMed] [Google Scholar]
- Buss L.F., Prete C.A., Jr, Abrahim C.M.M., Mendrone A., Jr, Salomon T., de Almeida-Neto C., et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science. 2021;371(6526):288–292. doi: 10.1126/science.abe9728. PMID: 33293339; PMCID: PMC7857406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.C., Lin H.H., Chang S.S., Lu T.P., Chang H.H. Health behavior monitoring of new life movement for COVID-19 disease prevention. 2020 Annual Conference of Taiwan Public Health Association on October 17, 2020 in Taipei, Taiwan. 2020 [Google Scholar]
- Chen Y.C., Chen P.J., Chang S.C., Kao C.L., Wang S.H., Wang L.H., et al. Infection control and SARS transmission among healthcare workers, Taiwan. Emerg Infect Dis. 2004;10(5):895–898. doi: 10.3201/eid1005.030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.H., Fang C.T. Combined interventions to suppress R0 and border quarantine to contain COVID-19 in Taiwan. J Formos Med Assoc. 2021;120(February (2)):903–905. doi: 10.1016/j.jfma.2020.08.003. PMID: 32855037; PMCID: PMC7413087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H.Y., Jian S.W., Liu D.P., Ng T.C., Huang W.T., Lin H.H., et al. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156–1163. doi: 10.1001/jamainternmed.2020.2020. JAMA Intern Med 2020;180(9):1264. PMID: 32356867; PMCID: PMC7195694., DOI: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.W.H., Chu J.T.S., Perera M.R.A., Hui K.P.Y., Yen H.-L., Chan M.C.W., et al. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(1) doi: 10.1016/S2666-5247(20)30003-3. e10–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W.T., Laporte R.P., Wu J. Determinants of Taiwan’s early containment of COVID-19 incidence. Am J Public Health. 2020;110(7):943–944. doi: 10.2105/AJPH.2020.305720. [DOI] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Li J.C.M., Fong M.W., et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everington K. NTU study finds antibodies in 4 people in central Taiwan. Taiwan News on Aug. 27, 2020. Available from: https://www.taiwannews.com.tw/en/news/3996009. [Accessed on 28 August 2020].
- Faranda D., Alberti T. Modeling the second wave of COVID-19 infections in France and Italy via a stochastic SEIR model. Chaos. 2020;30(11):111101. doi: 10.1063/5.0015943. [DOI] [PubMed] [Google Scholar]
- Feng S., Shen C., Xia N., Song W., Fan M., Cowling B.J. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8(5):434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlee F. Covid-19: transparency and communication are key. BMJ. 2020;371:m4764. doi: 10.1136/bmj.m4764. [DOI] [Google Scholar]
- Gondim J.A.M. Preventing epidemics by wearing masks: an application to COVID-19. Chaos Solitons Fractals. 2021;143:110599. doi: 10.1016/j.chaos.2020.110599. PMID: 33519118; PMCID: PMC7831736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Schmid M.B., Czypionka T., Bassler D., Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369:m1435. doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- He J., Guo Y., Mao R., Zhang J. Proportion of asymptomatic coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol. 2020;93(2):820–830. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.L., Wang F.Y., Lee H.R., Huang Y.L., Lai C.L., Jen W.C., et al. Seroprevalence of COVID-19 in Taiwan revealed by testing anti-SARS-CoV-2 serological antibodies on 14,765 hospital patients. Lancet Reg Health West Pac. 2020;3:100041. doi: 10.1016/j.lanwpc.2020.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Wang J., Cai J., Yao S., Chan P.K.S., Tam T.H., et al. Integrated vaccination and physical distancing interventions to prevent future COVID-19 waves in Chinese cities. Nat Hum Behav. 2021;(February) doi: 10.1038/s41562-021-01063-2. PMID: 33603201. [DOI] [PubMed] [Google Scholar]
- Jefferies S., French N., Gilkison C., Graham G., Hope V., Marshall J., et al. COVID-19 in New Zealand and the impact of the national response: a descriptive epidemiological study. Lancet Public Health. 2020;5(11):e612–e623. doi: 10.1016/S2468-2667(20)30225-5. PMID: 33065023; PMCID: PMC7553903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. Has COVID-19 fatigue caused a third wave in Europe? March 28, 2021. https://www.aljazeera.com/features/2021/3/28/has-covid-19-fatigue-caused-a-third-wave-in-europe.
- Kluge, HHP. COVID-19 case incidence on the rise as deaths edge towards 1 million, WHO Regional Director for Europe Press Statement on March 18, 2021. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/statements/statement-who-european-region-covid-19-case-incidence-on-the-rise-as-deaths-edge-towards-1-million.
- Lai A.C., Poon C.K., Cheung A.C. Effectiveness of facemasks to reduce exposure hazards for airborne infections among general populations. J R Soc Interface. 2012;9(70):938–948. doi: 10.1098/rsif.2011.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.C., Liu Y.H., Wang C.Y., Wang Y.H., Hsueh S.C., Yen M.Y., et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53:404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauring A.S., Hodcroft E.B. Genetic variants of SARS-CoV-2-what do they mean? JAMA. 2021;(January) doi: 10.1001/jama.2020.27124. Epub ahead of print. PMID: 33404586. [DOI] [PubMed] [Google Scholar]
- Lazarevic I., Pravica V., Miljanovic D., Cupic M. Immune evasion of SARS-CoV-2 emerging variants: what have we learnt so far? Viruses. 2021;13(June (7)):1192. doi: 10.3390/v13071192. PMID: 34206453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.I., Hsueh P.R. Emerging threats from zoonotic coronaviruses-from SARS and MERS to 2019-nCoV. J Microbiol Immunol Infect. 2020;53(3):365–367. doi: 10.1016/j.jmii.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lyu W., Wehby G.L. Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff (Millwood) 2020;39(8):1419–1425. doi: 10.1377/hlthaff.2020.00818. [DOI] [PubMed] [Google Scholar]
- Murray C.J.L. Forecasting the impact of the first wave of the COVID-19 pandemic on hospital demand and deaths for the USA and European Economic Area countries. medRxiv. 2020 doi: 10.1101/2020.04.21.20074732. Available from: https://www.medrxiv.org/content/10.1101/2020.04.21.20074732v1. [DOI] [Google Scholar]
- National Center for High-performance Computing (NCHC) 2021. Taiwan reports. Available from: https://covid-19.nchc.org.tw/dt_005-covidTable_taiwan.php. [Accessed on 16 January 2021] [Google Scholar]
- Olivera-La Rosa A., Chuquichambi E.G., Ingram G.P.D. Keep your (social) distance: pathogen concerns and social perception in the time of COVID-19. Pers Individ Dif. 2020;166 doi: 10.1016/j.paid.2020.110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida M., Nakamura I., Saito R., Nakaya T., Hanibuchi T., Takamiya T., et al. Adoption of personal protective measures by ordinary citizens during the COVID-19 outbreak in Japan. Int J Infect Dis. 2020;94:139–144. doi: 10.1016/j.ijid.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida M., Nakamura I., Saito R., Nakaya T., Hanibuchi T., Takamiya T., et al. How frequently do ordinary citizens practice hand hygiene at appropriate moments during the COVID-19 pandemic in Japan. Jpn J Infect Dis. 2021 doi: 10.7883/yoken.JJID.2020.631. [DOI] [PubMed] [Google Scholar]
- Pan S.C., Huang Y.S., Hsieh S.M., Chen Y.C., Chang S.Y., Chang S.C. A cross-sectional seroprevalence for COVID-19 among healthcare workers in a tertially care hospital in Taiwan. J Formos Med Assoc. 2021;(January) doi: 10.1016/j.jfma.2021.01.002. S0929-6646(21)00019-X. PMID: 33461841; PMCID: PMC7833685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet D., Hugonnet S., Harbarth S., Mourouga P., Sauvan V., Touveneau S., et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–1312. doi: 10.1016/s0140-6736(00)02814-2. [DOI] [PubMed] [Google Scholar]
- Pittet D., Donaldson L. Clean care is safer care: a worldwide priority. Lancet. 2005;366:1246–1247. doi: 10.1016/S0140-6736(05)67506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2018. R: A language and environment for statistical computing.https://www.R-project.org/ Available from: [Google Scholar]
- Reichardt C., Königer D., Bunte-Schönberger K., van der Linden P., Mönch N., Schwab F., et al. Three years of national hand hygiene campaign in Germany: what are the key conclusions for clinical practice? J Hosp Infect. 2013;83(Suppl. 1):S11–16. doi: 10.1016/S0195-6701(13)60004-3. [DOI] [PubMed] [Google Scholar]
- Rowan N.J., Moral R.A. Disposable face masks and reusable face coverings as non-pharmaceutical interventions (NPIs) to prevent transmission of SARS-CoV-2 variants that cause coronavirus disease (COVID-19): role of new sustainable NPI design innovations and predictive mathematical modelling. Sci Total Environ. 2021;772:145530. doi: 10.1016/j.scitotenv.2021.145530. PMID: 33581526; PMCID: PMC7848491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia J.L., Rivera D.N., Herrera V.L., Morwitzer M.J., Creager H.M., Santarpia G.W., et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 2020;10(1):12732. doi: 10.1038/s41598-020-69286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Cheng H.Y., Lin H.H., Barnardf L.T., Kvalsvigf A., Wilsona N., et al. Potential lessons from the Taiwan and New Zealand health responses to the COVID-19 pandemic. Lancet Reg Health West Pac. 2020;4(November) doi: 10.1016/j.lanwpc.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwan Centers for Disease Control (Taiwan CDC) 2020. Taiwan National Infectious Disease Statistics System.https://nidss.cdc.gov.tw/en/nndss/disease?id=19CoV Available from: [Google Scholar]
- Taiwan Centers for Disease Control (Taiwan CDC) 2020. Investigation into cluster infection involving naval crews of Dunmu fleet is complete; infection found only within naval crew members aboard Panshi.https://www.cdc.gov.tw/En/Bulletin/Detail/HrMDge9urbot2lFp1aLbWw?typeid=158 Available from: [Google Scholar]
- Taiwan Centers for Disease Control (Taiwan CDC) 2020. Coronavirus disease 2019 (COVID-19): digital learning courses. Hospital traffic control bundling in responding COVID-19.https://www.cdc.gov.tw/Category/Page/8rZJtd4HgGx65T43EgQiAg Available from: [Google Scholar]
- Taiwan Centers for Disease Control (Taiwan CDC) 2020. People must wear masks in eight public venues; local governments and competent authority may announce and impose penalties for violators if necessary.https://www.cdc.gov.tw/En/Bulletin/Detail/OGU5TvITYEKel0PH5Akg7g?typeid=158 Available from: [Google Scholar]
- Taiwan Centers for Disease Control (Taiwan CDC). Taiwan CECC reiterated that there were three opportunities to wear facemasks. 2020e. Available from: https://www.cdc.gov.tw/ Category/ListContent/EmXemht4IT-IRAPrAnyG9A?uaid= M3EZOYQonBr4EVTT5xwFqg.
- Taiwan Centers for Disease Control (Taiwan CDC) 2020. Letter no. 394 to medical professionals.https://www.cdc.gov.tw/Bulletin/Detail/KMJbFopTHhyHAmUNB2ElYA?typeid=48 Available from: [Google Scholar]
- Taiwan Ministry of Health and Welfare . 2020. Crucial policies for combating COVID-19. Central Epidemic Command Center.https://covid19.mohw.gov.tw/en/sp-timeline0-206.html Available from: [Google Scholar]
- Tirachini A., Cats O. COVID-19 and public transportation: current assessment, prospects, and research needs. J Public Trans. 2020;22(1) doi: 10.5038/2375-0901.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng W.P., Wu J.L., Wu C.C., Kuo K.T., Lin C.H., Chung M.Y., et al. Seroprevalence surveys for anti-SARS-CoV-2 antibody in different populations in Taiwan with low incidence of COVID-19 in 2020 and severe outbreaks of SARS in 2003. Front Immunol. 2021;(May) doi: 10.3389/fimmu.2021.626609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ellis S. 2020. Taiwan achieves record 200 days with no local coronavirus cases. Available from: https://time.com/5905129/taiwan-coronavirus-record/. [Accessed on 20 January 2021] [Google Scholar]
- Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. JAMA. 2020;323(14):1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9(October) doi: 10.7554/eLife.61312. PMID: 33112236; PMCID: PMC7723407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D.J. Braces, wheelchairs, and iron lungs: the paralyzed body and the machinery of rehabilitation in the polio epidemics. J Med Humanit. 2005;26(2–3):173–190. doi: 10.1007/s10912-005-2917-z. [DOI] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Yen M.Y., Schwartz J., Chen S.Y., King C.C., Yang G.Y., Hsueh P.R. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: implications for global prevention and control efforts. J Microbiol Immunol Infect. 2020;53(3):377–380. doi: 10.1016/j.jmii.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M.Y., Schwartz J., Shih C.L. Seventeen years after first implementation of traffic control bundling. J Microbiol Immunol Infect. 2021;54(1):1–3. doi: 10.1016/j.jmii.2020.12.006. PMID: 33518501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data for this study will be available upon request to the corresponding authors.