Abstract
The vast majority of drugs are not designed or developed for pediatric and infant populations. Peptide drugs, which have become increasingly relevant in the past several decades, are no exception. Unfortunately, nearly all of the 60+ approved peptide drugs are formulated for injection, a particularly unfriendly mode of administration for infants. Although four peptide drugs were recently approved for oral formulations, this major advance in peptide drug delivery is available only for adults. In this review, we consider the current challenges and opportunities for the oral formulation of peptide therapeutics, specifically for infant populations. We describe the strategies that enable oral protein delivery and the potential impact of infant physiology on those strategies. We also detail the limited but encouraging progress towards 1) adapting conventional drug development and delivery approaches to infants and 2) designing novel infant-centric formulations. Together, these efforts underscore the feasibility of oral peptide delivery in infants and provide motivation to increase attention paid to this underserved area of drug delivery and formulation.
Keywords: oral delivery, protein delivery, infant therapy, peptides, pediatrics, dosage forms
1. Introduction
Historically, drugs are not designed for children or infants. This is because children, as a population, present many challenges from a drug development perspective. For example, there are ethical concerns when testing medications in children and financial motivation is insufficient. Furthermore, children have unique needs in terms of drug dosage forms as well as physiology that is fundamentally different than that of adults.
These drug development challenges result in two major problems. The first is that medication simply is not available for many infant and childhood conditions, including necrotizing enterocolitis, childhood interstitial lung disease, and autosomal recessive polycystic kidney disease [1–3]. This, in turn, may account for the 10-fold lower enrollment of pediatric patients in clinical trials compared to adults [4]. The second problem is that when an appropriate medication does exist, it is often an adult medication that has been prescribed “off-label”. Such use can entail altering the approved dose, time period, dosage form, and/or route of administration. Unfortunately, off-label prescriptions lead to twice as many adverse drug reactions (e.g. vomiting, seizures) as licensed drugs [5, 6] and are associated with increased patient mortality, given the poorly understood physiological differences between adults and children [7]. Furthermore, dose adjustments are typically performed by educated guesswork based on weight or body surface area. Thus, there is a clear need for optimized drug formulations designed specifically for pediatric populations.
The oral route of drug delivery is considered to be the most patient-preferred because it results in the highest levels of compliance [8]. Oral formulations are easily administered outside of healthcare settings, ideal for longer-term use, and can be readily discontinued in the event of adverse reactions. Furthermore, tablets and other solid dosage forms facilitate longer shelf lives of the active pharmaceutical ingredients (API) compared to sterile and specialty pharmaceutical dosage forms [9]. Pediatric patients, in particular, benefit from oral medications because children tend to be more distressed by injections than adults. Although children sometimes have difficulty swallowing solid dosage forms, a number of antibiotics and non-steroidal anti-inflammatory drugs have been formulated as liquids to ease administration to pediatric patients [10]. Because very few drugs are formulated specifically for infants, pharmacists often extemporaneously dissolve, dilute, or resuspend formulations made for adults at different concentrations or in different media for infant use [11, 12]. This is a major safety concern, and fatal mistakes have been made due to the complexity of altering adult formulations with no clear guidance or standards [13].
Peptide and protein drugs are a unique and critical sector of the pharmaceutical market, with three new peptide drugs approved in 2019–2020 and an additional 150 in active development [14, 15]. Peptide drugs have a number of key advantages over small molecule drugs, including greater specificity and more sophisticated pharmacological mechanisms of action, which can be exploited for treating more complex diseases. Successful peptide drugs include insulin for the treatment of diabetes, and human growth hormone for treating growth disorders. However, peptide therapeutics suffer from the major disadvantage that they are difficult to formulate and deliver, particularly via the oral route, due to their physicochemical characteristics. Because these challenges have delayed the development and market approval of peptide drugs, they have also hindered the implementation of these drugs in pediatric populations.
In this review, we consider the current challenges and opportunities for the oral formulation of peptide therapeutics, specifically for infant populations. Infants are defined as being between 1 and 24 months of age [16]. An alternative review is available on drug delivery for neonates, which are babies less than one month of age [11]. In the first half of the review, we discuss the current status of the peptide drug pipeline for infants, and the challenges and solutions for oral formulation of peptide drugs. The second part of the review focuses on the distinct infant and adult gastrointestinal physiology that poses unique drug delivery challenges, highlighting the critical gaps of knowledge in this area. We also present the encouraging progress towards adapting conventional drug development and delivery approaches to infants.
2. The current peptide drug pipeline for pediatric patients
The clinical success of peptide drugs is due to their unique advantages compared to small molecules. These advantages include more specific interactions with their targets and biological activity that is inherently more limited, reducing off target effects. These attributes also make them particularly attractive for pediatric patients who are typically exposed to many medications, increasing the risk of potential drug-drug interactions [17]. Additionally, peptide drugs have more sophisticated pharmacological mechanisms than many small molecule drugs. As such, they are readily prescribed for diseases such as diabetes (insulin), osteoporosis (calcitonin), and acromegaly (octreotide), which are specifically treated in childhood.
However, of the many and diverse peptide and protein drugs currently in use, none of them are specifically indicated for pediatric use. This is despite the fact that the WHO’s list of essential medicines for children includes several peptide and protein drugs considered necessary for the basic needs of a healthcare system [18]. WHO-designated essential medicines include cyclosporine for treating organ transplant patients; several anti-cancer treatments including asparaginase, bleomycin, and dactinomycin; filgrastim, a protein used to treat low neutrophil count (often a result of chemotherapeutics); pancreatic enzymes for local intestinal enzyme replacement therapy; and insulin for the management of diabetes.
Encouragingly, as research into novel peptide and protein therapies has accelerated, some new peptide-based therapies for specific pediatric diseases have reached clinical trials in pediatric patients. For example, in 2016, Fouladi and coworkers published the results of a Phase I trial using a peptide drug to treat recurrent or progressive central nervous system tumors in pediatric patients [19]. In 2017, Carter et al. described the results of a clinical trial of the glucagon-like peptide 2 (GLP-2) analogue, teduglutide, which is being investigated for the treatment of short bowel syndrome [20]. This is a life-threatening condition that affects between 0.02–1.2% of newborns, making them reliant on total parenteral nutrition and greatly increasing mortality rates [21]. While these drugs are still being evaluated for efficacy, they are motivating examples for developing pediatric-specific oral formulations of peptide therapeutics. Both of the peptides described above would require long-term usage, and, thus, oral formulations of these drugs would improve ease of access and compliance.
Unfortunately, the inherent properties of peptide drugs, including gastric instability and poor intestinal permeability, have prohibited their oral formulation and reduced their value for infants. Currently, only four peptides have FDA-approved oral formulations, two of which were approved within the last two years of this writing. These are cyclosporine, a systemic immunosuppressant used to prevent organ rejection in transplant patients, semaglutide, a GLP-1 receptor agonist used to manage Type 2 diabetes, octreotide, a somatostatin analog for the treatment of acromegaly, and desmopressin, a vasopressin analog for treatment of nocturia and diabetes insipidus. Other oral peptide drugs in pre-/clinical development are summarized in Table 1. The four FDA-approved peptide drugs have rather unique properties that make them particularly amenable to oral formulation. These properties, and the challenges of developing oral formulation of peptide drugs in general, are described in detail below and we further discuss the strategies for oral peptide therapies translated to infants (Fig. 1).
Table 1.
–Peptide therapeutics in clinical trials or on the market
| Peptide | Molecular Weight (Da) | Indication | Manufacturer | Drug Name | Oral Delivery Technology | Trial Status | Relevancy to Pediatric Medicine |
|---|---|---|---|---|---|---|---|
| Cyclosporin | 1202.6 | Immunosuppressant (systemic) | Novartis | Neoral (previous form: Sandimmune) | Cyclic peptide, spontaneous microemulsion | FDA-Approved | Yes, studied in pediatric transplant patients aged 0.6–16 yrs (FDA doc) |
| AbbVie Inc | Gengraf | Sold as capsules or solution that contain the microemulsion | FDA-Approved | ||||
| Exenatide | 4186.6 | Glucagon-like peptide-1 analogue for T2D | Oramed Pharmaceuticals, Inc. | ORMD 0901 | Protein Oral Delivery (POD) technology: coated capsule, protease inhibitors, absorption enhancer (unspecified) | Preclinical/Investigational New Drug (Phase 1B); pharmacokinetic study completed in T2D patients, further bioavailability studies planned for 2020 | Trials for treatment of extreme obesity in older children (12–18) |
| Octreotide | 1019.2 | Synthetic somatostatin analog for treatment of acromegaly | Chiasma | Mycappsa (formerly Octreolin) | Transient Permeation Enhancer (TPE): multiple excipients create a lipophilic suspension of hydrophilic particles in hydrophobic medium | FDA-Approved | Case reports of treatment of GI bleeding, hyperinsulinism, hypothalamic obesity |
| Semaglutide | 4113.6 | GLP-1 receptor agonist for T2D | Novo Nordisk | Rybelsus | Eligen Technology licensed from Emisphere Technologies, Inc.: SNAC as a permeation enhancer | FDA-Approved | Investigated for weight management in obese adolescents |
| Insulin | 5808.0 | Regulate blood sugar for T1/T2 diabetes | Oramed | ORMD-0801 | POD technology | Phase 2 trials in progress for both T1D and T2D patients | T1D prevalence rate is 0.3% among children 0–19 years; insulin is standard treatment |
| Novo Nordisk (license acquired from Merrion Pharmaceuticals) | OI338GT | Gastro-Intestinal Permeation Enhancement Technology (GIPET): coated capsule with sodium caprate as a permeation enhancer | Phase 2 trials completed, but product development was discontinued due to high doses making it not commercially viable | ||||
| Oshadi Drug Administration, Ltd | Oshadi Icp | Silica nanoparticles with a branched polysaccharide and a suspension of insulin, proinsulin, and C-peptide in a mixture of oils | Phase 1 and 2 completed | ||||
| Biocon | Tregopil (IN-105) | Insulin analogue with covalent PEG modification for stability and solubility in the GI tract | FDA Phase 1 trial paused in 2018; Phase 2 and 3 trials ongoing in India | ||||
| Diasome | Oral-HDV insulin | Hepatic delivery vesicles (HDV): phospholipid nanocarriers with surface-bound insulin and specific hepatocyte targeting molecules | Phase 2b trial in progress | ||||
| Salmon calcitonin | 3431.9 | Hormone that reduces blood calcium for treatment of Paget’s disease, osteoporosis, hypercalcemia | R-Pharm JSC | TBRIA | Peptelligence (Enteris Biopharm) technology: citric acid | Phase 3 trial completed | Has been used in pediatric patients to treat hypercalcemia, but very rarely |
| Nordic Biosciences | SMCC021 | Eligen Technology from Emisphere Technologies, Inc.: 5-CNAC as a permeation enhancer | Phase 3 trial did not achieve primary endpoint of decreased incidence of bone fractures | ||||
| Leuprolide | 1209.4 | Hormone used to treat endometriosis, prostate cancer, premature puberty | Enteris Biopharma | Ovarest | Peptelligence (Enteris Biopharm) technology: surfactant permeation enhancer and citric acid | Phase 2 trial completed | Premature puberty affects 1 in 5–10,000 children and is often treated with injected hormones |
Abbreviations: FDA – US Food and Drug Administration, GI – gastrointestinal tract, GIPET – gastrointestinal permeation enhancing technology, PEG – Polyetholene glycol, POD™ – Protein Oral Delivery, SNAC – salcaprozate sodium, T1D – Type 1 diabetes, T2D – Type 2 diabetes, 5-CNAC - 8-(N-2-hydroxy-5-chloro-benzoyl)-amino-caprylic acid.
Fig. 1 –
The physiological barriers to delivering oral macromolecular drugs in infants differ from adults.
3. Challenges and solutions for oral formulation of peptide drugs
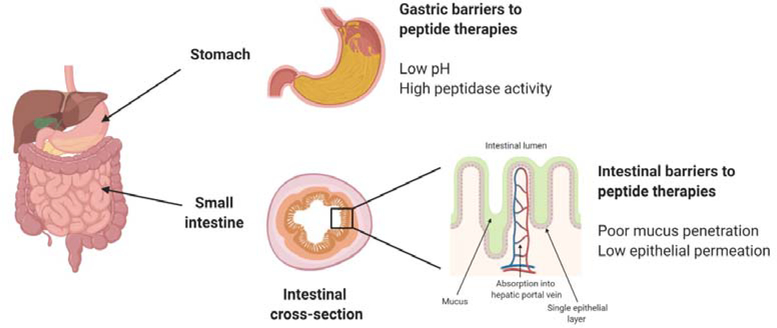
There are two key challenges that hinder the development of oral peptide drug formulations: their instability in the gastrointestinal tract, and their limited ability to cross the intestinal epithelium (Fig. 2). These challenges exist because the gastrointestinal tract was designed to break down nutritional proteins into individual amino acids for absorption, not to uptake intact protein drugs. Numerous strategies have been developed to overcome these issues, which are described below. However, these advances have been made largely within the context of adult physiology and require adaption for infants, as discussed in Section 4.
Fig. 2 –
The stomach and small intestine present unique biochemical and physical barriers to the oral delivery of peptide therapeutics.
3.1. Overcoming the gastric instability of peptide drugs
Peptide and protein drugs are prone to degradation in the acidic, enzyme-rich environment of the stomach. Specifically, peptides are highly susceptible to acid-catalyzed denaturation in the low pH of the stomach, and their complex structures are readily digested by proteases and peptidases in the intestinal lumen. Furthermore, their large size (generally >1 kDa) impedes uptake into the systemic circulation in therapeutic amounts because the small intestine is absorptive only to smaller molecules. This leads to many peptide therapeutics being categorized under the Biopharmaceutics Classification System (BCS) as either Class III (high solubility, low permeability) or Class IV (low solubility, low permeability) [22]. Strategies to improve stability generally fall into one of two categories: chemical modifications to the peptide that increase its resistance to digestive processes, and encapsulation methods that protect the drug cargo in the stomach and enable selective release in the absorptive region of the small intestine.
3.1.1. Molecular modifications of peptide drugs enhance gastric stability
In vitro digestion studies have suggested that cleavage by proteases such as pepsin is more problematic to peptide stability than the low pH in the stomach [23]. Thus, enhancing the stability of peptides to evade proteolysis by modifying their chemical and structural characteristics has been an active area of study. Several approaches have proven successful, including the introduction of cyclic structures and polymer conjugation, which are described in detail below.
Cyclosporine is one of the four peptide drugs with an FDA-approved oral formulation and one of two with a native cyclic structure, the other being cyclic octapeptide octreotide. This structure provides molecular rigidity and protection from endopeptidases, making it resistant to degradation in the gastrointestinal (GI) tract [24]. There are over forty cyclic peptide drugs available in parenteral formulations, with approximately one new cyclic peptide being approved annually [25]. Although many are isolated or derived from natural sources, cyclic structures have also been synthetically introduced into peptides that are not naturally cyclic to improve stability [26, 27].
A new approach for identifying structurally-stable peptide drugs was taken recently by Kong and colleagues. They used phage display technology to screen large libraries of so-called double-bridged peptides, which have increased resistance to proteolysis by GI proteases [28]. They identified a protease-resistant peptide that has the potential to treat inflammatory disorders of the digestive system such as Crohn’s disease. Another type of molecular stabilization that can be used to protect peptide drugs from proteolysis is the addition of hydrocarbon linkages or “staples” between amino acids on alpha helices. Bird et al. used this method to stabilize enfuvirtide, a 4.5 kDa peptide drug that can block HIV-1 entry in humans but is not widely used because it lacks the oral bioavailability of other anti-HIV-1 therapies [29]. Polymer conjugation is another well studied molecular approach used to increase the gastric stability of therapeutic peptides [30]. The addition of polymers, either by covalent conjugation or electrostatic attraction, increases stability in simulated gastric fluids as well as in animal models [31–33]. Many polymer chemistries have been used, and they are extensively reviewed in the literature [34].
Regarding use in infants, it is important to consider that infants metabolize many chemicals and drugs differently than adults. Altering the active pharmaceutical ingredient (API) – for example, by introducing a cyclic structure to improve bioavailability – may change how the drug is metabolized and excreted, which could have negative consequences. However, structurally modifying the API could also remove the need for additional excipients such as preservatives, which may also have undesirable effects in infants. This dilemma highlights the need for a better understanding of how pediatric patients process and tolerate different chemicals and drugs, which is discussed in Section 4.
3.1.2. Encapsulation methods protect peptides from gastric degradation
Another approach to protect peptide drugs from the proteolytic gastric environment is drug encapsulation with an enteric polymer that dissolves only after exiting the stomach. Several of the peptide drugs that have reached clinical trials utilize enteric-coated capsules or tablets (Table 1). The chemistry most commonly used is a system of acrylate/methacrylate and methacrylic acid copolymers [35, 36]. These polymers resist swelling and dissolution in the low pH environment of the stomach and release their cargo in the higher pH environment in the intestine. This release occurs because in pH 5–7 the carboxylic acid groups are transformed to carboxylate, causing the coating to dissolve. Eudragit® (Evonik) is a widely used, commercially available brand of enteric polymers, with co-polymer formulations that serve several different purposes. Eudragit® E masks the taste of drugs and nutritional supplements such as iron that have been encapsulated in microtablets for administration to infants and children [37]. Eudragit® E is cationic and soluble below pH 5.5, making it useful for taste masking, and Eudragit® L and S, which are soluble above pH 6 and 7 respectively, can be used to tailor release specifically to the small intestine or colon [38]. Other enteric polymers have been used in oral formulations to deliver pancrelipase to the small intestine for children cystic fibrosis, who need pancreatic enzyme supplementation to allow nutrient absorption for proper growth and development [39]. Enteric polymers are available in several forms, including aqueous dispersions, organic solutions, dry powder, and granules [38]. Capsugel® (Lonza) has developed capsules made out of intrinsically enteric polymers that eliminate the need for a coating step [40].
The flexibility of encapsulation technology means that it can be modified appropriately for infant populations. For example, drug formulations for infants are often dosed in fruit purees or yogurts and, therefore, the enteric polymer should accommodate a fed-state pH such as Eudragit® S. Additionally, there is an opportunity to develop pediatric-specific encapsulation polymers that are responsive to infant gastric and intestinal pH. Overall, the flexibility of encapsulation technologies is particularly helpful for infant-geared drug formulations, as unpleasant drug flavors can be taste-masked and release profiles can be optimized for the best pharmacokinetic outcome.
3.2. Enhancing peptide permeability in the intestine
In addition to degradation in the stomach, peptide drugs suffer poor oral bioavailability because they are not efficiently absorbed in the intestine. To reach systemic circulation, where most drugs take pharmacological effect, drugs need to first cross the mucus layer and then be absorbed across the intestinal epithelium. There are several commonly employed techniques that have overcome this barrier in clinical and pre-clinical studies of oral peptide formulations.
3.2.1. Evading the mucus trap
The mucus layer is a major barrier to macromolecule absorption in the intestine. Steric hindrance and electrostatic attraction of cationic atoms to the anionic mucus mesh network trap large molecules like proteins before they reach the absorptive surfaces of the epithelial cells [41, 42]. Many strategies have been employed to overcome the mucus barrier, including the use of mucolytic agents such as N-acetyl cysteine and polymeric carriers that alter the charge of the macromolecule and decrease mucus adherence [34, 42, 43]. It should be noted that infant intestinal mucus is more permeable than adult mucus (see Section 4 for more details), and it may be possible to take advantage of this unique physiological characteristic to avoid the use of mucus-penetrating agents in oral macromolecular drug formulations for young patients.
3.2.2. Stimulating transcellular/receptor-mediated uptake
The apical surface of intestinal epithelial cells is covered with transporters and receptors that facilitate drug and nutrient absorption. These have routinely been targeted to increase oral bioavailability of drugs [44]. For example, the di- and tripeptide transporter (PepT1), which has a broad range of specificity, was exploited to increase the absorption of peptidomimetic and pro-drug forms of antibiotics and antivirals [45–47]. Additionally, the neonatal Fc receptor pathway has facilitated oral peptide delivery in mice using insulin-loaded nanoparticles that were surface-modified with Fc fragments of IgG [48]. Another pathway with potential for oral protein delivery is the lactoferrin pathway. Lactoferrin is an iron-carrying protein, and there are receptors for its uptake on intestinal epithelial cells that can be used for a targeted method of uptake and transcytosis [49]. Recently, Han and coworkers formulated insulin in a zwitterionic micelle that was absorbed via the intestinal proton-assisted amino acid transporter 1 (PAT1), resulting in ~40% oral bioavailability in rats [50]. Although transcellular approaches have been successful, there is always a concern that the peptide drug will be degraded by intracellular peptidases if the drug is released too early or does not successfully cross the basolateral membrane.
For infantile delivery, it may be possible to tailor receptor-mediated approaches specifically for infant and pediatric applications due to relative expression of transporters. For example, we know that PepT1 expression and functionality is conserved between neonates through adulthood, and, therefore, that a PepT1-mediated uptake drug will be well absorbed in infants. Conversely, because the sodium-dependent imino transporter 1 (SIT1) has almost no expression in the newborn intestine, the uptake of proline and proline rich peptidomimetics is reduced compared to adults [51].
3.2.3. Enhancing intestinal permeability by the paracellular route
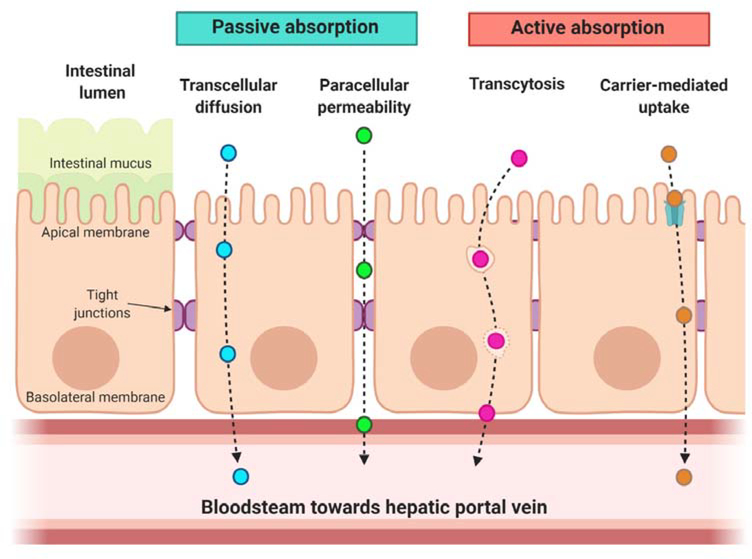
The paracellular route of transepithelial transport is regulated by a series of complex protein networks called tight junctions (Fig. 3) [52]. These limit the dilation of the paracellular space to prevent translocation of xenobiotics and, as such, hinder the absorption of larger peptide drugs. Many intestinal permeation enhancers take effect by transiently disrupting this network directly or indirectly through membrane fluidization, enabling drugs to be absorbed [53, 54]. Enhancers fall into classes such as: ionic and nonionic surfactants, bile salts, fatty acids, toxins, and other small molecules like heterocyclic amines [55–57]. Permeation enhancers have been extensively reviewed in the literature, but we will briefly present an overview of clinically successful enhancers as well as novel chemicals in pre-clinical development that have promise for oral delivery to infants [8, 58, 59]. Although we do not directly describe permeation enhancer mechanisms here, interested readers can refer to two recent reviews on this topic [60, 61].
Fig. 3 –
The complex mucus matrix and the intestinal epithelium pose transport barriers to the oral absorption of peptide therapeutics. A drug must diffuse through the intestinal mucus and then permeate the epithelial monolayer to reach systemic circulation. Transport across the epithelium can occur passively (via the transcellular or paracellular route) or actively (by transcytosis or receptor-mediated uptake).
Sodium caprate (C10) is perhaps the permeation enhancer with the most thoroughly investigated mechanism of action. Originally identified as a component of goat milk, C10 is a medium chain fatty acid that is an effective permeation enhancer in the small intestine with remarkably low toxicity compared to equally effective enhancers [53, 62–64]. C10 is the primary permeation enhancer used in GIPET® technology which was licensed by Novo Nordisk from Merrion Pharmaceuticals for use in an oral insulin formulation [65]. In addition to clinical trials for peptide oral delivery, C10 has also been investigated for the oral delivery of nucleotide-based drugs, increasing the available literature on its tolerability in humans [66]. Medium chain fatty acids, including C10, are the main fatty acid component of the solubilizing/permeation enhancing excipient Labrasol® from Gattefosse, along with triglycerides and PEGylated fatty acids [67, 68]. Chiasma’s oral formulation of octreotide (MYCAPPSA®) in an oily-suspension containing medium chain fatty acids (caprylate but not caprate) that act as intestinal permeation enhancers was approved by the FDA in 2020 [69, 70].
Another enhancer that has received significant attention is the medium chain fatty acid derivative, salcaprozate sodium (SNAC) [61]. Novo Nordisk developed an oral formulation of semaglutide using Eligen® Technology that includes SNAC as a stabilization agent and gastric permeation enhancer [71]. Fattah and colleagues have also shown that SNAC successfully improves the intestinal permeability of octreotide in isolated human intestinal tissue ex vivo [72]. Additionally, Peptelligence Technology developed by Enteris Biopharma uses citric acid (as a peptidase inhibitor) and acyl carnitine permeation enhancers in their in their delivery formulations of leuprolide and octreotide [73].
Chemical permeation enhancers are likely to be ubiquitous in oral peptide drug formulations going forward, based on the current status of marketed oral peptides as well as those in clinical trials. Fortunately, there are no data thus far that suggest that approved permeation enhancers pose significant physiological or toxicological effects in adults. Because it is unclear whether safety profiles will extend to infant and pediatric populations, chronic dosing will be required specifically in these populations prior to approval [74].
4. Infant and adult gastrointestinal physiology considerations for oral delivery
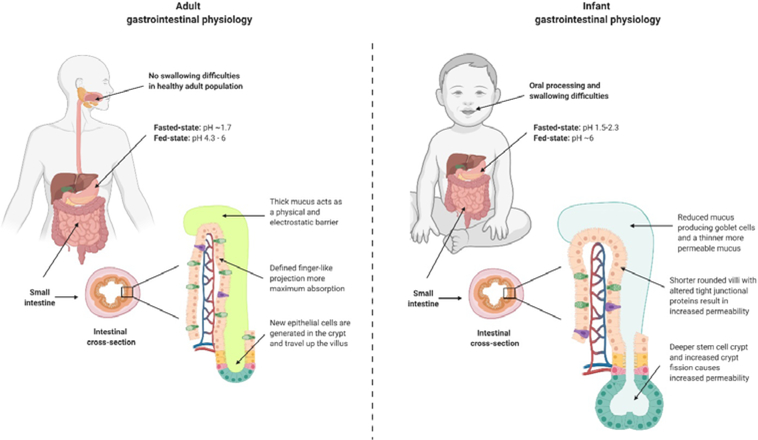
In the most basic terms, the barriers to oral drug delivery are the drug’s solubility and stability, and the absorptive capacity of the gastrointestinal tract. In pharmaceutics, there are standardized models for these processes based on research in adult subjects; however, the anatomy and physiology of infants and pediatric populations vary greatly from adults. Pharmacokinetic profiles are often extrapolated to children, but this is can be an oversimplification, as infants are not small adults from an adsorption, distribution, metabolism, and excretion (ADME) perspective. Here, we discuss the relevant physiological differences between infants (2 – 24 months of age) and adults (Fig. 1), and their implications for the oral delivery of peptide drugs.
4.1. Oral cavity and swallowing reflexes
Although the oral/buccal cavity is not a prominent barrier to oral drug delivery in adults [8], infants are different. Research has found that 25 – 45% of normally developing infants experience feeding difficulties [75]. In infants with cerebral palsy and dysphagia, this number climbs to 57 – 92%, making it a formidable barrier for oral drug delivery [76]. Infants initially develop the ability to swallow thin viscosity purees (4 – 6 months) and progress to more viscous foods with lumps or inconsistent texture (6 – 12 months) [77, 78]. Therefore, it is essential that delivery systems for younger infants are delivered in breastmilk, formulae, or a liquid of similar consistency to prevent swallowing difficulties [79]. Liquid dosage forms of vitamin and mineral supplements are used in 25% of infants [80]. Solid chewable or semi-solid gummy multivitamins are recommended only for children 2 years or older [81]. Such dosage forms are inappropriate for infants due to the risk of choking on small pieces while swallowing.
4.2. Gastric environment and transit time
The GI tract is a major biological barrier to oral drug delivery due to the harsh acidic environment and enzyme activity. Adults and infants differ in their gastric environment in that the pH of the infant stomach is higher than that of adults. In infants, gastric pH in the fed state is more readily buffered to higher pH (~ 6) than in adults (4 – 6) [82, 83], which is attributed to the high buffering capacity of breastmilk and formulae [84]. Another key attribute that affects oral delivery is gastric volume. The normalized gastric volume in fasted adults is ~ 0.3 ml/kg, while in infants it is slightly higher at 0.3 – 0.7 ml/kg [85, 86].
These physiological differences affect drug classifications, which serve as guides during the drug formulation process. Traditionally, the biopharmaceutical classification system (BCS) is used to categorize a drug based on the solubility in simulated gastric and intestinal fluids based on adult values. As such, the discrepancies between adults and infants have motivated the development of a harmonized pediatric BCS (pBCS). Recently, del Moral-Sanchez and colleagues utilized the WHO’s Essential Medicine List for children to map the pBCS and noted that almost a quarter of the drugs would switch from high to low solubility due to pediatric parameters [87]. The differences in gastric pH and volume can clearly impact the solubility of a drug and thereby the formulation approach should reply on the pBCS. Fortunately, infants and adults have similar gastric emptying rates and small intestine transit times [88, 89]. These factors are not expected to impact the translation of oral dosage forms from adults to infants.
4.3. Mucus composition and intestinal permeability
One of the first physical barriers that an orally delivered peptide drug must overcome is the intestinal mucus mesh. Small intestinal mucus is composed of water (~90%), electrolytes, lipids (1 – 2%), and mucin proteins (1 – 5%) [90]. Membrane bound mucin proteins form a densely packed network that anchors the secretory mucin-rich mucus to the epithelium [91]. This acts as both a steric and chemical barrier to the passage of particulates and macromolecules [42]. Goblet cells are “activated” after birth when they exposed to human milk bacteria and milk oligosaccharides initiating a life-long mucus barrier [92]. To our knowledge, although there are no studies on the compositional differences between human adult and infant mucus, there are some publications in pigs. The mucus in the piglet small intestine ex vivo is significantly less viscous than that of mature pigs, increasing its permeability to large objects (1 μm latex beads) [93]. This suggests that delivery vehicles and strategies that fail in adults due to mucoadhesion issues may still be a viable option for infants. Unfortunately, because the differences between human infant and adult mucus are unknown, fundamental studies on the properties of infant mucus are clearly needed to better understand this barrier.
The intestine is still developing when a newborn is delivered at full term. The intestinal epithelium is hyper-permeable in the first days of a neonate’s life to enable the translocation of colostral immunoglobulins such as IgA and IgM to develop the immune system [94]. Gut closure is believed to be initiated by growth factors in colostrum within the first 72 hours after birth in humans [95], which restricts the permeability of very large molecules (>150 kDa), while leaving it permeable to smaller molecules. There is an inverse relationship between age and permeability: human neonates have the highest intestinal permeability when using the oral lactulose:mannitol test (0.56 – 2.1), which drops two orders of magnitude as they enter infanthood (0.065 – 0.38) and another order of magnitude as they reach adulthood (0.013– 0.058) [96–102]. Similarly, we recently reported the increased oral absorption of insulin and lactoferrin in infant mice compared to adults resulted in pharmacodynamics effects of insulin without the aid of a permeation enhancer [103]. Unfortunately, it is unclear how this gradual change in mouse intestinal permeability relates to the passage of larger molecules such as peptide therapeutics in human infants.
The underlying mechanism of the enhanced permeability during infancy is likely related to altered expression of tight junction proteins, which are key regulators of the paracellular pathway in the intestinal epithelium [104]. As it is unethical to obtain biopsies from healthy infants, the expression profile of tight junctions is currently unknown. However, in rodent and porcine models, there is a clear correlation between maturation and barrier function relating to tight junction expression [105, 106]. Additionally, the morphology of the crypt-villus axis in infant intestinal physiology is dramatically different from adults. Infants have fewer villi, which are shorter and ridge-shaped, and elongated, deeper crypts (Fig. 1) than adults [107, 108]. It is possible that the immature intestinal villi in infants and the lower surface/volume ratio could lead to reduced drug absorption [109].
As with intestinal permeability in infants, there is also limited oral pharmacokinetic data available in infants. There have been a few studies on the pharmacokinetics of furosemide (330 Da), a commonly orally prescribed small molecule antidiuretic drug in pediatrics, which is absorbed in part paracellularly [109, 110]. When delivered to neonates, the oral bioavailability was 84%, but this dropped significantly when administered to children (56 ± 31%) and adults (49 ± 16) [111, 112]. However, in this study, the children were 2 to 15 years of age and this may overlook the enhanced permeability in infant-aged children. There are also bioavailability data in children of cyclosporine (1.2 kDa), an FDA- and EMEA-approved cyclic peptide immunosuppressant drug administered orally in pediatric populations. The oral bioavailability of cyclosporine in pediatric patients for heart and kidney transplant was equivalent to adults (24.7 ± 8%) [113]. Although there is a lack of substantive evidence, it is apparent that the intestinal barrier in healthy infants is more permeable than in adults. This provides an opportunity for the oral delivery of medications in this population but with the caveat that predicting the absorbed fraction is inherently more difficult.
4.4. Drug metabolism and clearance
Infants have increased drug metabolism and clearance. Intestinal and liver cytochrome P450 (CYPs) enzymes are the main drug metabolism enzymes. They are responsible for clearance of both small molecule drugs and peptide drugs after absorption, which limits their bioavailability [114]. Elevated duodenal CYP3A4 and CYP3A5 is reported in infants (0 – 12 months) and decreases as a function of age to mature levels after one year post birth [115, 116]. However, the degree to which expression reflects activity is unclear [117]. Instead, accelerated drug clearance in infants has been suggested to be due to the increased liver blood flow and larger ratio of liver to body mass in infants and young children [118]. Accordingly, there is a move towards allometric scaling for predicting drug dose administration in infants, which accounts for size and surface area differences. Tegenge et al. developed two different exponent models to better predict drug clearance in neonates and infants than previous more simplistic allometric approaches [119]. As more pharmacokinetic data become available for infant populations, these models will become even more robust and practical for dose selection.
There is a critical gap in our knowledge regarding appropriate dosing and pharmacokinetic profiling of drugs formulated for adults when used in infants. For example, oral administration of the anti-epileptic drug topiramate results in wildly different profiles for Cmax (1.8 vs 14.5 μg/ml) and t1/2 (41 vs 10 hours) in adults and infants respectively [120, 121]. Topiramate (marketed as TOPAMAX®) is noted by the FDA as having a 50% higher clearance rate in pediatric patients. What little data is available for drug clearance in infant populations is mostly restricted to small molecule drugs, which makes it challenging to predict the needs of a drug delivery system for peptide drugs. However, studies of injectable insulin in pediatric patients do highlight some additional challenges; for example, infants have less subcutaneous fat than adults, which will increase absorption [122]. Studies with cyclosporine are performed only in infants with severe organ failure, making it difficult to extend the data to predict absorption and clearance in the healthy infant population. Again, better predictive studies and appropriate models are needed for designing drug delivery systems specifically for infant and pediatric populations.
5. Adapting adult oral delivery approaches for infants
Given that infants pose unique physiological considerations when developing drug formulations and delivery strategies, conventional approaches need to be adapted to meet the needs of this population. This includes modifying formulation approaches and delivery devices and developing infant-relevant models for testing. Here, we highlight progress in these areas.
5.1. Formulation approaches for oral infant delivery
Liquid dosage forms offer a high degree of dose flexibility and ease of swallowing for infant patients. However, there are a number of fundamental limitations of liquid products, including palatability (children have a low tolerance for disagreeable taste and texture), lack of controlled release, dosage measurement accuracy and total volume, and peptide stability in solutions. The most appropriate vehicles to carry liquid dosage forms in infants are breastmilk, reconstituted milk formulae, and cow’s milk, due to their viscosity and improved palatability [79, 123]. However, when using milk as a vehicle, osmolality must be considered. The American Academy of Pediatrics recommended an osmolality limit of 400 mOsm kg−1 H2O for milk products for infants [124], and one study found that the addition of vitamin drops to human breastmilk doubled the osmolality to ~600 mOsm [125]. However, while osmolality is important for infants with gastrointestinal disorders due to the risk of perforation, it is not an issue for drug formulations for infants with healthy GI tracts. Infants who are > 6 months can be given liquid dosage forms that do not use milk as a carrier, and this has been successful for paracetamol (i.e. acetaminophen) and ibuprofen administration [126].
Peptide therapeutics are not as stable as small molecule drugs in liquid dosage forms and may require the addition of stabilizing excipients. Unfortunately, many preservatives and stabilizing agents that are safe or well-tolerated in adults are contraindicated for use in pediatric patients [127]. For example, sodium benzoate (a paraben preservative) causes hyperbilirubinemia in infants less than two months old, and other members of the paraben family have been reported to disrupt endocrine system development, causing the EMA to recommend that they be avoided in pediatric formulations [128, 129]. Polyethylene glycol is a commonly used drug solubilizer and is also approved as a safe treatment for constipation in children. However, its laxative effects are cause for concern in infants with healthy bowel movements [130].
Novartis’ oral cyclosporine solution NEORAL® is a combination of corn oil triglycerides (solubilizer), polyoxyl 40 stearate (surfactant and emulsifier), DL-α tocopherol (anti-oxidant), and propylene glycol (solvent). It is unclear if these excipients have negative effects, but as the patients require solid organ transplantation, it is expected that the therapeutic benefits far outweigh the concerns of excipient-induced toxicity. Research into safe excipients and their ability to stabilize oral liquid drugs for infants is ongoing, and it is likely that any given macromolecular drug will require specific formulation development [131]. There is evidence that several excipients are poor choices for inclusion in infant formulations. For example, sodium benzoate, which is often used in liquid dosage forms, can impair neonatal and infant development. Binson et al. showed that the compounding agent Syrspend® SF PH4 Dry successfully resuspended small molecule APIs such as dexamethasone [131]. Additionally, ethanol and propylene glycol are both excipient solvents that are considered harmful to pediatric populations and need to be avoided when formulating a liquid dosage form. It is clear that there is still much work to be done in the development of formulation solutions for an often-overlooked population.
The desire to avoid preservative use in infant formulations has led to a shift in focus to single-use solid oral dosage forms for pediatric patients. Solid dosage forms also overcome many of the concerns associated with delivering peptides in liquid form. For example, lyophilization is an effective stabilization strategy for peptide drugs and nanoformulations, especially when the cryoprotectant trehalose is used [132]. Trehalose is a naturally occurring D-glucose disaccharide with a history of use in humans and is considered a safe and appropriate excipient for infant formulations [133]. Lyophilization with trehalose is a simple way to turn a peptide drug into a powder that can then be processed into one of the many solid dosage forms currently being developed specifically for use in infants.
Some of the most promising of these solid dosage forms for peptide drug delivery in infants older than 6 months include mini-tablets and microparticle “sprinkles”/powders that can be added to age-appropriate semi-solid foods, and orodispersible tablets that disintegrate rapidly in the mouth [134]. Orbis Bioscience’s Optimμm® technology enables controlled release of peptide drugs and is available as a microparticle powder designed to enable food-mixing for pediatric drug delivery. Takeda Pharmaceutical’s SoluTab™ is an orodispersible tablet of lansoprazole prescribed for acid reflux in infants. Although used for local delivery, McNeil’s Pancrease MT® is an enteric-coated mini-tablet formulation of pancreatic enzymes for the treatment of chronic pancreatitis and cystic fibrosis. Overall, mini-tablets appear to be the most effective method to deliver an appropriate dose to an infant or younger child.
It is likely that permeation enhancers would be necessary to effectively deliver peptide drugs orally in infants. However, it may be possible to decrease their dose because of the increased intestinal permeability in infants, as discussed above. This could make peptide delivery easier from a safety standpoint, as the literature generally cautions against the overuse of permeation enhancers [74]. Permeation enhancers that are considered viable candidates for use in infants would be those derived either from components native to the gastrointestinal tract (bile salts) or components found in their diet (sodium caprylate and sodium caprate) [135]. However, the lack of physiologically-relevant models to study enhancers, excipients, and formulations in infants is one of the biggest barriers to development in the field (see Section 5.3).
5.2. Engineering devices for peptide delivery in infants
There are several drug delivery devices that have been specifically engineered for infants, and they are likely appropriate for protein and peptide drugs. JustMilk has developed a disposable silicone Nipple Shield Delivery System (NSDS) that can be loaded with an appropriate dispersible solid dosage form. The NSDS is placed on the breast, and the flow of milk solubilizes the drug, which is then consumed by the infant [136, 137]. The developers of the technology have formed focus groups to assess the use of the NSDS for anti-retroviral drug administration to infants in Kenya [138]. However, this technology is adapted specifically for breastfeeding infants, which accounts for only ~50% of infants and typically infants <6 months of age [139].
DS Technology’s XStraw™ meets the drug delivery needs of older infants and children with a pre-dosed straw. The granulated medication is pre-filled in the straw, and when the child drinks through the straw, it solubilizes the drug for easy oral administration. This type of technology is already on the market for flavored milks under “Milk Magic” and “Quick Milk,” and this similarity to novelty food products could improve compliance with children. Although not a device, 3D printing technology offers flexibility to produce small and intricately-designed delivery vehicles in a range of materials. A focus group of healthcare professionals was optimistic about the use of 3D printing for pediatric drug delivery, specifically for personalized doses and dosage forms [140]. Scoutaris et al. 3D-printed indomethacin in a polyethylene glycol and hypromellose acetate succinate matrix to imitate the popular Starmix® gummies, and this technology platform could be adapted for infant specific formulations [141]. Overall, these engineering applications are good examples of designing patient-focused delivery devices rather than forcing adult devices to work for infants and young children.
5.3. Models for predicting oral absorption in infants
One of the greatest difficulties in designing and adapting medications for children and infants is the lack of appropriate drug absorption models. Pediatric patients are enrolled in 10-times fewer trials that adults and notably in fewer drug trials compared to adults (48.7% vs. 65.8%), while they have higher enrollment in dietary supplement (5.3% vs. 2.7%) and biological therapies (15.4% vs. 6.1%) [4]. It appears that the adoption of an alternative formulation approach (oral vs. injectable) is uncommon in pediatric clinical trials, with some notable exceptions. For example, the Global Platform for the Prevention of Autoimmune Diabetes Primary Oral Insulin Trail (GPPAD-POInT) enrolled infants 4 to 7 months old who were at high risk of developing type 1 diabetes to receive oral insulin therapy and enrolled the first patient in 2018 [142].
The classic cellular model used in drug absorption studies is the Caco-2 cell model. This colonic adenocarcinoma cell line grows as a monolayer of polarized columnar epithelium and is easily adapted for paracellular and transcellular permeation studies [143]. Unfortunately, the cells are known for their heterogeneity in transporter expression and their poor in vitro-in vivo correlation [144]. In standard absorption studies, drug formulations are applied directly to the cell monolayer, which oversimplifies the complexity of the barriers for drug absorption in the intestine. A number of approaches have been taken to improve the physiological relevance of this model using mucus and altered tissue sources [145]. For example, co-cultures of Caco-2 cells and mucus producing HT29-MTX-E12 cells produce a more physiologically relevant model but still fail to mimic in vivo conditions [146]. Additionally, biosimilar mucus has been developed that reflects the steric and rheological properties of adult porcine mucus [147, 148]. Because it is likely that human infant mucus differs significantly from adult [93], there is a need to develop biosimilar mucus to reflect the rheological properties of infant mucus.
There are several models that recapitulate the infant intestine more faithfully than Caco-2 models. For example, the neonatal porcine jejunal IPEC-J2 cell line and intestinal organoids better reflect more permeable intestinal tissues [149, 150]. The developmental pathway involved in differentiating stem cells into intestinal organoids yields tissue that is similar to fetal intestinal tissue [151]. Additionally, the expression and function of uptake and efflux transporters in intestinal organoids are more reflective of in vivo biology [152]. Further investigation is needed to determine whether these systems are appropriate models to predict intestinal drug absorption in infant populations.
Unfortunately, animal models are also lacking in their prediction of drug absorption in infants. Drug absorption studies are commonly carried out on ex vivo tissue samples, with single pass intestinal perfusion/intestinal instillations, or via oral gavage in rodents [47, 67, 153]. Rodent intestinal development differs from human physiology; there is a lag in gut closure, and it is difficult to map infant mice or rats onto human infants. Researchers have calculated the relative infant development age in human years for mice (56 days = 1st human year) and rats (42 days = 1st human year) [154, 155]. Therefore, to conduct infant-relevant studies in rodents, the animals should be used before they are weaned from the dam at ~21 days [103]. Pigs and dogs are better predictors of human oral bioavailability, but there are considerable cost and logistical difficulties associated with these more advanced preclinical models [156]. In particular, the Göttingen Minipig model (3 to 6 weeks old) has a very similar gastrointestinal profile to human infants [157].
There is a critical need to understand the permeability and pharmacokinetics of various drug types in these infant models. This opinion has been reinforced by the European network on understanding gastrointestinal absorption-related processes (UNGAP). It has stated that the pediatric-specific gastrointestinal physiology has been overlooked during oral drug development, which has then made the prediction of absorption more difficult [158]. It is our opinion that improved in vitro models (organoid models) combined with in silico models will improve pediatric formulation design. For example, pediatric predictive absorption models using Certara’s Symcyp™ and Simulation Plus’ GastroPlus® have been successfully used for pediatric drug screening [159, 160].
6. Concluding opinions on the opportunities in infant oral drug delivery
Peptide therapeutics have revolutionized patient care since the discovery and isolation of insulin in the 1920s. Unfortunately, the majority of the 60+ approved peptide drugs and 150 peptides in active development are formulated as injectables [15]. Oral delivery of peptide drugs has been a consistent area of academic and industrial research that has yielded recent FDA approval for Novo Nordisk’s semaglutide, Chiasma’s octreotide, and Novartis’ cyclosporine, all of which are on the market as capsule formulations for use in adult patients. While there are limited data on oral peptide therapeutics in infants, some clinical trials have been carried out in infants including insulin (NCT01093638 [terminated 2016], and NCT03364868 [recruiting 2020]) and the cyclohexapeptide nepadutant for treatment of colic (NCT00655083 – completed 2011). The GPPAD-POInT trial (NCT03364868) is exciting, as it is one of the first trials that will provide insights into the efficacy of orally delivered peptides to infants, however, this trial is not due to complete until 2025. This trial, along with the approval of multiple oral peptide drugs, are reason for optimism when considering the future of oral peptide therapies for infants.
From a formulation and dosage-form perspective, significant progress has been made in terms of infant drug delivery. Anecdotally, giving an infant a liquid dosage form is a struggle for parents and healthcare providers, and the whole dose is rarely administered with ease. Mini-tablet and sprinkle formulations can enable mixing of the dosage form within food for older infants without the concern of choking. Age-appropriate devices have been designed to meet the needs of oral dosage in infants rather than attempting to adapt adult formulations. The rapid evolution of 3D printed technologies and their applicability in pharmacy and hospital settings will vastly improve our ability to adapt dosage forms for infants. We will be able to ad-hoc adapt the design, matrix, and dose in 3D printed solids, chewables, or thin-films to truly revolutionize patient-centric dosage forms. The most substantial remaining challenge is the access to appropriate absorption models for predictive pharmacokinetic profiling in infants.
Although the progress towards improving child-sized medicines is slow-moving, we are confident that the peptide therapeutic pipeline and advances in patient-centric delivery devices will improve the eventual translation of therapies to infants. Better incentives, either at the basic research level and/or for clinical translation, should be put in place by funding agencies and industry to catalyze progress. We believe that it is within the collective power of academia, industry, and healthcare to reduce off-label prescribing for infants by devoting more time and energy to the development of pediatric-appropriate drug formulations. As Nelson Mandela said, “There can be no keener revelation of a society’s soul than the way in which it treats its children”.
7. Acknowledgements
The authors acknowledge support from the National Institutes of Health (NIH) award #DP2-HD098860 and National Science Foundation (NSF) award #1807983. Graphics were created with BioRender.com.
8 References
- [1].Niño DF, Sodhi CP, Hackam DJ, Necrotizing enterocolitis: new insights into pathogenesis and mechanisms, Nature reviews. Gastroenterology & hepatology, 13 (2016) 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hime NJ, Zurynski Y, Fitzgerald D, Selvadurai H, Phu A, Deverell M, Elliott EJ, Jaffe A, Childhood interstitial lung disease: A systematic review, Pediatric Pulmonology, 50 (2015) 1383–1392. [DOI] [PubMed] [Google Scholar]
- [3].Büscher R, Büscher AK, Weber S, Mohr J, Hegen B, Vester U, Hoyer PF, Clinical manifestations of autosomal recessive polycystic kidney disease (ARPKD): kidney-related and non-kidney-related phenotypes, Pediatric nephrology, 29 (2014) 1915–1925. [DOI] [PubMed] [Google Scholar]
- [4].Pasquali SK, Lam WK, Chiswell K, Kemper AR, Li JS, Status of the pediatric clinical trials enterprise: an analysis of the US ClinicalTrials.gov registry, Pediatrics, 130 (2012) e1269–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Turner S, Nunn A, Fielding K, Choonara I, Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study, Acta Paediatrica, 88 (1999) 965–968. [DOI] [PubMed] [Google Scholar]
- [6].Pratico AD, Longo L, Mansueto S, Gozzo L, Barberi I, Tiralongo V, Salvo V, Falsaperla R, Vitaliti G, La Rosa M, Leonardi S, Rotondo A, Avola N, Sgarlata D, Damiano A, Tirantello M, Anzelmo G, Cipolla D, Rizzo A, Russo A, Ruggieri M, Salomone S, Drago F, Off-Label Use of Drugs and Adverse Drug Reactions in Pediatric Units: A Prospective, Multicenter Study, Current drug safety, 13 (2018) 200–207. [DOI] [PubMed] [Google Scholar]
- [7].Yackey K, Stukus K, Cohen D, Kline D, Zhao S, Stanley R, Off-label Medication Prescribing Patterns in Pediatrics: An Update, Hospital Pediatrics, 9 (2019) 186–193. [DOI] [PubMed] [Google Scholar]
- [8].Brown TD, Whitehead KA, Mitragotri S, Materials for oral delivery of proteins and peptides, Nature Reviews Materials, 5 (2020) 127–148. [Google Scholar]
- [9].Darji MA, Lalge RM, Marathe SP, Mulay TD, Fatima T, Alshammari A, Lee HK, Repka MA, Narasimha Murthy S, Excipient Stability in Oral Solid Dosage Forms: A Review, AAPS PharmSciTech, 19 (2018) 12–26. [DOI] [PubMed] [Google Scholar]
- [10].IWAI N, Drug compliance of children and infants with oral antibiotics for pediatric use, Pediatrics International, 39 (1997) 132–142. [DOI] [PubMed] [Google Scholar]
- [11].O’Brien F, Clapham D, Krysiak K, Batchelor H, Field P, Caivano G, Pertile M, Nunn A, Tuleu C, Making Medicines Baby Size: The Challenges in Bridging the Formulation Gap in Neonatal Medicine, International journal of molecular sciences, 20 (2019) 2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Batchelor HK, Marriott JF, Formulations for children: problems and solutions, British journal of clinical pharmacology, 79 (2015) 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rood JM, Engels MJ, Ciarkowski SL, Wagenknecht LD, Dickinson CJ, Stevenson JG, Variability in compounding of oral liquids for pediatric patients: a patient safety concern, Journal of the American Pharmacists Association : JAPhA, 54 (2014) 383–389. [DOI] [PubMed] [Google Scholar]
- [14].de la Torre BG, Albericio F, The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules, Molecules (Basel, Switzerland), 25 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lau JL, Dunn MK, Therapeutic peptides: Historical perspectives, current development trends, and future directions, Biorg. Med. Chem, 26 (2018) 2700–2707. [DOI] [PubMed] [Google Scholar]
- [16].EMA I, Topic E 11-Clinical Investigation of Medicinal Products in the Paediatric Population, EMEA, Editor, 2001. [Google Scholar]
- [17].Feinstein J, Dai D, Zhong W, Freedman J, Feudtner C, Potential Drug−Drug Interactions in Infant, Child, and Adolescent Patients in Children’s Hospitals, Pediatrics, 135 (2015) e99–e108. [DOI] [PubMed] [Google Scholar]
- [18].W.H. Organization., WHO Model List of Essential Medicines for Children: Explanatory notes, World Health Organization, Geneva, Switzerland, 2015. [Google Scholar]
- [19].Lulla RR, Goldman S, Yamada T, Beattie CW, Bressler L, Pacini M, Pollack IF, Fisher PG, Packer RJ, Dunkel IJ, Dhall G, Wu S, Onar A, Boyett JM, Fouladi M, Phase I trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study, Neuro-oncology, 18 (2016) 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Carter BA, Cohran VC, Cole CR, Corkins MR, Dimmitt RA, Duggan C, Hill S, Horslen S, Lim JD, Mercer DF, Merritt RJ, Nichol PF, Sigurdsson L, Teitelbaum DH, Thompson J, Vanderpool C, Vaughan JF, Li B, Youssef NN, Venick RS, Kocoshis SA, Outcomes from a 12-Week, Open-Label, Multicenter Clinical Trial of Teduglutide in Pediatric Short Bowel Syndrome, J Pediatr, 181 (2017) 102–111.e105. [DOI] [PubMed] [Google Scholar]
- [21].Kelly DG, Tappenden KA, Winkler MF, Short Bowel Syndrome: Highlights of Patient Management, Quality of Life, and Survival, Journal of Parenteral and Enteral Nutrition, 38 (2014) 427–437. [DOI] [PubMed] [Google Scholar]
- [22].Amidon GL, Lennernas H, Shah VP, Crison JR, A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability., Pharm. Res, 12 (1995) 413–420. [DOI] [PubMed] [Google Scholar]
- [23].Wang J, Yadav V, Smart AL, Tajiri S, Basit AW, Toward Oral Delivery of Biopharmaceuticals: An Assessment of the Gastrointestinal Stability of 17 Peptide Drugs, Mol. Pharm, 12 (2015) 966–973. [DOI] [PubMed] [Google Scholar]
- [24].Joo SH, Cyclic peptides as therapeutic agents and biochemical tools, Biomol Ther (Seoul), 20 (2012) 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abdalla MA, McGaw LJ, Natural Cyclic Peptides as an Attractive Modality for Therapeutics: A Mini Review, Molecules (Basel, Switzerland), 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gentilucci L, De Marco R, Cerisoli L, Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization, Curr. Pharm. Des, 16 (2010) 3185–3203. [DOI] [PubMed] [Google Scholar]
- [27].Purkayastha A, Kang TJ, Stabilization of Proteins by Covalent Cyclization, Biotechnology and Bioprocess Engineering, 24 (2019) 702–712. [Google Scholar]
- [28].Kong X-D, Moriya J, Carle V, Pojer F, Abriata LA, Deyle K, Heinis C, De novo development of proteolytically resistant therapeutic peptides for oral administration, Nature Biomedical Engineering, 4 (2020) 560–571. [DOI] [PubMed] [Google Scholar]
- [29].Bird GH, Madani N, Perry AF, Princiotto AM, Supko JG, He X, Gavathiotis E, Sodroski JG, Walensky LD, Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic, Proceedings of the National Academy of Sciences, 107 (2010) 14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Murata H, Cummings CS, Koepsel RR, Russell AJ, Polymer-Based Protein Engineering Can Rationally Tune Enzyme Activity, pH-Dependence, and Stability, Biomacromolecules, 14 (2013) 1919–1926. [DOI] [PubMed] [Google Scholar]
- [31].Lucius M, Falatach R, McGlone C, Makaroff K, Danielson A, Williams C, Nix JC, Konkolewicz D, Page RC, Berberich JA, Investigating the Impact of Polymer Functional Groups on the Stability and Activity of Lysozyme–Polymer Conjugates, Biomacromolecules, 17 (2016) 1123–1134. [DOI] [PubMed] [Google Scholar]
- [32].Fuhrmann G, Grotzky A, Lukić R, Matoori S, Luciani P, Yu H, Zhang B, Walde P, Schlüter AD, Gauthier MA, Leroux J-C, Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates, Nature Chemistry, 5 (2013) 582–589. [DOI] [PubMed] [Google Scholar]
- [33].Schulz JD, Patt M, Basler S, Kries H, Hilvert D, Gauthier MA, Leroux J-C, Site-Specific Polymer Conjugation Stabilizes Therapeutic Enzymes in the Gastrointestinal Tract, Adv. Mater, 28 (2016) 1455–1460. [DOI] [PubMed] [Google Scholar]
- [34].Schulz JD, Gauthier MA, Leroux J-C, Improving oral drug bioavailability with polycations?, Eur J Pharm Biopharm, 97 (2015) 427–437. [DOI] [PubMed] [Google Scholar]
- [35].Khan MZI, Prebeg Ž, Kurjaković N, A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers: I. Manipulation of drug release using Eudragit® L100–55 and Eudragit® S100 combinations, Journal of Controlled Release, 58 (1999) 215–222. [DOI] [PubMed] [Google Scholar]
- [36].Liu L, Yao W, Rao Y, Lu X, Gao J, pH-Responsive carriers for oral drug delivery: challenges and opportunities of current platforms, Drug Deliv, 24 (2017) 569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pratap Singh A, Siddiqui J, Diosady LL, Characterizing the pH-Dependent Release Kinetics of Food-Grade Spray Drying Encapsulated Iron Microcapsules for Food Fortification, Food and Bioprocess Technology, 11 (2018) 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Thakral S, Thakral NK, Majumdar DK, Eudragit: a technology evaluation, Expert Opin Drug Deliv, 10 (2013) 131–149. [DOI] [PubMed] [Google Scholar]
- [39].Van de Vijver E, Desager K, Mulberg AE, Staelens S, Verkade HJ, Bodewes FA, Malfroot A, Hauser B, Sinaasappel M, Van Biervliet S, Behm M, Pelckmans P, Callens D, Veereman-Wauters G, Treatment of infants and toddlers with cystic fibrosis-related pancreatic insufficiency and fat malabsorption with pancrelipase MT, J Pediatr Gastroenterol Nutr, 53 (2011) 61–64. [DOI] [PubMed] [Google Scholar]
- [40].Benameur H, Enteric capsule drug delivery technology—Achieving protection without coating, Drug Dev. Deliv, 15 (2015) 34–37. [Google Scholar]
- [41].Desai MA, Mutlu M, Vadgama P, A study of macromolecular diffusion through native porcine mucus, Experientia, 48 (1992) 22–26. [DOI] [PubMed] [Google Scholar]
- [42].Boegh M, Nielsen HM, Mucus as a barrier to drug delivery - understanding and mimicking the barrier properties, Basic & clinical pharmacology & toxicology, 116 (2015) 179–186. [DOI] [PubMed] [Google Scholar]
- [43].Ensign LM, Cone R, Hanes J, Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers, Adv Drug Deliv Rev, 64 (2012) 557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Anderson CM, Thwaites DT, Hijacking solute carriers for proton-coupled drug transport, Physiology (Bethesda, Md.), 25 (2010) 364–377. [DOI] [PubMed] [Google Scholar]
- [45].Ates M, Kaynak MS, Sahin S, Effect of permeability enhancers on paracellular permeability of acyclovir, The Journal of pharmacy and pharmacology, 68 (2016) 781–790. [DOI] [PubMed] [Google Scholar]
- [46].Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J, Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans, Antimicrob. Agents Chemother, 39 (1995) 2759–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gleeson JP, Frías JM, Ryan SM, Brayden DJ, Sodium caprate enables the blood pressure-lowering effect of Ile-Pro-Pro and Leu-Lys-Pro in spontaneously hypertensive rats by indirectly overcoming PepT1 inhibition, Eur J Pharm Biopharm, 128 (2018) 179–187. [DOI] [PubMed] [Google Scholar]
- [48].Pridgen EM, Alexis F, Kuo TT, Levy-Nissenbaum E, Karnik R, Blumberg RS, Langer R, Farokhzad OC, Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery, Sci Transl Med, 5 (2013) 213ra167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lönnerdal B, Jiang R, Du X, Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities, J Pediatr Gastroenterol Nutr, 53 (2011) 606–614. [DOI] [PubMed] [Google Scholar]
- [50].Han X, Lu Y, Xie J, Zhang E, Zhu H, Du H, Wang K, Song B, Yang C, Shi Y, Cao Z, Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions, Nature Nanotechnology, 15 (2020) 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Meier C, Camargo SM, Hunziker S, Moehrlen U, Gros SJ, Bode P, Leu S, Meuli M, Holland-Cunz S, Verrey F, Vuille-dit-Bille RN, Intestinal IMINO transporter SIT1 is not expressed in human newborns, American Journal of Physiology-Gastrointestinal and Liver Physiology, 315 (2018) G887–G895. [DOI] [PubMed] [Google Scholar]
- [52].Shen L, Weber CR, Raleigh DR, Yu D, Turner JR, Tight junction pore and leak pathways: A dynamic duo, Annu. Rev. Physiol, 73 (2011) 283–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brayden DJ, Gleeson JP, Walsh EG, A head-to-head multi-parametric high content analysis of a series of medium chain fatty acid intestinal permeation enhancers in Caco-2 cells, Eur J Pharm Biopharm, 88 (2014) 830–839. [DOI] [PubMed] [Google Scholar]
- [54].McCartney F, Rosa M, Brayden DJ, Evaluation of Sucrose Laurate as an Intestinal Permeation Enhancer for Macromolecules: Ex Vivo and In Vivo Studies, Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A, Zonula occludens toxin structure-function analysis: Identification of the fragment biologically active on tight junctions and the zonulin receptor binding domain, J. Biol. Chem, 276 (2001) 19160–19165. [DOI] [PubMed] [Google Scholar]
- [56].Gupta V, Hwang BH, Doshi N, Mitragotri S, A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine, Journal of controlled release : official journal of the Controlled Release Society, 172 (2013) 541–549. [DOI] [PubMed] [Google Scholar]
- [57].Fein KC, Lamson NG, Whitehead KA, Structure-Function Analysis of Phenylpiperazine Derivatives as Intestinal Permeation Enhancers, Pharm. Res, 34 (2017) 1320–1329. [DOI] [PubMed] [Google Scholar]
- [58].Maher S, Brayden DJ, Casettari L, Illum L, Application of Permeation Enhancers in Oral Delivery of Macromolecules: An Update, Pharmaceutics, 11 (2019) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gleeson JP, Ryan SM, Brayden DJ, Oral delivery strategies for nutraceuticals: Delivery vehicles and absorption enhancers, Trends in Food Science & Technology, 53 (2016) 90–101. [Google Scholar]
- [60].Brayden DJ, Hill TA, Fairlie DP, Maher S, Mrsny RJ, Systemic delivery of peptides by the oral route: Formulation and medicinal chemistry approaches, Advanced Drug Delivery Reviews, 157 (2020) 2–36. [DOI] [PubMed] [Google Scholar]
- [61].Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ, Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C(10)), Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lindmark T, Nikkila T, Artursson P, Mechanisms of absorption enhancement by medium chain fatty acids in intestinal epithelial Caco-2 cell monolayers, J. Pharmacol. Exp. Ther, 275 (1995) 958–964. [PubMed] [Google Scholar]
- [63].Gleeson JP, Heade J, Ryan SM, Brayden DJ, Stability, toxicity and intestinal permeation enhancement of two food-derived antihypertensive tripeptides, Ile-Pro-Pro and Leu-Lys-Pro, Peptides, 71 (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [64].Dahlgren D, Olander T, Sjöblom M, Hedeland M, Lennernäs H, Effect of paracellular permeation enhancers on intestinal permeability of two peptide drugs, enalaprilat and hexarelin, in rats, Acta Pharmaceutica Sinica B, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Halberg IB, Lyby K, Wassermann K, Heise T, Zijlstra E, Plum-Mörschel L, Efficacy and safety of oral basal insulin versus subcutaneous insulin glargine in type 2 diabetes: a randomised, double-blind, phase 2 trial, The Lancet Diabetes & Endocrinology, 7 (2019) 179–188. [DOI] [PubMed] [Google Scholar]
- [66].Tillman LG, Geary RS, Hardee GE, Oral delivery of antisense oligonucleotides in man, J. Pharm. Sci, 97 (2008) 225–236. [DOI] [PubMed] [Google Scholar]
- [67].McCartney F, Jannin V, Chevrier S, Boulghobra H, Hristov DR, Ritter N, Miolane C, Chavant Y, Demarne F, Brayden DJ, Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: Ex vivo and in vivo rat studies, Journal of Controlled Release, 310 (2019) 115–126. [DOI] [PubMed] [Google Scholar]
- [68].Shen Y, Lu Y, Jv M, Hu J, Li Q, Tu J, Enhancing effect of Labrasol on the intestinal absorption of ganciclovir in rats, Drug Dev. Ind. Pharm, 37 (2011) 1415–1421. [DOI] [PubMed] [Google Scholar]
- [69].Tuvia S, Atsmon J, Teichman SL, Katz S, Salama P, Pelled D, Landau I, Karmeli I, Bidlingmaier M, Strasburger CJ, Kleinberg DL, Melmed S, Mamluk R, Oral octreotide absorption in human subjects: Comparable pharmacokinetics to parenteral octreotide and effective growth hormone suppression, The Journal of Clinical Endocrinology & Metabolism, 97 (2012) 2362–2369. [DOI] [PubMed] [Google Scholar]
- [70].Tuvia S, Pelled D, Marom K, Salama P, Levin-Arama M, Karmeli I, Idelson G, Landau I, Mamluk R, A novel suspension formulation enhances intestinal absorption of macromolecules via transient and reversible transport mechanisms, Pharmaceutical research, 31 (2014) 2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, Madsen KG, Schéele SG, Alanentalo T, Kirk RK, Pedersen BL, Skyggebjerg RB, Benie AJ, Strauss HM, Wahlund P-O, Bjerregaard S, Farkas E, Fekete C, Søndergaard FL, Borregaard J, Hartoft-Nielsen M-L, Knudsen LB, Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist, Science translational medicine, 10 (2018) eaar7047. [DOI] [PubMed] [Google Scholar]
- [72].Fattah S, Ismaiel M, Murphy B, Rulikowska A, Frias JM, Winter DC, Brayden DJ, Salcaprozate sodium (SNAC) enhances permeability of octreotide across isolated rat and human intestinal epithelial mucosae in Ussing chambers, European Journal of Pharmaceutical Sciences, 154 (2020) 105509. [DOI] [PubMed] [Google Scholar]
- [73].Binkley N, Bolognese M, Sidorowicz-Bialynicka A, Vally T, Trout R, Miller C, Buben CE, Gilligan JP, Krause DS, A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial, J. Bone Miner. Res, 27 (2012) 1821–1829. [DOI] [PubMed] [Google Scholar]
- [74].McCartney F, Gleeson JP, Brayden DJ, Safety concerns over the use of intestinal permeation enhancers: A mini-review, Tissue Barriers, 4 (2016) e1176822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lau C, Development of Suck and Swallow Mechanisms in Infants, Ann Nutr Metab, 66 Suppl 5 (2015) 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sullivan PB, Lambert B, Rose M, Ford-Adams M, Johnson A, Griffiths P, Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study, Developmental medicine and child neurology, 42 (2000) 674–680. [DOI] [PubMed] [Google Scholar]
- [77].Arvedson JC, Brodsky L, Lefton-Greif MA, Pediatric swallowing and feeding: Assessment and management, Plural Publishing; 2019. [Google Scholar]
- [78].Cichero JAY, Introducing solid foods using baby-led weaning vs. spoon-feeding: A focus on oral development, nutrient intake and quality of research to bring balance to the debate, Nutrition Bulletin, 41 (2016) 72–77. [Google Scholar]
- [79].Frazier J, Chestnut AH, Jackson A, Barbon CEA, Steele CM, Pickler L, Understanding the Viscosity of Liquids used in Infant Dysphagia Management, Dysphagia, 31 (2016) 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Briefel R, Hanson C, Fox MK, Novak T, Ziegler P, Feeding Infants and Toddlers Study: do vitamin and mineral supplements contribute to nutrient adequacy or excess among US infants and toddlers?, J. Am. Diet. Assoc, 106 (2006) S52–65. [DOI] [PubMed] [Google Scholar]
- [81].Elliott C, Assessing Vitamins, Minerals and Supplements Marketed to Children in Canada, Int. J. Env. Res. Public Health, 16 (2019) 4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yu G, Zheng Q-S, Li G-F, Similarities and differences in gastrointestinal physiology between neonates and adults: a physiologically based pharmacokinetic modeling perspective, The AAPS journal, 16 (2014) 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dressman JB, Berardi RR, Dermentzoglou LC, Russell TL, Schmaltz SP, Barnett JL, Jarvenpaa KM, Upper gastrointestinal (GI) pH in young, healthy men and women, Pharm. Res, 7 (1990) 756–761. [DOI] [PubMed] [Google Scholar]
- [84].Guimarães M, Statelova M, Holm R, Reppas C, Symilllides M, Vertzoni M, Fotaki N, Biopharmaceutical considerations in paediatrics with a view to the evaluation of orally administered drug products – a PEARRL review, J. Pharm. Pharmacol, 71 (2019) 603–642. [DOI] [PubMed] [Google Scholar]
- [85].Mudie DM, Amidon GL, Amidon GE, Physiological parameters for oral delivery and in vitro testing, Molecular pharmaceutics, 7 (2010) 1388–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Litman RS, Wu CL, Quinlivan JK, Gastric volume and pH in infants fed clear liquids and breast milk prior to surgery, Anesthesia and analgesia, 79 (1994) 482–485. [DOI] [PubMed] [Google Scholar]
- [87].delMoral-Sanchez JM, Gonzalez-Alvarez I, Gonzalez-Alvarez M, Navarro A, Bermejo M, Classification of WHO Essential Oral Medicines for Children Applying a Provisional Pediatric Biopharmaceutics Classification System, Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bonner JJ, Vajjah P, Abduljalil K, Jamei M, Rostami-Hodjegan A, Tucker GT, Johnson TN, Does age affect gastric emptying time? A model-based meta-analysis of data from premature neonates through to adults, Biopharm Drug Dispos, 36 (2015) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Maharaj AR, Edginton AN, Examining Small Intestinal Transit Time as a Function of Age: Is There Evidence to Support Age-Dependent Differences among Children?, Drug Metab. Disposition, 44 (2016) 1080–1089. [DOI] [PubMed] [Google Scholar]
- [90].Paone P, Cani PD, Mucus barrier, mucins and gut microbiota: the expected slimy partners?, Gut, 69 (2020) 2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Johansson MEV, Ambort D, Pelaseyed T, Schütte A, Gustafsson JK, Ermund A, Subramani DB, Holmén-Larsson JM, Thomsson KA, Bergström JH, van der Post S, Rodriguez-Piñeiro AM, Sjövall H, Bäckström M, Hansson GC, Composition and functional role of the mucus layers in the intestine, Cell. Mol. Life Sci, 68 (2011) 3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, Lebrilla CB, Weimer BC, Mills DA, German JB, Sonnenburg JL, Bacteroides in the infant gut consume milk oligosaccharides via mucus-utilization pathways, Cell Host Microbe, 10 (2011) 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Macierzanka A, Mackie AR, Bajka BH, Rigby NM, Nau F, Dupont D, Transport of Particles in Intestinal Mucus under Simulated Infant and Adult Physiological Conditions: Impact of Mucus Structure and Extracellular DNA, PloS one, 9 (2014) e95274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hurley WL, Theil PK, Perspectives on immunoglobulins in colostrum and milk, Nutrients, 3 (2011) 442–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Vukavic T, Timing of the gut closure, J Pediatr Gastroenterol Nutr, 3 (1984) 700–703. [DOI] [PubMed] [Google Scholar]
- [96].Musa MA, Kabir M, Hossain MI, Ahmed E, Siddique A, Rashid H, Mahfuz M, Mondal D, Ahmed T, Petri WA, Haque R, Measurement of intestinal permeability using lactulose and mannitol with conventional five hours and shortened two hours urine collection by two different methods: HPAE-PAD and LC-MSMS, PloS one, 14 (2019) e0220397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Weaver LT, Laker MF, Nelson R, Intestinal permeability in the newborn, Arch. Dis. Child, 59 (1984) 236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Goto K, Chew F, Torún B, Peerson JM, Brown KH, Epidemiology of Altered Intestinal Permeability to Lactulose and Mannitol in Guatemalan Infants, Journal of Pediatric Gastroenterology and Nutrition, 28 (1999). [DOI] [PubMed] [Google Scholar]
- [99].Lunn PG, Northrop-Clewes CA, Downes RM, Intestinal permeability, mucosal injury, and growth faltering in Gambian infants, The Lancet, 338 (1991) 907–910. [DOI] [PubMed] [Google Scholar]
- [100].Colomé G, Sierra C, Blasco J, García MV, Valverde E, Sánchez E, Intestinal permeability in different feedings in infancy, Acta Paediatrica, 96 (2007) 69–72. [DOI] [PubMed] [Google Scholar]
- [101].Valentini L, Ramminger S, Haas V, Postrach E, Werich M, Fischer A, Koller M, Swidsinski A, Bereswill S, Lochs H, Schulzke J-D, Small intestinal permeability in older adults, Physiological Reports, 2 (2014) e00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Johnston SD, Smye M, Watson RG, McMillan SA, Trimble ER, Love AH, Lactulose-mannitol intestinal permeability test: a useful screening test for adult coeliac disease, Ann. Clin. Biochem, 37 ( Pt 4) (2000) 512–519. [DOI] [PubMed] [Google Scholar]
- [103].Gleeson JP, Fein KC, Chaudhary N, Doerfler R, Newby AN, Whitehead KA, The enhanced intestinal permeability of infant mice enables oral protein and macromolecular absorption without delivery technology, Int. J. Pharm, 593 (2021) 120120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Anderson JM, Van Itallie CM, Physiology and function of the tight junction, Cold Spring Harbor Perspectives in Biology, 1 (2009) a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM, Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns, Gene Expression Patterns, 6 (2006) 581–588. [DOI] [PubMed] [Google Scholar]
- [106].Pasternak JA, Kent-Dennis C, Van Kessel AG, Wilson HL, Claudin-4 Undergoes Age-Dependent Change in Cellular Localization on Pig Jejunal Villous Epithelial Cells, Independent of Bacterial Colonization, Mediators of Inflammation, 2015 (2015) 263629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Cummins AG, Catto-Smith AG, Cameron DJ, Couper RT, Davidson GP, Day AS, Hammond PD, Moore DJ, Thompson FM, Crypt fission peaks early during infancy and crypt hyperplasia broadly peaks during infancy and childhood in the small intestine of humans, J Pediatr Gastroenterol Nutr, 47 (2008) 153–157. [DOI] [PubMed] [Google Scholar]
- [108].Penna FJ, Hill ID, Kingston D, Robertson K, Slavin G, Shiner M, Jejunal mucosal morphometry in children with and without gut symptoms and in normal adults, J. Clin. Pathol, 34 (1981) 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Nicolas J-M, Bouzom F, Hugues C, Ungell A-L, Oral drug absorption in pediatrics: the intestinal wall, its developmental changes and current tools for predictions, Biopharm. Drug Disposition, 38 (2017) 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Flanagan SD, Takahashi LH, Liu X, Benet LZ, Contributions of saturable active secretion, passive transcellular, and paracellular diffusion to the overall transport of furosemide across adenocarcinoma (Caco-2) cells, Journal of pharmaceutical sciences, 91 (2002) 1169–1177. [DOI] [PubMed] [Google Scholar]
- [111].Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ, Raye JR, Furosemide pharmacokinetics in very low birth weight infants, J Pediatr, 112 (1988) 653–657. [DOI] [PubMed] [Google Scholar]
- [112].Prandota J, Clinical pharmacology of furosemide in children: a supplement, American journal of therapeutics, 8 (2001) 275–289. [DOI] [PubMed] [Google Scholar]
- [113].Cooney GF, Habucky K, Hoppu K, Cyclosporin pharmacokinetics in paediatric transplant recipients, Clin Pharmacokinet, 32 (1997) 481–495. [DOI] [PubMed] [Google Scholar]
- [114].Wacher VJ, Silverman JA, Zhang Y, Benet LZ, Role of P-Glycoprotein and Cytochrome P450 3A in Limiting Oral Absorption of Peptides and Peptidomimetics†, J. Pharm. Sci, 87 (1998) 1322–1330. [DOI] [PubMed] [Google Scholar]
- [115].Chen Y-T, Trzoss L, Yang D, Yan B, Ontogenic expression of human carboxylesterase-2 and cytochrome P450 3A4 in liver and duodenum: postnatal surge and organ-dependent regulation, Toxicology, 330 (2015) 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fakhoury M, Litalien C, Medard Y, Cavé H, Ezzahir N, Peuchmaur M, Jacqz-Aigrain E, Localization and mRNA expression of CYP3A and P-glycoprotein in human duodenum as a function of age, Drug metabolism and disposition: the biological fate of chemicals, 33 (2005) 1603–1607. [DOI] [PubMed] [Google Scholar]
- [117].Sadler NC, Nandhikonda P, Webb-Robertson B-J, Ansong C, Anderson LN, Smith JN, Corley RA, Wright AT, Hepatic Cytochrome P450 Activity, Abundance, and Expression Throughout Human Development, Drug Metab. Disposition, 44 (2016) 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Batchelor HK, Fotaki N, Klein S, Paediatric oral biopharmaceutics: key considerations and current challenges, Adv Drug Deliv Rev, 73 (2014) 102–126. [DOI] [PubMed] [Google Scholar]
- [119].Tegenge MA, Mahmood I, Jiang Z, Forshee R, Multistep Unified Models Using Prior Knowledge for the Prediction of Drug Clearance in Neonates and Infants, J. Clin. Pharmacol, 58 (2018) 877–884. [DOI] [PubMed] [Google Scholar]
- [120].Clark AM, Kriel RL, Leppik IE, Marino SE, Mishra U, Brundage RC, Cloyd JC, Intravenous topiramate: Comparison of pharmacokinetics and safety with the oral formulation in healthy volunteers, Epilepsia, 54 (2013) 1099–1105. [DOI] [PubMed] [Google Scholar]
- [121].Glauser TA, Miles MV, Tang P, Clark P, McGee K, Doose DR, Topiramate Pharmacokinetics in Infants, Epilepsia, 40 (1999) 788–791. [DOI] [PubMed] [Google Scholar]
- [122].Danne T, Phillip M, Buckingham BA, Jarosz-Chobot P, Saboo B, Urakami T, Battelino T, Hanas R, Codner E, ISPAD Clinical Practice Consensus Guidelines 2018: Insulin treatment in children and adolescents with diabetes, Pediatric Diabetes, 19 (2018) 115–135. [DOI] [PubMed] [Google Scholar]
- [123].Martir J, Flanagan T, Mann J, Fotaki N, Recommended strategies for the oral administration of paediatric medicines with food and drinks in the context of their biopharmaceutical properties: a review, J. Pharm. Pharmacol, 69 (2017) 384–397. [DOI] [PubMed] [Google Scholar]
- [124].Barness LA, Mauer AM, Holliday MA, Anderson AS, Dallman PR, Forbes GB, Goldbloom RB, Haworth JC, Jesse MJ, Scriver CR, Winick M, Kline OL, Miller RW, O’ Brien D, Commentary on breast-feeding and infant formulas, including proposed standards for formulas, Pediatrics, 57 (1976) 278–285. [PubMed] [Google Scholar]
- [125].Radmacher PG, Adamkin MD, Lewis ST, Adamkin DH, Milk as a vehicle for oral medications: hidden osmoles, Journal of Perinatology, 32 (2012) 227–229. [DOI] [PubMed] [Google Scholar]
- [126].Perrott DA, Piira T, Goodenough B, Champion GD, Efficacy and safety of acetaminophen vs ibuprofen for treating children’s pain or fever: a meta-analysis, Arch. Pediatr. Adolesc. Med, 158 (2004) 521–526. [DOI] [PubMed] [Google Scholar]
- [127].Fabiano V, Mameli C, Zuccotti GV, Paediatric pharmacology: remember the excipients, Pharmacol. Res, 63 (2011) 362–365. [DOI] [PubMed] [Google Scholar]
- [128].Green TP, Marchessault RP, Freese DK, Disposition of sodium benzoate in newborn infants with hyperammonemia, J Pediatr, 102 (1983) 785–790. [DOI] [PubMed] [Google Scholar]
- [129].EMA, Reflection paper on the use of methyl-and propylparaben as excipients in human medicinal products for oral use. Committee For Medicinal Products For Human Use., European Medicines Agency Science Medicines Health, (2015) 1–13. [Google Scholar]
- [130].Michail S, Gendy E, Preud’Homme D, Mezoff A, Polyethylene glycol for constipation in children younger than eighteen months old, J Pediatr Gastroenterol Nutr, 39 (2004) 197–199. [DOI] [PubMed] [Google Scholar]
- [131].Binson G, Beuzit K, Migeot V, Marco L, Troussier B, Venisse N, Dupuis A, Preparation and Physicochemical Stability of Liquid Oral Dosage Forms Free of Potentially Harmful Excipient Designed for Pediatric Patients, Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Emami F, Vatanara A, Park EJ, Na DH, Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals, Pharmaceutics, 10 (2018) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek AP, Waalkens-Berendsen DH, Shigoyuki A, Kurimoto M, Trehalose: a review of properties, history of use and human tolerance, and results of multiple safety studies, Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 40 (2002) 871–898. [DOI] [PubMed] [Google Scholar]
- [134].Trofimiuk M, Wasilewska K, Winnicka K, How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population, International journal of molecular sciences, 20 (2019) 3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Gleeson JP, Diet, food components and the intestinal barrier, Nutrition Bulletin, 42 (2017) 123–131. [Google Scholar]
- [136].Scheuerle RL, Bruggraber SFA, Gerrard SE, Kendall RA, Tuleu C, Slater NKH, Characterisation of zinc delivery from a nipple shield delivery system using a breastfeeding simulation apparatus, PloS one, 12 (2017) e0171624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Scheuerle RL, Gerrard SE, Kendall RA, Tuleu C, Slater NK, Mahbubani KT, Characterising the disintegration properties of tablets in opaque media using texture analysis, Int. J. Pharm, 486 (2015) 136–143. [DOI] [PubMed] [Google Scholar]
- [138].Hart CW, Israel-Ballard KA, Joanis CL, Baniecki ML, Thungu F, Gerrard SE, Kneen E, Sokal DC, Acceptability of a nipple shield delivery system administering antiviral agents to prevent mother-to-child transmission of HIV through breastfeeding, Journal of human lactation : official journal of International Lactation Consultant Association, 31 (2015) 68–75. [DOI] [PubMed] [Google Scholar]
- [139].Organisation WH., Global nutrition targets 2025: Breastfeeding policy brief, World Health Organisation., Geneva, Switzerland, 2014. [Google Scholar]
- [140].Rautamo M, Kvarnström K, Sivén M, Airaksinen M, Lahdenne P, Sandler N, Benefits and Prerequisites Associated with the Adoption of Oral 3D-Printed Medicines for Pediatric Patients: A Focus Group Study among Healthcare Professionals, Pharmaceutics, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Scoutaris N, Ross SA, Douroumis D, 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications, Pharm. Res, 35 (2018) 34. [DOI] [PubMed] [Google Scholar]
- [142].Ziegler A-G, Achenbach P, Berner R, Casteels K, Danne T, Gündert M, Hasford J, Hoffmann VS, Kordonouri O, Lange K, Elding Larsson H, Lundgren M, Snape MD, Szypowska A, Todd JA, Bonifacio E, group GS, Oral insulin therapy for primary prevention of type 1 diabetes in infants with high genetic risk: the GPPAD-POInT (global platform for the prevention of autoimmune diabetes primary oral insulin trial) study protocol, BMJ Open, 9 (2019) e028578–e028578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Artursson P, Ungell AL, Lofroth JE, Selective paracellular permeability in two models of intestinal absorption: cultured monolayers of human intestinal epithelial cells and rat intestinal segments, Pharm. Res, 10 (1993) 1123–1129. [DOI] [PubMed] [Google Scholar]
- [144].Pham-The H, Cabrera-Pérez M, Nam NH, Castillo-Garit JA, Rasulev B, Le-Thi-Thu H, Casañola-Martin GM, In Silico Assessment of ADME Properties: Advances in Caco-2 Cell Monolayer Permeability Modeling, Curr. Top. Med. Chem, 18 (2018) 2209–2229. [DOI] [PubMed] [Google Scholar]
- [145].Gleeson JP, McCartney F, Striving Towards the Perfect In Vitro Oral Drug Absorption Model, Trends Pharmacol. Sci, 40 (2019) 720–724. [DOI] [PubMed] [Google Scholar]
- [146].Lozoya-Agullo I, Araújo F, González-Álvarez I, Merino-Sanjuán M, González-Álvarez M, Bermejo M, Sarmento B, Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B Coculture Models To Predict Intestinal and Colonic Permeability Compared to Caco-2 Monoculture, Mol. Pharm, 14 (2017) 1264–1270. [DOI] [PubMed] [Google Scholar]
- [147].Birch D, Diedrichsen RG, Christophersen PC, Mu H, Nielsen HM, Evaluation of drug permeation under fed state conditions using mucus-covered Caco-2 cell epithelium, European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 118 (2018) 144–153. [DOI] [PubMed] [Google Scholar]
- [148].Boegh M, Baldursdóttir SG, Müllertz A, Nielsen HM, Property profiling of biosimilar mucus in a novel mucus-containing in vitro model for assessment of intestinal drug absorption, Eur J Pharm Biopharm, 87 (2014) 227–235. [DOI] [PubMed] [Google Scholar]
- [149].Zakrzewski SS, Richter JF, Krug SM, Jebautzke B, Lee I-FM, Rieger J, Sachtleben M, Bondzio A, Schulzke JD, Fromm M, Günzel D, Improved cell line IPEC-J2, characterized as a model for porcine jejunal epithelium, PloS one, 8 (2013) e79643–e79643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Gleeson JP, Estrada HQ, Yamashita M, Svendsen CN, Targan SR, Barrett RJ, Development of Physiologically Responsive Human iPSC-Derived Intestinal Epithelium to Study Barrier Dysfunction in IBD, Int J Mol Sci, 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Múnera JO, Sundaram N, Rankin SA, Hill D, Watson C, Mahe M, Vallance JE, Shroyer NF, Sinagoga KL, Zarzoso-Lacoste A, Hudson JR, Howell JC, Chatuvedi P, Spence JR, Shannon JM, Zorn AM, Helmrath MA, Wells JM, Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling, Cell Stem Cell, 21 (2017) 51–64.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Kasendra M, Tovaglieri A, Sontheimer-Phelps A, Jalili-Firoozinezhad S, Bein A, Chalkiadaki A, Scholl W, Zhang C, Rickner H, Richmond CA, Li H, Breault DT, Ingber DE, Development of a primary human Small Intestine-on-a-Chip using biopsy-derived organoids, Scientific Reports, 8 (2018) 2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Singh G, Pai RS, Atazanavir-loaded Eudragit RL 100 nanoparticles to improve oral bioavailability: optimization and in vitro/in vivo appraisal, Drug delivery, (2014) 1–8. [DOI] [PubMed] [Google Scholar]
- [154].Dutta S, Sengupta P, Men and mice: Relating their ages, Life Sci, 152 (2016) 244–248. [DOI] [PubMed] [Google Scholar]
- [155].Sengupta P, The Laboratory Rat: Relating Its Age With Human’s, Int J Prev Med, 4 (2013) 624–630. [PMC free article] [PubMed] [Google Scholar]
- [156].Henze LJ, Koehl NJ, O’Shea JP, Kostewicz ES, Holm R, Griffin BT, The pig as a preclinical model for predicting oral bioavailability and in vivo performance of pharmaceutical oral dosage forms: a PEARRL review, The Journal of pharmacy and pharmacology, 71 (2019) 581–602. [DOI] [PubMed] [Google Scholar]
- [157].Ayuso M, Buyssens L, Stroe M, Valenzuela A, Allegaert K, Smits A, Annaert P, Mulder A, Carpentier S, Van Ginneken C, Van Cruchten S, The Neonatal and Juvenile Pig in Pediatric Drug Discovery and Development, Pharmaceutics, 13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Vinarov Z, Abrahamsson B, Artursson P, Batchelor H, Berben P, Bernkop-Schnürch A, Butler J, Ceulemans J, Davies N, Dupont D, Flaten GE, Fotaki N, Griffin BT, Jannin V, Keemink J, Kesisoglou F, Koziolek M, Kuentz M, Mackie A, Meléndez-Martínez AJ, McAllister M, Müllertz A, O’Driscoll CM, Parrott N, Paszkowska J, Pavek P, Porter CJH, Reppas C, Stillhart C, Sugano K, Toader E, Valentová K, Vertzoni M, De Wildt SN, Wilson CG, Augustijns P, Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network, Advanced Drug Delivery Reviews, 171 (2021) 289–331. [DOI] [PubMed] [Google Scholar]
- [159].Lin W, Yan J-H, Heimbach T, He H, Pediatric Physiologically Based Pharmacokinetic Model Development: Current Status and Challenges, Current Pharmacology Reports, 4 (2018) 491–501. [Google Scholar]
- [160].Verscheijden LFM, Koenderink JB, Johnson TN, de Wildt SN, Russel FGM, Physiologically-based pharmacokinetic models for children: Starting to reach maturation?, Pharmacol. Ther, 211 (2020) 107541. [DOI] [PubMed] [Google Scholar]