In this issue, Schulte et al (1) report novel data demonstrating increased expression of GM-CSFR (IT) mRNA in peripheral blood cells of newborn infants with respiratory illness compared to those without respiratory signs and symptoms. Since GM-CSFR (IT) is a truncated receptor isoform that inhibits GM-CSF signaling in monocytes and macrophages, its expression may inhibit cellular processes dependent upon GM-CSF, including pulmonary surfactant catabolism and innate host defense in the lung. Increased levels of the inhibitory GM-CSF receptor fragment provide potential mechanisms to inhibit surfactant catabolism thereby enhancing surfactant pools as an adaptive response to respiratory illness in neonates. The findings serve to highlight some of the complex cellular and molecular mechanisms controlling surfactant homeostasis in the neonatal lung.
Establishing cellular and alveolar surfactant pool sizes in the newborn lung
Alveolar surfactant pool size represents the final output of a complex biological network integrating alveolar epithelial cell differentiation, surfactant lipid and protein biosynthesis, and the packaging, storage, secretion, assembly, and turnover of pulmonary surfactant. Each of the processes serves to establish the high levels of surfactant pools during the adaptation to breathing at birth (2, 3). Surfactant catabolism and recycling are initiated after birth, when alveolar macrophages differentiate and recycling by alveolar type 2 (AT2) cells begin to influence surfactant pool size. Of note, the lungs of newborn infants do not contain mature alveolar macrophages until after birth when activation of GM-CSF signaling causes expression and differentiation of macrophage progenitors. However, since peripheral air spaces of preterm infants have relatively low levels of surfactant, its catabolism may not have a substantial effect when pool sizes are low (4).
After birth, complex interactions between AT 2 cells and alveolar macrophages (AM) maintain alveolar surfactant concentrations. Surfactant proteins, which play primary roles in surfactant assembly and reuptake by AT2 cells (5), and GM-CSF, which recruits and activates alveolar macrophage progenitors begin the postnatal process of surfactant recycling and catabolism. GM-CSF produced by alveolar cells signals to alveolar macrophages to activate GM-CSF receptors (α and β chains) on alveolar macrophages to regulate both surfactant lipid catabolism and innate immune functions after birth (6, 7). Surfactant protein and lipid synthesis are highly active in late gestation in preparation for birth when surfactant pool sizes are at their highest (2, 3, 8). Synthetic rates of surfactant lipids are lower in preterm infants and term infants with severe respiratory disease (3, 6, 8). Regulation of surfactant recycling and catabolism begins after birth, thus the relatively large surfactant pool sizes present in the neonatal lung decrease slowly in the days and weeks after birth (9, 10). In the newborn rabbit, initial alveolar surfactant pool size normalizes to adult levels over two weeks (11). The dramatic increase in intracellular surfactant which occurs in late gestation in preparation for birth is regulated by a transcriptional network controlling AT2 cell differentiation, as well as surfactant lipid and protein synthesis (2, 12). After birth, reuptake, recycling, and secretion of surfactant by AT2 cells are influenced by surfactant protein D (SP-D) and GPR-116, a G-coupled receptor on AT2 cells (9, 13, 14). The lack of SP-D or GPR-116 signaling causes a marked accumulation of alveolar lipids and failure of normalization of postnatal alveolar surfactant to the lower levels characteristic of the mature lung (13, 14).
Loss of GM-CSF signaling as in GM-CSF receptor-deficient mice, patients with mutations in GM-CSF receptors (CSF2RB, CSF2RA), and patients with auto-antibodies neutralizing GM-CSF, cause pulmonary alveolar proteinosis (PAP) [for review (15)]. The lack of GM-CSF receptor signaling impairs surfactant catabolism resulting in the accumulation of toxic levels of cholesterol in alveolar macrophages. In PAP, alveolar surfactant accumulates despite the continued uptake of surfactant lipids and proteins by the GMCSF deficient macrophages. The slow, but massive accumulation of cholesterol produced by partial catabolism of surfactant lipid products is toxic, resulting in “foamy” macrophages and impaired innate immune functions of alveolar macrophages. GM-CSF signaling is required for alveolar macrophage differentiation, migration, phagocytosis, and the clearance of viral, fungal, and bacterial pathogens (7). Surfactant clearance and pulmonary innate immunity are also dependent on adequate numbers and differentiation of alveolar macrophages. After binding to GM-CSF receptors, GM-CSF initiates a transcriptional network mediated by Stat-5 phosphorylation, PPAR gamma, and PU.1 which regulate genes critical for alveolar macrophage differentiation and function, including cholesterol efflux needed to maintain AM function (7, 15).
Origins and recruitment of pulmonary macrophages
Present understanding of embryonic and postnatal origins of alveolar macrophages is based primarily on studies in the mouse (16, 17). These studies support the concept that alveolar macrophages, like other tissue macrophages, are derived from fetal liver progenitors, which colonize the lung and proliferate relatively late in gestation. AM differentiation occurs primarily after birth as the extracellular surfactant system is established and pulmonary innate defenses mature in response to the extrauterine environment and ventilation. Pulmonary alveolar macrophage progenitors are distinct from those of peripheral blood in monocytes (16, 17). After differentiation AMs reside and self-perpetuate primarily from local progenitors in a process remarkably specific to the lung and GM-CSF. The present observations by Schulte et al (1), that increased expression of GM-CSFRB (IT) RNA in neonates with respiratory illnesses raise the possibility that GM-CSFRV (IT) whether produced systematically, or within the alveolus may influence AM activity, in turn modulating surfactant catabolism, pool size, and innate immunity. Thus, mechanisms controlling transcription, splicing, and accessibility of B (IT) peptide and the role of the B (IT) in control of AM or monocyte GM-CSF signaling are of considerable interest during the perinatal period, when postnatal surfactant homeostasis is established to enable pulmonary responses to physiologic and environmental challenges. Future studies identifying GM-CSFR (IT) in the lung, its ability to influence alveolar macrophage differentiation and to regulate surfactant catabolism or innate defense will be of considerable interest in understanding the process of respiratory adaptation after birth.
Acknowledgement:
Figure 1 kindly provided by Paritha Arumugam, PhD and Bruce C. Trapnell, MS, MD, Translational Pulmonary Science Center, University of Cincinnati College of Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA. Erika Smith for Commentary preparation.
Figure:
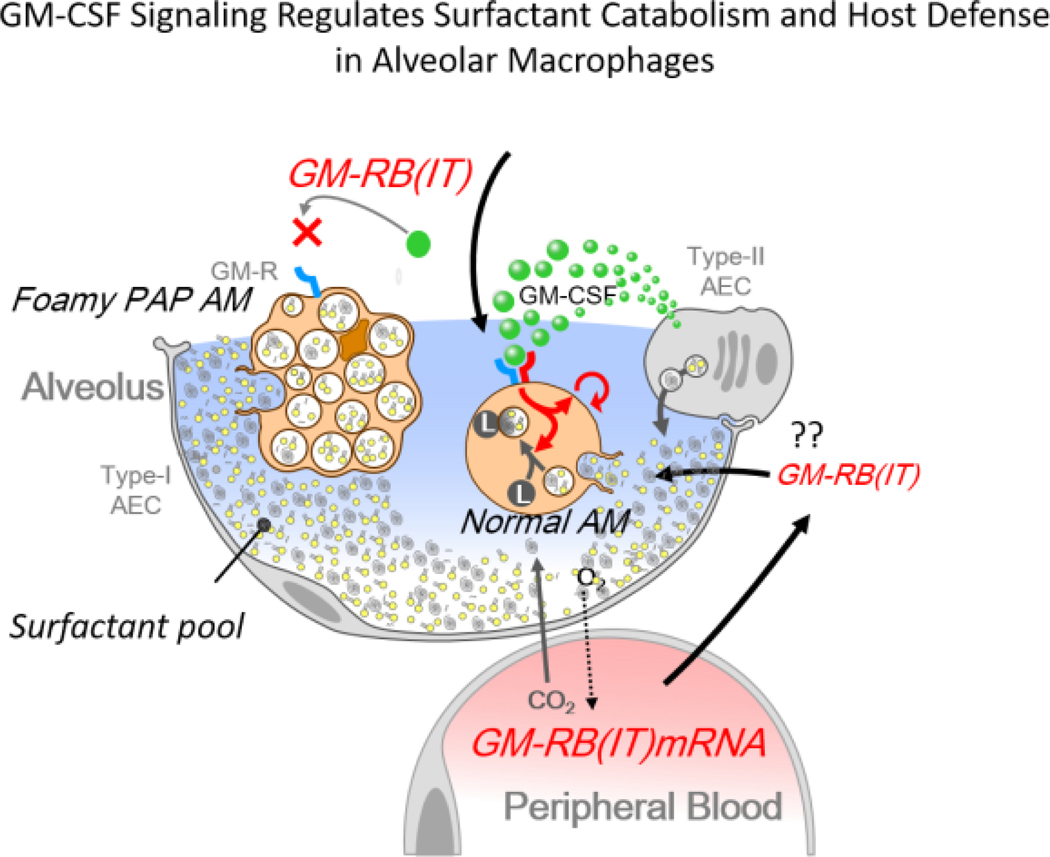
GM-CSF signaling and the regulation of surfactant pool size: a proposed role for GM-CSFRB (IT). Schulte et al (1) identified increased mRNA encoding GM-CSF receptor B (IT) or GM-RB (IT) in the peripheral blood of neonates. The GM-CSFRB (IT) mRNA encodes a truncated and therefore inhibitory “decoy” of the GM-CSF receptor, capable of inhibiting GM-CSF signaling. If GM-RB (IT) reaches the alveoli and inhibits GM-CSF signaling in alveolar macrophages, both surfactant catabolism and innate defense functions might be impaired. The prolonged lack of GM-CSF signaling in alveolar macrophages causes Pulmonary Alveolar Proteinosis (PAP) associated with the marked accumulation of surfactant lipids in the alveoli. Uptake and partial catabolism of surfactant lipids by GM-CSF deficient AMs causes intracellular accumulation of cholesterol causing foamy macrophages, further impairing alveolar macrophage function. Increased expression of GM-RB (IT) in the neonate with respiratory illness is proposed to inhibit GM-CSF signaling in alveolar macrophages and perhaps in circulating immune cells, impairing host defense and providing a potential mechanism by which surfactant pool sizes might be increased by the inhibition of surfactant catabolism and therefore serve as an adaptive response to respiratory illness in the infants.
Funding Source: NIH/NHLBI HL122642 to JAW
Footnotes
Conflict of Interest: Authors have no Conflict of Interest to disclose.
References
- 1.Schulte V, Sipol A, Burdach S,. The truncated splice variant of the GM-CSF receptor beta-chain in peripheral blood serves as severity biomarker of respiratory failure in newborns. Neonatology 2020. This issue. [DOI] [PubMed] [Google Scholar]
- 2.Guo M, Du Y, Gokey JJ, Ray S, Bell SM, Adam M, et al. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun. 2019;10(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmermann LJ, Janssen DJ, Tibboel D, Hamvas A, Carnielli VP. Surfactant metabolism in the neonate. Biol Neonate. 2005;87(4):296–307. [DOI] [PubMed] [Google Scholar]
- 4.Adams FH, Fujiwara T, Latta H. ‘Alveolar’ and whole lung phospholipids of premature newborn lambs. Correlations with surface tension, respiratory distress and pathology. Biol Neonate. 1971;17(3):198–218. [DOI] [PubMed] [Google Scholar]
- 5.Whitsett JA, Wert SE, Weaver TE. Diseases of pulmonary surfactant homeostasis. Annu Rev Pathol. 2015;10:371–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13(6):1455–76. [DOI] [PubMed] [Google Scholar]
- 7.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. [DOI] [PubMed] [Google Scholar]
- 8.Bohlin K, Merchak A, Spence K, Patterson BW, Hamvas A. Endogenous surfactant metabolism in newborn infants with and without respiratory failure. Pediatr Res. 2003;54(2):185–91. [DOI] [PubMed] [Google Scholar]
- 9.Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J Appl Physiol (1985). 2009;106(5):1545–52. [DOI] [PubMed] [Google Scholar]
- 10.Jobe AH, Ikegami M. Surfactant metabolism. Clin Perinatol. 1993;20(4):683–96. [PubMed] [Google Scholar]
- 11.Jobe A, Ikegami M, Jacobs H. Changes in the amount of lung and airway phosphatidylcholine in 0.5–12.5-day-old rabbits. Biochim Biophys Acta. 1981;664(1):182–7. [DOI] [PubMed] [Google Scholar]
- 12.Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and Regenerating the Lung Cell by Cell. Physiol Rev. 2019;99(1):513–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown K, Filuta A, Ludwig MG, Seuwen K, Jaros J, Vidal S, et al. Epithelial Gpr116 regulates pulmonary alveolar homeostasis via Gq/11 signaling. JCI Insight. 2017;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikegami M, Hull WM, Yoshida M, Wert SE, Whitsett JA. SP-D and GM-CSF regulate surfactant homeostasis via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L697–703. [DOI] [PubMed] [Google Scholar]
- 15.Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J, et al. Pulmonary alveolar proteinosis. Nat Rev Dis Primers. 2019;5(1):16. [DOI] [PubMed] [Google Scholar]
- 16.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210(10):1977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development. 2016;143(8):1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]