Abstract
Objective:
To describe the presentation and outcomes of patients with adrenal ganglioneuromas (AGN).
Design:
Single-center retrospective cohort study (January 1st, 1995 to December 31st, 2019) and systematic review of literature (January 1st, 1980 to November 19th, 2019).
Patients:
Diagnosed with histologically confirmed AGN.
Measurements:
Baseline clinical, imaging, and biochemical characteristics, recurrence rates, and mortality. Sub-group analysis was performed on tumors with histologic elements of ganglioneuroma and pheochromocytoma (i.e. composite tumors).
Results:
The cohort study included 45 patients with AGN, 20 (44%) of which had composite tumors. Compared to pure AGN, patients with composite tumor were older (median age, 62.5 vs. 35 years, P <0.001), had smaller tumors (median size, 3.9 vs 5.7 cm, P = 0.016), and were discovered incidentally less frequently (65% vs 84%, P = 0.009). No recurrences or ganglioneuroma-specific mortality occurred during follow-up (range, 0-266 months). The systematic review included 14 additional studies and 421 patients. The mean age of diagnosis was 39 years and 47% were women. AGNs were discovered incidentally in 72% of patients, were predominantly unilateral (99%), had a mean diameter of 5.8 cm, and an unenhanced CT attenuation of −118-49 Hounsfield units (HU). On imaging, 69% of AGNs were homogenous, 41% demonstrated calcifications, and 40% were lobulated.
Conclusions:
AGNs are rare benign tumors that present with variable imaging features including large size, unenhanced CT attenuation >20 HU, calcifications, and lobulated shape. Imaging characteristics can assist in establishing a diagnosis and avoiding an unnecessary adrenalectomy. The association of pheochromocytomas with AGNs is frequent. Diagnosis should include biochemical testing.
Keywords: adrenal mass, composite tumor, pheochromocytoma, adrenalectomy, adrenal imaging
Introduction
Adrenal tumors occur in approximately 5% of adults, however adrenal ganglioneuromas (AGN) are rare, representing 0.2-0.4% of all adrenal tumors1-3. Ganglioneuromas are benign neoplasms derived from neural crest cells and composed of a stroma consisting of mature Schwann cells, ganglion cells, and nerve fibers.4 The most frequently involved site for ganglioneuromas is the adrenal glands, representing approximately 30% of cases, followed by the mediastinum, retroperitoneum, and neck.5 Typically these tumors are found incidentally on imaging, but also have been reported in association with genetic disorders such as multiple endocrine neoplasia type 2 and neurofibromatosis type 1.6,7
Limited literature on AGNs suggests a slight female predominance and diagnosed more frequently in the fourth and fifth decades, however findings vary among studies.4,8-11 These tumors have a predilection for the right side, are usually asymptomatic and hormonally silent, and have an excellent prognosis.4,9,12-15 Mixed adrenal tumors composed of cells displaying more than one line of differentiation are commonly referred to as composite tumors, and such tumors with histologic elements of both ganglioneuroma and pheochromocytoma, myelolipoma, neuroblastoma, and/or nerve sheath tumor have also been reported.10,15,16
Pre-surgical diagnosis of AGN is challenging due to the variable clinical presentation and lack of pathognomonic imaging findings.5,8-15 Without surgical pathology, it is difficult to differentiate AGNs from other solid adrenal masses such as pheochromocytomas, adenomas, and neuroblastomas.4,11
We performed a single center retrospective cohort study and conducted a systematic review of the literature with the following objectives: 1) to describe and quantify baseline clinical characteristics of patients with AGN; 2) to investigate potential associations between the diagnosis of AGN and genetic syndromes; 3) to describe the imaging characteristics of AGN; 4) to determine the differences in presentation of patients with composite AGNs; and 5) to determine the rate of recurrence and mortality of patients with AGNs.
Methods
Given the rarity of AGN, we anticipated a small number of published cases and incomplete reporting of clinical and radiographic characteristics in the literature. Therefore, we supplemented the systematic review with a review of AGN cases evaluated at our large tertiary academic center. We followed the framework recommended by Lin et al.17
Single Center Retrospective Cohort Study
The study protocol was approved by the Mayo Clinic Institutional Review Board. We identified all patients with pathology confirmed AGN who were evaluated at Mayo Clinic in Rochester, Minnesota, between 1995 and 2019. Each patient’s medical record was reviewed for clinical information, biochemical and histopathologic data, imaging characteristics, and therapy. We excluded patients who did not have a histopathologic diagnosis of AGN. We defined composite tumors as tumors with intermixed elements displaying more than one cell type or line of differentiation. Specifically, tumors were reported as “composite” if histologic elements of both ganglioneuroma and pheochromocytoma were intermixed within a single mass on histopathology.
Systematic Review of Literature
The systematic review was performed based on a pre-determined protocol and reported according to standards set in the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.18 A comprehensive search of several databases from January 1st, 1980 to November 19th, 2019, in any language, was conducted. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Scopus. The search strategy was designed and conducted by a medical reference librarian (L.P.) with input from study investigators with experience in conducting systematic reviews (I.B., M.H.M.). Controlled vocabulary supplemented with keywords was used to search for studies of AGNs. The detailed strategy listing all search terms used and how they are combined is available in the Supplemental Table 1.
Selection of Studies
We excluded non-original studies, case reports, and studies reporting less than 10 patients. No language restrictions were used. When two studies included an overlapping patient cohort, the study with the larger number of patients or longer time interval was included.
Titles and abstracts of the identified studies were screened for eligibility by two reviewers (K.D., J.K.) working independently and in duplicate. At this phase of study selection most of the identified studies from our search were excluded. If reviewers disagreed on a study, the study progressed to the next phase. The two reviewers then screened full-text articles independently and in duplicate for eligibility for inclusion, Figure 1. Disagreements at this phase were resolved by consensus or a third reviewer (I.B.).
Figure 1:
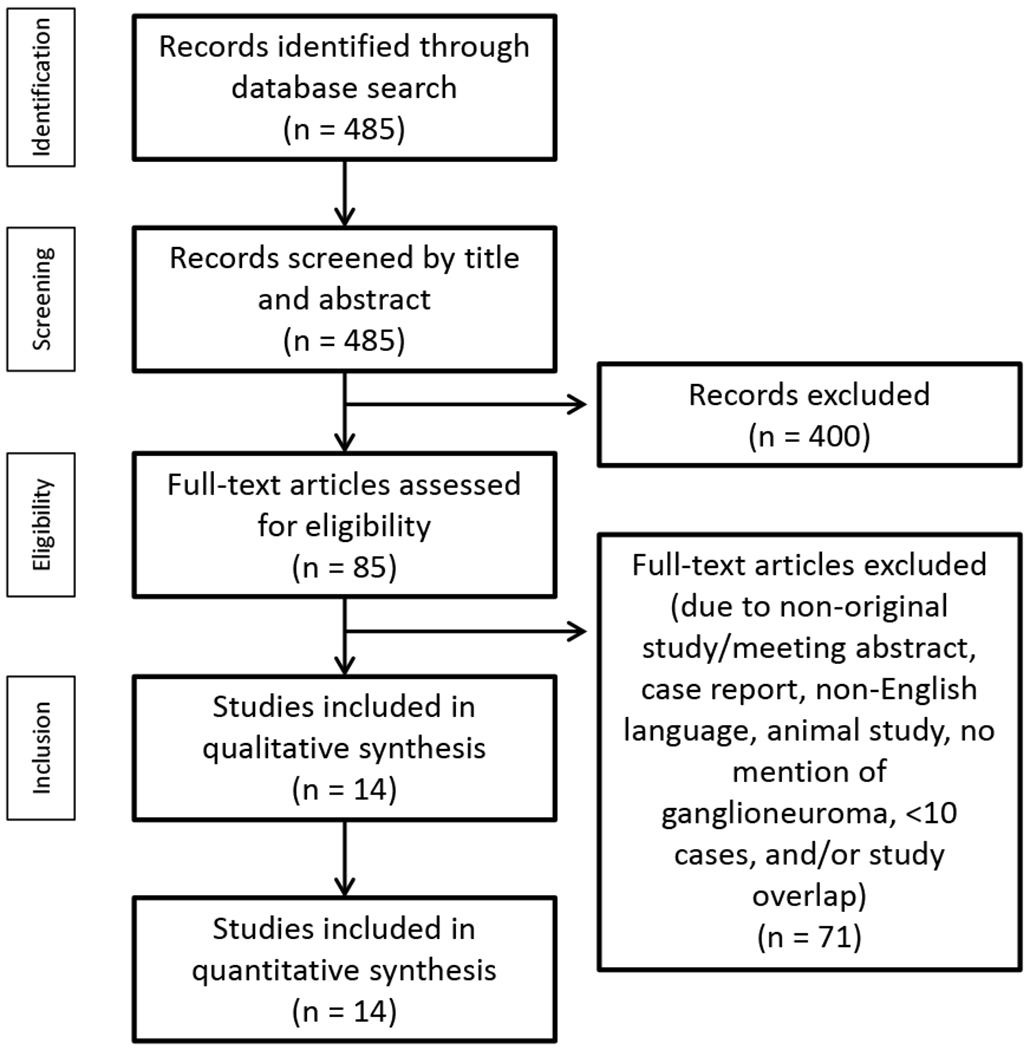
Literature search and selection of studies, PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flow diagram outlining the process of study selection.
Data Extraction and Management
Data extraction was performed independently and in duplicate by two reviewers to collect information from each eligible study. For each eligible study, the following data were extracted: first author, year of publication, study design, location, time interval of patient enrollment, patient age and gender, number of AGNs, tumor size and laterality, mode of discovery, composite tumors, imaging characteristics, intervention, duration of follow-up, recurrence, and mortality. The data extraction forms were then combined and disagreements between the two reviewers were resolved by referral to the full text of the study and consensus.
Methodological Quality
Two reviewers working independently and in duplicate assessed the methodological quality of the studies using a tool designed to appraise non-comparative case series, Figure 2.19 We evaluated the following domains, Supplemental Table 2: (i) whether the study sample represented the population of interest, (ii) whether histopathology obtained via adrenalectomy or biopsy was reported clearly, (iii) how the outcomes were assessed, and (iv) whether there was a sufficient follow-up period for the outcomes to occur (at least 5 years).
Figure 2:
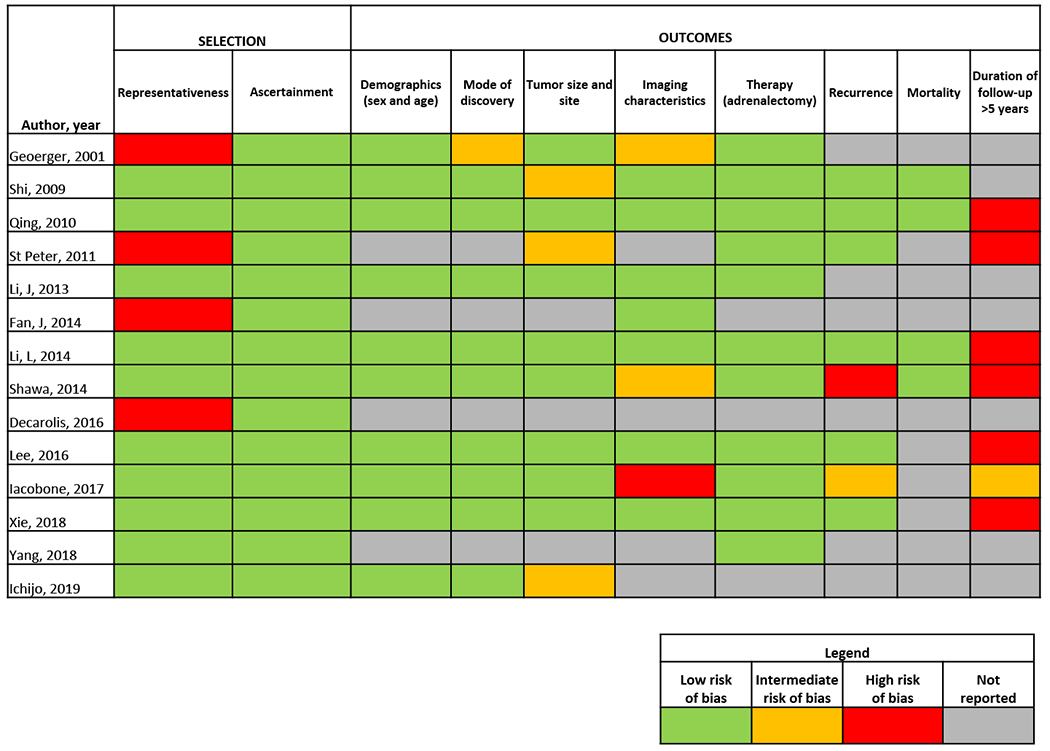
Methodological quality assessment of the included studies.
Statistical Analysis
For the single center study, a descriptive analysis was performed. Categorical data were summarized as counts and percentages and continuous data were summarized as median and ranges. A predefined subgroup analysis on composite tumors was also performed. Associations between variables were assessed using the Fisher exact test for categorical variables and the Wilcoxon/Kruskal–Wallis test for continuous variables. Statistical significance was defined as P-value less than 0.05. We performed statistical analysis using JMP software version 15.
For the systematic review of literature, descriptive statistics were extracted for each included study. Data were insufficient for meta-analysis. Data were summarized as total n (%) for categorical variables and range for continuous variables where available.
Results
Cohort Study
In our retrospective study of 45 patients, the median age at AGN diagnosis was 44 years (range, 6-87), and 25 (56%) were women, Table 1. The mode of AGN diagnosis was incidental in 34 (76%) patients, due to symptoms of mass effect in 5 (11%), due to symptoms of hormone excess in 5 (11%), and during the evaluation of a genetic syndrome in 1 (2%). AGNs had a median tumor size of 48 mm (range, 10-125) and were located in the right adrenal gland in 25 (56%) cases, on the left in 19 (42%), and bilateral in 1 (2%) patient.
Table 1:
Clinical, imaging, and biochemical presentation of patients with ganglioneuromas (single-center study)
| Variable | Total (N = 45) | Ganglioneuroma (N = 25) | Combined ganglioneuroma and pheochromocytoma (N = 20) | P value |
|---|---|---|---|---|
| Baseline Patient Characteristics | ||||
| Sex, n | ||||
| Female | 25 (56%) | 15 (60%) | 10 (50%) | 0.557 |
| Male | 20 (44%) | 10 (40%) | 10 (50%) | |
| Age at diagnosis, median (range) | 44 (6-87) | 35 (6-80) | 62.5 (20-87) | <0.001* |
| Race, n | ||||
| Caucasian | 40 (89%) | 22 (88%) | 18 (90%) | 0.456 |
| Asian | 1 (2%) | 0 (0%) | 1 (5%) | |
| Unknown | 4 (9%) | 3 (12%) | 1 (5%) | |
| Seen by endocrinologist at initial diagnosis and/or follow-up, n | 38 (84%) | 19 (76%) | 19 (95%) | 0.112 |
| Mode of Discovery, n | ||||
| Incidental | 34 (76%) | 21 (84%) | 13 (65%) | |
| Mass effect | 5 (11%) | 4 (16%) | 1 (5%) | 0.009* |
| Hormone excess | 5 (11%) | 0 (0%) | 5 (25%) | |
| Genetic syndrome | 1 (2%) | 0 (0%) | 1 (5%) | |
| Imaging Characteristics | ||||
| Tumor size | ||||
| Median (mm) | 48 | 57 | 39 | 0.016* |
| Range (mm) | 10-125 | 19-110 | 10-125 | |
| Laterality, n | ||||
| Right | 25 (56%) | 14 (56%) | 11 (55%) | 0.752 |
| Left | 19 (42%) | 11 (44%) | 8 (40%) | |
| Bilateral | 1 (2%) | 0 (%) | 1 (5%) | |
| Imaging performed prior to intervention, n = 40 | ||||
| Computed tomography | 36 (90%) | 20 (80%) | 16 (80%) | |
| Magnetic resonance imaging | 22 (55%) | 11 (44%) | 11 (55%) | - |
| Positron emission tomography | 4 (10%) | 2 (8%) | 2 (10%) | |
| Metaiodobenzylguanidine | 7 (18%) | 1 (4%) | 6 (30%) | |
| Not available | 5 (11%) | 4 (16%) | 1 (5%) | |
| Unenhanced CT available | 21 (47%) | 11 (44%) | 10 (50%) | |
| Homogeneous tumors | 18 (40%) | 11 (44%) | 7 (35%) | - |
| Median Hounsfield Units on CT, range | 30.3 (19-37.7) | 31 (23-37.7) | 26.3 (19-32) | |
| Heterogeneous tumors | 3 (7%) | 0 | 3 (15%) | |
| Range of Hounsfield Units | −118-49 | −118-49 | ||
| Enhanced CT available | 20 (44%) | 11 (44%) | 9 (45%) | |
| Homogeneous tumors | 9 (20%) | 7 (28%) | 2 (10%) | - |
| Median Hounsfield Units on CT, range | 40.7 (19-110.3) | 45.3 (35-110.3) | 25.6 (19-33.7) | |
| Heterogeneous tumors | 11 (24%) | 4 (16%) | 7 (35%) | |
| Range of Hounsfield Units | 19-142 | 23-103 | 19-142 | |
| Other imaging characteristics on CT | ||||
| Calcifications, n = 24 | 8 (33%) | 7/14 (50%) | 1/10 (10%) | 0.114 |
| Homogeneous, n = 21 | 18 (86%) | 11/11 (100%) | 7/10 (70%) | 0.09* |
| Shape, n = 24 | ||||
| Round | 8 (33%) | 2/14 (14%) | 6/10 (60%) | 0.059 |
| Multilobulated | 16 (67%) | 12/14 (86%) | 4/10 (40%) | |
| Appearance, n = 24 | ||||
| Cystic | 1 (4%) | 1/14 (7%) | 0/10 (0%) | 1 |
| Non-cystic | 23 (96%) | 13/14 (93%) | 10/10 (100%) | |
| Hormonal Work-up and Therapy | ||||
| Catecholamine profile, n = 36 tested a | ||||
| Adrenergic | 15 (42%) | 0 (0%) | 15 (75%) | |
| Noradrenergic | 3 (8%) | 0 (0%) | 3 (15%) | <0.001* |
| Non-functional | 18 (50%) | 17 (68%) | 1 (5%) | |
| Not tested | 9 (20%) | 8 (32%) | 1 (5%) | |
| Interventions | ||||
| Laparoscopic adrenalectomy | 26 (58%) | 12 (48%) | 14 (70%) | |
| Open adrenalectomy | 16 (36%) | 10 (40%) | 6 (30%) | 0.224 |
| Adrenal biopsy | 3 (6%) | 3 (12%) | 0 (0%) | |
| Follow-up | ||||
| Median follow-up time, months (range) | 5.5 (0-266) | 2.5 (0-266) | 9.5 (0-150) | 0.428 |
| Recurrence, n | 0 | 0 | 0 | 1 |
| Mortality, n b | 5 (11%) | 1 (4%) | 4 (20%) | 0.155 |
Tumors with levels of plasma or urine metanephrines/catecholamines within the reference ranges were classified as non-functional. Tumors hypersecreting primarily epinephrine/metanephrine or norepinephrine/normetanephrine were classified as adrenergic and noradrenergic, respectively.
Mortality was unrelated to AGN. Reasons for death in the AGN group: complications of Alzheimer’s dementia (1). Reasons for death in the composite group: cardiovascular event (1), non-adrenal malignancy (1), complications of Lewy body dementia (1), or unknown (1)
Statistically significant (P <0.05)
CT=Computed tomography
On imaging, the cohort of AGNs appeared mostly non-cystic (96%) and over two-thirds were multilobulated. Calcifications were present in 33% of tumors, Figure 3A, 3B. Unenhanced computed tomography (CT) images were available for 21 AGNs and of these, 18 (86%) were homogeneous in appearance and 3 (14%) were heterogeneous. Of the AGNs with a homogeneous appearance on unenhanced CT, the median unenhanced CT attenuation was 30.3 Hounsfield units (HU) (range, 19-37.7). Enhanced CT images were available for 20 AGNs and of these, 9 (45%) were homogeneous in appearance and 11 (55%) were heterogeneous. Of the AGNs with a homogeneous appearance on enhanced CT, the median enhanced CT attenuation of 40.7 HU (range, 19-110.3). Most patients underwent adrenalectomy (58% laparoscopic vs. 36% open adrenalectomy) due to indeterminate imaging and had no tumor recurrence or AGN-related mortality during a median follow-up time of 5.5 months (range, 0-266).
Figure 3:
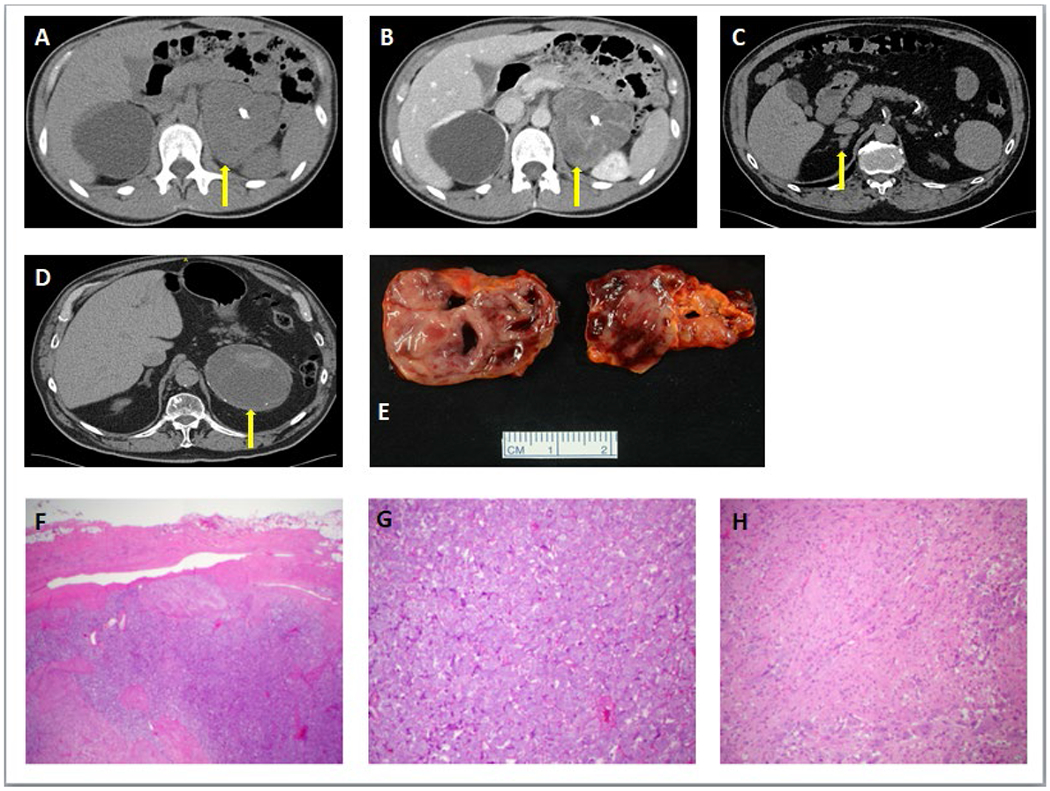
Computed Tomography imaging of selected patients with ganglioneuroma.
3A. On axial CT to evaluate a dilated right ureter, a 33-year-old woman had an incidentally discovered approximately 110 mm in maximal dimension, multi-lobulated, homogeneous left upper quadrant mass with internal calcifications, with unenhanced CT attenuation of 32 HU, and heterogenous appearance on post-contrast with a CT attenuation of 46-77 HU (3B). Following complete surgical resection of the left upper quadrant mass, pathology revealed a 136 × 96 × 81 mm left adrenal ganglioneuroma.
3C: In a 69-year-old man with an incidentally discovered right adrenal mass, axial CT showed an approximately 15 mm in maximal dimension, multi-lobulated, heterogeneous right adrenal mass without calcifications, with unenhanced CT attenuation of 25-44 HU, and post-contrast CT attenuation (not depicted) of 65-100 HU. Right adrenalectomy revealed a composite pheochromocytoma forming a 15 mm medullary tumor mass confined to the adrenal gland with negative surgical margins.
3D. In an 82-year-old man with an incidentally discovered left adrenal mass, axial CT showed an approximately 125 mm in maximal dimension, round, heterogeneous left adrenal mass with small rim calcifications, with unenhanced CT attenuation of 17-49 HU, and no post-contrast CT imaging was performed. Left adrenalectomy showed a composite tumor consisting of a ganglioneuroma with a pheochromocytoma.
3E. Gross pathology image of a composite adrenal ganglioneuroma and pheochromocytoma from a laparoscopic right adrenalectomy.
3F. Histopathology of a composite ganglioneuroma-pheochromocytoma showing the pheochromocytoma component on the right-hand side of the image and the ganglioneuroma component on the bottom-left of the image.
3G. High-power image of pheochromocytoma component showing the classic nested (zellballen) architecture of pheochromocytoma.
3H. High-power image of ganglioneuroma component showing a combination of Schwannian stroma and ganglion cells characteristic of ganglioneuroma.
Twenty patients (44%) had a composite tumor of AGN and pheochromocytoma (AGN-PHEO) (Table 1, Figure 3C-H). Patients diagnosed with a composite tumor were older (median age, 62.5 vs 35 years, P <0.001), had smaller tumors (median size, 39 vs 57 mm, P = 0.016), and were less likely to be discovered incidentally (65% vs 84%, P = 0.009) than patients with only AGN.
On imaging, composite tumors appeared non-cystic (100%) and mostly round (60%), compared to a non-cystic and round appearance in 93% and 14% of pure AGNs, respectively. Calcifications were present in 10% of composite tumors, whereas 50% of pure AGNs had calcifications. Of the composite tumors with a homogeneous appearance on CT, the median unenhanced CT HU were 26.3 (range, 19-32) compared to 25.6 HU (range, 19-33.7) on enhanced CT. Pure AGNs with a homogeneous appearance on CT had median unenhanced CT HU of 31 (range, 23-37.7) compared to 45.3 HU (range, 35-110.3) on enhanced CT, Table 1.
The limited number of magnetic resonance imaging (MRI) studies in our cohort prevented a direct statistical comparison of MRI characteristics between subjects with composite tumors and those with pure AGNs. However, in our cohort, MRI features common to both composite tumors and pure AGNs included hypointensity on T1-weighted images, hyperintensity on T2-weighted images, contrast enhancement, and lack of signal dropout on out of phase imaging. In contrast, septations were only seen in subjects with pure AGNs. One subject with a pure AGN had stellate, radially oriented septations and another had multiple enhancing septations. In the composite AGN-PHEO group, six patients had Metaiodobenzylguanidine (MIBG) scintigraphy (moderate to intense uptake in 5 patients, and no uptake in 1 patient) and two patients had Fluorodeoxyglucose-positron emission tomography (mild uptake with SUV max of 2-3.3).
The biochemical phenotype in the patients with composite tumors was adrenergic (n=15, 75%), noradrenergic (n=3, 15%), and nonfunctioning (n=1, 5%), Table 1.
Patients were treated with either laparoscopic (n=26) or open adrenalectomy (n=16). Reasons for open surgery included additional surgical indication (n=6, 38%), large size of tumor (n=2, 13%), concern for malignancy (n=1, 6%), additional surgical risk (n=2, 13 %), unknown (n=5, 31%). All patients with composite tumors were suspected to have pheochromocytoma prior to adrenalectomy, and therefore were treated with alpha-adrenergic blockade therapy prior to adrenalectomy. No perioperative complications were observed. The peri-operative outcome was uncomplicated in 18 patients in the composite group. One patient developed adrenal insufficiency after bilateral adrenalectomy, and peri-operative outcome was unknown in one patient. All patients underwent a clinical evaluation for personal or family history of genetic syndromes. However, only 3 patients with composite AGN underwent genetic testing, which was negative in 2 patients, and multiple endocrine neoplasia type 2B was detected in 1 patient. Overall, 21 patients were followed for at least one year at our institution, with the rest pursuing local follow up. Normalization of catecholamine excess was confirmed in 16 (80%) patients with composite tumors. There were no differences between sex, race, tumor laterality, tumor calcifications, homogeneity, shape, or appearance of AGNs compared to composite AGNs.
Systematic Review of Literature
From the initial search strategy, 485 records were identified for screening of titles and abstracts, Figure 1. Of these, 85 were selected for detailed, full-text assessment. Following full-text assessment of the 85 articles, 71 were excluded. The 14 studies included in the systematic review were published between 2001 and 2019, Table 2.4,8-11,14,20-27 The majority were retrospective cohort studies, with one being cross-sectional. Most were conducted in China (n=7). Included studies were determined to have adequate methodological quality, although 4 studies had limitations in the domains of representativeness of the cohort, and all studies had insufficient or unclear duration of follow-up, Figure 2. Demographics and mode of discovery were reported in 10 studies. Tumor size and site were sufficiently reported in eight studies, and imaging characteristics were reported in 10 studies.
Table 2:
Characteristics of included studies
| Author, year | Years of patient enrollment | Country of the study | Type of study | N | % Women | Mean age,years | Mode of discovery (n) | Composite, % | Histopathology, % | Recurrence (n) | Mortality (n) | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geoerger, 2001 | 1981-1999 | Germany | Retrospective | 10 | 30% | 7.3 ± 6.9 | Incidental (3), mass effect (4), othera (3) | 0% | Adrenalectomy, 100% | NR | NR | NR |
| Shi, 2009 | 1999-2007 | China | Retrospective | 18 | 44% | 41.2 (range 16-67) | Incidental (14), othera (4) | NR | Laparoscopic adrenalectomy, 89%; Open adrenalectomy, 11% | 0 | 0 | NR |
| Qing, 2010 | 1999-2009 | China | Retrospective | 17 | 71% | 39.2 (range 7-72) | Incidental (12), mass effect (4), othera (1) | 0% | Laparoscopic, 47%; Open, 53% | 0 | 0 | 41.8 (mean) |
| St Peter, 2011 | 2001-2011 | USA | Retrospective Multicenter | 22 | NR | NR | NR | NR | Laparoscopic, 100% | 0 | NR | 18 (median) |
| Li, J, 2013 | 1988-2011 | China | Retrospective | 29 | 38% | 35 | Incidental (12), mass effect (10), othera (7) | Unclear | Laparoscopic, 55%; Open, 45% | NR | NR | NR |
| Fan, 2014 | 2006-2013 | China | Retrospective | 35 | NR | NR | NR | NR | Surgical resection or biopsy, 100% | NR | NR | NR |
| Li, L, 2014 | 1997-2011 | China | Retrospective | 15 | 33% | 38.4 ± 7.9 | Incidental (14), mass effect (1) | 0% | Laparoscopic, 47%; Open, 53% | 0 | 0 | 64.8 (mean) |
| Shawa, 2014 | 1993-2012 | USA | Retrospective | 27 | 70% | 31 [median] (range 1.7-64) | Incidental (17), mass effect (9), othera (1) | 11% | Laparoscopic, 29%; Open, 71%; Biopsy 33% | 0 | 3b | 50 (median) |
| Decarolis, 2016 | 2000-2009 | Germany | Retrospective | 51 | NR | NR | NR | NR | Histopathology, >90% | NR | NR | NR |
| Lee, 2016 | 2002-2015 | South Korea | Retrospective | 35 | 60% | 33.4 ± 18.7 | Incidental (29), mass effect (6) | 0 | Laparoscopic, 51%; Open, 49% | 0 | NR | 19 (median) |
| Iacobone, 2017 | 1990-2015 | Italy | Retrospective | 10 | 60% | 37.8 ± 14.8 | Incidental (8), mass effect (2) | 0 | Laparoscopic, 60%; Open, 40% | 0 | NR | 114 (median) |
| Xie, 2018 | 2005-2016 | China | Retrospective | 42 | 31% | 35.3 (range 13-59) | Incidental (28), mass effect (20), othera (4) | 0 | Laparoscopic, 52%; Open, 48%; Biopsy 2% | 0 | NR | 76.5 (median) |
| Yang, 2018 | 2013-2018 | China | Retrospective | 15 | NR | NR | NR | NR | Adrenalectomy, 100% | NR | NR | NR |
| Ichijo, 2019 | 1999-2004 | Japan | Cross-sectional Multicenter | 50 | 36% | 48.3 ± 13.8 | Incidental (50) | 0 | Histopathology, 100% | NR | NR | NR |
| Current study, 2020 | 1995-2019 | USA | Retrospective | 45 | 56% | 47.4 ± 20.6 | Incidental (34), mass effect (5), othera (6) | 44% | Laparoscopic, 58%; Open, 35%; Biopsy, 7% | 0 | 5b | 33.7 (mean), 5.5 (median) |
| Pooled analysis | 1981-2019 | Asia:8 studies USA: 3 studies Europe: 3 studies | Retrospective : 14 studies Cross-sectional: 1 study | 421 | 47.3% | 39.2 | Incidental: 72% Mass effect: 20% Othera: 8% | Unclear | Laparoscopic: 58% Open: 41% Biopsy: 10% | No recurrence or mortality related to ganglioneuroma | Median of 5.5 – 114 months | |
aOther refers to additional reported modes of discovery that were unrelated to tumors diagnosed incidentally on imaging or after patient presentation with symptoms of mass effect.
bDeath due to causes unrelated to adrenal ganglioneuroma
NR=Not reported
Clinical and Radiographic Characteristics of Patients with Adrenal Ganglioneuroma
Overall, in the cohort study and systematic review, the mean age at diagnosis of AGN was 39 years, and 47% were women, Table 2. AGN were discovered incidentally in 72% of patients, due to symptoms of mass effect in 20%, and due to other symptoms in 8%. Most patients underwent adrenalectomy (58% laparoscopic, 41% open), and no recurrence or AGN-specific mortality was reported during a range of median follow-up of 5.5-114 months.
On imaging, AGN were mainly unilateral (59% right, 40% left, 1% bilateral), presenting with mean tumor size of 5.8 cm (range 1-20), Table 3. On unenhanced CT, CT attenuation was usually >20 HU (range −118-49). On imaging, 69% of tumors were homogenous, 41% presented with calcifications, 92% had well-defined margins. Composite ganglioneuromas were reported in 23/251 (9%) patients, most in our single center retrospective study, Table 2.
Table 3:
Imaging characteristics of ganglioneuromas
| Author, year | N | Mean tumor size, cm | Range tumor size, cm | Laterality Right/Left/Bilateral | CT available, n | Unenhanced CT, HU range | Post-contrast CT, HU range | Calcifications on imaginga, n | Homogeneous on imaginga, n | Well-defined margins on imaginga, n | Round appearance on imaginga, n | Cystic appearance on imaginga, n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Geoerger, 2001 | 10 | NR | NR | 5/5/0 | NR | NR | NR | NR | NR | NR | NR | NR |
| Shi, 2009 | 18 | 8.5 | 2.5-15 | NR | 18/18 | NR | NR | NR | 5/18 | 13/18 | 13/18 | NR |
| Qing, 2010 | 17 | 6.3 ± 3.1 | 1-13 | 13/4/0 | NR | NR | NR | 0/17 | 13/17 | 17/17 | 12/17 | NR |
| St Peter, 2011 | 22 | 5.5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Li, J, 2013 | 29 | NR | 2.5-12 | 17/11/1 | 29/29 | NR | NR | 9/29 | NR | 29/29 | NR | NR |
| Fan, J, 2014 | 35 | NR | NR | NR | NR | NR | NR | 21/35 | NR | NR | NR | NR |
| Li, L, 2014 | 15 | 6.9 ± 3.7 | 3-15 | 11/4/0 | 15/15 | NR | <30-40 | 0/15 | NR | NR | NR | NR |
| Shawa, 2014 | 27 | NR | 1.5-20 | 14/13/0 | NR | 25-46 | 27-114 | 6/15 | 14/15 | 14/15 | NR | NR |
| Decarolis, 2016 | 51 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Lee,2016 | 35 | 6.3 ± 3.3 | 1.5-16 | 20/15/0 | 35/35 | NR | NR | 28/35 | 23b | NR | NR | NR |
| Iacobone, 2017 | 10 | 5.5 ± 1.6 | 3-8 | 8/2/0 | 9/10 | NR | NR | NR | 9/10 | 9/10 | NR | NR |
| Xie, 2018 | 42 | 6 ± 2.6 | 2.2-17 | 22/20/0 | 37/42 | 21-39 | 30-54 | 14/42 | 23/37 | NR | 28/42 | NR |
| Yang, 2018 | 15 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Ichijo, 2019 | 50 | 5 | 1-12 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Current study, 2020 | 45 | 4.8 ± 2.5 | 1.5-12.5 | 25/19/1 | 36/40 | −118-49 | 19-142 | 8/24 | 18/21 | NR | 8/24 | 1/24 |
| Pooled analysis | 421 | 5.8 | 1-20 | 135/93/2 | 179 (95%) | −118-49 | 19-142 | 86 (41%) | 105 (69%) | 82 (92%) | 61 (60%) | 1 (4%) |
aAcross various imaging modalities, including CT, US, and MRI
bDenominator not specified
NR=Not reported; CT=Computed tomography; HU=Hounsfield Units
Discussion
The literature on AGNs reports that the most commonly described characteristics include well-demarcated borders, homogeneity, hypointensity on T1-weighted MRI and hyperintensity on T2-weighted MRI, and enhancement on post-contrast computed tomography (CT) imaging.4,11,15,28,29 In our single center retrospective cohort study of 45 patients and the systematic review of 14 studies, we characterized the clinical and imaging presentations of AGN. Imaging findings in this study appear to be consistent with the literature. We have demonstrated a high prevalence of composite AGN-PHEO tumors in the single center study compared to the low rate of composite tumors in the published literature.
We demonstrated that AGNs are rare benign tumors, often discovered incidentally. Overall, AGNs present around the 5th decade of life and are rarely associated with genetic syndromes. Imaging features of AGNs includes mostly unilateral with a very slight right-sided predominance, initial presentation with a large size of approximately 5 cm, median unenhanced CT attenuation around 30 HU, and typically enhance on CT contrast imaging. Calcifications can be seen in 41% of AGNs, and the vast majority are homogeneous appearing on unenhanced imaging. However, the ability to accurately diagnose AGNs on imaging and therefore subsequently avoid an unnecessary procedure or surgery remains challenging due to the variable imaging characteristics, inconsistencies in radiologist interpretation and reporting, and lack of universal adrenal imaging protocols.
We found that in patients with a histologically proven AGN, composite AGN-PHEO were frequent, occurring in nearly half of the patients in our single center cohort. In those patients with adrenergic/noradrenergic tumor profiles, the working diagnoses prior to surgery included pure pheochromocytoma or composite tumor with pheochromocytoma elements. All tumors with either adrenergic or noradrenergic catecholamine profiles were found to be composite AGN-PHEO. Interestingly, only one other study in our systematic review reported 3 out of 27 patients with AGN having composite AGN-PHEO.10 A recent systematic review of all-site ganglioneuromas that included both case series and case reports described a total of 43 non-neuroblastic composite ganglioneuromas out of 364 patients.30 This difference seen between the large number of composite tumors in our cohort compared to those included in our systematic review, as well as the systematic review by Fliedner et al. is noteworthy. Possible explanations for this difference include potential under-reporting, exclusion of composite tumors from previous studies, under-recognition of this association, or referral bias.
Patients in our single center and systematic review experienced no recurrence or AGN-specific mortality. A recent systematic review of patients with ganglioneuromas at any site also demonstrated that patients with these tumors have an excellent prognosis.30 This suggests that patients with pathologically proven AGN do not need a long-term follow up.
The strengths of this study are its large sample size and inclusion of a systematic review of the literature. The included single center study encompassed more than two decades of data and it included a comprehensive review of clinical and imaging presentation of patients with this rare adrenal mass. Moreover, our search strategy facilitated the inclusion of composite tumors. The limitations of this study are the retrospective design with selection and detection biases, incomplete patient data and follow up, and referral bias. Patients were evaluated with variable imaging protocols, not allowing characterization of imaging characteristics other than unenhanced CT. However, given the rarity of AGN, a retrospective design is often the only feasible method to collect and document disease characteristics. The systematic review was limited by high risk of bias in the representativeness of patients in a third of included studies, and incomplete clinical data, imaging characteristics, and follow up in some studies, Figure 2. The perioperative morbidity associated with unrecognized pheochromocytomas makes differentiating these tumors from AGN crucial prior to adrenalectomy. Since imaging characteristics of AGN and composite AGN-PHEO overlap, evaluation for catecholamine excess is essential in any indeterminate adrenal mass.
In conclusion, AGNs are rare, usually unilateral, large tumors, with variable imaging characteristics. Composite pheochromocytomas and AGNs are not uncommon and pre-surgical diagnosis can be challenging and requires biochemical evaluation in addition to imaging. Prognosis of patients with AGNs is excellent.
Supplementary Material
Acknowledgements
This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health USA under award K23 DK121888. The views expressed are those of the author(s) and not necessarily those of the National Institutes of Health USA.
Footnotes
Conflicts of Interest
Irina Bancos reports advisory board participation with Strongbridge and consulting with Sparrow Pharmaceutics outside the submitted work.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References:
- 1.Ebbehoj A, Li D, Kaur RJ, et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(11):894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reimondo G, Castellano E, Grosso M, et al. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J Clin Endocrinol Metab. 2020;105(4). [DOI] [PubMed] [Google Scholar]
- 3.Bancos I, Taylor AE, Chortis V, et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: a prospective test validation study. Lancet Diabetes Endocrinol. 2020;8(9):773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iacobone M, Torresan F, Citton M, Schiavone D, Viel G, Favia G. Adrenal ganglioneuroma: The Padua Endocrine Surgery Unit experience. Int J Surg. 2017;41 Suppl 1:S103–S108. [DOI] [PubMed] [Google Scholar]
- 5.Spinelli C, Rossi L, Barbetta A, Ugolini C, Strambi S. Incidental ganglioneuromas: a presentation of 14 surgical cases and literature review. J Endocrinol Invest. 2015;38(5):547–554. [DOI] [PubMed] [Google Scholar]
- 6.Liao CH, Chueh SC, Lai MK, Hsiao PJ, Chen J. Laparoscopic adrenalectomy for potentially malignant adrenal tumors greater than 5 centimeters. J Clin Endocrinol Metab. 2006;91(8):3080–3083. doi: 3010.1210/jc.2005-2420. Epub 2006 May 3023. [DOI] [PubMed] [Google Scholar]
- 7.Titos García A, Ramírez Plaza CP, Ruiz Diéguez P, Marín Camero N, Santoyo Santoyo J. [Ganglioneuroma as an uncommon cause of adrenal tumor]. Endocrinol Nutr. 2011;58(8):443–445. [DOI] [PubMed] [Google Scholar]
- 8.Fan H, Li HZ, Ji ZG, Shi BB, Zhang YS. [Diagnosis and treatment of adrenal ganglioneuroma: a report of 80 cases]. Zhonghua Wai Ke Za Zhi. 2017;55(12):938–941. [DOI] [PubMed] [Google Scholar]
- 9.Lee JH, Chai YJ, Kim TH, et al. Clinicopathological Features of Ganglioneuroma Originating From the Adrenal Glands. World J Surg. 2016;40(12):2970–2975. [DOI] [PubMed] [Google Scholar]
- 10.Shawa H, Elsayes KM, Javadi S, et al. Adrenal ganglioneuroma: features and outcomes of 27 cases at a referral cancer centre. Clin Endocrinol (Oxf). 2014;80(3):342–347. [DOI] [PubMed] [Google Scholar]
- 11.Xie J, Dai J, Zhou WL, Sun FK. Adrenal Ganglioneuroma: Features and Outcomes of 42 Cases in a Chinese Population. World J Surg. 2018;42(8):2469–2475. [DOI] [PubMed] [Google Scholar]
- 12.Chen CL, Huang ST, Chang PL, Ng KF. Adrenal ganglioneuroma: report of five cases. Chang Gung Med J. 2000;23(9):550–554. [PubMed] [Google Scholar]
- 13.Huang Z-M, Li H-Z, Xiao H, Ji Z-G. [Diagnosis and treatment for adrenal ganglioneuroma]. Chung Hua I Hsueh Tsa Chih. 2011;91(36):2561–2563. [PubMed] [Google Scholar]
- 14.Li J, Yang CH, Li LM. Diagnosis and treatment of 29 cases of adrenal ganglioneuroma. Eur Rev Med Pharmacol Sci. 2013;17(8):1110–1113. [PubMed] [Google Scholar]
- 15.Radin R, David CL, Goldfarb H, Francis IR. Adrenal and extra-adrenal retroperitoneal ganglioneuroma: imaging findings in 13 adults. Radiology. 1997;202(3):703–707. [DOI] [PubMed] [Google Scholar]
- 16.Lam KY, Lo CY. Composite Pheochromocytoma-Ganglioneuroma of the Adrenal Gland: An Uncommon Entity with Distinctive Clinicopathologic Features. Endocr Pathol. 1999;10(4):343–352. [DOI] [PubMed] [Google Scholar]
- 17.Lin JS, Murad MH, Leas B, et al. A Narrative Review and Proposed Framework for Using Health System Data with Systematic Reviews to Support Decision-making. J Gen Intern Med. 2020;35(6):1830–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Bmj. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decarolis B, Simon T, Krug B, et al. Treatment and outcome of Ganglioneuroma and Ganglioneuroblastoma intermixed. BMC Cancer. 2016;16:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91(10):1905–1913. [DOI] [PubMed] [Google Scholar]
- 22.Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Li L, Shao J, Gu J, Wang X, Qu L. Adrenal ganglioneuromas: experience from a retrospective study in a Chinese population. Urol J. 2014;11(2):1485–1490. [PubMed] [Google Scholar]
- 24.Qing Y, Bin X, Jian W, et al. Adrenal ganglioneuromas: a 10-year experience in a Chinese population. Surgery. 2010;147(6):854–860. [DOI] [PubMed] [Google Scholar]
- 25.Shi B-b, Li H-z, Chen C, et al. Differential diagnosis and laparoscopic treatment of adrenal pheochromocytoma and ganglioneuroma. Chin Med J. 2009;122(15):1790–1793. [PubMed] [Google Scholar]
- 26.St Peter SD, Valusek PA, Hill S, et al. Laparoscopic adrenalectomy in children: a multicenter experience. J Laparoendosc Adv Surg Tech A. 2011;21(7):647–649. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Cai S, Ma X, et al. Discrimination of histopathologic types of childhood peripheral neuroblastic tumors based on clinical and biological factors. Sci Rep. 2018;8(1):10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichikawa T, Ohtomo K, Araki T, et al. Ganglioneuroma: computed tomography and magnetic resonance features. Br J Radiol. 1996;69(818):114–121. [DOI] [PubMed] [Google Scholar]
- 29.Maweja S, Materne R, Detrembleur N, et al. Adrenal ganglioneuroma. Am J Surg. 2007;194(5):683–684. [DOI] [PubMed] [Google Scholar]
- 30.Fliedner SMJ, Winkelmann PER, Wesley R, Vonthein R, Lehnert H. Ganglioneuromas across age groups: Systematic review of individual patient data. Clin Endocrinol (Oxf). 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


