Abstract
Many lung transplant candidates and recipients are older and frailer compared to previous eras. Older patients are at increased risk for pre- and post-transplant mortality, but this risk is not explained by numerical age alone. This manuscript represents the product of the American Society of Transplantation (AST) conference on frailty. Experts in the field reviewed the latest published research on assessment of elderly and frail lung transplant candidates.
Physical frailty, often defined as slowness, weakness, low physical activity, shrinking, and exhaustion, and frailty evaluation is an important tool for evaluation of age-associated dysfunction. Another approach is assessment by cumulative deficits, and both types of frailty are common in lung transplant candidates. Frailty is associated with death or delisting before transplant, and may be associated with post-transplant mortality. Sarcopenia, cognitive dysfunction, depression, and nutrition are other important components for patient evaluation. Aging-associated inflammation, telomere dysfunction, and adaptive immune system senescence may also contribute to frailty.
Developing tools for frailty assessment and interventions holds promise for improving patient outcomes before and after lung transplantation.
Introduction
With the rise in the number of older lung transplant candidates and recipients, there is increasing interest in the study of frailty in transplantation. To this end, the American Society of Transplantation (AST) sponsored a consensus conference on frailty in kidney, liver, heart and lung transplantation in 2018 [1]. This conference brought together experts from the transplant community and provided an opportunity for exchange of ideas and discussion of best practices for frailty assessment. Given the special challenges regarding frailty in lung transplantation, a separate committee was convened to describe the approach to frailty in the evaluation of lung transplant candidates and recipients in greater detail.
Frailty is a concept initially associated with aging that has been defined in the field of geriatrics, and is commonly defined as an aging-associated physiological vulnerability to stressors leading to adverse health outcomes [2]. Although common in older patients, aging associated syndromes, including sarcopenia, impaired cognition, depression, and poor nutrition, have been shown to occur in all chronologic age groups. These aging-associated syndromes could be better described as associated with biological, as opposed to chronologic age (Figure 1). As a result, it has been observed that even younger patients with end stage organ disease can develop aging-associated syndromes, including frailty. Although the larger concept of frailty can be applied to wide range of aging-associated syndromes, for the purposes of this manuscript, we will use the term “frailty” for physical frailty or cumulative deficits and the term “aging associated syndromes” for the other components that are associated with frailty (i.e. cognition, depression, nutrition, sarcopenia).
Figure 1:
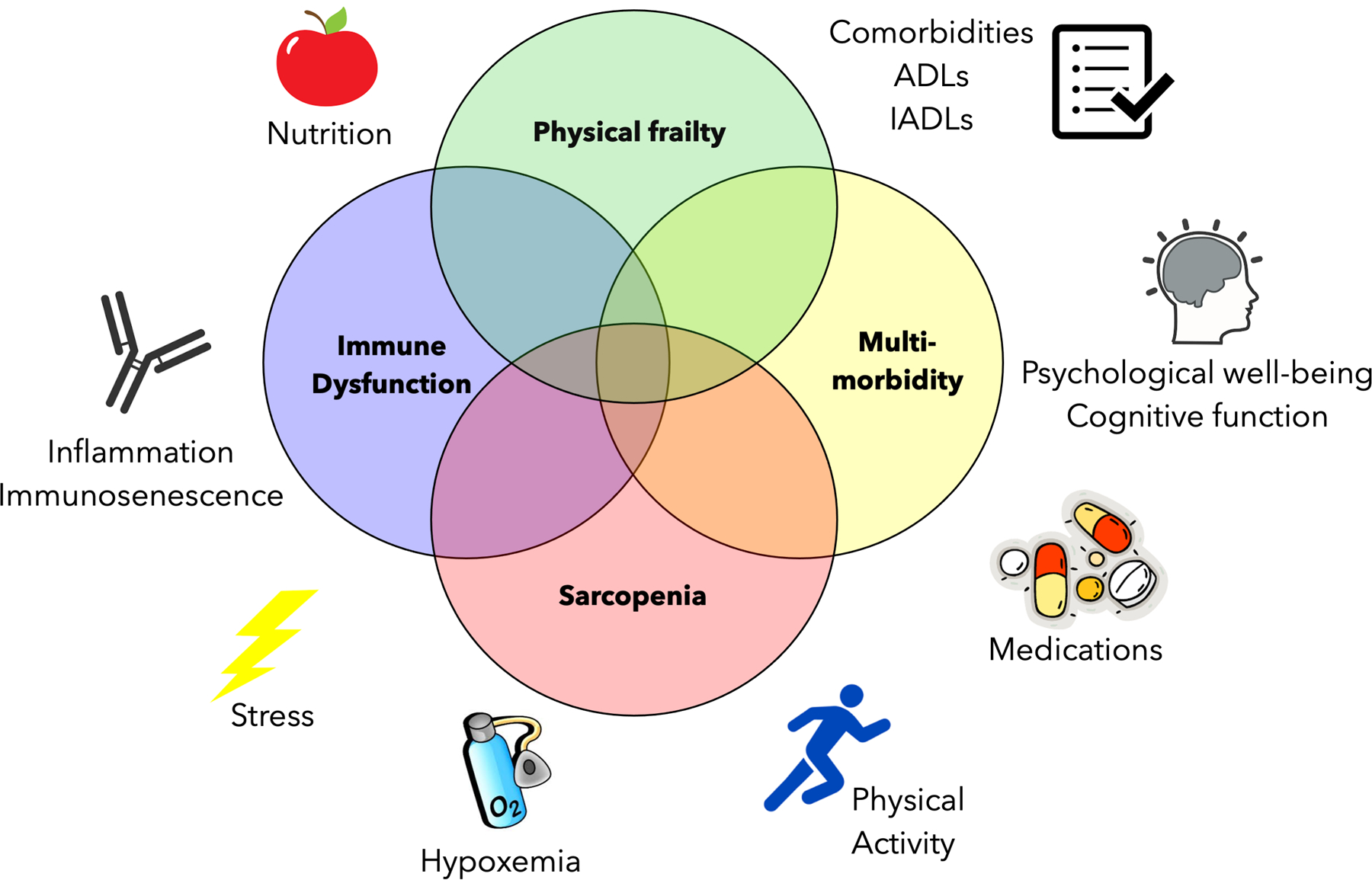
Conceptual Framework of Frailty and Aging-associated Dysfunction in Lung Transplant Candidates. Venn diagram demonstrates the age-related biologic constructs that may overlap with frailty, including multi-morbidity, sarcopenia, and immune system dysfunction, along with health-related issues that may contribute to these manifestations of aging.
The numbers of older patients undergoing transplantation each year is rising, with 35% who are 65 years of age or older, according to the 2018 the Organ Procurement and Transplant Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) Report [3]. Older patients undergoing lung transplantation clearly demonstrate a significant decrease in 1- and 5-year survival compared with patients under 60 years old. This increased burden of mortality in patients ≥ 60 years old is seen in the most recent International Society of Heart and Lung Transplantation (ISHLT) report [4]. With increased knowledge regarding frailty and aging associated syndromes combined with the increased number of older patients (≥ 60 years old) considered for lung transplantation, it is therefore crucial that the lung transplant community develop a comprehensive approach for patients undergoing lung transplantation. Prior to application of frailty testing, evaluation of frailty and other age-associated syndromes should be assessed in the context of end stage pulmonary disease, as patient characteristics and performance of frailty assessment would be predicted to differ between patients with lung disease compared with those with other types of end stage organ disease. This lung-focused approach should allow for development of tools to distinguish between frailty and other aging-associated syndromes in the context of the physical limitations related to advanced lung disease, which would be expected to improve after lung transplantation.
In reporting the results of this consensus panel discussion, we summarize the latest data, provide expert opinion on the assessment of frailty in lung transplantation, and develop a path forward to new areas in need of further investigation.
Methods
Approach to the design and execution of the AST conference on frailty has been described previously [1]. The in-person meeting took place in February 2018; this meeting was preceded by a series of conference calls among members of the lung transplant group to discuss topics related to frailty and aging associated syndromes in the evaluation of the lung transplant candidate and recipient. After publication of the Report from the AST on frailty in solid organ transplantation, the lung transplant group focused on frailty and aging associated syndromes that were specific to lung transplant candidates and recipients. Given the breadth of this topic, the team members felt that a secondary report that provided further data with a lung centered focus was necessary to fully inform the lung transplant community. To ensure the most up to date analysis would be presented, an updated literature review was performed through December 2019 to ensure inclusion of any peer-reviewed literature published after the date of the in-person meeting. This report represents a synthesis of these findings.
Results
“Frailty” assessment in lung transplantation
Frailty is not a uniform construct; various definitions and conceptual frameworks exist, and different approaches may be warranted depending upon the intended use of frailty assessments. Frailty instruments differ significantly in the assessed domains, inclusion of objective tests, and data sources, with each instrument having strengths and limitations [5]. The physical frailty model, also known as phenotypic or syndromic frailty, and the cumulative deficit frailty model, also known as stochastic frailty, are the predominant models of frailty [5]. Physical frailty is considered to be distinct from disability and comorbidity.
Tools for frailty assessment and their use in lung disease
Physical frailty is most commonly evaluated as an aggregate score of five features, often termed the Fried Physical Frailty Phenotype (FFP): low physical activity, slowness, weakness, shrinking, and exhaustion [5]. A modified version of the FFP was developed for advanced lung disease and transplant that better quantifies the low activity construct in the setting of lung disease by replacing the Minnesota Leisure Time Physical Activity (MLTA) questionnaire with the Duke Activity Status Index (DASI). This better quantification using DASI improved multiple tests of validity including improved prediction of transplant candidate waitlist delisting or death [6]. The Short Physical Performance Battery (SPPB) is a 3-component battery that includes gait speed, chair stands, and balance that measures lower extremity performance and has been utilized in several studies as a measurement of frailty [5]. Lower SPPB scores reflect increased impairment. Given its focus on objective functional measurements, the SPPB is also grounded in the physical frailty framework.
The cumulative deficits model conceptualizes frailty as an individual’s accumulation of age-associated deficits over time, which may include symptoms, signs, diseases, disabilities, or laboratory measurements [2]. Each deficit is identified and quantified where higher scores reflect increased frailty [5]. In this model, frailty may be defined as different sets of deficits and still exhibit consistent relationships with adverse outcomes such as mortality [2]. These indices, however, may be limited by the fact the list of deficits measured may vary in different applications of this tool. However, although possibly less responsive to rehabilitation or interventions targeting mechanisms of frailty, cumulative deficits measure may be more prognostic of outcomes.
Most frailty instruments have been used in cohort studies of community-dwelling older adults and were not developed specifically for patients with pulmonary disease [5], although a few studies have looked at other chronic pulmonary diseases, such as chronic obstructive pulmonary disease (COPD) with other types of chronic pulmonary disease [7]. In patients with pulmonary disease, physical disability may be secondary to physiologic limitations created by chronic lung disease. Frailty has been associated with shortness of breath and airflow limitation in patients with COPD, suggesting a potential interaction with the disease itself [7].
Individual factors in frailty assessments may drive the association between frailty and poor outcomes. For example, gait speed may be the best indicator of frailty in the FFP. It has been shown to predict risk of readmission in patients hospitalized for COPD exacerbation [8] and mortality in patients with idiopathic pulmonary fibrosis [9]. Other refinements of current frailty assessments may be necessary to better discriminate differences in patients with lung disease.
Performance of frailty testing in the context of lung transplantation
Emerging work has shown that both physical and cumulative deficit frailty are prevalent in lung transplant candidates. Depending on the measure used, 10–45% of lung transplant candidates are frail at the time of assessment [10–12]. Before transplant, both types of frailty are independently associated with worse exercise capacity by cardiopulmonary exercise testing, shorter distance walked in six minutes, and worse disability and health-related quality of life (HRQL) [10–13]. Pre-operative frailty is also associated with increased risk of death or delisting from the waitlist [10]. Pre-operative frailty has been associated with mortality after transplant; however, this association has not been observed uniformly in the literature [10–12,14,15]. In one single-center study, which included routine pre- and post-operative pulmonary rehabilitation, pre-transplant physical frailty was associated with greater improvements in HRQL and six-minute walk distance (6-MWD) with transplantation within the first 3–6 months post-transplant, without an increase in post-transplant mortality [12]
Data are more limited on the prevalence and predictive ability of frailty after lung transplantation. In the immediate post-transplant period, the incidence of frailty may rise, perhaps related to peri-operative complications, disuse atrophy, and malnutrition. Physical frailty at the time of hospital discharge from surgery was observed in approximately 50% of lung transplant recipients. Frailty at discharge was associated with increased risk of subsequent unplanned readmission [16].
Physical frailty appears dynamic in lung transplant patients. Pre-transplant physical frailty improves in most—but not all—lung transplant candidates within the first 6 months after transplant surgery [17]. This suggests that existing measures of physical frailty should not be used in isolation to exclude potential candidates being evaluated for lung transplantation. Frailty may also be modifiable. In a small pilot intervention trial targeting physical frailty in lung transplant candidates, use of a mobile health-technology enabled home-based intervention improved frailty, measured with the SPPB, in over half of the participants [18]. For transplant recipients who were frail at the time of discharge, an outpatient physical therapy program was associated with frailty resolution in the majority within the first two post-operative months [16].
Efforts to explore the pathobiology of frailty in lung transplant candidates are just underway. Compared with those who were not frail, frail lung transplant candidates had higher blood biomarker levels of interleukin-6 and tumor necrosis factor receptor-1 (systemic inflammation) and lower levels of leptin (wasting/sarcopenia) and insulin-like growth factor-1 (neurohormonal function) [10]. Recent work has established a strong association between adiposity—a proinflammatory phenotype associated with impaired muscle function—and frailty [19].
Other age-associated syndromes
While frailty assessment has been the primary approach for evaluation for older patients, other types of evaluation have also been applied for measurement of age-associated syndromes as described below.
Sarcopenia
Sarcopenia is the loss of skeletal muscle mass and function associated with aging [20]. To date, there is no established universal definition of sarcopenia measurement in lung transplant candidates [20]. Irrespective of the definition, there is significant overlap between sarcopenia and frailty with similar mechanisms including chronic inflammation, physical inactivity, malnutrition, and aging [7]. Thus, sarcopenia may be an important risk factor for development of frailty and interventions targeting skeletal muscle function may help prevent or mitigate frailty.
Several sarcopenia measurements have been utilized in lung transplant candidates as a predictor of outcomes. Skeletal muscle mass measurement has been the most common marker measured retrospectively using thoracic [21] and abdominal computed tomography scans [22,23]. Associations with transplant outcomes have been variable across studies with lower pre-transplant skeletal muscle mass associated with increased post-transplant mortality [22,24], whereas other investigations demonstrate only associations with intensive care unit days, ventilator days, or hospital length of stay [21,23,25]. To date, only one study evaluated the association of sarcopenia on early post-transplant outcomes combining all three elements of the definition (muscle mass, strength and physical function) [21]. Rozenberg et al. observed that increased number of skeletal muscle impairments and lower quadriceps strength were correlated with longer hospital length of stay post-transplant, but no association was seen with pre- or early post-transplant mortality. Thus, there is increasing evidence that individual measures of sarcopenia are associated with adverse pre- and post-transplant outcomes, but additional research is required to establish the optimal method of sarcopenia measurement and the clinical implications of sarcopenia in the presence of frailty.
Cognitive dysfunction
Mild cognitive impairment in a patient with physical frailty is sometimes termed cognitive frailty. As seen in the general population, the effects of cognition and physical frailty may have multiplicative effects on disability and mortality after lung transplantation [26]. Smith et al. showed that 45% of patients had neurocognitive impairment prior to lung transplantation [27]. Associations with lower neurocognitive function included non-CF pretransplant disease, age, lower education status, and perioperative delirium [27]. Cognitive impairment in lung transplant recipients may be multifactorial including lack of oxygen, comorbid depression, comorbid malnutrition, and immunosuppression medications.
Cognitive function may be reversible with appropriate interventions; therefore, early identification is important. For example, in a study of 24 lung transplant recipients with a past medical history of COPD, subjects who underwent a 3-week inpatient pulmonary rehab program had significant improvement in their memory, learning skills, and psychomotor speed [28].
There are multiple measurements of the various components of cognitive dysfunction. These include different aspects of cognitive dysfunction: 1) global cognition: Severe Impairment Battery and Mini Mental State Examination; 2) executive function: Verbal Fluency Test Category/Letters, Clock Drawing Test, and Trail Making Test-B; and 3) attention: Digit Span Forward, Digit Span Backward, and Trail Making Test-A [29]. In the lung transplant population, many of the measures have been employed [26,27,30,31]; however, no systematic review has demonstrated which instrument is the best to use in this specialized population.
Depression
In the general population above 60 years old, depression and frailty commonly co-exist. In a meta-analysis of 8023 people, those who met criteria for frailty were 2.6 (95% confidence interval (CI): 1.6–4.4) times more likely to have depression compared to those who were not frail after adjusting for confounders [32]. Additionally, patients with depression were at an increased risk of frailty (OR: 4.1 (95% CI: 1.9–8.6) [32]. There is significant overlap in the diagnostic criteria of depression and frailty, namely in decreased stamina and unintentional weight loss. Co-existing depression and frailty are associated with worse cognitive impairment and disability, and both entities are independently associated with increased morbidity and mortality [27]. Depression is a common psychiatric condition in lung transplant candidates and recipients [33]. Several studies suggest that pre-transplant depression is associated with increased mortality after lung transplantation [34–36]. Depression is speculated to increase mortality by lowering medical adherence, worsening coping strategies, and reducing engagement in pulmonary rehabilitation after transplantation [35]. These effects may result in increased hospitalizations, graft failure, infection, and mortality.
Given the implications of depression on outcomes after lung transplantation, it is important to identify those at risk. The 2019 ISHLT Consensus Statement on Psychosocial Evaluation outlines recommendations for a mental health history [37]. These include an assessment of 1) past and current mood, anxiety, or personality disorders; 2) severity and chronicity of symptoms; 3) receipt, adherence, and response to psychiatric treatment; willingness to seek treatment 4) current or past suicidal ideation; and 5) mental health history of immediate family members [37].
Nutrition
Frailty and malnutrition may be associated conditions in patients with chronic lung disease [38]. As lung disease becomes more severe, metabolic demands increase, which can result in a worsened catabolic and malnourished state. Severe malnourishment has significant post-operative implications after lung transplantation including an impaired immune response, delayed wound healing, sepsis, decreased diaphragm muscle strength, and increased risk of mortality [39].
Body mass index (BMI) is frequently used as a measure of nutritional status given its availability, accuracy, reproducibility, and low cost. A low pre-transplant BMI has been shown to be associated with a lower survival in lung transplant recipients in several studies [40,41]. In addition to BMI, a nutritional assessment tool called the Prognostic Nutritional Index (PNI) has been studied in lung transplant candidates [42]. The PNI is derived from a number of variables including the leukocyte count, albumin, transferrin and skin folds. Kim et al performed a receiver operator characteristics analysis to determine the optimal cut-point of the PNI of 41.14 in lung transplant candidates. In addition, it was shown that patients with a PNI of < 41.15 had a significantly lower survival compared to those with a PNI > 41.15 (p<0.001) [42]. Further research in a different cohort can help establish the external validity of the PNI.
Interventions to address frailty
Studies to date have been limited by the small numbers studied, but have suggested that frailty may improve after intervention in lung transplant candidates or recipients. Preliminary data suggests that exercise interventions may be helpful to treat or prevent elements of physical frailty prior to lung transplantation. For example, patients who underwent supervised aerobic and strength training for the entire pre-transplant period had preserved 6-MWD and experienced an increase in muscle training volumes and treadmill speed [43]. Patients with idiopathic pulmonary fibrosis awaiting lung transplant who completed all 36 sessions of supervised aerobic and strength training had a lower risk of death following lung transplant [44]. Additionally, a 23-session exercise and educational program significantly improved the 6-MWD for patients awaiting lung transplantation with the largest improvements seen in those with the lowest starting 6-MWD [45].
Data are similarly encouraging post-transplantation. Frailty is associated with an increased risk of mortality both on the waiting list and after lung transplantation. However, of 37 subjects who were frail prior to transplant and survived the first six months following transplant, 31 demonstrated frailty improvement (83.7%) [17]. Two studies showed an improvement in the trajectory of frailty in adults undergoing lung transplantation [16,17].
The immune system and the (biologically) older patient
Immune dysfunction associated with aging is another important issue of associated with adverse clinical outcomes including infections and impaired vaccine response, involving changes in the innate and adaptive arms of the immune system [46]. Cellular senescence is an important aspect of vulnerability to infection and impaired wound healing, and is also strongly associated with inflammation [47]. The most striking aspects of the impaired adaptive immune response, with decreased frequent of naïve T cells and increased frequency of senescent CD28- T cells, which are associated with impaired vaccine response but increased levels of inflammation [46]. B cells, and innate immune cells including neutrophils, macrophages, and NK cells are also affected by aging [46]. Despite the extensive amount of data regarding impaired immune response and aging, little is known in the context of chronic lung disease and lung transplantation.
Inflammation is a component of cellular senescence, sometimes termed “inflammaging”, and has been directly linked to physical frailty and vulnerability to age-associated morbidity and mortality [47,48]. Hallmarks of inflammation associated with frailty, sarcopenia, and cognitive dysfunction include cytokines such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor alpha (TNF-α) [48]. These frailty- and inflammation-associated biomarkers are associated with outcomes in lung transplant candidates and recipients including death on the waiting list, primary graft dysfunction, and ICU length of stay [10].
Another important aspect of immune dysfunction is telomere shortening, an age-associated change related to senescence and fibrosis associated with prolonged cellular replication [49]. Shortened telomere length in either lung transplant donors or recipients can be associated CLAD and shorter survival. Donor telomere dysfunction can drive airway remodeling and fibrosis directly, while recipient telomere dysfunction has been linked to impaired control of infection [49,50]. Telomere dysfunction may also impact alloimmune responses and tolerance of immunosuppression. The interaction between these aspects of immune dysfunction and physical frailty is an important area for future study.
Conclusion
Given the growing numbers of older lung transplant patients, many reports have appeared suggesting benefits of evaluation of transplant candidates. The presence of end stage lung disease can impair the performance of traditional approaches to patient assessment, making re-evaluation of previously studied methods for the assessment of frailty and other aging associated syndromes in the setting of lung transplantation critical for guidance in application of these tools. Our review of the literature reveals the importance of assessment from an interdisciplinary team to identify variables most predictive of clinical outcomes, including physical frailty, frailty by cumulative deficits, sarcopenia, cognitive assessment, psychological assessment, nutritional status, and immunologic alterations. An approach based on measurement of biologic age and immunologic competence through cellular function, inflammation, and telomere length may be beneficial to apply in the pre-transplant assessment. This approach could additionally be applied to post-transplant evaluation for monitoring and personalizing immune suppression.
The data presented here on frailty and sarcopenia evaluation as well as assessment of cognitive, nutritional, and immunologic status of lung transplant candidates suggests that these approaches could be utilized for patient risk stratification. Future work needs to focus on risk prediction, which would be able to assign individual patients an absolute risk for an event such as death, as compared to currently published work which has defined group level relative risk. The methodology needed for clinical prediction are different from approaches used in the frailty literature to date and would require derivation and validation cohorts, ideally from different centers. That would then enable centers to make patient-specific risk decisions and, importantly, would provide the patient important information so they can make informed decision making. There is currently active NIH-funded research to reassess the ideal platform for assessing frailty in this patient population. In addition to validating domains from existing frailty measures, multicenter center studies are currently ongoing to combine frailty domain assessment with imaging and blood-based biomarkers to develop a comprehensive prediction tool.
Developing a tool for assessing and diagnosing frailty specific to the lung transplant population with high level of discriminant validity would go a long way towards improving our ability to study and potentially intervene to reverse frailty and other aspects of aging-associated dysfunction to further improve clinical outcomes.
Acknowledgments / Funding:
We thank the American Society of Transplantation, the American Society of Transplant Surgeons, and the Canadian Society of Transplantation for sponsoring the frailty consensus conference. This work was supported in part by the Veterans Affairs Office of Research and Development (CX002011) and NIH (R01HL151552). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Dr. Kennedy is supported by National Institute of Health K23HL128859. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Dr. Greenland reports the following financial relationships, which are not related to this publication: Boehringer Ingelheim, Theravance, Genentech, Atara, bioMérieux (BioFire), Thermo Fisher. Dr. Rozenberg is supported by the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine. Dr. Schaenman is supported by the National Institute of Health R21 AG055879-01 and the Mendez National Institute of Transplantation Foundation.
Abbreviations
- AST
American Society of Transplantation
- OPTN/SRTR
Organ Procurement and Transplant Network/Scientific Registry of Transplant Recipients
- ISHLT
International Society of Heart and Lung Transplantation
- FFP
Fried Frailty Phenotype
- MLTA
Minnesota Leisure Time Physical Activity
- DASI
questionnaire with the Duke Activity Status Index
- SPPB
Short Physical Performance Battery
- COPD
chronic obstructive pulmonary disease
- HRQL
Health-Related Quality of Life
- CI
confidence interval
- OR
odds ratio
- CI
confidence interval
- BMT
body mass index
- PNI
Prognostic Nutritional Index
- CLAD
chronic lung allograft dysfunction
- CMV
cytomegalovirus
Footnotes
Disclosure: The authors have no relevant conflicts of interest to disclose.
References
- 1.Kobashigawa J, Dadhania D, Bhorade S, Adey D, Berger J, Bhat G, Budev M, Duarte-Rojo A, Dunn M, Hall S, et al. : Report from the American Society of Transplantation on frailty in solid organ transplantation. American Journal of Transplantation 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K: Frailty in elderly people. The Lancet 2013, 381:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valapour M, Lehr CJ, Skeans M, Smith JM, Uccellini K, Goff R, Foutz J, Israni A, Snyder JJ, Kasiske BL: OPTN/SRTR 2018 Annual Data Report: Lung. American Journal of Transplantation 2020, 20:427–508. [DOI] [PubMed] [Google Scholar]
- 4.ISHLT International Thoracic Organ Transplant Registry. Edited by: International Society for Heart and Lung Transplantation; 2018.
- 5.Dent E, Kowal P, Hoogendijk EO: Frailty measurement in research and clinical practice: A review. Eur J Intern Med 2016, 31:3–10. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin MR, Singer JP, Huang D, Sell J, Gonzalez WC, Pollack L, Maurer MS, D’Ovidio FF, Bacchetta M, Sonett JR, et al. : Refining Low Physical Activity Measurement Improves Frailty Assessment in Advanced Lung Disease and Survivors of Critical Illness. Annals of the American Thoracic Society 2017:AnnalsATS.201612–201008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer JP, Lederer DJ, Baldwin MR: Frailty in Pulmonary and Critical Care Medicine. Annals of the American Thoracic Society 2016, 13:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kon SS, Jones SE, Schofield SJ, Banya W, Dickson MJ, Canavan JL, Nolan CM, Haselden BM, Polkey MI, Cullinan P, et al. : Gait speed and readmission following hospitalisation for acute exacerbations of COPD: a prospective study. Thorax 2015, 70:1131–1137. [DOI] [PubMed] [Google Scholar]
- 9.Nolan CM, Maddocks M, Maher TM, Banya W, Patel S, Barker RE, Jones SE, George PM, Cullinan P, Man WD: Gait speed and prognosis in patients with idiopathic pulmonary fibrosis: a prospective cohort study. Eur Respir J 2019, 53. [DOI] [PubMed] [Google Scholar]
- 10.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, Dean MY, Hersh B, Wolters PJ, Tokman S, et al. : Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. American Journal of Respiratory and Critical Care Medicine 2015, 192:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson ME, Vakil AP, Kandel P, Undavalli C, Dunlay SM, Kennedy CC: Pretransplant frailty is associated with decreased survival after lung transplantation. Journal of Heart and Lung Transplantation 2016, 35:173–178. [DOI] [PubMed] [Google Scholar]
- 12.Rozenberg D, Mathur S, Wickerson L, Chowdhury NA, Singer LG: Frailty and clinical benefits with lung transplantation. J Heart Lung Transplant 2018, 37:1245–1253. [DOI] [PubMed] [Google Scholar]
- 13.Layton AM, Armstrong HF, Baldwin MR, Podolanczuk AJ, Pieszchata NM, Singer JP, Arcasoy SM, Meza KS, D’Ovidio F, Lederer DJ: Frailty and maximal exercise capacity in adult lung transplant candidates. Respir Med 2017, 131:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer JP, Diamond JM, Anderson MR, Katz PP, Covinsky K, Oyster M, Blue T, Soong A, Kalman L, Shrestha P, et al. : Frailty phenotypes and mortality after lung transplantation: A prospective cohort study. American Journal of Transplantation 2018, 18:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montgomery E, Macdonald PS, Newton PJ, Chang S, Jha SR, Hannu MK, Thomson C, Havryk A, Malouf M: Frailty as a Predictor of Mortality in Patients with Interstitial Lung Disease Referred for Lung Transplantation. Transplantation 2019. [DOI] [PubMed] [Google Scholar]
- 16.Courtwright AM, Zaleski D, Tevald M, Adler J, Singer JP, Cantu EE, C AB, Diamond JM: Discharge frailty following lung transplantation. Clin Transplant 2019, 33:e13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venado A, McCulloch C, Greenland JR, Katz P, Soong A, Shrestha P, Hays S, Golden J, Shah R, Leard LE, et al. : Frailty trajectories in adult lung transplantation: A cohort study. J Heart Lung Transplant 2019, 38:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer JP, Soong A, Bruun A, Bracha A, Chin G, Hays SR, Kukreja J, Rigler J, Golden JA, Greenland JR, et al. : A mobile health technology enabled home-based intervention to treat frailty in adult lung transplant candidates: A pilot study. Clin Transplant 2018, 32:e13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson MR, Kolaitis NA, Gao Y, Kukreja J, Greenland J, Hays S, Wolters P, Golden J, Diamond J, Palmer S, et al. : A nonlinear relationship between visceral adipose tissue and frailty in adult lung transplant candidates. Am J Transplant 2019, 19:3155–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rozenberg D, Wickerson L, Singer LG, Mathur S: Sarcopenia in lung transplantation_ A systematic review. Journal of Heart and Lung Transplantation 2014, 33:1203–1212. [DOI] [PubMed] [Google Scholar]
- 21.Rozenberg D, Mathur S, Herridge M, Goldstein R, Schmidt H, Chowdhury NA, Mendes P, Singer LG: Thoracic muscle cross-sectional area is associated with hospital length of stay post lung transplantation: a retrospective cohort study. Transpl Int 2017, 30:713–724. [DOI] [PubMed] [Google Scholar]
- 22.Kelm DJ, Bonnes SL, Jensen MD, Eiken PW, Hathcock MA, Kremers WK, Kennedy CC: Pre-transplant wasting (as measured by muscle index) is a novel prognostic indicator in lung transplantation. Clinical Transplantation 2016, 30:247–255. [DOI] [PubMed] [Google Scholar]
- 23.Weig T, Milger K, Langhans B, Janitza S, Sisic A, Kenn K, Irlbeck T, Pomschar A, Johnson T, Irlbeck M, et al. : Core Muscle Size Predicts Postoperative Outcome in Lung Transplant Candidates. Ann Thorac Surg 2016, 101:1318–1325. [DOI] [PubMed] [Google Scholar]
- 24.Pienta MJ, Zhang P, Derstine BA, Enchakalody B, Weir WB, Grenda T, Goulson R, Reddy RM, Chang AC, Wang SC, et al. : Analytic Morphomics Predict Outcomes After Lung Transplantation. Ann Thorac Surg 2018, 105:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoang V, Li GW, Kao CC, Dronavalli G, Parulekar AD: Determinants of pre‐transplantation pectoralis muscle area (PMA) and post‐transplantation change in PMA in lung transplant recipients. Clinical Transplantation 2017, 31. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman BM, Blumenthal JA, Carney RC, O’Hayer CV, Freedland K, Smith PJ, Babyak MA, Davis RD, Mathew JP, Martinu T, et al. : Changes in neurocognitive functioning following lung transplantation. Am J Transplant 2012, 12:2519–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith PJ, Rivelli S, Waters A, Reynolds J, Hoyle A, Flowers M, Davis RD, Palmer SM, Mathew J, Durheim M, et al. : Neurocognitive changes after lung transplantation. Ann Am Thorac Soc 2014, 11:1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrianopoulos V, Gloeckl R, Boensch M, Hoster K, Schneeberger T, Jarosch I, Koczulla RA, Kenn K: Improvements in functional and cognitive status following short-term pulmonary rehabilitation in COPD lung transplant recipients: a pilot study. ERJ Open Res 2019, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith PJ, Blumenthal JA, Hoffman BM, Davis RD, Palmer SM: Postoperative cognitive dysfunction and mortality following lung transplantation. American Journal of Transplantation 2017, 18:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith PJ, Blumenthal JA, Carney RM, Freedland KE, O’Hayer CVF, Trulock EP, Martinu T, Schwartz TA, Hoffman BM, Koch GG, et al. : Neurobehavioral functioning and survival following lung transplantation. Chest 2014, 145:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith PJ, Blumenthal JA, Hoffman BM, Davis RD, Palmer SM: Postoperative cognitive dysfunction and mortality following lung transplantation. Am J Transplant 2018, 18:696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtwright AM, Salomon S, Lehmann LS, Wolfe DJ, Goldberg HJ: The Effect of Pretransplant Depression and Anxiety on Survival Following Lung Transplant: A Meta-analysis. Psychosomatics 2016, 57:238–245. [DOI] [PubMed] [Google Scholar]
- 33.Parekh PI, Blumenthal JA, Babyak MA, Merrill K, Carney RM, Davis RD, Palmer SM, Investigators I: Psychiatric disorder and quality of life in patients awaiting lung transplantation. Chest 2003, 124:1682–1688. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberger EM, DiMartini AF, DeVito Dabbs AJ, Bermudez CA, Pilewski JM, Toyoda Y, Dew MA: Psychiatric Predictors of Long-term Transplant-Related Outcomes in Lung Transplant Recipients. Transplantation 2016, 100:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PJ, Blumenthal JA, Trulock EP, Freedland KE, Carney RM, Davis RD, Hoffman BM, Palmer SM: Psychosocial Predictors of Mortality Following Lung Transplantation. Am J Transplant 2016, 16:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PJ, Blumenthal JA, Snyder LD, Mathew JP, Durheim MT, Hoffman BM, Rivelli SK, Palmer SM: Depressive symptoms and early mortality following lung transplantation: A pilot study. Clin Transplant 2017, 31. [DOI] [PubMed] [Google Scholar]
- 37.Dew MA, DiMartini AF, Dobbels F, Grady KL, Jowsey-Gregoire SG, Kaan A, Kendall K, Young QR, Abbey SE, Butt Z, et al. : The 2018 ISHLT/APM/AST/ICCAC/STSW Recommendations for the Psychosocial Evaluation of Adult Cardiothoracic Transplant Candidates and Candidates for Long-term Mechanical Circulatory Support. Psychosomatics 2018, 59:415–440. [DOI] [PubMed] [Google Scholar]
- 38.Schwebel C, Pin I, Barnoud D, Devouassoux G, Brichon PY, Chaffanjon P, Chavanon O, Sessa C, Blin D, Guignier M, et al. : Prevalence and consequences of nutritional depletion in lung transplant candidates. Eur Respir J 2000, 16:1050–1055. [DOI] [PubMed] [Google Scholar]
- 39.Chamogeorgakis T, Mason DP, Murthy SC, Thuita L, Raymond DP, Pettersson GB, Blackstone EH: Impact of nutritional state on lung transplant outcomes. J Heart Lung Transplant 2013, 32:693–700. [DOI] [PubMed] [Google Scholar]
- 40.Lederer DJ, Wilt JS, D’Ovidio F, Bacchetta MD, Shah L, Ravichandran S, Lenoir J, Klein B, Sonett JR, Arcasoy SM: Obesity and underweight are associated with an increased risk of death after lung transplantation. Am J Respir Crit Care Med 2009, 180:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allen JG, Arnaoutakis GJ, Weiss ES, Merlo CA, Conte JV, Shah AS: The impact of recipient body mass index on survival after lung transplantation. J Heart Lung Transplant 2010, 29:1026–1033. [DOI] [PubMed] [Google Scholar]
- 42.Kim CY, Kim SY, Song JH, Kim YS, Jeong SJ, Lee JG, Paik HC, Park MS: Usefulness of the preoperative prognostic nutritional index score as a predictor of the outcomes of lung transplantation: A single-institution experience. Clin Nutr 2019, 38:2423–2429. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Mathur S, Chowdhury NA, Helm D, Singer LG: Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant 2013, 32:626–632. [DOI] [PubMed] [Google Scholar]
- 44.Florian J, Watte G, Teixeira PJZ, Altmayer S, Schio SM, Sanchez LB, Nascimento DZ, Camargo SM, Perin FA, Camargo JJ, et al. : Pulmonary rehabilitation improves survival in patients with idiopathic pulmonary fibrosis undergoing lung transplantation. Sci Rep 2019, 9:9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byrd R, Smith P, Mohamedaly O, Snyder LD, Pastva AM: A 1-Month Physical Therapy-Based Outpatient Program for Adults Awaiting Lung Transplantation: A Retrospective Analysis of Exercise Capacity, Symptoms, and Quality of Life. Cardiopulm Phys Ther J 2019, 30:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pritz T, Weinberger B, Grubeck-Loebenstein B: The aging bone marrow and its impact on immune responses in old age. Immunology Letters 2014, 162:310–315. [DOI] [PubMed] [Google Scholar]
- 47.Kirkland JL, Tchkonia T: Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcos-Perez D, Sanchez-Flores M, Maseda A, Lorenzo-Lopez L, Millan-Calenti JC, Gostner JM, Fuchs D, Pasaro E, Laffon B, Valdiglesias V: Frailty in Older Adults Is Associated With Plasma Concentrations of Inflammatory Mediators but Not With Lymphocyte Subpopulations. Front Immunol 2018, 9:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naikawadi RP, Green G, Jones KD, Achtar-Zadeh N, Mieleszko JE, Arnould I, Kukreja J, Greenland J, Wolters PJ: Airway Epithelial Telomere Dysfunction Drives Remodeling Similar to Chronic Lung Allograft Dysfunction. Am J Respir Cell Mol Biol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Popescu I, Mannem H, Winters SA, Hoji A, Silveira F, McNally E, Pipeling MR, Lendermon EA, Morrell MR, Pilewski JM, et al. : Impaired Cytomegalovirus Immunity in Idiopathic Pulmonary Fibrosis Lung Transplant Recipients with Short Telomeres. Am J Respir Crit Care Med 2019, 199:362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]


