Abstract
Objective
Interferon regulatory factor (IRF) 5 is a transcription factor known for promoting M1 type macrophage polarization in vitro. Given the central role of inflammatory macrophages in promoting atherosclerotic plaque progression, we hypothesize that myeloid cell-specific deletion of IRF5 is protective against atherosclerosis.
Methods
Female Apoe–/–LysmCre/+Irf5fl/fl and Apoe−/−Irf5fl/fl mice were fed a high-cholesterol diet for three months. Atherosclerotic plaque size and compositions as well as inflammatory gene expression were analyzed. Mechanistically, IRF5-dependent bone marrow-derived macrophage cytokine profiles were tested under M1 and M2 polarizing conditions. Mixed bone marrow chimeras were generated to determine intrinsic IRF5-dependent effects on macrophage accumulation in atherosclerotic plaques.
Results
Myeloid cell-specific Irf5 deficiency blunted LPS/IFNγ-induced inflammatory gene expression in vitro and in the atherosclerotic aorta in vivo. While atherosclerotic lesion size was not reduced in myeloid cell-specific Irf5-deficient Apoe–/– mice, plaque composition was favorably altered, resembling a stable plaque phenotype with reduced macrophage and lipid contents, reduced inflammatory gene expression and increased collagen deposition alongside elevated Mertk and Tgfβ expression. Irf5-deficient macrophages, when directly competing with wild type macrophages in the same mouse, were less prone to accumulate in atherosclerotic lesion, independent of monocyte recruitment. Irf5-deficient monocytes, when exposed to oxidized low density lipoprotein, were less likely to differentiate into macrophage foam cells, and Irf5-deficient macrophages proliferated less in the plaque.
Conclusion
Our study provides genetic evidence that selectively altering macrophage polarization induces a stable plaque phenotype in mice.
Keywords: Atherosclerosis; Interferon regulatory factor 5; Macrophage polarization (M1, M2); Plaque stabilization; Anti-inflammation; Aortic macrophages
Abbreviations: IRF5, Interferon regulatory factor 5; LYVE1, Lymphatic Vessel Endothelial Hyaluronidase; NEAA, Non-essential amino acids; MRC1, Mannose Receptor C-Type 1
Highlights
-
•
IRF5 is widely expressed in inflammatory, foamy, and resident-like macrophages in murine and human plaque.
-
•
IRF5 expression in macrophages destabilizes the atherosclerotic plaque.
-
•
IRF5 promotes mono to macro differentiation, macrophage proliferation, and foam cell formation in the plaque environment.
1. Introduction
Cardiovascular (CV) disease is the leading cause of morbidity and mortality. Despite optimal secondary prevention, CV deaths account for almost one third of all deaths worldwide [1]. Atherosclerosis is the underlying pathomechanism in most cases, considered a chronic, lipid-driven inflammatory disease [[2], [3], [4], [5], [6]].
In the developing plaque, monocytes infiltrate the arterial intima and differentiate into macrophages which proliferate locally, driven by oxidized low density lipoprotein (LDL) uptake [3,4,6]. Macrophages in the plaque are thought to preserve plasticity, changing their phenotype according to the cytokine composition in the environment [7]. Single-cell analyses have recently identified several populations of macrophages in the atherosclerotic aorta, most prominently a population of resident-like macrophages, “foamy”, lipid-rich macrophages expressing high levels of Triggering receptor expressed on myeloid cells (Trem) 2, and inflammatory macrophages [5,8]. Although macrophage heterogeneity in the plaque appears more complex than the traditional M1 and M2 type polarization states, the dichotomous concept of inflammation driving and resolving macrophage subsets prevails [[9], [10], [11]].
Interferon regulatory factor (IRF) family members are transcription factors, which are involved in the regulation of immune cell development, differentiation, and responses to pathogens. IRF3, 5, 7, and 8 mediate the response to viral RNA and DNA downstream of pathogen recognition receptors [12]. IRF4, 5, and 8 regulate myeloid cell development and phenotype [12]. IRF5 is predominantly expressed in B cells, monocytes, macrophages, and plasmacytoid dendritic cells (pDCs) [13,14]. However, IRF5 expression has also been reported in classic DCs, T-cells, fibroblasts, endothelial cells (ECs) and adipocytes, and thus in a plethora of atheroma-associated cell types [[15], [16], [17], [18], [19], [20]]. In macrophages, for example, IRF5 acts downstream of Myd88-associated Toll-like receptors (TLR) inducing expression of pro-inflammatory cytokines such as Interleukin (IL)-6, IL-12, Tumor Necrosis Factor (TNF)-α, Interferon gamma (IFN-γ) and IL-23, and repressing the expression of anti-inflammatory IL-10 and transforming growth factor (TGF)β [15,[20], [21], [22], [23], [24], [25]]. Therefore, transcription factor IRF5 is best known for mediating M1 polarization in macrophages, whereas IRF4 triggers M2 polarization while competing for the same binding sites at Myd88 [12,15,24,26,27]. In the plaque environment, high mobility group box 1 (HMGB1), heat shock protein (HSP)60, modified LDL and nucleic acid polymers activate Myd88-dependent TLR pathways upstream of IRF5 [[28], [29], [30]].
In this study, we show that selective Irf5 deficiency in macrophages is sufficient to generate a stable plaque phenotype in mice, providing a rationale for modulating macrophage phenotypes in order to combat atherosclerosis.
2. Materials and methods
2.1. Animals and diet
Wild type (WT) CD45.1+ (B6 CD45.1), Apoe–/–, Apoe–/– Irf5fl/fl and Apoe–/– LysmCre/+ Irf5fl/fl were housed under specific pathogen-free conditions. Myeloid cell-specific Irf5−/− (Apoe–/– LysmCre/+ Irf5fl/fl) and Irf5+/+ mice (Apoe–/– Irf5fl/fl) were generated through crossbreeding of Apoe–/– (B6·129P2-Apoetm1Unc/J), LysmCre/Cre (Bl.129P2-Lyz2tm1(Cre)lfo/J) and Irf5fl/fl (C57BL/6-Irf5tm1Ppr/J) mice (kindly provided by Klaus Ley, San Diego, CA, USA) (Figure 2A). Eight to 10-week-old female Apoe–/– Irf5fl/fl and Apoe–/– LysmCre/+ Irf5fl/fl mice were fed a high-cholesterol diet (HCD, 1.25% w/w cholesterol, D12108 mod., Sniff GmbH, Soest, Germany) ad libitum for 12 weeks to accelerate atherosclerotic plaque formation. Male Apoe–/– mice develop cutaneous ulcerations more frequently under HCD for which reason the Animal Care Committee of the University of Freiburg and the Regional Council, who approved all protocols, advised against the use of male mice in this study.
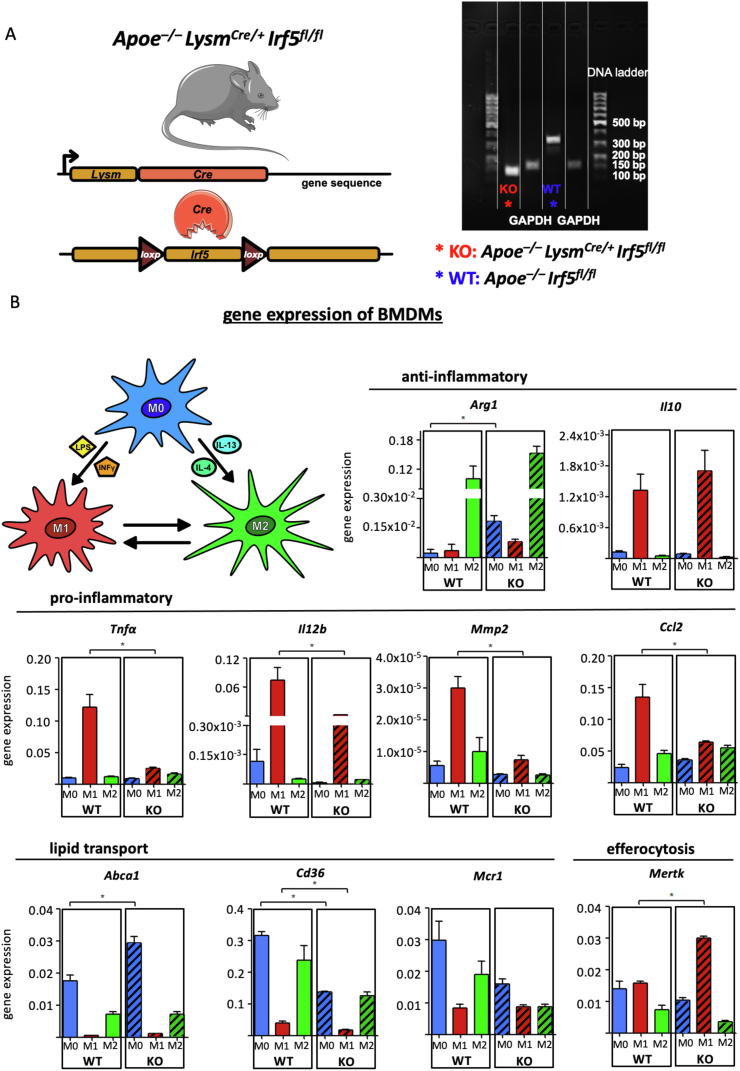
Figure 2.
(A) Confirmation of myeloid cell-specific Irf5 deficiency in Bone-marrow derived macrophages (BMDM) isolated from Apoe–/–LysmCre/+Irf5fl/flmice (KO) and WT controls (Apoe−/−Irf5fl/fl mice) by gel-electrophoresis of Irf5 PCR products. (B) BMDM generated from WT and KO bone marrow cells (M0) were polarized to M1 and M2 subsets in vitro through stimulation with LPS and IFNy or IL-4 and IL-13 for 12 h. Results of expressions for key macrophage-associated genes are presented as mean ± SEM. Gene expressions were analyzed using the ΔΔCq method and normalized to the expression of β-actin. ∗p < 0.05 denotes statistically significant differences between macrophage subtypes, Mann–Whitney, n = 4 per group. IFNγ = interferon, IL = interleukin, LPS = lipopolysaccharide, WT = wild type.
To generate mixed chimeras, 8 week-old female Apoe–/– mice were lethally irradiated 9.5 Gy, irradiator IBL637C γ-ray 137Cs (Schering Cis-Bio International, France) and reconstituted with 6 × 106 bone marrow cells from WT CD45.1+ Apoe–/– and CD45.2+ Apoe–/–LysmCre/+ Irf5fl/fl donor animals. Donor cells were mixed 1:1 and injected into the recipient Apoe–/– animals intravenously. Six weeks post bone marrow transplantation, the reconstituted mixed chimeric mice were fed a HCD for 12 weeks to accelerate atherogenesis.
2.2. Histology
Murine aortic roots were embedded in OCT Tissue Tek (Sakura Finetek, Tokyo, Japan) and cut into serial cryostat sections (5 μm) starting at the level of the aortic valve. Aortic arches were sectioned longitudinally, and plaques were analyzed along a 2 mm section of the lesser curvature. Sections were stained with Oil-red O (Sigma Aldrich, St. Louis, MO, USA), Masson Trichrome (Sigma Aldrich, St. Louis, MO, USA), anti-CD68 (clone FA-11, BioRad AbD Serotec, Purchheim, Germany), secondary antibodies rabbit-anti rat biotin conjugated (BA-4001) according to the manufacturers’ instructions (see [3]. Images were recorded with the Axioplan 2 imaging light-/fluorescence microscope (Carl Zeiss MicroImaging GmbH, Göttingen, Germany). Images were analyzed with Image Pro Premiere 9.2 (Media Cybernetics, Silver Springs, USA).
2.3. Flow cytometry
Murine aortic cells were retrieved through enzymatic digestion with collagenase I, collagenase XI, hyaluronidase, DNAse I and HEPES Solution (Sigma Aldrich, St. Louis, MO, USA) in a thermocycler for 45 min at 750 rpm and 37 °C. Murine blood samples were lysed in red blood cell lysis buffer (Biolegend, San Diego, CA, USA). Isolated cells from the blood and aorta were counted using a Neubauer Chamber (Marienfeld, Lauda-Königshofen, Germany).
Cells were stained with specific fluorescent antibodies as indicated in supplemental table 1. Bone marrow-derived macrophages (BMDMs) were stained with a viability dye (Fixable Viability Dye, eflour450, eBioscience, Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's protocol, prior to adding fluorescent antibodies. Ly6Chigh monocytes were identified as CD45+, CD11b+, Lin− (Lin = CD3, CD19, NK1.1, Ly6G), Ly6Chigh, CD115+, F4/80low. Macrophages were identified as CD45+, CD11b+, Lin−, Ly6Clow, F4/80high. Neutrophils were identified as CD45+, CD11b+, Ly6G+. T-cells as CD45+, CD11b−, CD3+, CD19- and B-cells as CD45+, CD11b−, CD19+, CD3-. Intracellular staining with anti-Ki67 and anti-active Caspase 3, BD Cytoxfix/Cytoperm (#554722, BD Biosciences, San Diego, CA, USA), BD Perm/Wash (#554723) and BD Permeabilization Buffer Plus (#561651) was conducted according to the manufacturer's instructions. Data were collected on a BD FACSCanto II Cell Analyzer (BD Bioscience, San Diego, CA, USA) and analyzed with FlowJo (Treestar, Ashland, OR, USA). Aortic macrophages were sorted into RLT-buffer/1% β-mercaptoethanol (Sigma Aldrich, St. Louis, MO, USA), (RLT-buffer out of RNeasy Micro Kit (Qiagen, Valencia, CA, USA)) by a BD FACSAria Fusion Cell Sorter (BD Bioscience, San Diego, CA, USA). Monocytes from blood and spleen were sorted in (Roswell Park Memorial Institute (RPMI)-1640, 10% fetal calf serum (FCS), 1% non-essential amino acids (NEAA), 1% Penicillin/Streptomycin (PenStrep)) for further cultivation.
2.4. Real-time qPCR
RNA was extracted from murine whole aortas using QIAzol and RNeasy Mini Kit (Qiagen, Valencia, CA, USA) or BMDMs using RNeasy Mini Kit or from sorted aortic macrophages using QIAshredder and RNeasy Micro Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Quantitative TaqMan-PCR was performed using a Bio-Rad CFX96 Touch Real-Time PCR System and TaqMan probes Mm00443258_m1 (Tnfα), Mm01336189_m1 (ProIl1b), Mm00439614_m1 (Il10), Mm01178820_m1 (Tgfb1), Mm00441242_m1 (Ccl2), Mm00496478_g1 (Irf5), Mm01320970_m1 (Vcam-1), Mm00434920_m1 (Mertk), Mm00485148_m1 (Mrc1), Mm00475988_m1 (Arg1), Mm00439498_m1 (Mmp2), Mm00516023_m1 (Icam-1), Mm01302427_m1 (Ccl5), Mm00434174_m1 (Il12), Mm01135198_m1 (Cd36), Mm00442646_m1 (Abca1) (Thermo Fisher Scientific, Waltham, MA, USA). Data were statistically analyzed using the 2-▵Ct method.
2.5. BMDMs
Bone marrow cells were isolated from myeloid cell-specific Irf5−/− (Apoe–/– LysmCre/+ Irf5fl/fl) and WT (Apoe–/– Irf5fl/fl) mice. Cells were stimulated with 30 ng/mL macrophage colony-stimulating factor (M-CSF) (Peprotech, Rocky Hill, NJ, USA) in normal culture media (RPMI-1640, 10% FCS, 1% NEAA, 1% PenStrep) and differentiated into macrophages for 5–7 days. Unstimulated M0 macrophages were polarized to either M1 or M2 macrophages by co-incubation with 100 ng/mL lipopolysaccharide (LPS) and 10 ng/mL Interferon gamma (IFN), or 20 ng/mL IL-4 and 20 ng/mL IL-13 (Peprotech, Rocky Hill, NJ, USA) in RPMI-1640/0.1% FCS/1% NEAA) for 12 h, respectively.
2.6. Monocyte to macrophage differentiation and oxidized (ox)LDL uptake
Monocytes were sorted from blood and spleen cell suspensions and cultivated in RPMI-1640, 10% FCS, 1% NEAA, 1% PenStrep with 30 ng/ml M-CSF overnight (16 h) for differentiation into F480high macrophages. During the final 4 h of macrophage differentiation, cells were incubated with 10 μg/mL human DiI-labeled medium oxidized LDL (DiI-oxLDL, Kalen Biomedical, Montgomery Village, MD, USA). Foam cell formation and differentiation was evaluated by flow cytometry (defined as Viability Dye– F4/80high Dil + macrophages).
2.7. Cholesterol assays
Murine plasma cholesterol levels were measured using Cholesterol FS 10′ Multi-purpose kit (DiaSys Diagnostic Systems GmbH, Holzheim, Germany) according to the manufacturer's instructions.
2.8. Bioinformatic integration of single-cell datasets and visualization of IRF5 expression
The expression matrix table and sample information were downloaded from Gene Expression Omnibus dataset GSE131776 [31] and generously provided by Dr. Dennis Wolf [32]. We only retained the TCF21 WT samples from Wirka et al. for the analysis. We used the R package Seurat (version 3.2) [33,34] to integrate the Apoe–/– mouse dataset from both publications. We first performed the standard Seurat workflow on each dataset separately. Briefly, after log-normalization with the scale factor of 10,000, the 2000 most variable genes selected with variance stabilizing transformation were used for principle component analysis. We applied the function RunTSNE to use the first 20 principle components for dimensionality reduction. Graph-based clustering was performed by FindNeighbors and FindClusters with a resolution of 0.65 and 1.2 for the Wirka et al. and Winkels et al. datasets, respectively. Cell types were assigned to clusters according to the signatures suggested in the original publications. The expression matrix and metadata with cell type information of both datasets were combined and used for another standard Seurat analysis. The parameters were kept the same except that the resolution was set at 0.8 for clustering. The two datasets were well integrated with macrophage and T cell clusters from each dataset overlapping. For the simplicity of presentation, we annotated the subclusters of smooth muscle cells and T cells as larger clusters. Sub-analysis of macrophages was performed by extracting the macrophage subset from the Seurat object and running another Seurat analysis with the same parameters as mentioned above. We annotated the sub-groups of macrophage according to the known macrophage subset markers —inflammatory (Tnf and Il1b), resident-like (Mannose Receptor C-Type1 (Mrc1) and Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (Lyve1)), and foamy (Triggering Receptor Expressed On Myeloid Cells 2 (Trem2) and ATP Binding Cassette Subfamily G Member 1 (Abcg1)) [8]. We applied the macrophage matrix and metadata with macrophage subset information for Seurat v3 integration. We used the functions, FindIntegrationAnchors and IntegrateData, as recommended by Seurat guidelines [35]. The same Seurat analysis procedure was performed on the human right coronary artery dataset. Cell type annotation was assigned according to the original publication. Analysis and annotation of macrophage subset were performed with the same settings and features that applied for mouse macrophage data analysis. The human dataset composed of samples from four patients was fairly well integrated but not as uniformly as the mouse dataset, likely due to the differences of cell composition and cell counts from each individual as well as larger variation between humans. Hence, we performed Seurat v3 integration for all cell types and macrophages from human with the same settings that were applied to mouse macrophage dataset. Expression of features and IRF5 was visualized by the Seurat tools, Featureplot and Vlnplot.
2.9. Statistics
Results are presented as mean +standard error of the mean (SEM). Differences between two groups from independent mice or samples were analyzed with the unpaired Student's t test (if n > 5 and passing D'Agostino and Pearson omnibus normality test) or with Mann–Whitney test (if n ≤ 5 or not normally distributed). To assess differences between more than 2 groups Kruskal Wallis (if n ≤ 5 or not normally distributed) and a subsequent Dunn's multiple comparisons test was applied. P values ≤ 0.05 denote significant changes. Gene expression comparison between subtypes of macrophages were performed with the Seurat tool, FindMarkers, using a Wilcoxon Rank–Sum test.
3. Results
3.1. Macrophages are the dominant IRF5-expressing cell type in atherosclerotic lesions
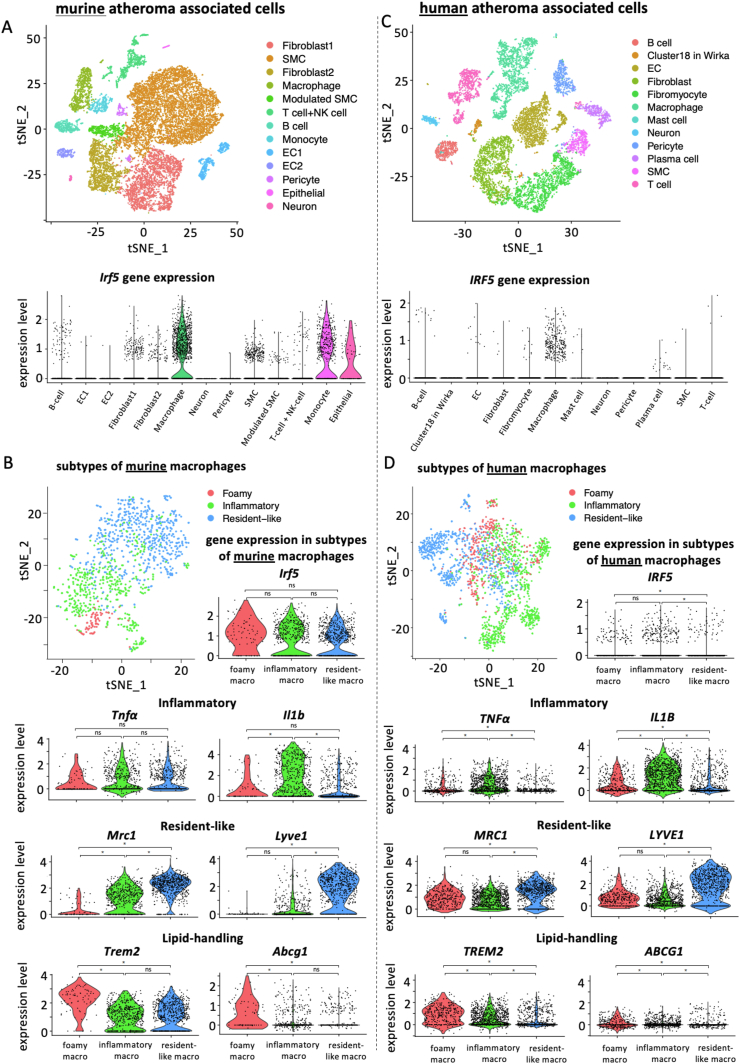
Irf5 expression was reported in monocytes, macrophages, DCs, B- and T-cells, fibroblasts and endothelial cells [[13], [14], [15], [16], [17], [18], [19], [20]] – cells that can be found in atherosclerotic lesions. To evaluate Irf5 expression in atheroma-associated cells directly, we inquired single-cell gene expression profiles from atherosclerotic aortic lesions in Apoe–/– mice [31,32]. While a minority of fibroblasts and vascular smooth muscle cells expressed Irf5, a large proportion of B cells, monocytes and macrophages, in particular, expressed Irf5 (Figure 1A). Irf5 expression increased in the population of aortic macrophages during HCD-induced atherosclerosis formation in Apoe–/– mice (Supplemental Fig. 1A). Single-cell analysis and subclustering of macrophages into inflammatory, resident-like, and foamy subsets showed that Irf5 expression was wide-spread and not significantly enriched in a specific macrophage subtype in mice (Figure 1B and Supplemental Fig. 1B). Notably, single-cell RNAseq analysis of human plaques identified a similar IRF5 expression pattern in- and outside macrophages, and IRF5 expression being highest in foamy and inflammatory macrophages (Figure 1C, D Supplemental Fig. 1D).
Figure 1.
scRNA-Seq datasets from murine (Winkels et al., 2018 + Wirka et al., 2019, data integrated) and human (Wirka et al., 2019) atherosclerotic lesions were analyzed for Irf5 expression. (A, C) tSNE plots of mouse and human atheroma associated cell types and Irf5 expression herein (violin plots). (B, D) tSNE plots depicting foamy, inflammatory, and resident-like subtypes of murine (Winkels et al., 2018 + Wirka et al., 2019, data integrated) and human macrophages (Wirka et al., 2019) in atherosclerotic lesions. Violin plots showing expression levels of Irf5 and subtype defining genes in the respective macrophage subtypes. ∗p < 0.05 denotes statistically significant differences between macrophage subsets, adjusted p-value, Wilcoxon Rank–Sum test.
To study the functional relevance of Irf5 expression in macrophages for the development of atherosclerosis, we generated a selective Irf5 knockout (KO) in monocytes and macrophages of Apoe–/– mice (Figure 2A).
3.2. Irf5-dependent macrophage polarization in vitro
First, macrophages were generated from bone marrow cells isolated from Apoe–/– LysmCre/+ Irf5fl/fl (KO) and Apoe–/– Irf5fl/fl (WT) mice, studying, in vitro, how the loss of Irf5 under M1 and M2 polarizing conditions affected the expression of genes linked to key macrophage functions in the context of atherosclerosis: inflammation, efferocytosis and lipid transport (Figure 2B). Stimulation of BMDM with LPS and IFNγ-induced expression of M1-associated genes Tnfa, Il12, matrix metalloproteinase (Mmp) 2 and CC chemokine ligand (Ccl) 2, and suppressed gene expression of scavenger receptor CD36 and cholesterol exporter ATP-binding cassette transporter (Abca) 1. Irf5 deficiency blunted the increase in inflammatory M1 marker expression and suppressed CD36 expression in polarized BMDM (Figure 2B). Irf5-deficient BMDM doubled the expression of Mertk when stimulated with LPS and IFNγ compared to Irf5+/+ BMDM. M2 polarization, by stimulating BMDM with IL-4 and IL-13, induced Arginase (Arg)1 expression, which remained unaffected by Irf5 deficiency. However, mature Irf5−/− BMDM already expressed elevated Arg1 and Abca1 levels without stimulation (M0) compared to Irf5+/+ BMDM. In summary, Irf5 promoted inflammatory gene expression in macrophages in vitro. Next, we tested the effect of myeloid cell-specific IRF5 deficiency on atherogenesis in vivo.
3.3. Myeloid cell-specific Irf5 deficiency promotes a stable plaque phenotype
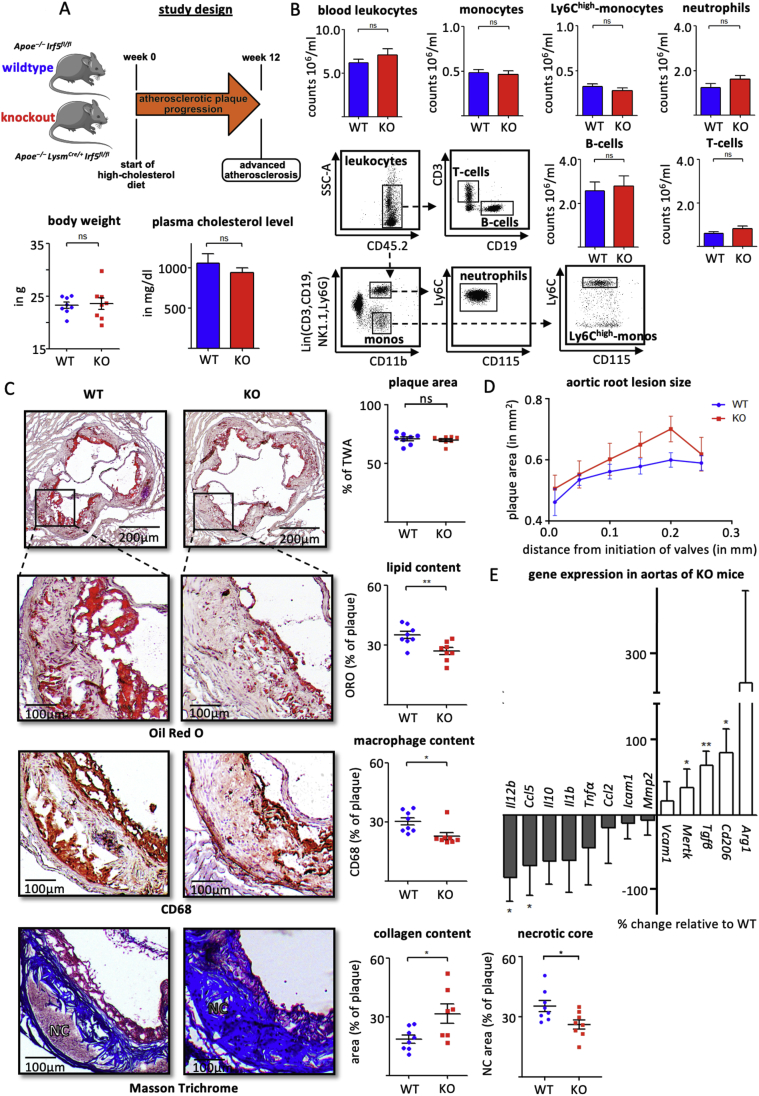
Following 12 weeks of HCD, body weights, plasma cholesterol levels and leukocyte counts in blood, spleen and bone marrow (Figure 3 A, B and Supplemental Fig. 2) of myeloid cell-specific Irf5−/− mice (Apoe–/– LysmCre/+ Irf5fl/fl; KO) did not differ from those in Irf5+/+ mice (Apoe–/– Irf5fl/fl; WT). Atherosclerotic lesion size and area at the aortic root level and aortic arch were similar in both groups, but plaque composition was altered (Figure 3 C, D, and Supplemental Fig. 3). Lipid and macrophage contents and necrotic core size were significantly reduced, and collagen contents increased in atherosclerotic lesions of KO mice compared to WT controls (Figure 3C and Supplemental Fig. 3). Accordingly, gene expression profiles of atherosclerotic aortas from KO mice showed a relative increase in Tgfb, Mertk, and Cd206, while Ccl5 and Il12 expressions were significantly reduced, with other inflammatory markers pointing towards the same direction (Figure 3E). Taken together, myeloid cell-specific Irf5 deficiency promoted a stable plaque phenotype.
Figure 3.
(A) Myeloid cell-specific Irf5 deficient Apoe–/– mice (KO) and WT controls were fed a high cholesterol diet for 12 weeks. Body weight and plasma cholesterol levels are presented as mean ± SEM, n = 8 per group. ns = non-significant, t-test. (B) Representative dot plots and cell counts for leukocytes, neutrophils, monocytes, Ly6Chigh-monocytes in particular, T-cells and B-cells in the blood are presented as mean ± SEM, n = 8 per group. ns = non-significant, t-test. (C) Representative histologic images and quantification of plaque area, lipid- (Oil Red O), macrophage- (CD68), collagen-contents and necrotic core (Masson Trichrome), and necrotic core (NC) in aortic root sections. The plaque area represents the intimal lesion area as a fraction of the total wall area (encompassing intima and media). Areas within lesions filled with lipids, macrophages, collagen and the necrotic core are related to the intimal lesion area. Results are presented as mean percentage ± SEM, n = 8 per group, ∗p < 0.05 denotes statistically significant differences between WT and KO mice, t-test. (D) Absolute aortic root lesion size measured in 6 serial sections at 50 μm intervals starting from valve initiation. Results are presented as mean ± SEM, n = 8 per group. ns = non-significant, t-test. (E) Change in gene expressions in aortas from myeloid cell-specific Irf5 KO-mice relative to expression levels in WT mice. Gene expressions were analyzed using the ΔΔCq method and normalized to the expression of β-actin. Results are presented as mean ± SEM, n = 8 per group. ∗p < 0.05 denotes statistically significant differences between WT and KO, t-test and Mann–Whitney. ORO=Oil red O, TWA = total wall area, NC = necrotic core.
3.4. Multifactorial attenuation of Irf5-deficient macrophage accumulation in the plaque
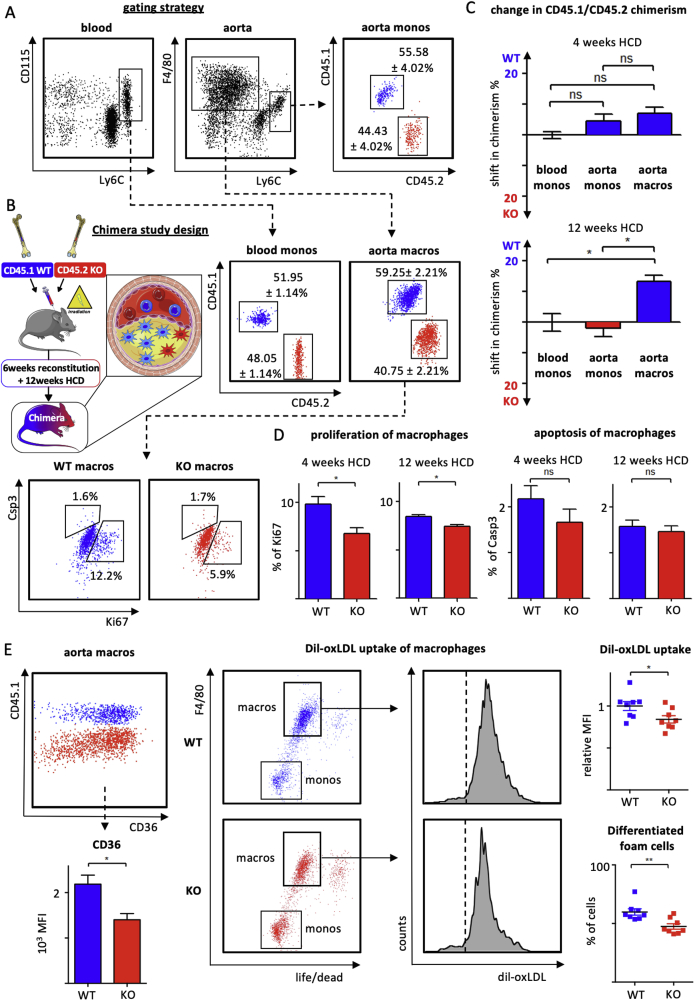
Next, we generated mixed bone-marrow chimeras by reconstituting lethally irradiated Apoe–/– mice with a mixture of CD45.1 Apoe–/– (WT) and CD45.2 Apoe −/− myeloid cell-specific Irf5−/− (KO) bone marrow cells (Figure 4A,B). In these mice, Irf5-deficient and competent monocytes compete directly for accumulation in atherosclerotic lesions, and allow for assessing the intrinsic impact of Irf5 deficiency in monocytes and their progeny, macrophages, in the plaque. Chimerism of Irf5 WT and KO cells was determined by flow cytometric staining for CD45.1 and CD45.2 in the blood and aortic tissue cell suspensions (Figure 4A) as previously described [3,6]. In the blood of reconstituted Apoe–/– mice fed a HCD for 4 weeks, 48.05 ± 1.14% of circulating Ly6Chigh monocytes were CD45.2+ and thus derived from the Irf5 KO bone marrow, while Irf5 WT bone marrow gave rise to CD45.1+ Ly6Chigh monocytes (51.95 ± 1.14%). Interestingly, CD45.1/CD45.2 chimerism among Ly6Chigh monocytes in the atherosclerotic aorta resembled the chimerism determined in the blood, indicating that Irf5 KO monocytes were as likely as Irf5 WT monocytes to infiltrate atherosclerotic lesions (Figure 4A,C). At 4 weeks of HCD, chimerism among aortic macrophages had already shifted slightly, yet not significantly, towards the CD45.1 WT population (+7.15 ± 1.95%). With further plaque progression, following 12 weeks of HCD, the CD45.1+ Irf5 WT macrophage population had outcompeted the CD45.2+ Irf5 KO population significantly by 13.24 ± 2.08%, while monocyte chimerism in the aorta still resembled CD45.1/CD45.2 chimerism in the blood (Figure 4C). These data suggest that Irf5 deficiency impaired macrophage accumulation in the plaque, in situ, independent of monocyte recruitment as plaque development progressed. When determining local macrophage proliferation and apoptosis by flow cytometric staining for intracellular Ki67 and active Caspase 3, the frequency of proliferating macrophages was reduced in IRF5-deficient (CD45.2+) compared to Irf5-expressing cells (CD45.1+), whereas apoptosis remained unaffected (Figure 4D). Similarly, cell surface expression levels of CD36 were quantified by flow cytometry measuring mean fluorescent intensity in Irf5-deficient (CD45.2+) and Irf5-expressing (CD45.1+) aortic macrophages. Expression of the lipid uptake receptor CD36 was relatively reduced in Irf5−/− macrophages (Figure 4E). To determine whether Irf5 expression might influence monocyte to macrophage differentiation in the atherosclerotic plaque environment, we sorted Ly6Chigh monocytes from peripheral blood and spleens of Irf5 WT and KO mice and differentiated them into macrophage foam cells, in vitro, in the presence of oxidized (ox)LDL. Both the fraction of F4/80high macrophages and the uptake of DiI-labelled oxLDL were reduced in IRFrf5-deficient cells (Figure 4F). In summary, Irf5 deficiency within macrophages limited their accumulation in progressing plaques by hampering local macrophage differentiation, foam cell formation and proliferation.
Figure 4.
(A, B) Lethaly irradiated Apoe–/– mice were reconstituted with a mixture of Apoe–/– CD45.1+ bone marrow cells (WT) and CD45.2+Apoe–/–LysmCre/+Irf5fl/fl bone marrow cells (KO) to create mixed chimeric mice. CD45.1 and CD45.2 chimerism was analyzed in monocytes and macrophages in the blood and aortic tissue cell suspension in mixed chimeras fed a high cholesterol diet for 4 weeks. Results are presented as mean ± SEM, n = 4 mixed chimeras. (C) Relative change in CD45.1 and CD45.2 chimerism (%) between monocytes in the blood and aorta, and macrophages in the aorta is presented following 4 and 12 weeks of HCD feeding, Results are presented as mean ± SEM, n = 4–5 mixed chimeras, ∗p < 0.05 denotes statistically significant differences between cell populations, Kruskal–Wallis and Dunn's multiple comparisons test. (D) Quantification of proliferation (Ki67) and apoptosis (Casp3) among aortic macrophages in mixed chimeras following 4 and 12 weeks of HCD. Results are presented as mean ± SEM, n = 4–5 mixed chimeras, ∗p < 0.05 denotes statistically significant intraindividual differences between CD45.1 (blue) and CD45.2 (red) macrophages, Mann Whitney. (E) Quantification of CD36 surface expression on aortic macrophages in mixed chimeras following 12 weeks of HCD based on mean fluorescent intensities (MFI) as illustrated in the representative dot plot. CD45.1+Irf5+/+ macrophages are shown in blue and CD45.2+Irf5−/− macrophages are shown in red. Results are presented as mean ± SEM, n = 4 mixed chimeras, ∗p < 0.05 denotes statistically significant intraindividual differences between CD45.1 (blue) and CD45.2 (red) macrophages, Mann Whitney. (F) Monocytes isolated from WT and KO mice were cultured with M-CSF for 16 h to induce macrophage differentiation, subsequently stimulated with Dil-labeled oxLDL for 4 h generating macrophage foam cells. Quantification of the fraction of differentiated F4/80high foam cells and oxLDL uptake by flow cytometry (representative dot plots and histogram with dashed line representing unstimulated control). Results are presented as mean ± SEM, n = 8 per group. ∗p < 0.05 denotes statistically significant differences between WT and KO, t-test. Dil-oxLDL = dil-labeled oxidized low density lipoprotein. HCD = high-cholesterol diet.
4. Discussion
Our study shows that Irf5 expression is highly prevalent in, but not limited to, macrophages in atherosclerotic lesions in mice and men. Moreover, single-cell RNA sequencing documents that Irf5 expression is not limited to one particular subcluster of macrophages in atherosclerotic aortas in Apoe–/– mice and humans, although in human plaques, resident-like macrophages express lower levels of IRF5 compared to both foamy and inflammatory subsets. A previous study, using immunofluorescent co-staining, showed that IRF5 protein expression associated with CD68 and CD11c but not alpha-smooth muscle actin (αSMA) in aortic root lesion of Apoe–/– mice [36]. CD11c has been referred to as a M1 marker in atherosclerotic plaques [36,37], and our single-cell analysis confirms that CD11c expression is enriched in inflammatory and foamy macrophage clusters compared to M2/resident-like macrophages (Supplemental Fig. 1B). Having said this, Irf5 expression does not correlate with CD11c expression in lesional macrophages according to our scRNA seq analysis (Supplemental Fig. 1C, E). The lack of co-localization of IRF5 expression and αSMA in atherosclerotic lesions, shown by Seneviratne et al., does not preclude that IRF5 can function in vascular smooth muscle cells (VSMC) in atherosclerotic lesions. VSMC that infiltrate the intima lose αSMA expression when transdifferentiating to macrophage-like cells [38], and VSMC in the media impact lesion formation by cell death, being replaced with extracellular matrix, and by increasing LDL deposition in the plaque [39,40]. Fibroblasts, mostly located in the adventitia, are sources of inflammatory cytokines and growth factors [41,42], as are B-cells, which also serve as antigen-presenting cells secreting antibodies [43,44]. Our single-cell analysis reveals that IRF5 is expressed in these atheroma-associated cell types besides monocytes and macrophages, albeit at lower levels. To study the impact of IRF5 expression on atherogenesis selectively in monocytes and macrophages, we generated Apoe–/– LysmCre/+ Irf5fl/fl mice. First, we confirmed that bone marrow-derived macrophages in these mice expressed lower levels of inflammatory genes under M1 polarizing conditions. When fed an HCD to accelerate the formation of atherosclerotic lesions, these plaques featured an altered phenotype with reduced macrophage and lipid contents and increased collagen depositions. These phenotypic changes in female mice resembled those described by Seneviratne and colleagues, who studied systemic IRF5-deficient Apoe–/– male mice on a chow diet [36]. As lesions progressed, the authors did not observe differences in lesion size at the aortic root level but detected smaller necrotic core areas. Because we fed Apoe–/– mice a HCD, atherosclerotic lesions were more advanced and two to three times larger in our study. Moreover, in our study, the overall macrophage content was significantly reduced in plaques of myeloid cell-specific Irf5-deficient Apoe–/– mice in contrast to the study by Seneviratne. Reduced CCL5 production in atherosclerotic aortas and attenuated monocyte to macrophage foam cell differentiation, in situ proliferation and oxLDL uptake likely contributed to the phenotype we present. Expression of Cd36, an oxLDL uptake mediating scavenger receptor, was reduced in M0-and M1-polarized BMDM in the absence of Irf5, and cholesterol exporter Abca1 expression was increased, providing a mechanistic explanation for decreased foam cell formation and lipid deposition in the atherosclerotic plaques of myeloid cell-specific Irf5-KO mice. Notably, lipid uptake stimulates macrophage proliferation in the plaque, and CD36 cell surface expression was relatively reduced in Irf5−/− aortic macrophages compared to Irf5+/+ macrophages in our chimeras [3,6]. Whether, in addition, IRF5 can regulate the transcription of cell cycle genes in macrophages, directly, as reported in B cells [45], is unknown. Seneviratne et al. reported that Irf5 deficiency did not impact foam cell formation in GMCSF-cultured BMDM following incubation with acetylated (ac)LDL. Of note, acLDL is mainly taken up via macrophage scavenger receptor 1 (Msr1), but not CD36, we reported recently [3]. Msr1 expression was not significantly suppressed in Irf5 deficient BMDM in our experiments, which may explain the discrepancies in the foam cell assay outcomes between the studies. Mertk expression was elevated in Irf5-deficient M1-polarized BMDM and in atherosclerotic aortas of myeloid cell-specific Irf5-deficient mice. Mertk is an essential efferocytosis-mediating receptor on macrophages, a process minimizing necrotic core formation in atherosclerotic lesions which become more fibrotic, as we show. Increased aortic Tgfb1 expression in KO mice further supported collagen deposition, resulting in a stabilized plaque phenotype. Improved efferocytosis capacity was also postulated by Seneviratne et al. as the key mechanism for the favorable plaque phenotype observed in male Apoe–/– Irf5−/− mice on chow diet. Increased Tgfb1 expression in visceral adipose tissue was reported in myeloid cell-specific Irf5−/− mice consuming a high-fat diet [20].
Our results contrast with another study investigating the role of Irf5 deficiency in lupus-associated atherosclerosis in Apoe–/– mice carrying a gld point mutation in the gene encoding Fas ligand, which results in spontaneous autoimmune disease [46]. Although Irf5 deficiency ameliorated lupus disease severity, atherosclerotic plaque formation exacerbated significantly. Contrasting with our study and the one by Seneviratne et al., Irf5 deficiency augmented serum cholesterol levels in the Apoe-deficient lupus mouse model. Notably, in these mice, both Irf5-deficient bone marrow-derived and non-bone marrow-derived cells contributed to the phenotype, confirming that IRF5 can also function in cells other than macrophages in the context of atherosclerosis.
This is why generating Apoe–/– LysmCre/+ Irf5fl/fl mice was essential to investigate the role of IRF5 selectively in monocytes and macrophages in the context of atherosclerosis. Our study provides a rationale for exploiting modulation of macrophage polarization, therapeutically, by showing that altering macrophage phenotypes can change plaque phenotypes.
Acknowledgments
This work was supported by the German Research Foundation to I.H. (HI1573/2 and Collaborative Research Center SFB1425 grant 422681845) and to A.E. (grant 413517907, IMM-PACT program for Clinician Scientists, Department of Medicine II, Medical Center – University of Freiburg and Faculty of Medicine, University of Freiburg). J.L. was supported by the Otto-Hess- Scholarship from the German Cardiac Society.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2021.101250.
Contributor Information
Julia Leipner, Email: julia.leipner@universitaets-herzzentrum.de.
Tsai-Sang Dederichs, Email: tsai-sang.dederichs@uniklinik-freiburg.de.
Alexander von Ehr, Email: alexander.von.ehr@uniklinik-freiburg.de.
Simon Rauterberg, Email: simon.rauterberg@uniklinik-freiburg.de.
Carolin Ehlert, Email: carolin.ehlert@universitaets-herzzentrum.de.
Julian Merz, Email: Julian.merz@universitaets-herzzentrum.de.
Bianca Dufner, Email: bianca.dufner@universitaets-herzzentrum.de.
Natalie Hoppe, Email: natalie.hoppe@universitaets-herzzentrum.de.
Katja Krebs, Email: katja.krebs@uniklinik-freiburg.de.
Timo Heidt, Email: timo.heidt@universitaets-herzzentrum.de.
Constantin von zur Muehlen, Email: constantin.vonzurmuehlen@universitaets-herzzentrum.de.
Peter Stachon, Email: peter.stachon@universitaets-herzzentrum.de.
Klaus Ley, Email: klaus@liai.org.
Dennis Wolf, Email: dennis.wolf@universitaets-herzzentrum.de.
Andreas Zirlik, Email: andreas.zirlik@medunigraz.at.
Christoph Bode, Email: christoph.bode@universitaets-herzzentrum.de.
Ingo Hilgendorf, Email: ingo.hilgendorf@universitaets-herzzentrum.de.
Carmen Härdtner, Email: carmen.haerdtner@universitaets-herzzentrum.de.
Conflict of interest
None declared
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Al-Mallah M.H., Sakr S., Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Current Atherosclerosis Reports. 2018;20(1):1. doi: 10.1007/s11883-018-0711-4. [DOI] [PubMed] [Google Scholar]
- 2.Schaftenaar F., Frodermann V., Kuiper J., Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Current Opinion in Lipidology. 2016;27(3):209–215. doi: 10.1097/MOL.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 3.Härdtner C., Kornemann J., Krebs K., Ehlert C.A., Jander A., Zou J. Inhibition of macrophage proliferation dominates plaque regression in response to cholesterol lowering. Basic Research in Cardiology. 2020;115(6):78. doi: 10.1007/s00395-020-00838-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindau A., Härdtner C., Hergeth S.P., Blanz K.D., Dufner B., Hoppe N. Atheroprotection through SYK inhibition fails in established disease when local macrophage proliferation dominates lesion progression. Basic Research in Cardiology. 2016;111 doi: 10.1007/s00395-016-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willemsen L., de Winther M.P. Macrophage subsets in atherosclerosis as defined by single-cell technologies. The Journal of Pathology. 2020;250(5):705–714. doi: 10.1002/path.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robbins C.S., Hilgendorf I., Weber G.F., Theurl I., Iwamoto Y., Figueiredo J.-L. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature Medicine. 2013;19(9):1166–1172. doi: 10.1038/nm.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett T.J. Macrophages in atherosclerosis regression. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40(1):20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alma Zernecke, Holger Winkels, Clément Cochain, Williams Jesse W., Dennis Wolf, Oliver Soehnlein. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circulation Research. 2020;127(3):402–426. doi: 10.1161/CIRCRESAHA.120.316903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J.-D., Nishi H., Poles J., Niu X., Mccauley C., Rahman K. Single-cell analysis of fate-mapped macrophages reveals heterogeneity, including stem-like properties, during atherosclerosis progression and regression. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.124574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinetti-Gbaguidi G., Colin S., Staels B. Macrophage subsets in atherosclerosis. Nature Reviews Cardiology. 2015;12(1):10–17. doi: 10.1038/nrcardio.2014.173. [DOI] [PubMed] [Google Scholar]
- 11.Cardilo-Reis L., Gruber S., Schreier S.M., Drechsler M., Papac-Milicevic N., Weber C. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Molecular Medicine. 2012;4(10):1072–1086. doi: 10.1002/emmm.201201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jefferies C.A. Regulating IRFs in IFN driven disease. Frontiers in Immunology. 2019;10:325. doi: 10.3389/fimmu.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren J., Chen X., Chen Z.J. IKKβ is an IRF5 kinase that instigates inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(49):17438–17443. doi: 10.1073/pnas.1418516111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Pelaez M., Lamont D.J., Peggie M., Shpiro N., Gray N.S., Cohen P. Protein kinase IKKβ-catalyzed phosphorylation of IRF5 at Ser462 induces its dimerization and nuclear translocation in myeloid cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(49):17432–17437. doi: 10.1073/pnas.1418399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krausgruber T., Blazek K., Smallie T., Alzabin S., Lockstone H., Sahgal N. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunology. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 16.Lazear H.M., Lancaster A., Wilkins C., Suthar M.S., Huang A., Vick S.C. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathogens. 2013;9(1) doi: 10.1371/journal.ppat.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mai L.T., Smans M., Silva-Barrios S., Fabié A., Stäger S. IRF-5 expression in myeloid cells is required for splenomegaly in L. Donovani infected mice. Frontiers in Immunology. 2020;10 doi: 10.3389/fimmu.2019.03071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffau P., Menn-Josephy H., Cuda C.M., Dominguez S., Aprahamian T.R., Watkins A.A. Interferon regulatory factor 5 promotes inflammatory arthritis. Arthritis & Rheumatology (Hoboken, N.J.) 2015;67(12):3146–3157. doi: 10.1002/art.39321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weihrauch D., Krolikowski J.G., Jones D.W., Zaman T., Bamkole O., Struve J. An IRF5 decoy peptide reduces myocardial inflammation and fibrosis and improves endothelial cell function in tight-skin mice. PloS One. 2016;11(4) doi: 10.1371/journal.pone.0151999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalmas E., Toubal A., Alzaid F., Blazek K., Eames H.L., Lebozec K. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nature Medicine. 2015;21(6):610–618. doi: 10.1038/nm.3829. [DOI] [PubMed] [Google Scholar]
- 21.Hedl M., Yan J., Abraham C. IRF5 and IRF5 disease-risk variants increase glycolysis and human M1 polarization by regulating proximal signaling and Akt2 activation. Cell Reports. 2016;16(9):2442–2455. doi: 10.1016/j.celrep.2016.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caplan I.F., Maguire-Zeiss K.A. Toll-like receptor 2 signaling and current approaches for therapeutic modulation in synucleinopathies. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ban T., Sato G.R., Tamura T. Regulation and role of the transcription factor IRF5 in innate immune responses and systemic lupus erythematosus. International Immunology. 2018;30(11):529–536. doi: 10.1093/intimm/dxy032. [DOI] [PubMed] [Google Scholar]
- 24.Negishi H., Ohba Y., Yanai H., Takaoka A., Honma K., Yui K. Negative regulation of Toll-like-receptor signaling by IRF-4. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(44):15989–15994. doi: 10.1073/pnas.0508327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature Reviews Immunology. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 26.Günthner R., Anders H.-J. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators of Inflammation. 2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss M., Blazek K., Byrne A.J., Perocheau D.P., Udalova I.A. IRF5 is a specific marker of inflammatory macrophages in vivo. Mediators of Inflammation. 2013:245804. doi: 10.1155/2013/245804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh G.B., Zhang Y., Boini K.M., Koka S. High mobility group box 1 mediates TMAO-induced endothelial dysfunction. International Journal of Molecular Sciences. 2019;20(14):3570. doi: 10.3390/ijms20143570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habich C., Baumgart K., Kolb H., Burkart V. The receptor for heat shock protein 60 on macrophages is saturable, specific, and distinct from receptors for other heat shock proteins. The Journal of Immunology. 2002;168(2):569–576. doi: 10.4049/jimmunol.168.2.569. [DOI] [PubMed] [Google Scholar]
- 30.Curtiss L.K., Tobias P.S. Emerging role of Toll-like receptors in atherosclerosis. Journal of Lipid Research. 2009;50:S340–S345. doi: 10.1194/jlr.R800056-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirka R.C., Wagh D., Paik D.T., Pjanic M., Nguyen T., Miller C.L. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nature Medicine. 2019;25(8):1280–1289. doi: 10.1038/s41591-019-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkels H., Ehinger E., Vassallo M., Buscher K., Dinh H.Q., Kobiyama K. Atlas of the immune cell repertoire in mouse atherosclerosis defined by single-cell RNA-sequencing and mass cytometry. Circulation Research. 2018;122(12):1675–1688. doi: 10.1161/CIRCRESAHA.117.312513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M. Comprehensive integration of single cell data. BioRxiv. 2018:460147. doi: 10.1101/460147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nature Biotechnology. 2018;36(5):411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–1902. doi: 10.1016/j.cell.2019.05.031. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seneviratne A.N., Edsfeldt A., Cole J.E., Kassiteridi C., Swart M., Park I. Interferon regulatory factor 5 controls necrotic core formation in atherosclerotic lesions by impairing efferocytosis. Circulation. 2017;136(12):1140–1154. doi: 10.1161/CIRCULATIONAHA.117.027844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono Y., Nagai M., Yoshino O., Koga K., Nawaz A., Hatta H. CD11c+ M1-like macrophages (MΦs) but not CD206+ M2-like MΦ are involved in folliculogenesis in mice ovary. Scientific Reports. 2018;8 doi: 10.1038/s41598-018-25837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basatemur G.L., Jørgensen H.F., Clarke M.C.H., Bennett M.R., Mallat Z. Vascular smooth muscle cells in atherosclerosis. Nature Reviews Cardiology. 2019;16(12):727–744. doi: 10.1038/s41569-019-0227-9. [DOI] [PubMed] [Google Scholar]
- 39.Hamczyk M.R., Villa-Bellosta R., Gonzalo P., Andrés-Manzano M.J., Nogales P., Bentzon J.F. Vascular smooth muscle-specific progerin expression accelerates atherosclerosis and death in a mouse model of hutchinson-gilford progeria syndrome. Circulation. 2018;138(3):266–282. doi: 10.1161/CIRCULATIONAHA.117.030856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamczyk M.R., Andrés V. Accelerated atherosclerosis in HGPS. Aging. 2018;10(10):2555–2556. doi: 10.18632/aging.101608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tillie R.J.H.A., van Kuijk K., Sluimer J.C. Fibroblasts in atherosclerosis: heterogeneous and plastic participants. Current Opinion in Lipidology. 2020;31(5):273–278. doi: 10.1097/MOL.0000000000000700. [DOI] [PubMed] [Google Scholar]
- 42.Xu F., Ji J., Li L., Chen R., Hu W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: a novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis. Medical Hypotheses. 2007;69(4):908–912. doi: 10.1016/j.mehy.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 43.Sage A.P., Tsiantoulas D., Binder C.J., Mallat Z. The role of B cells in atherosclerosis. Nature Reviews Cardiology. 2019;16(3):180–196. doi: 10.1038/s41569-018-0106-9. [DOI] [PubMed] [Google Scholar]
- 44.Hilgendorf I., Theurl I., Gerhardt L.M.S., Robbins C.S., Weber G.F., Gonen A. Innate response activator B cells aggravate atherosclerosis by stimulating T helper-1 adaptive immunity. Circulation. 2014;129(16):1677–1687. doi: 10.1161/CIRCULATIONAHA.113.006381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De S., Zhang B., Shih T., Singh S., Winkler A., Donnelly R. B cell-intrinsic role for IRF5 in TLR9/BCR-induced human B cell activation, proliferation, and plasmablast differentiation. Frontiers in Immunology. 2017;8:1938. doi: 10.3389/fimmu.2017.01938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watkins A.A., Yasuda K., Wilson G.E., Aprahamian T., Xie Y., Maganto-Garcia E. IRF5 deficiency ameliorates lupus but promotes atherosclerosis and metabolic dysfunction in a mouse model of lupus-associated atherosclerosis. The Journal of Immunology. 2015;194(4):1467–1479. doi: 10.4049/jimmunol.1402807. Baltimore, Md. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.