Abstract
Aromatase converts androgens into estrogens in the brain of vertebrates including humans. This enzyme is also expressed in other tissues where its action may result in negative effects on human health (e.g., promotion of tumor growth). To prevent these effects, aromatase inhibitors were developed and are currently used to block human estrogen-dependent tumors. In vertebrates including quail, aromatase is expressed in a highly conserved set of interconnected brain nuclei known as the social behavior network. This network is directly implicated in the expression of a large range of social behaviors. The primary goal of this study was to characterize in Japanese quail the potential impact of brain aromatase on sexual behavior, aggressiveness and social motivation (i.e., tendency to approach and stay close to conspecifics). An additional goal was to test the feasibility and effectiveness of long-term delivery of an aromatase inhibitor directly into the third ventricle via Alzet™ osmotic minipumps using male sexual behavior as the aromatase dependent measure. We demonstrate that this mode of administration results in the strongest inhibition of both copulatory behavior and sexual motivation ever observed in this species, while other social behaviors were variably affected. Sexual motivation and the tendency to approach a group of conspecifics including females clearly seem to depend on brain aromatase, but the effects of central estrogen production on aggressive behavior and on the motivation to approach males remain less clear.
Keywords: aromatase, neurosestrogen, sexual behavior, aggressive behavior, social motivation, bird
1. INTRODUCTION
The enzyme aromatase catalyzes the conversion of androgens into estrogens. It is expressed in the brain of vertebrates from fishes to mammals including humans (Naftolin et al., 1971; Naftolin et al., 1975; Biegon et al., 2010; Schlinger & Balthazart, 2012; Biegon, 2016). Brain derived estrogens have been associated with the regulation of multiple physiological or behavioral processes including sexual differentiation (McCarthy, 2012), activation of reproductive behaviors (Balthazart & Ball, 2012), neuroprotection (Duncan & Saldanha, 2019), cognition and memory (Bakoyiannis et al., 2016; Navarro-Pardo et al., 2017; Rosenfeld et al., 2018; Marbouti et al., 2020; Taxier et al., 2020) and mood (Fink et al., 1998; Halbreich & Kahn, 2001; Grigoriadis & Kennedy, 2002). In addition, aromatase is found in other tissues (Simpson, 2004), including adipose tissue and estrogen-dependent breast tumors where its action via an overproduction of estrogens results in negative effects on human health by increasing body weight (Cohen, 2001) or by promoting tumor growth respectively (Zahid et al., 2016).
To prevent these negative actions of estrogens, powerful aromatase inhibitors have been developed and are currently used in humans namely as first line therapy of breast cancer in postmenopausal women (Cuzick et al., 2010; Regan et al., 2011). Aromatase inhibitors are also administered in pediatrics to treat a variety of syndromes, including follicular ovarian cyst hyperandrogenism, pubertal gynecomastia and short stature and/or pubertal delay in boys (Shulman et al., 2008; de Ronde & de Jong, 2011; Wit et al., 2011) as well as in adults to treat late-onset hypogonadism, obesity-related hypogonadotropic hypogonadism, gynecomastia, or male subfertility (Singh, 2013; Tan et al., 2014). Additionally, aromatase inhibitors are diverted from their medical use by bodybuilders or athletes as supplement to doping with androgens or anabolic steroids in order to block gynecomastia induced by the excess of estrogens derived from conversion of the administered exogenous androgens (Vari et al., 2016). However, the potential effects of continuous aromatase inhibitor administration on brain function are still poorly understood (Gervais et al., 2019).
In vertebrates, aromatase is expressed in multiple parts (Foidart et al., 1995; Wu et al., 2009; Stanić et al., 2014) of a highly conserved set of interconnected brain nuclei known as the social behavior network (Newman, 1999; Goodson, 2005). This network is directly implicated in the expression of a large range of social behaviors (i.e., behaviors involving interactions between at least two conspecifics) including sexual behavior and sexual motivation, parental behavior, various forms of aggressive behavior, and social motivation (Goodson, 2005; Robinson et al., 2008; Goodson & Kabelik, 2009a; O’Connell & Hofmann, 2011, 2012). The function of aromatase in each of these nuclei is largely unknown with the exception of the medial preoptic area, where local androgen aromatization is key for the activation of male sexual behavior (Clancy et al., 1995; Balthazart et al., 2004).
Evidence from aromatase knockout (ArKO) mice models and from pharmacological studies based on the systemic administration of aromatase inhibitors confirms that brain aromatase plays a key role in the expression of male copulatory behavior. Indeed, male sexual behavior of ArKO mice is characterized by markedly increased latencies to mount and decreased numbers of mounts of a female stimulus (Honda et al., 1998; Bakker et al., 2002; Bakker et al., 2004; Brooks et al., 2020). Inhibition of sexual behavior following administration of aromatase inhibitors has also been described in monkeys (Zumpe et al., 1993), ferrets (Carroll et al., 1988), musk shrews (Rissman et al., 1996), rats (Bonsall et al., 1992; Clancy et al., 1995; Vagell & McGinnis, 1997) and birds such as quail (Alexandre & Balthazart, 1986; Balthazart et al., 1990a; Taziaux et al., 2004). Some limited evidence also suggests a role of aromatase in the expression of aggressive behavior in mice (Toda et al., 2001) and songbirds (Soma et al., 2000; Heimovics et al., 2018). However, the putative role of brain aromatase in other types of social behaviors remains poorly characterized.
The Japanese quail (Coturnix japonica) is an excellent experimental model to analyze the functions of brain aromatase. Males of this species exhibit a dense expression of aromatase in most nuclei of the social behavior network (Foidart et al., 1995) and they display a large range of social behaviors in laboratory settings (Mills et al., 1997; Ball & Balthazart, 2010). Systemic injections of aromatase inhibitors were shown to decrease both sexual motivation and copulatory behavior (Balthazart et al., 1990a; Taziaux et al., 2004). More specifically, it was demonstrated that estrogens synthetized in the brain (neuroestrogens) are a key element in the activation of male sexual behavior by testosterone (Balthazart & Surlemont, 1990b). Indeed, implantation of an aromatase inhibitor into the medial preoptic nucleus (POM) inhibits male copulatory behavior (Balthazart et al., 1990a; de Bournonville et al., 2019). Likewise, initiation of sexual behavior was delayed by implants in the BST (de Bournonville et al., 2019). A measure of sexual motivation, the frequency of rhythmic cloacal sphincter movements (RCSM) produced in response to the view of a female was however not significantly affected by any of these brain implants (de Bournonville et al., 2019). Yet, a previous study had shown that RCSM are decreased by a systemic treatment with an aromatase inhibitor (Taziaux et al., 2004). Given that this behavioral inhibition only developed fully after multiple tests with a female, it was impossible to determine whether this effect resulted from a direct effect of the treatment on sexual motivation or whether it indirectly resulted from a decreased value of the female as a sexual stimulus due to the inability of males to copulate. Alternatively, the lack of effect on RCSM of aromatase inhibition in POM could be due to an insufficient dose of inhibitor being released from the implant or from the fact that this behavior is controlled by aromatase in another brain nucleus.
The role of brain aromatase in the control of social behaviors other than sexual behavior is also poorly known in quail. Yet, the neuroanatomical distribution of aromatase largely overlaps with the distribution of vasotocin (Balthazart et al., 1997a) and mesotocin (Bons, 1980) in brain regions where these nonapeptides, or their mammalian equivalent vasopressin and oxytocin, play a role in the control of social behaviors (Kelly & Goodson, 2014). While the interaction of estrogens and mesotocin has not been studied in quail, the expression of vasotocin in regions involved in social behavior depends on estrogens (Viglietti-Panzica et al., 2001). A role for neuroestrogens in the control of social behaviors in quail thus seems likely but has never been investigated. Systemic administration of aromatase inhibitors had been found to reduce behaviors potentially reflecting aggressiveness in male quail (Schlinger & Callard, 1990a, 1990b) but to our knowledge the role of aromatase had never been investigated in other social behaviors.
The main goal of this study was thus to assess the role of brain aromatase in the expression of social behaviors. Experiment 1 was set out to test the impact of chronic central delivery of the aromatase inhibitor vorozole via an intracerebroventricular cannula connected to an osmotic pump on sexual behavior, since this behavior is well known to depend on brain aromatase. Then, we went on to test the effect of the aromatase inhibition on partner preference, aggressive behavior, and social motivation. Using gonadally intact males with a limited sexual experience, this experiment demonstrated that this delivery mode prevents the expression of sexual behavior and indicated that central aromatase inhibition blocks the motivation to join a mixed sex group but failed to answer the question of the role of the aromatase on aggressive behavior. This experiment also did not identify any robust preference to spend time with a male or female conspecific. Experiment 2 was then conducted to extend our conclusions about efficacy of the treatment on the sexual behavior of fully experienced males thus exploring the ability of the treatment to inhibit a fully established behavior and further assess the role of neuroestrogens on aggressive behavior and on the motivation to join a group composed of males only. To insure that the observed effects reflected direct actions the aromatase inhibitor on the circuits underlying social behaviors rather than an indirect effect via alteration of the negative feedback on gonadal secretion, experiment 2 was conducted in castrated males chronically treated with exogenous testosterone. This treatment resulted in a very powerful inhibition of both copulatory behavior and sexual motivation, while other social behaviors were variably affected.
2. MATERIAL AND METHODS
2.1. Experimental animals
Two experiments were performed to analyze the effects of central administration of the aromatase inhibitor vorozole (VOR) on a range of social behaviors in male Japanese quail (Coturnix japonica). In experiment 1, birds were obtained from the Faculty of Veterinary Medicine of Liège at the age of approximately ten weeks and were immediately housed in individual cages. In experiment 2, birds were hatched at our own colony and raised in mixed sex groups kept in cages enriched with a cardboard box filled with wood chips until the age of 6 weeks when they were transferred to individual cages (Central Animal Facility from the University of Liège, agreement number: LA1610002). All subjects were maintained under a photoperiod simulating long summer days (16h light and 8h dark) and provided with food and water ad libitum.
Body mass as a measure of health and cloacal gland size as a measure of circulating androgen concentrations (Sachs, 1967) were periodically collected during these experiments. No significant changes in these measures related to the aromatase inhibitor were detected following the experimental treatments and these data will therefore not be reported in detail here. All experimental procedures were in agreement with the Belgian laws on the “Protection of Experimental Animals” and were approved by the Ethics Committee for the Use of Animals at the University of Liège (Protocol #1442).
2.2. Surgeries and drug administration
In experiments 1 and 2, adult male quail were implanted with a 200 μl osmotic pump (Alzet®, Model 2004, 0.25 μl per hour, 28 day expected duration) connected by a 25 cm vinyl cannula tubing (C312VT; 0.69 × 1.14 mm; PlasticsOne®) to a single guide osmotic brain cannula (3220P/SPC, Cut 8 mm below pedestal; PlasticsOne®) implanted in the third ventricle.
The osmotic pumps were loaded with either the aromatase inhibitor, vorozole (R76713 or VOR, Janssen Pharmaceutica, Beerse, Belgium; 50 μg/μl in pure propylene glycol) or with the vehicle, pure propylene glycol (P4347, Sigma-Aldrich) under sterile conditions. The choice of this dose was based on the previous observation that a single injection of 50 μg of vorozole inhibited the frequency of rhythmic cloacal sphincter movements (RCSM) within 30 min and for maximum 4 hours (Seredynski et al., 2015). An administration of 300 μg/day would thus be necessary to obtain the same result throughout a day. As the approximate delivery rate of the pumps is 0.33 μl/hr (7.92 μl/day), pumps were loaded with 50 μg/μl yielding an approximate daily dose of 396 μg/day to produce a robust and lasting blockade of the enzyme. Although it is difficult to estimate and compare the brain concentrations obtained locally following peripheral injections and constant delivery in the third ventricle, this dose probably represents a high dose given that the maximal daily dose ever tested in the periphery was 2 mg/kg (approximately 0.6mg per bird; (Balthazart et al., 1990a)).
Prefilled pumps were connected to one end to the vinyl tubing already filled with VOR or vehicle and the other extremity of the tubing was inserted into a 1 mL Eppendorf Tubes®. To verify the expelled volume as an indicator of proper functioning, the pumps were placed in sterile 0.9% saline at 37°C for 40 hours before implantation to prime diffusion as recommended by the manufacturer. This procedure confirmed that all pumps were working properly.
All birds were fasted starting in the evening prior to surgery and then anesthetized with 5% isoflurane (in oxygen; Isovet, Verdifarm), subsequently decreased to 2%. A primed osmotic pump was first introduced into the abdominal cavity through a single incision on the left side of the body. The vinyl tubing connected to the osmotic pump was then inserted under the skin from the abdominal cavity to the neck to be connected to a prefilled brain cannula on the other end. The bird was placed in a stereotaxic apparatus (Kopf Instruments; Tujunga, CA, USA), the skull was exposed by a medial skin incision and a square was drilled in the skull to visualize the venous sinus located in medial position at the surface of the brain. The cannula was then implanted in the third ventricle using an angular approach (10° away from the vertical) to avoid the venous sinus. Coordinates of the cannula tip were 1.80 mm anterior, 2.80 mm dorsal, and 0.00 mm lateral to the zero-reference point (center of the interaural axis). Finally, the cannula was secured to the skull with dental cement and skin was sutured.
The duration of release from the osmotic pump was calculated considering the reservoir volume (233 ± 1 μL depending on batch) and the mean body temperature of the species (± 42°C in Japanese quail; Gilbreath and Ko, 1970; Feuerbacher and Prinzinger, 1981) resulting in a duration of approximatively 29 days at 0.33 μl/hr. At the end of the experiment, the brain was extracted from the skull, frozen on dry ice and stored at −80°C. Post-mortem analyses of Nissl-stained brain sections confirmed that the cannula was in the third ventricle in all birds.
2.3. Experimental designs
Experiment 1
initially tested in gonadally intact males the feasibility and effectiveness of central VOR administration via Alzet® osmotic pumps in quail by assessing effects of this treatment on sexual behaviors that were already known to depend on brain aromatization (Balthazart et al., 1990a; Taziaux et al., 2004; Seredynski et al., 2013; de Bournonville et al., 2019). In a second step, the same subjects were used to determine whether central VOR administration could affect other social behaviors besides sexual behavior.
Subjects had been raised in groups until they were transferred to our laboratory at about 10 weeks of age. Since sexual maturity is reached in quail around the age of 6–7 weeks (Ottinger & Brinkley, 1979), they probably had some sexual experience before the beginning of the experiment. Males were assigned to two groups matched based on mean body mass and cloacal gland size. After surgery, vehicle- (n=8) and VOR-treated males (n=9) were repeatedly tested for various behaviors. Four partner preference tests (PPT) were given starting on day 3 and repeated every 6 days until day 21 (PPT1 to 4; day 3, 9, 15 and 21). Two copulatory tests were performed between successive partner preference tests until day 23 totaling 7 tests (T1 to 7: Day 5, 7, 11, 13, 17, 19 and 23). These tests were followed by a test of social motivation on day 23, a test of sexual motivation (rhythmic cloacal sphincter movements (RCSM) on day 24 and a test of aggressiveness on day 25 during which RCSM produced in response to a gonadally intact male were also measured (see next section for details of procedures).
Experiment 2
was designed to further investigate the effects of central aromatase inhibition on social behaviors including sexual behavior and try to answer questions raised by results of experiment 1. Males were castrated at the age of 3 weeks and implanted with one 20 mm long Silastic™ capsule (Silclear® tubing, 1.57 mm i.d; 2.41 mm o.d.; Degania silicone, Ref: 20301502431) filled with crystalline testosterone (Sigma, B6500) at the age of 5 weeks. This size of testosterone implant maintains circulating concentrations that are in the physiological range usually observed in gonadally intact sexually mature male quail (Balthazart et al., 1983; Balthazart et al., 1990c). Males remained in mixed sex groups for one week before their transfer to individual cages. Since the effects of testosterone typically take one or two weeks to develop (Balthazart et al., 1983; Balthazart et al., 1990c), it is unlikely they had acquired much sexual experience during this time. At the age of 8 weeks, males were given a series of copulatory pretests to give them extensive experience with the testing procedures, to ensure that all subjects were sexually active and to establish their baseline socio-sexual performance. All subjects were given on different days 8 tests for male copulatory behavior, one test measuring RCSM in response to the view of a female and one test of social motivation.
Males were assigned to two groups (7 vehicle- and 10 VOR-treated males) matched based on the mean body mass, cloacal gland size, and behavior displayed during the pre-test phase including RCSM frequency, mount attempts and cloacal contact movements frequency and latency and mean group preference score (see details of behavioral tests in next section).
After the implantation of the osmotic pump (day 0), males were tested for copulatory behavior (on day 4, 6, 8, 11, 13, 15), for RCSM frequency (on day 2 and 18), for social motivation (on day 21) and for aggressive behavior (on day 23).
2.4. Quantification of socio-sexual behaviors
All behavioral tests assessing in a quantitative manner a range of socio-sexual behaviors were performed by an investigator blind to the treatment of each subject. Most male and female stimuli were gonadally intact. Stimulus females were gained sexual experience as they interacted with the experimental males during (pre)tests. Male stimuli that participated in the aggressiveness and social motivation tests (Exp. 2) were castrated and as a consequence did not develop sexual experience.
2.4.1. Sexual behavior
Male sexual behavior is classically divided into an appetitive phase, indicative of the motivation to mate, and a consummatory phase, the actual sexual performance (Beach, 1956; review in Balthazart & Ball, 1998).
2.4.1.1. Consummatory sexual behavior
The frequency and latency of neck grabs (NG), mount attempts (MA), mounts (M) and cloacal contact movements (CCM; detailed description in Adkins & Alder, 1972; Hutchison, 1978) were scored in real time during 5 min periods immediately following the introduction of the experimental male in a small arena (60 [length] x 40 [width] x 50 [height] cm) containing a gonadally intact female. Birds who did not display a given behavior were assigned a latency of 300 seconds (5 min) for statistical purposes. As NG and M frequencies and latencies were nearly identical to MA and CCM frequencies and latencies respectively, only data for MA and CCM will be presented.
2.4.1. 2. Appetitive sexual behavior - Rhythmic cloacal sphincter movements (RCSM)
The frequency of RCSM produced in response to the view of a female is commonly used as a measure of sexual motivation (Ball & Balthazart, 2010). RCSM were quantified here by placing the experimental male quail in a glass aquarium (50 [length] x 30 [width] x 40 [height] cm). The experimental device was covered on top with wire mesh and virtually divided in two unequal compartments separated by a transparent glass partition and an adjacent vertically sliding cardboard that initially prevented the birds located on each side from seeing each other. The gonadally intact stimulus quail was placed in the small compartment of the aquarium (16.7 cm long). The experimental bird was placed in the larger compartment (33.4 cm long) that was virtually divided in two equal parts allowing a measure of the time spent either away or close to the stimulus. The experimental device was positioned on an elevated transparent platform with a mirror placed underneath at a 45° angle to provide an unobstructed view of the cloacal area of the experimental bird (Balthazart & Ball, 1998; Seiwert & Adkins-Regan, 1998).
During the first phase of the test, the experimental male was placed for 150 sec in the large compartment of the aquarium, while the view of the stimulus bird located in the other side was obstructed by the cardboard. In the second phase, the cardboard was lifted, allowing visual access to the stimulus for another 150 sec although physical interaction was still prevented by the glass partition.
The number of RCSM was quantified in real time during the two 150 sec periods before (phase 1 “no view”) and during (phase 2 “view”) exposure to the view of the stimulus.
2.4.3. Aggressive behavior
During the RCSM tests of experiment 1 measuring the responses to a male stimulus, an indirect measure of aggressive behavior was obtained by quantifying the time spent near the glass partition separating the experimental and stimulus gonadally intact males and counting the number of pecks to the glass partition during the two phases of the test. These behaviors were described by Schlinger and colleagues (1987) as markers of aggressive behavior. Experiment 2 used more direct measures of aggressiveness adapted from previous studies (Tsutsui & Ishii, 1981; Ishii, 1984; Caliva et al., 2017; Voigt et al., 2018). These measures were obtained by scoring during 5 min the behavior of the experimental male during physical interactions with a castrated male stimulus (which does not respond to fights) in a large arena (90 [length] x 90 [width] x 50 [height] cm). In these tests, the numbers of pecks as well as NG, MA, M ad CCM performed by the experimental male were recorded.
2.4.4. Sex partner preference
This test was designed to evaluate the preference of sex partner. In this procedure, the experimental animal was offered the choice between a gonadally intact female stimulus and a gonadally intact male stimulus tethered at the two ends of a long corridor (233 [length] x 23 [width] x 15 [height] cm). The side where each stimulus was placed was counterbalanced between subjects within and between tests. The corridor was divided by Plexiglas partitions into five compartments (C1–5) of equal length closed on the top by wire mesh and whose floor was covered with wood chips. The test was conducted in three phases. During the first phase, the experimental quail was placed for 2 min in the central compartment (C3) for habituation. During the second phase, the two transparent partitions limiting C3 were removed by means of a string and pulley system controlled from a distance by the experimenter to allow the bird to freely move for 5 min in the three compartments (C2 to C4) and get closer visual access to the stimuli placed in the two last compartments (C1 and C5). During the third phase, the last two transparent partitions were removed allowing the experimental bird to physically interact with the male and female stimuli during 2 min. The full test was videotaped to allow further analysis.
The time spent in each compartment was recorded with the help of a customized program designed for this test. A preference score was calculated by subtracting the time spent during phase 2 (5 min) in the compartment near the male (C2) from the time spent in the compartment near the female (C4). We also analyzed the time spent in C1+C2 versus C4+C5 when the last partitions had been removed (2 min of phase 3). The frequencies of sexual behaviors (NG, MA, M and CCM) displayed when the experimental bird freely interacted with the stimuli were also scored on videotaped recordings. These copulatory behaviors matched what was observed during copulatory tests. Vehicle birds displayed the full copulatory sequence toward stimulus females and attempted to mount the stimulus males who tried to avoid them; vorozole-treated birds almost never showed these behaviors. These data are therefore redundant with the results of copulatory tests and will not be presented here.
2.4.5. Social motivation
This test, adapted from studies conducted on social reinstatement or flocking behavior (Launay et al., 1991; Goodson et al., 2009b), assessed the motivation of a male to approach a group of conspecifics in the same long corridor used for the partner preference tests. In this procedure, the experimental subject was offered the choice between a group of three gonadally intact females plus one gonadally intact male versus an empty compartment (Experiment 1) or a group of three castrated males (to limit fights between males) plus one gonadally intact male versus an empty compartment (Experiment 2).
Stimuli were located at the two ends (in C1 and C5) of the long corridor (233×23×15cm). The test was conducted in two phases. In the first phase, the experimental quail was placed for 2 min (Experiment 1) or 10 sec (Experiment 2) in the central compartment (C3) for habituation. During the second phase, the two transparent partitions defining C3 were removed, and the experimental quail could freely move within compartments C2 to C4. The full test was videotaped to allow further analysis. The time spent in each compartment was recorded with a customized software designed for this test. A preference score was calculated by subtracting from the time spent in the compartment adjacent to the group the time spent in the compartment adjacent to the empty compartment. The sides in which stimuli were located were counterbalanced between subjects within one test.
2.5. Statistical analyses
Sexual behavior frequencies and latencies during each test did not meet the criteria for parametric data and were thus analyzed by separate non-parametric Mann-Whitney U tests to assess differences between vehicle and VOR infused males. A Bonferroni correction for multiple comparisons was applied considering the number of tests and all p values were multiplied by the number of comparisons (corrected p or pcor in the rest of the paper). Other behaviors were analyzed by parametric student t test or two-way ANOVA followed by Sidak’s post-hoc tests when significant interactions were observed. Preference scores were compared between vehicle and vorozole-treated males with Mann-Whitney U-tests. RCSM frequency when males were exposed to different stimuli and preference scores at different time points were analyzed with Wilcoxon signed-rank tests. Some preference scores were also analyzed by comparison to a hypothetical zero mean (H0=mean equal 0) using a one-sample two-tailed t-test.
All analyses were performed with GraphPad Prism 6.0 for Windows 10. All effect sizes (Cohen d) were evaluated using calculators present on the websites https://www.psychometrica.de/effect_size.htm and http://www.campbellcollaboration.org/. Results were considered significant for p (or pcor) <0.05) and are presented by their mean ± standard error of mean.
3. RESULTS
3.1. Consummatory sexual behavior
3.1.1. Effect of central aromatase inhibition in gonadally intact males (Exp.1)
Males used in this experiment had gained some degree of sexual experience before being isolated in individual cages (see methods). Yet males treated with the vehicle improved their sexual performance over the first few tests as illustrated in figure 1. The intracerebroventricular infusion of the aromatase inhibitor VOR in adult males drastically prevented the expression of copulatory behavior as compared to the vehicle infused males. Separate Mann-Whitney U tests analyzing data for each behavioral test revealed significant differences in MA frequencies from test 5 to 7 (U≤5.50, p≤0.0012, pcor≤0.0108, d≥2.027; Fig. 1A), in CCM frequencies for T3 and from T5 to T7 (U=9.00, p=0.0023, pcor=0.0207, d=1.623; Fig. 1B), in MA latencies from T4 to T7 (U≤10.00, p≤0.01, pcor≤0.05, d≥1.527; Fig. 1C), and in CCM latencies for T3 and from T5 to T7 (U=9.00, p=0.0023, pcor=0.0207, d=1.623; Fig. 1D). Thus, central aromatase blockade profoundly prevented the expression and full acquisition of male sexual behavior.
Figure 1.
Effect of central inhibition of aromatization on the expression of copulatory behavior in gonadally intact males. Data from each test (T1 to T7) were analyzed by separate Mann-Whitney U test followed by Bonferroni correction: (*)pcor<0.10, *pcor<0.05, **pcor<0.01. MA: mount attempt, CCM: cloacal contact movement.
3.1.2. Effect of central aromatase inhibition in testosterone treated castrates (Exp.2)
In previous experiment, males were not fully sexually experienced at the initiation of treatment. Moreover, they were gonadally intact and we cannot exclude that the effects of treatment were not indirect, resulting from an action on the central control of gonadal secretions. In experiment 2, all birds were castrated and chronically treated with testosterone, had already acquired a stable expression of copulatory behavior prior to treatment initiation and the two experimental groups were matched based on a variety of morphological and behavioral measures (see methods). Copulatory behavior was first tested 4 days after treatment onset (T1) and then every other day for 15 days (T2 to T6).
As illustrated in Fig.2, copulatory frequencies decreased as early as 4 days after implantation of the osmotic pumps containing the aromatase inhibitor, while their latencies increased over the six copulatory tests in VOR-treated males. By contrast, copulatory frequencies and latencies remained relatively stable in the vehicle-treated birds. Accordingly, separate Mann-Whitney U tests identified significant differences between the VOR and vehicle groups for MA frequencies (from T1 to T6: U≤8.50, p≤0.01, pcor≤0.05, d≥1.611; Fig. 2A), CCM frequencies (on T1 and from T4 to T6: U≤8.00, p≤0.01, pcor≤0.05, d≥1.662; Fig. 2B), MA latencies (from T2 to T6: U≤7.50; p≤0.01, pcor≤0.05, d≥1.715; Fig. 2C), and CCM latencies (from T2 to T6: U≤8.50, p≤0.01, pcor≤0.05, d≥1.611; Fig. 2D).
Figure 2.
Effect of central inhibition of aromatization on the expression of copulatory behavior in castrated males chronically treated with testosterone. Data are presented for the 8 pretests and 6 tests performed after osmotic pump implantation. Data from each test were analyzed by separate Mann-Whitney U test followed by Bonferroni correction: (*) pcor<0.10, *pcor<0.05, **pcor<0.01, ***pcor<0.001. MA: mount attempt, CCM: cloacal contact movement.
Together, these results revealed that aromatase inhibitors not only affect the acquisition of copulatory behavior as males rarely displayed sexual behavior in the presence of a female stimulus (experiment 1), but also drastically reduced the expression of copulatory behavior in sexually experienced males (experiment 2).
3.2. Appetitive sexual behavior
3.2.1. Effect of central aromatase inhibition in gonadally intact males (Exp.1)
As previously shown (Cornil & Ball, 2010), males produced more cloacal contractions when the female was visible (Figure 4A). Chronic central aromatase inhibition reduced RCSM frequency in the presence of the female, as supported by the two-way repeated measures ANOVA that revealed a significant effect of the treatment (F1, 30=15.38, p=0.0005) and the test conditions (F1, 30=32.06, p<0.0001) as well as a significant interaction between the two factors (F1, 30=9.269, p=0.0048). This interaction is explained by the lower RCSM frequency of the VOR group compared to the vehicle group in the presence of the female (Sidak post hoc test; p<0.001) as well as the lower RCSM frequency of both groups of males while they could not see the female compared to RCSM frequency in vehicle males exposed to the view of the female (both p values<0.001; Fig. 3A).
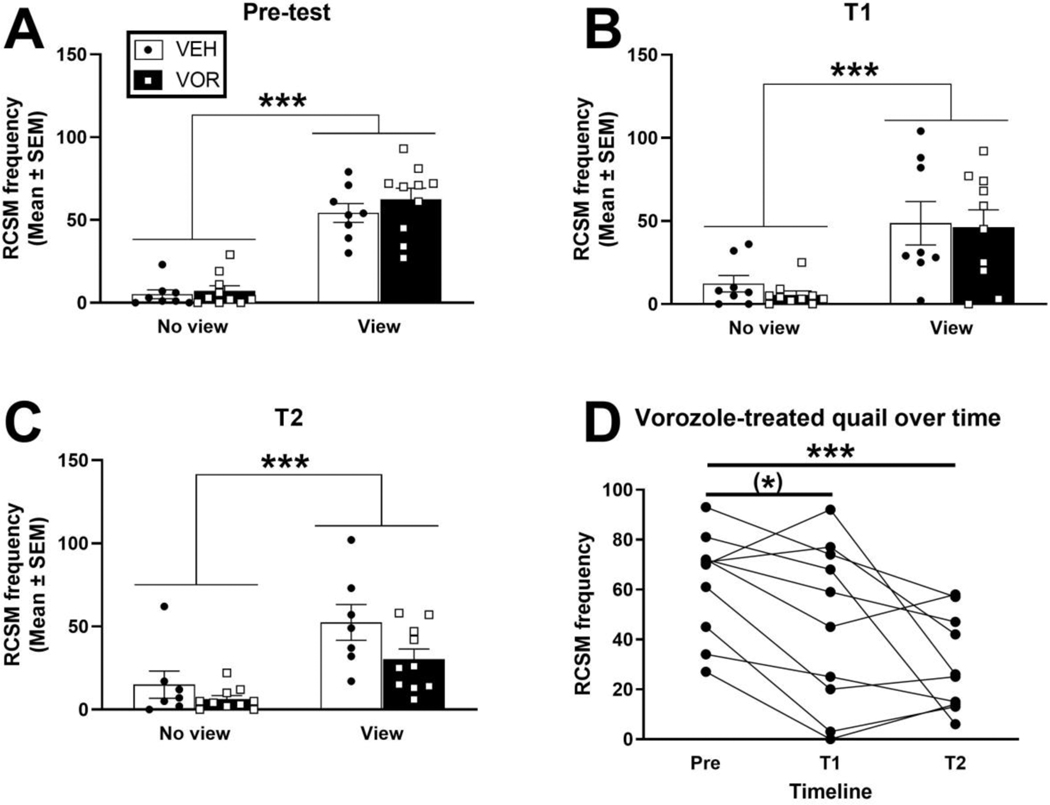
Figure 4.
Effect of central inhibition of aromatization on the rhythmic cloacal sphincter movement (RCSM) frequency in response to the view of a female stimulus in castrated testosterone-treated males. Tests were performed before implantation of the osmotic pumps filled with vehicle or vorozole (VOR) (Pre-test, A) and 2 (Test 1 (T1; B) or 18 (T2; C) days post-surgery. Individual variations for the vorozole treated birds were also plotted over time (D). Data from each test (A to C) were analyzed by two-way repeated measures ANOVA. Data from panel D were analyzed by one-way repeated measure ANOVA followed by Sidak’s post-hoc tests. (*)p<0.10, ***p<0.001.
Figure 3.
Effect of central inhibition of aromatization on the rhythmic cloacal sphincter movement (RCSM) frequency in response to the view of a female (A) or a male (B) stimulus and comparison of the RCSM produced by the vehicle males in response to the two stimuli (C). Data in A and B were analyzed by two-way ANOVA with the two phases of the test as a repeated measure and the treatment as an independent factor followed by Sidak’s post-hoc tests when the interaction was significant. Data in C were analyzed by paired t test. (*)p<0.10, **p<0.01,***p<0.001.
Surprisingly, experimental males also responded to the view of another stimulus male by more frequent RCSM and this response was blocked by central aromatase inhibition as supported by the two-way repeated measures ANOVA which revealed significant effects of the treatment (F1, 30=13.05, p=0.0011) and test conditions (F1, 30=32.78, p<0.0001) as well as a significant interaction (F1, 30=5.050, p=0.0321). The Sidak’s post hoc analysis confirmed that this interaction resulted from a lower RCSM frequency in VOR infused males compared to vehicle males when seeing the male (p<0.01) and from the lower RCSM frequency of both groups when the male stimulus was hidden compared to the vehicle group when the male stimulus was in sight (both p values <0.001; Fig. 3B).
The aromatase inhibitor thus induced a reduction of RCSM frequency when males were exposed to the view of a male or a female. As found in previous studies (Seiwert & Adkins-Regan 1998; Cornil et al. 2010), vehicle males performed more RCSM when viewing a female as compared to a male but a paired t test comparing the RCSM frequency displayed by the vehicle infused males in response to the view of a female or of a male only detected a statistical trend (t(7)=2.204, p=0.0634, d= −0.751; Fig. 3C).
3.2.2. Effect of central aromatase inhibition in testosterone treated castrates (Exp.2)
In experiment 1, RCSM were only quantified after 24 days of exposure to the aromatase inhibitor when birds had been repeatedly tested for copulatory behavior. It was thus not possible to determine whether the observed effects resulted from a direct inhibition of sexual motivation by the treatment or whether it was an indirect effect due to a devaluation of the reinforcing properties of the females resulting in a reduced sexual motivation. In this experiment, males were first tested for RCSM frequency prior to treatment onset (pre-test) and two RCSM tests were performed following treatment: one after two days after treatment onset (T1, day 2) to assess the direct effect of treatment on sexual motivation and second one two days after the last copulatory test (T2; day 18) to assess the long-term effects.
As observed before, the view of the female markedly increased the number of RCSM in the three tests (test conditions: F>22.73, p<0.001; Fig. 4A–C). RCSM frequencies in response to the view of the female were on average decreased by the VOR treatment on the second test (day 18), but not yet on the first (day 2). Accordingly, the ANOVA revealed no effect of treatment and no interaction with the condition on test 1 (respectively F1,16=0.244, p=0.628 and F1,16=0.072, p=0.792; Fig. 4A and B). On test 2, there was still no effect of treatment or interaction due to the large individual variations even if results suggested a weak inhibitory effect of the treatment (respectively F1,15=4.038, p=0.063 and F1,15=1.366, p=0.261; Fig. 4C).
However, it is noteworthy that RCSM frequencies in response to the view of the female were on average decreased in the VOR-treated animals on both test 1 and test 2, while it did not vary over time in vehicle-treated animals. A one-way repeated measure ANOVA comparing RCSM frequency within VOR treated males confirmed an effect of time related to the treatment (F1.405,12.64=9.801, p=0.0049; Fig. 4D) although this effect was more pronounced in test 2 than in test 1 as supported by Sidak’s post hoc tests that revealed a significant effect of vorozole for test 2 (p=0.0009) but only a trend for test 1 (p=0.0842) compared to the pre-test.
3.3. Effect of central aromatase inhibition on putative markers of aggressiveness
Experiment 1 assessed the time spent near a glass partition separating the experimental male from a male stimulus and the frequency of pecks to the glass partition separating both males as a marker of aggressive behavior (Schlinger et al., 1987). These behaviors were quantified during two periods when the stimulus male was or was not visible (Fig. 5). A two-way repeated measure ANOVA of the time spent near the glass partition identified no effect of the treatment (F1,30=0.0, p>0.999), the test conditions (F1,30=0.8342, p=0.3683) or their interaction (F1,30=2.039, p=0.1636; Fig. 5A). Overall, very few pecks at the glass partition were observed in these conditions and the pecking frequency was unaffected by the two factors and their interaction (treatment: F1,30=0.0569, p=0.8131; test conditions: F1,30=3.768, p=0.0617; interaction: F1,30=0.2921, p=0.5929, Fig. 5B). Therefore, these data do not allow us to conclude on an effect of the VOR treatment on the pecking behavior.
Figure 5.
Effect of central inhibition of aromatization on two markers of aggressiveness: the time spent near a glass partition located in front of a male stimulus (A) and the pecking frequency produced in response to the view of a male (B). Data were analyzed by two-way repeated measure ANOVA that identified no significant effect of the treatments, the test conditions and their interaction.
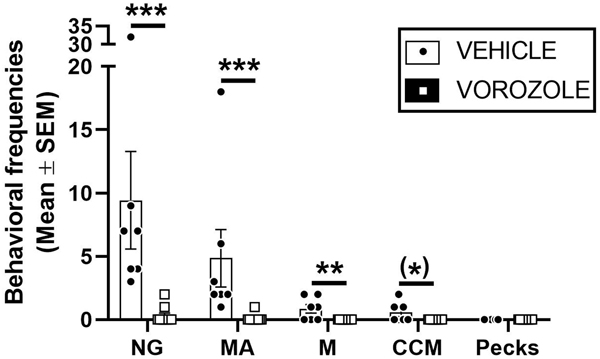
As most of vehicle males did not show the expected pecking behavior in experiment 1, experiment 2 used another approach to assess the effect of the aromatase inhibitor on aggressiveness. Experimental males were allowed to freely interact with a castrated male and the behaviors displayed during these interactions were quantified. These behaviors consisted of pecks, neck grabs (NG), mount attempts (MA), mounts (M) and more rarely contact cloacal movements (CCM), which have been considered by others to provide measures of aggressive behavior in this context (Tsutsui & Ishii, 1981; Ishii, 1984; Caliva et al., 2017; Voigt et al., 2018).
In the present experiment, vehicle infused males exhibited the behavioral sequence characterized by NG, MA, M and sometimes CCM toward the castrated male who tried to avoid contacts with the experimental male (Fig.6). All vehicle males exhibited body, head and neck grabs followed by pursuits, or MA (=7/7), while only three (n=3/7) reached CCM. Only two of the VOR males showed at least one NG and MA (n=2/10) and none of them displayed M or CCM. No peck was performed by birds of either group. Mann Whitney tests accordingly indicated that frequencies of NG (p<0.001), MA (p<0.001) and M (p=0.014) were significantly higher in vehicle than in VOR treated males. This difference did not reach significance for CCM (p=0.051). Castrated male stimuli rarely responded to contacts (only 3 NG observed over 17 tests), but all of them seemed to resist against NG and MA.
Figure 6.
Effect of central inhibition of aromatization on the expression of male-male behavioral interactions. Males infused with vorozole (VOR) rarely interacted with the castrated male stimulus, while vehicle infused males (VEH) exhibited neck grabs (NG), mount attempts (MA), mounts (M) and occasionally contact cloacal movements (CCM). Mann-Whitney U-test: (*)p<0.10, **p<0.01,***p<0.001.
3.4. Effect of central aromatase blockade on sexual partner preference of males
In experiment 1, four sexual partner preference tests were performed to evaluate the evolution of sexual attraction of the experimental males towards females over time (see methods). The first test (T1) was performed 3 days after treatment onset and before any copulatory test. The following tests (T2–4) were conducted at an interval of 6 days during which males received 2 copulatory tests. The preference scores for females exhibited by gonadally intact males when seeing the stimuli behind a glass partition (phase 2: C4-C2, see methods) did not differ between vehicle and VOR-treated males on day 3 (T1), 9 (T2), 15 (T3) and 21 (T4) (Mann-Whitney U test: U>15.50, p>0.0495, pcor>0.297, d<1.09; Fig. 7). The same conclusions were drawn following the analysis of the total time near or with a given stimulus when the last partitions separating the stimulus and experimental subjects had been removed (preference scores during phase 3, (C4+C5)-(C1+C2); Mann-Whitney U-test: U>22.00, p>0.1996, pcor>0.999, d<0.691) or in the analysis of the cumulative times during phase 2 and 3 (time near or with the stimulus; Mann-Whitney U-test: U>14.00, p>0.036, pcor>0.216, d<1.197; graphs not showed to avoid redundancy).
Figure 7.
Effect of the inhibition of central aromatization on measures of the sex partner preference of male quail. The preference was quantified during 4 tests (T1 to T4) and preference scores observed during phase 2 of each test (5 min) when the experimental male could approach either the female or the male stimuli were compared by Mann Whitney tests that identified no significant effect of treatment.
It must also be noted, however, that the vehicle infused males did not show a stable preference for the female side in the corridor. Preference scores of the vehicle group were compared to a hypothetical null mean (H0: mean equal 0) using a one-sample two-tailed t test. The preference scores exhibited by the vehicle male, when seeing the female behind a glass partition (phase 2), did not reach significance over tests (T1 to T4, all p>0.1021, all pcor>0.4084). The same conclusions were obtained in the analysis of the total time near or with a female when the last partitions had been removed (preference scores during phase 3; T1, T2 and T4, all p>0.2902, pcor>0.999) or in the analysis of the cumulative times during phase 2 and 3 (time near or with the female; T1, T2 and T4, all p>0.7860, pcor>0.999), although a tendency was observed during test 3 before Bonferroni correction (T3: p=0.0913 [phase 2] and 0.0970 [phase 3], pcor>0.3652).
As no clear sexual preference was showed by the vehicle infused males in experiment 1, the sexual partner preference test was not used in experiment 2.
3.5. Effect of central inhibition of aromatization on measures of social motivation
Experiment 1 tested the effects of aromatase inhibition on social motivation (i.e., tendency to approach and stay close to conspecifics) by quantifying the time spent by an experimental subject near a group of three female plus one gonadally intact male compared to the time spent near an empty compartment in the 5-compartment corridor. As shown in figure 8A, the VOR infused males exhibited a lower preference score for the group compared to vehicle infused males (Mann-Whitney U test, U=9.00, p=0.0079, d=1.623). In fact, most of the vehicle infused males spent nearly all their time near the group located behind a glass partition.
Figure 8.
Effect of central aromatase inhibition on social motivation. The experiments assessed the time spent near a group of three gonadally intact females and one gonadally intact male versus an empty compartment (Exp. 1; A) and the time spent near a group of three castrated males and one gonadally intact male versus an empty compartment (Exp. 2; B). In experiment 2 (panel B), birds were tested before and after being implanted with the osmotic pump delivering vorozole (VOR) or its vehicle (VEH). Mann-Whitney U-test: **p<0.01
The preference score measured in experiment 1 demonstrated that experimental subjects were clearly able to exhibit a preference for the group, but this preference could obviously be influenced by the presence of females in the group. It was thus impossible to separate social from sexual motivation. In an attempt to discriminate between these interpretations, experiment 2 tested the preference for a group of one gonadally intact male and three castrated males (to limit fights between males) compared to an empty compartment. A pretest had been performed before the implantation of the osmotic pumps to assess the initial preference of all males and the two groups (vehicle vs. VOR) had been matched based on the observed preference scores. During this pretest, nearly all males spent more time near the group than near the empty compartment (Fig. 8B). Once the birds received the VOR or control treatment, most males still showed a preference for the group, although a few subjects no longer showed this preference and spent more time near the empty compartment. This change in behavior concerned only a subset of subjects and did not result in a significant difference between pretest and tests after surgery (Wilcoxon test; vehicle group: W= −16.00, p=0.2188, VOR group Z= −9.00, p=0.6953). Moreover, such a slight change in preference was observed as frequently in the vehicle as in the VOR groups. Thus, males infused with vehicle did not differ from males infused with VOR during the pretest (Mann-Whitney U-test, U=32.00, p=0.8011, pcor>0.999, d=0.142) nor after implantation of the pumps (U = 34.00, p= 0.9252, pcor>0.999, d=0.047). In contrast with what had been observed in experiment 1, aromatase inhibition thus did not seem to affect the preference scores, suggesting that the measured preference in experiment 1 was a proxy for sexual motivation rather than gregariousness per se.
4. DISCUSSION
The first aim of this study was to determine whether aromatase inhibition decreases various measures of social behaviors, including aggressive behavior, partner preference, and the motivation to interact with a group, as it inhibits male copulatory behavior. To achieve this goal, we first had to validate tests to quantify these behaviors and validate the capacity of osmotic pumps to deliver effective doses of aromatase inhibitors directly in the third ventricle in order to reliably inhibit behaviors. The osmotic pump procedure was demonstrated to be an extremely effective way for delivering aromatase inhibitors based on the robust inhibition of male sexual behavior. Additional tests suggested that aromatase inhibition might also affect other types of social behaviors even if interpretation of these results is often complex and will thus require further experiments.
4.1. Copulatory behavior
We showed that the infusion of an aromatase inhibitor into the third ventricle prevents the expression of copulatory behavior in males who are not fully experienced and almost completely blocks the expression of copulatory behavior in fully experienced males. This observation is not only in line with previous studies based on systemic injections (Balthazart et al., 1990a; Cornil et al., 2004; Taziaux et al., 2004; Cornil et al., 2006) or stereotaxic implantation of aromatase inhibitors in the preoptic area (Balthazart et al., 1990a; de Bournonville et al., 2019), but it also illustrates the strongest inhibition of copulatory behavior ever observed in quail. The contrast with studies following systemic administration of the drug is likely explained by the higher exposure of the brain to the aromatase inhibitor obtained by central administration. Indeed, it is likely that this delivery method allows to reach a local concentration of the inhibitor that was similar to or higher than the concentration reached following peripheral treatment with the daily dose of 2mg/kg, the highest dose tested in quail (Balthazart et al., 1990a). This mode of delivery probably results in a constant inhibition of the enzymatic activity of aromatase while the daily or twice daily injections likely cause fluctuations in this enzymatic activity. Such high local concentrations probably also explain the faster effects observed here compared to previous studies. In both experiments, maximal effects on copulatory behavior were already reached on the first test that took place 5 (Exp.1) or 4 days (Exp.2) after the treatment was initiated. Along with the demonstration that a single intraperitoneal injection of a very high dose of vorozole (30 mg/kg; approximately 10 mg per bird) completely inhibits brain aromatase activity within 30 min (Cornil et al., 2006), this observation suggests that the present mode of administration and dose must have inhibited aromatase activity very rapidly to allow the disappearance of the estrogenic effects, some of which are known to be relatively long lasting. The striking difference between the large amplitude of the present effects compared to the effects observed following stereotaxic administration within the POM specifically may suggest that aromatization in other brain areas (not blocked by VOR in POM but blocked after ICV infusion) contributes to the activation of copulatory behavior. Alternatively, it is also possible that this discrepancy only reflects a difference in the dosage of the drug or that the site-targeted aromatase inhibition did not block the enzyme throughout the entire volume of the POM. These possibilities are not mutually exclusive and may all contribute to explain the differences between studies.
Another interesting aspect of the present study lies in the fact that the inhibition of aromatase was observed in two separate experiments using gonadally intact males and castrated males chronically treated with testosterone. That the inhibition was so pronounced in gonadally intact males where circulating testosterone is still regulated by the negative feedback highlights the great efficacy of the inhibition produced by osmotic pump delivery. It should also be noted that the extensive inhibition of copulatory behavior was first observed in birds that had not been pretested for sexual behavior and had thus not developed a full sexual experience. In this experiment, even if males treated with vorozole rarely exhibited copulatory behaviors, stable group differences were only observed when control males had acquired sufficient experience and their sexual performance had reached a plateau. The second experiment confirmed the pronounced effect of the treatment on two groups of males who had received extensive sexual experience and had been matched for their pre-experimental behavior, which excludes all possibilities of a pre-existing bias.
4.2. Sexual motivation
In agreement with previous studies (Taziaux et al., 2004; Seredynski et al., 2013), aromatase inhibitors reduced the appetitive sexual behavior measured by the frequency of rhythmic cloacal sphincter movements (RCSM) when males were exposed to the view of a female. Somewhat surprisingly, a similar inhibition was also observed when the experimental male was presented with the view of a male (Fig. 3 A and B). On average vehicle males produced more RCSM in response to a female than a male, but in contrast to previous studies (Seiwert & Adkins-Regan, 1998; Cornil & Ball, 2010), this difference did not reach full statistical significance (p<0.10, Fig. 3C).
The present experiments also addressed the question of whether estrogens derived from aromatization directly control the expression of RCSM as a marker of sexual motivation or whether aromatase inhibition indirectly decreases RCSM frequency as a consequence of the suppression of copulatory behavior. Previous work based on systemic injections of an inhibitor had shown that RCSM frequencies was inhibited only after males had been repeatedly tested for copulatory behavior (Taziaux et al., 2004) and this was also the case here during experiment 1. As central aromatase inhibition strongly inhibits copulatory behavior, it could be speculated that the reinforcing value of the female was decreased in VOR treated males since they were no longer copulating. A decrease in RCSM would thus only be observed after multiple copulatory tests. Such an effect would be similar to the extinction observed in the social proximity response, another measure of sexual motivation, following systemic aromatase inhibition (Balthazart et al., 1997b). Therefore, in experiment 2, RCSM were quantified before (day 2) as well as after (day 18) males had a chance to copulate with a female. The results indicated that vorozole decreased RCSM frequency on day 18 but not on day 2. This observation could thus support the notion that sexual motivation is reduced following a devaluation of the female as a sexual stimulus. However, the analysis of RCSM frequency over time within the VOR group revealed a trend towards an effect already on day 2 and a fully significant effect by day 18, which tends to support existence of an early direct action of central aromatase inhibition in the control of sexual motivation. This interpretation is supported by a previous observation of an acute inhibition of RCSM frequency within 15 min of central blockade of estrogen production or action (Seredynski et al., 2013; Seredynski et al., 2015).
Taken together the present experiments confirm that aromatase inhibition blocks both the appetitive and consummatory components of sexual behavior. The observation of an increased RCSM frequency in response to the view of a male which is also inhibited by central aromatase blockade somewhat contradicts previous observations and would deserve further study.
4.3. Aggressive behavior
We also analyzed the impact of central aromatase inhibition on the expression of behaviors that have been previously reported to represent measures of aggressive behavior. In experiment 1, the quantification of pecks to a window separating the experimental subject from a male stimulus and the measure of the time spent near that window did not reveal sufficient behavioral displays in vehicle males to be able to detect an effect of the treatment on this response. The number of pecks measured during this test was well below the number previously reported by Schlinger and colleagues (1990a) despite the fact that our animals were sexually active and exposed to testosterone for weeks. To circumvent this issue, it was then decided to test another measure of aggressiveness by simply looking at the behaviors displayed during a physical encounter between experimental males and a castrated male. Using this approach, experiment 2 revealed a drastic effect of vorozole on the number of NG, MA and M directed to the castrated male stimulus, but no pecks were observed regardless of the treatment.
The substantial overlap between male aggressive and sexual behaviors in Japanese quail makes it complicated to unambiguously interpret this inhibition of NG, MA and M behaviors. In our opinion, there is to date no behavioral test described as providing a clear measure of aggressiveness in quail. Pecks at the window have been used in previous papers as a measure of aggressive behavior (Schlinger & Callard, 1990a, 1990b), as have been the frequencies of NG, MA, M and CCM directed towards another male (Tsutsui & Ishii, 1981; Ishii, 1984; Caliva et al., 2017; Voigt et al., 2018). Previous studies had reported that males show higher NG, MA and M frequencies when they interact with males rather than females (Ishii, 1984). However, behavioral frequencies were not analyzed separately and did not consider the response of the stimulus. It is possible that the higher behavioral frequencies displayed by the experimental male in presence of a gonadally intact male stimulus reflect the difficulty to express aggressive or sexual behaviors towards a non-receptive male stimulus which tends to avoid and resist physical contacts, whereas female stimulus are usually receptive in the presence of a male. Attempts to minimize the avoidance and non-receptive behaviors were made by using male stimuli exposed to short days to inhibit testicular development (Caliva et al., 2017) or surgically castrated male stimulus as in experiment 2. In the present study, male-male interactions were not associated with obvious aggressive pecks toward the castrated stimulus contrasting with previous observations (Caliva et al., 2017). Moreover, there was no effect on pecks at the window toward a gonadally intact male, these pecks being detected in much smaller frequencies than in previous studies from Schlinger and Callard (1990a,b). One can therefore wonder whether the effects observed here specifically concern aggressiveness independently of sexual behavior.
From an evolutionary perspective, aggressiveness between males was hypothesized to provide a greater reproductive success to the “dominant” individual. However, the reproductive success of “dominant” males during male-male (sexual) interactions measured by NG, MA, M and CCM was shown to be without effect on subsequent mating and fertilization success with a female (Adkins-Regan, 2014). Hence, mounting behavior directed towards males may be a by-product of a high sexual motivation rather than a way to establish social dominance (Adkins-Regan, 2014). The interpretation of the inhibition of NG, MA and M behaviors thus remains complicated.
Interestingly, two recent studies showed increased expression of immediate early genes following male-male interactions (Voigt et al., 2018; Caliva et al., 2019) in brain areas that are distinct from those activated by interactions with females (Charlier et al., 2005; Taziaux et al., 2006). To our knowledge, a systematic study comparing with similar methods the same brain regions after expression of sexual or aggressive behavior is however not available. It is also remarkable that the medial preoptic nucleus (POM) has been considered as a key region involved in the aggressive behavior of Japanese quail (Schlinger & Callard, 1989; Schlinger & Callard, 1990a), while other studies indicate a strong causal link of the POM to male sexual behavior (Balthazart et al., 1990a; Balthazart & Surlemont, 1990b; Balthazart et al., 1992; de Bournonville et al., 2019).
Finally, multiple studies have indicated that aggressive behavior is increased in male quail exposed to endogenous or exogenous testosterone (reviewed in Mills et al., (1997)), but, as mentioned already, work on aggressiveness is based on behavioral tests whose specificity is questionable. Horward and Bermant (1967) actually noted that, in their studies, aggressive behavior may have been confounded with “homosexual” copulation attempts (see Mills et al., 1997 for additional discussion). It must therefore be concluded that based on available evidence, there are still no data that would univocally support the idea that brain aromatase has specific effects on male aggressive behavior, independently from the effects on sexual behavior.
4.4. Sex partner preference
We also attempted to develop a test to identify the preferred sex partner in male quail and then test whether this preference is influenced by estrogens derived from brain aromatization. As clearly illustrated by the results of four successive tests that were performed in experiment 1, the 5-compartment corridor failed to identify a clear tropism toward a female partner, as was a priori expected in sexually active adult males. One could therefore wonder whether male quail are able to recognize males from females based only on visual (and possibly auditory and olfactory) stimuli, but sex recognition was actually described by experiments in which males were trained to spend more time in front of a window providing visual access to females, and less time in front of a window revealing males (Domjan & Hall, 1986; Nash et al., 1989). More importantly, there is ample evidence demonstrating the ability of quail to discriminate and recognize specific characteristics in individual conspecifics, such as plumage color (Nash et al., 1989; Nash & Domjan, 1991; Riters & Balthazart, 1998). Nevertheless, the sex recognition was described in very specific conditions that required the tested males to be repeatedly exposed to copulatory experience with females but non-copulatory interactions with males. It must therefore be concluded that the 5-compartment corridor test, as used in our experimental design, does not provide a reliable test for sex partner preference. No specific conclusion can therefore be formulated at this point concerning a putative role of brain aromatase in the determination of the sex partner preference.
4.5. Social motivation
Tests of social motivation have never been described in Japanese quail. Social motivation could however be closely related to social reinstatement behavior that has been extensively studied in quail (Launay et al., 1991, 1993; Mills et al., 1995; François et al., 1998; Francois et al., 1999). Social reinstatement behavior partly depends on genetics as illustrated by studies comparing different lines of quail that had been selected for multiple generations based on the approach behavior they displayed at the age of one week towards another group of chicks in a treadmill test. After 20 generations, the mean distance travelled by the highly social line was 20 meters, whereas the low sociality line only ran for 3 meters on the conveyer belt moving them away from the group (Mills & Faure, 1990). These two lines selected based on this social reinstatement response were later found to differ in a number of behavioral traits ((Launay et al., 1991; Jones et al., 1996; François et al., 1998; Francois et al., 1999); reviewed in Mills et al. (1997)). There is no evidence to our knowledge that social reinstatement motivation relates to brain aromatase and it was our intention here to test this idea in adult animals using non-genetically selected quail.
To determine whether the motivation to join a group depends on brain aromatase, a choice apparatus inspired by studies of gregarious songbird species (Goodson et al., 2009b) was used to test adult male quail. In two distinct experiments, birds were offered the choice between either a group of conspecifics or an empty compartment (which might reflect a preference for social isolation). Most quail preferred to spend most of their time near a group of mixed- or same-sex conspecifics rather than an empty compartment. The composition of the group played however a crucial role on the impact of aromatase inhibition on this preference. When the group was composed of three females and one male, more than one half of males treated centrally with the aromatase inhibitor lost interest towards the group, while untreated males spent more than two third of their time near the group (Fig. 8A). However, when the group was composed of males only, the interest of the experimental male for the group tended to decrease over tests in some subjects regardless of their treatment (Fig. 8B). The inhibition by vorozole of the preference score of males in response to a group including females (Fig. 8A), but not males (Fig. 8B), suggests that female stimuli played a crucial role in this measure of social motivation. Consequently, this measure might be considered as a marker of sexual motivation rather than gregariousness. The higher preference for a group containing females rather than males incidentally confirms that male quail are indeed able to recognize the sex of a conspecific behind a glass partition (see discussion in section 4.4.). Together, these data suggest that central aromatization does not play a prominent role in the expression of social motivation per se.
5. CONCLUSION
In summary, the present experiments demonstrate that brain aromatase can be reliably blocked by infusion of an inhibitor via osmotic pumps attached to a cannula implanted in the third ventricle. This blockade results in a nearly complete suppression of copulatory behavior tested in various experimental conditions. Inhibitory effects were also observed on some other social behaviors, but these effects were less prominent and were only observed in specific testing conditions. The behavioral tests assessing other social behaviors are therefore less reproducible. Sexual motivation and the tendency to approach a group of females clearly seem to depend on brain aromatase, but the effects of the central production of estrogens on aggressive behavior and on the social motivation remain less clear. Finding a definitive answer to this question would require development and validation of test conditions that would quantify these behaviors in a more specific manner.
ACKNOWLEDGEMENTS
This research was supported by NIH/NIMH grant R01 MH50388, FNRS Research Credit J.0025.19 and the “Fondation Léon Frédéricq”. CAC is a F.R.S.-FNRS Senior Research Associate. LC is a FRIA PhD student (F.R.S-FNRS). The authors would like to thank Simon Uyttendaele for the design of the behavior recording program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adkins-Regan E. (2014). Male-male sexual behavior in Japanese quail: being “on top” reduces mating and fertilization with females. Behav Processes, 108, 71–79. doi: 10.1016/j.beproc.2014.09.027 [DOI] [PubMed] [Google Scholar]
- Adkins EK, & Alder NT (1972). Hormonal control of behavior in the Japanese quail. Journal of comparative and physiological psychology, 81(1), 27. [DOI] [PubMed] [Google Scholar]
- Alexandre C, & Balthazart J. (1986). Effects of metabolism inhibitors, antiestrogens and antiandrogens on the androgen and estrogen induced sexual behavior in Japanese quail. Physiology & behavior, 38(4), 581–591. doi: 10.1016/0031-9384(86)90429-4 [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, & Balthazart J. (2002). Sexual partner preference requires a functional aromatase (cyp19) gene in male mice. Horm Behav, 42(2), 158–171. doi: 10.1006/hbeh.2002.1805 [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, & Balthazart J. (2004). Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Hormones and Behavior, 46(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Bakoyiannis I, Tsigka EA, Perrea D, & Pergialiotis V. (2016). The Impact of Endocrine Therapy on Cognitive Functions of Breast Cancer Patients: A Systematic Review. Clin Drug Investig, 36(2), 109–118. doi: 10.1007/s40261-015-0364-9 [DOI] [PubMed] [Google Scholar]
- Ball GF, & Balthazart J. (2010). Japanese quail as a model system for studying the neuroendocrine control of reproductive and social behaviors. ILAR journal, 51(4), 310–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Viglietti-Panzica C, & Panzica GC (1997a). Vasotocinergic innervation of areas containing aromatase-immunoreactive cells in the quail forebrain. Journal of neurobiology, 33(1), 45–60. [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, & Ball GF (2004). Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiology & behavior, 83(2), 247–270. [DOI] [PubMed] [Google Scholar]
- Balthazart J, & Ball G. (2012). Brain Aromatase, Estrogens, and Behavior: Oxford University Press. [Google Scholar]
- Balthazart J, & Ball GF (1998). The Japanese quail as a model system for the investigation of steroid-catecholamine interactions mediating appetitive and consummatory aspects of male sexual behavior. Annu Rev Sex Res, 9, 96–176. [PubMed] [Google Scholar]
- Balthazart J, Castagna C, & Ball GF (1997b). Aromatase inhibition blocks the activation and sexual differentiation of appetitive male sexual behavior in Japanese quail. Behav Neurosci, 111(2), 381–397. [PubMed] [Google Scholar]
- Balthazart J, De Clerck A, & Foidart A. (1992). Behavioral demasculinization of female quail is induced by estrogens: studies with the new aromatase inhibitor, R76713. Hormones and Behavior, 26(2), 179–203. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Evrard L, & Surlemont C. (1990a). Effects of the Nonsteroidal Inhibitor R76713 on Testosterone-Induced Sexual Behavior in the Japanese Quail (Coturnix coturnix japonica). Hormones and Behavior, 24(4), 510–531. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, & Hendrick JC (1990c). The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiology & behavior, 47(1), 83–94. doi: 10.1016/0031-9384(90)90045-6 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, & Ottinger MA (1983). Sexual differences in the Japanese quail: behavior, morphology, and intracellular metabolism of testosterone. Gen Comp Endocrinol, 51(2), 191–207. [DOI] [PubMed] [Google Scholar]
- Balthazart J, & Surlemont C. (1990b). Copulatory Behavior is Controlled by the Sexually Dimorphic Nucleus of the Quail POA. Brain research bulletin, 25(1), 7–14. [DOI] [PubMed] [Google Scholar]
- Beach FA (1956). Characteristics of masculine “sex drive”. In Jones MR (Ed.), Nebraska symposium on motivation (Vol. 4, pp. 1–32). Lincoln: University of Nebraska Press. [Google Scholar]
- Biegon A. (2016). In vivo visualization of aromatase in animals and humans. Front Neuroendocrinol, 40, 42–51. doi: 10.1016/j.yfrne.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, King P, Logan J, Muench L, Pareto D, Schlyer D, Shea C, Telang F, Wang GJ, Xu Y, & Fowler JS (2010). Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse, 64(11), 801–807. doi: 10.1002/syn.20791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N. (1980). The topography of mesotocin and vasotocin systems in the brain of the domestic mallard and japanese quail: Immunocytochemical identification. Cell Tissue Res, 213(1), 37–51. doi: 10.1007/BF00236919 [DOI] [PubMed] [Google Scholar]
- Bonsall RW, Clancy AN, & Michael RP (1992). Effects of the nonsteroidal aromatase inhibitor, Fadrozole, on sexual behavior in male rats. Hormones and Behavior, 26(2), 240–254. doi: 10.1016/0018-506X(92)90045-W [DOI] [PubMed] [Google Scholar]
- Brooks DC, Coon VJ, Ercan CM, Xu X, Dong H, Levine JE, Bulun SE, & Zhao H. (2020). Brain Aromatase and the Regulation of Sexual Activity in Male Mice. Endocrinology, 161(10). doi: 10.1210/endocr/bqaa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliva JM, Falkenburger Melleu F, Marino-Neto J, Marin RH, & Kembro JM (2019). Expression of aggressiveness modulates mesencephalic c-fos activation during a social interaction test in Japanese quail (Coturnix japonica). Behav Brain Res, 367, 221–229. doi: 10.1016/j.bbr.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Caliva JM, Kembro JM, Pellegrini S, Guzman DA, & Marin RH (2017). Unexpected results when assessing underlying aggressiveness in Japanese quail using photocastrated stimulus birds. Poult Sci, 96(12), 4140–4150. doi: 10.3382/ps/pex258 [DOI] [PubMed] [Google Scholar]
- Carroll RS, Weaver CE, & Baum MJ (1988). Evidence implicating aromatization of testosterone in the regulation of male ferret sexual behavior. Physiol Behav, 42(5), 457–460. doi: 10.1016/0031-9384(88)90176-x [DOI] [PubMed] [Google Scholar]
- Charlier TD, Ball GF, & Balthazart J. (2005). Sexual behavior activates the expression of the immediate early genes c-fos and Zenk (egr-1) in catecholaminergic neurons of male Japanese quail. Neuroscience, 131(1), 13–30. doi: 10.1016/j.neuroscience.2004.09.068 [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, & Michael RP (1995). Intracerebral infusion of an aromatase inhibitor, sexual behavior and brain estrogen receptor-like immunoreactivity in intact male rats. Neuroendocrinology, 61(2), 98–111. doi: 10.1159/000126830 [DOI] [PubMed] [Google Scholar]
- Cohen PG (2001). Aromatase, adiposity, aging and disease. The hypogonadal-metabolicatherogenic-disease and aging connection. Med Hypotheses, 56(6), 702–708. doi: 10.1054/mehy.2000.1169 [DOI] [PubMed] [Google Scholar]
- Cornil CA, & Ball GF (2010). Effects of social experience on subsequent sexual performance in naïve male Japanese quail (Coturnix japonica). Hormones and Behavior, 57(4–5), 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Holloway KS, Taziaux M, & Balthazart J. (2004). The effects of aromatase inhibition on testosterone-dependent conditioned rhythmic cloacal sphincter movements in male Japanese quail. Physiol Behav, 83(1), 99–105. doi: 10.1016/j.physbeh.2004.07.011 [DOI] [PubMed] [Google Scholar]
- Cornil CA, Taziaux M, Baillien M, Ball GF, & Balthazart J. (2006). Rapid effects of aromatase inhibition on male reproductive behaviors in Japanese quail. Hormones and Behavior, 49(1), 45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, & Forbes JF (2010). Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. Lancet Oncol, 11(12), 1135–1141. doi: 10.1016/s1470-2045(10)70257-6 [DOI] [PubMed] [Google Scholar]
- de Bournonville MP, Vandries LM, Ball GF, Balthazart J, & Cornil CA (2019). Site-specific effects of aromatase inhibition on the activation of male sexual behavior in male Japanese quail (Coturnix japonica). Horm Behav, 108, 42–49. doi: 10.1016/j.yhbeh.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ronde W, & de Jong FH (2011). Aromatase inhibitors in men: effects and therapeutic options. Reproductive Biology and Endocrinology : RB&E, 9, 93–93. doi: 10.1186/1477-7827-9-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M, & Hall S. (1986). Determinants of social proximity in Japanese quail (Coturnix coturnix japonica): male behavior. Journal of Comparative Psychology, 100(1), 59. [PubMed] [Google Scholar]
- Duncan KA, & Saldanha CJ (2019). Central aromatization: A dramatic and responsive defense against threat and trauma to the vertebrate brain. Front Neuroendocrinol, 100816. doi: 10.1016/j.yfrne.2019.100816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerbacher I, & Prinzinger R. (1981). The effects of the male sex-hormone testosterone on body temperature and energy metabolism in male japanese quail (Coturnix coturnix japonica). Comparative Biochemistry and Physiology Part A: Physiology, 70(2), 247–250. [Google Scholar]
- Fink G, Sumner BE, McQueen JK, Wilson H, & Rosie R. (1998). Sex steroid control of mood, mental state and memory. Clin Exp Pharmacol Physiol, 25(10), 764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, & Balthazart J. (1995). Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. Journal of chemical neuroanatomy, 8(4), 267–282. [DOI] [PubMed] [Google Scholar]
- François N, Mills A, & Faure J. (1998). Place preferences of Japanese quail given a permanent choice between a social or a non-social but enriched situation. Behavioural processes, 43(2), 163–170. [DOI] [PubMed] [Google Scholar]
- Francois N, Mills AD, & Faure JM (1999). Inter-individual distances during open-field tests in Japanese quail (Coturnix japonica) selected for high or low levels of social reinstatement behaviour. Behav Processes, 47(2), 73–80. doi: 10.1016/s0376-6357(99)00050-9 [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, & Lacreuse A. (2019). Adverse Effects of Aromatase Inhibition on the Brain and Behavior in a Nonhuman Primate. J Neurosci, 39(5), 918–928. doi: 10.1523/jneurosci.0353-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbreath JC, & Ko R-C (1970). Sex Differential for Body Temperature in Japanese Quail. Poultry science, 49(1), 34–36. doi: 10.3382/ps.0490034 [DOI] [PubMed] [Google Scholar]
- Goodson JL (2005). The vertebrate social behavior network: Evolutionary themes and variations. Hormones and Behavior, 48(1), 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, & Kabelik D. (2009a). Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Frontiers in Neuroendocrinology, 30(4), 429–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, & Kingsbury MA (2009b). Mesotocin and Nonapeptide Receptors Promote Estrildid Flocking Behavior. Science, 325(5942), 862–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis S, & Kennedy SH (2002). Role of estrogen in the treatment of depression. Am J Ther, 9(6), 503–509. doi: 10.1097/00045391-200211000-00008 [DOI] [PubMed] [Google Scholar]
- Halbreich U, & Kahn LS (2001). Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs, 15(10), 797–817. doi: 10.2165/00023210-200115100-00005 [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Merritt JR, Jalabert C, Ma C, Maney DL, & Soma KK (2018). Rapid effects of 17beta-estradiol on aggressive behavior in songbirds: Environmental and genetic influences. Horm Behav, 104, 41–51. doi: 10.1016/j.yhbeh.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Harada N, Ito S, Takagi Y, & Maeda S. (1998). Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem Biophys Res Commun, 252(2), 445–449. [DOI] [PubMed] [Google Scholar]
- Howard ES, & Bermant G. (1967). Hormonal Control of Aggressive Behavior in Japanese Quail (Coturnix coturnix japonica). Behaviour, 28(3/4), 255–268. [DOI] [PubMed] [Google Scholar]
- Hutchison RE (1978). Hormonal differentiation of sexual behavior in Japanese quail. Horm Behav, 11(3), 363–387. [DOI] [PubMed] [Google Scholar]
- Ishii S. (1984). Endocrine control of aggressive behavior in male and female Japanese quail. In Aoki K, Ishii S, & Morita H. (Eds.), Animal behavior: Neurophysiological and ethological approaches (pp. 223–234). Tokyo: Japan Scientific Societies Press. [Google Scholar]
- Jones RB, Mills AD, & Faure JM (1996). Social discrimination in Japanese quail Coturnix japonica chicks genetically selected for low or high social reinstatement motivation. Behav Processes, 36(2), 117–124. doi: 10.1016/0376-6357(95)00024-0 [DOI] [PubMed] [Google Scholar]
- Kelly AM, & Goodson JL (2014). Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol, 35(4), 512–529. doi: 10.1016/j.yfrne.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Launay F, Mills AD, & Faure JM (1991). Social motivation in Japanese quail coturnix coturnix japonica chicks selected for high or low levels of treadmill behaviour. Behavioural processes, 24(2), 95–110. doi: 10.1016/0376-6357(91)90002-H [DOI] [PubMed] [Google Scholar]
- Launay F, Mills AD, & Faure JM (1993). Effects of test age, line and sex on tonic immobility responses and social reinstatement behaviour in Japanese quail Coturnix japonica. Behav Processes, 29(1–2), 1–16. doi: 10.1016/0376-6357(93)90023-k [DOI] [PubMed] [Google Scholar]
- Marbouti L, Zahmatkesh M, Riahi E, & Sadr SS (2020). Inhibition of brain 17β-estradiol synthesis by letrozole induces cognitive decline in male and female rats. Neurobiol Learn Mem, 175, 107300. doi: 10.1016/j.nlm.2020.107300 [DOI] [PubMed] [Google Scholar]
- McCarthy MM (2012). Sexual Differentiation of Brain and Behavior. In Fink G, Pfaff DW, & Levine JE (Eds.), Handbook of Neuroendocrinology (pp. 393–413): Academic Press. [Google Scholar]
- Mills AD, Crawford LL, Domjan M, & Faure JM (1997). The behavior of the japanese or domestic quail Coturnix japonica. Neuroscience & Biobehavioral Reviews, 21(3), 261–281. doi: 10.1016/S0149-7634(96)00028-0 [DOI] [PubMed] [Google Scholar]
- Mills AD, & Faure J-M (1990). The treadmill test for the measurement of social motivation in Phasianidae chicks. Medical Science Research, 18(5), 179–180. [Google Scholar]
- Mills AD, Jones RB, & Faure JM (1995). Species specificity of social reinstatement in Japanese quail Coturnix japonica genetically selected for high or low levels of social reinstatement behaviour. Behav Processes, 34(1), 13–22. doi: 10.1016/0376-6357(94)00044-h [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, & Wolin L. (1975). The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res, 31, 295–319. doi: 10.1016/b978-0-12-571131-9.50012-8 [DOI] [PubMed] [Google Scholar]
- Naftolin F, Ryan KJ, & Petro Z. (1971). Aromatization of androstenedione by limbic system tissue from human foetuses. J Endocrinol, 51(4), 795–796. doi: 10.1677/joe.0.0510795 [DOI] [PubMed] [Google Scholar]
- Nash S, & Domjan M. (1991). Learning to discriminate the sex of conspecifics in male Japanese quail (Coturnix coturnix japonica): Tests of “biological constraints.”. Journal of Experimental Psychology: Animal Behavior Processes, 17(3), 342–353. doi: 10.1037/0097-7403.17.3.342 [DOI] [PubMed] [Google Scholar]
- Nash S, Domjan M, & Askins M. (1989). Sexual-discrimination learning in male Japanese quail (Coturnix coturnix japonica). Journal of Comparative Psychology, 103(4), 347. [DOI] [PubMed] [Google Scholar]
- Navarro-Pardo E, Holland CA, & Cano A. (2017). Sex Hormones and Healthy Psychological Aging in Women. Front Aging Neurosci, 9, 439. doi: 10.3389/fnagi.2017.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW (1999). The Medial Extended Amygdala in Male Reproductive Behavior: A Node in the Mammalian Social Behavior Network. Annals of the New York Academy of Sciences, 877(1), 242–257. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol, 519(18), 3599–3639. doi: 10.1002/cne.22735 [DOI] [PubMed] [Google Scholar]
- O’Connell LA, & Hofmann HA (2012). Evolution of a vertebrate social decision-making network. Science, 336(6085), 1154–1157. doi: 10.1126/science.1218889 [DOI] [PubMed] [Google Scholar]
- Ottinger MA, & Brinkley HJ (1979). Testosterone and Sex Related Physical Characteristics during the Maturation of the Male Japanese Quail (Coturnix coturnix japonica). Biology of reproduction, 20(4), 905–909. doi: 10.1095/biolreprod20.4.905 [DOI] [PubMed] [Google Scholar]
- Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Lang I, Wardley A, Rabaglio M, Price KN, Gelber RD, Coates AS, & Thurlimann B. (2011). Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8.1 years median follow-up. Lancet Oncol, 12(12), 1101–1108. doi: 10.1016/s1470-2045(11)70270-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman EF, Harada N, & Roselli CE (1996). Effect of vorozole, an aromatase enzyme inhibitor, on sexual behavior, aromatase activity and neural immunoreactivity. J Neuroendocrinol, 8(3), 199–210. doi: 10.1046/j.1365-2826.1996.04505.x [DOI] [PubMed] [Google Scholar]
- Riters LV, & Balthazart J. (1998). Behavioral evidence for individual recognition in Japanese quail. Behaviour, 135(5), 551–578. [Google Scholar]
- Robinson GE, Fernald RD, & Clayton DF (2008). Genes and Social Behavior. Science, 322(5903), 896–900. doi: 10.1126/science.1159277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Shay DA, & Vieira-Potter VJ (2018). Cognitive Effects of Aromatase and Possible Role in Memory Disorders. Front Endocrinol (Lausanne), 9, 610. doi: 10.3389/fendo.2018.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD (1967). Photoperiodic Control of the Cloacal Gland of the Japanese Quail. Science, 157(3785), 201–203. doi: 10.1126/science.157.3785.201 [DOI] [PubMed] [Google Scholar]
- Schlinger BA, & Balthazart J. (2012). Aromatase and Behavior: Concepts Gained from Studies of Aromatase in the Avian Brain. In Balthazart J. & Ball G. (Eds.), Brain Aromatase, Estrogens, and Behavior: Oxford University Press. [Google Scholar]
- Schlinger BA, & Callard GV (1989). Aromatase Activity in Quail Brain: Correlation with Aggressiveness. Endocrinology, 124(1), 437–443. doi: 10.1210/endo-124-1-437 [DOI] [PubMed] [Google Scholar]
- Schlinger BA, & Callard GV (1990a). Aromatization Mediates Aggressive Behavior in Quail. General and comparative endocrinology, 79(1), 39–53. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, & Callard GV (1990b). Aggressive Behavior in Birds: An Experimental Model for Studies of Brain-Steroid Interactions. Comparative Biochemistry and Physiology Part A: Physiology, 97(3), 307–316. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Palter B, & Callard GV (1987). A Method to Quantify Aggressiveness in Japanese Quail (Coturnix c. japonica). Physiology & behavior, 40(3), 343–348. [DOI] [PubMed] [Google Scholar]
- Seiwert CM, & Adkins-Regan E. (1998). The foam production system of the male Japanese quail: characterization of structure and function. Brain Behav Evol, 52(2), 61–80. doi: 10.1159/000006553 [DOI] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Ball GF, & Cornil CA (2015). Estrogen Receptor beta Activation Rapidly Modulates Male Sexual Motivation through the Transactivation of Metabotropic Glutamate Receptor 1a. J Neurosci, 35(38), 13110–13123. doi: 10.1523/jneurosci.2056-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seredynski AL, Balthazart J, Christophe VJ, Ball GF, & Cornil CA (2013). Neuroestrogens rapidly regulate sexual motivation but not performance. Journal of Neuroscience, 33(1), 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman DI, Francis GL, Palmert MR, & Eugster EA (2008). Use of Aromatase Inhibitors in Children and Adolescents With Disorders of Growth and Adolescent Development. Pediatrics, 121(4), e975–e983. doi: 10.1542/peds.2007-2081 [DOI] [PubMed] [Google Scholar]
- Simpson ER (2004). Aromatase: Biologic Relevance of Tissue-Specific Expression. Semin Reprod Med, 22(01), 11–23. doi: 10.1055/s-2004-823023 [DOI] [PubMed] [Google Scholar]
- Singh SK (2013). Aromatase inhibitors in male sex. Indian journal of endocrinology and metabolism, 17(Suppl 1), S259–261. doi: 10.4103/2230-8210.119594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma KK, Sullivan KA, Tramontin AD, Saldanha CJ, Schlinger BA, & Wingfield JC (2000). Acute and chronic effects of an aromatase inhibitor on territorial aggression in breeding and nonbreeding male song sparrows. Journal of Comparative Physiology A, 186(7), 759–769. doi: 10.1007/s003590000129 [DOI] [PubMed] [Google Scholar]
- Stanić D, Dubois S, Chua HK, Tonge B, Rinehart N, Horne MK, & Boon WC (2014). Characterization of aromatase expression in the adult male and female mouse brain. I. Coexistence with oestrogen receptors α and β, and androgen receptors. PLoS One, 9(3), e90451-e90451. doi: 10.1371/journal.pone.0090451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan RB, Guay AT, & Hellstrom WJ (2014). Clinical Use of Aromatase Inhibitors in Adult Males. Sex Med Rev, 2(2), 79–90. doi: 10.1002/smrj.23 [DOI] [PubMed] [Google Scholar]
- Taxier LR, Gross KS, & Frick KM (2020). Oestradiol as a neuromodulator of learning and memory. Nature Reviews Neuroscience, 21(10), 535–550. doi: 10.1038/s41583-020-0362-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, & Balthazart J. (2004). Aromatase inhibition blocks the expression of sexually-motivated cloacal gland movements in male quail. Behav Processes, 67(3), 461–469. doi: 10.1016/j.beproc.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Taziaux M, Cornil CA, Dejace C, Arckens L, Ball GF, & Balthazart J. (2006). Neuroanatomical specificity in the expression of the immediate early gene c-fos following expression of appetitive and consummatory male sexual behaviour in Japanese quail. Eur J Neurosci, 23(7), 1869–1887. doi: 10.1111/j.1460-9568.2006.04719.x [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, & Shizuta Y. (2001). A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19). Journal of Endocrinology, 168(2), 217–220. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, & Ishii S. (1981). Effects of sex steroids on aggressive behavior of adult male Japanese quail. General and comparative endocrinology, 44(4), 480–486. doi: 10.1016/0016-6480(81)90336-1 [DOI] [PubMed] [Google Scholar]
- Vagell ME, & McGinnis MY (1997). The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol, 9(6), 415–421. doi: 10.1046/j.1365-2826.1997.00598.x [DOI] [PubMed] [Google Scholar]
- Vari C-E, Ősz B-E, Miklos A, Berbecaru-Iovan A, & Tero-Vescan A. (2016). Aromatase inhibitors in men-off-label use, misuse, abuse and doping. Farmacia, 64, 813–818. [Google Scholar]
- Viglietti-Panzica C, Balthazart J, Plumari L, Fratesi S, Absil P, & Panzica GC (2001). Estradiol mediates effects of testosterone on vasotocin immunoreactivity in the adult quail brain. Horm Behav, 40(4), 445–461. doi: 10.1006/hbeh.2001.1710 [DOI] [PubMed] [Google Scholar]
- Voigt C, Hirschenhauser K, & Leitner S. (2018). Neural activation following offensive aggression in Japanese quail. Biol Open, 7(12). doi: 10.1242/bio.038026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit JM, Hero M, & Nunez SB (2011). Aromatase inhibitors in pediatrics. Nat Rev Endocrinol, 8(3), 135–147. doi: 10.1038/nrendo.2011.161 [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S-I, Harada N, & Shah NM (2009). Estrogen Masculinizes Neural Pathways and Sex-Specific Behaviors. Cell, 139(1), 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid H, Simpson ER, & Brown KA (2016). Inflammation, dysregulated metabolism and aromatase in obesity and breast cancer. Curr Opin Pharmacol, 31, 90–96. doi: 10.1016/j.coph.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Zumpe D, Bonsall RW, & Michael RP (1993). Effects of the nonsteroidal aromatase inhibitor, fadrozole, on the sexual behavior of male cynomolgus monkeys (Macaca fascicularis). Horm Behav, 27(2), 200–215. doi: 10.1006/hbeh.1993.1015 [DOI] [PubMed] [Google Scholar]