Abstract
It is unclear whether women have higher brain tau pathology. The objective of this study was to examine whether women have higher tau burden than men, and whether tau differences are independent of amyloid β (Aβ) burden. We conducted a cross-sectional analysis of a multi-ethnic sample of 252 non-demented late middle-aged (mean age: 64.1 years) adults with tau and amyloid Positron Emission Tomography (PET) data. Tau burden was measured as global standardized uptake value ratio (SUVR) in the middle/inferior temporal gyri and medial temporal cortex with 18F-MK-6240 PET. Aβ was measured as global SUVR with 18F-Florbetaben PET. Women had higher middle/inferior temporal gyri tau SUVR compared to men. However, no sex differences in the medial temporal cortex were observed. Women had higher brain Aβ SUVR compared to men. Continuous Aβ SUVR was positively correlated with medial temporal cortex and middle/inferior temporal gyri tau SUVR. However, there was no evidence of effect modification by Aβ SUVR on sex and tau. Compared with men, women in late middle age show higher tau burden, independent of Aβ.
Keywords: sex differences, neuroimaging, epidemiology, Alzheimer’s disease
1. INTRODUCTION
There has been conflicting evidence from epidemiologic studies on sex differences in the prevalence and incidence of dementia due to Alzheimer’s disease (AD). Data suggest that almost two-thirds of those diagnosed with AD dementia are women (Hebert et al., 2013). The Aging, Demographics, and Memory Study reported that among individuals 71 years and older, 16% of women have AD dementia compared to 11% of men (Plassman et al., 2007). Women also have a greater lifetime risk of AD dementia. In the Framingham Heart Study, lifetime risk for AD dementia at age 45 was 20% in women and 10% in men (Chene et al., 2015). It has been hypothesized that sex differences in AD may be attributable to differences in brain structure and function, biochemistry (mainly hormones levels), and susceptibility to developing AD in response to genetics and other factors (Mazure and Swendsen, 2016).
Hallmark characteristics of AD include amyloid β (Aβ) plaques, neurofibrillary tangles, and neurodegeneration (Jack et al., 2018). It is unclear whether sex differences in dementia are due to sex differences in AD neuropathology. We previously reported that compared to men, women had higher Aβ in a community-based sample of 266 participants (Luchsinger et al., 2020b). In a subsidiary analysis of a subsample of 75 participants, women had higher tau levels in the middle/inferior temporal gyri compared to men (Luchsinger et al., 2020b). To date, most studies of in-vivo tau neuropathology have been conducted in clinic referral samples and elderly community-based samples. Moreover, there continues to be a paucity of data on in-vivo tau neuropathology in non-White samples (Jack et al., 2018). Therefore, we sought to update our preliminary findings of sex differences in tau burden by adding additional tau data, in a multi-ethnic sample, to further examine whether women have a higher burden of in-vivo tau neuropathology as compared to men. We also assessed whether associations were independent of Aβ burden.
2. METHODS
2.1. Study Design and Population
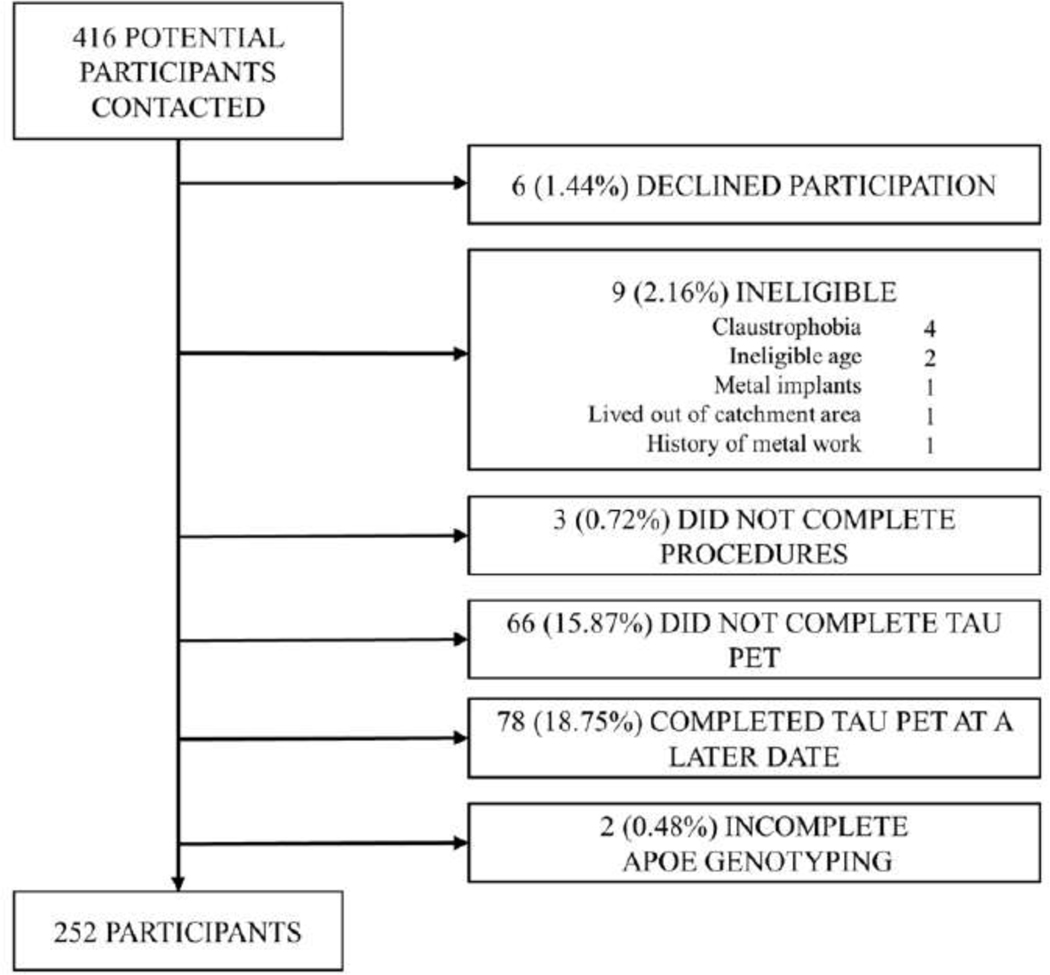
This was a cross-sectional analysis of 252 middle-aged adults in New York City with measured in-vivo AD neuropathology, recruited between 07/01/2017 and 10/31/2019. This study was based at Columbia University Irving Medical Center (CUIMC). Inclusion criteria were any sex; aged 55–69 years; willing and able to undergo phlebotomy, clinical and neuropsychological assessments; 3T brain magnetic resonance imaging (MRI); and positron emission tomography (PET) imaging with injection of radioligands 18F-Florbetaben for amyloid and 18F-MK-6240 for tau. Exclusion criteria were: self-reported diagnosis of dementia, cancer other than non-melanoma skin cancer, and MRI contraindications. A total of 416 participants were screened of which 6 (1.44%) declined study participation, 9 (2.16%) were ineligible, and 3 (0.72%) were eligible but did not complete study procedures (Figure 1). A total of 254 of 398 participants who completed MRI and amyloid PET imaging also underwent tau PET imaging between 07/01/2018 and 10/31/2019. Two participants had incomplete APOE-ε4 genotyping resulting in a final analytic sample of 252 participants (Figure 1). The interval between MRI and amyloid PET was 35.54 ± 64.62 days. The interval between MRI and tau PET was 96.64 ± 96.96 days. This study was approved by the Institutional Review Board and the Joint Radiation Safety Commission at Columbia University. All study participants provided written informed consent.
Figure 1. Flow chart of participant recruitment.
2.2. Exposure: Sex
The exposure was sex, ascertained at the time of screening by self-report with the question “Are you female or male?”. Possible responses included: female, male, unknown, decline to answer, or not applicable. We used the response categories of “female” and “male” to classify sex in this report as “women” and “men”, respectively. All participants reported being either women or men (Table 1).
Table 1.
Demographic and clinic characteristics, overall and by sex subgroups (n=252).
| Total Sample (n = 252) | Women (n = 173) | Men (n = 79) | p-value | |
|---|---|---|---|---|
| Age, mean (SD), years | 64.06 (3.27) | 63.88 (3.31) | 64.47 (3.15) | 0.18 |
| Ethnicity, No. (%) | ||||
| Hispanic | 222 (88.10) | 155 (89.60) | 67 (84.81) | 0.28 |
| Dominican | 183 (72.62) | 131 (75.72) | 52 (65.82) | |
| Other Caribbean Hispanic | 18 (7.14) | 13 (7.51) | 5 (6.33) | |
| South American | 14 (5.56) | 7 (4.05) | 7 (8.86) | |
| Central American | 4 (1.59) | 3 (1.73) | 1 (1.27) | |
| Unspecified Hispanic | 3 (1.19) | 1 (0.58) | 2 (2.53) | |
| Non-Hispanic Black | 19 (7.54) | 10 (5.78) | 9 (11.39) | |
| Non-Hispanic White | 11 (4.37) | 8 (4.62) | 3 (3.80) | |
| Education, mean (SD), years | 10.98 (4.15) | 10.83 (4.05) | 11.30 (4.37) | 0.40 |
| APOE-ε4 Genotyping, No. (%) | 83 (32.94) | 55 (31.79) | 28 (35.44) | 0.57 |
| ε2ε2 | 1 (0.40) | 0 (0.00) | 1 (1.27) | |
| ε2ε3 | 30 (11.90) | 22 (8.73) | 8 (10.13) | |
| ε2ε4 | 4 (1.59) | 2 (0.79) | 2 (2.53) | |
| ε3ε3 | 138 (54.76) | 96 (38.10) | 42 (53.16) | |
| ε3ε4 | 71 (28.17) | 48 (19.05) | 23 (29.11) | |
| ε4ε4 | 8 (3.17) | 5 (1.98) | 3 (3.80) | |
| Hemoglobin A1c, mean (SD), % | 6.23 (1.31) | 6.21 (1.24) | 6.28 (1.44) | 0.69 |
| Fasting glucose, mean (SD), mg/dL | 107.13 (33.15) | 105.64 (30.92) | 110.38 (37.57) | 0.29 |
| Body mass index, mean (SD), kg/m2 | 29.09 (4.92) | 29.42 (5.15) | 28.35 (4.33) | 0.11 |
| Waist circumference, mean (SD), cm | 96.98 (11.45) | 95.29 (11.42) | 100.72 (10.66) | 0.0005 |
| Triglycerides, mean (SD), mg/dL | 110.52 (54.59) | 111.13 (56.22) | 109.20 (51.16) | 0.80 |
| High density lipoprotein, mean (SD), mg/dL | 53.65 (14.73) | 55.87 (13.92) | 48.78 (15.35) | 0.0003 |
| Low density lipoprotein, mean (SD), mg/dL | 106.55 (33.56) | 108.37 (32.46) | 102.63 (35.73) | 0.21 |
| Statin use, No. (%) | 100 (39.68) | 68 (39.31) | 32 (40.51) | 0.86 |
| Systolic blood pressure, mean (SD), mmHg | 133.97 (17.99) | 132.52 (17.97) | 137.11 (17.76) | 0.060 |
| Diastolic blood pressure, mean (SD), mmHg | 82.89 (9.97) | 81.77 (9.56) | 85.33 (10.46) | 0.0083 |
| Metabolic syndrome score, mean (SD) | 2.40 (1.32) | 2.47 (1.31) | 2.23 (1.32) | 0.17 |
| Metabolic syndrome, No. (%) | 117 (46.43) | 87 (50.29) | 30 (37.97) | 0.070 |
| WMH volume, mean (SD), cm2 | 5.93 (8.61) | 5.53 (7.90) | 6.82 (10.00) | 0.27 |
| Brain infarcts, No. (%) | 66 (26.19) | 40 (23.12) | 26 (32.91) | 0.10 |
| Global brain Aβ SUVR, mean (SD) | 1.16 (0.14) | 1.18 (0.14) /\N | 1.14 (0.14) | 0.037 |
| Aβ positivity, No. (%) | 18 (7.14) | 12 (6.94) | 6 (7.59) | 0.85 |
| Tau SUVR | ||||
| Medial Temporal Cortex, mean (SD) | 0.98 (0.21) | 0.95 (0.22) | 0.99 (0.21) | 0.13 |
| Middle/Inferior T emporal Gyri, mean (SD) | 1.20 (0.18) | 1.13 (0.18) | 1.23 (0.17) | <0.0001 |
| Elevated tau (1 SD threshold), No. (%) | 39 (15.48) | 31 (17.92) | 8 (10.13) | 0.11 |
| Elevated tau (2 SD threshold), No. (%) | 18 (7.14) | 14 (8.09) | 4 (5.06) | 0.39 |
SD = standard deviation; WMH = white matter hyperintensity; SUVR = standardized uptake value ratio
2.3. Outcome: In-vivo Tau and Amyloid Beta (Aβ)
The primary outcome was tau burden ascertained as tau standardized uptake value ratio (SUVR) measured continuously in the middle/inferior temporal gyri and medial temporal cortex measured with 18F-MK-6240 PET obtained from 3T brain MRI. Tau aggregation is limited to medial temporal cortex in older adults and next spreads to basal and lateral temporal cortex, with involvement of further neocortical regions after AD symptoms become apparent (Shah and Catafau, 2014). Thus, we used medial temporal cortex and middle/inferior temporal gyri for the primary analysis of tau imaging. We also examined interactions by brain amyloid burden, measured as global cortical brain amyloid beta (Aβ) SUVR using 18F-Florbetaben PET. We used the same methodology for amyloid and tau PET as reported in previous studies (Luchsinger et al., 2015; Luchsinger et al., 2020b; Palta et al., 2020).
Amyloid PET:
The dose of 18F-Florbetaben was 300 MBq (8.1 mCi), maximum 30 mcg mass dose, administered as a single slow intravenous bolus. Images were acquired over 20 minutes starting 90 minutes after injection. Dynamic PET frames (4 scans) were aligned to the first frame using rigid-body registration and a static PET image was obtained by averaging the four registered frames. Information on the amyloid PET scan processing protocol has been previously published (Tahmi et al., 2019). The standardized uptake value (SUV), defined as the decay-corrected brain radioactivity concentration normalized for injected dose and body weight, was calculated in all Freesurfer regions. The SUV in each region, as well as each voxel, was also normalized to the SUV in cerebellar gray matter to derive the regional and voxel-wise SUVR. We used a standard approach to calculating global Aβ burden using Alzheimer’s Disease Neuroimaging Initiative (ADNI) defined regions of interests (ROIs) to generate four lobar SUVRs (frontal, temporal, cingulate and parietal cortex) (Landau et al., 2016). An overall mean SUVR value of Aβ burden across all ROIs was derived.
Tau PET:
The 18F-MK-6240 injected activity was 185 MBq (5 mCi), and images were acquired 80–100 minutes post-injection. PET was performed without arterial sampling. Dynamic PET frames (4 scans) were aligned to the first frame using rigid-body registration and a static tau PET image was obtained by averaging the four registered frames. The static tau PET image was then co-registered to the corresponding static amyloid PET image space. The same Freesurfer-derived ROIs from the amyloid PET processing steps above were then applied to the tau PET image. Regional concentration of radioactivity was then extracted from the static tau PET images. 18F-MK-6240 SUVRs were calculated using an eroded cerebellar gray matter reference region, consisting of posterior cerebellum to avoid spill-over of the 18F-MK-6240 signal from tentorium cerebelli and ventral temporal/occipital cortex (Betthauser et al., 2019).
2.4. Covariates
Primary models adjusted for age only (measured in years). In secondary analyses, we further adjusted for potential vascular risk factor burden in two ways: (1) adjustment for the metabolic syndrome score, an aggregate measure of vascular risk factors, and (2) adjustment for white matter hyperintensity (WMH) burden and cerebral infarcts in the brain as a surrogate marker of cerebrovascular disease. We chose the metabolic syndrome as the primary aggregate vascular risk factor measure because it accounts for adiposity, hypertension, dyslipidemia, and dysglycemia. We estimated the metabolic syndrome score in our study using the NIH definition, as previously described (Palta et al., 2021). In addition in a secondary model, we compared and adjusted for WMH volume and presence/absence of cerebral infarcts, as the measure of vascular risk factor burden, between men and women to account more directly for cerebrovascular burden. WMH volume and cerebral infarcts may better capture the long-term impact of hypertension and other cardiovascular risk factors on the brain. MRI methods for the measurement of WMH volume and cerebral infarcts in this study have been previously described (Luchsinger et al., 2020a).
The metabolic syndrome was included in the model as a continuous score ranging from 0–5. The following components and definitions are included in the metabolic syndrome: elevated blood pressure (systolic blood pressure ≥130 and/ or diastolic blood pressure ≥ 85 mmHg or use of anti-hypertensive medication use), high fasting glucose (≥100 mg/dL or diabetes medication use), high waist circumference (women: ≥35 inches or ≥ 89 centimeters; men: ≥40 inches or ≥102 centimeters), high triglycerides level (≥150 mg/dL (1.7 mmol/L) or triglyceride medication use), and abnormal high density lipoprotein ([HDL]; women: <50 mg/dL (1.3 mmol/L); men: <40 mg/dL (1.04 mmol/L) or low HDL medication use). HDL and triglycerides were measured on an automated immunochemistry analyzer, Integra 400 plus (Roche Diagnostics, Indianapolis, IN) using an enzymatic colorimetric assay with a lower limit of quantitation of 3.09 mg/dL for HDL and 0.1mmol/L for triglycerides. Glucose is measured in plasma with NaF preservative using an enzymatic reference method on the automated analyzer, Integra 400 plus (Roche Diagnostics, Indianapolis, IN). All reagents, calibrators and controls were purchased from Roche. The lower limit of detection of the assay was 0.24mmol/L. The intra-assay precision was 0.7% and inter-assay precision was 1.3%.
In sensitivity analyses, APOE-ε4 genotype and intracranial volume were considered as covariates. APOE-ε4 genotype was selected as a secondary covariate given that it is the strongest risk factor for sporadic dementia due to AD (Buckley et al., 2019), and also the strongest determinant of amyloid burden (Jansen et al., 2015). APOE-ε4 genotyping was conducted by LGC genomics (Beverly, MA) using single nucleotide polymorphisms rs429358 and rs7412. Participants were classified as APOE-ε4 carriers if they were homozygous or heterozygous for APOE-ε4. We accounted for differences in head size between men and women by adjusting for intracranial volume.
2.5. Statistical Analysis
Tau (middle/inferior temporal gyri and medial temporal cortex) was analyzed continuously in primary models. In a secondary analysis, we examined elevated tau status (T +/−) in the medial temporal cortex by defining an elevated threshold at 1 (SUVR=1.11) and 2 (SUVR=1.26) standard deviations above the mean for participants classified as amyloid negative according to the k-means clustering method (n=234, Table 1) (Tahmi et al., 2020). This method to define elevated tau status has been previously published in the Wisconsin Registry for Alzheimer’s Prevention (Betthauser et al., 2020).
Global Aβ was analyzed continuously and classified as a binary variable for Aβ positivity. We determined Aβ positivity according to a k-means clustering algorithm (SUVR=1.367), as previously described (Tahmi et al., 2020). Briefly, to classify two groups, this algorithm starts by identifying two Aβ SUVR values from our data at random and subsequently assigns all other observations to the group with the closest mean Aβ SUVR. Once this process has been completed for all observations, it will continue to reiterate, checking if observations should be reclassified to the other cluster, until all group assignments are stable. The final classifications should minimize each within-cluster variance, equivalent to the sum of the squared Euclidean distance between each Aβ SUVR value and its within-cluster mean (MacQueen, 1967).
Comparisons between men and women were made with analysis of variance for continuous variables, and chi-squared for categorical variables. Pearson correlations were also used to evaluate the relationship between brain Aβ SUVR and tau SUVR measured continuously. Adjusted mean differences in tau SUVR levels comparing men and women were estimated using analysis of covariance (ANCOVA) adjusted for covariates. Sex differences in elevated tau SUVR in the medial temporal cortex were examined by odds ratios (OR) obtained from multivariable logistic regression models. Model 1 was unadjusted and model 2 was adjusted for age. We additionally adjusted for vascular risk factor burden, according to (1) the metabolic syndrome score (primary model) or (2) WMH burden and presence of cerebral infarcts in a secondary model. Sensitivity analyses additionally considered APOE-ε4 and intracranial volume as covariates. We examined effect modification by Aβ by including an interaction term between sex and Aβ (measured continuously and categorically as described above) in age-adjusted ANCOVA models. Given that the sample is predominantly (n=222, 88%) Hispanic, we also conducted an analysis restricted to the Hispanic sample only. All analyses were performed using SAS version 9.4m5 and R version 3.6.0.
3. RESULTS
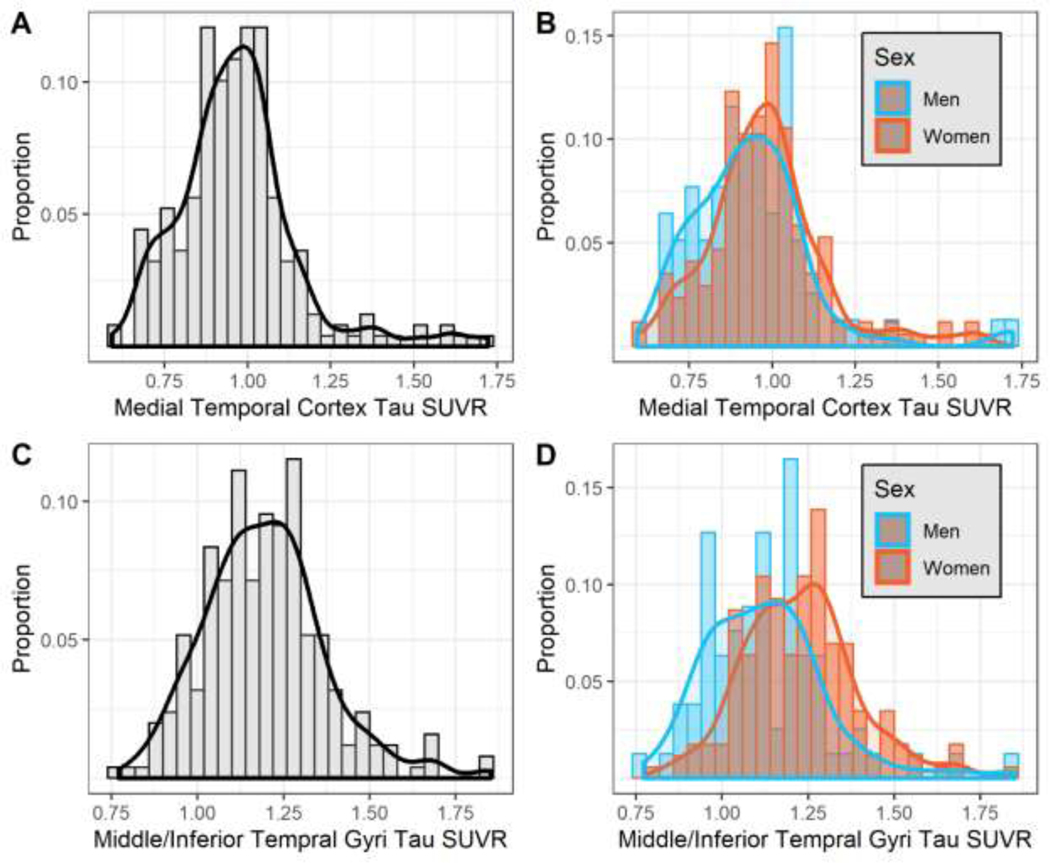
The unadjusted distribution of medial temporal cortex and middle/inferior temporal gyri tau SUVR, overall and by sex subgroups, is provided in Figure 2. Demographic and clinical characteristics are reported in Table 1 overall and by sex subgroups. Only unadjusted middle/inferior temporal gyri tau SUVR levels were higher among women compared to men. There were no differences in age, education, APOE-ε4 carrier status, or the frequency of elevated tau between men and women. Women had higher HDL cholesterol, lower waist circumference, and lower diastolic blood pressure compared to men. Women also had higher continuous brain Aβ SUVR compared to men (1.18 ± 0.14 vs. 1.14 ± 0.14; p = 0.037). However, there were no sex differences in Aβ positivity when using the k-means clustering method. APOE-ε4 was associated with continuous Aβ (Beta=0.08, 95% Confidence Interval (CI): 0.05, 0.12) and Aβ positivity (Odds Ratio (OR)=9.29, 95% CI: 2.89, 29.83). APOE-ε4 was associated with continuous medial temporal cortex tau SUVR (Beta=0.08, 95% CI: 0.03, 0.14), but only elevated tau based on the 2 SD threshold (OR: 6.41, 95% CI: 2.18, 18.86). APOE-ε4 was not associated with the middle/inferior temporal gyri tau SUVR (Beta=0.01, 95% CI: −0.03, 0.06).
Figure 2. Distribution of tau SUVR, overall and by sex subgroups.
Black lines in Panels A and C represent non-parametric kernel density estimations for the corresponding tau SUVR measure in the overall sample. Red and blue lines in Panels B and D represent non-parametric kernel density estimations for the corresponding sex within each tau SUVR measure.
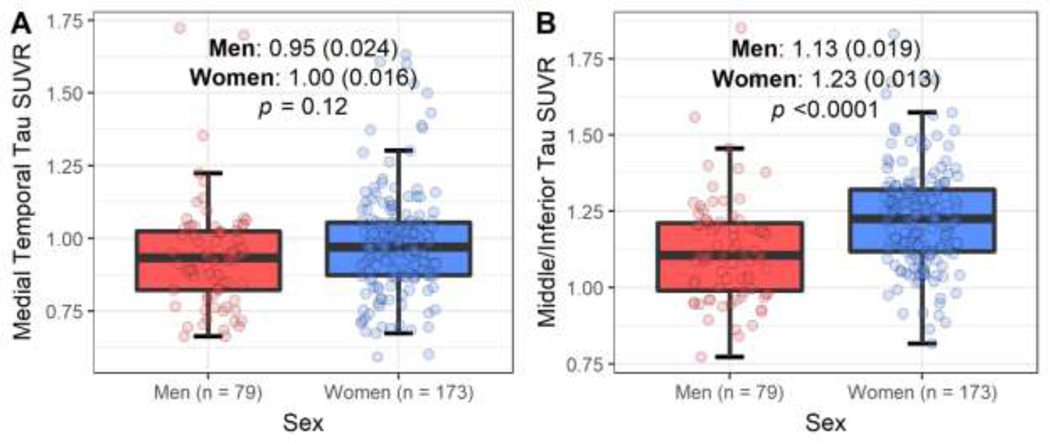
Women had higher middle/inferior temporal gyri tau SUVR compared to men (1.23 ± 0.013 vs. 1.13 ± 0.019; p <0.0001; Figure 3, Panel B), adjusted for age. Results were robust to adjustment for vascular risk factor burden using the metabolic syndrome score (Table 2) or WMH burden and presence/absence of cerebral infarcts (data not shown). No sex differences in the medial temporal cortex measured continuously (Figure 3, Panel A) or categorically as elevated tau (Table 2) were observed.
Figure 3. Adjusted Mean Differences in Tau SUVR regions of interest, by sex subgroups.
Boxplots comparing the adjusted means (standard deviation) for tau SUVR from ANCOVA for men and women. Models are adjusted for age.
Table 2.
Unadjusted and adjusted associations of sex and elevated tau SUVR in the medial temporal cortex (n=252).
| Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|
| Sex | No. | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value |
| Elevated medial temporal cortex tau SUVR using 2 standard deviation threshold | |||||||
| Men | 79 | Reference | Reference | Reference | |||
| Women | 173 | 1.65 (0.53, 5.19) | 0.39 | 1.66 (0.53, 5.23) | 0.39 | 1.69 (0.53, 5.35) | 0.37 |
| Elevated medial temporal cortex tau SUVR using 1 standard deviation threshold | |||||||
| Men | 79 | Reference | Reference | Reference | |||
| Women | 173 | 1.94 (0.85, 4.43) | 0.12 | 1.92 (0.84, 4.40) | 0.12 | 1.87 (0.81, 4.30) | 0.14 |
Elevated tau SUVR was defined by both one (SUVR=1.11) and two (SUVR=1.26) standard deviations above the mean medial temporal cortex tau SUVR in amyloid negative participants (n=234).
Model 1: unadjusted
Model 2: adjusted for age
Model 3: Model 2 + metabolic syndrome score
SD = standard deviation
SUVR= standardized uptake value ratio
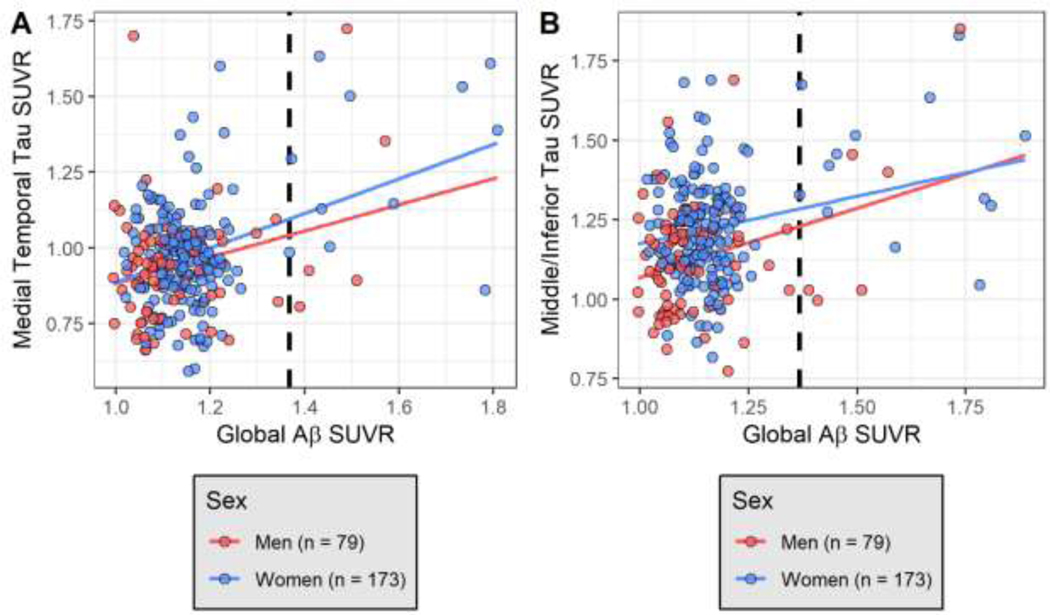
Continuous global Aβ SUVR was positively correlated with both the medial temporal cortex (r = 0.52; p <0.0001) and middle/inferior temporal gyri (r = 0.30; p <0.0001) tau SUVR. Bivariate unadjusted associations between continuous global Aβ SUVR and continuous medial temporal cortex and middle/inferior temporal gyri tau SUVR are shown in Figure 4 stratified by sex. Higher Aβ positivity was associated with higher medial temporal cortex and middle/inferior temporal gyri tau SUVR (p <0.0001). However, there was no evidence of effect modification by Aβ SUVR on the relationship between sex and tau SUVR regions of interest (data not shown). All interactions between sex and Aβ SUVR on tau SUVR regions of interest were not significant (p>0.05) for continuous Aβ SUVR or Aβ positivity.
Figure 4. Unadjusted bivariate association between (A) medial temporal tau SUVR and Global Aβ SUVR and (B) middle/inferior tau SUVR and Global Aβ SUVR, stratified by sex subgroups.
Red line indicates unadjusted regression association between tau SUVR measures and Global Aβ SUVR among men. Blue line indicates unadjusted regression association between tau SUVR measures and Global Aβ SUVR among women. Dashed line is Aβ positivity according to a k-means clustering algorithm (SUVR=1.367).
Results and inferences were unchanged in sensitivity analyses adjusting for APOE-ε4 carrier status and intracranial volume. All results were also robust to sensitivity analyses restricting the analytic sample to Hispanics only (data not shown).
4. DISCUSSION
We observed that women had higher tau burden, in the middle/inferior temporal gyri but not the medial temporal cortex, as compared to men in this cross-sectional analysis of a multi-ethnic community-based sample of late middle-aged adults in New York City. Women also had higher Aβ burden, as we had previously reported (Luchsinger et al., 2020b), but this did not seem to be driving differences in tau, as evidenced by a lack of statistical interaction of sex and Aβ burden. We also replicated the observation of higher tau SUVR levels among women compared to men that were previously published in a secondary analysis of a subsample of 75 participants from this study (Luchsinger et al., 2020b). Discrepant associations between sex and tau measured in specific regions may be due to differences in tau binding in the middle/inferior temporal gyri and medial temporal cortex. Prior data suggests that tau aggregation in the medial temporal lobe often occurs with normal aging and simultaneously with neurodegeneration (Braak and Del Tredici, 2015; Crary et al., 2014; Harrison et al., 2018). In contrast, tau aggregation in the middle/inferior temporal gyri is often accelerated and shows the earliest signs of AD pathology (Insel et al., 2020), which has been documented as more common among women compared to men (Barnes et al., 2005). However, our results suggest that the higher tau aggregation in the middle/inferior temporal gyri in women as compared with men that we observed was independent of sex differences in Aβ burden.
It has been hypothesized that sex hormones, specifically estrogen and estradiol, may be implicated in the development of AD neuropathology and the downstream risk of AD, and may therefore contribute to disparities in dementia risk between women and men. In human clinical studies, lifetime estrogen exposure has been associated with better cognitive performance and lower AD risk (Matyi et al., 2019; Paganini-Hill and Henderson, 1994). However, in the Women’s Health Initiative Memory Study (WHIMS), hormone replacement therapy in older adulthood was associated with a higher risk of dementia (Shumaker et al., 2003) and greater brain atrophy (Resnick et al., 2009). An additional observational study of women from the Kaiser Permanente Medical Care Program of Northern California confirmed these results, but also showed differential benefits of estrogen tied to the age of hormonal treatment—specifically that hormone replacement therapy in mid-life may be protective, but administration in late-life may have harmful effects (Whitmer et al., 2011). The adverse effects of hormone replacement therapy on dementia risk in WHIMS therefore may be attributable to the timing of treatment, specifically that most participants in WHI initiated treatment at a mean age of 78 years, which may be too late for cerebral protection. Despite these findings from WHIMS, estradiol is hypothesized to protect against tau hyperphosphorylation. At the time of menopause, typically occurring between the ages of 40 and 50 and closely linked to middle adulthood, there is a significant reduction in estradiol and estrogen levels, which may contribute to elevated levels of AD neuropathology and therefore potentially be counteracted with hormone therapy. Therefore, sex hormonal effects on tau phosphorylation may play a role in the differences in tau levels between women and men. No participants in our study reported hormone replacement therapy use to test the hypothesis that hormone treatment may be implicated in the development of AD neuropathology. Despite this hypothesis, Wisch and colleagues recently reported on differences in the association between sex and tau levels by hormone therapy use and found that although hormone therapy use accounted for a small reduction in tau levels, men still had lower levels of tau overall compared to women (Wisch et al., 2020), suggesting that other factors beyond hormonal differences may also play a role in the higher burden of tau pathology in women as compared to men. Further investigation is needed to understand these pathways.
Prior data from studies including cerebrospinal fluid (CSF) measures of tau have documented higher CSF tau levels in women compared to men. Specifically, in a multicohort study of 10 longitudinal cohorts of normal aging and AD (n=1798, mean age: 70 years, 48% women), female APOE-ε4 carriers had higher CSF tau levels compared to male APOE-ε4 carriers, but these associations were only statistically supported among participants classified as Aβ positive only (Hohman et al., 2018). Our results differ from those recently published by the Harvard Aging Brain Study (HABS), which examined a sample of 193 cognitively normal participants (age range: 55–92 years, 61% women) to estimate the association between sex and tau, measured with PET imaging and the [18F] Flortaucipir radioligand (Buckley et al., 2019). They also tested for interactions by Aβ and APOE-ε4 in these associations. Contrary to our data showing that the association between sex and tau is independent of amyloid, this study, with a slightly older mean age but similar distribution of sex, found that women only exhibited higher levels of entorhinal cortical tau compared to men among those participants with high Aβ burden only. Although of similar age and proportion of women to our study, the discrepant findings may be due to differences in the radioligand tau tracers used or characteristics of the study sample. Specifically, our study had a narrower age range and included late middle-aged adults rather than primarily older adults suggesting that participants in the Harvard Aging Brain Study may have more advanced neuropathology compared to participants in our study. Second, the Harvard Aging Brain Study was a clinic-based sample, which may be more subject to recruitment and sampling biases than a community-based cohort. Lastly, the Harvard Aging Brain Study included primarily non-Hispanic white adults, whereas our study is one of the first to report on sex differences in in-vivo tau burden in a predominantly Hispanic sample.
Our findings, however, are consistent with those reported by Wisch and colleagues using cross-sectional data from 148 cognitively normal participants (mean age: 70 years, 58% female) recruited from the Knight Alzheimer’s Disease Research Center (Wisch et al., 2020). In this study, tau PET levels across 10 regions (cuneus, frontal pole, inferior parietal, lateral occipital, middle temporal, pars orbitalis, pars triangularis, rostral middle frontal, superior temporal and temporal pole) and as a summary score were both higher in women compared with men, and all associations were independent of amyloid burden.
Our study has some limitations to consider. First, this study’s main limitation is the cross-sectional design, and therefore we cannot infer causality. Second, we do not have information on family history of dementia to determine whether differences in family history between men and women may explain the observed associations. Related, our assessment of dementia at the time of recruitment was based on self-report only. Third, despite the unique aspect of this cohort with regards to the multi-ethnic composition, due to the small number of non-Hispanic blacks (7.5%) and whites (4.4%) in the study population, we do not have sufficient power to examine differences by race/ethnicity. Fourth, we do not have information on history of obstetric or gynecologic exposures to examine as an explanatory factor in the observed associations. Lastly, we do not have continent-of-origin genotype information for any information that would inform the degree of racial admixture in this cohort. The main strength of our study, however, is the availability of state-of-the-art biomarkers of tau and amyloid in a multi-ethnic community-based sample, predominantly Hispanic, in which there is a paucity of information on in-vivo AD neuropathology (Jack et al., 2018). The study sample is also homogenous with respect to age, limiting potential confounding effects of age. Furthermore, our sample size of 252 participants with tau SUVR burden measures is larger than most other studies examining these biomarkers in middle age.
In conclusion, our data show that non-demented women have higher tau burden compared to men that is independent of amyloid burden. These differences, evident in middle age, may in part explain the higher dementia risk in elderly women compared to men.
Highlights.
Unclear if sex differences in dementia is due to sex differences in neuropathology
Females had higher middle/inferior temporal gyri tau SUVR compared to males
No sex differences in medial temporal cortex tau SUVR were observed
No evidence of effect modification by Aβ SUVR on sex and tau
Funding
This work was supported by United States National Institutes of Health grants R01AG050440 and RF1AG051556. Partial support was also provided by grants K24AG045334, P30AG059303, ULT1TR001873. Dr. Palta is in part supported by grant R00AG052830 from the National Institute on Aging. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute of Aging; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Footnotes
Conflict of Interest/Disclosures
JA Luchsinger receives a stipend from Wolters Kluwer, N.V. as Editor in Chief of the journal Alzheimer’s Disease and Associated Disorders, and has served as a paid consultant to vTv therapeutics, Inc. and Recruitment Partners. AM Brickman is a paid consultant for Regeneron Pharmaceuticals and Cognition Therapeutics, Inc and owns equity in Mars Holding Limited. The other authors have no other conflicts of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA, 2005. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62(6), 685–691. [DOI] [PubMed] [Google Scholar]
- Betthauser TJ, Cody KA, Zammit MD, Murali D, Converse AK, Barnhart TE, Stone CK, Rowley HA, Johnson SC, Christian BT, 2019. In Vivo Characterization and Quantification of Neurofibrillary Tau PET Radioligand 18F-MK-6240 in Humans from Alzheimer Disease Dementia to Young Controls. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 60(1), 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betthauser TJ, Koscik RL, Jonaitis EM, Allison SL, Cody KA, Erickson CM, Rowley HA, Stone CK, Mueller KD, Clark LR, Carlsson CM, Chin NA, Asthana S, Christian BT, Johnson SC, 2020. Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 143(1), 320–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2015. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138(Pt 10), 2814–2833. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ, Jacobs HIL, Papp KV, Amariglio RE, Properzi MJ, Schultz AP, Kirn D, Scott MR, Hedden T, Farrell M, Price J, Chhatwal J, Rentz DM, Villemagne VL, Johnson KA, Sperling RA, 2019. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured by Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurol 76(5), 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chene G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, Seshadri S, 2015. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 11(3), 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT, 2014. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128(6), 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, Mellinger TJ, Edwards L, Pham J, Miller BL, Rabinovici GD, Jagust WJ, 2018. Longitudinal tau accumulation and atrophy in aging and Alzheimer’s disease. Ann Neurol 85, 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80(19), 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B, Gifford KA, Bush WS, Chibnik LB, Mukherjee S, De Jager PL, Kukull W, Crane PK, Resnick SM, Keene CD, Montine TJ, Schellenberg GD, Haines JL, Zetterberg H, Blennow K, Larson EB, Johnson SC, Albert M, Bennett DA, Schneider JA, Jefferson AL, Alzheimer’s Disease Genetics C, the Alzheimer’s Disease Neuroimaging, I., 2018. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA Neurol 75(8), 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel PS, Mormino EC, Aisen PS, Thompson WK, Donohue MC, 2020. Neuroanatomical spread of amyloid beta and tau in Alzheimer’s disease: implications for primary prevention. Brain Commun 2(1), fcaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, 2018. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 14(4), 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ, and the Amyloid Biomarker Study, G., 2015. Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-analysisPrevalence of Cerebral Amyloid Pathology in Persons Without DementiaPrevalence of Cerebral Amyloid Pathology in Persons Without Dementia. JAMA 313(19), 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Horng A, Fero A, Jagust WJ, Alzheimer’s Disease Neuroimaging I, 2016. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 86(15), 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Cabral R, Eimicke JP, Manly JJ, Teresi J, 2015. Glycemia, Diabetes Status, and Cognition in Hispanic Adults Aged 55–64 Years. Psychosom Med 77(6), 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Palta P, Rippon B, Sherwood G, Soto L, Ceballos F, Laing K, Igwe K, He H, Razlighi Q, Teresi J, Moreno H, Brickman AM, 2020a. Pre-Diabetes, but not Type 2 Diabetes, Is Related to Brain Amyloid in Late Middle-Age. J Alzheimers Dis 75(4), 12411252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Palta P, Rippon B, Soto L, Ceballos F, Pardo M, Laing K, Igwe K, Johnson A, Tomljanovic Z, He H, Reitz C, Kreisl W, Razlighi Q, Teresi J, Moreno H, Brickman AM, 2020b. Sex Differences in in vivo Alzheimer’s Disease Neuropathology in Late Middle-Aged Hispanics. J Alzheimers Dis 74(4), 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen J, 1967. Some methods for classification and analysis of multivariate observations. Proceedings of the Berkeley Symposium on Mathematical Statistics and Probability 1(14), 281. [Google Scholar]
- Matyi JM, Rattinger GB, Schwartz S, Buhusi M, Tschanz JT, 2019. Lifetime estrogen exposure and cognition in late life: the Cache County Study. Menopause 26(12), 1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazure CM, Swendsen J, 2016. Sex differences in Alzheimer’s disease and other dementias. The Lancet. Neurology 15(5), 451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganini-Hill A, Henderson VW, 1994. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol 140(3), 256–261. [DOI] [PubMed] [Google Scholar]
- Palta P, Rippon B, Reitz C, He H, Sherwood G, Ceballos F, Teresi J, Razlighi Q, Moreno H, Brickman AM, Luchsinger JA, 2020. Apolipoprotein E genotype and in vivo amyloid burden in middle-aged Hispanics. Neurology 95(15), e2086–e2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palta P, Rippon B, Tahmi M, Sherwood G, Soto L, Ceballos F, Laing K, He H, Reitz C, Razlighi Q, Teresi JA, Moreno H, Brickman AM, Luchsinger JA, 2021. Metabolic syndrome and its components in relation to in vivo brain amyloid and neurodegeneration in late middle age. Neurobiol Aging 97, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB, 2007. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology 29(1–2), 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C, 2009. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology 72(2), 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Catafau AM, 2014. Molecular Imaging Insights into Neurodegeneration: Focus on Tau PET Radiotracers. J Nucl Med 55(6), 871–874. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J, Investigators W, 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289(20), 2651–2662. [DOI] [PubMed] [Google Scholar]
- Tahmi M, Bou-Zeid W, Razlighi QR, 2019. A fully automatic technique for precise localization and quantification of Amyloid-β PET scans. J Nucl Med 60(12), 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmi M, Rippon B, Palta P, Soto L, Ceballos F, Pardo M, Sherwood G, Hernandez G, Arevalo R, He H, Sedaghat A, Arabshahi S, Teresi J, Moreno H, Brickman AM, Razlighi QR, Luchsinger JA, 2020. Brain Amyloid Burden and Resting-State Functional Connectivity in Late Middle-Aged Hispanics. Front Neurol 11, 529930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Quesenberry CP, Zhou J, Yaffe K, 2011. Timing of hormone therapy and dementia: the critical window theory revisited. Ann Neurol 69(1), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisch JK, Meeker KL, Gordon BA, Flores S, Dincer A, Grant EA, Benzinger TL, Morris JC, Ances BM, 2020. Sex-related Differences in Tau Positron Emission Tomography (PET) and the Effects of Hormone Therapy (HT). Alzheimer Dis Assoc Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]