Abstract
MRSA enterocolitis is under-recognized in the setting of PCR testing. In this case report, we describe risk factors, the importance of stool culture, and the third published case of MRSA enterocolitis in a patient with leukemia. In addition, we performed a retrospective analysis of all stool cultures at our institution that have grown Staphylococcus aureus, and we describe an additional five cases. We also report the diagnostic yield of organisms detected by culture, but not on the FilmArray panel. While rare, these cases demonstrate that MRSA in stool may indicate a severe and potentially life-threatening infection, particularly in immunocompromised persons.
Keywords: Staphylococcus aureus, Enterocolitis, Immunocompromised, Stool cultures
1. Introduction
Confirmed fatal infection with enterocolitis from methicillin resistant Staphylococcus aureus (MRSA) has rarely been reported in the US. The present case demonstrates the importance of recognizing MRSA as a potential pathogen in the setting of neutropenic enterocolitis during induction chemotherapy and the continued importance of stool culture in the era of molecular diagnostics.
2. Case report
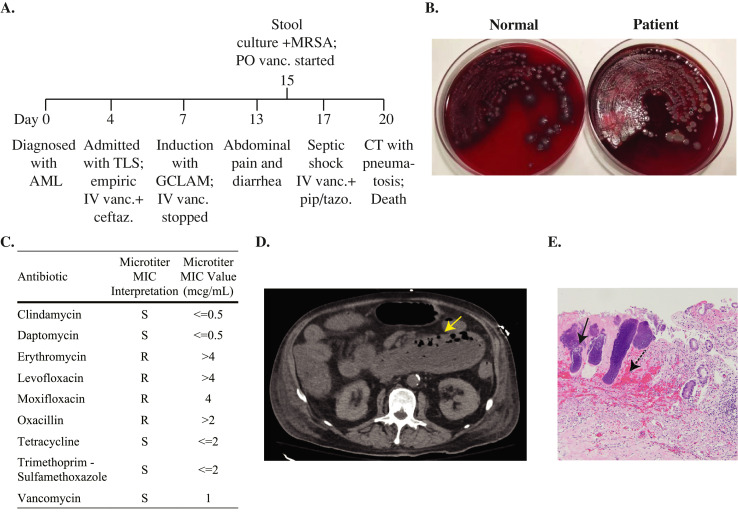
A 72-year-old man with a history of clinically quiescent Crohn's disease presented to the emergency department with fever, fatigue, altered mental status, and nausea/vomiting four days after a new diagnosis of acute myelogenous leukemia (AML) (Fig. 1A). He had tumor lysis syndrome with acute kidney injury (AKI) and hyperleukocytosis and required intensive care unit (ICU) admission. Given the initial diagnostic uncertainty regarding whether his multi-organ failure was related to tumor lysis or infection, he was also treated empirically with ceftazidime and vancomycin for possible septic shock. Antibiotic infusions were separated by over 12 h so that co-mixing was avoided [1]. Blood and urine cultures were sterile. Computed tomography (CT) of the chest, abdomen, and pelvis was unremarkable. He became neutropenic after cytoreductive therapy with hydroxyurea and cytarabine, and defervesced after receiving dose-reduced G-CLAM (filgrastim, cladribine, cytarabine, and mitoxantrone). Ceftazidime was continued for neutropenic prophylaxis, and vancomycin was stopped given the lack of evidence for active infection. Five days later, he again fevered. The following day, he reported mild abdominal pain and new diarrhea. In the setting of febrile neutropenia, an enteric pathogen panel was obtained. This panel consisted of a rapid PCR using the Biofire FilmArray GI panel for 13 bacteria, 4 parasites, and 5 viruses, plus a blood agar plate, primarily for the detection of Aeromonas spp. Reflexive susceptibility testing for Salmonella, Campylobacter, Shigella, and Vibrio species is performed if these organisms are detected on the PCR panel. No pathogens were detected by PCR, and loperamide was started. Two days later, the blood agar plate showed 4+ MRSA with reduced normal fecal flora (Fig. 1B and C). Due to his profound immunocompromised status, oral vancomycin was started for possible MRSA enterocolitis. Gram-negative and anaerobic coverage was also broadened to piperacillin-tazobactam. The following day, he developed respiratory distress, was intubated, and had recurrence of AKI and shock. Vancomycin IV was added. CT of the abdomen and pelvis showed diffuse small bowel wall edema, thickening, dilatation measuring up to 6.2 cm, and pneumatosis without pneumoperitoneum (Fig. 1D). No surgical interventions were available, and the patient was transitioned to comfort care, expiring that same day. Postmortem evaluation revealed gross dilatation of the duodenum and jejunum with dusky discoloration, mucosal erythema and congestion, and Gram positive cocci (Fig. 1E). Focal mucosal erosions were noted in the stomach and cecum. No pseudomembranes were found. Cause of death was attributed to septic shock with multiorgan failure, secondary to MRSA enterocolitis.
Fig. 1.
Clinical, microbiologic, radiographic, and histopathologic findings in a case of MRSA enterocolitis. (A) Timeline of the clinical course from diagnosis to death. (B) Stool cultures from a healthy individual (left) and from the patient (right) showing yellow colonies surrounded by hemolysis. (C) Antibiotic susceptibility testing of the Staphylococcus aureus isolate. (D) CT scan of the abdomen with the yellow arrow indicating an area of pneumatosis. (E) Postmortem histopathology of small bowel mucosa. The black arrow points to an area of Gram-positive cocci. The dotted arrow points to an area of submucosal necrosis and hemorrhage. Abbreviations: IV, intravenous; PO, oral; Vanc, vancomycin; Pip/tazo, piperacillin/tazobactam; MIC, minimum inhibitory concentration; AML, acute myeloid leukemia; CT, computed tomography; GCLAM, filgrastim, cladribine, cytarabine, and mitoxantrone; MIC, minimum inhibitory concentration; MRSA, methicillin resistant Staphylococcus aureus; TLS, tumor lysis syndrome.
3. Discussion
Staphylococcus aureus enterocolitis has been recognized as a cause of antibiotic associated colitis since the mid-20th century[2]. However, when attention shifted to Clostridioides difficile as the major infectious cause for antibiotic-associated diarrhea, reports of Staphylococcus aureus enterocolitis in English scientific literature declined, and awareness diminished. Staphylococcus aureus is now often disregarded when isolated from stool culture [3]. Most recent reports published on Staphylococcus aureus enterocolitis have been from Japan [2], [3], [4]. These and other reports identified gastric resection, older age, longer hospitalization, intestinal carriage, and prior antibiotic exposure as risk factors for Staphylococcus aureus enterocolitis [5], [6], [7].
Patients with hematologic malignancies are also at risk for neutropenic enterocolitis and Clostridioides difficile infection (CDI) due to cytotoxic chemotherapy and antibiotic exposure, but reports of MRSA enterocolitis are rare [8,9]. Although targeted and less cytotoxic therapies against AML are being increasingly studied, intestinal complications, including neutropenic enterocolitis, remain problematic [10], [11], [12], [13]. To our knowledge, there are only three cases of MRSA enterocolitis in the setting of acute leukemia reported in the literature. This is possibly due to underdiagnosis as stool cultures are not often obtained and now supplanted by PCR testing [14,15]. While our laboratory utilizes a blood agar plate primarily for the detection of Aeromonas species, we report the presence of other organisms of unclear pathogenic potential if they are predominant in the culture (Table 1). Interdisciplinary discussion between the microbiology lab and clinical teams can be helpful in determining if MRSA enterocolitis, in the absence of other more common enteric pathogens, fits with the clinical presentation.
Table 1.
Organisms recovered from blood agar, not detected by Biofire FilmArray (7/2018–6/2020).
| Organism group | N (%)* | Comments |
|---|---|---|
| Aeromonas spp. | 30 (0.42%) | A. caviae (N = 7) |
| A. hydrophila (N = 8) | ||
| A. veronii (N = 10) | ||
| Not determined (N = 5) | ||
| Bacillus spp. | 7 (0.10%) | |
| Beta hemolytic streptococci | 4 (0.06%) | |
| Pseudomonas spp. | 38 (0.53%) | |
| Staphylococcus aureus | 4 (0.06%) | |
| Grimontia hollisae | 1 (0.01%) | |
| Fungi | 112 (1.56%) | Aspergillus fumigatus (N = 2) |
| Mucorales (N = 3) | ||
| Yeast (N = 107) |
Percent is out of N = 7179 total enteric panels performed.
Inflammatory bowel disease and other gastrointestinal disorders have been associated with Staphylococcus aureus intestinal carriage [16]. In this case, Crohn's disease may have played a role in the development of MRSA enterocolitis [17]. This, coupled with chemotherapy, neutropenia, and antibiotic exposure may have predisposed to mucosal damage and dysbiosis allowing for MRSA overgrowth and enterotoxin susceptibility [4,18,19]. Although MRSA enterocolitis can present similarly to CDI, it more frequently affects the small bowel, is associated with fever and vomiting, results in more profuse diarrhea, and mucosal invasion is often observed on histopathology [4,20]. It can be difficult to clinically discern whether a positive stool culture for MRSA is due to colonization or disease, but, in this case, a postmortem examination confirmed the histopathologic diagnosis of MRSA enterocolitis, demonstrating the continued importance of autopsy to understand infectious diseases.
Gut microbiome analyses have shown that molecular signatures of baseline microbial diversity might be predictive of infectious risk following induction therapy for AML [21]. Although a comprehensive 16 s rRNA analysis of the gut microbiome was not performed prior to induction chemotherapy, data from the stool culture, which is an assay widely available to clinicians, showed overgrowth of MRSA that was likely a risk factor for invasive disease and sepsis. MRSA enterocolitis is underrecognized and should be considered in patients with hematologic malignancies, particularly those with severe or prolonged antibiotic associated diarrhea, neutropenic enterocolitis, and negative CDI testing [5,20]. Stool cultures can be used to diagnose this rare entity since many commercially available enteric PCR panels do not detect S. aureus. In reviewing stool cultures accompanying the Biofire enteric panel at our institution from January 2014 to October 2020, only seven were positive for S. aureus, including the case described (Table 2). Three cases involved immunocompromised hosts with malignancies. At the time of the stool cultures, only one patient (this case) was thought to have S. aureus enterocolitis, but in retrospect, it is possible that this entity may have contributed to febrile neutropenia in the other two immunocompromised patients. S. aureus was likely a colonizer in the other cases. All patients except for the case described recovered. Together, these results demonstrate that S. aureus is rarely found in the stool as the predominant organism. However, its presence can indicate either colonization or infection, with immunocompromised hosts being more likely to suffer from severe disease.
Table 2.
Review of Staphylococcus aureus positive stool cultures from 1/2014–10/2020*.
| Age group (years) | Biofire | Discharge diagnosis by clinician | Comorbidities during diagnosis | Imaging | Antibiotics | Outcome |
|---|---|---|---|---|---|---|
| 5–10 | Norovirus | Norovirus | none | none | none | Outpatient |
| 20–29 | Negative | Laxative-induced | none | none | none | Outpatient |
| 30–39 | Negative | Neutropenic enterocolitis (S. aureus not contributory) | Relapsed metastatic neuroblastoma status-post autologous hematopoietic stem cell transplant complicated by febrile neutropenia | CT: small bowel thickening | Vancomycin IV, cefepime IV, metronidazole IV | Discharged home with resolution |
| 30–39 | Campylobacter | Campylobacter | none | none | Azithromycin | Outpatient |
| 30–39 | Negative | Laxative-induced | Diabetic ketoacidosis, Staphylococcus aureus bacteremia complicated by epidural abscess and sepsis | none | Vancomycin IV | Discharged home with resolution |
| 50–59 | Negative | Neutropenic enterocolitis (S. aureus not contributory) | Myelodysplastic syndrome status-post allogeneic hematopoietic stem cell transplant complicated by febrile neutropenia | CT: small bowel thickening | Vancomycin IV, cefepime IV, metronidazole IV | Discharged home with resolution |
| 70–79 | Negative | Neutropenic enterocolitis (attributed to S. aureus) | Acute myelogenous leukemia status-post induction chemotherapy complicated by febrile neutropenia, clinically quiescent Crohn's disease | CT: small bowel thickening and dilatation, pneumatosis | Piperacillin-tazobactam, vancomycin IV, vancomycin PO | Died of septic shock secondary to MRSA enterocolitis with severe neutropenia |
Cultures obtained at the University of Washington Medical Center and Seattle Cancer Care Alliance.
In a review of the literature on MRSA enterocolitis, therapy with oral vancomycin is recommended [3,4,20,22]. In our case, at the time of diagnosis and initiation of oral vancomycin, disease was already severe with evidence of pneumatosis. MRSA enterocolitis has been most frequently described in immunocompetent patients [22], and further studies of its role in hematologic malignancy patients with antibiotic associated diarrhea or neutropenic enterocolitis is needed. Non-classic stool pathogens, particularly MRSA, should not be universally disregarded as colonization. These cases demonstrate that MRSA can be a rare cause of neutropenic enterocolitis in patients with hematologic malignancies after induction chemotherapy and demonstrates the continued utility of stool culture in the era of molecular diagnostics.
Funding
This work was supported by the Fred Hutchinson Joel Meyer's Endowment (J.B.), a new investigator award from the American Society for Transplantation and Cell Therapy (J.B.), and the Host Defense Training in Allergy and Infectious Diseases 5T32AI007044–45 (V.A.).
CRediT authorship contribution statement
Pooja Bhattacharyya: Data curation, Writing – original draft. Andrew Bryan: Data curation, Writing – original draft, Formal analysis. Vidya Atluri: Writing – original draft. Jimmy Ma: Writing – original draft. Lindsey Durowoju: Formal analysis. Anshu Bandhlish: Formal analysis. Jim Boonyaratanakornkit: Data curation, Writing – original draft.
Declaration of Competing Interest
The authors declare no conflicts of interest.
References
- 1.Meyer K., Santarossa M., Danziger L.H., Wenzler E. Compatibility of ceftazidime-avibactam, ceftolozane-tazobactam, and piperacillin-tazobactam with vancomycin in dextrose 5% in water. Hosp. Pharm. 2017;52(3):221–228. doi: 10.1310/hpj5203-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotler D.P., Sordillo E.M. A case of staphylococcus aureus enterocolitis: a rare entity. Gastroenterol. Hepatol. (N Y) 2010;6(2):117–119. [PMC free article] [PubMed] [Google Scholar]
- 3.Iwata K., Doi A., Fukuchi T., Ohji G., Shirota Y., Sakai T., Kagawa H. A systematic review for pursuing the presence of antibiotic associated enterocolitis caused by methicillin resistant Staphylococcus aureus. BMC Infect. Dis. 2014;14:247. doi: 10.1186/1471-2334-14-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Z., Kotler D.P., Schlievert P.M., Sordillo E.M. Staphylococcal enterocolitis: forgotten but not gone? Dig. Dis. Sci. 2010;55(5):1200–1207. doi: 10.1007/s10620-009-0886-1. [DOI] [PubMed] [Google Scholar]
- 5.Boyce J.M., Havill N.L. Nosocomial antibiotic-associated diarrhea associated with enterotoxin-producing strains of methicillin-resistant Staphylococcus aureus. Am. J. Gastroenterol. 2005;100(8):1828–1834. doi: 10.1111/j.1572-0241.2005.41510.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa Y., Saraya T., Koide T., Kikuchi K., Ohkuma K., Araki K., Makino H., Yonetani S., Takizawa H., Goto H. Methicillin-resistant Staphylococcus aureus enterocolitis sequentially complicated with septic arthritis: a case report and review of the literature. BMC Res. Notes. 2014;7:21. doi: 10.1186/1756-0500-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acton D.S., Plat-Sinnige M.J., van Wamel W., de Groot N., van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 2009;28(2):115–127. doi: 10.1007/s10096-008-0602-7. [DOI] [PubMed] [Google Scholar]
- 8.Chubachi A., Nishimura S., Endo Y., Miura A.B. [Methicillin-resistant staphylococcal enterocolitis developed after induction chemotherapy in a case of acute promyelocytic leukemia] Rinsho Ketsueki. 1989;30(8):1319–1320. [PubMed] [Google Scholar]
- 9.Rothman A., Lio J., Lee Y., Colitis M.R.S.A. An under-recognized cause of septic shock. Abstr. Am. Thoracic Soc. 2020 May 22. [Google Scholar]
- 10.Short N.J., Konopleva M., Kadia T.M., Borthakur G., Ravandi F., DiNardo C.D., Daver N. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 2020;10(4):506–525. doi: 10.1158/2159-8290.CD-19-1011. [DOI] [PubMed] [Google Scholar]
- 11.Xia R., Zhang X. Neutropenic enterocolitis: a clinico-pathological review. World J. Gastrointest. Pathophysiol. 2019;10(3):36–41. doi: 10.4291/wjgp.v10.i3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorschluter M., Mey U., Strehl J., Schmitz V., Rabe C., Pauls K., Ziske C., Schmidt-Wolf I.G., Glasmacher A. Invasive fungal infections in neutropenic enterocolitis: a systematic analysis of pathogens, incidence, treatment and mortality in adult patients. BMC Infect. Dis. 2006;6:35. doi: 10.1186/1471-2334-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ueda M., El-Jurdi N., Cooper B., Caimi P., Baer L., Kolk M., Brister L., Wald D.N., Otegbeye F., Lazarus H.M., Sandmaier B.M., William B., Saunthararajah Y., Woost P., Jacobberger J.W., de Lima M. Low-dose azacitidine with DNMT1 level monitoring to treat post-transplantation acute myelogenous leukemia or myelodysplastic syndrome relapse. Biol. Blood Marrow Transpl. 2019;25(6):1122–1127. doi: 10.1016/j.bbmt.2018.12.764. [DOI] [PubMed] [Google Scholar]
- 14.Siegel D.L., Edelstein P.H., Nachamkin I. Inappropriate testing for diarrheal diseases in the hospital. JAMA. 1990;263(7):979–982. [PubMed] [Google Scholar]
- 15.Beal S.G., Tremblay E.E., Toffel S., Velez L., Rand K.H. A Gastrointestinal PCR panel improves clinical management and lowers health care costs. J. Clin. Microbiol. 2018;56(1) doi: 10.1128/JCM.01457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kates A.E., Thapaliya D., Smith T.C., Chorazy M.L. Prevalence and molecular characterization of Staphylococcus aureus from human stool samples. Antimicrob Resist Infect Control. 2018;7:42. doi: 10.1186/s13756-018-0331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Hertogh G., Geboes K. Crohn's disease and infections: a complex relationship. Med. Gen. Med. 2004;6(3):14. [PMC free article] [PubMed] [Google Scholar]
- 18.Hueso T., Ekpe K., Mayeur C., Gatse A., Joncquel-Chevallier Curt M., Gricourt G., Rodriguez C., Burdet C., Ulmann G., Neut C., Amini S.E., Lepage P., Raynard B., Willekens C., Micol J.B., De Botton S., Yakoub-Agha I., Gottrand F., Desseyn J.L., Thomas M., Woerther P.L., Seguy D. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: the role of mucosal strengthening. Gut. Microbes. 2020;12(1) doi: 10.1080/19490976.2020.1800897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashidi A., Zhu Z., Kaiser T., Manias D.A., Holtan S.G., Rehman T.U., Weisdorf D.J., Khoruts A., Dunny G.M., Staley C. Vancomycin-resistance gene cluster, vanC, in the gut microbiome of acute leukemia patients undergoing intensive chemotherapy. PLoS ONE. 2019;14(10) doi: 10.1371/journal.pone.0223890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane A.B., Copeland N.K., Onmus-Leone F., Lawler J.V. Methicillin-resistant staphylococcus aureus as a probable cause of antibiotic-associated enterocolitis. Case Rep. Infect. Dis. 2018;2018 doi: 10.1155/2018/3106305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galloway-Pena J.R., Shi Y., Peterson C.B., Sahasrabhojane P., Gopalakrishnan V., Brumlow C.E., Daver N.G., Alfayez M., Boddu P.C., Khan M.A.W., Wargo J.A., Do K.A., Jenq R.R., Kontoyiannis D.P., Shelburne S.A. Gut microbiome signatures are predictive of infectious risk following induction therapy for acute myeloid leukemia. Clin. Infect. Dis. 2020;71(1):63–71. doi: 10.1093/cid/ciz777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergevin M., Marion A., Farber D., Golding G.R., Levesque S. Severe MRSA enterocolitis caused by a strain harboring enterotoxins D, G, and I. Emerg. Infect. Dis. 2017;23(5):865–867. doi: 10.3201/eid2305.161644. [DOI] [PMC free article] [PubMed] [Google Scholar]