Abstract
BRCA1-associated protein 1 (BAP1) is emerging as an intensively studied cancer-associated gene. Germline mutations in BAP1 lead to a cancer syndrome, and somatic loss is found in several cancer types. BAP1 encodes a deubiquitinase enzyme, which plays key roles in cell cycle regulation, cell death, and differentiation. Recent studies have demonstrated that BAP1 is also involved in several aspects of cellular metabolism, including metabolic homeostasis, glucose utilization, control of ferroptosis, and stress response. A better knowledge of the metabolic roles of cancer-associated genes is important to understanding tumor initiation and progression, as well as highlighting potential therapeutic avenues. With this review, we summarize the current knowledge regarding BAP1-mediated regulation of metabolic activities that may support new strategies to treat BAP1-mutated cancers.
Keywords: BAP1, metabolism, cancer, tumor suppressor, ubiquitination
BAP1, a tumor suppressor
BRCA1-associated protein 1 (BAP1) is a tumor suppressor gene located on chromosome 3p21.1 (1). The encoded product, BAP1, was initially identified as a protein that binds to BRCA1 (2), but now is more widely recognized as a deubiquitinating enzyme (DUB). It’s N-terminal ubiquitin carboxyl hydrolase domain is linked to a C-terminal region that includes a ubiquitin-like domain, two nuclear localization signals, and binding motifs that mediate interactions with host cell factor 1 (HCF-1), BARD1 and ASXL1/2 (3–5) . BAP1 exerts tumor suppressive functions on, cell cycle control, DNA damage repair, and differentiation (4–7).
Metabolism reprogramming is a hallmarks of cancer (8). Studies have demonstrated that oncogene activation and/or tumor suppressor gene inactivation alter cancer cell metabolism for tumor formation and progression (8,9). For example, TP53 mutations contribute to increased glycolysis and redox homeostasis (10). Recent findings highlight BAP1 roles in cancer cell metabolism. Here, we briefly review the BAP1 loss in cancer types and its effects as a DUB and a transcriptional regulator, and refer readers to Carbone and colleague’s recent review (11) for more in depth coverage. Rather, we mainly focus our attention of BAP1 effects on cellular metabolism.
Genetic alterations of BAP1 in cancer
Somatic genetic lesions that alter BAP1 expression or its protein activity contribute to the development and progression of several cancers. BAP1 loss come from chromosomal deletions and/or inactivating mutations, including deletion, frameshift, nonsense, and missense mutations (2,12,13). Both whole-gene copy loss and mutations have been identified in BAP1-deficient malignancies, which commonly appear with a single copy loss (loss of heterozygosity), and a truncating or inactivating point mutation (14,15) (Figure 1A). There is also a strong association between germline BAP1 mutations and cancer susceptibilities including uveal melanoma (UM), malignant mesothelioma (MM), renal cell carcinoma (RCC), cutaneous melanoma (CM) and other tumors, that defined as “BAP1 cancer syndrome” (11,16,17).
Figure 1. BAP1 somatic alterations and BAP1 roles in cellular processes.
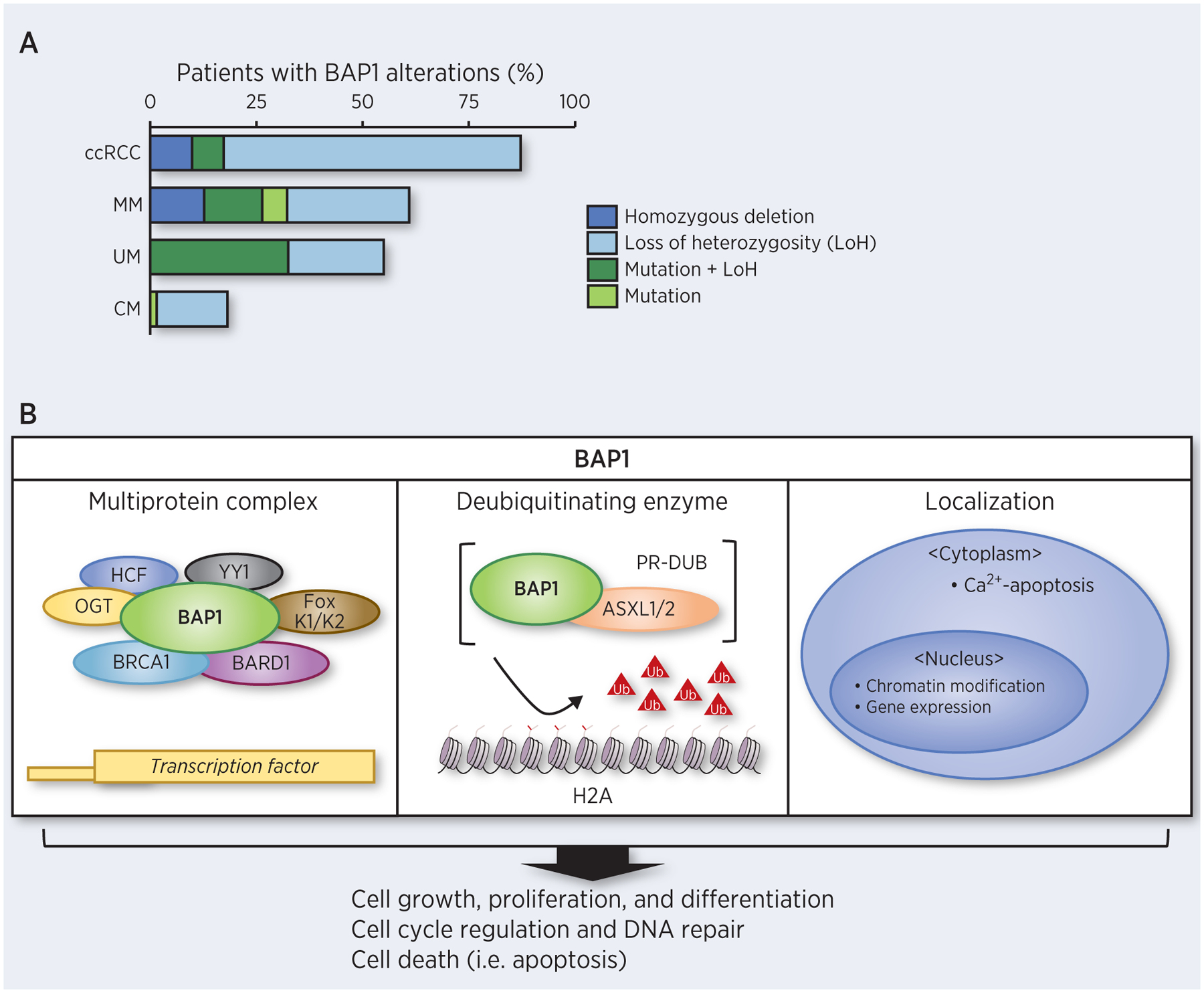
A. Frequency of patients with BAP1 alterations from TCGA Firehose cohort data (14) for clear cell renal cell carcinoma (ccRCC), malignant mesothelioma (MM), uveal melanoma (UM) and cutaneous melanoma (CM). Dark colors represent alterations that resulted in no functional copies. B. BAP1 is involved in different cellular pathways controlling cell growth, differentiation, and survival.
Uveal melanoma:
Metastatic UM is the most lethal intraocular cancer in adults. Oncogenic mutations in guanine nucleotide-binding proteins (GNAQ/GNA11) occur early in UM. Monosomy 3, which reduces BAP1 copy number, and inactivating BAP1 mutations are highly associated with advanced UM and poor prognosis (18,19). A compelling case study of BAP1 cancer syndrome can be found in UM, where both germline and somatic BAP1 mutations have discovered (20). UM can be divided into two prognostic subsets, either class 1 (low metastatic risk) or class 2 (high metastatic risk) (21). Approximately 80% of metastasized UM carry BAP1 mutations and BAP1 mutations are strongly associated with poor prognosis in UM (13,22). This correlation between BAP1 mutations and metastatic UM has been linked to various potential underlying molecular mechanisms (23,24). For example, BAP1 deletion in UM cells induced stem-like features and loss of differentiation (24). When BAP1-deficient UM cells are treated with histone deacetylase inhibitors, ubiquitinated H2A levels increase and tumor features become switched towards a class 1-like phenotype (23). Additionally, BAP1 loss is related to DNA methylation repatterning in aggressive UM (25). Furthermore, BAP1 loss alone or aberrations in BAP1 copy number are associated with significant changes in the transcriptome of advanced UM (26), potentially implicating BAP1 in transcriptional regulatory events that contribute to UM progression and metastasis.
Malignant mesothelioma:
Another cancer type where BAP1 mutations are important is malignant mesothelioma (MM). Similar to UM, BAP1 deletion and mutation lead to inactivation of both copies of the BAP1 gene in MM. Approximately 50% of MM patients harbor somatic BAP1 biallelic mutations, including single nucleotide mutations and larger deletions (27,28). BAP1 alteration is involved in early tumor development in MM, whereas in UM, BAP1 loss likely occurs after the acquisition of either a GNAQ or GNA11 mutation (19). BAP1 mutations themselves are strong factors for tumorigenesis; however, in BAP1-related cancers such as MM (29,30), they are significantly correlated with environmental carcinogens (i.e. asbestos), suggesting the importance of gene-environmental factor interactions (11,17,31). BAP1+/− mice show a high incidence of MM after exposure to low doses of asbestos that rarely causes MM in wild type mice (29).
The presence of germline BAP1 mutations are associated with improved survival of MM patients (11,27,32), highlighting the prognostic importance of BAP1 germline mutations in MM. Additionally, pleural MM patients with germline BAP1 mutations show a higher sensitivity to chemotherapy (33). The possibility of BAP1 serving as a predictive biomarker of immunotherapy in MM has been suggested (11,34,35). Moreover, BAP1 mutations may elevate tumor responsiveness to immunotherapy (36). In a recent clinical application, early detection screening for germline BAP1 mutations is now being utilized to identify individuals and their family members who carry BAP1 germline mutations and to predict risk of MM (37,38).
Renal cell carcinoma(RCC):
RCC encompasses a heterogeneous group of cancers, with clear cell RCC (ccRCC) constituting the most common subtype (39). Approximately 15% of ccRCC patients carry somatic BAP1 mutations, which are associated with high tumor grade and poor prognosis (40,41). Similar to UM, changes in aneuploidy for chromosome 3 occur in ccRCC; however, the 3p arm is typically lost rather than the whole chromosome. In RCC, inactivating BAP1 mutations are associated with mTORC1 activation (40). Patients with BAP1 mutations identified from primary tumors had an inferior response to mTOR inhibitor as a treatment for metastatic disease (39). Also, inactivating BAP1 mutations induce chromosomal instability and aneuploidy by decreasing expression of microspherule protein 1 (42). In general, RCC responds poorly to radiation therapy but BAP1 loss sensitizes RCC cells to chemoradiation and PARP inhibitors, thereby improving therapeutic response (40).
Cutaneous melanoma, melanocytic tumors and other skin tumors:
The frequency of cutaneous melanoma (CM) development is likely to increase with germline BAP1 mutations and tumor-promoting effects of these BAP1 mutations are strongly influenced by co-occurrence of associated oncogenes such as mutant BRAF (2,43). Kumar and colleagues discovered that distinct and apparently opposing effects of BAP1 on cell growth are cell type-dependent (44). In melanoma cells, genetic deletion of BAP1 decreases cell proliferation and tumor growth in vivo; however, BAP1 overexpression in non-transformed melanocytes likewise decreases proliferation (44). The molecular mechanisms responsible for these seemingly contradictory effects of BAP1 on cell proliferation remain unknown.
Another common and benign cancer type coming from germline BAP1 mutations is melanocytic BAP1-associated intradermal tumors (MBAITs) (16). MBAITs display specific molecular and histological features compared to atypical Spitz tumors and other melanocytic lesions (16) and screening for MBAITs provides the chance to identify the possibility of germline BAP1 mutations of patients (45). In addition, BAP1 mutations also cause basal cell carcinoma (BCC, 6.3% frequency) and squamous cell carcinoma (SCC, 1.1% frequency) in BAP1+/− carriers (11).
Cellular roles of BAP1 in cancer
BAP1-dependent multi-protein complexes:
The majority of cellular BAP1 is present in multiprotein complexes that link BAP1 to transcription factors (TFs) and other regulators (Figure 1B) (46). Complex formation between BAP1 and the cell cycle regulator, HCF-1, bridges HCF-1 to histone-modifying complexes to regulate cell growth (2,4). HCF-1 mediates these effects, at least in part, through activation of E2F-TFs, regulating cell cycle progression (47,48). BAP1 loss induces a modest over-accumulation of HCF-1, leading G1 to S phase cell cycle transition, promoting cell proliferation (4). Additionally, BAP1/HCF-1 complex can recruit either O-linked N-acetylglucosamine transferase (OGT), the Ying Yang 1 transcriptional repressor (YY1), or forkhead transcription factors (FOXK1/K2) to form distinct multiprotein complexes (6,7,49). Within the BAP1/HCF-1/OGT complex, HCF1 is modified by O-GlcNacylation, the addition of single O-linked N-acetylglucosamine moiety to specific serine or threonine residues (50,51). O-GlcNacylated HCF-1 recruits OGT and BAP1 to activate peroxisome proliferator activator receptor gamma coactivator 1α (PGC1α), stimulating gluconeogenesis (49). Alternatively, BAP1/HCF-1/YY1 complexes regulate cellular proliferation by binding to and regulating the promoter of COX7C, encodes mitochondrial respiratory chain components (6). Ternary protein complexes of BAP1, HCF-1, and FOXK1/K2 regulates transcriptional regulatory programs in cell proliferation and cell cycle control (7).
As part of a multiprotein complex, BAP1 also functions in DNA damage repair through both BRCA1-dependent and –independent mechanisms (2). BAP1 binds to the BRCA1-associated RING domain protein 1 (BARD1) and inhibits E3 ubiquitin ligase activity of the BRCA1/BARD1 complex, which modulates the DNA damage response (5). Contrasting reports indicate that BAP1-associated cell growth repression is independent of wild-type BRCA1 (2,52), although the molecular mechanisms underlying this effect have yet to be fully identified.
BAP1 deubiquitinating function:
The removal of ubiquitin moieties from proteins alters stability, activity and/or intracellular localization. Through its catalytic activity as a DUB, BAP1 influences diverse cellular processes. BAP1 is required for the Polycomb repressive complex (PRC) and Polycomb group repressive deubiquitinase complex (PR-DUB) formation (53). In the nucleus, BAP1 interacts with ASXL1/2 to form the PR-DUB, leading chromatin modification for epigenetic gene regulation through deubiquitination of histone H2A at Lys119 and HCF-1 (3,54). Cancer-associated BAP1 mutations lead loss of DUB activity and impair function of the BAP1-ASXL1/2 complex, causing an elevation in H2A ubiquitination levels, and subsequent cell cycle progression dysregulation (3). Additionally, BAP1 deubiquitinates the lysine residues of HCF-1 to recruit multiprotein complex partners (i.e. OGT and PGC1α) and stabilize these partners, thereby potentiating transcriptional regulatory effects (49).
Subcellular localization-dependent BAP1 activities:
The subcellular localization of BAP1 may important in its tumor suppressive activity (52,55). Originally, the tumor suppressive effects of BAP1 were linked to its nuclear localization, indicating that BAP1 DUB activity predominantly regulates nuclear targets that are involved in gene transcription and related cellular processes (52). BAP1 was recently shown to act as tumor suppressor in the cytoplasm by participating in cellular calcium release (55). This observation indicates that BAP1 might exert distinct tumor suppressive effects in a context-dependent manner within different subcellular compartments.
Functions of BAP1 in metabolism and tumorigenesis
Given the increased evidence of metabolic reprogramming playing an important role tumor development and progression, and therapeutic responses in cancer (56), it is critical to understand BAP1 functions in cellular metabolism. We categorize the roles of BAP1 in normal and cancer cell metabolism into four main themes (Figure 2A). Within each theme, we highlight current gaps in knowledge and challenges for the field.
Figure 2. The diverse metabolic functions of BAP1.
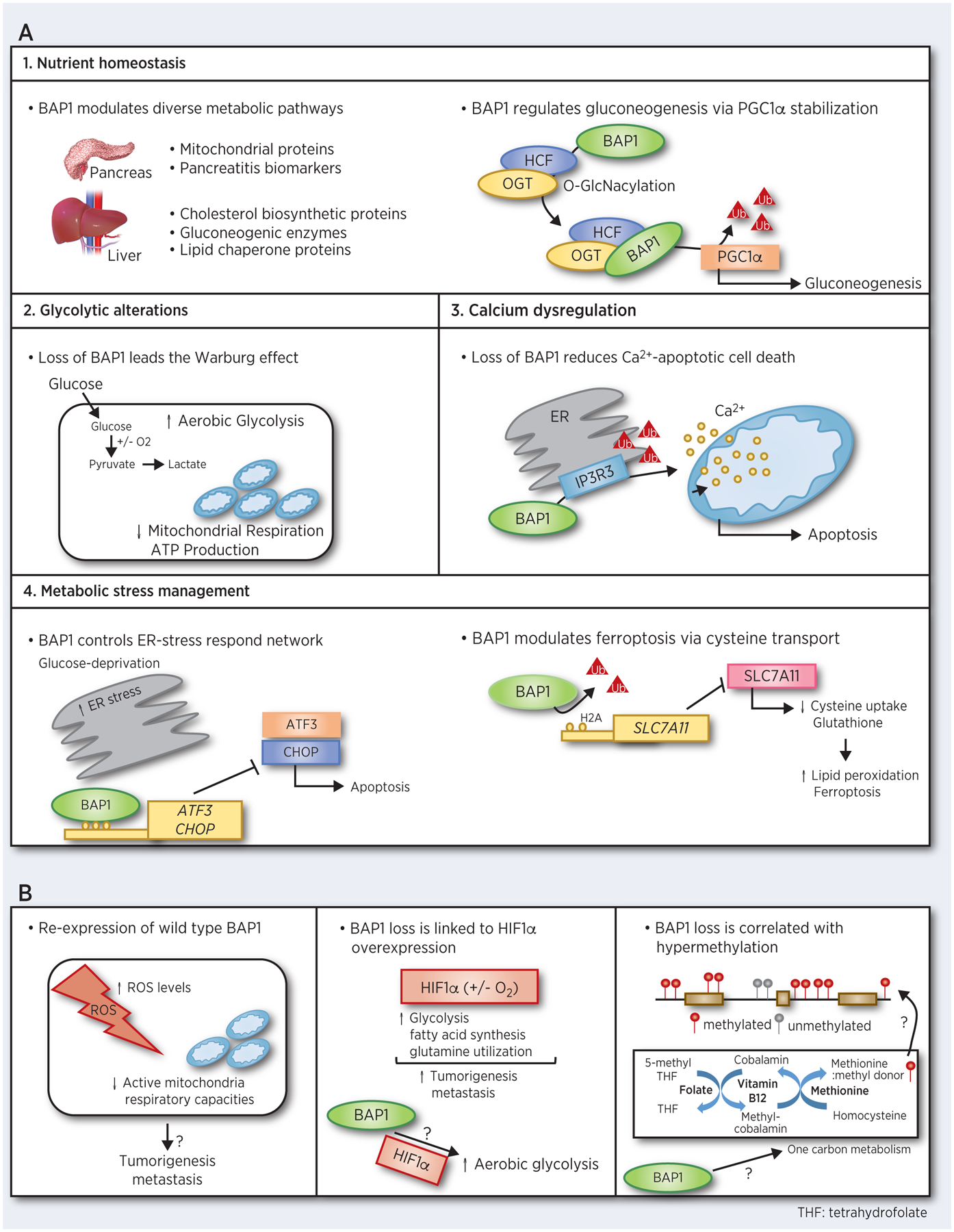
A. BAP1 plays role in multiple metabolic pathways. B. Additional potential BAP1 involvement in metabolic pathways that may explain the effects of BAP1 loss.
Nutrient homeostasis:
The precise regulation of nutrients is important to maintain healthy cellular conditions; thus, imbalances develop disease including cancer (56,57). A genetically engineered inducible Bap1 knockout model demonstrated that Bap1 regulates glucose and cholesterol metabolism in the pancreas and liver (58). Minimal overlap in the observed metabolic protein alterations caused by Bap1 deletion was observed in different tissues (58). In the pancreas, knockdown of Bap1 diminished mitochondrial protein levels (e.g. Cox6c, Trap1, and Suox) and upregulated pancreatitis biomarkers (e.g. Reg family members), leading to dysregulation of pancreatic acinar cell maintenance. Whereas in the liver, Bap1 loss increased cholesterol biosynthetic protein expression (e.g. Hsd17b7, mgcr, and Cyp51a1), and decreased gluconeogenic enzymes (e.g. Pklr, Fbp1, and Aldob) and lipid chaperone protein levels, leading hypercholesterolemia, hypoglycemia, and loss of hepatic lipid contents, respectively (58). These findings imply that BAP1 metabolic functions in tumorigenesis vary greatly, perhaps due to effects of BAP1 on tissue-specific metabolic enzyme expression and/or tissue-specific microenvironments. Also, Bap1 deletion led to elevated histone H2A ubiquitination and mitochondrial outer membrane proteins in liver (58), implying that the metabolic functions of Bap1 might be dependent upon its enzymatic activity as a DUB.
O-GlcNacylation is a post-transcriptional protein modification that is regulated by nutrient availability within the cell (49,59). Abnormal O-GlcNacylation links alterations of cancer cell metabolism to tumor initiation (60,61). O-GlcNacylation of the HCF-1/OGT complex helps recruits BAP1 to PGC1α, thereby deubiquitinating and inhibiting PGC1α degradation to promote gluconeogenesis (49). An intriguing observation from this study is that diabetic mice or mice fed a high fat diet exhibit elevated Bap1 expression suggesting that BAP1 and nutrient homeostasis might have a bidirectional, positive-feedback relationship. In conclusion, BAP1 makes key contributions to nutrient homeostasis in a tissue-specific manner, primarily due to its role as a DUB.
Glycolytic alterations:
In addition to regulating nutrient homeostasis in normal cells and tissues, BAP1 loss increases cancer susceptibility by regulating cellular metabolism (55,62). The Warburg effect is characterized by increased aerobic glycolysis and lactate production in cancer cells (63). Through an integrated metabolomic and molecular analysis of patient samples coupled with an in vitro model, Bononi and colleagues showed that primary human fibroblasts from carriers of BAP1 mutations exhibit key metabolic alterations typical of the Warburg effect and suppressed mitochondrial respiration (62). Moreover, metabolic modifications caused by BAP1 deletion were specifically associated with the DUB catalytic function of BAP1 (62). In support of these findings, BAP1 mutations were enriched in ccRCC tumor samples with elevated glycolysis level (64).
Calcium dysregulation:
Most functions of BAP1 are assigned to its nuclear localization; however, a study discovered that BAP1 regulates intracellular calcium (Ca2+) flux. Indeed, cytoplasmic BAP1 localized to the endoplasmic reticulum (ER), the main site for intracellular Ca2+ storage (55,65). Dysregulation of intracellular Ca2+ levels is associated with apoptosis-resistance and metastasis (66). At the ER, BAP1-dependent deubiquitination of the type 3 inositol-1,4,5-trisphosphate receptor (IP3R3) is necessary to sustain the IP3R3 ER channel integrity (11,55). The IP3R3 channel mediates the Ca2+ release into the cytosol and mitochondria, which subsequently triggers apoptosis (55). Along with maintenance of IP3R3 ER channel integrity, BAP1-dependent deubiquitination of IP3R3 may change the threshold for IP3R3 activation by Ca2+ itself or IP3. BAP1 deletion increased tumorigenesis by decreasing levels of IP3R3, Ca2+ flux, and apoptosis upon stimulation by various stressors (55). Taken together, these observations suggest that reprogramming of Ca2+ metabolism by BAP1 loss may serve as an underlying mechanism contributing to the elevated tumor susceptibilities seen in BAP1-mutated individuals.
Metabolic stress management:
Coping with metabolic stress is critical for cancer cells to survive and metastasize (56,67). Environments that cause metabolic stress (i.e. nutrient and/or oxygen depletion) can trigger cell death (68). To cope, cancer cells activate their own survival mechanisms under metabolic stress, including remodeling of cell death pathways and the unfolded protein response (UPR) (8,69). Elucidating the underlying mechanisms of metabolic stress management is important to understand tumor development and to explore new potential therapeutic strategies.
Recent evidence highlights that BAP1 suppresses metabolic stress-induced death in cancer cells (70,71). BAP1 repressed glucose deprivation-induced ER stress cell death by inhibiting transcription of two major UPR-TFs, activating transcription factor 3 (ATF3) and C/EBP homologous protein (CHOP) (70). The observed functions of BAP1 in ATF3 and CHOP transcriptional repression appears to be independent of its DUB activity. Moreover, BAP1 plays a role in ferroptosis, a type of metabolic stress-associated non-apoptotic cell death, further highlighting its myriad of unappreciated roles as a tumor suppressor (71). BAP1 deubiquitinates histone 2A in the regulatory chromatin of the of SLC7A11 gene, which encodes, a key transporter of extracellular cysteine. In this manner, BAP1 reduces SLC7A11 expression and cysteine uptake, leading lipid peroxidation upregulation and the ferroptosis induction (71). Thus, the observed tumor suppressive functions of BAP1 are due, at least in part, to its involvement in metabolic stress pathway management.
Potential roles of BAP1 in other metabolic pathways
Several studies raise the possibility that BAP1 protein has additional roles in cell metabolism that might be relevant to tumorigenesis and/or cancer progression. The potential BAP1 involvement in additional metabolic pathways is outlined in Figure 2B.
ROS and mitochondrial metabolism:
Moderate elevation of reactive oxygen species (ROS) is associated with tumorigenesis and metastasis (72). By integrating gene expression and molecular analyses, Hebert and colleagues observed wild type BAP1 re-expression increases intracellular ROS levels and decreases respiratory capacities (73). ROS homeostasis (74) and mitochondrial respiration (75) are important aspects of cancer cell metabolism that, when better understood, might elucidate the BAP1 involvement, as yet unappreciated aspects of tumorigenesis and metastasis.
HIF1α-dependent metabolism:
Bioinformatics interrogation of The Cancer Genome Atlas (TCGA) data have associated BAP1 alterations with changes in hypoxia-inducible factor-1 alpha (HIFα) expression (25,76,77). BAP1 loss was significantly correlated with HIF1α overexpression (77,78). Overexpression of HIF1α level is a major feature of cancer cells, and HIF1α induces metabolic reprogramming of cancer cells, including the Warburg effect to promote tumorigenesis and metastasis (79,80). The strong association between BAP1 and HIF1α might determine why BAP1 loss increases the risk of metastasis in certain cancer types such UM.
One carbon metabolism:
One carbon metabolism provides the methyl group involved in the DNA and histone methylation (81) and is considered as a vital cellular process by which nutrients play a epigenetic role in DNA and histone modifications (82). BAP1 loss is associated with remodeling of histone and global DNA methylation levels (25,76). The mechanisms underlying these effects are unclear; however, the observations suggest potential BAP1 involvement in one carbon metabolism and/or related nutrient pathways, including methionine and folate to induce changes in histone and DNA methylation. Other tumor suppressors,TP53, regulate one carbon metabolism pathways by promoting serine synthesis pathways, leading to tumorigenesis (83). Therefore, the study opens up uncharted venues for exploring novel roles for BAP1 in distinct metabolic processes and how these metabolic pathways might relate to one another, perhaps generating new insights into the links between cancer cell metabolism and epigenetics.
Concluding remarks and future directions
BAP1 has rightly attracted much interest as a potential tumor suppressor gene in a variety of cancers. Previous studies regarding the disparate BAP1 functions have mainly focused on cellular processes (11,84); however, recently, BAP1 roles in cellular metabolism have emerged . We have summarized metabolic roles of BAP1 in four main categories: i) nutrient homeostasis; ii) glycolytic alterations; iii) calcium dysregulation, and iv) metabolic stress management (Figure 2A). BAP1 appears to play role in nutrient homeostasis in a tissue-specific manner. Consistent with a widespread role in cell metabolism, BAP1 is involved in the development and/or maintenance of multiple different tissues and the metabolic disease developments (49,58). With respect to cancer biology, BAP1 loss confers metabolic advantages to cancer cells that might partially account for how BAP1 mutations increase the susceptibility of tumorigenesis (62,65). Lastly, BAP1 exerts its tumor suppressor effects by managing metabolic stress, a promising target for therapeutic modalities aimed at enhancing the apoptosis and elimination of cancer cells (70,71). Collectively, these studies emphasize the important emerging roles of BAP1 in cancer cell metabolism. However, much work remains to be done to fully understand the underlying mechanisms of how BAP1 exerts its effects. Further investigations are required to understand the tissue-dependent BAP1 metabolic activities. Since BAP1 location is different between humans (chromosome 3) and mice (chromosome 14), the outcomes of BAP1 mutations could be different. For instance, BAP1 mutations are linked to myeloid tumor development in mice while, it induces a different tumor types in humans (11).Therefore, possible different results of BAP1 loss in cellular metabolism between humans and mice should be considered. These efforts may clarify the functional link between BAP1 mutations and the specific cancer development, and open new avenues for the future development of therapeutic options to target cell metabolism in BAP1 mutant cancers.
The DUB activity of BAP1 accounts for many of its roles in cellular processes (3,7,48), and plays a major role in its metabolic functions (49,65,71). Identifying the full range of BAP1 DUB direct targets in UM represents an important goal. In contrast, some studies have shown that BAP1 can directly bind TFs to exert its metabolic roles independent of its DUB activity (70). Identifying the key gene regulatory regions of chromatin that are bound by BAP1 and its transcriptional co-factors remains a critical avenue of investigation for future studies. Furthermore, it has discovered that the subcellular localization of BAP1 influences its pleiotropic functions, emphasizing that more studies are required to clarify the distinct functional roles of BAP1 in the nucleus, cytoplasm, and even discrete subcellular sites such as the ER.
It has been reported that non-metastatic and metastatic cells have substantially different properties in terms of their cellular metabolism, suggesting that changes in cancer cell metabolism might contribute to metastasis (85,86). BAP1 mutations are highly associated with UM metastasis (13). A recent study showed that BAP1 mutant UM has increased oxidative phosphorylation gene expression compared with BAP1 wild type UM (87), raising the possibility that BAP1 mutations increase metastatic risk through metabolic alterations. There are some initial advances in understanding genomic differences between primary and metastatic UM (26).
New roles of BAP1 in cellular metabolism have discovered and are being characterized in terms of their effects on specific pathways. Nevertheless, further studies need to understand the detailed mechanisms, define the critical metabolic functions of BAP1, and clarify their potential links with metastasis. Future efforts will lay the foundation for better informed exploration of novel interventions to target cell metabolism in BAP1 mutant cancers. Advances in this area will ultimately broaden the available therapeutic options for patients who currently face bleak disease outcomes for several BAP1- associated cancers.
Acknowledgments
We thank all members of the Aplin lab for feedback on this review. This work was supported by grants R01 CA196278, R01 CA253977 and P01 CA114046 from the National Institutes of Health (NIH)/National Cancer Institute (NCI) and by a Melanoma Research Alliance team science award (#559058) to A.E. Aplin. Additional support was provided by the 2020 AACR-Ocular Melanoma Foundation Fellowship, in honor of Robert C. Allen, MD, Grant Number 20-40-39-HAN, awarded to A. Han.
Footnotes
Conflict of interest: A.E. Aplin reports receiving a commercial research grant from Pfizer Inc. (2013–2017) and has ownership interest in patent number 9880150. No potential conflicts of interest are disclosed by the other authors.
References
- 1.Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene 1998;16:1097–112 [DOI] [PubMed] [Google Scholar]
- 2.Murali R, Wiesner T, Scolyer RA. Tumours associated with BAP1 mutations. Pathology 2013;45:116–26 [DOI] [PubMed] [Google Scholar]
- 3.Daou S, Hammond-Martel I, Mashtalir N, Barbour H, Gagnon J, Iannantuono NV, et al. The BAP1/ASXL2 histone H2A deubiquitinase complex regulates cell proliferation and is disrupted in cancer. J Biol Chem 2015;290:28643–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Misaghi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, et al. Association of C-terminal ubiquitin hydrolase BRCA1-associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol 2009;29:2181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res 2009;69:111–9 [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol 2010;30:5071–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okino Y, Machida Y, Frankland-Searby S, Machida YJ. BRCA1-associated protein 1 (BAP1) deubiquitinase antagonizes the ubiquitin-mediated activation of FoxK2 target genes. J Biol Chem 2015;290:1580–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74 [DOI] [PubMed] [Google Scholar]
- 9.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv 2016;2:e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruiswijk F, Labuschagne CF, Vousden KH. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol 2015;16:393. [DOI] [PubMed] [Google Scholar]
- 11.Carbone M, Harbour JW, Brugarolas J, Bononi A, Pagano I, Dey A, et al. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov 2020;10:1103–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhattacharya S, Hanpude P, Maiti TK. Cancer associated missense mutations in BAP1 catalytic domain induce amyloidogenic aggregation: a new insight in enzymatic inactivation. Sci Rep 2015;5:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center BITGDA. Analysis-ready standardized TCGA data from Broad GDAC Firehose 2016_01_28 run. Broad Institute of MIT and Harvard; 2016 [Google Scholar]
- 15.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. AACR; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbone M, Ferris LK, Baumann F, Napolitano A, Lum CA, Flores EG, et al. BAP1 cancer syndrome: malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med 2012;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carbone M, Arron ST, Beutler B, Bononi A, Cavenee W, Cleaver JE, et al. Tumour predisposition and cancer syndromes as models to study gene–environment interactions. Nat Rev Cancer 2020:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua V, Aplin AE. Novel therapeutic strategies and targets in advanced uveal melanoma. Curr Opin Oncol 2018;30:134–41 [DOI] [PubMed] [Google Scholar]
- 19.Shain AH, Bagger MM, Yu R, Chang D, Liu S, Vemula S, et al. The genetic evolution of metastatic uveal melanoma. Nat Genet 2019;51:1123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewens K, Lalonde E, Richards-Yutz J, Shields C, Ganguly A. Comparison of germline versus somatic BAP1 mutations for risk of metastasis in uveal melanoma. BMC Cancer 2018;18:1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res 2004;64:7205–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun 2020;11:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res 2012;18:408–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matatall KA, Agapova OA, Onken MD, Worley LA, Bowcock AM, Harbour JW. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer 2013;13:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field MG, Kuznetsov JN, Bussies PL, Cai LZ, Alawa KA, Decatur CL, et al. BAP1 loss is associated with DNA Methylomic Repatterning in highly aggressive class 2 Uveal melanomas. Clin Cancer Res 2019;25:5663–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson J, Nilsson LM, Mitra S, Alsén S, Shelke GV, Sah VR, et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat Commun 2020;11:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, Pagano I, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J Clin Oncol 2018;36:3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshikawa Y, Emi M, Hashimoto-Tamaoki T, Ohmuraya M, Sato A, Tsujimura T, et al. High-density array-CGH with targeted NGS unmask multiple noncontiguous minute deletions on chromosome 3p21 in mesothelioma. PNAS 2016;113:13432–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napolitano A, Pellegrini L, Dey A, Larson D, Tanji M, Flores EG, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2016;35:1996–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung M, Kadariya Y, Talarchek J, Pei J, Ohar JA, Kayaleh OR, et al. Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene–environment interaction. Cancer Lett 2015;369:261–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carbone M, Klein G, Gruber J, Wong M. Modern criteria to establish human cancer etiology. Cancer Res 2004;64:5518–24 [DOI] [PubMed] [Google Scholar]
- 32.Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan R, Morrow B, Thomas A, Walsh T, Lee MK, Gulsuner S, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. PNAS 2019;116:9008–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray SG, Mutti L. Immunotherapy for mesothelioma: a critical review of current clinical trials and future perspectives. Transl Lung Cancer Res 2020;9:S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shrestha R, Nabavi N, Lin Y-Y, Mo F, Anderson S, Volik S, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med 2019;11:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mansfield AS, Peikert T, Vasmatzis G. Chromosomal rearrangements and their neoantigenic potential in mesothelioma. Transl Lung Cancer Res 2020;9:S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kittaneh M, Berkelhammer C. Detecting germline BAP1 mutations in patients with peritoneal mesothelioma: benefits to patient and family members. J Transl Med 2018;16:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobrinski DA, Yang H, Kittaneh M. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res 2020;9:S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, et al. Renal cell carcinoma. Nature reviews Disease primers 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 2012;44:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S-S, Gu Y-F, Wolff N, Stefanius K, Christie A, Dey A, et al. Bap1 is essential for kidney function and cooperates with Vhl in renal tumorigenesis. PNAS 2014;111:16538–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng J, Ma J, Li W, Mo R, Zhang P, Gao K, et al. Stabilization of MCRS1 by BAP1 prevents chromosome instability in renal cell carcinoma. Cancer Lett 2015;369:167–74 [DOI] [PubMed] [Google Scholar]
- 43.Wiesner T, Obenauf AC, Murali R, Fried I, Griewank KG, Ulz P, et al. Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011;43:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar R, Taylor M, Miao B, Ji Z, Njauw JC, Jönsson G, et al. BAP1 has a survival role in cutaneous melanoma. J Invest Dermatol 2015;135:1089–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haugh AM, Njauw C-N, Bubley JA, Verzì AE, Zhang B, Kudalkar E, et al. Genotypic and phenotypic features of BAP1 cancer syndrome: a report of 8 new families and review of cases in the literature. JAMA dermatology 2017;153:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carbone M, Yang H, Pass HI, Krausz T, Testa JR, Gaudino G. BAP1 and cancer. Nat Rev Cancer 2013;13:153–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eletr ZM, Wilkinson KD. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem Biophys 2011;60:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem 2009;284:34179–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruan H-B, Han X, Li M-D, Singh JP, Qian K, Azarhoush S, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab 2012;16:226–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forma E, Jóźwiak P, Bryś M, Krześlak A. The potential role of O-GlcNAc modification in cancer epigenetics. Cell Mol Biol Lett 2014;19:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dey A, Seshasayee D, Noubade R, French DM, Liu J, Chaurushiya MS, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science 2012;337:1541–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ventii KH, Devi NS, Friedrich KL, Chernova TA, Tighiouart M, Van Meir EG, et al. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008;68:6953–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang A, Papneja A, Hyrcza M, Al-Habeeb A, Ghazarian D. BAP1: gene of the month. J Clin Pathol 2016:jclinpath-2016–203866 [DOI] [PubMed] [Google Scholar]
- 54.Fukuda T, Tsuruga T, Kuroda T, Nishikawa H, Ohta T. Functional link between BRCA1 and BAP1 through histone H2A, heterochromatin and DNA damage response. Curr Cancer Drug Targets 2016;16:101–9 [DOI] [PubMed] [Google Scholar]
- 55.Bononi A, Giorgi C, Patergnani S, Larson D, Verbruggen K, Tanji M, et al. BAP1 regulates IP3R3-mediated Ca 2+ flux to mitochondria suppressing cell transformation. Nature 2017;546:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science 2020;368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strzyz P. Maintenance of nutrient homeostasis. Nat Rev Mol Cell Biol 2015;16:703- [Google Scholar]
- 58.Baughman JM, Rose CM, Kolumam G, Webster JD, Wilkerson EM, Merrill AE, et al. NeuCode proteomics reveals Bap1 regulation of metabolism. Cell Rep 2016;16:583–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanover JA, Krause MW, Love DC. Linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol 2012;13:312–21 [DOI] [PubMed] [Google Scholar]
- 60.Hanover JA, Chen W, Bond MR. O-GlcNAc in cancer: An Oncometabolism-fueled vicious cycle. J Bioenerg Biomembr 2018;50:155–73 [DOI] [PubMed] [Google Scholar]
- 61.Ferrer CM, Lynch TP, Sodi VL, Falcone JN, Schwab LP, Peacock DL, et al. O-GlcNAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Mol Cell 2014;54:820–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bononi A, Yang H, Giorgi C, Patergnani S, Pellegrini L, Su M, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ 2017;24:1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen GK, Mellnick VM, Yim AK-Y, Salter A, Ippolito JE. Synergy of sex differences in visceral fat measured with CT and tumor metabolism helps predict overall survival in patients with renal cell carcinoma. Radiology 2018;287:884–92 [DOI] [PubMed] [Google Scholar]
- 65.Berridge MJ. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 2016;96:1261–96 [DOI] [PubMed] [Google Scholar]
- 66.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta pharmaceutica sinica B 2017;7:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreuzaler P, Panina Y, Segal J, Yuneva M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol Metab 2020;33:83–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Green DR, Galluzzi L, Kroemer G. Metabolic control of cell death. Science 2014;345:1250256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev 2009;23:537–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai F, Lee H, Zhang Y, Zhuang L, Yao H, Xi Y, et al. BAP1 inhibits the ER stress gene regulatory network and modulates metabolic stress response. PNAS 2017;114:3192–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol 2018;20:1181–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perillo B, Di Donato M, Pezone A, Di Zazzo E, Giovannelli P, Galasso G, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med 2020:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hebert L, Bellanger D, Guillas C, Campagne A, Dingli F, Loew D, et al. Modulating BAP1 expression affects ROS homeostasis, cell motility and mitochondrial function. Oncotarget 2017;8:72513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chio IIC, Tuveson DA. ROS in cancer: the burning question. Trends Mol Med 2017;23:411–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Viale A, Corti D, Draetta GF. Tumors and mitochondrial respiration: a neglected connection. Cancer Res 2015;75:3687–91 [DOI] [PubMed] [Google Scholar]
- 76.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017;32:204–20. e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brouwer NJ, Wierenga A, Gezgin G, Marinkovic M, Luyten GP, Kroes WG, et al. Ischemia is related to tumour genetics in uveal melanoma. Cancers (Basel) 2019;11:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mennerich D, Kubaichuk K, Kietzmann T. Dubs, hypoxia, and cancer. TRENDS CANCER 2019;5:632–53 [DOI] [PubMed] [Google Scholar]
- 79.Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-modified cancer cell metabolism. Front Cell Dev Biol 2019;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rankin EB, Nam J-M, Giaccia AJ. Hypoxia: signaling the metastatic cascade. TRENDS CANCER 2016;2:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mentch SJ, Locasale JW. One carbon metabolism and epigenetics: understanding the specificity. Ann N Y Acad Sci 2016;1363:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y-P, Lei Q-Y. Metabolic recoding of epigenetics in cancer. Cancer Commun 2018;38:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosenzweig A, Blenis J, Gomes AP. Beyond the Warburg effect: how do cancer cells regulate one-carbon metabolism? Front Cell Dev Biol 2018;6:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Nunno V, Frega G, Santoni M, Gatto L, Fiorentino M, Montironi R, et al. BAP1 in solid tumors. Future Oncol 2019;15:2151–62 [DOI] [PubMed] [Google Scholar]
- 85.Tasdogan A, Faubert B, Ramesh V, Ubellacker JM, Shen B, Solmonson A, et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 2020;577:115–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pascual G, Domínguez D, Benitah SA. The contributions of cancer cell metabolism to metastasis. Dis Model Mech 2018;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han A, Purwin TJ, Bechtel N, Liao C, Chua V, Seifert E, et al. BAP1 mutant uveal melanoma is stratified by metabolic phenotypes with distinct vulnerability to metabolic inhibitors. Oncogene 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


