Abstract
The suffering from organ dysfunction due to damaged or diseased tissue/bone has been globally on the rise. Current treatment strategies for non-union bone defects include: the use of autografts, allografts, synthetic grafts and free vascularized fibular grafts. Bone tissue engineering has emerged as an alternative for fracture repair to satisfy the current unmet need of bone grafts and to alleviate the problems associated with autografts and allografts. The technology offers the possibility to induce new functional bone regeneration using synergistic combination of functional biomaterials (scaffolds), cells, and growth factors. Bone scaffolds are typically made of porous biodegradable materials that provide the mechanical support during repair and regeneration of damaged or diseased bone. Significant progress has been made towards scaffold materials for structural support, desired osteogenesis and angiogenesis abilities. Thanks for innovative scaffolds fabrication technologies, bioresorbable scaffolds with controlled porosity and tailored properties are possible today. Despite the presence of different bone scaffold fabrication methods, pore size, shape and interconnectivity have not yet been fully controlled in most of the methods. Moreover, scaffolds with tailored porosity for specific defects are still difficult to manufacture. Nevertheless, such scaffolds can be designed and fabricated using three dimensional (3D) printing approaches. 3D printing technology, as an advanced tissue scaffold fabrication method, offers the opportunity to produce complex geometries with distinct advantages. The technology has been used for the production of various types of bodily constructs such as blood vessels, vascular networks, bones, cartilages, exoskeletons, eyeglasses, cell cultures, tissues, organs and novel drug delivery devices. This review focuses on 3D printed scaffolds and their application in bone repair and regeneration. In addition, different classes of biomaterials commonly employed for the fabrication of 3D nano scaffolds for bone tissue engineering application so far are briefly discussed.
Keywords: Biomaterials, Bone tissue engineering, Nanofiber scaffolds, Three dimensional printing, Nanohydroxyapitite
Highlights
-
•
Various 3D printed scaffolds used for bone tissue regeneration are discussed.
-
•
Different biomaterials commonly employed for fabrication of 3D scaffolds are discussed.
-
•
3D printing and its advantage over the other scaffolds is discussed.
-
•
Commonly used biomaterials for 3D printed scaffolds for bone regeneration are addressed.
1. Introduction
1.1. Bone tissue engineering
Each year, the number of people suffering from organ dysfunction or organ failure due to damaged or diseased tissue is increasing, because of the aging global population, traumas and illnesses such as; heart attacks, strokes and joint degeneration. The existing pharmacological treatment options are also incapable to adequately repair tissue damage and may also drastically reduce the quality of life of the victims. This lack of therapeutic intervention is principally due to the fact that current treatments focuses mostly on prevention or reducing further tissue damage rather than contributing to the repair or regeneration of the damaged tissue [1]. Associated with increasing global population mobility, aging, accidents, war and displacement, trauma and organ/tissue damages such as, organ degeneration, bone fracture and maxillofacial complications have been still rampant [2]. In addition to the physical trauma and functional impairment, such damages do have critical psychosocial and emotional implications [3,4]. Transplantation using donated organs has been practiced in different settings in addition to available pharmacological and supportive treatment modalities. However, inadequate access to donated organs and immunological complications has been major challenges in the field [[5], [6], [7]].
Encompassing multidisciplinary experts including molecular biology, chemistry, cell biology, biomaterial science, immunology, engineering and medicine, tissue engineering has played indispensable roles in the design, fabrication and transplantation of 3D tissues constructs by combining scaffolds, cells and other bioactive materials [8].
Actually, bone is well known for its self-healing abilities; however, in case of large-scale bone defects, self-healing may be extensively delayed or may not be possible at all and external intervention is needed to restore normal tasks [[9], [10], [11]]. Non-union fractures are commonly characterized by a substantial gap between the fractured bone ends. To bridge this distance, a platform is necessary and also serve as a temporary support at the defect zone [12]. Current treatment strategies for non-union bone defects include: the use of autografts, allografts, synthetic grafts, free vascularized fibular grafts, osteoconductive scaffolds, osteoprogenitor cells and growth factor [13]. Autografting remains to be the gold standard strategy, because of its osteoconductive and osteoinductive environment and non-immunogenicity [14,15]. However, limited quantities for harvest and donor morbidity has been the limiting factors for the use of autografting driving the search for alternative solutions [16]. While allografts and synthetic grafts could avoid the mentioned limitations compared to autografts, they do not provide the necessary osteoinductive signals and vascularity required for bone healing [17]. In addition, possibility of graft rejection by the host immune system and disease transmission from donor to host are problems associated with allografts [14]. Synthetic grafts on the other hand are subjected to fatigue and wear upon aging [18]. Most grafts used in clinical settings have also limitations because of the lack of integration with bone substitution at the ends of grafts leading to non-unions, and late graft fracture occurring in as high as 60% of the cases at 10 years [19,20].
Bone tissue engineering has emerged as an alternative for fracture repair to satisfy the current unmet need of bone grafts and to alleviate the problems associated with autografts and allografts [2,18]. It focuses on methods to synthesize and/or regenerate bone to restore, maintain or improve its functions in vivo [21].
The technology offers to possibility to induce new functional bone regeneration using synergistic combination of functional biomaterials, cells, and growth factors [22]. As a result of its desirable attributes, bone tissue engineering is gaining momentum in orthopedic and regenerative medical practices. According to a review by Amini and Laurencin [2012], over half a million people in the US receive bone defect repairs yearly with an estimated cost of 2.5 billion, a figure predicted to be doubled by 2020 [23]. In the US, bone is the second most transplanted tissue next to blood, and the over increasing demand for bone grafts and substitutes was estimated to be $3.3 billion of revenues by 2013, with a compound annual growth rate of 13.8% between 2006 and 2013 [2].
Globally, approximately 15 million fracture incidences are reported (of which up to 10% are complicated by non-unions [18,24]. USA contributes 48.6% of the global market revenue to tissue engineering solutions and is the leading country by investing about 60% of the global tissue engineering expenditure to research and development (R&D) [2]. The process of tissue engineering often begins with the development of a three-dimensional (3D) scaffold to be supported in the medium and essential for the appropriate proliferation and differentiation of cells embedded in or infiltrating within it [1].
1.2. Bone scaffolds
Tissue engineering is a multidisciplinary science employing the principles of engineering and biological sciences for the fabrication of functional constructs used to restore, maintain or improve tissue functions [25,26]. The technology requires three basic elements: cells, factors for tissue induction, and a matrix for seeding a cell. This combination is designed to grow tissue in vitro, prior to implantation in to the subject [25]. The design of the scaffold prior to exposure to cells is of vital importance. The scaffold must present a surface that promotes cell attachment, growth and differentiation, while providing a porous network for tissue growth [27].
When designing a scaffold, the material of choice is of a great concern. For optimum tissue regeneration, biocompatible scaffolds with comparable degradation rate to tissue regeneration are best recommended [28]. After implanted, the scaffold must have the mechanical properties required to temporarily offer structural support until the formation of a new tissue. In addition to being biocompatible and biodegradable, the scaffold must possess key morphological characteristics; it must be highly porous and offer a suitable path for nutrient transmission and tissue ingrowths. To achieve these requirements, tissue engineering scaffolds are often designed to mimic the structure of the naturally occurring extracellular matrix (ECM) [29,30].
Bone scaffolds are typically made of porous biodegradable materials that provide the mechanical support during repair and regeneration of damaged or diseased bone [31]. Researches on bone tissue engineering over the past decades have encouraged innovation in new materials, new processing techniques, and applications. Significant progress has been made toward scaffold materials for structural support, desired osteogenesis and angiogenesis abilities [32,33]. Nowadays, biodegradable scaffolds with controlled porosity and tailored properties are possible as a result of innovation in scaffold fabrication using advanced technologies. The exceptional mechanical properties of natural bone are derived from an architectural design that spans nanoscale to macroscopic dimensions, with precisely and carefully engineered interfaces [34]. Different research groups have tried to manipulate the mechanical properties (stiffness, strength, and toughness) of scaffolds through the design of nanostructures (the inclusion of nanoparticles or nanofiber reinforcements in polymer matrices) to mimic the natural nanocomposite architecture of bone [34].
There has been an increasing interest in scaffold-based strategies for bone tissue engineering as represented by the exponential rise in the number of scientific articles over the past decade (Fig. 2A). Various scaffolds used in conjunction with stem cells and gene therapy strategies have demonstrated promising results of new bone formation and repair of segmental defects in both small and large animal studies [2], justifying that scaffolds are an integral part of bone tissue engineering. Scaffolds are 3D biocompatible structures which can mimic the ECM properties (such as mechanical support, cellular activity and protein production through biochemical and mechanical interactions), and provide a template for cell attachment and stimulate bone tissue formation in vivo [21,35]. In addition to chemistry, other critical parameters which define the performance of a scaffold's are: pore size, pore volume and mechanical strength. At an early phase, bone ingrowth takes place at the periphery of scaffolds with a negative gradient in mineralization toward the inner parts. For continuous ingrowth of bone tissue, interconnected porosity is important as it can allow nutrients and molecules to transport to inner parts of a scaffold to facilitate cell ingrowth, vascularization, as well as waste material removal [36,37].
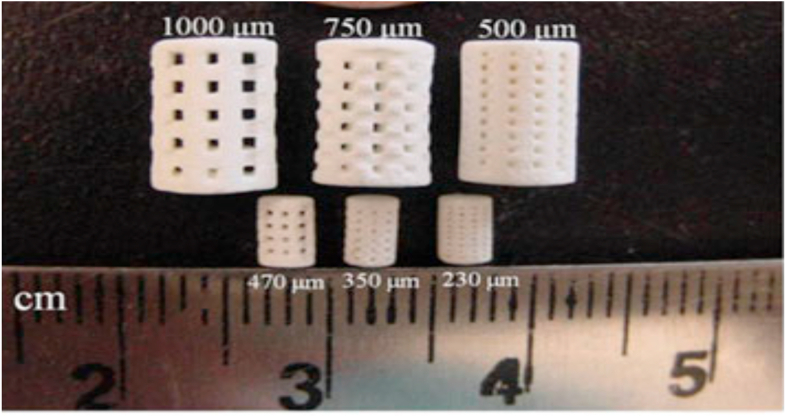
Fig. 2.
Photograph of the sintered 3D printed β-TCP scaffolds for mechanical strength and in vivo testing (small samples) [99].
As surface area per unit volume can be increased with higher porosity, the biodegradation kinetics of scaffolds can be influenced by varying pore parameters. Biodegradation through a cell mediated process or chemical dissolution are both important to ascertain stabilized repair and scaffold replacement with new bone without any remnant [37]. A minimum pore size between 100 and 150 μm is needed for bone formation [38,39]. However, enhanced bone formation and vascularization are reported for scaffolds with pore size larger than 300 μm [40,41]. The permeability of nutrients through the scaffold can also be controlled by pore volume and the mechanical properties. Besides the biological performance, the initial mechanical properties and strength, degradation rate should match that of the host tissue for optimum bone healing [42]. The pore size, geometry, and strut orientation with respect to the loading direction can highly affect the degradation kinetics of porous scaffolds [43]. Finally, surface properties such as chemistry, surface charge and topography also influence hydrophilicity and in turn cell–material interactions for bone tissue ingrowths [44].
There are different methods that can be applied for bone scaffold fabrication. Chemical/gas foaming [45], solvent casting, particle/salt leaching [46], freeze drying [47], thermally induced phase separation [48], foam-gel technology [49] and electrospinning [50] are some of the extensively used techniques in the field. But, it is not possible to fully control, pore size, shape, and its interconnectivity in these approaches. Moreover, scaffolds with tailored porosity for specific defects are difficult to manufacture with most of these approaches [51]. Such scaffolds can be designed and fabricated using additive manufacturing (AM) approaches. A variety of AM methods, including: 3D printing (3DP), solid freeform fabrication (SFF), rapid prototyping (RP), are approaches that allow complex shapes for scaffolds’ fabrication directly from a computer-aided design (CAD) file [9]. This review focuses on 3D printed scaffolds and their application in bone repair and regeneration.
1.3. Three-dimensional (3D) printing
Over the last three decades, significant advances have been made on 3D printing technology and it has being used in various industries including; electronics, robotics and healthcare [52]. 3D printing follows an additive principle where by solid objects can be created by printing successive layers using a computer aided modeling. Three-dimensional (3D) printing is becoming a research and development focus in many field including both traditional industries advanced biomedicine as it can quickly and accurately fabricate any desired 3D model only if its size is appropriate. It is conceptually defined as a method for direct digital manufacturing that provides capabilities for creating a wide range of object geometries (including internal channels) using a broad variety of materials such as ceramic, metal, metal-ceramic composite, and polymeric materials [2].
The conventional techniques used for the fabrication of tissue scaffold such as solvent casting, gas foaming, phase separation, particulate leaching, and freeze drying lack the unique features of native tissue responsible for coordination of specialized cell and tissue functions. 3D printing technology, as an advanced tissue scaffold fabrication method offers the opportunity to produce complex geometries with distinct advantages such as fitting into irregular defect sites and mimicking tissue complexity through precise positioning [53,54].
1.3.1. What is 3D printing?
Three-dimensional printing (3DP) is a manufacturing process in which objects are made by fusing or depositing materials such as; plastic, metal, ceramics, powders, liquids, or even living cells in layers to produce a 3D object [55]. This process is also referred to as additive manufacturing (AM), rapid prototyping (RP), or solid free-form technology (SFF). 3D printers function in a similar fashion to traditional inkjet printers; however, three-dimensional objects are built in 3D unlike printing layers of ink on papers [56]. 3D printing is expected to revolutionize the healthcare technology through the provision advanced diagnostic, imaging and therapeutic options [55,57]. Despite the presence of dozens of 3D printing process with varying technological platforms, resolution capacities, production efficiencies and input material requirement, all can build a 3D object in almost any shape imaginable as defined in a computer-aided design (CAD) file [58,59]. 3D printing process is founded on the development of virtual blueprints of objects using CAD which can later be scanned by the printing machine, built on from a series of layers and finally fused to generate the desired shape. The instructions from the CAD system guides the movement of the 3D print head along the x-y-z plane to build the object vertically layer by layer. The technology offers the opportunity to convert two-dimensional (2D) radiographic images such as x-rays, magnetic resonance imaging (MRI), or computerized tomography (CT) scans to digital 3D print files for creating complex, customized anatomical and medical structures [60,61].
1.3.2. History of 3D bioprinting
The conception of 3D printing, also referred to as additive manufacturing (AM), rapid prototyping (RP), or solid-freeform technology (SFF), was first developed by Charles Hull in 1980s and he patented the first 3D printing technology, stereolithography in 1986 by the US Patent Office [62,63]. His basic training in physics and his work on hotopolymers for the production of plastic objects at the Ultra Violet Products company helped him develop the technology [64].
Under the stereolithography technology, an STL file format is used to interpret the date from CAD and that can be electronically communicated to the printers for the manufacturing of 3D objects with the desired color, texture, and thickness [64,59].
The initial technology had several limitations including lengthy fabrication process and design imperfections. Subsequently, Hull and other investigators have made significant developments like the STL file format, the CAD software and data transmission systems. Hull also developed the first commercial 3D printer, commonly referred as “Stereolithography Apparatus”. 3D printing technology was further revolutionized following the development of Fused Deposition Modeling (FDM) by Scott Crump at Stratasys in 1990 [65]. Michael Cima and Emanuel Sachs from MIT also patented the first apparatus termed “3D printer” in 1993 to print plastic, metal, and ceramic parts [66]. Companies such as Helisys Organovo have developed technologies to print objects from living human tissue [67].
3D bioprinting is based on the same principles of earlier 3D printing technologies, but has been customized to manufacture permanent implants, biomimetic scaffolds and drug delivery platforms using cells, growth factors and biomaterials as input materials. The technology can produce objects with controlled morphology and internal structure having highly similar structure to the human body [68,69]. Currently, the technology is used in various aspects of tissue engineering and regenerative medicines applications including hard and soft tissue printing, cartilage printing, skin printing and tumorous tissue model printing [69]. The commonly used printing technologies for 3D bioprinting include laser printing, inkjet printing and extrusion printing with unique features as presented under Table 1. The control and optimization of key factors such as input materials properties, scaffold structure, printing precision and environmental control are essential elements for successful 3D bioprinting [[70], [71], [72]].
Table 1.
Unique features of the major 3D bioprinting technologies [73].
| Print methods | Bioinks | Resolution | Cell viability | Cell density | Print speed | Target tissue |
|---|---|---|---|---|---|---|
| Laser-assisted Printing | Fibrinogen, collagen, GelMA | 1–50 μm | 97% | 108 cells/ml | 100–1600 mm/s | Skin, vesse |
| Inkjet Printing | Collagen, poly(ethylene glycol) dimethacrylate (PEGDMA), fibrinogen, alginate, GelMA | 50–500 μm | 85–98% | <5 × 106 cells/ml | 1000–5000 droplets/s | Skin, cartilage, bone, tumor, liver |
| Extrusion Printing | Gelatin, poly-caprolactone (PCL),polyethyleneglycol (PEG), alginate, hyaluronic acid (HA), polyamide(PA), polydimethyl-siloxane (PDMS) dECM, nanocellulose | >50 μm | 80–96% | Cell spheroid | 5–20 mm/s | Skin, cartilage, vessel, bone, muscle, tumor, heart |
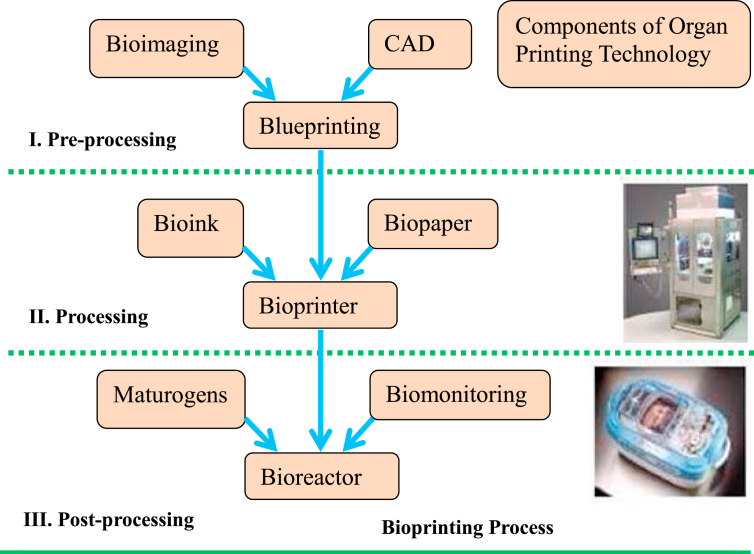
According to the comprehensive review by Ventola [59], the major steps in 3D bioprinting include: (i) creating a blueprint of the desired organ with its vascular architecture; (ii) generating a bioprinting process plan; (iii) isolating stem cells and differentiating them into organ-specific cells; (iv) preparing bioink reservoirs with organ-specific cells, blood vessel cells, and support medium to be loaded into the printer; (v) bioprinting the required product; and (vi) placing the bioprinted organ in a bioreactor prior to transplantation (Fig. 1).
Fig. 1.
Scheme of main 3 steps in organ printing technology [74].
2. Medical application of 3D printing
The medical applications of 3D printing date back to the early 2000s following the production and use of dental implants and prosthetics [75]. Since then, the medical applications for 3D printing have evolved considerably. Medical uses for 3D printing, both actual and potential, can be organized into several broad categories, including: tissue and organ fabrication; creation of customized prosthetics, implants, and anatomical models; and pharmaceutical research regarding drug dosage forms, delivery, and discovery [76]. Some reviews describe the use of 3D printing to produce bones, ears, exoskeletons, windpipes, a jaw bone, eyeglasses, cell cultures, stem cells, blood vessels, vascular networks, tissues, and organs, as well as novel dosage forms and drug delivery devices [77]. Its application in medicine can provide many benefits, including: the customization and personalization of medical products, drugs, and equipment; cost-effectiveness; increased productivity; the democratization of design and manufacturing; and enhanced collaboration [78].
Following the development of fast and precise prototyping 3D printing machines, companies have started commercialization of various medical technology products. Availability of open access software technology sources helped rapid proliferation and commercialization of the platform for medical application [64]. The growing application of 3D printing in medicine is primarily associated with the efficient manufacturing process, and the flexibility to manufacture medical products with customized size and shape. The technology has been used for the production of various types bodily constructs such as blood vessels, vascular networks, bones, cartilages, exoskeletons, eyeglasses, cell cultures, tissues, organs and novel drug delivery devices [55,79]. Generally, the medical applications of 3D printing technology can be broadly categorized as: (i) tissue and organ fabrication; (ii) manufacturing of prosthetics, implants and anatomical models; and (iii) development of novel drug delivery platforms and advanced dosage forms [59]. Because of the increasing aging population, natural and manmade crisis, accidents, birth defects and related medical problems, the demand for replacement and transplant products is significantly growing. In the light of increasing demand and dire shortage of donors, 3D bioprinting will continue to play major roles in the field [80,81].
As the latest advancement in tissue engineering, 3D bioprinting has been predicted to revolutionize the production of tissue constructed using cell-based material inputs and well-established bioink technologies. Because of their desired features such as; greater differentiation capacity and self-renewal, stem cells are gaining momentum for 3D biofabrication of precise tissue scaffolds and replacements organ constructs [82,83].
2.1. 3D printed bone nanoscaffolds
As a scaffolding tissue, bone is responsible for support, protection, load bearing and hematopoietic functions. The human bone is well known for its ability to continuously remodel and rebuild itself [84]. However, large scale defects, degenerations or inflammations caused by accidents, physical traumas, musculoskeletal maladies, infections or tumors may often be difficult to be healed by the natural process demanding for external interventions [85,86]. On the other hand, growing shortage of donors, transplant rejection, donor site morbidity, failure upon aging and mechanical wearing associated with the conventional tissue transplantation and use prosthetic supportive implants calls for cytocompatible and lasting solutions [87,88]. Advances in biomaterial sciences and nanotechnology enabled the application of 3D printing in tissue engineering and regenerative medical practices with better flexibility and clinical outcomes [2,89].
3D bioprinting method was used to fabricate more ideal structural scaffolds with better control of pore morphology, pore size, and porosity. The technology is being used for fabrication of versatile solid free-form structures that can offer an unprecedented flexibility in both material selection and geometry to produce customized scaffolds for growing irregular tissues [90]. While engineering hard tissues like bone, a high degree of porosity together with high mechanical strength is critical which can be difficult to attain using traditional techniques [91]. An ideal 3D scaffold is expected to have important features such as: high porosity, well-interconnected pore networks, and consistent and adequate pore size which can augment cell migration and infiltration [92]. These parameters are essential elements of scaffold geometry which determine the level of access to cell recruitment, vascularization and nutrients which intern influence cell adhesion, proliferation and distribution. There are different views by researchers on the size range of these parameters of which some do have contradicting views where pore sizes ranging from 20 μm to 1500 μm have been reported [39,40,93]. A pore size range of 100–135 μm has been recommended as optimum for effective bone growth [94,95]. Degree of porosity in excess of 90% was recommended for adequate nutrient diffusion and cell–biomaterial interactions [96]; however, it should not be extremely high as it may affect the desired mechanical properties of the scaffold [95]. The selection of input materials is hence a critical component in 3D bioprinting. The different class of biomaterials commonly employed for the fabrication of 3D nanoscaffolds for bone tissue engineering application are presented in Table 2.
Table 2.
Different 3D printed bone tissue engineered scaffolds.
| Types of Materials | References |
|---|---|
| 1. Calcium phosphate (CaPs) based bioactive ceramic scaffolds | |
|
[97] |
|
[98] |
|
[98] |
|
[98] |
|
[99] |
|
[100] |
|
[101] |
|
[100] |
|
[101] |
|
[101] |
|
[102] |
|
[91] |
| 2. Polymer based Scaffolds | |
|
[103] |
|
[104] |
|
[105] |
|
[106] |
|
[107] |
|
[108] |
|
[109] |
| 3. Composite scaffolds | |
|
[100] |
|
[110] |
|
[100] |
|
[111] |
|
[112] |
|
[113] |
Because of the relative similarity with body proteins and receptors, the fabrication such biomaterials as nanoscaffolds could enable them to freely interact with receptors and easily integrate with the membrane matrix structures. Investigations have shown that nanoscaffolds demonstrated better cell functionality than micro and macro level constructs [114]. This is associated with the fact that bone is composed of different proteins like collagen, fibronectin, laminin, and vitronectin which form soft hydrogel nanocomposites in the presence of water; and the bioactive nanoscaffolds can easily mimic the natural environment and augment osteocyte differentiation and mobility pathways [115]. Silk fibroin-hydroxybutyl chitosan blended nanofibers, apatite-collagen-polycaprolactone nanocomposites, Bone Morphogenetic Protein 2 (BMP-2) based gold nanoarrays, BMP-2 based silk fibroin/chitosan/Nanohydroxyapatite nanocomposites have demonstrated promising results in animal models [116,117].
2.1.1. Calcium phosphate (CaPs) based bioactive ceramic scaffolds
Calcium phosphate (CaP) ceramics are commonly used in bone tissue engineering because of their biocompatibility, excellent bioactivity, osteoconductivity, availability and cost-effectiveness [9,114]. The incorporation of biomimetic CaP nanomaterials such as nano-hydroxy apatite (nHA), TCP and CaP are at the forefront of 3D printing research. As a result of its excellent cytocompatibility, osteoconductive and bioactive nature, nHA has been targeted as the future bone nanomaterial to be considered in 3D printing systems, and even used as the main constituent. In addition to nHA, TCP is also utilized in 3D printing. The fine powder form of nHA-TCP were used for the fabrication of nanoscaffolds by a novel 3D sintering method. Sintered TCP scaffolds are characterized by higher compressive strength and more optimal microporosity to macroporosity ratio which in turn can facilitate the formation of new bone in vivo [51]. Controlled biodegradability is an essential feature of bone scaffolds as it can progressively create space for new tissue growth during regeneration. In this regard, CP scaffolds, particularly TCPs care capable of tunable bioresorption [95]. The degradation products of CP scaffolds also participate in biomineralization and can facilitate bone formation and bioactivity [ [119,120]]. The limitation of CP scaffolds; however, is weak and brittle property associated with the porosity limiting their use only in none load-bearing bone repairs. In addition, CP scaffolds lack osteoinductive activity which is important in bone healing process [121]. Combination of CP ceramics with biopolymers has demonstrated improved mechanical and biochemical performances [ [[122], [123], [124]]].
During the process of bone and tissue repair, capillaries and vessel formation, and homogeneous osteoconduction can be enhanced from central channels [9,97]. The effect of pore size on human fetal osteoblasts (hFOB) was studied with 3D-printed TCP scaffolds [99]. The decrease in designed pore size from 1000 to 750 and 500 μm resulted in an increase in proliferated cell density. As can be depicted in Fig. 2, the 3D printed and microwave sintered β-TCP scaffolds show interconnected macro porosity across the sample. The study done by Bose et al.(2013) on the morphologies of hFOB cells on scaffold surfaces and pore walls showed good cell adherence and cell ingrowth into the pores, suggesting that the scaffolds were non-toxic [9].
New bone formation was observed at the implant/host bone interface and inside the interconnected macro and intrinsic micro pores after 4 and 8 weeks in both pure and doped TCP. Tartrate resistant acid phosphatase (TRAP) staining, lacunae formation and microscopic images conducted using osteoclasts also confirmed monocytes differentiation to multinuclear osteoclast-like cells on a wide range of compositions ensuring the biocompatibility of the scaffolds [98]. HA scaffolds with high surface areas demonstrated cytocompatibility and adequate cell adhesion with MC3T3-E1 fibroblast cells in vitro [125]. In vivo biocompatibility and osteoconductivity of 3D-printed scaffolds showed that, the 3Dprinted brushite and monetite cements with controlled open porosity increased osteoconduction in vivo in a goat model [37]. It has been shown that the use of phosphoric acid instead of polymeric binders can improve both resolution and compressive strength [100].
Biphasic calcium phosphate (BCP) and β-TCP hydroxyapatite scaffolds at different concentration of stable phases have been better recommended because of their controlled bioactivity and better balancing between resorption and solubilization which can maintain biomaterial stability while enhancing bone ingrowth [118]. 3D-printed TCP samples with micro and macro-porosity were also facilitated osteogenesis in a rat femur model [126]. To further improve the mechanical properties of the scaffolds, Khalyfa, et al. (2007) have utilized tow post-fabrication procedures: sintering and a polymer infiltration on TTCP/β-TCP and TTCP/calcium sulfate dehydrate bone cements, and demonstrated cytocompatibility on MC3T3-E1 cells model. According to their report, the shortest hardening time obtained was between 20 and 40% for citric acid, and 30–40% for lactic acid while used as binders. It was also reported that lower binder concentration in the presence of sodium hydrogen phosphate and phosphoric acid can prolong the hardening time for the cements [101]. 3D printed mesoporous bioactive glasses (MBG) modified β-tricalcium phosphate (MBG-β-TCP) scaffolds with hierarchical pore structure and functional strut surfaces also demonstrated better compressive strength and apatite-mineralization ability with enhanced new bone formation in vivo as compared to BG-β-TCP and β-TCP scaffolds [117].
2.1.2. Polymer based 3D printed bone scaffolds
Bone scaffolds are designed to offer a number of desired functions including: (i) promoting cell–scaffold interactions, cell adhesion and ECM deposition, (ii) facilitating transport of gases, nutrients and regulatory factors that are essential for cell survival, proliferation and differentiation; and (iii) provoking minimal degree of inflammation or toxicity. Biodegradation at a controllable rate approximately matching the rate of tissue regeneration is also an important attribute for bone scaffolds [127]. In consideration such important attributes, the selection of input materials for the fabrication of bone and tissue scaffolds requires at most care. Biodegradable and biocompatible natural and synthetic polymers are among the most extensively used materials in tissue engineering and regenerative medicine [30,128]. Such polymers have been used for the fabrication of customized and patient-specific medical devices, such as implants, prostheses, bone and tissue scaffolds, anatomical models, and surgical guides. Collagen, gelatin, alginate, hyaluronic acid, polyvinyl alcohol, polyethylene glycol and polylactides are among the most extensively investigated polymers for the purpose [21,129,130].
An investigation by Suwanprateeb et al. [103] 3D-printed polyethylene (PE) scaffolds with 22.3–49.7% porosity demonstrated a tensile strength up to 4 MPa with no toxicity to human osteoblasts. The Oxford Performance Materials Company also used SLS and a proprietary poly (ether-keytone-ketone) biomimetic polymer to create a bone substitute for craniofacial defects. The product was approved by FDA in 2013 as one of the first 3D printed polymer implants. This product, designated by the company as “OPEKK-IG” was reported to osteoconductive, mechanically strong, with textured surface, and with the capacity to maintain cell proliferation without exhausting metabolic demands on the cells [109]. Similarly, Wang et al. [111] fabricated poly (propylene fumarate) porous scaffolds which were suitable for bone tissue reengineering with characteristic degradation for over 224 days. 3D printed poly(lactic acid) (PLA) scaffolds with a mussel inspired surface coating prepared by Kao et al. [106] also demonstrated significantly better cell adhesion, proliferation and higher alkaline phosphatase activity when tested on human adipose-derived stem cells (hADSCs) [106].
2.1.3. Composite scaffolds
Even though a variety of materials including ceramics, polymers and hydrogels have shown promising results for the fabrication of bone and tissues scaffolds, each of them has limitations in giving all required material attributes, and also in mimicking the natural processes of bone growth when used individually. Combination of materials in the form of 3D composite scaffolds has been widely used with to address the stated limitations [131]. Composites are materials made from two or more constituents with significantly different physical or chemical properties, and produce superior characteristics when combined than the individual constituents [31,132]. Biodegradable composites should have mechanical competence characterized by suitable fracture strength and elastic modulus values, as well as controlled strength and modulus degradation to provide the necessary support for cell attachment and proliferation. Ideally, composite scaffolds are required to have an approximate compressive strength of 100–230 MPa; elastic modulus closer to 7–30 GPa; tensile strength of 50–151 MPa; porosity between 60% and 90%; and an average pore size of >150 μm [133,134]. Besides, smart combination of biodegradable polymers and bioactive ceramics have the ability to control fast autocatalytic degradation effect of acidic end groups resulting from hydrolysis of some polymer chains through buffering the pH of the surrounding solution [135].
Combination of polymers with bioceramics or bio glasses have been widely used as composite scaffolds in bone tissue engineering [21,136]. Schantz et al. [137] fabricated a biodegradable polymer-ceramic scaffold via 3D printing and tested in vitro using human MSCs within fibrin glue. The results revealed that the cells were able to attach, migrate and osteogenically differentiated within the biomimetic bone scaffold. 3D printed mineral trioxide aggregate/polycaprolactone (MTA/PCL) hybrid scaffolds with controlled size, high-porosity (70%), and a compressive strength of 4.5 MPa showed effective adhesion, proliferation, and differentiation on human dental pulp cells with excellent physical and chemical properties suitable for bone tissue engineering (hDPCs). These scaffolds have not only excellent physical and chemical properties but also enhanced osteogenic differentiation, making them useful for bone tissue engineering [90]. Similarly, Yao et al. [111] demonstrated improved cell adhesion, proliferation and chondrogenic differentiation by 3D polycaprolactone-hydroxyapatite scaffolds upon in vitro and in vivo testing. 3D-printed PCL/PLGA/β-TCP scaffolds also showed enhanced osteogenic potential when tested on human nasal inferior turbinate tissue-derived mesenchymal stromal cells [112]. 3D printed Biodentine/polycaprolactone composite scaffolds with controlled macropore sizes and structures fabricated using extrusion technology for orthopedic and dental applications were proved to have good apatite-forming ability, and enhancing cell proliferation and differentiation when tested on human dental pulp cells [113]. 3D printed scaffolds made from calcium sulfate hemihydrate powder (CaSO4-1/2 H2O), transformed into hydroxyapatite (HAp) by treatment with a hydrothermal reaction in an NH4H2PO4 solution and coated with a ε-polycaprolactone (PCL) polymer solution (5 and 10 w/v showed significantly increased compressive strength by about 2-fold and 4-fold, respectively, compared with uncoated scaffolds. In another study, 3D scaffolds coated with PCL improved MG-63 cells adhesion and proliferated with improved osteoblast differentiation [110]. Similarly, β-tricalcium phosphate (β-TCP) and polycaprolactone (PCL) composites scaffolds, at 50:50 and 70:30 composition showed an improved cell adhesion and proliferation, and higher alkaline phosphates activity making them ideal for dental applications or regeneration therapies [138].
3. Conclusion
Advances in biomaterial sciences and nanotechnology enabled the application of 3D printing in tissue engineering and regenerative medical practices with better flexibility and clinical outcomes. Over the last decades, 3D bioprinting method has been used to fabricate more ideal structural scaffolds with better control of pore morphology, pore size, and porosity. The selection of input materials is a critical component in 3D bioprinting. Different classes of biomaterials were employed for the fabrication of 3D nanoscaffolds for bone tissue engineering application. Calcium phosphate based bioactive ceramic scaffolds, polymer based scaffolds, and composite of Calcium phosphate based bioactive ceramic and polymer scaffolds have been extensively exploited so far. Among calcium phosphate based bioactive ceramics, nano-hydroxy apatite (nHA), TCP and CaP are at the forefront of 3D printing research. By virtue of its excellent cytocompatibility, osteoconductive and bioactive nature, nHA has been targeted as the future bone nanomaterial to be considered in 3D printing systems, and even used as the main constituent. From polymer based materials, 3D printed poly(lactic acid) (PLA) scaffolds with a mussel-inspired surface coating have demonstrated significantly better osteogenesis. Even though ceramics and polymers have shown promising results for the fabrication of bone and tissues scaffolds, each of them has limitations in giving all required material attributes, and also in mimicking the natural processes of bone growth when used individually. Combination of materials in the form of 3D composite scaffolds has been widely used to address the stated limitations. 3D printed MTA/PCL hybrid scaffolds showed effective adhesion, proliferation, and differentiation on human dental pulp cells with excellent physical and chemical properties suitable for bone tissue engineering. These scaffolds have not only excellent physical and chemical properties but also enhanced osteogenic differentiation, making them useful for future bone tissue engineering application. On the other hand, the development of less invasive methods/technologies with improved tissue repair and regeneration; the capability for consistent delivery of cells needed for multiple tissue regeneration; and associated ethical and regulatory issues are yet to be explored further.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Do A.V., Khorsand B., Geary S.M., Salem A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015;4:1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu W., Li Y., Liu J., Niu X., Wang Y., Li D. Application and performance of 3D printing in nanobiomaterials. J Nanomater. 2013;13 doi: 10.1155/2013/681050. ID 681050. [DOI] [Google Scholar]
- 3.Patrick C.W. Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263:361–366. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 4.Unnithan A.R., Sasikala A.R.K., Thomas S.S., Nejad A.G., Cha Y.S., Park C.H. Strategic design and fabrication of biomimetic 3d scaffolds: unique architectures of extracellular matrices for enhanced adipogenesis and soft tissue reconstruction. Nature Sci. Rep. 2018;8:5696. doi: 10.1038/s41598-018-23966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans R.W., Manninen D.L., Garrison L.P.J., Maier A.M. Donor availability as the primary determinant of the future of heart transplantation. J Am Med Assoc. 1986;255:1892–1898. [PubMed] [Google Scholar]
- 6.Wood K.J., Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93:1–10. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 7.Maggiore U., Oberbauer R., Pascual J., Viklicky O., Dudley C., Budde K. Strategies to increase the donor pool and access to kidney transplantation: an international perspective. Nephrol. Dial. Transpl. 2015;30:217–222. doi: 10.1093/ndt/gfu212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castells-Sala C., Alemany-Ribes M., Fernández-Muinos T., Recha-Sancho L., Lopez-Chicon P., Aloy- Reverte C. Current applications of tissue engineering in biomedicine. J. Biochip. Tissue. Chip. 2013;S2 doi: 10.4172/2153-0777.S2-004. [DOI] [Google Scholar]
- 9.Bose S., Vahabzadeh S., Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16:496–504. [Google Scholar]
- 10.Gao G., Schilling A.F., Yonezawa T., Wang J., Dai G., Cui X. Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells. Biotechnol J. 2014;9:1304–1311. doi: 10.1002/biot.201400305. [DOI] [PubMed] [Google Scholar]
- 11.Garcia P., Histing T., Holstein J.H., Klein M., Laschke M.W., Matthys R. Rodent animal models of delayed bone healing and non-union formation: a comprehensive review. Eur Cell Mater. 2013;26:1–14. doi: 10.22203/ecm.v026a01. [DOI] [PubMed] [Google Scholar]
- 12.Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 13.Emara K.M., Diab R.A., Emara A.K. Recent biological trends in management of fracture non-union. World J. Orthop. 2015;6:623–628. doi: 10.5312/wjo.v6.i8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose F.R., Oreffo R.O. Bone tissue engineering: hope vs hype. Biochem. Bioph. Res. Co. 2002;292:1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder J.E., Mosheiff R. Tissue engineering approaches for bone repair: concepts and evidence. Injury. 2011;42:609–613. doi: 10.1016/j.injury.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Swetha M., Sahithi K., Moorthi A., Srinivasan N., Ramasamy K., Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010;47:1–4. doi: 10.1016/j.ijbiomac.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Lane J.M., Tomin E., Bostrom M.P. Biosynthetic bone grafting. Clin Orthop Relat Res. 1999;367:S107–S117. doi: 10.1097/00003086-199910001-00011. [DOI] [PubMed] [Google Scholar]
- 18.Salgado A.J., Coutinho O.P., Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler D.L., Enneking W.F. Allograft bone decreases in strength in vivo over time. Clin. Orthop. Relat. R. 2005;435:36–42. doi: 10.1097/01.blo.0000165850.58583.50. [DOI] [PubMed] [Google Scholar]
- 20.Soucacos P.N., Dailiana Z., Beris A.E., Johnson E.O. Vascularised bone grafts for the management of non-union. Injury. 2006;37:S41–S50. doi: 10.1016/j.injury.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 21.Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 22.Akter F., Ibanez J. Bone and cartilage tissue engineering. In: Akter J., editor. Tissue engineering made easy. Elsevier Inc.; Cambridge: 2016. pp. 77–97. [Google Scholar]
- 23.Amini A.R., Laurencin C.T., Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40:363–408. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Keefe R.J., Mao J. Bone tissue engineering and regeneration: from discovery to the clinic-an overview. Tissue Eng. Part B: Rev. 2011;17:389–392. doi: 10.1089/ten.teb.2011.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacanti J.P., Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354:S32–S34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 26.Langer R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng. 2007;13:1–2. doi: 10.1089/ten.2006.0219. [DOI] [PubMed] [Google Scholar]
- 27.Liu X., Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 28.Lee K.Y., Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 29.Smith I.O., Liu X.H., Smith L.A., Ma P.X. Nanostructured polymer scaffolds for tissue engineering and regenerative medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009;1:226–236. doi: 10.1002/wnan.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar D.S. Polymeric scaffolds in tissue engineering application: a review. Int. J. Polym. Sci. 2011 doi: 10.1155/2011/290602. [DOI] [Google Scholar]
- 31.Bose S., Roy M., Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–554. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanczler J.M., Oreffo R.O.C. Osteogenesis and angiogenesis: the potential for engineering bone. Eur Cell Mater. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Chen S., Kawazoe N., Chen G. Promoted angiogenesis and osteogenesis by dexamethasone-loaded calcium phosphate nanoparticles/collagen composite scaffolds with microgroove networks. Nature Sci. Rep. 2018;8:14143. doi: 10.1038/s41598-018-32495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong T., Xie J., Liao J., Zhang T., Lin S., Lin Y. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029. doi: 10.1038/boneres.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller B., Deyhlea H., Fierza F.C., Irsenc S.H., Yoona J.Y., Mushkolaja S. Bio-mimetic hollow scaffolds for long bone replacement. Proc SPIE Int Soc Opt Eng. 2009;7401 74010D-1. [Google Scholar]
- 36.Jones A.C., Arns C.H., Sheppard A.P., Hutmacher D.W., Milthorpe B.K., Knackstedt M.A. Assessment of bone ingrowth into porous biomaterials using MICRO-CT. Biomaterials. 2007;28:2491–2504. doi: 10.1016/j.biomaterials.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 37.Habibovic P., Gbureck U., Doillon C.J., Bassett D.C., van Blitterswijk C.A., Barralet J. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–953. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Mour M., Das D., Winkler T., Hoenig E., Mielke G., Morlock M.M. Advances in porous biomaterials for dental and orthopaedic applications. Materials. 2010;3:2947–2974. [Google Scholar]
- 39.Murphy C.M., O'Brien F.J. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adh. Migr. 2010;4:377–381. doi: 10.4161/cam.4.3.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Otsuki B., Takemoto M., Fujibayashi S., Neo M., Kokubo T., Nakamura T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials. 2006;27:5892–5900. doi: 10.1016/j.biomaterials.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Bandyopadhyay A., Bernard S., Xue W., Bose S. Calcium phosphate-based resorbable ceramics: influence of MgO, ZnO, and SiO2 dopants. J Am Ceram Soc. 2006;89:2675–2688. [Google Scholar]
- 43.Bose S., Tarafder S., Banerjee S.S., Davies N.M., Bandyopadhyay A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO 2 doped β-TCP. Bone. 2011;48:1282–1290. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarafder S., Banerjee S., Bandyopadhyay A., Bose S. Electrically polarized biphasic calcium phosphates: adsorption and release of bovine serum albumin. Langmuir. 2010;26:16625–16629. doi: 10.1021/la101851f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kucharska M., Butruk B., Walenko K., Brynk T., Ciach T. Fabrication of in-situ foamed chitosan/β-TCP scaffolds for bone tissue engineering application. Mater Lett. 2012;85:124–127. [Google Scholar]
- 46.Stoppato M., Carletti E., Sidarovich V., Quattrone A., Unger R.E., Kirkpatrick C.J. Influence of scaffold pore size on collagen I development: a new in vitro evaluation perspective. J. Bioact. Compat. Pol. 2013;28:16–32. [Google Scholar]
- 47.Im O., Li J., Wang M., Zhang L.G., Keidar M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int. J. Nanomedicine. 2012;7:2087. doi: 10.2147/IJN.S29743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 49.Seyedjafari E., Soleimani M., Ghaemi N., Shabani I. Nanohydroxyapatite-coated electrospun poly (l-lactide) nanofibers enhance osteogenic differentiation of stem cells and induce ectopic bone formation. Biomacromolecules. 2010;11:3118–3125. doi: 10.1021/bm1009238. [DOI] [PubMed] [Google Scholar]
- 50.Amjadian S., Seyedjafari E., Zeynali B., Shabani I. The synergistic effect of nano-hydroxyapatite and dexamethasone in the fibrous delivery system of gelatin and poly (l-lactide) on the osteogenesis of mesenchymal stem cells. Int. J. Pharm. 2016;507:1–11. doi: 10.1016/j.ijpharm.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 51.O'Brien C.M., Holmes B., Faucett S., Zhang L.G. Three-dimensional printing of nanomaterial scaffolds for complex tissue regeneration. Tissue Eng. Part B: Rev. 2014;21:103–114. doi: 10.1089/ten.teb.2014.0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munoz-Abraham A.S., Rodriguez-Davalos M., Bertacco A., Wengerter B., Geibel J.P., Mulligan D.C. 3D printing of organs for transplantation: where are we and where are we heading? Curr. Transpl. Rep. 2016;3:93–99. [Google Scholar]
- 53.Oberpenning F., Meng J., Yoo J.J., Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 54.Temple J.P., Hutton D.L., Hung B.P., Huri P.Y., Cook C.A., Kondragunta R. Engineering anatomically shaped vascularized bone grafts with hASCs and 3D-printed PCL scaffolds. J Biomed Mater Res A. 2014;102:4317–4325. doi: 10.1002/jbm.a.35107. [DOI] [PubMed] [Google Scholar]
- 55.Schubert C., van Langeveld M.C., Donoso L.A. Innovations in 3D printing: a 3D over view from optics to organs. Br J Ophthalmol. 2014;98:159–161. doi: 10.1136/bjophthalmol-2013-304446. [DOI] [PubMed] [Google Scholar]
- 56.Attaran M. The rise of 3-D printing: the advantages of additive manufacturing over traditional manufacturing. Bus Horiz. 2017;60:677–688. [Google Scholar]
- 57.Brown C. 3D printing set to revolutionize medicine. CMAJ (Can Med Assoc J) 2017;189:E973–E974. doi: 10.1503/cmaj.1095442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lipson H. New world of 3-D printing offers" completely new ways of thinking": Q&A with author, engineer, and 3-D printing expert Hod Lipson. IEEE pulse. 2013;4:12–14. doi: 10.1109/MPUL.2013.2279615. [DOI] [PubMed] [Google Scholar]
- 59.Ventola C.L. Medical applications for 3D printing: current and projected uses. Pharm. Ther. 2014;39:704–711. [PMC free article] [PubMed] [Google Scholar]
- 60.Cui X., Boland T., D'Lima D.D., Lotz K.M. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012;6:149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ursan I.D., Chiu L., Pierce A. Three-dimensional drug printing: a structured review. J. Am. Pharm. Assoc. 2013;53:136–144. doi: 10.1331/JAPhA.2013.12217. [DOI] [PubMed] [Google Scholar]
- 62.Hull C.W. USPaT Office; United States: 1986. Apparatus for production of three-dimensional objects by stereolithography.https://www.lens.org/lens/patent/022-138-245-291-118 [Google Scholar]
- 63.Whitaker M. The history of 3D printing in healthcare: how an emergent technology is changing the way we treat patients. The Bulletin. 2018:17–19. doi: 10.1308/147363514X13990346756481. [DOI] [Google Scholar]
- 64.Gross, Erkal J.L., Lockwood S.Y., Chen C., Spence D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86:3240–3253. doi: 10.1021/ac403397r. [DOI] [PubMed] [Google Scholar]
- 65.S.S. Crump . U.S. Patent and Trademark Office; Washington, DC: 1992. Apparatus and method for creating three-dimensional objects, U.S. Patent No. 5,121,329.https://patents.google.com/patent/US5121329A/en [Google Scholar]
- 66.Dimitrov D., Schreve K., de Beer N., Christiane P. Three dimensional printing in the South African industrial environment. S Afr J Ind Eng. 2008;19:195–213. [Google Scholar]
- 67.Jones R., Haufe P., Sells E., Iravani P., Olliver V., Palmer C. RepRap-the replicating rapid prototyper. Robotica. 2011;29:177–191. [Google Scholar]
- 68.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 69.Ozbolat I.T., Peng W.J., Ozbolat V. Application areas of 3D bioprinting. Drug Discov Today. 2016;21:1257–1271. doi: 10.1016/j.drudis.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Lee M., Dunn J.C.Y., Wu B.M. Scaffold fabrication by indirect three-dimensional printing. Biomaterials. 2005;26:4281–4289. doi: 10.1016/j.biomaterials.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 71.Buyukhatipoglu K., Jo W., Clyne A.M. The role of printing parameters and scaffold biopolymer properties in the efficacy of a new hybrid nano-bioprinting system. Biofabrication. 2009;1 doi: 10.1088/1758-5082/1/3/035003. [DOI] [PubMed] [Google Scholar]
- 72.Skardal A., Atala A. Biomaterials for integration with 3-D bioprinting. Ann Biomed Eng. 2015;43:730–746. doi: 10.1007/s10439-014-1207-1. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B., Luo Y., Ma L., Gao L., Li Y., Xue Q. 3D bioprinting: an emerging technology full of opportunities and challenges. Biodes. Manuf. 2018 doi: 10.1007/s42242-018-0004-3. [DOI] [Google Scholar]
- 74.Rezende R.A., Kasyanov V., Mironov V., da Silva J.V.L. Organ Printing as an information technology. Procedia Eng. 2015;110:151–158. [Google Scholar]
- 75.Jo W., Lee J.S., Lee H.J., Moon M.W. 3D printed tactile pattern formation on paper with thermal reflow method. Rsc. Advances. 2014;4:31764–31770. [Google Scholar]
- 76.Klein G.T., Lu Y., Wang M.Y. 3D printing and neurosurgery-ready for prime time? World Neurosurg. 2013;80:233–235. doi: 10.1016/j.wneu.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Banks J. Adding value in additive manufacturing: researchers in the United Kingdom and Europe look to 3D printing for customization. IEEE pulse. 2013;4:22–26. doi: 10.1109/MPUL.2013.2279617. [DOI] [PubMed] [Google Scholar]
- 78.Mertz L. Dream it, design it, print it in 3-D: what can 3-D printing do for you? IEEE pulse. 2013;4:15–21. doi: 10.1109/MPUL.2013.2279616. [DOI] [PubMed] [Google Scholar]
- 79.Bartlett S. Printing organs on demand. Lancet Respir. Med. 2013;1:684. doi: 10.1016/S2213-2600(13)70239-X. [DOI] [PubMed] [Google Scholar]
- 80.Ozbolat I.T., Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60:691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 81.Derakhshanfar S., Mbeleck R., Xu K., Zhang X., Zhong W., Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact. Mater. 2018;3:144–156. doi: 10.1016/j.bioactmat.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tricomi B.J., Dias A.D., Corr D.T. Stem cell bioprinting for applications in regenerative medicine. Ann. NY. Acad. Sci. 2016;1383:115–124. doi: 10.1111/nyas.13266. [DOI] [PubMed] [Google Scholar]
- 83.Leberfinger A.N., Ravnic D.J., Hawan A., Ozbolat I.T. Concise review: bioprinting of stem cells for transplantable tissue fabrication, Stem Cells Transl. Med. 2017;6:1940–1948. doi: 10.1002/sctm.17-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuchs R., Warden S., Turner C. Bone anatomy, physiology and adaptation to mechanical loading. In: Planell J.A., Best S.M., Lacroix D., editors. Bone repair Biomaterials. Woodhead Publishing Limited and CRC Press LLC; Cambridge: 2009. p. 25. [Google Scholar]
- 85.Mourino V., Boccaccini A.R. Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface. 2010;7:209–227. doi: 10.1098/rsif.2009.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yi H., Rehman F.U., Zhao C., Liu B., He N. Recent advances in nanoscaffolds for bone repair. Bone Res. 2016;4:16050. doi: 10.1038/boneres.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giannoudis P.V., Dinopoulos H., Tsiridis E. Bone substitutes: an update. Injury. 2005;36:S20–S27. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 88.Zhang L., Webster T.J. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today. 2009;4:66–80. [Google Scholar]
- 89.Gu B.K., Choi D.J., Park S.J., Kim M.S., Kang C.M., Kim C.H. 3-dimensional bioprinting for tissue engineering applications. Biomater Res. 2016;20:12. doi: 10.1186/s40824-016-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chiu Y.C., Fang H.Y., Hsu T.T., Lin C.Y., Shie M.Y. The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endodont. 2017;43:923–929. doi: 10.1016/j.joen.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Y., Xia L., Zhai D., Shi M., Luo Y., Feng C. Mesoporous bioactive glass nanolayer-functionalized 3D-printed scaffolds for accelerating osteogenesis and angiogenesis. Nanoscale. 2015;7:19207–19221. doi: 10.1039/c5nr05421d. [DOI] [PubMed] [Google Scholar]
- 92.An J., Teoh J.E.M., Suntornnond R., Chua C.K. Design and 3D printing of scaffolds and tissues. Engineering. 2015;1:261–268. doi: 10.15302/J-ENG-2015061. [DOI] [Google Scholar]
- 93.Loh Q.L., Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. Part B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harley B.A.C., Kim H.D., Zaman M.H., Yannas I.V., Lauffenburger D.A., Gibson L.J. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction Interactions. Biophys J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Velasco M.A., Narváez-Tovar C.A., Garzón-Alvarado D.A. Design, materials, and mechanobiology of biodegradable scaffolds for bone tissue engineering. BioMed Res Int. 2015 doi: 10.1155/2015/729076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polo-Corrales L., Latorre-Esteves M., Ramirez-Vick J.E. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14:15–56. doi: 10.1166/jnn.2014.9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Becker S.T., Bolte H., Schünemann K., Seitz H., Bara J.J., Beck-Broichsitter B.E. Endocultivation: the influence of delayed vs. simultaneous application of BMP-2 onto individually formed hydroxyapatite matrices for heterotopic bone induction. Int. J. Oral Max. Surg. 2012;41:1153–1160. doi: 10.1016/j.ijom.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 98.Detsch R., Schaefer S., Deisinger U., Ziegler G., Seitz H., Leukers B. In vitro-osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J Biomater Appl. 2011;26:359–380. doi: 10.1177/0885328210373285. [DOI] [PubMed] [Google Scholar]
- 99.Tarafder S., Balla V.K., Davies N.M., Bandyopadhyay A., Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regen. M. 2013;7:631–641. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vorndran E., Klarner M., Klammert U., Grover L.M., Patel S., Barralet J.E. 3D powder printing of β-tricalcium phosphate ceramics using different strategies. Adv Eng Mater. 2008;10:B67–B71. [Google Scholar]
- 101.Khalyfa A., Vogt S., Weisser J., Grimm G., Rechtenbach A., Meyer W. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J Mater Sci: Mater Med. 2007;18:909–916. doi: 10.1007/s10856-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 102.Klammert U., Gbureck U., Vorndran E., Rödiger J., Meyer-Marcotty P., Kübler A.C. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J. Cranio. Maxill. Surg. 2010;38:565–570. doi: 10.1016/j.jcms.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 103.Suwanprateeb J., Thammarakcharoen F., Wongsuvan V., Chokevivat W. Development of porous powder printed high density polyethylene for personalized bone implants. J. Porous. Mat. 2012;19:623–632. [Google Scholar]
- 104.Giordano R.A., Wu B.M., Borland S.W., Cima L.G., Sachs E.M., Cima M.J. Mechanical properties of dense polylactic acid structures fabricated by three dimensional printing. J. Biomat. Sci-Polym. E. 1997;8:63–75. doi: 10.1163/156856297x00588. [DOI] [PubMed] [Google Scholar]
- 105.Kao C.T., Lin C.C., Chen Y.W., Yeh C.H., Fang H.Y., Shie M.Y. Poly (dopamine) coating of 3D printed poly (lactic acid) scaffolds for bone tissue engineering. Mater Sci Eng C. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 106.Wang M.O., Piard C.M., Melchiorri A., Dreher M.L., Fisher J.P. Evaluating changes in structure and cytotoxicity during in vitro degradation of three-dimensional printed scaffolds. Tissue Eng. Part A. 2015;21:1642–1653. doi: 10.1089/ten.tea.2014.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lam C.X.F., Mo X.M., Teoh S.H., Hutmacher D.W. Scaffold development using 3D printing with a starch-based polymer. Mater Sci Eng C. 2002;20:49–56. [Google Scholar]
- 108.Suwanprateeb J., Suvannapruk W., Wasoontararat K., Leelapatranurak K., Wanumkarng N., Sintuwong S. Preparation and comparative study of a new porous polyethylene ocular implant using powder printing technology. J. Bioact. Compat. Pol. 2011;26:317–331. [Google Scholar]
- 109.Ganey T. Confidential OPM Report-March.; 2011. Cell proliferation and vitality determination of osteoblasts on different materials and surface characteristics; Interpretation of laboratory data. [Google Scholar]
- 110.Kim B.S., Yang S.S., Park H., Lee S.H., Cho Y.S., Lee J. Improvement of mechanical strength and osteogenic potential of calcium sulfate-based hydroxyapatite 3-dimensional printed scaffolds by ε-polycarbonate coating. J Biomater Sci Polym Ed. 2017;28:1256–1270. doi: 10.1080/09205063.2017.1312059. [DOI] [PubMed] [Google Scholar]
- 111.Yao Q., Wei B., Guo Y., Jin C., Du X., Yan C. Design, construction and mechanical testing of digital 3D anatomical data-based PCL–HA bone tissue engineering scaffold. J. Mater. Sci-Mater. M. 2015;26:51. doi: 10.1007/s10856-014-5360-8. [DOI] [PubMed] [Google Scholar]
- 112.Pati F., Song T.H., Rijal G., Jang J., Kim S.W., Cho D.W. Ornamenting 3D printed scaffolds with cell-laid extracellular matrix for bone tissue regeneration. Biomaterials. 2015;37:230–241. doi: 10.1016/j.biomaterials.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 113.Ho C.C., Fang H.Y., Wang B., Huang T.H., Shie M.Y. The effects of Biodentine/polycaprolactone 3D-scaffold with odontogenesis properties on human dental pulp cells. Int Endod J. 2017 doi: 10.1111/iej.12799. [DOI] [PubMed] [Google Scholar]
- 114.Katz E., Willner I. Integrated nanoparticle-biomolecule hybrid systems: synthesis, properties, and applications. Angew. Chem. Int. Ed. Engl. 2004;43:6042–6108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 115.Balasundaram G., Storey D.M., Webster T.J. Novel nano-rough polymers for cartilage tissue engineering. Int. J. Nanomedicine. 2014;9:1845–1853. doi: 10.2147/IJN.S55865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang K., Qian Y., Wang H., Fan L., Huang C., Mo X. Electrospun silk fibroin-hydroxybutyl chitosan nanofibrous scaffolds to biomimic extracellular matrix. J Biomater Sci Polym Ed. 2011;22:1069–1082. doi: 10.1163/092050610X498204. [DOI] [PubMed] [Google Scholar]
- 117.Wang J., Wu D., Zhang Z., Li J., Shen Y., Wang Z. Biomimetically ornamented rapid prototyping fabrication of an apatite-collagen-polycaprolactone composite construct with nano-micro-macro hierarchical structure for large bone defect treatment. ACS Appl Mater Interfaces. 2015;7:26244–26256. doi: 10.1021/acsami.5b08534. [DOI] [PubMed] [Google Scholar]
- 118.Lobo S.E., Arinzeh T.L. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials. 2010;3:815–826. [Google Scholar]
- 119.Tay B.K., Patel V.V., Bradford D.S. Calcium sulfate-and calcium phosphate-based bone substitutes: mimicry of the mineral phase of bone. Orthop. Clin. North Am. 1999;30:615–623. doi: 10.1016/s0030-5898(05)70114-0. [DOI] [PubMed] [Google Scholar]
- 120.Wang L., Nancollas G.H. Pathways to biomineralization and biodemineralization of calcium phosphates: the thermodynamic and kinetic controls. Dalton Trans. 2009;15:2665–2672. doi: 10.1039/b815887h. [DOI] [PubMed] [Google Scholar]
- 121.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He F., Li J., Ye J. Improvement of cell response of the poly(lactic-co-glycolic acid)/calcium phosphate cement composite scaffold with unidirectional pore structure by the surface immobilization of collagen via plasma treatment. Colloids Surf B Biointerfaces. 2013;103:209–216. doi: 10.1016/j.colsurfb.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 123.Nguyen T.B., Lee B.T. A combination of biphasic calcium phosphate scaffold with hyaluronic acid-gelatin hydrogel as a new tool for bone regeneration. Tissue Eng. Part A. 2014;20:1993–2004. doi: 10.1089/ten.tea.2013.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun H., Yang H.L. Calcium phosphate scaffolds combined with bone morphogenetic proteins or mesenchymal stem cells in bone tissue engineering. Chin. Med. J. Engl. 2015;128:1121–1127. doi: 10.4103/0366-6999.155121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leukers B., Gülkan H., Irsen S.H., Milz S., Tille C., Seitz H. Biocompatibility of ceramic scaffolds for bone replacement made by 3D printing. Materialwiss. Werkst. 2005;36:781–787. [Google Scholar]
- 126.Kalita S.J., Bose S., Hosick H.L., Bandyopadhyay A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater Sci Eng C. 2003;23:611–620. [Google Scholar]
- 127.Langer R., Tirrell D.A. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 128.Tappa K., Jammalamadaka U. Novel biomaterials used in medical 3d printing techniques: Review. J Funct Biomater. 2018;9:1–16. doi: 10.3390/jfb9010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rhee S., Puetzer J.L., Mason B.N., Reinhart-King C.A., Bonassar L.J. 3D Bioprinting of sspatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2:1800–1805. doi: 10.1021/acsbiomaterials.6b00288. [DOI] [PubMed] [Google Scholar]
- 130.Tan Z., Parisi C., di Silvio L., Dini D., Forte A.E. Cryogenic 3D printing of super soft hydrogels. Sci Rep. 2017;7:16293. doi: 10.1038/s41598-017-16668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Turnbull G., Clarke J., Picard F., Riches P., Jia L., Han F. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018;3:278–314. doi: 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harris B. The Institute of Materials; London: 1999. Engineering composite materials.https://pdfs.semanticscholar.org/6818/f30049385b7dd0959435087cf52eb8493e61.pdf retrieved from. [Google Scholar]
- 133.Mastrogiacomo M., Muraglia A., Komlev V., Peyrin F., Rustichelli F., Crovace A. Tissue engineering of bone: search for a better scaffold. Orthod. Craniofacial Res. 2005;8:277–284. doi: 10.1111/j.1601-6343.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 134.Chen Q., Zhu C., Thouas G.A. Progress and challenges in biomaterials used for bone tissue engineering: bioactive glasses and elastomeric composites. Prog Biomater. 2012;1:2. doi: 10.1186/2194-0517-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daryasari M.P., Telgerd M.D., Karami M.H., Zandi-Karimi A., Akbarijavar H., Khoobi M. Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Artif Cells Nanomed Biotechnol. 2019;47:4020–4029. doi: 10.1080/21691401.2019.1658594. [DOI] [PubMed] [Google Scholar]
- 136.Gloria A., De Santis R., Ambrosio L. Polymer-based composite scaffolds for tissue engineering. J Appl Biomater Biomech. 2010;8:57–67. [PubMed] [Google Scholar]
- 137.Schantz J.T., Brandwood A., Hutmacher D.W., Khor H.L., Bittner K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J. Mater. Sci-Mater. M. 2005;16:807–819. doi: 10.1007/s10856-005-3584-3. [DOI] [PubMed] [Google Scholar]
- 138.Park J., Lee S.J., Jo H.H., Lee J.H., Kim W.D., Lee J.Y. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J Ind Eng Chem. 2017;46:175–181. [Google Scholar]