Abstract
Substance use disorder (SUD) is characterized, in part by behavior biased toward drug use and away from natural sources of reward (e.g. social interaction, food, sex). The neurobiological underpinnings of SUDs reveal distinct brain regions where neuronal activity is necessary for the manifestation of SUD-characteristic behaviors. Studies that specifically examine how these regions are involved in behaviors motivated by drug versus natural reward allow determinations of which regions are necessary for regulating seeking of both reward types, and appraisals of novel SUD therapies for off-target effects on behaviors motivated by natural reward. Here, we evaluate studies directly comparing regulatory roles for specific brain regions in drug versus natural reward. While it is clear that many regions drive behaviors motivated by all reward types, based on the literature reviewed we propose a set of interconnected regions that become necessary for behaviors motivated by drug, but not natural rewards. The circuitry necessary selectively for drug seeking includes an Action/Reward subcircuit, comprising nucleus accumbens, ventral pallidum, and ventral tegmental area, a Prefrontal subcircuit comprising prelimbic, infralimbic, and insular cortices, a Stress subcircuit comprising the central nucleus of the amygdala and the bed nucleus of the stria terminalis, and a Diencephalon circuit including lateral hypothalamus. Evidence was mixed for nucleus accumbens shell, insular cortex, and ventral pallidum. Studies for all other brain nuclei reviewed supported a necessary role in regulating both drug and natural reward seeking. Finally, we discuss emerging strategies to further disambiguate necessity of brain regions in drug- versus natural reward-associated behaviors.
Keywords: Nucleus Accumbens, Drug Seeking, Natural Reward, Addiction, Reward Circuit, Stress Circuit
Graphical Abstract:
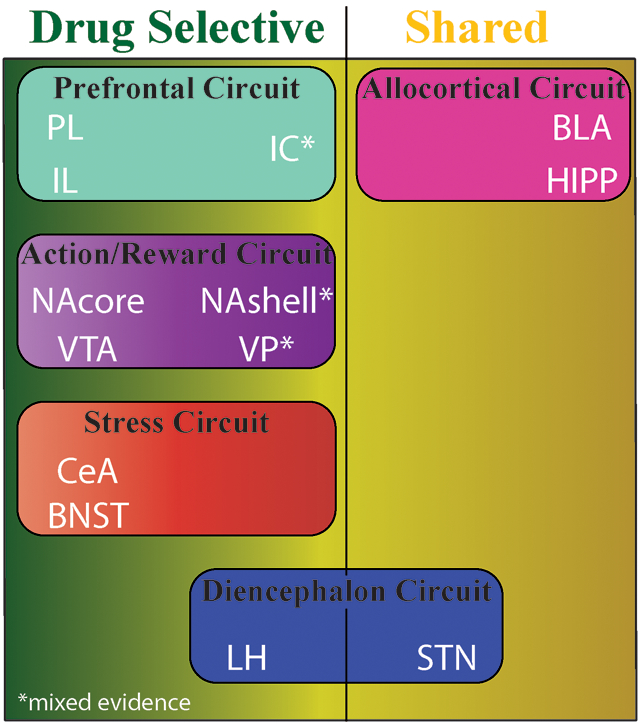
We evaluated the literature and propose circuitry necessary for drug but not natural reward seeking. Circuitry selectively necessary for drug rewards includes an Action/Reward subcircuit: nucleus accumbens core (NAcore), ventral tegmental area (VTA), Prefrontal subcircuit: prelimbic (PL), infralimbic (IL) cortices, Stress subcircuit: central nucleus of the amygdala (CeA), bed nucleus of stria terminalis (BNST), and Diencephalon subcircuit: lateral hypothalamus (LH). Evidence was mixed for nucleus accumbens shell (NAshell), insular cortex (IC), and ventral pallidum (VP). Circuitry necessary for both drug and natural reward: subthalamic nucleus (STN), basolateral amygdala (BLA), and hippocampus (HIPP). We discuss future strategies to expand this circuitry.
Substance use disorder (SUD) is a significant and widespread burden on public and private health, incurring an estimated annual cost of $740 billion and affecting over 20 million people in the United States (Center for Behavioral Health Statistics and Quality, 2015). Pharmacological and behavioral treatments are partially effective at reducing the amount of substance used, but relapse following treatment is common. For example, the pharmacological smoking cessation aid varenicline is effective at helping smokers initiate abstinence, but the effects are transient with around 60% of smokers relapsing within 1 year of treatment (Agboola et al., 2015). Likewise, contingency management is among the most effective treatments for SUDs (Prendergast et al., 2006), however, approximately 60% of patients relapse following treatment (McLellan et al., 2000). Indeed, SUDs are characterized by a difficulty in refraining from drug use and by chronic episodes of relapse (American Psychiatric Association, 2013) induced by exposure to drug-associated cues or contexts following abstinence (Volkow et al., 2012).
Another hallmark of SUDs is the persistent procurement and consumption of abused substances at the expense of non-drug or natural sources of reinforcement (e.g. food, sex, social interaction). This tendency is reflected in several of the diagnostic criteria for SUDs: Spending considerable time obtaining the drug; Repeated failure to carry out major obligations at work, school, or home due to drug use; Stopping or reducing important social, occupational, or recreational activities due to drug use (American Psychiatric Association, 2013). Further, the fact that drug and natural rewards largely activate overlapping brain nuclei complicates research designed to disambiguate the neurobiological underpinnings of behaviors motivated by drugs and natural rewards. However, we argue here that evidence of neuronal activity in a brain nucleus or circuit is not necessarily commensurate with its necessity for reward seeking. Thus, studies showing causal relationships of particular nuclei allowed us to identify a circuit necessary selectively for behaviors motivated by drug but not natural reward. Understanding which brain subcircuits are selectively required for drug seeking, but not seeking of natural rewards, will help identify the brain sites harboring neurobiological adaptations critical for SUDs, and assist in developing selective therapies with less off-target effects on seeking of natural rewards.
Extant understanding of the neurobiological mechanisms underlying SUDs is largely based on preclinical studies using animal models. The gold standard among animal models of addiction is the drug self-administration (SA) model, in which animals first perform operant responses to earn access to drug. Subsequently, drug seeking is reduced by removing the animal from the experimental context (i.e. forced abstinence) or no longer providing access to drug upon seeking responses (i.e. extinction). After drug seeking has been reduced, animals are commonly re-exposed to drugs (i.e. primed reinstatement), drug-associated cues (i.e. cued reinstatement), drug-associated contexts (i.e. contextual reinstatement), or stress (stress-induced reinstatement) to reinitiate drug seeking behaviors (for reviews see, Crombag et al., 2008; Everitt et al., 2018; Kuhn et al., 2019; Marchant et al., 2013; Venniro et al., 2016). Increasing the duration of forced abstinence periods often increases drug seeking upon return to the experimental context (i.e. incubation of craving; Li et al., 2015). Another common model to examine drug seeking is conditioned place preference (CPP; McKendrick & Graziane, 2020), in which animals are exposed to drug in one distinct context and to vehicle or another reward in a different distinct context. Preference is indicated by time spent seeking in one or the other context when both contexts are accessible.
Investigators have identified specific adaptations and cellular activity patterns related to drug use, seeking, and refraining from seeking by manipulating and observing activity at various points in the protocols of these preclinical models (Kuhn et al., 2019). Importantly, analogous studies have been conducted where a natural reward, often sucrose, is used as the reinforcer (e.g., Bobadilla et al., 2017a; Grimm, 2020; Nair et al., 2006). However, to date it is uncommon for investigators to include both drug and natural reinforcers in the same study, and direct comparisons between these two reinforcers within the same animal are even more rare. Yet, studies that directly compare the neurobiological and behavioral effects of drug versus natural rewards are uniquely poised to clarify if observed treatment effects or adaptations and cellular activity patterns in a given region are selective for drug seeking. This rationale has likely inspired the recent increase in preclinical studies which include multiple reward modalities (see for discussion; Kuhn et al., 2019; Venniro et al., 2020).
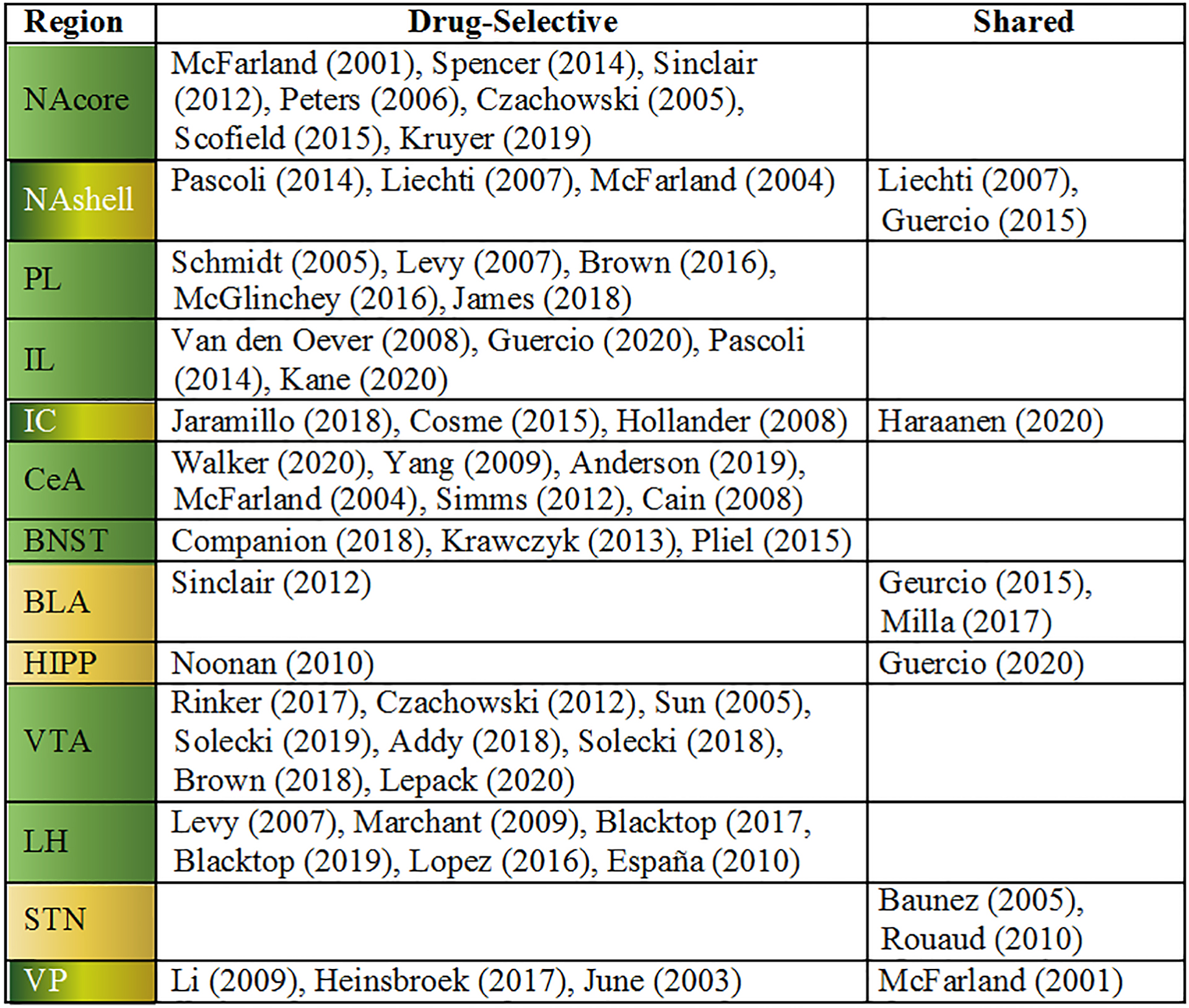
Here we review the current body of published research that specifically examines both drug and natural reward within the same study in an effort to map the brain circuits that are necessary for seeking behaviors motivated by drug or natural reward. Because a large body of work has identified drug-specific adaptations in the nucleus accumbens (NAc) as critical for expressing seeking behaviors, we begin our analysis of the literature with studies focused on the role of the NAc in drug versus natural reward seeking. Then, we move to other brain regions that, together with the NAc, comprise the circuits driving drug and/or natural reward seeking behaviors. We recognize that different classes of addictive drugs and natural rewards may produce different neuroadaptations in given brain regions. While we make note of some of these, our primary goal is to examine whether activity in a given region is necessary for seeking. As such, we focus our review on studies that manipulate activity within each brain region. While many regions are activated by both drug and natural rewards, examining studies that manipulate activity within a given region allows the identification of regions where adaptations occur that leave those regions necessary for drug seeking, but not seeking of natural reward. We refer to these regions as “drug-selective” to indicate that while they may be involved in behaviors motivated by both drug and natural rewards, they exhibit selective plasticity and behavioral consequences in response to drugs of abuse. Table 1 provides a summary of which reports found a particular region to be selectively involved in drug-motivated behavior (i.e. drug-selective) or for both drug- and natural-reward-motivated behaviors (i.e. shared). Then, we discuss promising experimental strategies for discriminating between the neural bases of behaviors motivated by drug and natural rewards. Finally, we discuss a potential circuitry between these regions.
Table 1:
Evidence for drug-selective and shared circuitry in NAc and NAc-connected region
|
Evidence for necessity of reviewed regions for drug and natural reward behaviors. Dark green = drug-selective (100% studies showed drug selectivity), Yellow/Green gradient = mixed (>50% studies showed drug selectivity), yellow = shared (≤50% studies showed drug selectivity). Abbreviations: BLA = basolateral amygdala, BNST = bed nucleus of the stria terminalis, CeA = central nucleus of the amygdala, HIPP = hippocampus, IL = infralimbic cortex, IC = insular cortex, LH = lateral hypothalamus, NAcore = nucleus accumbens core, NAshell = nucleus accumbens shell, PL = prelimbic cortex, STN = subthalamic nucleus, VP = ventral pallidum, VTA = ventral tegmental area.
NAc Structure, Connectivity and Function
NAc Structure:
The NAc is a key structure of the ventral striatum that is traditionally divided into central core and surrounding shell regions (NAcore and NAshell, respectively) based on heterogeneity in structure, function, and connectivity (Scofield et al., 2016; Záborszky et al., 1985; Zahm, 1999). Most neurons within the NAc (~90–95%) are GABAergic medium spiny neurons (MSNs) that can be differentiated by expression of dopamine D1- or D2-receptor expression (Gerfen & Surmeier, 2011). Remaining neurons include interneurons expressing parvalbumin, calretinin, and choline acetyltransferase, as well as those that co-express somatostatin, neuropeptide Y, and neuronal nitric oxide synthase (Burke et al., 2017; Tepper et al., 2010). Finally, the NAc contains glia that regulate extracellular glutamate and influence synaptic plasticity (Jarvis et al., 2020; Kalivas, 2009; Kruyer & Kalivas, 2021; McGrath & Briand, 2019).
NAc Connectivity:
Connectivity differs between the NAcore and NAshell. The NAcore integrates inputs from cortical and allocortical structures involved in reward processing, goal-directed behavior to earn reward, and reinstatement (Scofield et al., 2016). The majority of NAcore inputs arise from glutamatergic afferents including the prefrontal cortex (PFC), the insular cortex (IC), the basolateral amygdala (BLA), the hippocampus (HIPP) and midline thalamus (Brog et al., 1993; Sesack et al., 1989), whereas, the ventral tegmental area (VTA), ventral pallidum (VP), and to a minor extent PFC, provide GABAergic input (Lee et al., 2014; Scofield et al., 2016). Finally, VTA and substantia nigra (SN) provide dopaminergic inputs to NAcore (Brog et al., 1993). Reciprocal projections from the NAcore innervate the SN and VP, and to a lesser extent, lateral VTA (Bernal-Gamboa et al., 2017; Groenewegen et al., 1999; Heimer et al., 1991; Tripathi et al., 2010).
The NAshell integrates inputs from a variety of brain regions central to reward learning and motivation (Castro & Bruchas, 2019). Glutamatergic afferents of the NAshell include cortical (anterior insular cortex, dorsal peduncular cortex, infralimbic cortex, orbitofrontal cortex, and prelimbic cortex) and allocortical (BLA and ventral HIPP) regions, as well as a input from the paraventricular thalamus, with quantitatively smaller glutamatergic input from the VTA (Beckstead et al., 1979; Brog et al., 1993; Mcdonald, 1991; Sesack et al., 1989). The NAshell also receives dopaminergic input from the VTA, and GABAergic inputs from the central nucleus of the amygdala (CeA), lateral hypothalamus (LH), VP, and VTA (Brog et al., 1993; Groenewegen et al., 1999). NAshell D2-MSNs project primarily to the VP, but also innervate the VTA and LH, while D1-MSNs project to the VP, VTA and LH (Gibson et al., 2018; O’Connor et al., 2015).
NAc Function:
Several functions within the NAc have direct relevance for understanding SUDs. The rewarding and reinforcing effects of different abused substances (Di Chiara & Imperato, 1988; Volkow et al., 2011c) and natural rewards (Volkow et al., 2011b; Wise, 2006) have long been associated with increased NAc dopamine release. However, individuals with SUDs often show paradoxical blunting of NAc dopamine in response to abused substances relative to nonaddicted individuals (Volkow et al., 1997, 2014) but increased NAc dopamine responses to drug-paired stimuli which correlates with drug craving (Volkow et al., 2014). This finding is supported by a study in rats where NAc lesions reduced drug SA mediated by conditioned stimuli, but had little effect on maintenance of SA without cues (Ito et al., 2004). This increased propensity for drug-paired stimuli to drive behavior has been termed incentive salience and is thought to contribute to the prepotency of drug seeking behavior (Koob & Volkow, 2016). NAc sensitivity to drug stimuli is of particular interest to SUD research due to the prominent role of drug-associated stimuli in occasioning drug craving and relapse.
NAc D2-MSNs are implicated in refraining from drug seeking (Roberts-Wolfe et al., 2018, 2019), and D2 receptor availability is reduced in individuals with SUDs (Volkow et al., 2002). Further, D2 receptor downregulation in individuals with SUDs is associated with decreased activity in cortical areas associated with decision making (Volkow et al., 2011a), and increases or decreases in D2 expression in animals results in reduced or increased sensitivity to abused substances, respectively (Bello et al., 2011; Thanos et al., 2001, 2008). These findings indicate a potential role for NAc in regulating compulsive seeking of abused substances, another hallmark symptom of SUDs.
Whether the nature of drug seeking is primarily goal-directed or habitual in individuals with SUDs is an area of growing contention. Drug seeking tends to prevail over other non-drug-related behaviors in individuals with SUDs, despite the finding that addicted individuals often describe a reduced desire to consume drugs (Berridge & Robinson, 2016). Further, drug seeking shows relative insensitivity to aversive consequences, devaluation, and reward unavailability in animal models, leading many to conclude that drug seeking becomes habitual in nature, such that drug-related stimuli drive a habitual response pattern that terminates in procuring and using drugs (Everitt & Robbins, 2016). However, a recent study indicates that drug seeking in humans tends to be highly flexible, potentially detracting from habit-based accounts of addiction (Hogarth, 2020). Regardless of the outcome of this debate, the NAc is likely to play a prominent role in the maintenance of persistent drug seeking. The NAc is involved both in habitual responding for drugs (Belin et al., 2013; Everitt et al., 2008) and in complex tasks related to outcome ambiguity or uncertainty (e.g. Mascia et al., 2019), and is thought to aid in response selection based on relative salience of inputs (Floresco, 2015). Thus, the NAc is likely involved in persistent drug-seeking behaviors regardless of whether they are described as habitual or goal-directed.
Finally, while the NAc has been shown to regulate behavior motivated by both drug and natural rewards (Kelley, 2004; Robinson & Berridge, 2000), drug-specific adaptations that occur within the NAc over the course of drug SA, extinction/abstinence, and relapse are not seen in identical sucrose SA experiments. Many studies have characterized drug-specific adaptations in pre- and post-synaptic compartments, extracellular matrix, and astroglia (i.e. the tetrapartite synapse) that lead to changes in glutamate signaling and in turn regulate drug-seeking behaviors (for detailed reviews see, Bobadilla et al., 2017b; Kruyer et al., 2020; Neuhofer & Kalivas, 2018). Taken together, existing data strongly suggest that the NAc is a critical brain nucleus for regulating the behavioral symptoms of SUDs.
Drug vs. Natural Reward in Nucleus Accumbens Core:
Drug-induced adaptations in the NAcore are necessary for the expression of seeking behaviors related to SUDs (Scofield et al., 2016). Because the NAcore is also involved in behaviors motivated by natural reward (Berridge, 2009; Salamone et al., 2003), investigations directly comparing adaptations and the effects of manipulations on drug versus natural reward in NAcore are especially beneficial for understanding how drugs, but not natural rewards lead to pathological behaviors in SUDs. Alasmari et al. (2018) found adaptations in glial glutamate regulation in the NAcore induced by ethanol and nicotine, but not sucrose. They also demonstrated that nicotine + sucrose, nicotine + ethanol, and ethanol alone decreased expression of glutamate transporter (GLT-1) and cystine glutamate antiporter (xCT) and increased expression of metabotropic glutamate receptor (mGluR1) in the NAcore relative to sucrose only or water controls. These results are consistent with the important role of the NAcore glutamate in relapse (Kalivas, 2009), as well as the role of glia in regulating glutamate in drug, but not sucrose seeking (Kruyer et al., 2020). Martin et al. (2006) found that cocaine but not food SA inhibited long-term depression in both the NAcore and NAshell after only 1 day of forced abstinence, but selectively in the NAcore after 21 days of abstinence, suggesting that adaptations specifically in the NAcore may contribute to incubation of drug craving. Moreover, Cameron and Carelli (2012) used in vivo electrophysiological recordings to demonstrate that neurons in the NAcore exhibited mostly reward-specific phasic activity in a multiple schedule of cocaine and sucrose reinforcement. Re-exposure to the multiple schedule after 30d of abstinence revealed that most neurons remained selectively activated, but that the percentage coding for cocaine-motivated behaviors increased, and the percentage coding for sucrose-motivated behavior decreased. Further, while the relative increase in the number of neurons coding for cocaine and decrease in neurons coding for sucrose occurred in both the NAcore and NAshell, increased cocaine coding was more dramatic in the NAcore while decreased sucrose coding was more dramatic in the NAshell. A recent study by Bobadilla et al. (2020) used a c-Fos-TRAP (Targeted Recombination in Active Populations) strategy to investigate drug-specific NAcore neuronal ensembles alongside a within-subject behavioral model in which mice were trained to self-administer cocaine and sucrose across alternating sessions. This study showed that cued reinstatement of cocaine or sucrose seeking formed NAcore ensembles that were mostly distinct, overlapping by only ~30%, and that ensembles primarily consisted of D1-expressing neurons during cued reinstatement and D2-expressing neurons during extinction. Further, while cocaine and sucrose seeking ensembles were similar in size during cued reinstatement when each reward was administered independently, mice exposed to both rewards exhibited a larger cocaine-seeking than sucrose-seeking ensemble, and the magnitude of cued reinstatement was greater for cocaine than sucrose when both cues were simultaneously available. They also noted that the size of reward-specific ensembles was significantly correlated with the magnitude of reinstated behavior. These results suggest that D1- and D2-expressing NAcore neurons encode seeking and refraining from seeking, respectively, and that mostly non-overlapping neuronal ensembles in the NAcore code for cocaine versus sucrose seeking. Finally, these data indicate that differences in recruitment to reward-specific ensembles may explain preferential relapse effects for drug compared to natural reward. Together, the studies by Alasmari et al. (2018), Bobadilla et al. (2020), Cameron and Carelli (2012), and Martin et al. (2006) suggest that drugs of abuse can lead to adaptations in the NAcore that are not shared with natural rewards, that these adaptations correspond with behavior, and that adaptations within NAc subregions may differ for drug versus natural rewards.
Consistent with the findings by Cameron and Carelli (2012), other data indicate that substances of abuse induce adaptations in the NAcore during cued reinstatement (Koya et al., 2006; Madsen et al., 2012) contextual reinstatement (Edwards et al., 2011) and CPP (Mattson & Morrell, 2005; Zombeck et al., 2008) that are not recapitulated by natural reward. Likewise, several studies show that perturbations of NAcore activity preferentially affect responding motivated by drug but not natural reward. McFarland and Kalivas (2001) found that GABA agonism within the NAcore prevented primed reinstatement of cocaine but not sucrose seeking. Spencer et al. (2014) showed that reducing excitatory transmission in NAc by inhibiting voltage-gated calcium channels attenuated primed but not cued reinstatement of cocaine seeking, and did not affect primed sucrose seeking. Sinclair et al. (2012) found that blocking NAcore glutamate receptor mGluR5 prevented cued reinstatement of alcohol but not sucrose seeking. Similarly, Peters and Kalivas (2006) showed that while mGluR2/3 antagonism reduced primed reinstatement of both cocaine and sucrose seeking, cocaine primed reinstatement was more substantially reduced than sucrose reinstatement. Czachowski (2005) showed that NAcore administration of serotonin1B agonist decreased ongoing alcohol seeking, but had little effect on consumption of freely-available alcohol. Conversely, a serotonin1A agonist decreased alcohol consumption, but not ongoing seeking. Importantly, neither agonist had an effect on sucrose consumption or seeking. Consistent with the findings of Alasmari et al. (2018) discussed above, Scofield et al. (2015) found that chemogenetic activation of NAcore glia inhibited cued reinstatement of cocaine but not sucrose seeking. Building on this data, Kruyer et al. (2019) discovered that glia were retracted from NAcore synapses following extinction of heroin but not sucrose seeking, and that cued reinstatement of heroin, but not sucrose seeking produced a transient reassociation of glia with synapses. Further, blocking glial reassociation with synapses reduced cued heroin seeking. Taken together, these results strongly indicate that the necessity of NAcore is drug selective, and support the hypothesis that the NAcore is an essential nucleus for regulating the behavioral symptomology of SUDs.
Drug vs. Natural Reward in Nucleus Accumbens Shell:
Contrary to the drug selectivity of the NAcore, studies examining the NAshell produced mixed results. Some data indicate drug selective adaptations (Crombag et al., 2005, 2008; Mattson & Morrell, 2005) and others indicate similar adaptations between drug and natural reward (Madsen et al., 2012; Zombeck et al., 2008). Manipulation studies also find mixed results, with some studies demonstrating a necessity for the NAshell in regulating behaviors motivated by drug but not natural reward. Pascoli et al. (2014) found that neurons projecting from infralimbic cortex to NAshell showed cocaine-evoked plasticity (i.e. reduced AMPA/NMDA ratio and increased rectification mediated by GluA2 lacking AMPA receptors). Optogenetic reversal of plasticity in these neurons prior to testing eliminated cue-induced reinstatement of cocaine but not sucrose seeking. Liechti et al. (2007) found downregulation of mGluR2/3 in NAshell following nicotine but not sucrose SA, and mGluR2/3 antagonism reduced ongoing nicotine but not sucrose seeking. McFarland et al. (2004) found that GABA agonism in NAshell reduced stress-primed cocaine seeking but not food-primed food seeking. However, other data indicate that the NAshell is necessary for both drug- and natural-reward-motivated behaviors. Liechti et al. (2007) reported that mGluR2/3 antagonism in the NAshell decreased cued reinstatement of both nicotine and sucrose seeking. Similarly, Guercio et al. (2015) found that deep brain stimulation of the NAshell attenuated cued reinstatement of both cocaine and sucrose seeking. Thus, the role of the NAshell in governing drug versus natural-reward motivated behaviors is not entirely clear. One potential explanation for these mixed results could be that only particular NAshell subcircuits, cell types, or neurotransmitter systems show drug selectivity. This possibility is supported by the drug selectivity of IL-NAshell (Pascoli et al., 2014) but not BLA-NAshell (Millan et al., 2017; reviewed below) projections in regulating cocaine seeking. Another potential explanation is that differences in methodology (e.g. deep brain stimulation vs. reversible inactivation, differences in behavioral paradigms) are responsible for discrepant findings.
Drug Selectivity in Circuitry in which NAc is Embedded
Prefrontal Cortex:
The prefrontal cortex is critical for decision making and executive control, and regulates reward seeking in part via glutamatergic projections to the NAc (Kalivas et al., 2005). Involvement of the PFC in SUDs is well established, where deficits associated with attribution of salience, impulsivity, motivational arousal, and self-control are accompanied by increased PFC activation by drugs or drug cues, blunted PFC activation by natural reward, and poor performance on PFC-mediated cognitive tasks (Goldstein & Volkow, 2011). Further, individuals with an extended recreational cocaine-use history but not a diagnosable SUD show increased PFC grey matter volume while individuals with cocaine use disorder show reduced PFC grey matter volume relative to non-drug-using controls (Ersche et al., 2013a). These results have led some to suggest that variations in PFC functionality may confer vulnerability or resilience to developing SUD following exposure to abused substances (Ersche et al., 2013b, 2020). Recent animal studies provide some support for this hypothesis. PFC-dependent task performance and PFC orexin receptor expression prior to beginning methamphetamine SA correlated with future methamphetamine preference (Tavakkolifard et al., 2020). Similarly, De Laat et al. (2018) found that pre-SA performance on a cognitive task and levels of PFC glutamate and glycine were associated with future rates of cocaine intake. Together these studies indicate a potential role for PFC in conferring vulnerability or resilience to the development of SUDs.
The PFC has also been described as a critical component of the final common pathway for relapse of drug seeking (Kalivas & Volkow, 2005). A serial pathway from dorsomedial PFC, to NAcore and VP is necessary for drug seeking, as perturbations of this circuit reduce seeking across different types of triggering events and drug classes (e.g. Cordie & McFadden, 2019; Doncheck et al., 2020; Hernandez et al., 2020; Ma et al., 2014; Palombo et al., 2017; Struik et al., 2019), highlighting the importance of PFC for the expression of relapse to drug seeking. Overall, these studies strongly suggest that PFC mediates pathological behaviors associated with SUDs.
Other studies have identified the role of heterogeneous regions of the prefrontal cortex in drug seeking and relapse. While a complete characterization of the role of these subregions in SUDs is beyond the scope of this review, a brief overview is provided here to aid in the discussion of drug selective effects below. PFC subregions with a notable role in SUDs and in which studies investigating drug vs natural reward exist include medial PFC prelimbic (PL) and infralimbic (IL) cortices, and the lateral prefrontal insular cortex (IC). In general, existing data implicate PL in relapse to drug seeking (Moorman et al., 2015). IL is involved in suppression of drug seeking (Peters et al., 2008), and experimentally manipulating IL activity affects relapse of drug seeking (Augur et al., 2016; Ma et al., 2014; Muller Ewald & LaLumiere, 2018). Further, Warren et al. (2016) showed that distinct ensembles of IL neurons control food SA and extinction, and the same lab later showed that distinct ensembles regulating cocaine SA and extinction are mostly composed of IL-NAcore and IL-NAshell projection neurons, respectively. Finally, IC is thought to be involved in interoception and drug craving (Paulus & Stewart, 2014) and is strongly implicated in nicotine craving in smokers (Droutman et al., 2015; Kenny, 2011). Overall, these data suggest that heterogeneous cortical subregions are important for regulating drug-related behaviors in individuals with SUDs.
Drug selective adaptations have been noted in medial prefrontal cortex regions (mPFC; PL, IL). Koya et al. (2006) found increased immediate early gene (IEG) expression in mPFC following cued reinstatement for heroin but not sucrose relative to extinction controls. Wedzony et al. (2003) also noted increased Fos expression in mPFC following protracted abstinence from alcohol but not sucrose SA, specifically near the border of PL and IL (i.e. ventromedial PFC). In addition, Crombag et al. (2005) found increased spine density in the mPFC following amphetamine SA relative to sucrose self-administering and untreated controls. Using an innovative in vivo single-cell calcium imaging approach (discussed below), Siciliano et al. (2019) characterized activity patterns in neurons projecting from the mPFC to the dorsal periaqueductal grey that predicted susceptibility to compulsive alcohol drinking. These observations led the authors to optogenetically manipulate the circuit and conclude that compulsive alcohol seeking is likely driven by reduced aversion signaling. Importantly, they showed that manipulating this circuit had selective effects on alcohol but not water seeking. Because drug selective adaptations occur in several mPFC subregions, and manipulations of neurons in the mPFC affect behaviors motivated by drug but not natural reward, we will next explore the whether mPFC subregions are selectively necessary for behaviors motivated by drug but not natural rewards.
Drug vs. Natural Reward in Prelimbic Cortex:
Data from PL strongly support drug selectivity. Data suggest that drug selective adaptations occur in PL during SA and extinction (Parrilla-Carrero et al., 2018), cued reinstatement (McGlinchey et al., 2016; Schmidt et al., 2005) and CPP (Mattson & Morrell, 2005). Experiments manipulating PL activity also demonstrate the necessity of PL in regulating behaviors motivated by drug but not natural reward. Schmidt et al. (2005) found that intra-PL infusions of muscimol + baclofen potentiated cued reinstatement of heroin but not sucrose seeking. Levy et al. (2007) showed that electrical stimulation in PL reduced cocaine- but not sucrose-primed reinstatement. Brown et al. (2016) reported that infusions of orexin-1 receptor antagonist into PL attenuated cued reinstatement of alcohol but not sucrose seeking. Other studies specifically implicate PL-NAcore projections in regulating drug seeking. McGlinchey et al. (2016) blocked dopamine signaling in PL and glutamate signaling in NAcore and found that inactivating this pathway attenuated cued reinstatement of cocaine but not sucrose seeking. Similarly, James et al. (2018) found that activation in PL projections innervating contralateral NAcore is correlated with cued reinstatement of cocaine but not sucrose seeking, and that blocking dopamine D1/D2 receptors in PL reduced cocaine but not sucrose seeking. Taken together, the above studies strongly indicate that PL becomes essential for drug seeking and relapse but not for behaviors motivated by natural reward.
Drug vs. Natural Reward in Infralimbic Cortex:
In opposition to the drug selective response invigorating effects of the PL, the IL is largely involved in refraining from reward seeking, though the potentiation by PL and inhibition by IL is not perfectly distinguished (see, Moorman et al., 2015). While immediate early gene activity in IL is sometimes similar during drug and natural reward seeking during extinction (Schmidt et al., 2005), cued reinstatement (Schmidt et al., 2005) and CPP (Mattson & Morrell, 2005), manipulation data strongly indicate that the IL is necessary for behaviors motivated by drug but not natural reward. Van den Oever et al. (2008) showed that inhibiting endocytosis of AMPA receptor subunit GluR2 in ventromedial PFC (i.e. IL) reduced cue-induced reinstatement of heroin but not sucrose seeking. Guercio et al. (2020) found that deep brain stimulation of the IL during testing attenuated cued reinstatement of cocaine but not sucrose seeking. As discussed above, Pascoli et al. (2014) found that optogenetic reversal of plasticity in IL-NAshell neurons eliminated reinstatement of cocaine but not sucrose seeking induced by cue-exposure following forced abstinence. These findings are supported by a recent innovative study by Kane and colleagues (2020) in which rats self-administered cocaine and sucrose across alternating sessions before a 7d abstinence period. Following abstinence, rats were briefly exposed to a cued seeking task to induce Fos activation of neuronal ensembles in ventromedial PFC (i.e. IL) encoding either cocaine or sucrose. The ensembles were then selectively inactivated before a second cued reinstatement test under extinction conditions. Testing revealed that inactivation of the sucrose ensemble had no effect on cocaine or sucrose seeking, but that inactivation of the cocaine ensemble selectively attenuated cocaine seeking. Collectively, these results suggest that IL selectively regulates relapse of drug but not natural reward seeking, and that IL effects may in part be enacted by drug-encoding neuronal ensembles projecting to NAshell.
Drug vs. Natural Reward in Insular Cortex:
Prior work indicates that IC is involved in drug induced devaluation of natural rewards (Moschak et al., 2018), drug craving (Naqvi et al., 2014), context-induced relapse (Arguello et al., 2017), relapse after extended withdrawal (Campbell et al., 2019), and even relapse in a novel model of contingency management (Venniro et al., 2017). The IC likely influences behavior via its projections to the NAcore (Rogers-Carter et al., 2019) and to the extended amygdala (Centanni et al., 2019; Venniro et al., 2017). Although studies examining drug versus natural rewards in IC are less common, extant data indicate that both drug and natural rewards produce IC activity (Liu et al., 2013; Tomasi et al., 2015). Some inactivation studies indicate that IC is necessary for behaviors motivated by drug but not natural reward. Hollander et al. (2008) showed that blocking orexin/hypocretin receptors with intra-IC infusions of a hypocretin receptor antagonist dose-dependently reduced responding for nicotine but not sucrose seeking under a progressive ratio schedule. Jaramillo et al. (2018) found that chemogenetically silencing IC-NAcore projections selectively decreased alcohol but not sucrose SA. Similarly, Cosme et al. (2015) found that muscimol + baclofen inactivation of dorsal anterior IC reduced cued but not primed reinstatement of cocaine seeking, an effect that was mimicked by intra-IC blockade of CRF-1 receptors, but inactivation did not influence cued, primed, or cued + primed reinstatement of food seeking. However, Haaranen et al. (2020) found that chemogenetically activating anterior IC reduced consumption of freely-available alcohol and sucrose, while inactivation had no effect. Thus some results in IC (Cosme et al., 2015; Hollander et al., 2008; Jaramillo et al., 2018) suggest IC is necessary and/or sufficient for behaviors motivated by drug but not natural rewards, and provide clues for IC involvement in specific forms of relapse and specific neurotransmitter systems. However, it is difficult to form a strong hypothesis with so few studies comparing drug versus natural reward and with the contrasting evidence by Haaranen et al. (2020). One potential explanation for these mixed findings could be that differences in experimental design (i.e. operant responding versus free consumption) or manipulation of different IC circuits (e.g. IC-NAc versus IC-hypothalamus) produced the discrepant findings. Another possibility is that these mixed findings arise from differences in IC function, as IC is involved in both interoceptive awareness of drug craving (see, Tomasi et al., 2015) and in processing salient drug events as a critical node in the salience network (e.g., Grodin et al., 2017). A recent study has defined a salience network in rats that includes projections from the ventral anterior IC to anterior cingulate cortex, as in the human salience network (Tsai et al., 2020). Further, dorsal anterior and ventral anterior portions of the IC project to the NAcore and lateral NAshell, respectively (Brog et al., 1993; Sesack et al., 1989). Thus, future work interrogating specific subregions of IC may provide more conclusive evidence regarding the necessity for IC in behaviors motivated by drug and natural reward. Collectively, these mixed data indicate that the necessity for IC in regulating behavior is mostly drug selective.
Amygdala:
The amygdala (AMY) is critical for memory-processing, emotional responses, decision making, and drug seeking via connections to the NAc and PFC (Peters et al., 2009). The amygdala has been implicated in addiction, with particular roles in negative affect during withdrawal and preoccupation with abused substances (Koob & Volkow, 2016). Indeed, simultaneous downregulation of PFC and increased activity in AMY likely confer vulnerability and contribute to negative affect and relapse (Ruisoto & Contador, 2019). Like NAc and PFC, activity in AMY is increased when individuals with SUDs are exposed to cues related to abused substances (Jasinska et al., 2014) and resting state functional connectivity is increased in chronic heroin users relative to non-using controls (Ma et al., 2009). Finally, a review of neuroimaging studies by Mihov and Hurlemann (2012) revealed consistent increases in AMY activity during abstinence from nicotine, greater reactivity to nicotine-paired cues than neutral cues, reduced AMY activation by harm signals, and increased AMY activity during relapse prevention therapy in smokers. These findings led the authors to suggest nicotine cue-reactivity and decreased sensitivity to harm-signals as potential vulnerability biomarkers for smoking relapse. Taken together, the studies above suggest that AMY plays a central role in the pathological behaviors associated with SUDs, with particular involvement in stress and emotional processing. Importantly, these effects are mediated by different subregions within AMY, with components of the extended amygdala (i.e. central nucleus of the amygdala [CeA], bed nucleus of the stria terminalis [BNST]) controlling negative affect and stress reactivity (Centanni et al., 2019), and the basolateral amygdala mediating cue reactivity (See et al., 2003) and reward valuation (Wassum & Izquierdo, 2015). Thus, we will explore the necessity of these subregions for regulating behaviors motivated by drug and natural reward next.
Drug vs. Natural Reward in the Central Nucleus of the Amygdala:
Subregions in AMY differ with respect to drug selectivity. The central nucleus of the amygdala (CeA) is implicated in threat detection, regulation of mood and affect, reward valence, and is critically involved in behavioral symptoms of SUDs (Centanni et al., 2019). Studies also strongly indicate drug selectivity in CeA, and CeA Fos activation is increased following cued reinstatement of drug but not natural-reward seeking (Madsen et al., 2012). Walker et al. (2020) reported that blocking CART (cocaine and amphetamine regulated transcript) signaling via antibody infusions into CeA attenuated stress-induced reinstatement of alcohol but not sucrose seeking. Yang et al. (2009) found that normalizing reduced levels of substance P in CeA of alcohol preferring rats (relative to non-preferring rats) reduced ongoing SA of alcohol but not sucrose, suggesting a specific role for CeA substance P signaling in alcohol-motivated behaviors. Similarly, Anderson et al. (2019) found that systemic and intra-CeA administration of a kappa opioid agonist increased and intra-CeA antagonist administration decreased binge like alcohol consumption, but systemic administration of neither drug affected sucrose consumption in the same model. McFarland et al. (2004) showed that GABA agonism in CeA resulted in reductions in stress-induced reinstatement of cocaine seeking, but not primed reinstatement of food seeking. Simms et al. (2012) found that intra-CeA infusions of glucocorticoid antagonist selectively attenuated stress-induced reinstatement of alcohol, but not sucrose seeking, suggesting a specific role for CeA in mediating stress-induced reinstatement of drug seeking. Cain, Denehy, and Bardo (2008) classified rats as high or low responders (HR & LR, respectively) based on activity in an inescapable novel environment, a task related to sensation seeking which is positively correlated with substance use in humans. They found that intra-CeA infusion of GABAA agonist reduced amphetamine seeking only in HR rats, while sucrose seeking was not differentially affected between HR and LR rats. Thus, CeA appears to be integral in expression of high-rate drug seeking in high-sensation seeking animals. Altogether, these results strongly suggest that CeA in necessary selectively for drug-related behaviors.
Drug vs. Natural Reward in the Bed Nucleus of the Stria Terminalis:
CeA is densely interconnected with the bed nucleus of stria terminalis (BNST), and this connection is implicated in several behaviors associated with SUDs, including drug intake, escalation of drug use, and relapse (Centanni et al., 2019). BNST plays a major role in aversive learning and memory, and serves as an interface between reward and aversion systems by integrating inputs including PFC and AMY with outputs to brainstem regions governing response to harm signals and to the VTA, a key node for the mesocorticolimbic reward pathway (Stamatakis et al., 2014). Due to its role in regulating both aversion and reward, the BNST is likely to be critically involved in the neural circuits that underlie SUD. Accordingly, studies comparing the role of BNST in regulating behaviors motivated by drug and natural reward find drug selectivity. Studies have described drug selective adaptations in BNST (Lee et al., 2015; Shalev et al., 2001), and BNST manipulations produce drug selective results. Companion and Thiele (2018) found that silencing BNST to VTA projections disrupted ongoing alcohol but not sucrose drinking. Krawczyk et al. (2013) found that D1-receptor-mediated long term potentiation (LTP) of GABAA in the oval region of the lateral BNST (ovBNST) was associated with prolonged SA of cocaine but not sucrose. Further, blocking this effect with intra-ovBNST infusions of a D1 antagonist reduced progressive ratio breakpoints for cocaine but had no effect of breakpoints for sucrose. These results indicate a role for D1-mediated LTP in ovBNST in regulating enhanced motivation for drug rewards in SUDs. Pleil et al. (2015) found a role for BNST neuropeptide Y receptors in controlling binge drinking of alcohol but not sucrose. Using a drinking in the dark paradigm, they found that agonism of Y1R in BNST reduced alcohol drinking and Y1R antagonism increased alcohol drinking. Importantly, neither manipulation affected sucrose drinking in the same paradigm. Further, they isolated this effect to CRF neurons, suggesting a drug selective role for neuropeptide Y signaling in BNST CRF neurons. Thus, evidence supports drug selectivity in BNST, with a particular emphasis on affect-related behaviors.
Drug vs. Natural Reward in Basolateral Amygdala:
BLA is an allocortical region thought to integrate stimulus sensory information and affective valence, encode various aspects of reward, including history, value, and cost, and influence behavioral symptoms of SUDs by mediating habit and drug-induced changes in reward valuation (Wassum & Izquierdo, 2015). Evidence indicates that BLA is involved in behaviors motivated by both drug and natural reward, with studies revealing increased BLA activity following cued reinstatement (Koya et al., 2006; Madsen et al., 2012) and CPP expression (Mattson & Morrell, 2005) of both drug and natural reward. BLA manipulations also mostly produce effects on both drug- and natural-reward motivated responding. Guercio et al. (2015) showed that deep brain stimulation in BLA reduced primed reinstatement of both cocaine and sucrose seeking. Similarly, Milla, Kim, and Janak (2017) found that optogenetic activation of BLA neurons projecting to NAshell similarly reduced cued reinstatement of alcohol and sucrose seeking. However, Sinclair et al. (2012) found that intra-BLA blockade of mGluR5 eliminated cued reinstatement of alcohol but not sucrose seeking. Thus, future studies will need to carefully compare behaviors motivated by drug and natural rewards across experimental phases to determine if drug selective effects occur in BLA. At present, extant data suggest that BLA has a common role in regulating behavior motivated by both drug and natural reward.
Hippocampus.
The hippocampus (HIPP) is heavily implicated in learning and memory and sends glutamatergic projections to NAshell (ventral HIPP), and to a lesser extent to NAcore (dorsal HIPP; Britt et al., 2012; Groenewegen et al., 1987; Kelley & Domesick, 1982). HIPP is also involved in SUDs, where increased activity is associated with the formation of salient drug-stimulus associations and decreased activity is associated with drug withdrawal, potentially contributing to relapse (Kutlu & Gould, 2016). In addition, several classes of abused substances can affect neurogenesis in HIPP, which is thought to contribute to inflexible decision making, negative affect, and relapse in SUDs (Canales, 2012). Indeed, blocking neurogenesis in HIPP increased cocaine SA and cued reinstatement in mice (Deroche-Gamonet et al., 2019). A recent review emphasized the role of HIPP in stress-, context-, and cue-induced relapse, and suggested that HIPP may regulate drug SA and relapse via inputs to PFC (Goode & Maren, 2019). In addition, ventral HIPP projections to NAshell regulate cocaine seeking after abstinence (Pascoli et al., 2014). Overall, these findings indicate that HIPP plays an important role in the behavioral symptomology of SUDs.
Drug vs. Natural Reward in the Hippocampus:
Despite the role in regulating substance use and seeking discussed above, most studies reveal that the HIPP function is necessary for both drug and natural reward seeking. Data indicate both drug selective (Alasmari et al., 2018; De Laat et al., 2018) and shared adaptations (Crombag et al., 2005; Madsen et al., 2012) in HIPP, and manipulating neuronal activity in HIPP reveals involvement in both drug and sucrose seeking. Guercio et al. (2020) found that deep brain stimulation in HIPP reduced primed-reinstatement of both cocaine and sucrose seeking. Alternatively, Noonan et al. (2010) used irradiation to suppress HIPP neurogenesis and observed increased SA, progressive ratio breakpoints, resistance to extinction, and context-induced reinstatement in cocaine versus sucrose self-administering animals. The mixed outcomes of these studies suggest future work is needed to confidently determine whether drug selective effects occur in HIPP. Further, manipulation studies specifically investigating distinct HIPP subregions and projections could be beneficial for understanding how HIPP contributes to behaviors motivated by drug and natural reward. Together, these data indicate that necessity of HIPP is shared between behaviors motivated by drug and natural rewards.
Ventral Tegmental Area:
The ventral tegmental area (VTA) is the major source of mesocorticolimbic dopamine, is critical for reward processing, motivational salience, and learning, and sends inputs to the NAc (Russo & Nestler, 2013). The role of VTA in drug- (Lüscher & Malenka, 2011) and natural-reward-motivated behavior (Morales & Margolis, 2017) is well established, and abused substances modulate both excitatory and inhibitory effects within VTA (Oliva & Wanat, 2016). In humans, Gu et al. (2011) showed that VTA resting state functional connectivity was reduced in cocaine users relative to healthy controls, and notably VTA connectivity to NAc was reduced. While these results indicate a role for VTA in SUDs, it is important to assess drug selectivity of VTA due to its known involvement in mediating behavior motivated by natural reward.
Drug vs. Natural Reward in the Ventral Tegmental Area:
Existing reports largely indicate drug selectivity in VTA. Data indicate that adaptations in VTA are drug selective (Wang et al., 2012), as are the effects of VTA manipulations. Rinker et al. (2017) showed that intra-VTA antagonism of CRF-1 and activation of CRF-2 receptors attenuated binge-like drinking of alcohol but not sucrose. Further, chemogenetic inhibition of BNST-VTA projecting neurons expressing CRF selectively reduced alcohol seeking. Czachowski et al. (2012) demonstrated that while tetrodotoxin inactivation of VTA decreased seeking of both alcohol and sucrose, intra-VTA glutamate antagonism selectively reduced alcohol seeking at high doses, demonstrating a reward- and dose-dependent effect. Further, neither manipulation affected consumption of either reward, indicating a specific role for VTA glutamate in regulating motivation to seek alcohol. Relatedly, Sun et al. (2005) found that blocking VTA glutamate receptors attenuated primed reinstatement of cocaine but not sucrose seeking. In addition, several other studies have shown drug selective effects of VTA on relapse. Solecki et al. (2019) found that optogenetic inhibition of VTA dopamine neurons reduced cued reinstatement of cocaine but not food seeking. Addy et al. (2018) found that intra-VTA infusions of calcium channel blocker selectively attenuated cue-induced cocaine-seeking, without altering cocaine reinforcement nor cue-induced sucrose-seeking. Solecki et al. (2018) showed that VTA noradrenergic signaling selectively regulates cued cocaine seeking, as evidenced by decreases in cued reinstatement of cocaine but not sucrose seeking following intra-VTA infusions of α1 and α2 antagonists. Brown et al. (2018) demonstrated a drug selective role for inflammatory signaling in VTA on relapse by blocking inflammatory signaling in VTA, which reduced primed reinstatement of cocaine but not sucrose seeking. Finally, preventing the dopaminylation of histone H3 in the VTA prevents reinstatement of cocaine but not food seeking following re-exposure to cues after extended forced abstinence (Lepack et al., 2020). In sum, extant literature strongly suggests that VTA shows drug selectivity during SA and relapse.
Diencephalon (thalamus and hypothalamus):
The thalamus mediates cognitive function as well as goal-directed and motor behaviors via projections to the PFC and striatum which also establish a role for the thalamus in reward circuitry (Huang et al., 2018). A recent review by Huang et al. (2018) highlights several adaptations in the thalamus of individuals with SUDs. First, grey matter is reduced and this reduction is correlated with drug craving, length of substance use, time in abstinence, and relapse. Differences in thalamic activity were observed with a variety of methods, and differences in functional connectivity were noted between the thalamus and other regions discussed here, including AMY, NAc and PFC. Thalamic activity was reduced in response inhibition tasks, and this decrease was correlated with SUD severity. Finally, thalamic activity was increased in response to drug-paired stimuli in individuals with SUDs relative to healthy controls.
The hypothalamus also plays an important role in regulating behaviors characteristic of SUDs. Orexin/hypocretin is a neuropeptide originating the lateral hypothalamus (LH) and orexin has been shown to play an important role in highly-motivated responding (including for drugs) and in negative affect, stress, and anxiety (Hopf, 2020). Interestingly, orexin neurons appear to be activated preferentially by cocaine but not highly palatable food in one study (Matzeu & Martin-Fardon, 2018), but other studies find mixed evidence for activation of orexin neurons by natural reward (for discussion see, Hopf, 2020). Finally, Orisni et al. (2018) reported that increased functional connectivity during early abstinence from cocaine vs. sucrose seeking in rats varied with respect to subregions of the thalamus and hypothalamus. Thus, regions within the diencephalon appear to play a role in characteristic behaviors of SUDs. We explore drug selectivity in some of these regions of the diencephalon next.
Drug vs. Natural Reward in Diencephalon:
The lateral hypothalamus (LH) is involved in modulating motivation, reward, and satiety, and projects to nodes in the mesocorticolimbic reward pathway (Castro et al., 2015). The studies that have directly compared drug and natural reward in LH find drug selectivity. While Fos activation is similar following cued reinstatement of drug and natural rewards (Madsen et al., 2012), manipulation studies in LH suggest selective involvement in drug-motivated behaviors. Levy et al. (2007) showed that deep brain stimulation in LH reduced cued reinstatement of cocaine but not sucrose seeking. Similarly, Marchant et al. (2009) found that inactivation of LH with muscimol + baclofen reduced contextual reinstatement of alcoholic beer seeking, but not sucrose seeking. Moreover, they found evidence indicating that these effects were likely dependent on projections to NAshell. Perineuronal nets in the dorsal zone of the LH are necessary for cocaine, but not food CPP, cocaine, but not food SA, and cue-induced reinstatement of cocaine but not sucrose seeking (Blacktop et al., 2017; Blacktop & Sorg, 2019). In addition, the LH derived neuropeptide orexin has been shown to potently reduce alcohol but not sucrose drinking (Lopez et al., 2016), and orexin is necessary for cocaine but not sucrose SA in sated rats (España et al., 2010). Thus, extant studies strongly indicate drug selectivity within LH, with a particular role for LH-derived orexin in controlling drug motivated responding.
The subthalamic nucleus (STN) is implicated in cognition, motivation, and emotion and regulates motor action via connections with ventral pallidum, substantia nigra and globus pallidus via the canonical indirect pathway, and via direct cortical inputs (i.e. the hyperdirect pathway; Bonnevie & Zaghloul, 2019). STN also shows interesting effects with respect to addiction, in that it bidirectionally controls motivated seeking of drug versus natural reward (Hamani et al., 2017). Baunez et al. (2005) found no effect of lesions in STN when each cocaine or sucrose response was reinforced (i.e. fixed ratio 1 schedule). However, STN lesions increased responding for sucrose and decreased responding for cocaine under progressive ratio conditions. They also found that STN lesioned animals increased preference for a food-paired compartment and decreased preference for a cocaine-paired compartment (relative to non-rewarded compartments) in a CPP assay. Similarly, Rouaud et al. (2010) found that deep brain stimulation in STN increased place preference and progressive ratio breakpoints in sucrose-seeking animals, and decreased place preference and progressive ratio breakpoints in cocaine-seeking animals. Further, they showed that STN deep brain stimulation reduced compensatory increases in cocaine seeking following decreasing doses relative to non-stimulated controls. While these data excitingly suggest that STN bidirectionally controls responding motivated by drug vs. natural reward, they also indicate a role for STN in regulating behaviors motivated by both drug and natural reward. As such, these data indicate a shared role for STN in regulating behaviors motivated by both drug and natural reward.
Ventral Pallidum:
The ventral pallidum (VP) has reciprocal connections with NAc and VTA and sends outputs directly to the thalamus (Smith et al., 2009). VP is implicated in both hedonic “liking” and incentive motivational “wanting” of reward (Smith et al., 2009), as well as drug seeking, stimulus discrimination, working memory, and relapse (Root et al., 2015). Thus, VP is an important nucleus in regulating behaviors motivated by both drug and natural reward. Heinsbroek et al. (2020) reported that specific cell types in VP are involved in refraining from drug seeking and cued reinstatement. Specifically, in vivo single-cell calcium imaging of VP glutamate neurons revealed the highest activity during extinction, and chemogenetic stimulation of these neurons attenuated cued reinstatement of cocaine seeking. VP GABA and enkephalin neurons were most active during cued reinstatement, and chemogenetic stimulation of these neurons reinstated cocaine seeking. Similarly, Creed et al. (2016) showed that VP synapses from NAc D1-neurons were potentiated while synapses from NAc D2-neurons were depressed following cocaine exposure. Further, they found that reversing potentiation of NAc D1-neuron synapses in VP reduced cocaine sensitization, and reversal of depression in NAc D2-neuron synapses in VP increased motivation and decreased negative affective responses to natural reward (i.e. orofacial responses to sucrose). Together, these studies establish that VP is critically involved in mediating behavioral symptoms of SUDs.
Drug vs. Natural Reward in the Ventral Pallidum:
Evaluations comparing the effects of VP manipulations on drug versus natural reward are mixed, but mostly show drug selectivity. Li et al. (2009) found that systemic, intra-VP, or intra-NAc infusion of mGluR7 agonist AMN082 reduced cocaine seeking while systemic infusions had no effect on sucrose seeking. Further, they showed that pre-treatment with AMN082 blocked cocaine-induced decreases in extracellular GABA concentrations in VP. Intra-VP or intra-NAc infusions of mGluR7 antagonist MMPIP blocked the effect of AMN082, leading the authors to conclude that mGluR7s in the NAc-VP GABAergic pathway are involved in selectively mediating ongoing cocaine seeking. Similarly, Heinsbroek et al. (2017) found that chemogenetic stimulation of D1-MSNs in NAcore potentiated cued reinstatement of cocaine but not sucrose seeking. Though this manipulation occurred in NAc, they showed that simultaneous chemogenetic stimulation of NAc D1-MSNs and inhibition of VP reversed potentiation of cued reinstatement, indicating that the effect was dependent on D1 NAc-VP projections. June et al. (2003) found that infusing a GABAA1-receptor-specific ligand into the VP selectively reduced alcohol but not sucrose seeking in two alcohol preferring rat strains. However, McFarland and Kalivas (2001) found that injections of GABA agonists muscimol + baclofen into VP reduced primed reinstatement of both cocaine and food seeking. Together, these studies mostly suggest that VP, and particularly the NAc-VP pathway show drug selectivity. However, it is difficult to confidently assert that VP shows drug selectivity with so few studies, and some contrary evidence. One potential explanation for these divergent findings lies in the methodology. McFarland and Kalivas (2001) assessed primed reinstatement, while Heinsbroek et al. (2017) examined cued reinstatement. Because VP is known to regulate hedonic responses to abused substances, it is possible that deactivating VP reduced the perceived value of both cocaine and sucrose, thereby blunting priming-induced reinstatement. VP is also a heterogeneous structure, consisting of distinct subregions, and containing different cell types and output pathways to AMY, LH, NAc, STN, PFC, VTA, diencephalon, and brain stem (see, Prasad et al., 2020; Root et al., 2015). Thus, another hypothesis is that manipulations in different subregions within VP may influence different output pathways, and there is some support for differential roles of VP output pathways (Prasad et al., 2020) and cell types (Heinsbroek et al., 2020) in regulating drug-motivated behavior. Regardless of the reason for these mixed results, the NAcore-VP pathway appears to mostly show drug selectivity.
Limitations and Promising Technology for Future Studies
Limitations:
One limitation of the current review is that the extant studies examined only comparisons between drug reward and non-pathological behaviors motivated by natural reward. A growing literature is directed at understanding the similarities and differences in neurobiological factors contributing to so-called behavioral addictions (e.g. gambling disorders, eating disorders, exercise addiction) and SUDs (e.g., Chamberlain et al., 2016; Hadad & Knackstedt, 2014; James & Tunney, 2017). While recent discussions have drawn parallels between the characteristic behaviors and neurobiological mechanisms of both pathologies (e.g., Fletcher & Kenny, 2018; Kuhn et al., 2019), studies employing protocols for directly comparing pathological natural and drug reward seeking do not yet exist.
An obvious limitation is that there exist relatively few studies directly comparing the necessity of a brain nucleus or circuit in drug and natural reward. Below we outline behavioral paradigms that will facilitate this comparison in future studies. The vast majority of the work we discuss compared reinstated seeking of a drug (most often cocaine) in one group versus sucrose in another group and employed relatively broad manipulation techniques to assess the necessity of the specified region for controlling behavior. Thus, future studies utilizing more finely-grained analyses are needed to refine the proposed drug selective circuitry we compile in Figure 1C. In the following sections, we discuss promising protocols and technologies that will allow investigators to draw more nuanced conclusions regarding the role of brain circuits in regulating drug versus natural reward seeking.
Behavioral Models:
Polysubstance use is highly prevalent among people suffering from substance use disorders (Crummy et al., 2020; Liu et al., 2018). The rise of the opioid epidemic in the United States has indeed precipitated polydrug-induced overdoses, notably due to the use of opiates as drug-cutting ingredients (Meier et al., 2020; Nolan et al., 2019). Besides polysubstance abuse, humans have complex lives comprising many sources of non-drug rewards, such as food, water, social interaction, or sex. Like drugs, these rewards drive and influence behavior constantly. It is therefore important for future studies to determine how the simultaneous use of multiple drugs or competing rewards affects the brain. Mounting evidence indicates that chronic exposure to unmixed drugs of abuse induces drug selective effects that may occur only at particular stages of addiction (e.g., Cameron & Carelli, 2012; De Laat et al., 2018; Yager et al., 2019), in particular modalities of relapse (e.g., Spencer et al., 2014; Wunsch et al., 2017), or in particular subsets of subjects (e.g., Cain et al., 2008; Hernandez & Moorman, 2020). Thus, strategies that allow for drug:drug and drug:natural-reward comparisons in the same animal across experimental phases will shed light on how individual differences and drug-induced adaptations in neurobiology selectively regulate drug reward, drug seeking, and refraining from drug seeking. Making comparisons between drugs of different classes or drugs and natural rewards in animal models can be challenging due to differences in routes of administration (e.g. oral versus intravenous), onset of action (e.g. quick for cocaine, slow for alcohol), and direct effects different rewards on behavior (e.g. increased activity by stimulants and decreased activity by depressants). However, these difficulties can be minimized with careful arrangement of experimental paradigms and validation of interesting findings in more societally-relevant paradigms. Indeed, any interesting findings with the potential to further understanding of the neurobiological underpinnings of SUDs or contribute to the development of novel therapies should be validated using procedures high in face validity.
To increase the face validity of animal models of SUDs, preclinical models designed to investigate specific endophenotypes underlying SUDs have recently emerged, including drug-induced alterations in motivation for natural reward (e.g., Creed et al., 2016; Hart et al., 2018), relapse following analogues of human behavioral interventions using natural rewards (e.g., Nall et al., 2018; Venniro et al., 2019), and resistance to aversive effects of drugs (e.g., Marchant et al., 2019; Nall & Shahan, 2020). However, there is currently little work directly comparing drug versus natural reward using these preclinical models. Thus, while polyreward models are more complex than non-mixed reward paradigms, they more closely approximate key features of SUDs in humans. Future work using these behavioral models along with the finely-grained biological assays discussed below could be fruitful for determining how drugs of abuse, but not natural reward, lead to pathological behaviors indicative of SUDs.
Cellular Ensembles and Subpopulations:
The majority of the studies discussed in this review compared natural reward with drug reward for purposes of controlling for effects mediated by the behavioral protocol. However, it is becoming increasingly apparent that groups of neurons (i.e. neuronal ensembles) within the same brain nucleus of an animal may encode both drug and natural rewards, while other ensembles may selectively motivate responding for one or another reward (Bobadilla et al., 2020; Cameron & Carelli, 2012; Carelli et al., 2000; Cruz et al., 2013; DeNardo & Luo, 2017; Kane et al., 2020; Pfarr et al., 2018). While some studies indicate a relatively small overlap in drug and natural-reward ensembles (e.g., Bobadilla et al., 2020; Cameron & Carelli, 2012), others indicate larger overlap (e.g., Pfarr et al., 2018). Ensembles may also differentially code behaviors related to SA and refraining from seeking during extinction. Indeed, there is evidence that self-administration and extinction of both cocaine (Warren et al., 2019) and food seeking (Warren et al., 2016) depend, in part, on mostly independent ensembles of IL neurons. Further, cocaine SA and extinction ensembles are composed primarily of IL-NAcore and IL-NAshell projection neurons, respectively (Warren et al., 2019). As such, comparisons of individual neuron activity within-subject could provide particularly precise data regarding drug selectivity and differential control over drug-related behaviors. Further, a variety of approaches, such as TRAP (DeNardo et al., 2019) or Calcium Modulated Photoactivatable Ratiometric Integrator (CaMPARI; Moeyaert et al., 2018) strategies have been developed that allow these neuronal ensembles to be tagged and manipulated in vivo, providing rich data on how specific cellular ensembles control motivated behavior (for discussion see, Cruz et al., 2013; Whitaker & Hope, 2018).
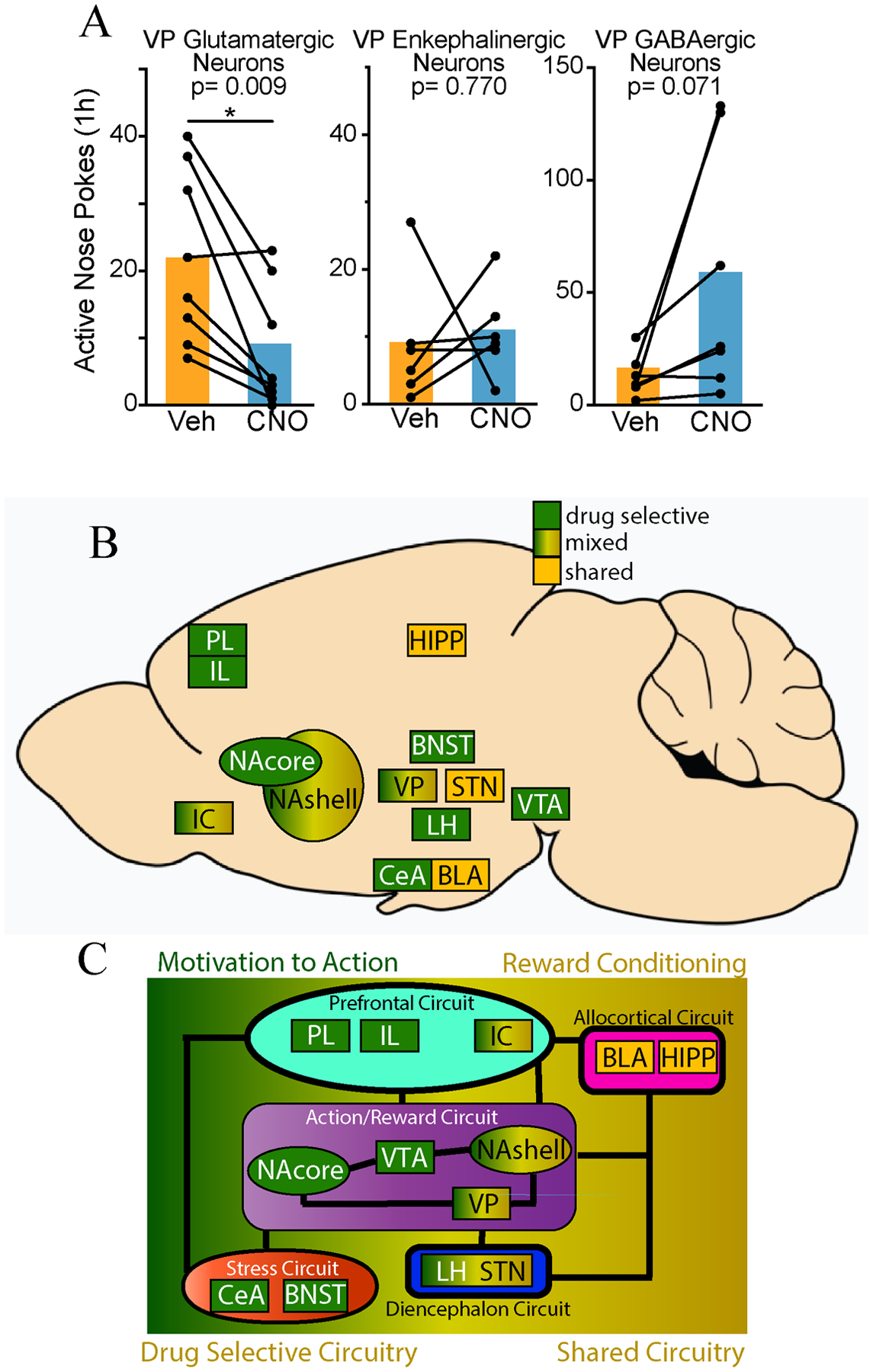
Individual cell types can also differentially control behavior. For example, a growing body of evidence indicates differential roles for D1- and D2-MSNs in NAcore in regulating behavior, with specific D1-MSN circuits mostly promoting drug seeking and D2-MSN circuits mostly inhibiting seeking (e.g., Bock et al., 2013; Gibson et al., 2018; Heinsbroek et al., 2017; Kravitz et al., 2012; Lobo & Nestler, 2011; Roberts-Wolfe et al., 2018). The dual-reward study discussed above by Bobadilla and colleagues (2020) revealed that reward- seeking ensembles were mostly comprised of D1-MSNs within NAcore during seeking, and mostly D2-MSNs during extinction, showing that opposing control over behavior by NAcore D1- and D2-MSNs is also recapitulated in recruitment of those cell types to specific cellular ensembles. Recent evidence also indicates that individual cell types in VP differentially control behavior. As detailed above, Heinsbroek et al (2020) used a calcium imaging approach to demonstrate that activity in glutamatergic VP cells was high while activity in GABAergic or an enkephalin-expressing subpopulation of GABAergic VP cells was low during extinguished cocaine seeking. Chemogenetic stimulation of VP glutamate cells attenuated extinguished cocaine seeking, while stimulation of VP GABA and enkephalin cells reinstated cocaine seeking. Importantly, recent follow-up experiments showed that while activation of VP glutamate cells also inhibited sucrose seeking, activation of enkephalin neurons did not augment sucrose seeking and activating GABA neurons produced mixed results on sucrose seeking (Figure 1A). These data also highlight the power of the relatively new in vivo single-cell calcium imaging approach, that can provide information on cellular activity in real time across different phases of drug-seeking (see also, Siciliano & Tye, 2019). Further, calcium imaging allows for tracking cells over time, and clustering of individual cells into ensembles based on similar patterns of calcium activity. Such analyses can provide rich within-subject data regarding changes in ensemble activity across experimental phases and in response to both drug and natural reward. Together, the studies discussed in this section indicate the utility of studying drug selective and nonselective involvement of brain circuits in neuronal subpopulations within each nucleus or in subpopulations that have distinct axon terminal fields or unique activity patterns. Ideally, these more nuanced analyses would be utilized in conjunction with a within-subjects protocol (see above) to directly compare behaviors motivated by drug and natural rewards across experimental phases.
Discussion
SUDs are characterized, in part, by biased behavior toward substances of abuse and away from natural rewards, as reflected by many of the symptoms/diagnostic criteria of SUDs. Studies that examine the effects of drug versus natural reward within brain nuclei provide insight on where addictive drugs might produce neuroadaptations that facilitate drug seeking without altering natural reward seeking, a potentially critical distinction in developing therapies to selectively target drug seeking. The studies reviewed here examined the role of NAc and the circuitry in which NAc is embedded in governing behaviors motivated by drug versus natural rewards. Table 1 provides a summary of studies that found specific regions to be necessary selectively for drug-motivated behaviors (i.e. drug selective) or for both drug and natural reward motivated behaviors (i.e. shared). Based on the reviewed studies that examined drug selectivity we characterized the role of each region as drug selective or shared between natural and drug reward (Figure 1B), and then clustered nuclei into larger functional groups for discussion purposes (Figure 1C).
Manipulations in the NAcore, PL, IL, LH, VTA, CeA and BNST selectively altered drug- but not natural-reward motivated behavior (i.e. the Motivation to Action Circuit, Figure 1C). Drug selective necessity of the NAcore and VTA (i.e. Action/Reward subcircuit) as well as PL and IL is perhaps unsurprising, as these many of these regions are major components in the mesocorticolimbic reward pathway (Cooper et al., 2017), which is heavily implicated in behavioral symptomology of SUDs. Indeed nodes in this circuit are responsible for increased salience of drug cues and reward (Berridge & Robinson, 2016). Moreover, the PL and IL (i.e. Prefrontal subcircuit) were selectively necessary in drug seeking, as another of the major pathologies of SUDs is altered executive functioning, which is characterized by behavioral symptoms such as impulsivity (Jupp & Dalley, 2014) and drug-biased decision making (Paulus, 2007), and driven by regions in the Prefrontal subcircuit (Goldstein & Volkow, 2011). Further, interplay between the Action/Reward and Prefrontal subcircuits is carried out by glutamatergic PL-NAcore and dopaminergic VTA-NAcore circuits and critical for behavioral symptoms of SUDs (e.g., Shen et al., 2014). Drug selectivity in CeA and BNST (i.e. Stress subcircuit) is also predictable given the role of the extended amygdala in controlling the negative, stressful aspects of SUDs, including negative affect, blunting of natural reward, and withdrawal (Centanni et al., 2019). Finally, drug selective necessity of LH is consistent with recent literature suggesting that orexin/hypocretin neurons originating in LH and projecting widely across cortical and allocortical structures (i.e. Diencephalon subcircuit) play an important role in many of the behavioral symptoms of SUDs including enhanced motivation for abused substances, stress/anxiety, and compulsive drug seeking (Hopf, 2020). We certainly recognize that each subcircuit could be further parsed into functionally independent subcircuits. For example, the Action/Reward subcircuit contains NAcore and NAshell, which are well documented to serve different functions in several paradigms(Bossert et al., 2007; Cartoni et al., 2016; Di Chiara, 2002; Floresco et al., 2008). However, future research is needed to develop more nuanced understanding of the role of these interconnected regions in controlling behaviors motivated by drug versus natural reward. Altogether, the drug selectivity of nuclei within these subregions is likely to govern many of the behavioral symptoms of SUDs.
Data from other regions canonically thought to respond to abused substances and regulate SUD-associated behaviors indicated a shared necessity for regulating behaviors motivated by both drug and natural reward. These included BLA, HIPP, and STN (i.e. Reward Conditioning circuit). The shared involvement of the BLA and HIPP (i.e. Allocortical subcircuit) can be predicted based on their critical roles in fundamental learning processes necessary for mammals to learn and recall associations between rewards and environmental stimuli. Thus, inactivation of these regions eliminates recall of discrete or contextual information associated with either drug or natural rewards (Cador et al., 1989; Fuchs et al., 2005; Grimm & See, 2000; Riaz et al., 2017; Rogers & See, 2007; Stefanik & Kalivas, 2013). The STN (part of the Diencephalon subcircuit) is well established as a primary output within the extrapyramidal motor system and densely innervated by the globus pallidus and ventral pallidum (e.g., Root et al., 2015; Shink et al., 1996; Tillage et al., 2020). As an integral component of motor behavior, it is perhaps not surprising that STN is needed to execute behaviors motivated by both natural and drug reward. However, it is worth highlighting that the unique bi-directional enhancement of natural-reward motivated behaviors and diminution of drug-motivated behaviors in STN does provide a potential avenue for future SUD therapies (e.g., Pelloux & Baunez, 2013; Wang et al., 2018). Overall, regions of the Reward Conditioning circuit are critically involved in both drug and natural-reward motivated behaviors.
Mixed-involvement was found for three regions: NAshell, IC, and VP. NAshell integrates a variety of cortical and allocortical inputs with varying levels of drug selectivity. For instance, the IL-NAshell projection showed drug selectivity (Pascoli et al., 2014) but the BLA-NAshell pathway did not (Millan et al., 2017). Further, mostly individual cellular ensembles within NAshell code for drug and natural reward (e.g. Cameron & Carelli, 2012). Thus, it is likely that the mixed data in NAshell arise from differential connectivity or effects on specific cell types/ensembles. IC and VP are heterogeneous structures, but little animal research has simultaneously compared the role of subregions in IC and VP on behaviors motivated by drug and natural reward. Indeed, the dorsal anterior and ventral anterior portions of the IC project to the NAcore and lateral aspect of NAshell, respectively (Brog et al., 1993; Sesack et al., 1989). Given that NAcore showed drug selectivity, but NAshell data were mixed, it is worth investigating drug selectivity within these specific IC-NAc projections. Similarly, VP subregions also have different projection targets (e.g., Groenewegen et al., 1993). Ventromedial VP projects to both VTA and LH, regions that showed drug selectivity. By contrast, dorsolateral VP projects to STN, which did not show drug selectivity in the studies reviewed here. Finally, cell types and projections also differ along the rostral-caudal axis of VP (Groenewegen et al., 1993; Kupchik & Kalivas, 2013), and VP cell types are differentially implicated in drug-seeking behavior across experimental phases (Heinsbroek et al., 2020). Thus, further investigations into NAshell, IC, and VP using the finely-grained approaches discussed above would seem likely to reveal distinct subregions or populations within each nucleus that are drug selective or shared between drug and natural rewards.
Conclusions
This review summarized findings from investigations on the role of canonical addiction circuitry in regulating behavior motivated by drug versus natural reward. Brain regions were characterized as part of a drug selective circuit (i.e., the given nucleus was necessary for the initiation or execution of behavior motivated by drug but not natural reward), or the brain region was included in a shared circuit (i.e., the given region was necessary for both drug and natural reward seeking). Evidence for drug selectivity was found in NAcore, PL, IL, BNST, CeA, VTA and LH while shared circuitry included the BLA, HIPP and STN. Additionally the NAshell, IC and VP were considered mixed, presumably because these brain regions contain a connectomic or topographic mixture of both circuits that was not specifically targeted in the studies to date. Based on these findings and extant work in the addiction field, we proposed a preliminary circuit hypothesized to be necessary selectively for drug-motivated behavior. Together, drug selective neuroplasticity and functional dysregulation in the drug selective circuitry can account for many of the behavioral symptoms used to diagnose SUDs in humans. Thus, this circuitry provides a rich testing arena in which to reveal the neurobiological mechanisms of SUDs and evaluate novel therapeutic approaches for SUDs that may not influence the adaptive behaviors engendered by natural rewards. We also reviewed promising behavioral and biological techniques that will advance our understanding of how drugs of abuse, but not natural rewards, produce pathological behaviors characteristic of SUDs. More specifically, these techniques promise to reveal how even though each nucleus likely encodes both natural and drug reward in distinct subcircuits and neuronal ensembles, drug use modifies the drug selective circuits and ensembles to promote a cardinal symptom of SUDs, choosing drug reward over natural reward.
Funding Sources and Conflict of Interest Disclosure:
NIH DA046522, P20GM121310 (A-CB); DP5 OD026407 (JAH); DA003906, DA012513, DA046373, BX004727 (PWK). The authors have no conflicting interests to disclose.
Abbreviations:
- ACC
anterior cingulate cortex
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMY
amygdala
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- CPP
conditioned place preference
- CRF
corticotropin releasing factor
- GABA
gamma-aminobutyric acid
- GLT
glutamate transporter
- HIPP
hippocampus
- HR
high responder
- IC
insular cortex
- IL
infralimbic cortex
- LH
lateral hypothalamus
- LR
low responder
- mGluR
metabotropic glutamate receptor
- LTP
long-term potentiation
- mPFC
medial prefrontal cortex
- MSN
medium spiny neuron
- NAc
nucleus accumbens
- NAcore
nucleus accumbens core
- NAshell
nucleus accumbens shell
- NMDA
n-methyl-d-aspartate
- OFC
orbitofrontal cortex
- ovBNST
oval bed nucleus of the stria terminalis
- PFC
prefrontal cortex
- PL
prelimbic cortex
- SA
self-administration
- SN
subtantia nigra
- STN
subthalamic nucleus
- SUD
substance use disorder
- VP
ventral pallidum
- VTA
ventral tegmental area
- xCT
cystine glutamate antiporter
References
- Addy NA, Nunes EJ, Hughley SM, Small KM, Baracz SJ, Haight JL, & Rajadhyaksha AM (2018). The L-type calcium channel blocker, isradipine, attenuates cue-induced cocaine-seeking by enhancing dopaminergic activity in the ventral tegmental area to nucleus accumbens pathway. Neuropsychopharmacology, 43(12), 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agboola SA, Coleman T, McNeill A, & Leonardi-Bee J (2015). Abstinence and relapse among smokers who use varenicline in a quit attempt-a pooled analysis of randomized controlled trials. Addiction, 110(7), 1182–1193. [DOI] [PubMed] [Google Scholar]
- Alasmari F, Bell RL, Rao PSS, Hammad AM, & Sari Y (2018). Peri-adolescent drinking of ethanol and/or nicotine modulates astroglial glutamate transporters and metabotropic glutamate receptor-1 in female alcohol-preferring rats. Pharmacol Biochem Behav, 170(May), 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders. American Psychiatric Pub; (4th ed., T). Washington DC. [Google Scholar]
- Anderson RI, Lopez MF, Griffin WC, Haun HL, Bloodgood DW, Pati D, Boyt KM, Kash TL, & Becker HC (2019). Dynorphin-kappa opioid receptor activity in the central amygdala modulates binge-like alcohol drinking in mice. Neuropsychopharmacology, 44(6), 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello AA, Wang R, Lyons CM, Higginbotham JA, Hodges MA, & Fuchs RA (2017). Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats. Psychopharmacology (Berl), 234(16), 2431–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston-Jones G, Kalivas PW, & Peters J (2016). Chemogenetic activation of an extinction neural circuit reduces cue-induced reinstatement of cocaine seeking. J Neurosci, 36(39), 10174–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Dias C, Cador M, & Amalric M (2005). The subthalamic nucleus exerts opposite control on cocaine and “natural” rewards. Nat Neurosci, 8(4), 484–489. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, & Nauta WJH (1979). Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res, 175(2), 191–217. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, & Everitt BJ (2013). Addiction: Failure of control over maladaptive incentive habits. Curr Opin Neurobiol, 23(4), 564–572. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, & Rubinstein M (2011). Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D 2 autoreceptors. Nat Publ Gr, 14(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Gamboa R, G??mez AM, & Nieto J (2017). Reducing spontaneous recovery and reinstatement of operant performance through extinction-cues. Behav Processes, 135, 1–7. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2009). “Liking” and “wanting” food rewards: Brain substrates and roles in eating disorders. Physiol Behav, 97(5), 537–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson T (University of M. (2016). Liking, wanting and the incentive salience theory of addiction. Am Psychol, 71(8), 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, & Sorg BA (2019). Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior. Neuropsychopharmacology, 44(5), 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Todd RP, & Sorg BA (2017). Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology, 118, 124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Dereschewitz E, Vaccaro L, Heinsbroek JA, Scofield MD, & Kalivas PW (2020). Cocaine and sucrose rewards recruit different seeking ensembles in the nucleus accumbens core. Mol Psychiatry, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Garcia-Keller C, Heinsbroek JA, Scofield MD, Chareunsouk V, Monforton C, & Kalivas PW (2017a). Accumbens mechanisms for cued sucrose seeking. Neuropsychopharmacology, 42(12), 2377–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobadilla AC, Heinsbroek JA, Gipson CD, Griffin WC, Fowler CD, Kenny PJ, & Kalivas PW (2017b). Corticostriatal plasticity, neuronal ensembles, and regulation of drug-seeking behavior. Progress in Brain Research (1st ed., Vol. 235). Elsevier B.V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, & Alvarez VA (2013). Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. [DOI] [PMC free article] [PubMed]
- Bonnevie T, & Zaghloul KA (2019). The Subthalamic Nucleus: Unravelling New Roles and Mechanisms in the Control of Action HHS Public Access. Neuroscientist, 25(1), 48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, & Shaham Y (2007). Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci, 27(46), 12655–12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, & Bonci A (2012). Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron, 76(4), 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY, Zahm DS, Sesack SR, Deutch AY, Roth RH, & Bunney BS (1993). The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro‐gold. J Comp Neurol, 290(2), 255–278. [DOI] [PubMed] [Google Scholar]
- Brown KT, Levis SC, O’Neill CE, Northcutt AL, Fabisiak TJ, Watkins LR, & Bachtell RK (2018). Innate immune signaling in the ventral tegmental area contributes to drug-primed reinstatement of cocaine seeking. Brain Behav Immun, 67, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Kim AK, Khoo SYS, Kim JH, Jupp B, & Lawrence AJ (2016). Orexin-1 receptor signalling in the prelimbic cortex and ventral tegmental area regulates cue-induced reinstatement of ethanol-seeking in IP rats. Addict Biol, 21(3), 603–612. [DOI] [PubMed] [Google Scholar]
- Burke DA, Rotstein HG, & Alvarez VA (2017). Striatal local circuitry: a new framework for lateral inhibition. Neuron, 96(2), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cador M, Robbins TW, & Everitt BJ (1989). Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience, 30(1), 77–86. [DOI] [PubMed] [Google Scholar]
- Cain ME, Denehy ED, & Bardo MT (2008). Individual differences in amphetamine self-administration: The role of the central nucleus of the amygdala. Neuropsychopharmacology, 33(5), 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, & Carelli RM (2012). Cocaine abstinence alters nucleus accumbens firing dynamics during goal-directed behaviors for cocaine and sucrose. Eur J Neurosci, 35(6), 940–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EJ, Flanagan JPM, Walker LC, Hill MKRI, Marchant NJ, & Lawrence AJ (2019). Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. [DOI] [PMC free article] [PubMed]
- Canales JJ (2012). Deficient plasticity in the hippocampus and the spiral of addiction: Focus on adult neurogenesis. Curr Top Behav Neurosci, 15, 293–312. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, & Crumling AJ (2000). Evidence That Separate Neural Circuits in the Nucleus Accumbens Encode Cocaine Versus “Natural” (Water and Food) Reward. [DOI] [PMC free article] [PubMed]
- Cartoni E, Balleine B, & Baldassarre G (2016). Appetitive Pavlovian-instrumental Transfer: A review. Neurosci Biobehav Rev, 71, 829–848. [DOI] [PubMed] [Google Scholar]
- Castro DC, & Bruchas MR (2019). A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell. Neuron, 102(3), 529–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Cole SL, & Berridge KC (2015). Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Front Syst Neurosci, 9(June), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni SW, Bedse G, Patel S, & Winder DG (2019). Driving the Downward Spiral: Alcohol-Induced Dysregulation of Extended Amygdala Circuits and Negative Affect. Alcohol Clin Exp Res, 43(10), 2000–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health. (HHS Pulication No SMA 15–4927, NSDUH Ser H-50, 64. Retrieved from http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf%5Cnhttp://www.samhsa.gov/data/ [Google Scholar]
- Chamberlain SR, Lochner C, Stein DJ, Goudriaan AE, van Holst RJ, Zohar J, & Grant JE (2016). Behavioural addiction-A rising tide? Eur Neuropsychopharmacol, 26(5), 841–855. [DOI] [PubMed] [Google Scholar]
- Companion MA, & Thiele TE (2018). Assessment of ventral tegmental area-projecting GABAergic neurons from the bed nucleus of the stria terminalis in modulating binge-like ethanol intake. Eur J Neurosci, 48(11), 3335–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S, Robison AJ, & Mazei-Robison MS (2017). Reward Circuitry in Addiction. Neurotherapeutics, 14(3), 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordie R, & McFadden LM (2019). Optogenetic inhibition of the medial prefrontal cortex reduces methamphetamine-primed reinstatement in male and female rats. Behav Pharmacol, 30(6), 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosme CV, Gutman AL, & LaLumiere RT (2015). The Dorsal Agranular Insular Cortex Regulates the Cued Reinstatement of Cocaine-Seeking, but not Food-Seeking, Behavior in Rats. Neuropsychopharmacology, 40(10), 2425–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, & Lüscher C (2016). Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron, 92(1), 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, & Shaham Y (2008). Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci, 363(1507), 3233–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, & Robinson TE (2005). Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex, 15(3), 341–348. [DOI] [PubMed] [Google Scholar]
- Crummy EA, O’Neal TJ, Baskin BM, & Ferguson SM (2020, June 16). One Is Not Enough: Understanding and Modeling Polysubstance Use. Front Neurosci. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz FC, Koya E, Guez-Barber DH, Bossert JM, Lupica CR, Shaham Y, & Hope BT (2013). New technologies for examining the role of neuronal ensembles in drug addiction and fear. Nat Rev Neurosci, 14(11), 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL (2005). Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol and sucrose seeking and intake. Alcohol Clin Exp Res, 29(7), 1146–1155. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ, & Pope JD (2012). Behavioral and Neurotransmitter Specific Roles for the Ventral Tegmental Area in Reinforcer-Seeking and Intake. Alcohol Clin Exp Res, 36(10), 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laat B, Weerasekera A, Leurquin-Sterk G, Bormans G, Himmelreich U, Casteels C, & Van Laere K (2018). Glutamatergic biomarkers for cocaine addiction: A longitudinal study using MR spectroscopy and mGluR5 PET in self-administering rats. J Nucl Med, 59(6), 952–959. [DOI] [PubMed] [Google Scholar]
- DeNardo LA, Liu CD, Allen WE, Adams EL, Friedmann D, Fu L, Guenthner CJ, Tessier-Lavigne M, & Luo L (2019). Temporal evolution of cortical ensembles promoting remote memory retrieval. Nat Neurosci, 22(3), 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo L, & Luo L (2017). Genetic strategies to access activated neurons. Curr Opin Neurobiol, 45, 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Revest JM, Fiancette JF, Balado E, Koehl M, Grosjean N, Abrous DN, & Piazza PV (2019). Depleting adult dentate gyrus neurogenesis increases cocaine-seeking behavior. Mol Psychiatry, 24(2), 312–320. [DOI] [PubMed] [Google Scholar]
- Di Chiara G (2002). Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav Brain Res, 137(1–2), 75–114. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, & Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A, 85(14), 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doncheck EM, Liddiard GT, Konrath CD, Liu X, Yu L, Urbanik LA, Herbst MR, DeBaker MC, Raddatz N, Van Newenhizen EC, Mathy J, Gilmartin MR, Liu Q. song, Hillard CJ, & Mantsch JR (2020). Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology, (April). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droutman V, Read SJ, & Bechara A (2015). Revisiting the role of the insula in addiction. Trends Cogn Sci, 19(7), 414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Bachtell RK, Guzman D, Whisler KN, & Self DW (2011). Emergence of context-associated GluR 1 and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol, 16(3), 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, & Robbins TW (2013a). Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biol Psychiatry, 74(2), 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Meng C, Ziauddeen H, Stochl J, Williams GB, Bullmore ET, & Robbins TW (2020). Brain networks underlying vulnerability and resilience to drug addiction. Proc Natl Acad Sci U S A, 117(26), 15253–15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, & Bullmore ET (2013b). Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol, 23(4), 615–624. [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, & Jones SR (2010). The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci, 31(2), 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, & Robbins TW (2008). Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci, 363(1507), 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, & Belin D (2018). Addictive behaviour in experimental animals: Prospects for translation. Philos Trans R Soc B Biol Sci, 373(1742). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, & Robbins TW (2016). Drug addiction: Updating actions to habits to compulsions ten years on. Annu Rev Psychol, 67, 23–50. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, & Kenny PJ (2018). Food addiction: a valid concept? Neuropsychopharmacology, 43(13), 2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB (2015). The nucleus accumbens: An interface between cognition, emotion, and action. Annu Rev Psychol, 66, 25–32. [DOI] [PubMed] [Google Scholar]
- Floresco SB, McLaughlin RJ, & Haluk DM (2008). Opposing roles for the nucleus accumbens core and shell in cue-induced reinstatement of food-seeking behavior. Neuroscience, 154(3), 877–884. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, & See RE (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology, 30(2), 296–309. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, & Surmeier DJ (2011). Modulation of Striatal Projection Systems by Dopamine. Annu Rev Neurosci, 34(1), 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GD, Prasad AA, Jean-Richard-dit-Bressel P, Yau JOY, Millan EZ, Liu Y, Campbell EJ, Lim J, Marchant NJ, Power JM, Killcross S, Lawrence AJ, & McNally GP (2018). Distinct Accumbens Shell Output Pathways Promote versus Prevent Relapse to Alcohol Seeking. Neuron, 98(3), 512–520.e6. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, & Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci, 12(11), 652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2019, January 1). Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology (Berl). Springer Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW (2020). Incubation of food craving in rats: A review. J Exp Anal Behav, 113(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, & See RE (2000). Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology, 22(5), 473–479. [DOI] [PubMed] [Google Scholar]
- Grodin EN, Cortes CR, Spagnolo PA, & Momenan R (2017). Structural deficits in salience network regions are associated with increased impulsivity and compulsivity in alcohol dependence. Drug Alcohol Depend, 179(July), 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, & Haber SN (1993). Organization of the output of the ventral striatopallidal system in the rat: Ventral pallidal efferents. Neuroscience, 57(1), 113–142. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, der Zee E. V.Van, te Kortschot A, & Witter MP (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience, 23(1), 103–120. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AVJ, & Voorn P (1999). Convergence and segregation of ventral striatal inputs and outputs. In Annals of the New York Academy of Sciences (Vol. 877, pp. 49–63). [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, & Stein EA (2011). as Demonstrated by Resting State Functional Connectivity, 53(2), 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio LA, Schmidt HD, & Pierce RC (2015). Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of both cocaine and sucrose seeking in rats. Behav Brain Res, 281, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guercio LA, Wimmer ME, Schmidt HD, Swinford-Jackson SE, Pierce RC, & Vassoler FM (2020). Deep brain stimulation of the infralimbic cortex attenuates cocaine priming-induced reinstatement of drug seeking. Brain Res, 1746(May), 147011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaranen M, Scuppa G, Tambalo S, Järvi V, Bertozzi SM, Armirotti A, Sommer WH, Bifone A, & Hyytiä P (2020). Anterior insula stimulation suppresses appetitive behavior while inducing forebrain activation in alcohol-preferring rats. Transl Psychiatry, 10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad NA, & Knackstedt LA (2014). Addicted to palatable foods: Comparing the neurobiology of Bulimia Nervosa to that of drug addiction. Psychopharmacology (Berl). Springer Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Florence G, Heinsen H, Plantinga BR, Temel Y, Uludag K, Alho E, Teixeira MJ, Amaro E, & Fonoff ET (2017). Subthalamic nucleus deep brain stimulation: Basic concepts and novel perspectives. ENeuro, 4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Gerson JO, & Izquierdo A (2018). Persistent effect of withdrawal from intravenous methamphetamine self-administration on brain activation and behavioral economic indices involving an effort cost. Neuropharmacology, 140, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, & Wohltmann C (1991). Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience, 41(1), 89–125. [DOI] [PubMed] [Google Scholar]
- Heinsbroek JA, Bobadilla AC, Dereschewitz E, Assali A, Chalhoub RM, Cowan CW, & Kalivas PW (2020). Opposing Regulation of Cocaine Seeking by Glutamate and GABA Neurons in the Ventral Pallidum. Cell Rep, 30(6), 2018–2027.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla AC, Kupchik YM, & Kalivas PW (2017). Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. J Neurosci, 37(4), 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JS, Binette AN, Rahman T, Tarantino JD, & Moorman DE (2020). Chemogenetic Inactivation of Orbitofrontal Cortex Decreases Cue-induced Reinstatement of Ethanol and Sucrose Seeking in Male and Female Wistar Rats. Alcohol Clin Exp Res, 44(9), 1769–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JS, & Moorman DE (2020). Orbitofrontal cortex encodes preference for alcohol. ENeuro, 7(4), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L (2020). Addiction is driven by excessive goal-directed drug choice under negative affect: translational critique of habit and compulsion theory. Neuropsychopharmacology, 45(5), 720–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, & Kenny PJ (2008). Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A, 105(49), 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW (2020). Recent perspectives on orexin/hypocretin promotion of addiction-related behaviors. Neuropharmacology, 168(January), 108013. [DOI] [PubMed] [Google Scholar]
- Huang AS, Mitchell JA, Haber SN, Alia-Klein N, & Goldstein RZ (2018). The thalamus in drug addiction: From rodents to humans. Philos Trans R Soc B Biol Sci, 373(1742). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, & Everitt BJ (2004). Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci, 7(4), 389–397. [DOI] [PubMed] [Google Scholar]
- James MH, Mcglinchey EM, Vattikonda A, Mahler SV, & Aston-Jones G (2018). Cued Reinstatement of Cocaine but Not Sucrose Seeking Is Dependent on Dopamine Signaling in Prelimbic Cortex and Is Associated with Recruitment of Prelimbic Neurons That Project to Contralateral Nucleus Accumbens Core. Int J Neuropsychopharmacol, 21(1), 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RJE, & Tunney RJ (2017, March 1). The need for a behavioural analysis of behavioural addictions. Clin Psychol Rev. Elsevier Inc. [DOI] [PubMed] [Google Scholar]
- Jaramillo AA, Randall PA, Stewart S, Fortino B, Van Voorhies K, & Besheer J (2018). Functional role for cortical-striatal circuitry in modulating alcohol self-administration. Neuropharmacology, 130, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis R, Tamashiro-Orrego A, Promes V, Tu L, Shi J, & Yang Y (2020). Cocaine Self-administration and Extinction Inversely Alter Neuron to Glia Exosomal Dynamics in the Nucleus Accumbens. Front Cell Neurosci, 13(January), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, & Yalachkov Y (2014). Factors modulating neural reactivity to drug cues in addiction: A survey of human neuroimaging studies. Neurosci Biobehav Rev, 38(1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJA, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PVVS, … Skolnick P (2003). The Reinforcing Properties of Alcohol are Mediated by GABAAI Receptors in the Ventral Pallidum. Neuropsychopharmacology, 28(12), 2124–2137. [DOI] [PubMed] [Google Scholar]
- Jupp B, & Dalley JW (2014). Convergent pharmacological mechanisms in impulsivity and addiction: insights from rodent models. Br J Pharmacol, 171(20), 4729–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW (2009). The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 10(8), 561–572. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, & Volkow ND (2005). The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry, 162(8), 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, & Seamans J (2005). Unmanageable motivation in addiction: A pathology in prefrontal-accumbens glutamate transmission. Neuron, 45(5), 647–650. [DOI] [PubMed] [Google Scholar]
- Kane L, Venniro M, Quintana-Feliciano R, Madangopal R, Rubio FJ, Bossert JM, Caprioli D, Shaham Y, Hope BT, & Warren BL (2020). Fos-expressing neuronal ensemble in rat ventromedial prefrontal cortex encodes cocaine seeking but not food seeking in rats. Addict Biol, (July), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE (2004). Ventral striatal control of appetitive motivation: Role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev, 27(8), 765–776. [DOI] [PubMed] [Google Scholar]
- Kelley AE, & Domesick VB (1982). The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: An anterograde and retrograde-horseradish peroxidase study. Neuroscience, 7(10), 2321–2335. [DOI] [PubMed] [Google Scholar]
- Kenny PJ (2011, February 1). Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol Biochem Behav. Elsevier Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry, 3(8), 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer ANM, De Vries TJ, & Smit AB (2006). Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem, 98(3), 905–915. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, & Kreitzer AC (2012). Distinct roles for direct and indirect pathway striatal neurons in reinforcement, 15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk M, Mason X, Debacker J, Sharma R, Normandeau CP, Hawken ER, Di Prospero C, Chiang C, Martinez A, Jones AA, Doudnikoff É, Caille S, Bézard E, Georges F, & Dumont ÉC (2013). D1 dopamine receptor-mediated LTP at GABA synapses encodes motivation to self-administer cocaine in rats. J Neurosci, 33(29), 11960–11971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, Chioma VC, & Kalivas PW (2020). The Opioid-Addicted Tetrapartite Synapse. Biol Psychiatry, 87(1), 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, & Kalivas PW Astrocytes as cellular mediators of cue reactivity in addiction, 56 Current Opinion in Pharmacology § (2021). Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruyer A, Scofield MD, Wood D, Reissner KJ, & Kalivas PW (2019). Heroin Cue–Evoked Astrocytic Structural Plasticity at Nucleus Accumbens Synapses Inhibits Heroin Seeking. Biol Psychiatry, 86(11), 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn BN, Kalivas PW, & Bobadilla AC (2019). Understanding Addiction Using Animal Models. Front Behav Neurosci, 13(November), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, & Kalivas PW (2013). The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: An electrophysiological study. Brain Struct Funct, 218(6), 1487–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, & Gould TJ (2016, October 1). Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: Contributions to development and maintenance of addiction. Learn Mem. Cold Spring Harbor Laboratory Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, & Sohal VS (2014). A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci, 34(35), 11519–11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coehlo M, McGregor HA, Waltermire RS, & Szumlinski KK (2015). Binge alcohol drinking elicits persistent negative affect in mice. Behav Brain Res, 291, 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, Smith ACW, Ramakrishnan A, Lyu Y, Bastle RM, Martin JA, Mitra S, O’Connor RM, Wang ZJ, Molina H, Turecki G, Shen L, … Maze I (2020). Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science (80- ), 368(6487), 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, & Zangen A (2007). Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci, 27(51), 14179–14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Caprioli D, & Marchant NJ (2015, September 1). Recent updates on incubation of drug craving: A mini-review. Addict Biol. Blackwell Publishing Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li J, Peng XQ, Spiller K, Gardner EL, & Xi ZX (2009). Metabotropic glutamate receptor 7 modulates the rewarding effects of cocaine in rats: Involvement of a ventral pallidal gabaergic mechanism. Neuropsychopharmacology, 34(7), 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, & Markou A (2007). Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci, 27(34), 9077–9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Chefer S, Lu H, Guillem K, Rea W, Kurup PK, Yang Y, Peoples L, & Stein EA (2013). Dorsolateral caudate nucleus differentiates cocaine from natural reward-associated contextual cues. Proc Natl Acad Sci U S A, 110(10), 4093–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Williamson V, Setlow B, Cottler LB, & Knackstedt LA (2018, November 1). The importance of considering polysubstance use: lessons from cocaine research. Drug Alcohol Depend. Elsevier Ireland Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, & Nestler EJ (2011). The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. [DOI] [PMC free article] [PubMed]
- Lopez MF, Moorman DE, Aston-Jones G, & Becker HC (2016). The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res, 1636, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, & Malenka RC (2011). Drug-Evoked Synaptic Plasticity in Addiction: From Molecular Changes to Circuit Remodeling. Neuron, 69(4), 650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, Xu H-S, Fu X-M, Hu X, & Zhang D-R (2009). Addiction Related Alteration in Resting-state Brain Connectivity. [DOI] [PMC free article] [PubMed]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, & Dong Y (2014). Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron, 83(6), 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Brown RM, Short JL, & Lawrence AJ (2012). Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. J Physiol, 590(10), 2427–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Pelloux Y, Bossert JM, & Shaham Y (2019). Context-induced relapse after extinction versus punishment: similarities and differences. Psychopharmacology (Berl), 236(1), 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, & McNally GP (2009). Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci, 29(5), 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, & Shaham Y (2013). Recent developments in animal models of drug relapse. Curr Opin Neurobiol, 23(4), 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, & Bonci A (2006). Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci, 9(7), 868–869. [DOI] [PubMed] [Google Scholar]
- Mascia P, Neugebauer NM, Brown J, Bubula N, Nesbitt KM, Kennedy RT, & Vezina P (2019). Exposure to conditions of uncertainty promotes the pursuit of amphetamine. Neuropsychopharmacology, 44(2), 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, & Morrell JI (2005). Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience, 135(2), 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, & Martin-Fardon R (2018, August 28). Drug seeking and relapse: New evidence of a role for orexin and dynorphin co-transmission in the paraventricular nucleus of the thalamus. Front Neurol. Frontiers Media S.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald AJ (1991). Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience, 44(1), 15–33. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, & Kalivas PW (2004). Limbic and Motor Circuitry Underlying Footshock-Induced Reinstatement of Cocaine-Seeking Behavior. J Neurosci, 24(7), 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, & Kalivas PW (2001). The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci, 21(21), 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C, & Aston-Jones G (2016). Prelimbic to Accumbens Core Pathway Is Recruited in a Dopamine-Dependent Manner to Drive Cued Reinstatement of Cocaine Seeking. J Neurosci, 36(33), 8700–8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath AG, & Briand LA (2019, December 1). A potential role for microglia in stress- and drug-induced plasticity in the nucleus accumbens: A mechanism for stress-induced vulnerability to substance use disorder. Neurosci Biobehav Rev. Elsevier Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendrick G, & Graziane NM (2020). Drug-Induced Conditioned Place Preference and Its Practical Use in Substance Use Disorder Research. Front Behav Neurosci, 14(September), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, & Kleber HD (2000). Drug Dependence, a Chronic Medical Illness: Implications for Treatment, Insurance, and Outcomes Evaluation. J Am Med Assoc, 284(13), 1689–1695. [DOI] [PubMed] [Google Scholar]
- Meier A, Moore SK, Saunders EC, McLeman B, Metcalf SA, Auty S, Walsh O, & Marsch LA (2020). Understanding the increase in opioid overdoses in New Hampshire: A rapid epidemiologic assessment. Drug Alcohol Depend, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihov Y, & Hurlemann R (2012). Altered amygdala function in nicotine addiction: Insights from human neuroimaging studies. Neuropsychologia, 50(8), 1719–1729. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, & Janak PH (2017). Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience, 360, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeyaert B, Holt G, Madangopal R, Perez-Alvarez A, Fearey BC, Trojanowski NF, Ledderose J, Zolnik TA, Das A, Patel D, Brown TA, Sachdev RNS, Eickholt BJ, Larkum ME, Turrigiano GG, Dana H, Gee CE, … Schreiter ER (2018). Improved methods for marking active neuron populations. Nat Commun, 9(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, & Aston-Jones G (2015). Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res, 1628, 130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, & Margolis EB (2017). Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci, 18(2), 73–85. [DOI] [PubMed] [Google Scholar]
- Moschak TM, Wang X, & Carelli RM (2018). A Neuronal Ensemble in the Rostral Agranular Insula Tracks Cocaine-Induced Devaluation of Natural Reward and Predicts Cocaine Seeking. [DOI] [PMC free article] [PubMed]
- Muller Ewald VA, & LaLumiere RT (2018). Neural systems mediating the inhibition of cocaine-seeking behaviors. Pharmacol Biochem Behav, 174, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Gray SM, & Ghitza UE (2006). Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol Behav, 88(4–5), 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall RW, Craig AR, Browning KO, & Shahan TA (2018). Longer treatment with alternative non-drug reinforcement fails to reduce resurgence of cocaine or alcohol seeking in rats. Behav Brain Res, 341, 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall RW, & Shahan TA (2020). Resurgence of Punishment-Suppressed Cocaine Seeking in Rats. Exp Clin Psychopharmacol, 28(3), 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, & Bechara A (2014). The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci, 1316(1), 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhofer DN, & Kalivas PW (2018). Metaplasticity at the addicted tetrapartite synapse: A common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiol Learn Mem, 154, 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan ML, Shamasunder S, Colon-Berezin C, Kunins HV, & Paone D (2019). Increased Presence of Fentanyl in Cocaine-Involved Fatal Overdoses: Implications for Prevention. J Urban Heal, 96(1), 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, & Eisch AJ (2010). Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci, 30(1), 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, & Lüscher C (2015). Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron, 88(3), 553–564. [DOI] [PubMed] [Google Scholar]
- Oliva I, & Wanat MJ (2016). Ventral tegmental area afferents and drug-dependent behaviors. Front Psychiatry, 7(MAR), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Colon-Perez LM, Heshmati SC, Setlow B, & Febo M (2018). Functional connectivity of chronic cocaine use reveals progressive neuroadaptations in neocortical, striatal, and limbic networks. ENeuro, 5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo P, Leao RM, Bianchi PC, de Oliveira PEC, Planeta C. da S., & Cruz FC (2017). Inactivation of the Prelimbic Cortex Impairs the Context-Induced Reinstatement of Ethanol Seeking. Front Pharmacol, 8(OCT), 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrilla-Carrero J, Buchta WC, Goswamee P, Culver O, McKendrick G, Harlan B, Moutal A, Penrod R, Lauer A, Ramakrishnan V, Khanna R, Kalivas P, & Riegel AC (2018). Restoration of Kv7 channel-mediated inhibition reduces cued-reinstatement of cocaine seeking. J Neurosci, 38(17), 4212–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O’connor EC, & Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature, 509(7501), 459–464. [DOI] [PubMed] [Google Scholar]
- Paulus MP (2007). Decision-making dysfunctions in psychiatry - Altered homeostatic processing? Science (80- ), 318(5850), 602–606. [DOI] [PubMed] [Google Scholar]
- Paulus MP, & Stewart JL (2014). Interoception and drug addiction. Neuropharmacology, 76(PART B), 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, & Baunez C (2013). Deep brain stimulation for addiction: Why the subthalamic nucleus should be favored. Curr Opin Neurobiol, 23(4), 713–720. [DOI] [PubMed] [Google Scholar]
- Peters J, & Kalivas PW (2006). The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl), 186(2), 143–149. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, & Quirk GJ (2009). Extinction circuits for fear and addiction overlap, (787), 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, & Kalivas PW (2008). Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci, 28(23), 6046–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfarr S, Schaaf L, Reinert JK, Paul E, Herrmannsdörfer F, Roßmanith M, Kuner T, Hansson AC, Spanagel R, Körber C, & Sommer WH (2018). Choice for drug or natural reward engages largely overlapping neuronal ensembles in the infralimbic prefrontal cortex. J Neurosci, 38(14), 3507–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Rinker JA, Lowery-Gionta EG, Mazzone CM, McCall NM, Kendra AM, Olson DP, Lowell BB, Grant KA, Thiele TE, & Kash TL (2015). NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat Neurosci, 18(4), 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, Xie C, Chaichim C, Nguyen JH, McClusky HE, Killcross S, Power JM, & McNally GP (2020). Complementary roles for ventral pallidum cell types and their projections in relapse. J Neurosci, 40(4), 880–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast ML, Podus D, Finney J, Greenwell L, & Roll JM (2006). Contingency management for treatment of substance use disorders: A meta-analysis. Addiction, 101(11), 1546–1560. [DOI] [PubMed] [Google Scholar]
- Riaz S, Schumacher A, Sivagurunathan S, Van Der Meer M, & Ito R (2017). Ventral, but not dorsal, hippocampus inactivation impairs reward memory expression and retrieval in contexts defined by proximal cues. Hippocampus, 27(7), 822–836. [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, Kash TL, Navarro M, & Thiele TE (2017). Extended Amygdala to Ventral Tegmental Area Corticotropin-Releasing Factor Circuit Controls Binge Ethanol Intake. Biol Psychiatry, 81(11), 930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Bobadilla AC, Heinsbroek JA, Neuhofer DN, & Kalivas PW (2018). Drug refraining and seeking potentiate synapses on distinct populations of accumbens medium spiny neurons. J Neurosci, 38(32), 7100–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Heinsbroek JA, Spencer SM, Bobadilla AC, Smith ACW, Gipson CD, & Kalivas PW (2019). Transient synaptic potentiation in nucleus accumbens shell during refraining from cocaine seeking. Addict Biol, (March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2000). The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction, 95(8). [DOI] [PubMed] [Google Scholar]
- Rogers-Carter MM, Djerdjaj A, Gribbons KB, Varela JA, & Christianson JP (2019). Insular cortex projections to nucleus accumbens core mediate social approach to stressed juvenile rats. J Neurosci, 39(44), 8717–8729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, & See RE (2007). Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem, 87(4), 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, & Napier TC (2015). The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol, 130, 29–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, & Baunez C (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci U S A, 107(3), 1196–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruisoto P, & Contador I (2019). The role of stress in drug addiction. An integrative review. Physiol Behav, 202(January), 62–68. [DOI] [PubMed] [Google Scholar]
- Russo SJ, & Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci, 14(9), 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, & Weber SM (2003, April 1). Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: Implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. J Pharmacol Exp Ther. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Voorn P, Binnekade R, Schoffelmeer ANM, & De Vries TJ (2005). Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci, 22(9), 2347–2356. [DOI] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, & Kalivas PW (2015). Gq-DREADD selectively initiates glial glutamate release and inhibits cue-induced cocaine seeking. Biol Psychiatry, 78(7), 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer SM, Smith ACW, Roberts-Wolfe DJ, & Kalivas PW (2016). The nucleus accumbens: Mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev, 68(3), 816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Fuchs R. a, Ledford CC, & McLaughlin J (2003). Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci, 985, 294–307. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, & Bunney BS (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract‐tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol, 290(2), 213–242. [DOI] [PubMed] [Google Scholar]
- Shalev U, Morales M, Hope B, Yap J, & Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl), 156(1), 98–107. [DOI] [PubMed] [Google Scholar]
- Shen HW, Gipson CD, Huits M, & Kalivas PW (2014). Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology, 39(5), 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Bevan MD, Bolam JP, & Smith Y (1996). The subthalamic nucleus and the external pallidum: Two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience, 73(2), 335–357. [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang CJ, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, Kimchi EY, Heiman M, & Tye KM (2019). A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science (80- ), 366(6468), 1008–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, & Tye KM (2019). Leveraging calcium imaging to illuminate circuit dysfunction in addiction. Alcohol, 74, 47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Haass-Koffler CL, Bito-Onon JJ, Li R, & Bartlett SE (2012). Mifepristone in the Central Nucleus of the Amygdala Reduces Yohimbine Stress-Induced Reinstatement of Ethanol-Seeking. Neuropsychopharmacology, 37(4), 906–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair CM, Cleva RM, Hood LE, Olive MF, & Gass JT (2012). mGluR5 receptors in the basolateral amygdala and nucleus accumbens regulate cue-induced reinstatement of ethanol-seeking behavior. Pharmacol Biochem Behav, 101(3), 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, & Berridge KC (2009). Ventral pallidum roles in reward and motivation. Behav Brain Res, 196(2), 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solecki WB, Szklarczyk K, Pradel K, Kwiatkowska K, Dobrzański G, & Przewłocki R (2018). Noradrenergic signaling in the VTA modulates cocaine craving. Addict Biol, 23(2), 596–609. [DOI] [PubMed] [Google Scholar]
- Solecki WB, Wilczkowski M, Pradel K, Karwowska K, Kielbinski M, Drwięga G, Zajda K, Blasiak T, Soltys Z, Rajfur Z, Szklarczyk K, & Przewłocki R (2019). Effects of brief inhibition of the ventral tegmental area dopamine neurons on the cocaine seeking during abstinence. Addict Biol, (November 2018), 1–13. [DOI] [PubMed] [Google Scholar]
- Spencer SM, Brown RM, Quintero GC, Kupchik YM, Thomas CA, Reissner KJ, & Kalivas PW (2014). A2Δ-1 Signaling in Nucleus Accumbens Is Necessary for Cocaine-Induced Relapse. J Neurosci, 34(25), 8605–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, Mcelligott ZA, Decot H, & Stuber GD (2014). Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology, 76(PART B), 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, & Kalivas PW (2013). Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front Behav Neurosci, 7(DEC), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struik RF, Marchant NJ, de Haan R, Terra H, van Mourik Y, Schetters D, Carr MR, van der Roest M, Heistek TS, & De Vries TJ (2019). Dorsomedial prefrontal cortex neurons encode nicotine-cue associations. Neuropsychopharmacology, 44(12), 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, & Rebec GV (2005). Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology, 30(11), 2073–2081. [DOI] [PubMed] [Google Scholar]
- Tavakkolifard M, Vousooghi N, Mahboubi S, Golab F, Ejtemaei Mehr S, & Zarrindast MR (2020). Evaluation of the relationship between the gene expression level of orexin-1 receptor in the rat blood and prefrontal cortex, novelty-seeking, and proneness to methamphetamine dependence: A candidate biomarker. Peptides, 131(July), 170368. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koós T, & Ibáñez-Sandoval O (2010). Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat, 4(DEC), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Umegaki H, & Volkow ND (2008). D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse, 62(7), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, & Hitzemann R (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem, 78(5), 1094–1103. [DOI] [PubMed] [Google Scholar]
- Tillage RP, Wilson GE, Cameron Liles L, Holmes PV, & Weinshenker D (2020). Chronic environmental or genetic elevation of galanin in noradrenergic neurons confers stress resilience in mice. J Neurosci, 40(39), 7464–7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, & Volkow ND (2015). Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: Association to striatal D2/D3 receptors Act. Hum Brain Mapp, 36(1), 120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Cebrían C, & Mengual E (2010). Axonal branching patterns of nucleus accumbens neurons in the rat. J Comp Neurol, 518(22), 4649–4673. [DOI] [PubMed] [Google Scholar]
- Tsai P-J, Keeley RJ, Carmack SA, Vendruscolo JCM, Lu H, Gu H, Vendruscolo LF, Koob GF, Lin C-P, Stein EA, & Yang Y (2020). Converging Structural and Functional Evidence for a Rat Salience Network. Biol Psychiatry, (16), 1–12. [DOI] [PubMed] [Google Scholar]
- Van Den Oever MC, Goriounova NA, Wan Li K, Van Der Schors RC, Binnekade R, Schoffelmeer ANM, Mansvelder HD, Smit AB, Spijker S, & De Vries TJ (2008). Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci, 11(9), 1053–1058. [DOI] [PubMed] [Google Scholar]
- Venniro M, Banks ML, Heilig M, Epstein DH, & Shaham Y (2020). Improving translation of animal models of addiction and relapse by reverse translation. Nat Rev Neurosci, 21(11), 625–643. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2016). Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Progress in Brain Research (1st ed., Vol. 224). Elsevier B.V. [DOI] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, & Shaham Y (2019, January 1). Novel models of drug relapse and craving after voluntary abstinence. Neuropsychopharmacology. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Caprioli D, Zhang M, Whitaker LR, Zhang S, Warren BL, Cifani C, Marchant NJ, Yizhar O, Bossert JM, Chiamulera C, Morales M, & Shaham Y (2017). The Anterior Insular Cortex→Central Amygdala Glutamatergic Pathway Is Critical to Relapse after Contingency Management. Neuron, 96(2), 414–427.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, & Goldstein RZ (2011a). Addiction: Pulling at the Neural Threads of Social Behaviors. Neuron, 69(4), 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, & Goldstein RZ (2002). Role of dopamine, the frontal cortex and memory circuits in drug addiction: Insight from imaging studies. Neurobiol Learn Mem, 78(3), 610–624. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, Fowler JS, Wong CJ, Yin P, & Du C (2014). Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry, 19(9), 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, & Baler RD (2011b). Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, & Pappas N (1997). Decreased striatal dopaminergic responsiveness in detoxified cocaine- dependent subjects. Nature, 386(6627), 830–833. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, & Tomasi D (2012). Addiction circuitry in the human brain. Annu Rev Pharmacol Toxicol, 52(1), 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, & Telang F (2011c). Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci U S A, 108(37), 15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Hand LJ, Letherby B, Huckstep KL, Campbell EJ, & Lawrence AJ (2020). Cocaine and amphetamine regulated transcript (CART) signalling in the central nucleus of the amygdala modulates stress-induced alcohol seeking. Neuropsychopharmacology, (July). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Tai F, Yu P, & Wu R (2012). Reinforcing properties of pups versus cocaine for fathers and associated central expression of Fos and tyrosine hydroxylase in mandarin voles (Microtus mandarinus). Behav Brain Res, 230(1), 149–157. [DOI] [PubMed] [Google Scholar]
- Wang TR, Moosa S, Dallapiazza RF, Elias WJ, & Lynch WJ (2018). Deep brain stimulation for the treatment of drug addiction. Neurosurg Focus, 45(2), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Kane L, Venniro M, Selvam P, Richard Quintana-Feliciano X, Mendoza MP, Madangopal R, Komer L, Whitaker LR, Javier Rubio F, Bossert JM, Caprioli D, Shaham Y, & Hope BT (2019). Separate vmPFC Ensembles Control Cocaine Self-Administration Versus Extinction in Rats. J Neurosci, 39(37), 7394–7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Mendoza MP, Cruz FC, Leao RM, Caprioli D, Rubio FJ, Whitaker LR, McPherson KB, Bossert JM, Shaham Y, & Hope BT (2016). Distinct fos-expressing neuronal ensembles in the ventromedial prefrontal cortex mediate food reward and extinction memories. J Neurosci, 36(25), 6691–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, & Izquierdo A (2015). The basolateral amygdala in reward learning and addiction. Neurosci Biobehav Rev, 57, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Koros E, Czyrak A, Chocyk A, Czepiel K, Fijal K, Mackowiak M, Rogowski A, Kostowski W, & Bienkowski P (2003). Different pattern of brain c-Fos expression following re-exposure to ethanol or sucrose self-administration environment. Naunyn Schmiedebergs Arch Pharmacol, 368(5), 331–341. [DOI] [PubMed] [Google Scholar]
- Whitaker LR, & Hope BT (2018). Chasing the addicted engram: Identifying functional alterations in Fos-expressing neuronal ensembles that mediate drug-related learned behavior. Learn Mem, 25(9), 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2006). Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc B Biol Sci. Royal Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch AM, Yager LM, Donckels EA, Le CT, Neumaier JF, & Ferguson SM (2017). Chemogenetic inhibition reveals midline thalamic nuclei and thalamo-accumbens projections mediate cocaine-seeking in rats. Eur J Neurosci, 46(3), 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Donckels EA, & Ferguson SM (2019). Chemogenetic inhibition of direct pathway striatal neurons normalizes pathological, cue-induced reinstatement of drug-seeking in rats. Addict Biol, 24(2), 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ARST, Heon SY, Mamczarz J, June HL, Hwang BH, & June HL (2009). Deficits in substance P mRNA levels in the CeA are inversely associated with alcohol-motivated responding. Synapse, 63(11), 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Záborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, & Palkovits M (1985). Cholecystokinin innervation of the ventral striatum: A morphological and radioimmunological study. Neuroscience (Vol. 14). 10.1016/0306-4522(85)90302-1 [DOI] [PubMed] [Google Scholar]
- Zahm DS (1999). Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann N Y Acad Sci, 877(1 ADVANCING FRO), 113–128. [DOI] [PubMed] [Google Scholar]
- Zombeck JA, Chen GT, Johnson ZV, Rosenberg DM, Craig AB, & Rhodes JS (2008). Neuroanatomical specificity of conditioned responses to cocaine versus food in mice. Physiol Behav, 93(3), 637–650. [DOI] [PubMed] [Google Scholar]


