Abstract
Over the past decade, organs-on-a-chip and microphysiological systems have emerged as a disruptive in vitro technology for biopharmaceutical applications. By enabling new capabilities to engineer physiological living tissues and organ units in the precisely controlled environment of microfabricated devices, these systems offer great promise to advance the frontiers of basic and translational research in biomedical sciences. Here, we review an emerging body of interdisciplinary work directed towards harnessing the power of organ-on-a-chip technology for reproductive biology and medicine. The focus of this topical review is to provide an overview of recent progress in the development of microengineered female reproductive organ models with relevance to drug delivery and discovery. We introduce the engineering design of these advanced in vitro systems and examine their applications in the study of pregnancy, infertility, and reproductive diseases. We also present two case studies that use organ-on-a-chip design principles to model placental drug transport and hormonally regulated crosstalk between multiple female reproductive organs. Finally, we discuss challenges and opportunities for the advancement of reproductive organ-on-a-chip technology.
Keywords: Organ-on-a-chip, Microphysiological systems, Microfluidics, Reproductive system, Reproductive biology and medicine, Pregnancy, IVF, Preterm birth
Graphical Abstract
1. Introduction
Reproduction is a fundamental process of life that plays an essential role in the survival and propagation of all species. Production of viable offspring is a highly complex event coordinated by the specialized tissues, organs, and hormones of the reproductive system that together enable fertilization and support the subsequent process of embryonic and fetal development. Over the last decades, great strides have been made in investigating the molecular basis of reproduction to define biological mechanisms responsible for the development and physiological function of the male and female reproductive systems [1]. Advances in our fundamental understanding have also led to the development of new technologies for clinical intervention of reproductive disorders, such as infertility [2].
Despite considerable efforts in this active area of basic and translational research, much remains to be learned about the inner workings of human reproduction. Direct investigation of the complex reproductive process in humans is fundamentally challenging due to major ethical issues associated with human subject research in which the health of both the mother and the developing fetus must be considered [3–6]. Medical imaging technologies such as ultrasound have enabled researchers to overcome some of these challenges by providing a means for clinical investigation of human reproduction without disrupting the fetus [7–9]. However, this approach intended primarily for detection and visualization of organ-scale reproductive events has limited potential for in-depth studies of cellular and molecular processes that contribute to the health and disease of the reproductive system. Combined with the fact that pregnant subjects are usually excluded from clinical trials due to ethical considerations, the limitations of existing research methodologies also pose significant challenges to the development of efficacious and safe drugs for reproductive and fetal disorders [5,6]
These problems, in turn, have provided an impetus for the development of new experimental approaches and surrogate models for the study of human reproduction. Among the conventional techniques is to use tissue explants from human reproductive organs. This method has proven particularly useful for laboratory investigation of the placenta [10,11], but often suffers from the difficulty of sourcing human materials and establishing complex ex vivo models that are highly prone to failure during long-term culture [12]. As is the case with other areas of biomedicine, in vivo studies using laboratory animals remain the mainstay of research in reproductive sciences and continue to make key contributions to advancing our knowledge of reproduction [13]. Animal experimentation, however, presents its own set of challenges due to the significant species-specific differences in the anatomical and functional characteristics of the reproductive system [14–16]. For example, the estrous cycle in mice lasts approximately 4–5 days, which is substantially shorter than a menstrual cycle of 28 days in humans [17]. It is also known that the type and relative abundance of key surface receptors and transporters expressed by reproductive organs (e.g., glucose transporters in the placenta) vary significantly between rodents and humans [18]. These interspecies disparities have come under increased scrutiny in recent years, questioning the scientific validity of using animal models to study human reproduction [19].
Importantly, the limitations of existing model systems have motivated new efforts to exploit engineering technologies to facilitate research advances in reproductive biology and medicine [20]. This trend has led to a new wave of in vitro models capable of recapitulating the complexity of the human reproductive system in ways not possible in traditional cell cultures [21–26]. As the most recent advance in this area, research has demonstrated the feasibility of adapting microfabrication techniques developed for the microelectronics industry to create microengineered three-dimensional (3D) cell culture systems, collectively known as organs-on-a-chip or microphysiological systems, for in vitro modelling of human reproduction. Our review aims to examine this emerging approach by providing a comprehensive survey of organ-on-a-chip systems that have been developed over the last decade to model various organs of the human female reproductive systems. The focus of our discussion will be on the demonstrated capabilities and potential of these models for i) emulating maternal-fetal interactions during pregnancy, ii) advancing assisted reproductive technology, and iii) investigating pathophysiological conditions of the reproductive system. Using select examples from the recent literature, we also present case studies to delve into how reproductive organ-on-a-chip technology can be leveraged for applications in drug development.
2. Basic principles of constructing organs-on-a-chip
Organs-on-a-chip refer to advanced in vitro models of multicellular tissue complexes or functional organ units created in microfabricated cell culture devices [27–32]. The term, organ-on-a-chip, is used synonymously with tissue-on-a-chip or microphysiological systems. Constructing these specialized model systems represents an engineering design process that follows the principle of reductionism and exploits the precision of microfabrication technologies for cell culture.
To elaborate on this process, let us consider the development of a human placenta-on-a-chip as an example (Figure 1). Production of organ-on-a-chip models typically begins with the process of decomposing the organ of interest to its most basic, self-repeating anatomical elements responsible for specialized organ-specific physiological function [28]. As the essential organ of pregnancy that functions to support fetal development, the placenta contains lobular structures known as cotyledons, each consisting of chorionic villi that extend from the outermost membrane of the fetus into the intervillous space (Figure 1A) [33,34]. All branches of the villi are in direct contact with the maternal blood and contain fetal capillary networks that arise from the umbilical blood vessels (Figure 1B) [35]. The outer surface of the villi is covered with a layer of trophoblast cells, which is separated from the vascular endothelium of fetal capillaries by a thin interstitium (Figure 1C) [34,35]. Importantly, this multilayered tissue structure is known as the placental barrier and serves as an essential organ unit that regulates the exchange of nutrients, oxygen, waste products, and other materials between the maternal and fetal blood circulating in the intervillous space and fetal capillaries, respectively [36]. As demonstrated in this analysis, detailed examination of organ anatomy allows us to identify i) the functional unit of our target organ (placental barrier), ii) its cellular constituents (trophoblast cells, endothelial cells), iii) its characteristic structural organization (closely apposed bi-layers of cells), and iv) the key features of its biomechanical/biochemical microenvironment (blood flow).
Figure 1.
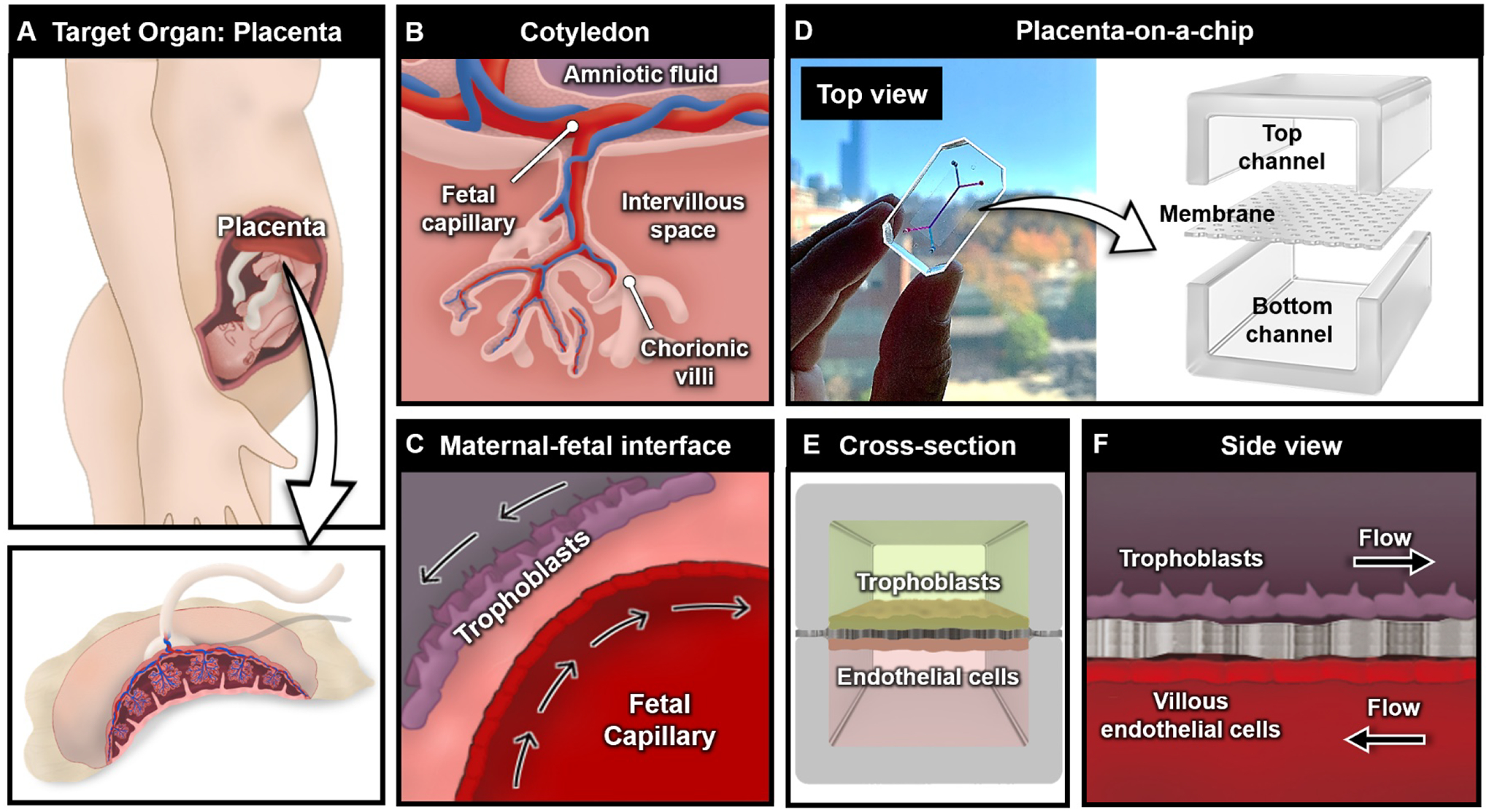
Design of placenta-on-a-chip. (A) Organ-on-a-chip utilizes a reductionist design approach to identify the functional unit of the organ of interest. The human placenta is shown as a representative example. (B) The cotyledon contains chorionic villi, which is a key unit to establish the maternal-fetal interface at the cellular level. (C) Trophoblasts and fetal endothelial cells are separated by a thin interstitium, which forms a barrier for material exchange between the fetus and mother. (D) The placental barrier is recapitulated in a microdevice by designing top and bottom channels separated by a thin porous membrane, which allows for the crosstalk between cell types. (E) Cross-sectional and (F) side views of the placenta-on-a-chip.
The next step in the model development process is to design a microdevice that makes it possible to replicate the identified key features. Among the most common device designs implemented in achieving this goal is to use two parallel, overlapping microchannels separated by a thin semipermeable membrane (Figure 1D) [27–30]. This configuration enables co-culture of two different cell types while permitting their biological crosstalk through the membrane pores, providing a means to emulate tissue compartmentalization and tissue-tissue interactions observed in virtually all organs in vivo. The number, geometry, and size of the microchannels can be adjusted depending on the architecture of the target organ and the desired complexity of the model. In our example, the two-chamber design can be used to grow trophoblast cells and fetal endothelial cells on either side of the membrane to mimic the microarchitecture of the placental barrier (Figure 1E). Moreover, the upper and lower culture chambers equipped with separate fluidic access ports allow for individually addressable fluid flow in the trophoblast and endothelial compartments to simulate the hemodynamic microenvironment of the placental barrier in vivo (Figure 1F).
Following the design step is the fabrication of organ-on-a-chip devices, which is often accomplished by soft lithography that enables replica molding of microscale cell culture chambers using transparent, biocompatible, and gas-permeable elastomers such as poly(dimethylsiloxane) (PDMS) [37]. This approach has proven instrumental for the purposes of rapid-prototyping organ-chip models, especially in academic research settings. It should be noted, however, that there is an increasing need for alternative materials and fabrication strategies due to the undesirable property of PDMS to absorb hydrophobic small molecules [38,39], which has raised concerns regarding the applicability of PDMS devices to drug testing. Nevertheless, significant progress has been made over the last decade in developing organ-on-a-chip models of various human physiological systems and demonstrating their potential for biomedical and pharmaceutical applications. We note that comprehensive surveys of recent work investigating the value of organ-on-a-chip technology from the general standpoint of drug development have been provided elsewhere [27,32] and that our review focuses on a subset of these studies that involve in vitro modeling of the reproductive system.
3. Applications of organ-on-a-chip technology in reproductive sciences
While organs-on-a-chip provide in vitro platforms for studying various aspects of human reproduction, modeling pregnancy and its related disorders has been central to an emerging body of research aiming to explore the potential of this technology for reproductive biology and medicine. Studies over the last decade have also demonstrated the feasibility of developing organ-on-a-chip models of intractable reproductive diseases, such as cancer and endometriosis, as a way to improve our understanding of their underlying pathophysiology and facilitate clinical and pharmaceutical interventions. On the basis of this recent trend, we examine in this section representative examples of microengineered in vitro systems developed for the study of three main topics – pregnancy, assisted reproductive technology (ART), and reproductive diseases.
3.1. Organ-on-a-chip models of pregnancy
Pregnancy is a highly coordinated process during which the female reproductive system undergoes a sequence of complex, interdependent physiological events. Beginning with fertilization of the embryo and decidualization of the uterus, pregnancy is divided into three trimesters, each contributing to the development of the placenta and fetus [40]. Modeling these different stages of pregnancy represents an important goal in reproductive research that has major clinical implications. In early pregnancy, for example, significant efforts are being made to advance our understanding of cellular and molecular events underlying abnormal implantation and placental remodeling that play a causative role in the development of pregnancy complications such as preeclampsia, intrauterine growth restriction (IUGR), and fetal developmental delays [41,42]. The ability to simulate and probe the process of decidualization and implantation at the earliest stages of pregnancy could make great contributions to developing new drugs and treatment strategies for these serious conditions. Similarly, modeling fetal-maternal crosstalk in the placenta at later stages of pregnancy [33,43] is important for various clinically relevant situations, such as assessment of the delivery and safety of maternally administered pharmaceutical products and treatment of late stage preeclampsia and preterm birth [44–46]. This section focuses on demonstrating how organ-on-a-chip technology has been used to address technical challenges associated with in vitro modeling of these complex reproductive processes.
3.1.1. Modeling decidualization
During the luteal phase of the menstrual cycle, elevated progesterone levels drive dynamic cellular and vascular changes in the endometrium, leading to the formation of a specialized mucosal layer on the uterine wall, termed the decidua (Figure 2A) [47]. The decidualization of the maternal tissue is the critical first step of pregnancy that serves to create a receptive environment for embryo implantation. Researchers have demonstrated the proof-of-principle of applying organ-on-a-chip approaches to modeling this female reproductive process that represents an essential requirement for the successful establishment of pregnancy.
Figure 2.
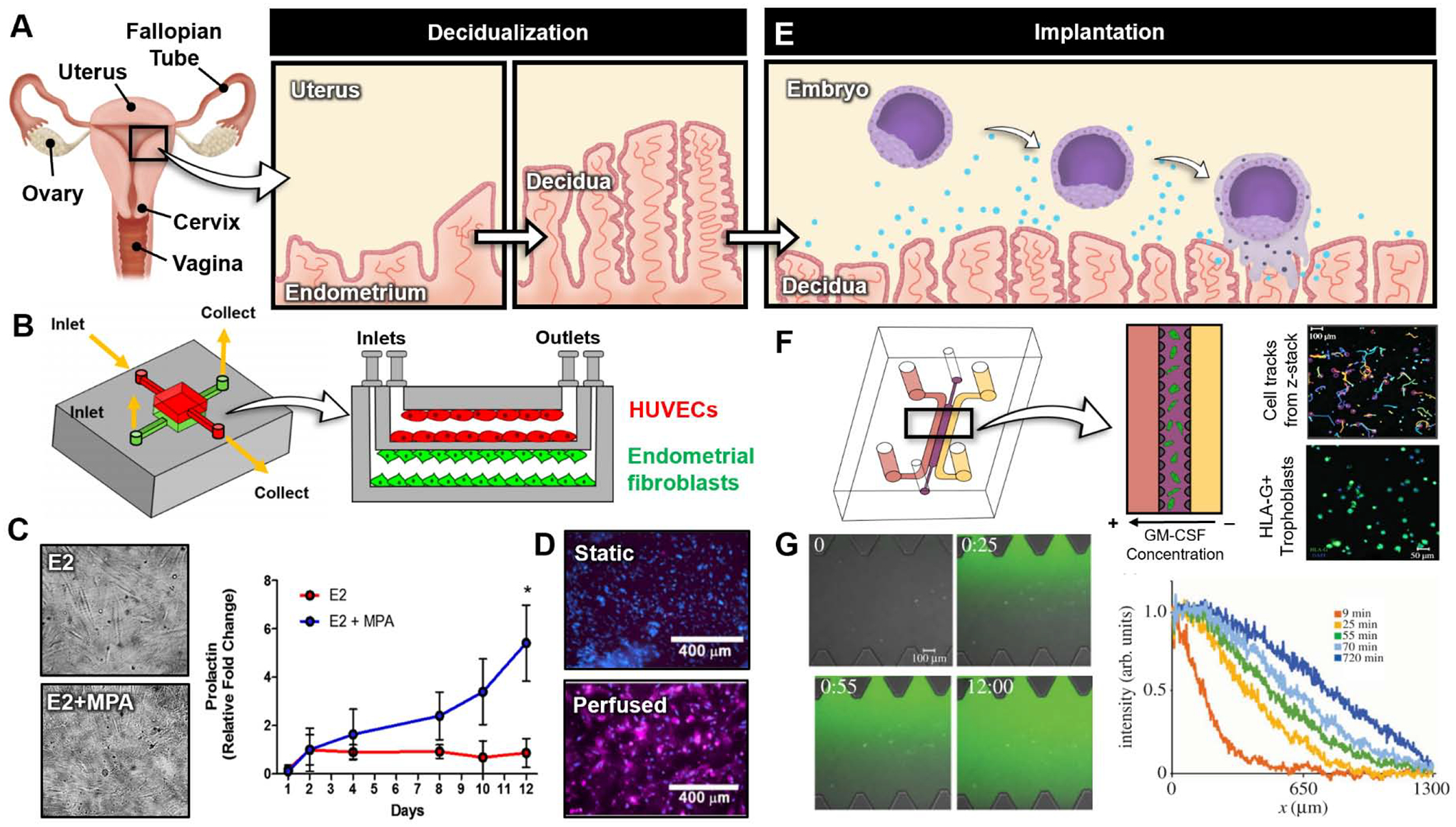
Organ-on-a-chip models of decidualization and implantation. (A) During decidualization, the inner most tissue layer of the uterus, called the endometrium, thickens in response to rising progesterone levels to form the decidua. (B) PDMS microdevice developed by Gnecco et al. [49]. The cross-section of device shows endothelial cells and endometrial stromal fibroblasts cultured in the top and bottom channels, respectively. (C) Stromal fibroblasts cultured with estradiol (E2) alone (top) and estradiol (E2) mixed with medroxyprogesterone acetate (MPA). The plot shows stromal fibroblast prolactin production over time under the E2 and E2+MPA culture conditions. (D) Endothelial cell expression of cyclooxygenase-2 (COX-2) under static (top) and perfused (bottom) culture conditions. (E) Implantation occurs 5–6 following fertilization and involves complex signaling between the decidua and embryo. Once attached to the decidua, trophoblasts stream out of the embryo, invading the maternal decidua and endometrium. (F) Microdevice developed by Abbas et al. [53]. Isolated first trimester extravillous trophoblasts (EVTs) are embedded in Matrigel in the central channel. Constant flow media with and without human granulocyte–macrophage colony-stimulating factor (GM-CSF) is applied to the left and right side channels, respectively, to create a cytokine gradient. Cell tracks were generated from time lapse microscopy. EVT purity is confirmed with human leukocyte antigen-G+ (HLA-G+) stain (green). (G) Characterization of chemical gradient through hydrogel using fluorescein dextran with similar molecular weight to GM-CSF as a model compound. The plot shows fluorescein intensity across device. All figures adapted with permissions from original publications.
For example, Gnecco et al. developed a microengineered in vitro model of the human endometrium for the study of decidualization. Specifically, a multilayered microdevice was created that enabled co-culture of primary human endometrial fibroblasts and human umbilical vein endothelial cells (HUVECs) on the opposite sides of a thin membrane with 2 μm pores to mimic the interface between the endometrial stroma and the vasculature in the maternal decidua (Figure 2B) [48]. The compartmentalized microfluidic design allowed for controlled delivery of ovarian hormones to the stromal cells through the vascular compartment, making it possible to simulate the temporal changes in the levels of estrogen and progesterone during a 28-day menstrual cycle. Importantly, under the culture conditions approximating the physiological hormonal microenvironment, the stromal fibroblasts underwent morphological transformation into cuboidal cells over a period of 10 days that resembled decidual stromal cells in vivo (Figure 2C). These changes were accompanied by increased production of decidual markers such as prolactin, validating the capabilities of the model to reproduce the key morphological and biochemical characteristics of decidualization (Figure 2C).
In a separate study, the same research group exploited the co-culture configuration of this system to investigate vascular contributions to the decidualization of endometrial stromal cells [49]. In comparison to static control, continuous perfusion of the vascular compartment lined with primary uterine microvascular endothelial cells was found to have significant effects on the differentiation of the stromal cells and promote their decidualization. Through further analysis, this work revealed that fluid shear stress generated by vascular perfusion activates the expression of cyclooxygenase-2 (COX-2) by the endothelial cells (Figure 2D) and causes concomitant increases in endothelial production of prostaglandin E2 (PGE2) and prostacyclin (PGl2), which can bind to their respective receptors on endometrial stromal cells to enhance decidualization.
3.1.2. Modeling implantation and placentation
Approximately 5–6 days after fertilization, the developing embryo enters the uterus from the fallopian tube and rolls on the surface of the uterine wall until it establishes connection with the decidualized, receptive endometrium that expresses adhesion molecules (Figure 2E). This key reproductive event is known as embryo implantation, which is marked by the firm attachment of the blastocyst to the endometrial epithelium and its invasion into the underlying stroma [50]. After adhesion, the outermost layer of the blastocyst gives rise to cytotrophoblasts that undergo branching morphogenesis to form placental villi that anchor the embryo to the maternal decidua [42]. During the progression of placentation, the trophoblasts at the anchoring villi differentiate into invasive cells known as extravillous trophoblasts (EVTs) that migrate deep into the endometrial stroma to reach maternal blood vessels and remodel them in order to establish the uteroplacental circulation that provides vascular supply to the growing fetus.
To study the invasive behavior of EVTs in this early stage of pregnancy, Abbas et al. created a microdevice consisting of three parallel chambers separated by microfabricated pillars (Figure 2F). The pillars were designed as surface tension-dependent capillary valves [51,52] to confine a Matrigel solution in the central compartment and form a 3D ECM scaffold that contained primary EVTs isolated from human placental samples between 7 and 12 weeks of gestation. The 3D microphysiological system was used as a microfluidic assay to examine how soluble factors produced by resident immune cells in the maternal decidua affect the directional migration of EVTs during placental development. The specific focus of this study was to measure EVT migration in response to spatial gradients of granulocyte-macrophage colony-stimulating factor (GM-CSF) – a cytokine produced by decidual natural killer (dNK) cells [53] – across the hydrogel scaffold (Figure 2G). Through real-time high-resolution imaging, this paper demonstrated directional 3D migration of trophoblasts towards the source of GM-CSF. The results of the study also showed that activation of the dNK cell receptor, KIR2DS1, and the resultant increase in the production of GM-CSF are the key molecular mechanism responsible for the chemotactic behavior of EVTs, supporting previous in vivo and in vitro findings of enhanced trophoblast invasion due to GM-CSF secreted by activated dNK cells [54].
3.1.3. Modeling maternal-fetal crosstalk during fetal development
Pregnancy is maintained by the placenta that plays an indispensable role in supporting fetal development. The main function of this specialized organ is to regulate transport of oxygen and nutrients to the fetus from the mother, as well as removal of metabolic waste products from the fetal circulation [34]. The placenta also provides a highly selective barrier that protects the fetus from harmful pathogens and potentially toxic materials circulating in the maternal blood. As discussed in Section 2, these critical physiological functions are carried out by a multilayered membranous structure known as the placental barrier that makes up the wall of the chorionic villi in the intervillous space and physically separates the maternal circulation from the fetal blood (Figure 1C). Modeling this maternal-fetal interface and its function has been an area of increasing interest in recent efforts to demonstrate the potential of organ-on-a-chip technology for the study of pregnancy.
By implementing the design process outlined in Figure 1, several research groups have developed placenta-on-a-chip models capable of generating multilayered tissue structures reminiscent of the human placental barrier [55–60]. A representative example can be found in the work of Blundell et al. [56] in which the two chamber design was used to co-culture the BeWo human choriocarcinoma cell line and primary human placental villous endothelial cells (HPVECs) on a thin polycarbonate membrane sandwiched between two overlapping PDMS chambers perfused with culture media (Figure 3A). Interestingly, during the formation of the microengineered placental barrier, fluid shear stress due to media perfusion induced the formation of dense microvilli on the apical surface of the trophoblast cells (Figure 3B), which is an important physiological phenotype for transport function of the placenta. The model was also capable of recapitulating physiological remodeling of the placenta barrier due to trophoblast syncytialization, which was shown by chemically induced fusion of the cultured trophoblast cells and the formation of multinucleated syncytium (Figure 3C). Notably, this study investigated nutrient transport in the microphysiological system and demonstrated the ability of the engineered barrier to support maternal-to-fetal transfer of glucose mediated by glucose transporter 1 (GLUT 1) (Figure 3D) at rates closely approximating those measured in ex vivo models of the human placenta.
Figure 3.
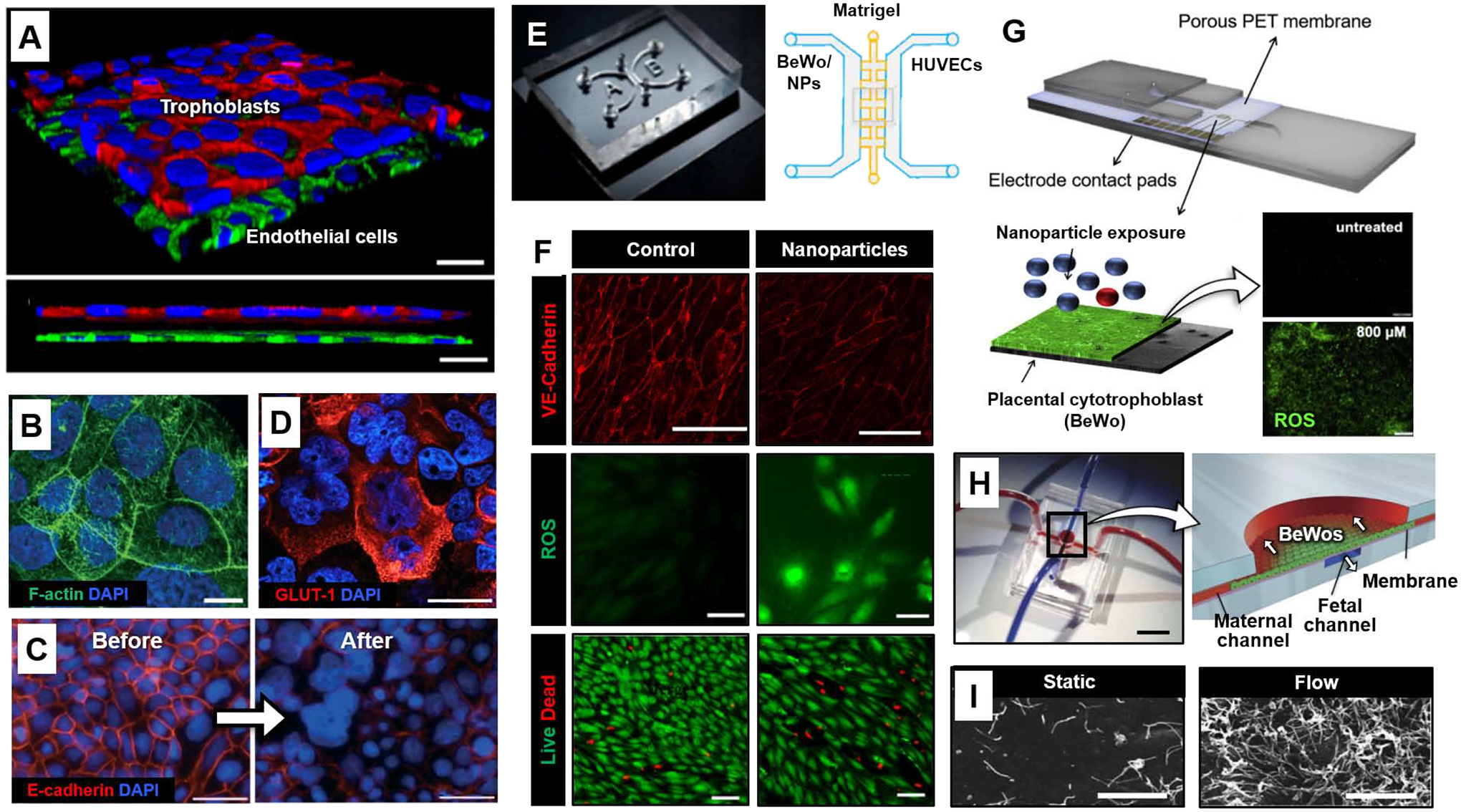
Organ-on-a-chip models of placental barrier function. (A) 3D rendered confocal image of the microengineered placental barrier [56]. BeWo trophoblast and endothelial cells are stained for E-cadherin (red) and VE-cadherin (green), respectively. Nuclei are shown in blue. Scale bars: 30 μm. (B) The surface of BeWo cells cultured under dynamic flow is covered with a dense layer of microvilli (green). Blue shows nuclear staining. Scale bars: 10 μm. (C) E-cadherin (red) and nuclear (blue) staining prior before (left) and after (right) forskolin treatment. Scale bars: 100 μm. (D) Trophoblasts of the microengineered placental barrier express high levels of GLUT-1 (red). Scale bars: 10 μm. (E) Microdevice developed by Yin et al. [58]. Matrigel is seeded in the middle matrix microchannel with BeWo cells and HUVCEs cultured and perfused separately in each side channel. Environmental exposure to nanoparticles (NPs) was modeled by introducing nanoparticles to the maternal (BeWo) side of the chip. (F) HUVEC VE-cadherin expression (red, top), reactive oxygen species (ROS) production (green, middle), and live/dead stain (green/red, bottom) after maternal exposure to titanium dioxide nanoparticles (TiO2-NPs) in the microdevice compared to control. Scale bars: 200, 50, and 100 μm, for VE-cadherin, ROS, and Live/Dead, respectively. (G) Microdevice developed by Schuller et al. [59]. Interdigitated impedance biosensors are located on top of a free-standing porous PET membrane separating BeWo cells and endothelial cells. The rest of the device is fabricated from glass and adhesive layers. BeWo ROS production (green) after 24 hours of exposure to 800 μM zinc oxide (ZnO) particles (bottom) compared to control (top). Scale bar: 200 μm. (H) PDMS device fabricated by Mirua et al. [60]. Red and blue dye were infused into microchannels to visualize the maternal and fetal compartments, respectively (left). Scale bar: 1 cm. Cross-section of device showing the apical maternal channel seeded with BeWo cells and basolateral fetal channel separated by a vitrified collagen membrane (right). The arrows indicate direction of flow in the maternal and fetal channels. (I) Scanning electron micrographs of microvilli protrusions on the apical surface of BeWo cells cultured overnight under static or flow (5 μl/min) conditions. Scale bars: 5 μm. All figures adapted with permissions from original publications.
Similar models have been reported that utilize the same microfluidic design to replicate the microarchitecture and transport function of the maternal-fetal interface [26,55,57]. Among these models is the microphysiological system developed by Pemathilaka et al. to support microfluidic co-culture of BeWo cells and HUVECs [57]. This placenta-on-a-chip was tested as an in vitro platform to examine caffeine transport across the human placental barrier. Using liquid chromatography mass spectrometric analysis of device effluent, this study showed that the rate of caffeine transport from the maternal to fetal compartment increased over time and peaked after 3 hours of continuous perfusion. Within 6.5 hours, the caffeine concentration reached a steady-state in both chambers, yielding approximately 2% of maternal caffeine in the fetal compartment.
Motivated by rapidly increasing use of engineered nanomaterials and their unknown adverse impact on pregnancy, recent studies have also demonstrated the application of placenta-on-a-chip technology to investigating maternal-to-fetal transport of nanomaterials. For instance, Yin et al. created a microengineered placental model to examine the effect of titanium dioxide nanoparticles (TiO2 NP) widely used in consumer products such as sunscreen [58]. This system employed a unique design in which BeWo cells and HUVECs were grown on the opposite sides of a Matrigel scaffold formed between two parallel culture chambers to mimic the basement membrane and interstitium of the placental barrier in vivo (Figure 3E). When the maternal compartment was perfused with high concentrations of TiO2 NP under near-physiological flow conditions, the fetal endothelium was seen with significant loss of cell viability and increased production of reactive oxygen species (ROS) (Figure 3F). Additionally, the model showed reduced expression of intercellular junctions and increased permeability of the microengineered placental barrier, raising the possibility that maternal TiO2 NP exposure may exert detrimental effects on the fetus.
In another example, Schuller et al. introduced a more advanced placental model containing a porous polyester membrane micropatterned with interdigitated electrodes that enabled non-invasive real-time analysis of impedance across a monolayer of BeWo cells (Figure 3G) [59]. This integrated system was used to monitor the permeability of the tissue barrier during exposure to various types of nanoparticles, including silicon dioxide (SiO2), titanium dioxide (TiO2), and zinc oxide (ZnO). The data showed no changes in normalized impedance due to SiO2 and TiO2, indicating no loss in barrier integrity. In contrast, a decrease in normalized impedance was observed in the presence of ZnO, providing evidence for its deleterious potential to compromise placental barrier function. This result was validated by demonstrating increased ROS and cell death due to ZnO (Figure 3G).
As outlined here, modeling material transfer across the maternal-fetal interface has been the primary focus of recent studies in microengineered analogs of the human placenta. However, efforts have also been made to exploit these systems for in-depth investigation of key regulatory mechanisms that control placental structure and function. The work of Miura et al. provides a good example of such mechanistic studies that delved into the effect of hemodynamic forces on microvilli formation in placental cells [60], which is recognized as the essential feature of differentiated trophoblasts that increases effective surface area for placental transport [61]. Using microfluidic monoculture of BeWo cells or primary human villous trophoblasts grown on a vitrified collagen membrane (Figure 3H), the authors demonstrated that fluid shear stress produced by media flow over the apical surface of the trophoblasts triggered the formation of microvilli at substantially higher density than was observed in static culture (Figure 3I). Increased microvilli formation under flow conditions also led to increased expression and activity of glucose transporters. Further characterization of the model discovered that this mechanosensitive response was mediated by the influx of extracellular calcium ion (Ca2+) into trophoblasts. Importantly, this study identified transient receptor potential, vanilloid family type-6 (TRPV6) calcium ion channel as molecular machinery that mediates the flow-induced Ca2+ influx and the resultant Akt-ezrin phosphorylation necessary for microvilli formation.
3.2. Organ-on-a-chip technology for infertility
Major advances have been made over the last decades in the development and clinical application of assisted reproductive technology (ART), which refers to laboratory techniques and procedures used to treat infertility, including in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) [62–64]. Emerging efforts to exploit organ-on-a-chip technology for ART stem from early work that has explored the use of microengineered systems for precise handling and manipulation of germ cells and embryos required for the success of ART. These studies have demonstrated microfluidic devices that, for example, take advantage of multiple laminar streams to sort and isolate motile sperm from semen samples [65–67] or enable perfusion culture of embryos [68–73] for IVF applications. With recent progress in organ-on-a-chip technology, researchers are beginning to develop more advanced in vitro platforms that can recapitulate the complex process of human reproduction with increased fidelity towards the goal of improving the clinical outcome of ART.
3.2.1. Embryo culture in physiological microenvironment
Following fertilization, the embryo travels through a specialized tubular organ of the reproductive system called the oviduct (also known as the fallopian tube) for 3–4 days until it reaches the uterus (Figure 4A). The inner surface of the oviduct is composed predominantly of epithelial cells with hormonally regulated motile cilia that, along with rhythmic contraction of the oviductal smooth muscle, direct the transport of the embryo towards the uterus [74,75]. The oviductal epithelium also contains secretory cells responsible for producing nutrient-rich fluid that lubricates the epithelial surface and provides nourishment and protection to the embryo [76]. While the underlying mechanisms remain to be elucidated, studies have suggested that biophysical and biochemical cues generated by this specialized microenvironment may play a crucial role in embryo development at the earliest stages of pregnancy [77]. Increasing attention is being paid to the possibility of leveraging this knowledge for the benefit of ART but such efforts have been greatly challenged by the difficulty of mimicking the dynamic, complex environment of the oviduct in vitro.
Figure 4.
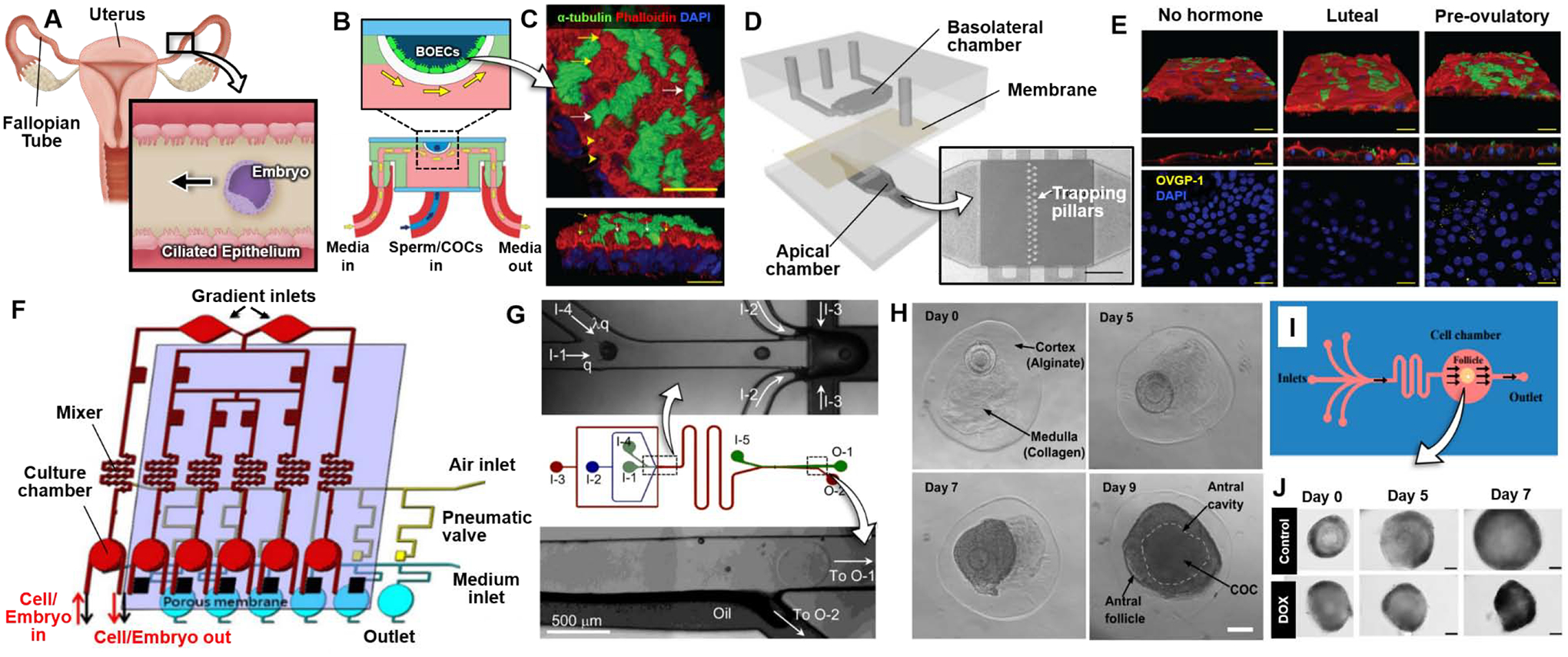
Organ-on-a-chip technology for assisted reproductive technology (ART). (A) After fertilization, the embryo travels down the fallopian tube towards the uterus. The ciliated epithelium along the fallopian tube beat at a synchronized frequency to drive directional embryo movement. (B) Side view (bottom) and zoomed-in view (top) of bovine oviductal epithelial cells (BOECs) in the 3D printed oviduct-on-a-chip developed by Ferraz et al. [78]. Chambers were separately perfused, as indicated by blue and yellow arrows. (C) Acetylated α-tubulin (green), phalloidin (red), and DAPI (blue) staining to label cilia, actin filaments, and nuclei, respectively in BOECs in 3D culture at an air-liquid interface for 28 days. Ciliated cells marked by white arrows and actin rich secretory protrusions marked by yellow arrows. Scale bars: 25 μm. (D) PDMS oviduct-on-a-chip developed by Ferraz et al. [79]. The basolateral compartment (top layer) was perfused with hormone supplemented media to simulate the phases of the menstrual cycle, while the apical compartment (bottom layer) contained BOECs perfused with culture media to support differentiation. The layers were separated by a porous polycarbonate membrane. The apical chamber contained cell-trapping pillars (micrograph). Scale bar: 1 mm. (E) Acetylated α-tubulin (green), phalloidin (red), and DAPI (blue) stains to label cilia, actin filaments, and nuclei, respectively in BOECs stimulated with hormones to mimic the luteal and pre-ovulatory phase. Oviductal glycoprotein 1 (OVGP1) is stained yellow in bottom figures. Scale bars: 10 μm. (F) Schematic of womb-on-a-chip developed by Chang et al [80]. A concentration gradient generator is integrated with a mixer and cell culture chamber in the top layer. Pneumatic valves are incorporated under the gradient channel to stop flow from the gradient channel to the culture chamber. Endometrial stromal cells are cultured on top of a porous membrane in the culture chamber with media perfusion under the membrane. (G) Schematic of top view of the flow-focusing microfluidic device developed by Choi et al. [86], with zoom-in view of junction for encapsulation of ovarian follicles (OFs) in a core-shell microcapsule (top) and exit of the extraction channel (bottom), respectively. I-1, I-2, I-3, I-4, and I-5 are inlets of core, shell, mineral oil, dispatching, and extraction flows, respectively. O-1 and O-2 are outlets for the aqueous and oil flows, respectively. (H) Brightfield images of in vitro development of early secondary preantral follicles to the antral stage over 9 days in the microcapsule containing a collagen core and non-oxidized alginate shell. Antral follicle defined by cumulus-oocyte complex (COC) inside fluid-filled cavity (dashed line). Scale bar: 100 μm. (I) Top-down view of PDMS microfluidic device for ovarian follicle culture, developed by Aziz et al. [89]. (J) Images show DOX treated follicles compared to control over 7 days of culture. Scale bars: 50 μm. All figures adapted with permissions from original publications.
Motivated by this challenge, Ferraz et al. developed an oviduct-on-a-chip capable of generating physiological oviductal tissues that retain their native phenotype and support key reproductive events [78]. This system was constructed by 3D printing a photo-curable resin to create U-shaped microfluidic chambers for air-liquid interface (ALI) culture of primary bovine oviduct epithelial cells (Figure 4B). During 28-day culture, the polarized fluidic environment of the microdevice enabled the production of a fully differentiated columnar pseudostratified epithelium that contained both ciliated and secretory epithelial cells (Figure 4C). The ciliated, polarized epithelium was stably maintained for more than 6 weeks, which was in contrast to rapid loss of differentiated phenotype in conventional 2D culture. Importantly, the microenvironment of this model permitted sperm penetration and on-chip fertilization of oocytes without parthenogenic activation or polyspermy often observed in conventional IVF. These key findings were attributed to the presence of secretory products and conditioning factors released by the differentiated epithelium that enabled optimal interaction of sperm and oocytes to enhance monospermic fertilization, which was suggested as a potentially useful approach to improving the outcome of IVF.
In a follow-up study, the same group introduced a modified oviduct-on-a-chip system for the study of cellular differentiation during early embryo development in vitro [79]. This platform consisted of two PDMS flow-through chambers representing the basolateral and apical compartments of the oviduct epithelium, which were separated by a porous membrane (Figure 4D). In this design, the basolateral chamber was perfused with media containing estrogen and progesterone at varying concentrations to simulate the different phases of the menstrual cycle, while the apical chamber was used to culture and differentiate primary bovine oviduct epithelial cells under flow conditions. Using this model, the authors showed that perfusion of the device enhanced cell differentiation and allowed the engineered oviduct epithelium to exhibit physiological responses to cycling hormones, as exemplified by increased expression of oviductal glycoprotein 1 (Figure 4E) and genes involved in ciliogenesis, ciliary beating, and immune responses during the pre-ovulatory phase simulated by estrogen stimulation. The benefit of the in vivo-like environment in the oviduct-on-a-chip was further evidenced by RNA-sequencing of zygotes produced by on-chip IVF. The results of this analysis revealed that embryos in the microdevice recapitulated transcriptomic signatures and global methylation patterns of their native counterparts more closely than in conventional IVF, highlighting the benefit of incorporating living oviduct epithelium and its specialized microenvironment for successful in vitro embryo production and development.
Recent work has also demonstrated advanced instrumentation of microphysiological embryo culture systems using integrated microfluidic devices equipped with sophisticated means of controlling the culture microenvironment. As a representative example, Chang et al. developed a “womb-on-a-chip” platform for in vitro embryo development in a dynamically regulated physiological fluidic environment [80]. This system was designed to seamlessly integrate individually addressable six embryo culture chambers with microfluidic mixers and gradient generators on the same device to dynamically control the concentration of steroid hormones delivered to mouse embryos cultured on primary human endometrial stromal cells (Figure 4F). Using this integrated system, the authors demonstrated in vitro development of 8-cell embryos into hatching blastocysts within 48 hours, which is similar to the timeline of mouse embryonic development in vivo. The rate which this process occurred increased in the presence of endometria stromal cells and was found to be significantly higher than that in conventional 96-well static co-cultures, illustrating the important role of physiological cellular cross-talk and mechanical stimulation in embryo development in vitro. By leveraging the advanced capabilities for precise, dynamic control of the hormonal environment, this study also showed a trend of enhanced blastocyst formation with increasing concentrations of progesterone.
3.2.2. Microengineered culture for ovarian follicle development
The ovarian follicle (OF) is the functional unit of the ovary responsible for supporting the development of an immature egg into a mature oocyte and its release into the fallopian tube for fertilization [81]. Development of OFs, commonly referred to as folliculogenesis, represents a complex biological process in which an oocyte undergoes structural and functional maturation through an orchestrated sequence of hormonal stimulation [82–84]. In the context of ART, in vitro culture of immature follicles isolated from the ovary has been proposed as a promising strategy to preserve fertility in clinical situations involving polycystic ovarian syndrome, premature ovarian failure, or oncotherapy [83,85]. Early studies have demonstrated that encapsulation of immature OFs in alginate matrix can improve the OF maturation process prior to cryopreservation and serve as a tool for in vitro investigation of folliculogenesis [82]. Microengineering technologies are beginning to further these 3D culture systems and make important contributions to realizing the potential of this ART approach by enabling the development of new tools for in vitro growth, maturation, and manipulation of ovarian follicles.
The work of Choi et al. provides an example of recent studies that demonstrates the use of microfluidic devices to engineer a more supportive environment for ovarian follicle development in vitro [86]. Specifically, this system used a microfluidic flow focusing technique to encapsulate early secondary preantral ovarian follicles in microcapsules composed of a soft collagen core and hard alginate shell that mimicked the ovarian medullary and cortical tissues, respectively (Figure 4G). The 3D heterogeneous microtissues improved follicular development from the pre-antral to antral stage (Figure 4H). Following antral stage development, the microtissues were treated with luteinizing hormone (LH) and epidermal growth factor (EGF) to simulate the initiation of ovulation. In this experiment, 1 out of 6 antral follicles treated with LH and EGF ovulated, while ovulation was observed for all untreated follicles. The authors suggested that LH surges might suppress ovulation to allow for only a few oocytes to be ovulated per estrous cycle.
Current efforts to develop new techniques for in vitro folliculogenesis rely heavily on the use of mouse models. Given the well-documented interspecies differences in the size of follicles and their developmental dynamics [85], a question remains whether the findings of small animal studies are translatable to large mammalian species or humans. On the basis of this question, recent work used a microfabricated device to culture ovarian follicles of large mammals with the goal of understanding their growth patterns and identifying optimal culture conditions [87]. Using domestic dog and cat follicles embedded in ovarian explants or individually isolated from the ovarian cortex, this study focused specifically on investigating the effect of flow on the viability of the follicles. The presented data showed that microfluidic perfusion culture was effective for sustaining the viability of cat follicles in cortical explants without requiring agarose gel blocks and cell encapsulation used in conventional techniques, suggesting dynamic microfluidic culture as an alternative strategy for in vitro maintenance of ovarian follicles. Interestingly, this study also revealed significant species-specific differences in follicular responses to flow by showing cellular disorganization or loss in microfluidic culture of dog follicles perfused at the same flow rates.
Moving beyond applications in ART, microengineered culture has also proven instrumental for developing in vitro assays to investigate responses of ovarian follicles to physiologically or clinically relevant external stimuli. A good example is provided by microfluidic culture of human ovarian follicles established by Aziz et al for the study of chemotherapy-induced toxicity [88,89]. This system was created in a multilayered elastomeric device in which a single human ovarian follicle was housed in a microfabricated chamber (Figure 4I). When the device was perfused with clinically relevant concentrations of an anticancer drug, doxorubicin (DOX), for 7 days, the cultured follicles showed arrested growth or significant decreases in their size (Figure 4J), which was accompanied by the activation of apoptotic pathways and reduced secretion of estrogen. Furthermore, this study demonstrated enhanced toxicity on the development of the follicles due to co-treatment of DOX with protein kinase inhibitors (Src and PIM inhibitors) commonly used in combination therapies.
3.3. Modeling reproductive diseases in organs-on-a-chip
Modeling complex human disease has become as an area of intense research interest in the field of organ-on-a-chip. Driven by this trend, efforts are being made to harness the potential of organ-on-a-chip technology to overcome the limitations of conventional animal and in vitro models for the study of human reproductive diseases [22]. Recent work has demonstrated the possibility of using microphysiological systems to model, interrogate, and pharmacologically modulate the pathophysiology of intractable diseases in the human reproductive system. In this section, we examine this emerging body of studies by focusing on representative organ-chip models of pathophysiological processes involved in the following three conditions that represent major clinical challenges in reproductive medicine – preterm birth, ovarian cancer, and endometriosis.
3.3.1. Preterm birth / placental inflammation
Preterm birth is defined as birth prior to 37 weeks of gestation and accounts for an estimated 1 million infant deaths every year [90,91]. While the pathogenesis of preterm birth remains poorly understood, clinical research has indicated that the development of this condition is often closely associated with intrauterine inflammation [92]. During pregnancy, intrauterine inflammation commonly presents as a condition called chorioamnionitis, which is inflammation of the chorion, amnion, and placenta usually caused by bacterial infection. Chorioamnionitis causes an inflammatory cascade within the placenta, stimulating prostaglandin production and matrix metalloprotease (MMPs) synthesis and release. Prostaglandins stimulate uterine contractions and MMPs cause cervical ripening and degradation of the chorioamnionic membranes, leading to rupture and delivery [93].This pathophysiological condition also poses major threats to fetal development through the release of proinflammatory mediators, some of which may be transferred across the placental barrier to the fetal circulation [93]. Many fetuses exposed to chorioamnionitis develop a systemic inflammatory response known as the fetal inflammatory response syndrome (FIRS) [94]. The most pronounced effects of FIRS present in the neonatal respiratory and neurological systems, however recent work has descried negative outcomes in other organ systems as well [93,94].
To examine maternal-fetal crosstalk during inflammation, a recent study by Zhu et al. established a microengineered model of bacterial infection in the human placenta [95], which is the most common identified cause of preterm birth [96]. The system was designed to mimic the placental barrier by using two opposing monolayers of BeWo cells and HUVECs grown on either side of a microporous membrane (Figure 5A). When the maternal compartment was incubated with E. coli to simulate infection, both the trophoblast and endothelial populations were seen with significant cell death. In case of BeWo cells, bacterial infection also led to substantially increased expression of inflammatory cytokines including several interleukins and tumor necrosis factor-α (TNFα). The inflammatory phenotype of this model was further illustrated by increased trophoblast adhesion of THP-1 monocytes added to the maternal channel (Figure 5B). Importantly, infection also disrupted the integrity of the placental barrier and elicited inflammatory responses in the fetal endothelium as evidenced by its increased expression of proinflammatory genes, indicating the deleterious potential of maternal inflammation to induce fetal complications during pregnancy.
Figure 5.
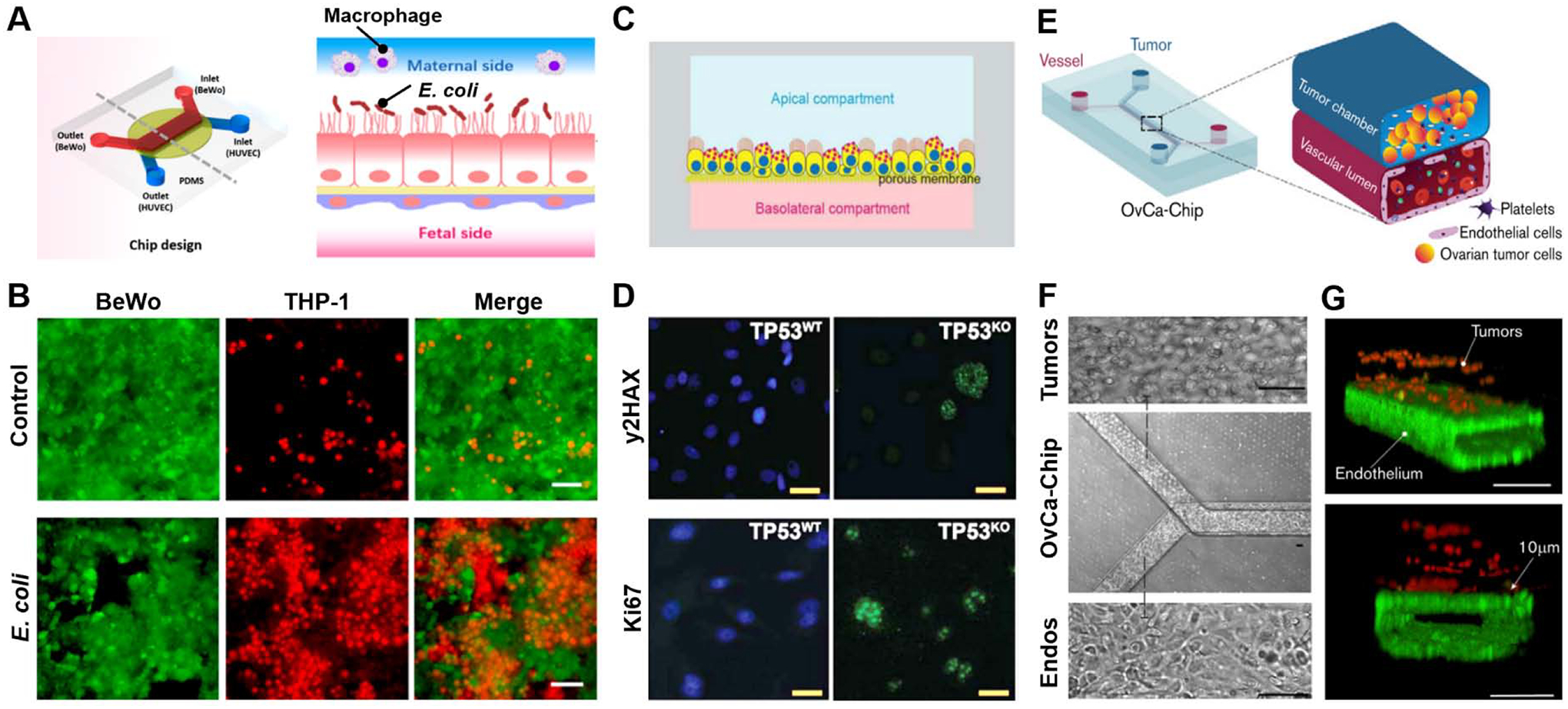
Organ-on-a-chip models of reproductive disease. (A) PDMS device developed by Zhu et al. [95]. BeWo trophoblast cells are cultured on the apical side and HUVECs are cultured on the basolateral side of the porous membrane (left). Schematic representation of cross-sectional view of the device (right). The BeWo cells on the maternal side are exposed to E. coli., and THP-1 monocytes are then added to the maternal side to represent maternal macrophages at the maternal-fetal interface during infection. (B) BeWo cells (green) incubated with E. coli for 6 hours and then co-cultured with THP-1 macrophages (red) for 30 min. Following E. coli exposure, maternal macrophages appear to adhere to the BeWo cell layer in the device. Scale bars: 100 μm. (C) Schematic representation of cross-section of oviduct-on-a-chip developed by Ferraz et al. to model high grade serous ovarian carcinoma (HGSC) [99]. Dog oviduct epithelium is grown on the membrane with independently perfused apical and basolateral compartments. (D) CRISPR-Cas9 edited (TP53KO) dog oviductal epithelial cells are compared to unedited (TP53WT) cells by immunofluorescence stains for double strand breaks (yH2AX, green), cell proliferation (Ki67, green) and nuclei (blue). Scale bars: 10 μm. (E) Schematic representation of OvCa-Chip developed by Saha et al. [101]. The cutout shows two fluidic chambers: the top channel is perfused with ovarian cancer cells (blue) and the bottom channel mimic a vascular lumen, containing a perfused endothelial lining. (F) Bright field microscopy of OvCa-Chip (center) showing zoom-in views of ovarian cancer cells (top) and primary endothelial cells (bottom). Scale bars: 100 μm. (G) 3D reconstruction of confocal fluorescence micrographs showing the cross-section of the OvCa-Chip. Spheroidal ovarian cancer cells are stained for anti-ZO1 (red) and primary endothelial cells stained for VE-cadherin (green). All 4 sides of the lower microchannel are covered with endothelial cell monolayers to form a blood-perfusable vessel. Platelets are perfused into the vascular lumen of the chip. Scale bars: 100 μm. All figures adapted with permissions from original publications.
3.3.2. Ovarian cancer
Ovarian cancer is the most common type of malignancy in the female reproductive system. This deadly disease is divided into multiple histological subtypes with distinct pathophysiological features [97]. As the most commonly diagnosed epithelial ovarian cancer, high-grade serous carcinoma (HGSC) has been suggested to arise from the fallopian tube on the basis of similarities in cell morphology and patterns of gene and protein expression [98]. This finding has led researchers to study the transformation of the fallopian tube epithelium in order to understand the development of ovarian cancer.
Following this line of investigation, Ferraz et al. developed an oviduct-on-a-chip system to model serous tubal intraepithelial carcinoma (STIC), which is considered the earliest manifestation of HGSC (Figure 5C) [99]. The microengineered model utilized dog oviductal epithelial cells cultured and differentiated at an air-liquid interface on a semipermeable membrane separating basolateral and apical chambers. Based on the observation that 96% of cancerous lesions in the fallopian tube and their metastatic form are marked by mutant p53 [100], the authors used the CRISPR-Cas9 gene editing technique to silence the canine TP53 gene in the oviduct epithelial cells (TP53KO). This genetic manipulation induced loss of epithelial polarization in TP53KO and caused the cells to become larger and flattened. Many of these cells were also seen with reduced cilia and multinucleated morphology, as well as increased proliferation and DNA double-stranded breaks (Figure 5D). The ability of this gene-edited model to recapitulate the molecular profiles of native tumors was verified by mRNA analysis that revealed a set of differentially regulated genes whose expression patterns matched those measured in HGSC tumors in vivo.
Another recent study demonstrated a similar microengineering approach to modeling platelet extravasation and infiltration into ovarian tumor tissue [101], which has been shown to promote the proliferation of ovarian cancer cells and the development of their resistance to chemotherapy [102,103]. By employing a common 3-layer design consisting of the upper and lower chambers bonded to an interstitial microporous PDMS membrane, this study established microfluidic co-culture of A2780 human ovarian cancer cells with HUVECs (Figures 5E, 5F). Importantly, continuous perfusion of the endothelialized lower chamber with human platelets led to endothelial adhesion of the platelets and their extravasation into the tumor microenvironment (Figure 5G), which was not observed in control established using benign cells. Further analysis revealed that increased production of proinflammatory cytokines (e.g., IL-6, IL-8, TNF-α) by ovarian cancer cells and the activation of endothelial signaling pathways implicated in loss of barrier function were key contributors to platelet activation, adhesion, and extravasation observed in the model. The authors also demonstrated the potential of this system as a preclinical drug testing platform by showing that atorvastatin administered into the vascular compartment preserved the integrity of the endothelial barrier and reduced cytokine production to prevent platelet recruitment.
3.3.3. Endometriosis
Endometriosis is a chronic disorder of the female reproductive system characterized by the presence of endometrial-like tissue outside the uterus, usually occurring in the peritoneal cavity [104]. Much remains to be learned about the etiology of this disease, but the majority of patients exhibit retrograde menstruation in which menstrual blood flows back into the fallopian tube [105]. Based on this clinical observation, it has been hypothesized that cells and tissue fragments contained in the backward menstrual flow may enter the peritoneal cavity, implant, and invade the pelvic tissue to form endometriotic lesions [106]. Investigating this disease process in animal models has been particularly challenging due to the inability of rodents to develop endometriosis spontaneously [107]. As an alternative approach, organ-on-a-chip models of endometriosis are being developed using patient-derived primary cells that are relatively easy to obtain [108].
A representative example is given by a simple micropatterned co-culture platform created by Chen et al. [109]. Using a microfabricated device containing two separate culture chambers, the authors generated spatially defined, juxtaposed islands of primary human endometrial stromal cells (ESCs) and human peritoneum mesothelial cells (HPMCs). These patterns were used to investigate the interaction between the two cell types during the invasion of endometrial cells into peritoneal tissues. The microengineered assay showed in vivo-like directional invasive migration of ESCs towards HPMCs derived from endometriosis patients. Interestingly, this response was not observed when the endometriotic cells were replaced with HPMCs from healthy subjects, suggesting disease-specific cellular crosstalk mediated by soluble factors. Similar studies are underway using more advanced organ-on-a-chip systems [48,110] to model and elucidate complex endometrial-stromal interactions during the development and progression of endometriosis.
4. Case studies of reproductive organs-on-a-chip for pharmaceutical applications
As discussed above, organ-on-a-chip technology provides a potentially powerful in vitro approach for reconstructing the complexity of the human reproductive system and its essential physiological and pathophysiological processes. Emerging as the focus of recent efforts is to leverage the advanced capabilities of microengineered reproductive organ models for applications in drug development. In particular, modeling drug responses of female reproductive organs in human cell-based microphysiological systems has been suggested as a promising preclinical strategy ideally suited to tackle the challenging ethical issues of clinical trials involving pregnant women and increasing scientific concerns regarding the translatability of animal data to human conditions [111]. Here we present a focused review of two recent papers as case studies to highlight this research trend and discuss important technical advances towards the development of reproductive organ-a-chip technology for drug screening.
4.1. Placental drug transport-on-a-chip
Reproductive toxicity is an important regulatory consideration for pharmaceutical development. In particular, assessing the hazardous potential of maternally administered drugs to interfere with fetal development during pregnancy has been a central research theme in reproductive toxicology [112,113]. With the increasing use of over the counter and prescription drugs by pregnant women, fetal exposure to medications is becoming a significant health concern. Unfortunately, preclinical efforts to address this issue have met little success due primarily to our limited ability to simulate maternal-fetal interactions in the human placenta. Despite advances in the development of animal and explant-based ex vivo models, it remains a major challenge to accurately predict drug transfer from the maternal to fetal circulation.
Recent studies have developed a promising strategy to tackle this challenge that relies on the advanced capabilities of organs-on-a-chip to model drug transport in the human placenta. The proof-of-concept of this approach was demonstrated by the work of Blundell et al. that utilized the placenta-on-a-chip platform shown in Figure 1D–1F to investigate maternal-to-fetal drug transfer across the human placental barrier (Figure 6A) [114]. Using the microengineered maternal-fetal interface constructed by co-culture of syncytialized BeWo trophoblasts and human villous endothelial cells, the research team examined fetal translocation of heparin, which is an anti-coagulant agent for the treatment of deep vein thrombosis and embolism during pregnancy. The results of this study showed that the bi-layer tissue provided a nonpermeable barrier to heparin transport and protected the fetal compartment from the drug (Figure 6B), consistent with previous findings that the passage of heparin across the placental barrier is prohibited due to its large size.
Figure 6.
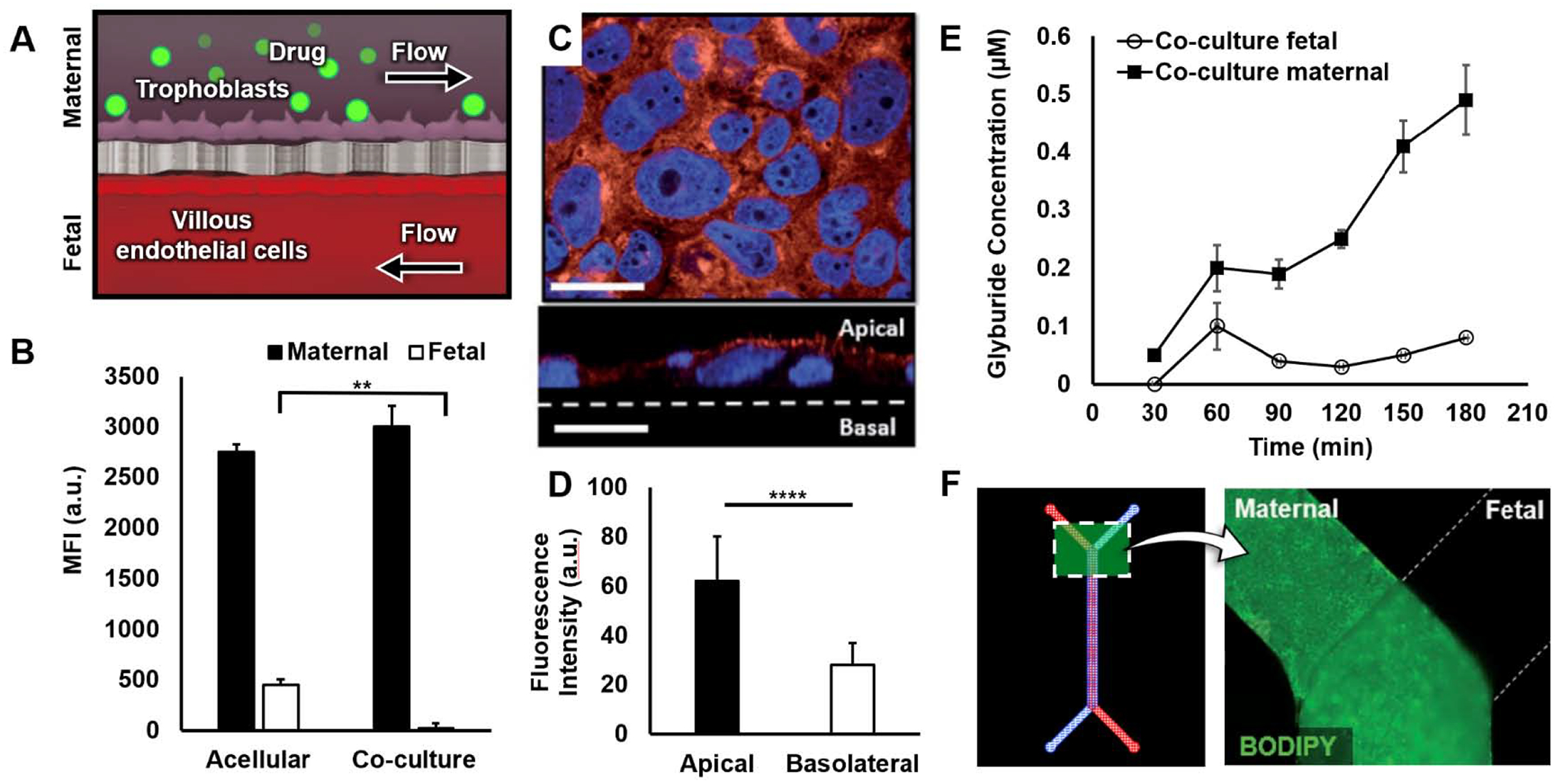
Placental drug transport-on-a-chip [114]. (A) Schematic of device cross-section. BeWo trophoblast cells in the maternal channel are exposed to drug molecules to examine transport across the microengineered placental barrier. (B) Transfer of fluorescein-heparin from the maternal to fetal compartments evaluated by measuring MFI of perfusate collected from the fetal channel. The placental barrier (co-culture) blocks heparin transport. (C) Breast cancer resistance protein (BCRP, red) expression in BeWo cells in the placenta-on-a-chip (top). Nuclei stained in blue. Cross-sectional view of BCRP expression (bottom). Scale bars: 36 μm. (D) Graph shows BCRP localization to microvillous membrane of BeWo cells. (E) Plot of glyburide concentration in fetal (circle) and maternal (square) perfusates over time following glyburide administration. (F) Glyburide accumulation in the BeWo monolayer (maternal compartment) with no visible fluorescence in human placental villous endothelial cells (HPVEC) population (fetal). Adapted with permissions from original publication.
Importantly, this paper also presented a placenta-on-a-chip model of active drug transport in the human placenta. The focus of this study was to demonstrate the physiological protective function of breast cancer resistance protein (BCRP), an efflux transporter responsible for shuttling drug molecules internalized by syncytiotrophoblasts back into the maternal circulation to inhibit fetal drug exposure. The BeWo trophoblast cells cultured in this model expressed high levels of BCRP on their apical surface, similar to the spatial localization of BCRP in the human placental barrier in vivo (Figures 6C, 6D). To investigate the activity of the transporter, the authors used BODIPY-labeled glyburide – a common medication used for pregnant women with gestational diabetes – as a model compound and monitored its concentration following drug administration on the maternal side. The key finding of this study was a continuous, gradual yet significant increase in the maternal level of glyburide over time (Figure 6E). Through further analysis using an inhibitor of BCRP, the authors showed that this was due to the efflux of absorbed glyburide from the trophoblasts into the maternal compartment by action of BCRP. By comparing the rate of glyburide transport in cell-coated and acellular devices, the authors also investigated unwanted drug absorption into PDMS to indicate that a loss of drug compounds due to this problem was not significant under the tested conditions. Importantly, despite the increased concentration gradient across the placental barrier, drug transfer into the fetal compartment was not detected (Figure 6F). These results validated the ability of the microengineered placental barrier to recapitulate the specialized role of its in vivo counterpart in protecting the developing fetus from maternal drugs.
4.2. EVATAR
Analysis of pharmacokinetics (PK) and pharmacodynamics (PD) is essential for accurate assessment of drug efficacy and safety. During preclinical studies, predicting the PK/PD profiles of drug compounds requires the ability to model the sequential process of drug absorption, distribution, metabolism, and elimination (ADME) that takes place in the integrated context of the whole body [115]. This critical requirement of preclinical drug testing has provided an impetus for long-standing efforts in the organ-on-a-chip field to develop integrated in vitro models termed ‘body-on-a-chip’ [116,117]. These systems are constructed by connecting microengineered models of different organs to enable simulation and prediction of systems-level drug responses that account for physiological multiorgan interactions [118,119]. Building upon the successful demonstration of body-on-a-chip technology for various organ systems, researchers are now attempting to develop integrated models of the reproductive system.
The work of Xiao et al. provides a representative example of using the multiorgan-on-a-chip approach to recapitulate functional interactions between the key organs in the female reproductive tract [120]. In this study, the authors created an integrated cell culture platform by linking five microfluidic organ modules containing murine ovary and human tissue explants isolated from the fallopian tube, endometrium, ectocervix, and liver (Figure 7A). This system termed EVATAR was also equipped with electromagnetically actuated micropumps and valves for precise control of fluid flow between and within the organ modules to permit dynamic biological interactions between the cultured tissues.
Figure 7.
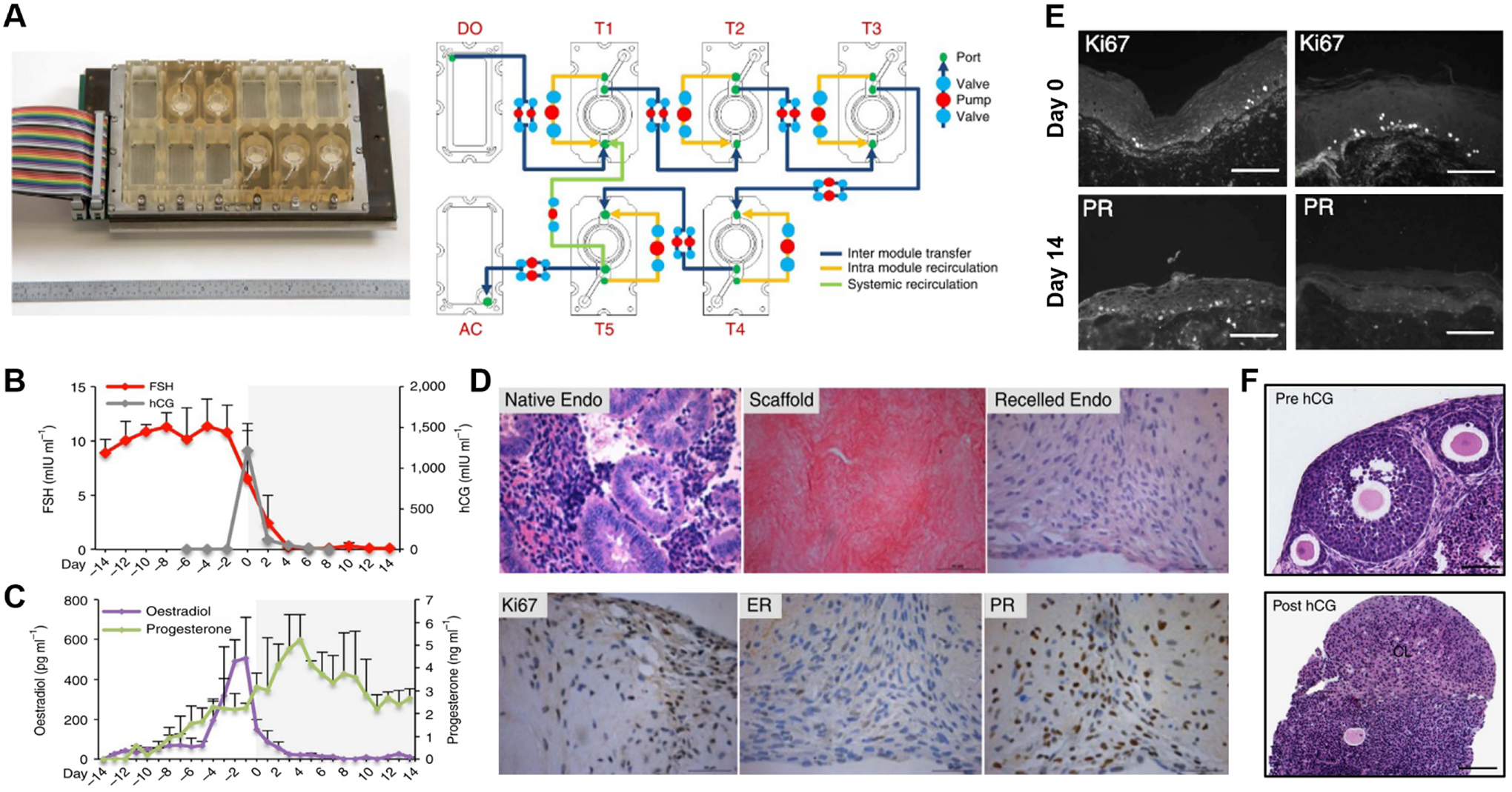
EVATAR [120]. (A) Device photo (left) and schematic representation of interconnected culture modules (right). Dark blue, yellow, and green lines show inter module transfer, intra module recirculation, and systemic recirculation, respectively. DO = donor module, AC = acceptor module, T= tissue module. (B) Follicle stimulating hormone (FSH) and human chorionic gonadotropin (hCG) concentration and (C) oestradiol and progesterone concentration in the device over 28 days of culture. (D) Human endometrium tissue before and after decellularization, and after recellularization (top). Immunohistochemical staining of Ki67, estrogen receptors (ER), and progesterone receptors (PR) in recellularized endometrial scaffolds on day 14 (28 days of microfluidic culture). (E) Ki67 and PR staining in ectocervical tissue at the end of the follicular (day 0) and luteal (day 14) phases. Scale bars: 10 μm. (F) Histology of ovarian tissue on day 0 (Pre hCG, top) and day 8 (Post hCG, bottom) showing corpus luteum (CL) formation. Scale bars: 100 μm. Adapted with permissions from original publication.
Using the integrated platform, this paper demonstrated a hormonally coupled in vitro analog of the female reproductive tract. A physiological hormonal environment was established in this model by controlling the concentration of follicle-stimulating hormone (FSH) and human chorionic gonadotropin (hCG) circulating through the microfluidic modules to reproduce the temporal profiles of pituitary hormones during the 28-day human menstrual cycle (Figure 7B). Importantly, this condition allowed the ovarian tissue cultured in the system to model the secretory function of its native counterpart as shown by the production of estrogen and progesterone that reached a peak in the follicular and luteal phases of microfluidic culture, respectively (Figure 7C). Human fallopian tube explants connected in the downstream of the ovary module exhibited high cell viability and ciliary activity during long-term culture and underwent physiological phenotypic changes in epithelial morphology and gene expression. EVATAR also supported human endometrial explants grown in decellularized uterine tissue scaffolds by maintaining the proliferative capacity of uterine stromal cells and their expression of estrogen and progesterone receptors (Figure 7D). Similarly, human ectocervix tissue in this multi-organ model retained its native morphology and physiological responses to estrogen as evidenced by the robust epithelial and stromal expression of progesterone receptor at the end of the simulated follicular phase, which was not detected at the end of the luteal phase when estrogen concentrations decreased (Figure 7E).Another key demonstration of this paper was to model pregnancy in EVATAR by supplementing media with hCG during the luteal phase to simulate hCG production by fertilized embryos and developing placenta at the beginning of pregnancy. Under this condition, the ovarian explants were seen with the corpus luteum (CL) during the entire period of 14-day culture following ovulation in the device (Figure 7F), recapitulating CL rescue and maintenance in vivo that plays an essential role in early pregnancy [121]. The physiological endocrine function of CL was verified by significantly higher levels of progesterone in the hCG-treated model in comparison to the untreated control. Although not directly relevant to reproductive physiology, this study also demonstrated long-term viability and secretory function of human liver spheroids maintained in the integrated system, suggesting the possibility of using EVATAR to model liver metabolism for pharmacological and toxicological studies of the reproductive system.
5. Future prospects: challenges and opportunities
Research in reproductive sciences has long suffered from a paucity of physiologically relevant, predictive preclinical models of human reproduction. Despite its nascence, the idea of leveraging advances in precision engineering technologies to tackle this major issue is emerging as a new research trend that has gained considerable traction in recent years. By making it possible to reproduce 3D structural organization and complex, dynamic physiological environment of tissue and organ units using human-derived cells, microphysiological systems offer the promise to overcome the limitations of conventional cell culture models and expand our capabilities for in vitro modeling and analysis of the reproductive system. While studies reviewed here (Table 1) represent the success and promise of this approach, it is important to note that significant challenges remain in realizing the full potential of organ-on-a-chip technology for reproductive biology and medicine.
Table 1.
Summary of existing reproductive-on-a-chip models.
| Application | Physiology modeled | Cell types | Fabrication method/materials Used | References |
|---|---|---|---|---|
| Pregnancy modeling | Decidualization & implantation | HUVECs, Stromal fibroblasts, Primary EVTs, uNKs | Soft lithography, PDMS | [39], [44] |
| Maternal-fetal crosstalk | BeWo b30, JEG-3, HUVEC, HPVEC | Soft lithography, PDMS, vitrified collagen, PET, polycarbonate | [47], [49], [50], [51], [52] | |
| ART | Embryo development | BOEC, bovine oocytes, bull sperm, human primary stromal cells, mouse embryos | Soft lithography, 3D printing, PDMS, photo-cured resin | [70], [71], [72], |
| Ovarian follicle development | Mouse, cat and dog ovarian follicles | Soft lithography, micromachining, PMMA, PDMS | [77], [78], [79], [80] | |
| Spermatogenesis | Mouse testis tissue | Soft lithography, PDMS, polycarbonate | [84], [85] | |
| Disease modeling | Preterm birth / placental inflammation | BeWo b30, HUVECs | Soft lithography, PDMS, polycarbonate | [93] |
| Ovarian cancer | Dog oviduct epithelial, A2780, HUVECs, | Soft lithography, PDMS | [97], [99] | |
| Endometriosis | Primary human endometrial stromal and peritoneum mesothelial | Soft lithography, PDMS | [107] |
Among the critical barriers to progress is the difficulty of sourcing primary human cells that are amenable to in vitro culture in microphysiological systems. Although published protocols exist for establishing primary cultures of human reproductive cells [122–127], setting up these systems is often challenged by ethical and legal issues associated with obtaining cells from pregnant women and fetal tissues. This problem has also led to limited availability of standardized culture protocols and commercial sources of primary cells, which has become a major obstacle to in vitro investigation of human reproduction. As demonstrated by several papers examined in this review, immortalized or transformed cells (e.g. BeWo, JEG-3, HTR-8) have been used successfully for in vitro studies. This approach, however, is restricted by the limited selection of available cell types and often faces the question of whether cell lines are capable of adequately representing in vivo physiology. To address this problem, increasing attention is being paid to induced pluripotent stem cells (iPSCs) as a sustainable source of human reproductive cells [128]. In light of recent success in the development of iPSC-derived organ-chip systems [129–134], studies are needed to explore the extension of this approach to creating microphysiological models of human reproductive organs.
On a related note, as discussed in this review, microphysiological systems have garnered great attention as a novel platform to culture gametes and embryos and to support their development in vitro for applications in ART. Regardless of the species, the process of sourcing, handling, and analyzing such cells in microdevices represents a technical challenge that needs to be further investigated. Equally important is to give special consideration to ethical issues associated with these types of studies involving the formation and early growth of life – in-depth discussion of this topic is provided elsewhere [3,135–137]. To advance the potential of organ-on-a-chip approaches in this area, the development of new in vitro technologies should be accompanied by collective efforts towards establishing ethical guidelines for proper use and analysis of embryonic materials in microengineered devices and model systems.
Model validation is another major challenge. As discussed in the Introduction, the significant interspecies difference in the structure and function of the reproductive system is becoming an increasingly important issue that questions the validity of using animal data to verify the in vivo relevance of human cell-based microphysiological systems. As alternatives, ex vivo models based on living human tissue explants are available but these materials are often hard to obtain at the gestation stages required for direct comparison [12]. Additionally, establishing conventional ex vivo models (e.g., perfused placental explants) is burdened by high failure rates and poor reproducibility and long-term viability, making it difficult to generate sufficient amounts of reliable data needed for model validation. Overcoming this challenge will require concerted efforts between model developers and clinical scientists to maximize the utility and potential of clinical human data and specimens for accurate assessment of in vitro-in vivo correlation. It is believed that these efforts will be greatly facilitated by recent research initiatives (e.g., NIH’s Human Placenta Project) aimed at developing new technologies to directly probe and examine human reproductive organs during their development and function [138].
Despite considerable progress, much remains to be accomplished in reconstructing the complexity of native reproductive tissues and organ units in microengineered cell culture devices. For example, work is required to improve our ability to recreate the complex hormonal environment in the reproductive system and model its critical role as a key regulator of reproductive health and disease. Reproductive organs, especially those involved in pregnancy, are known to undergo substantial structural and functional changes over time but mimicking these dynamic, spontaneous processes in engineered systems has been technically challenging. Culture conditions optimized for maintaining existing models also differ from the in vivo cellular microenvironment – for instance, the atmospheric oxygen tension (20%) commonly used in organ-chip systems is substantially higher than that measured in native reproductive organs (lower than 10%) [139]. It has proven difficult to replicate dynamic changes in oxygen tension and hormone levels commonly encountered in the reproductive system in vivo, particularly at various stages of pregnancy [140–142]. In addition, existing organ-chip models still rely heavily on 2D monolayer culture to mimic the maternal-fetal interface. As demonstrated by a recent report of a placental barrier model containing 3D vasculature and underlying connective tissue [143], efforts should be made to recapitulate 3D structural organization of native tissues towards the goal of developing more predictive and realistic model systems. In essence, addressing in vitro-in vivo differences is often a design challenge and will require the development and engineering of more advanced cell culture systems.
Although discussed frequently in other reviews [27–32], the current dominance of PDMS in the fabrication of organ-on-a-chip systems is problematic especially for drug testing due to its intrinsic properties to absorb small-molecule drug compounds [38,39]. The unwanted chemical absorption has been shown to have a significant impact on the bioavailability of drugs in PDMS cell culture devices [39,144], posing major challenges to PK/PD analysis. Studies have also revealed partitioning of various hormones into PDMS [145], which is of great concern for modeling the hormonal regulation of the reproductive system in organ-chip devices. Recent progress has been made in developing potential solutions to this problem that rely on the chemical passivation of PDMS surfaces to reduce the absorption of small hydrophobic molecules [146,147]. Efforts are also underway to find replacements for PDMS (e.g., polyurethane, cyclic olefin polymers, tetrafluoroethylene-propylene elastomer) and develop new microfabrication techniques based on the alternative materials [148–151].
Despite these challenges and technical limitations, organs-on-a-chip and microphysiological systems hold great promise to advance the frontiers of research in reproductive biology and medicine. In the specific context of drug development, as suggested by several examples in this review, microengineered in vitro models provide a basis for new preclinical strategies that complement traditional animal studies and enable accurate simulation and reliable prediction of drug efficacy and toxicity in the human reproductive system. Organs-on-a-chip also represent an enabling platform technology for drug delivery research. By providing a means to mimic the complex structural organization of functionally associated multiple tissue types in a realistic, organ-specific microenvironment, these advanced model systems may allow researchers to overcome the long-standing challenge of simulating delivery, transport, cellular uptake, and clearance of drugs and pharmaceutical products in the integrated physiological context of the human reproductive system. Precise parametric control and direct visualization of cells and their microenvironment are also the key strength of organs-on-a-chip that may provide unique advantages over animal-based approaches for preclinical modeling and quantitative analysis of drug delivery in reproductive and other related organs.
Another promising area of investigation is to explore the possibility of incorporating organoids derived from human reproductive organs into microphysiological systems. This idea stems from the emerging efforts to develop organoid-on-a-chip technology that synergistically combines the controllability of precisely engineered organ-chip platforms with the inherent capacity of stem cells to self-organize into complex organ-like structures [28,152]. In reproductive biology, great progress has recently been made in establishing organoid models of various female organs in healthy and diseased states, including the fallopian tube [153–155], endometrium [156,157], placenta [158,159], ovary [160,161], and cervix [162]. While much work remains to be done to further characterize and optimize these organoids, growing them in the tightly regulated environment of microengineered systems may enable the development of complex and realistic in vitro models not achievable using conventional organ-chip or organoid approaches alone. With further development, this integrative strategy may lead to more advanced and predictive preclinical platforms with the potential for broad and significant impact on drug discovery and delivery research.
The models discussed in the review focus on the female reproductive physiology and pathophysiology. However, organ-on-a-chip technology may also provide a potentially powerful approach to develop advanced culture platforms for male reproductive anatomy. Recent work by Komeya et al. shows the promise of this strategy [163,164]. While causes of male infertility vary, most common is idiopathic primary testicular dysfunction with abnormal spermatogenesis [165]. For decades, researchers have attempted to recapitulate this essential male reproductive process in vitro in order to treat male infertility or preserve fertility for prepubescent patients undergoing gonadotoxic therapies, such as chemotherapy [166]. Motivated by the challenges associated with these types of studies, the authors exploited microengineering techniques to improve the conventional methods of inducing spermatogenesis in air-liquid interface culture of mouse testis [167,168]. Through direct visualization, this study showed repeated emergence and disappearance of GFP-positive cells in the cultured tissue constructs, indicating the cyclic nature of spermatogenesis that has been described in vivo. The reproductive capacity of sperm generated in this system was demonstrated by the production of viable offspring following intracytoplasmic sperm injection (ICSI) procedures.
Finally, microphysiological reproductive models may provide a fertile ground for exploring the integration of microengineered cell culture with bioanalytical systems. Among the defining features of the reproductive system is its highly complex, dynamically regulated secretome that plays an essential role in physiological homeostasis, as well as various disease processes [169]. Creating realistic reproductive organ-on-a-chip models will require the ability to not only capture this complexity but also measure, characterize, and validate the complex soluble environment of the engineered constructs in a quantitative manner. One of the promising strategies to meet this important requirement is to integrate bioanalytical tools into microfluidic cell culture for in-line real-time monitoring of cells and their microenvironment. Recent studies have shown the feasibility of this approach by demonstrating organ-on-a-chip platforms equipped with integrated electrochemical and optical biosensors for rapid on-chip analysis of various biological functions, such as cytokine/chemokine production [170], barrier function [171], and neuronal activity [172]. Similar efforts will be beneficial for in vitro modeling of reproductive organs and make great contributions to improving our ability to emulate and delineate the dynamic biochemical basis of human reproduction or integrated responses of reproductive tissues to external stimuli. For example, incorporation of electrochemical biosensors to the placenta-on-a-chip model discussed in our case studies would allow for real-time measurement of placental barrier permeability to examine how the dynamics of maternal-fetal interactions change during clinically relevant situations such as infection or drug treatment. Similar strategies could also be used to capture and dynamically interrogate secretory products in reproductive-on-a-chip models, which may enable the identification of novel biomarkers associated with reproductive health and disease for clinical and pharmaceutical applications.
6. Concluding remarks
Remarkable progress in life science and technology in the past century has advanced our fundamental understanding of the human reproductive system beyond our imagination. However, the ever-increasing knowledge in this active area of research has done surprisingly little to improve our ability to emulate the complexity of human reproduction in experimental model systems. The marriage of organs-on-a-chip with reproductive sciences discussed here has enabled a major undertaking to address this fundamental problem. Reproductive organ-on-a-chip technology is still in its infancy but we believe that research advances highlighted in this review represent important first steps towards the ultimate goal of transforming the way in which we study arguably the most essential process of life.
Acknowledgements
We thank H Shin, C Blundell, JY Park, and G Al for their input. This work was supported by the National Institutes of Health (1R01ES029275), National Science Foundation Graduate Research Fellowship under (Grant No. 2018266781), and the Chan-Zuckerberg Initiative Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
D.H. is a co-founder of Vivodyne Inc. and holds equity in Vivodyne Inc. and Emulate Inc.
References
- [1].Rojas J, Chávez-Castillo M, Olivar LC, Calvo M, Mejías J, Rojas M, Morillo J, Bermúdez V, Physiologic Course of Female Reproductive Function: A Molecular Look into the Prologue of Life, J. Pregnancy (2015). 10.1155/2015/715735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Matzuk MM, Lamb DJ, The biology of infertility: research advances and clinical challenges, Nat. Med 14 (2008) 1197–1213. 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McCullough LB, Chervenak FA, The fetus as a patient and the ethics of human subjects research: Response to commentaries on “an ethically justified framework for clinical investigation to benefit pregnant and fetal patients,” Am. J. Bioeth 11 (2011) W3–W7. 10.1080/15265161.2011.576939. [DOI] [PubMed] [Google Scholar]
- [4].Chervenak FA, McCullough LB, The ethics of maternal–fetal surgery, Semin. Fetal Neonatal Med. 23 (2018) 64–67. 10.1016/j.siny.2017.09.008. [DOI] [PubMed] [Google Scholar]
- [5].Sheffield JS, Siegel D, Mirochnick M, Heine RP, Nguyen C, Bergman KL, Savic RM, Long J, Dooley KE, Nesin M, Designing drug trials: Considerations for pregnant women, Clin. Infect. Dis 59 (2014) S437–S444. 10.1093/cid/ciu709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mazure CM, Jones DP, Twenty years and still counting: Including women as participants and studying sex and gender in biomedical research, BMC Womens. Health 15 (2015) 1–16. 10.1186/s12905-015-0251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bottomley C, Van Belle V, Mukri F, Kirk E, Van Huffel S, Timmerman D, Bourne T, The optimal timing of an ultrasound scan to assess the location and viability of an early pregnancy, Hum. Reprod 24 (2009) 1811–1817. 10.1093/humrep/dep084. [DOI] [PubMed] [Google Scholar]
- [8].Jauniaux E, Johns J, Burton GJ, The role of ultrasound imaging in diagnosing and investigating early pregnancy failure, Ultrasound Obstet. Gynecol. 25 (2005) 613–624. 10.1002/uog.1892. [DOI] [PubMed] [Google Scholar]
- [9].Farooq M, Ma′ajeni E, Messawa M, Ayaz A, Daghistani M, The role of doppler ultrasound in high risk pregnancy: A comparative study, Niger. Med. J 53 (2012) 116. 10.4103/0300-1652.104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schneider H, Panigel M, Dancis J, Transfer across the perfused human placenta of antipyrine, sodium, and leucine, Am. J. Obstet. Gynecol 114 (1972) 822–828. 10.1016/0002-9378(72)90909-X. [DOI] [PubMed] [Google Scholar]
- [11].Grafmüller S, Manser P, Krug HF, Wick P, von Mandach U, Determination of the transport rate of xenobiotics and nanomaterials across the placenta using the ex vivo human placental perfusion model, J. Vis. Exp (2013) 1–7. 10.3791/50401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Conings S, Amant F, Annaert P, Van Calsteren K, Integration and validation of the ex vivo human placenta perfusion model, J. Pharmacol. Toxicol. Methods 88 (2017) 25–31. 10.1016/j.vascn.2017.05.002. [DOI] [PubMed] [Google Scholar]
- [13].Grigsby PL, Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy, Semin. Reprod. Med 34 (2016) 11–16. 10.1055/s-0035-1570031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schmidt A, Morales-Prieto DM, Pastuschek J, Fröhlich K, Markert UR, Only humans have human placentas: Molecular differences between mice and humans, J. Reprod. Immunol 108 (2015) 65–71. 10.1016/j.jri.2015.03.001. [DOI] [PubMed] [Google Scholar]
- [15].Dilworth MR, Sibley CP, Review: Transport across the placenta of mice and women, Placenta. 34 (2013) S34–S39. 10.1016/j.placenta.2012.10.011. [DOI] [PubMed] [Google Scholar]
- [16].Carter AM, Enders AC, Pijnenborg R, The role of invasive trophoblast in implantation and placentation of primates, Philos. Trans. R. Soc. B Biol. Sci 370 (2015). 10.1098/rstb.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Caligioni C, Assessing Reproductive Status/Stages in Mice, Curr Protoc Neurosci. (2009) 1–11. 10.1002/0471142301.nsa04is48.Assessing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hahn T, Barth S, Weiss U, Mosgoeller W, Desoye G, Sustained hyperglycemia in vitro down regulates the GLUT1 glucose transport system of cultured human term placental trophoblast: a mechanism to protect fetal development?, FASEB J. 12 (1998) 1221–1231. 10.1096/fasebj.12.12.1221. [DOI] [PubMed] [Google Scholar]
- [19].Ackerman IV WE, Carter AM, De Mestre AM, Golos TG, Jeschke U, Kusakabe K, Laurent LC, Parast MM, Roberts RM, Robinson JM, Rutherford J, Soma H, Takizawa T, Ui-Tei K, Lash GE, IFPA Meeting 2012 Workshop Report I: Comparative placentation and animal models, advanced techniques in placental histopathology, human pluripotent stem cells as a model for trophoblast differentiation, Placenta. 34 (2013) S3–S5. 10.1016/j.placenta.2012.11.006. [DOI] [PubMed] [Google Scholar]
- [20].Gargus ES, Rogers HB, McKinnon KE, Edmonds ME, Woodruff TK, Engineered reproductive tissues, Nat. Biomed. Eng 4 (2020). 10.1038/s41551-020-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arumugasaamy N, Rock KD, Kuo CY, Bale TL, Fisher JP, Microphysiological systems of the placental barrier, Adv. Drug Deliv. Rev (2020). 10.1016/j.addr.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nawroth J, Rogal J, Weiss M, Brucker SY, Loskill P, Organ-on-a-Chip Systems for Women’s Health Applications, Adv. Healthc. Mater 7 (2018) 1–21. 10.1002/adhm.201700550. [DOI] [PubMed] [Google Scholar]
- [23].Kashaninejad N, Shiddiky MJA, Nguyen N-T, Advances in Microfluidics-Based Assisted Reproductive Technology: From Sperm Sorter to Reproductive System-on-a-Chip, Adv. Biosyst 2 (2018) 1700197. 10.1002/adbi.201700197. [DOI] [Google Scholar]
- [24].Weng L, IVF-on-a-Chip: Recent Advances in Microfluidics Technology for In Vitro Fertilization., SLAS Technol. 24 (2019) 373–385. 10.1177/2472630319851765. [DOI] [PubMed] [Google Scholar]
- [25].Smith GD, Takayama S, Application of microfluidic technologies to human assisted reproduction, Mol. Hum. Reprod 23 (2017) 257–268. 10.1093/molehr/gaw076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pemathilaka RL, Reynolds DE, Hashemi NN, Drug transport across the human placenta: Review of placenta-on-a-chip and previous approaches, Interface Focus. 9 (2019). 10.1098/rsfs.2019.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Esch EW, Bahinski A, Huh D, Organs-on-chips at the frontiers of drug discovery, Nat. Rev. Drug Discov 14 (2015) 248–260. 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Park SE, Georgescu A, Huh D, Organoids-on-a-chip, Science (80-. ). 364 (2019) 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bhatia SN, Ingber DE, Microfluidic organs-on-chips, Nat. Biotechnol 32 (2014) 760–772. 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- [30].Huh D, Hamilton GA, Ingber DE, From 3D cell culture to organs-on-chips, Trends Cell Biol. 21 (2011) 745–754. 10.1016/j.tcb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE, Microengineered physiological biomimicry: Organs-on-Chips, Lab Chip. 12 (2012) 2156–2164. 10.1039/c2lc40089h. [DOI] [PubMed] [Google Scholar]
- [32].Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA, Organs-on-chips: into the next decade, Nat. Rev. Drug Discov (2020). 10.1038/s41573-020-0079-3. [DOI] [PubMed] [Google Scholar]
- [33].Syme MR, Paxton JW, a Keelan J, Drug Transfer and Metabolism by the Human Placenta, Clin. Pharmacokinet 43 (2004) 487–514. [DOI] [PubMed] [Google Scholar]
- [34].Gude NM, Roberts CT, Kalionis B, King RG, Growth and function of the normal human placenta, Thromb. Res 114 (2004) 397–407. 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- [35].Huppertz B, The anatomy of the normal placenta, J. Clin. Pathol 61 (2008) 1296–1302. 10.1136/jcp.2008.055277. [DOI] [PubMed] [Google Scholar]
- [36].Griffiths SK, Campbell JP, Placental structure, function and drug transfer, Contin. Educ. Anaesthesia, Crit. Care Pain 15 (2015) 84–89. 10.1093/bjaceaccp/mku013. [DOI] [Google Scholar]
- [37].Qin D, Xia Y, Whitesides GM, Soft lithography for micro- and nanoscale patterning, Nat. Protoc 5 (2010) 491–502. 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- [38].Berthier E, Young EWK, Beebe D, Engineers are from PDMS-land, biologists are from polystyrenia, Lab Chip. 12 (2012) 1224–1237. 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- [39].van Meer BJ, de Vries H, Firth KSA, van Weerd J, Tertoolen LGJ, Karperien HBJ, Jonkheijm P, Denning C, IJzerman AP, Mummery CL, Small molecule absorption by PDMS in the context of drug response bioassays, Biochem. Biophys. Res. Commun 482 (2017) 323–328. 10.1016/j.bbrc.2016.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].The American College of Obstetricians and Gynecologists, Your Pregnancy and Childbirth Month to Month, 6th ed., American College of Obstetricians and Gynecologists, 2015. [Google Scholar]
- [41].Sankaran S, Kyle PM, Aetiology and Pathogenesis of IUGR, Best Pract. Res. Clin. Obstet. Gynaecol 23 (2009) 765–777. 10.1016/j.bpobgyn.2009.05.003. [DOI] [PubMed] [Google Scholar]
- [42].Knöfler M, Pollheimer J, IFPA Award in Placentology Lecture: Molecular regulation of human trophoblast invasion, Placenta. 33 (2012) 55–62. 10.1016/j.placenta.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vähäkangas K, Myllynen P, Drug transporters in the human blood-placental barrier, Br. J. Pharmacol 158 (2009) 665–678. 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].ACOG Committee on Practice Bulletins--Obstetrics No. 33, Diagnosis and management of preeclampsia and eclampsia, Obstet. Gynecol 99 (2002) 159–167. 10.1097/00006250-200201000-00028. [DOI] [PubMed] [Google Scholar]
- [45].Arumugasaamy N, Gudelsky A, Hurley-Novatny A, Kim PCW, Fisher JP, Model Placental Barrier Phenotypic Response to Fluoxetine and Sertraline: A Comparative Study, Adv. Healthc. Mater 8 (2019) 1–11. 10.1002/adhm.201900476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Arumugasaamy N, Hurley-Novatny A, Lembong J, Kim PCW, Fisher JP, Assessing SSRIs’ effects on fetal cardiomyocytes utilizing placenta-fetus model, Acta Biomater. 99 (2019) 258–268. 10.1016/j.actbio.2019.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Henriet P, Gaide Chevronnay HP, Marbaix E, The endocrine and paracrine control of menstruation, Mol. Cell. Endocrinol 358 (2012) 197–207. 10.1016/j.mce.2011.07.042. [DOI] [PubMed] [Google Scholar]
- [48].Gnecco JS, Pensabene V, Li DJ, Ding T, Hui EE, Bruner-Tran KL, Osteen KG, Compartmentalized Culture of Perivascular Stroma and Endothelial Cells in a Microfluidic Model of the Human Endometrium, Ann. Biomed. Eng 45 (2017) 1758–1769. 10.1007/s10439-017-1797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG, Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium, Hum. Reprod 34 (2019) 702–714. 10.1093/humrep/dez003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Norwirz ER, Schust DJ, Fisher SJ, Implantation and the survival of early pregnancy, N. Engl. J. Med 345 (2001) 1400–1408. [DOI] [PubMed] [Google Scholar]
- [51].Whisler JA, Chen MB, Kamm RD, Control of perfusable microvascular network morphology using a multiculture microfluidic system, Tissue Eng. - Part C Methods 20 (2014) 543–552. 10.1089/ten.tec.2013.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Park YK, Tu TY, Lim SH, Clement IJM, Yang SY, Kamm RD, In vitro microvessel growth and remodeling within a three-dimensional microfluidic environment, Cell. Mol. Bioeng 7 (2014) 15–25. 10.1007/s12195-013-0315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Abbas Y, Oefner CM, Polacheck WJ, Gardner L, Farrell L, Sharkey A, Kamm R, Moffett A, Oyen ML, A microfluidics assay to study invasion of human placental trophoblast cells, J. R. Soc. Interface 14 (2017). 10.1098/rsif.2017.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xiong S, Sharkey AM, Kennedy PR, Gardner L, Farrell LE, Chazara O, Bauer J, Hiby SE, Colucci F, Moffett A, Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation, J. Clin. Invest 123 (2013) 4264–4272. 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, Huh D, Placenta-on-A-chip: A novel platform to study the biology of the human placenta, J. Matern. Neonatal Med. 29 (2016) 1046–1054. 10.3109/14767058.2015.1038518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Blundell C, Tess ER, Schanzer ASR, Coutifaris C, Su EJ, Parry S, Huh D, A microphysiological model of the human placental barrier, Lab Chip. 16 (2016) 3065–3073. 10.1039/c6lc00259e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pemathilaka RL, Caplin JD, Aykar SS, Montazami R, Hashemi NN, Placenta-on-a-Chip: In Vitro Study of Caffeine Transport across Placental Barrier Using Liquid Chromatography Mass Spectrometry, Glob. Challenges 3 (2019) 1800112. 10.1002/gch2.201800112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yin F, Zhu Y, Zhang M, Yu H, Chen W, Qin J, A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier, Toxicol. Vitr 54 (2019) 105–113. 10.1016/j.tiv.2018.08.014. [DOI] [PubMed] [Google Scholar]
- [59].Schuller P, Rothbauer M, Kratz SRA, Höll G, Taus P, Schinnerl M, Genser J, Bastus N, Moriones OH, Puntes V, Huppertz B, Siwetz M, Wanzenböck H, Ertl P, A lab-on-a-chip system with an embedded porous membrane-based impedance biosensor array for nanoparticle risk assessment on placental Bewo trophoblast cells, Sensors Actuators, B Chem. 312 (2020) 127946. 10.1016/j.snb.2020.127946. [DOI] [Google Scholar]
- [60].Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S, Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6, Nat. Commun 6 (2015). 10.1038/ncomms9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lager S, Powell TL, Regulation of nutrient transport across the placenta, J. Pregnancy 2012 (2012). 10.1155/2012/179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Boulet SL, Mehta A, Kissin DM, Warner L, Kawwass JF, Jamieson DJ, Trends in Use of and Reproductive Outcomes Associated With Intracytoplasmic Sperm Injection, JAMA. 313 (3015) 255–263. 10.1001/jama.2014.17985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Palermo G, Joris H, Devroey P, Van Steirteghem AC, Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte, Lancet. 340 (1992) 17–18. 10.1016/0140-6736(92)92425-F. [DOI] [PubMed] [Google Scholar]
- [64].Biggers JD, IN VITRO FERTILIZATION AND EMBRYO TRANSFER IN HUMAN BEINGS, N. Engl. J. Med 304 (1981) 336–342. 10.1056Liu/NEJM198102053040607. [DOI] [PubMed] [Google Scholar]
- [65].Cho BS, Schuster TG, Zhu X, Chang D, Smith GD, Takayama S, Passively driven integrated microfluidic system for separation of motile sperm, Anal. Chem 75 (2003) 1671–1675. 10.1021/ac020579e. [DOI] [PubMed] [Google Scholar]
- [66].Schuster TG, Cho B, Keller LM, Takayama S, Smith GD, Isolation of motile spermatozoa from semen samples using microfluidics, Reprod. Biomed. Online 7 (2003) 75–81. 10.1016/S1472-6483(10)61732-4. [DOI] [PubMed] [Google Scholar]
- [67].Riordon J, Tarlan F, You JB, Zhang B, Graham PJ, Kong T, Wang Y, Lagunov A, Hannam T, Jarvi K, Sinton D, Two-dimensional planar swimming selects for high DNA integrity sperm, Lab Chip. 19 (2019) 2161–2167. 10.1039/c9lc00209j. [DOI] [PubMed] [Google Scholar]
- [68].Chen CF, Chang KW, Yueh TR, Huang HY, Liu CS, A microfluidic device for automatic embryo trapping and coculture with stromal cells in vitro, in: 9th IEEE Int. Conf. Nano/Micro Eng. Mol. Syst. IEEE-NEMS 2014, IEEE, 2014: pp. 281–284. 10.1109/NEMS.2014.6908808. [DOI] [Google Scholar]
- [69].Heo YS, Cabrera LM, Bormann CL, Shah CT, Takayama S, Smith GD, Dynamic microfunnel culture enhances mouse embryo development and pregnancy rates, Hum. Reprod 25 (2010) 613–622. 10.1093/humrep/dep449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kimura H, Nakamura H, Akai T, Yamamoto T, Hattori H, Sakai Y, Fujii T, On-chip single embryo coculture with microporous-membrane-supported endometrial cells, IEEE Trans. Nanobioscience 8 (2009) 318–324. 10.1109/TNB.2009.2035275. [DOI] [PubMed] [Google Scholar]
- [71].Ma R, Xie L, Han C, Su K, Qiu T, Wang L, Huang G, Xing W, Qiao J, Wang J, Cheng J, In vitro fertilization on a single-oocyte positioning system integrated with motile sperm selection and early embryo development, Anal. Chem 83 (2011) 2964–2970. 10.1021/ac103063g. [DOI] [PubMed] [Google Scholar]
- [72].Heo YS, Cabrera LM, Bormann CL, Smith GD, Takayama S, Real time culture and analysis of embryo metabolism using a microfluidic device with deformation based actuation, Lab Chip. 12 (2012) 2240–2246. 10.1039/c2lc21050a. [DOI] [PubMed] [Google Scholar]
- [73].Kim MS, Bae CY, Wee G, Han YM, Park JK, A microfluidic in vitro cultivation system for mechanical stimulation of bovine embryos, Electrophoresis. 30 (2009) 3276–3282. 10.1002/elps.200900157. [DOI] [PubMed] [Google Scholar]
- [74].Shi D, Komatsu K, Uemura T, Fujimori T, Analysis of ciliary beat frequency and ovum transport ability in the mouse oviduct, Genes to Cells. 16 (2011) 282–290. 10.1111/j.1365-2443.2011.01484.x. [DOI] [PubMed] [Google Scholar]
- [75].Lyons RA, Saridogan E, Djahanbakhch O, The reproductive significance of human Fallopian tube cilia, Hum. Reprod. Update 12 (2006) 363–372. 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- [76].Lloyd RE, Romar R, Matás C, Gutiérrez-Adán A, Holt WV, Coy P, Effects of oviductal fluid on the development, quality, and gene expression of porcine blastocysts produced in vitro, Reproduction. 137 (2009) 679–687. 10.1530/REP-08-0405. [DOI] [PubMed] [Google Scholar]
- [77].Li S, Winuthayanon W, Oviduct: Roles in fertilization and early embryo development, J. Endocrinol 232 (2017) R1–R26. 10.1530/JOE-16-0302. [DOI] [PubMed] [Google Scholar]
- [78].Ferraz MAMM, Henning HHW, Costa PF, Malda J, Melchels FP, Wubbolts R, Stout TAE, Vos PLAM, Gadella BM, Improved bovine embryo production in an oviduct-on-a-chip system: prevention of poly-spermic fertilization and parthenogenic activation, Lab Chip. 17 (2017) 905–916. 10.1039/c6lc01566b. [DOI] [PubMed] [Google Scholar]
- [79].Ferraz MAMM, Rho HS, Hemerich D, Henning HHW, Van Tol HTA, Hölker M, Besenfelder U, Mokry M, Vos PLAM, Stout TAE, Le Gac S, Gadella BM, An oviduct-on-a-chip provides an enhanced in vitro environment for zygote genome reprogramming, Nat. Commun 9 (2018). 10.1038/s41467-018-07119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chang KW, Chang PY, Huang HY, Li CJ, Tien CH, Yao DJ, Fan SK, Hsu W, Liu CH, Womb-on-a-chip biomimetic system for improved embryo culture and development, Sensors Actuators, B Chem. 226 (2016) 218–226. 10.1016/j.snb.2015.11.004. [DOI] [Google Scholar]
- [81].Rimon-Dahari N, Yerushalmi-Heinemann L, Alyagor L, Dekel N, Ovarian Folliculogenesis, in: Piprek RP (Ed.), Mol. Mech. Cell Differ. Gonad Dev, Volume 58, Springer, Cham, 2016: pp. 167–190. 10.1007/978-3-319-31973-5_7. [DOI] [PubMed] [Google Scholar]
- [82].Pangas SA, Saudye H, Shea LD, Woodruff TK, Novel Approach for the Three-Dimensional Culture of Granulosa Cell-Oocyte Complexes, Tissue Eng. 9 (2003) 1013–1021. 10.1089/107632703322495655. [DOI] [PubMed] [Google Scholar]
- [83].Xu M, Barrett SL, West-Farrell E, Kondapalli LA, Kiesewetter SE, Shea LD, Woodruff TK, In vitro grown human ovarian follicles from cancer patients support oocyte growth, Hum. Reprod 24 (2009) 2531–2540. 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Brito IR, Lima B IMT, Xu C M, Shea LD, Woodruff TK, Figueiredo JR, Three-dimensional systems for in vitro follicular culture: overview of alginate-based matrices, Reprod. Fertil. Dev 26 (2014) 915–930. 10.1071/RD12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Picton HM, Harris SE, Muruvi W, Chambers EL, The in vitro growth and maturation of follicles, Reproduction. 136 (2008) 703–715. 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- [86].Choi JK, Agarwal P, Huang H, Zhao S, He X, The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue, Biomaterials. 35 (2014) 5122–5128. 10.1016/j.biomaterials.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Nagashima JB, El Assal R, Songsasen N, Demirci U, Evaluation of an ovary-on-a-chip in large mammalian models: Species specificity and influence of follicle isolation status, J. Tissue Eng. Regen. Med 12 (2018) e1926–e1935. 10.1002/term.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Aziz AUR, Fu M, Deng J, Geng C, Luo Y, Lin B, Yu X, Liu B, A microfluidic device for culturing an encapsulated ovarian follicle, Micromachines. 8 (2017). 10.3390/mi8110335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Aziz A. ur R., Yu X, Jiang Q, Zhao Y, Deng S, Qin K, Wang H, Liu B, Doxorubicin-induced toxicity to 3D-cultured rat ovarian follicles on a microfluidic chip, Toxicol. Vitr 62 (2019) 104677. 10.1016/j.tiv.2019.104677. [DOI] [PubMed] [Google Scholar]
- [90].Goldenberg RL, Culhane JF, Iams JD, Romero R, Epidemiology and causes of preterm birth, Lancet. 371 (2008) 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Phillips C, Velji Z, Hanly C, Metcalfe A, Risk of recurrent spontaneous preterm birth: A systematic review and meta-analysis, BMJ Open. 7 (2017) 1–6. 10.1136/bmjopen-2016-015402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Redline RW, Placental inflammation, Semin. Neonatol 9 (2004) 265–274. 10.1016/j.siny.2003.09.005. [DOI] [PubMed] [Google Scholar]
- [93].Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJM, The consequences of chorioamnionitis: Preterm birth and effects on development, J. Pregnancy 2013 (2013). 10.1155/2013/412831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gantert M, Been JV, Gavilanes AWD, Garnier Y, Zimmermann LJI, Kramer BW, Chorioamnionitis: A multiorgan disease of the fetus?, J. Perinatol 30 (2010) 21–30. 10.1038/jp.2010.96. [DOI] [PubMed] [Google Scholar]
- [95].Zhu Y, Yin F, Wang H, Wang L, Yuan J, Qin J, Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection, ACS Biomater. Sci. Eng 4 (2018) 3356–3363. 10.1021/acsbiomaterials.8b00653. [DOI] [PubMed] [Google Scholar]
- [96].Goldenberg RL, Hauth JC, Andrews WW, Intrauterine infection and preterm delivery, N. Engl. J. Med 342 (2000) 1500–1507. 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- [97].Soslow RA, Histologic subtypes of ovarian carcinoma: An overview, Int. J. Gynecol. Pathol 27 (2008) 161–174. 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- [98].Lin SF, Gerry E, Shih IM, Tubal origin of ovarian cancer – the double-edged sword of haemoglobin, J. Pathol 242 (2017) 3–6. 10.1002/path.4875. [DOI] [PubMed] [Google Scholar]
- [99].de Almeida Monteiro Melo Ferraz M, Nagashima JB, Venzac B, Le Gac S, Songsasen N, A dog oviduct-on-a-chip model of serous tubal intraepithelial carcinoma, Sci. Rep 10 (2020) 1–11. 10.1038/s41598-020-58507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yamamoto Y, Ning G, Howitt BE, Mehra K, Wu L, Wang X, Hong Y, Kern F, Wei TS, Zhang T, Nagarajan N, Basuli D, Torti S, Brewer M, Choolani M, McKeon F, Crum CP, Xian W, In vitro and in vivo correlates of physiological and neoplastic human Fallopian tube stem cells, J. Pathol 238 (2016) 519–530. 10.1002/path.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Saha B, Mathur T, Handley KF, Hu W, Afshar-Kharghan V, Sood AK, Jain A, OvCa-chip microsystem recreates vascular endothelium-mediated platelet extravasation in ovarian cancer, Blood Adv. 4 (2020) 3329–3342. 10.1182/bloodadvances.2020001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hu Q, Hisamatsu T, Hammerle M, Cho MS, Pradeep S, Rupaimoole R, Rodriguez-aguayo C, Lopez-berestein G, Wong TC, Sood AK, Afshar-kharghan V, Role of platelet-derived Tgfβ1 in the progression of ovarian cancer, Clin. Cancer Res 23 (2017) 5611–5621. 10.1158/1078-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Haemmerle M, Taylor ML, Gutschner T, Pradeep S, Cho MS, Sheng J, Lyons YM, Nagaraja AS, Dood RL, Wen Y, Mangala LS, Hansen JM, Rupaimoole R, Gharpure KM, Rodriguez-Aguayo C, Yim SY, Lee JS, Ivan C, Hu W, Lopez-Berestein G, Wong ST, Karlan BY, Levine DA, Liu J, Afshar-Kharghan V, Sood AK, Platelets reduce anoikis and promote metastasis by activating YAP1 signaling, Nat. Commun 8 (2017). 10.1038/s41467-017-00411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Giudice LC, Kao LC, Endometriosis, Lancet. 364 (2004) 1789–1799. [DOI] [PubMed] [Google Scholar]
- [105].Parasar P, Ozcan P, Terry K, Endometriosis: Epidemiology, Diagnosis and Clinical Management, Curr. Obstet. Gynecol. Rep 6 (2017) 34–41. 10.1007/s13669-017-0187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Malvezzi H, Marengo EB, Podgaec S, Piccinato CDA, Endometriosis: Current challenges in modeling a multifactorial disease of unknown etiology, J. Transl. Med 18 (2020) 1–21. 10.1186/s12967-020-02471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Grümmer R, Animal models in endometriosis research, Hum. Reprod. Update 12 (2006) 641–649. 10.1093/humupd/dml026. [DOI] [PubMed] [Google Scholar]
- [108].Mancini V, Pensabene V, Organs-On-Chip Models of the Female Reproductive System, Bioengineering. 6 (2019) 103. 10.3390/bioengineering6040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Chen Z, Dai Y, Dong Z, Li M, Mu X, Zhang R, Wang Z, Zhang W, Lang J, Leng J, Jiang X, Co-cultured endometrial stromal cells and peritoneal mesothelial cells for an in vitro model of endometriosis, Integr. Biol 4 (2011) 1090–1095. 10.1039/c2ib00172a. [DOI] [PubMed] [Google Scholar]
- [110].Gnecco JS, Brown AT, Kan EL, Baugh L, Ives C, Loring M, Griffith LG, Physiomimetic Models of Adenomyosis, Semin. Reprod. Med 1 (2020). 10.1055/s-0040-1719084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Eddie SL, Kim JJ, Woodruff TK, Burdette JE, Microphysiological modeling of the reproductive tract: A fertile endeavor, Exp. Biol. Med 239 (2014) 1192–1202. 10.1177/1535370214529387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Chahoud I, Ligensa A, Dietzel L, Faqi AS, Correlation between maternal toxicity and embryo/fetal effects, Reprod. Toxicol 13 (1999) 375–381. 10.1016/S0890-6238(99)00035-0. [DOI] [PubMed] [Google Scholar]
- [113].Farrar HC, Blumer JL, Fetal effects of maternal drug exposure, Annu. Rev. Pharmacol. Toxicol 31 (1991) 525–547. 10.1146/annurev.pa.31.040191.002521. [DOI] [PubMed] [Google Scholar]
- [114].Blundell C, Yi YS, Ma L, Tess ER, Farrell MJ, Georgescu A, Aleksunes LM, Huh D, Placental Drug Transport-on-a-Chip: A Microengineered In Vitro Model of Transporter-Mediated Drug Efflux in the Human Placental Barrier, Adv. Healthc. Mater 7 (2018). 10.1002/adhm.201700786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gerlowski LE, Jain RK, Physiologically Based Pharmacokinetic Modeling: Principles and Applications, J. Pharm. Sci 72 (1983) 1103–1127. 10.1111/joa.12538. [DOI] [PubMed] [Google Scholar]
- [116].Sung JH, Kam C, Shuler ML, A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip, Lab Chip. 10 (2010) 446–455. 10.1039/b917763a. [DOI] [PubMed] [Google Scholar]
- [117].Esch MB, King TL, Shuler ML, The Role of Body-on-a-Chip Devices in Drug and Toxicity Studies, Annu. Rev. Biomed. Eng 13 (2011) 55–72. 10.1146/annurev-bioeng-071910-124629. [DOI] [PubMed] [Google Scholar]
- [118].Esch MB, Smith AST, Prot JM, Oleaga C, Hickman JJ, Shuler ML, How multi-organ microdevices can help foster drug development, Adv. Drug Deliv. Rev 69–70 (2014) 158–169. 10.1016/j.addr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wang YI, Oleaga C, Long CJ, Esch MB, McAleer CW, Miller PG, Hickman JJ, Shuler ML, Self-contained, low-cost Body-on-a-Chip systems for drug development, Exp. Biol. Med 242 (2017) 1701–1713. 10.1177/1535370217694101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, Mckinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK, A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle, Nat. Commun 8 (2017) 1–13. 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yuan W, Wang XN, Greenwald GS, Follicle-stimulating hormone, human chorionic gonadotropin, and prolactin receptors in hamster corpora lutea or dispersed luteal cells during pregnancy, Biol. Reprod 52 (1995) 313–319. 10.1095/biolreprod52.2.313. [DOI] [PubMed] [Google Scholar]
- [122].Petroff MG, Phillips TA, Ka H, Pace JL, Hunt JS, Isolation and Culture of Term Human Trophoblast Cells BT - Placenta and Trophoblast: Methods and Protocols Volume 1, in: Soares MJ, Hunt JS (Eds.), Placenta Trophobl. Methods Mol. Med, Humana Press, Totowa, NJ, 2006: pp. 203–217. 10.1385/1-59259-983-4:201. [DOI] [PubMed] [Google Scholar]
- [123].Graham CH, Lysiak JJ, McCrae KR, Lala PK, Localization of transforming growth factor-β at the human fetal-maternal interface: Role in trophoblast growth and differentiation, Biol. Reprod 46 (1992) 561–572. 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- [124].Jividen K, Movassagh MJ, Jazaeri A, Li H, Two methods for establishing primary human endometrial stromal cells from hysterectomy specimens, J. Vis. Exp (2014). 10.3791/51513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Fan T, Li X, Li Y, Zhi Y, Rong S, Cheng G, Zhang X, An improved method for primary culture of normal cervical epithelial cells and establishment of cell model in vitro with HPV-16 E6 gene by lentivirus, J. Cell. Physiol (2017) 2773–2780. 10.1002/jcp.25978. [DOI] [PubMed] [Google Scholar]
- [126].Karast AM, Drapkin R, Primary culture and immortalization of human fallopian tube secretory epithelial cells, Nat. Protoc 7 (2012) 1755–1764. 10.1038/nprot.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Stromberg K, Azizkhan JC, Speeg KV, Isolation of Functional Human Trophoblast Cells and Their Partial Characterization in Primary Cell Culture, In Vitro. 14 (1978) 631–638. [DOI] [PubMed] [Google Scholar]
- [128].Laronda MM, Burdette JE, Kim JJ, Woodruff TK, Recreating the female reproductive tract in vitro using iPSC technology in a linked microfluidics environment, Stem Cell Res. Ther 4 (2013). 10.1186/scrt374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Abulaiti M, Yalikun Y, Murata K, Sato A, Sami MM, Sasaki Y, Fujiwara Y, Minatoya K, Shiba Y, Tanaka Y, Masumoto H, Establishment of a heart-on-a-chip microdevice based on human iPS cells for the evaluation of human heart tissue function, Sci. Rep 10 (2020) 1–12. 10.1038/s41598-020-76062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ramme AP, Koenig L, Hasenberg T, Schwenk C, Magauer C, Faust D, Lorenz AK, Krebs AC, Drewell C, Schirrmann K, Vladetic A, Lin GC, Pabinger S, Neuhaus W, Bois F, Lauster R, Marx U, Dehne EM, Autologous induced pluripotent stem cell-derived four-organ-chip, Futur. Sci. OA 5 (2019). 10.2144/fsoa-2019-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE, Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip, Nat. Biomed. Eng 1 (2017). 10.1038/s41551-017-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Abaci HE, Guo Z, Coffman A, Gillette B, Lee WH, Sia SK, Christiano AM, Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells, Adv. Healthc. Mater 5 (2016) 1800–1807. 10.1002/adhm.201500936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJA, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT, Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies, Nat. Med 20 (2014) 616–623. 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sances S, Ho R, Vatine G, West D, Laperle A, Meyer A, Godoy M, Kay PS, Mandefro B, Hatata S, Hinojosa C, Wen N, Sareen D, Hamilton GA, Svendsen CN, Human iPSC-Derived Endothelial Cells and Microengineered Organ-Chip Enhance Neuronal Development, Stem Cell Reports. 10 (2018) 1222–1236. 10.1016/j.stemcr.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Niemiec E, Howard HC, Ethical issues related to research on genome editing in human embryos, Comput. Struct. Biotechnol. J 18 (2020) 887–896. 10.1016/j.csbj.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Munsie M, Gyngell C, Ethical issues in genetic modification and why application matters, Curr. Opin. Genet. Dev 52 (2018) 7–12. 10.1016/j.gde.2018.05.002. [DOI] [PubMed] [Google Scholar]
- [137].Haimes E, Taylor K, Researching the relationships between tissue providers, clinicians, and stem cell scientists, Cell Stem Cell. 8 (2011) 613–615. 10.1016/j.stem.2011.05.005. [DOI] [PubMed] [Google Scholar]
- [138].Weinberg D, The Human Placenta : A short-lived organ , with a long-lasting impact Dr David Weinberg from the Human Placenta Project , a program of the Eunice Kennedy Shriver National Institute of, Openaccessgovernment.Org. (2018) 1–4. [Google Scholar]
- [139].Ng KYB, Mingels R, Morgan H, Macklon N, Cheong Y, In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: A systematic review, Hum. Reprod. Update 24 (2018) 15–34. 10.1093/HUMUPD/DMX028. [DOI] [PubMed] [Google Scholar]
- [140].Genbacev O, Zhou Y, Ludlow JW, Fisher SJ, Regulation of Human Placental Development by Oxygen Tension, Science (80-. ). 277 (1997) 1669–1672. 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- [141].Javam M, Audette MC, Iqbal M, Bloise E, Gibb W, Matthews SG, Effect of oxygen on multidrug resistance in term human placenta, Placenta. 35 (2014) 324–330. 10.1016/j.placenta.2014.02.010. [DOI] [PubMed] [Google Scholar]
- [142].Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ, Onset of maternal arterial blood flow and placental oxidative stress: A possible factor in human early pregnancy failure, Am. J. Pathol 157 (2000) 2111–2122. 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nishiguchi A, Gilmore C, Sood A, Matsusaki M, Collett G, Tannetta D, Sargent IL, McGarvey J, Halemani ND, Hanley J, Day F, Grant S, Murdoch-Davis C, Kemp H, Verkade P, Aplin JD, Akashi M, Case CP, In vitro placenta barrier model using primary human trophoblasts, underlying connective tissue and vascular endothelium, Biomaterials. 192 (2019) 140–148. 10.1016/j.biomaterials.2018.08.025. [DOI] [PubMed] [Google Scholar]
- [144].Toepke MW, Beebe DJ, PDMS absorption of small molecules and consequences in microfluidic applications, Lab Chip. 6 (2006) 1484–1486. 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- [145].Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-zelski J, Stephanie, Murphy WL, Schuler LA, Alarid ET, Beebe DJ, Biological implications of polydimethylsiloxane-based microfluidic cell culture, Lab Chip. 9 (2009) 2132–2139. 10.1039/b903043c.Biological. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sasaki H, Onoe H, Osaki T, Kawano R, Takeuchi S, Parylene-coating in PDMS microfluidic channels prevents the absorption of fluorescent dyes, Sensors Actuators, B Chem. 150 (2010) 478–482. 10.1016/j.snb.2010.07.021. [DOI] [Google Scholar]
- [147].Riaz A, Gandhiraman RP, Dimov IK, Basabe-Desmonts L, Ducrée J, Daniels S, Ricco AJ, Lee LP, Reactive deposition of nano-films in deep polymeric microcavities, Lab Chip. 12 (2012) 4877–4883. 10.1039/c2lc40296c. [DOI] [PubMed] [Google Scholar]
- [148].Domansky K, Leslie DC, McKinney J, Fraser JP, Sliz JD, Hamkins-Indik T, Hamilton GA, Bahinski A, Ingber DE, Clear castable polyurethane elastomer for fabrication of microfluidic devices, Lab Chip. 13 (2013) 3956–3964. 10.1039/c3lc50558h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nunes PS, Ohlsson PD, Ordeig O, Kutter JP, Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications, Microfluid. Nanofluidics 9 (2010) 145–161. 10.1007/s10404-010-0605-4. [DOI] [Google Scholar]
- [150].Derencsenyi TT, FLUOROELASTOMER COMPOST ON AND ARTICLE, 4,568,716, 1986. [Google Scholar]
- [151].Sano E, Mori C, Matsuoka N, Ozaki Y, Yagi K, Wada A, Tashima K, Yamasaki S, Tanabe K, Yano K, Torisawa YS, Tetrafluoroethylene-propylene elastomer for fabrication of microfluidic organs-on-chips resistant to drug absorption, Micromachines. 10 (2019) 1–13. 10.3390/mi10110793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Takebe T, Zhang B, Radisic M, Synergistic Engineering: Organoids Meet Organs-on-a-Chip, Cell Stem Cell. 21 (2017) 297–300. 10.1016/j.stem.2017.08.016. [DOI] [PubMed] [Google Scholar]
- [153].Xie Y, Park ES, Xiang D, Li Z, Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube, Stem Cell Res. 32 (2018) 51–60. 10.1016/j.scr.2018.08.021. [DOI] [PubMed] [Google Scholar]
- [154].Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M, Sehouli J, Fotopoulou C, Meyer TF, The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids, Nat. Commun 6 (2015) 1–11. 10.1038/ncomms9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Yucer N, Holzapfel M, Jenkins Vogel T, Lenaeus L, Ornelas L, Laury A, Sareen D, Barrett R, Karlan BY, Svendsen CN, Directed Differentiation of Human Induced Pluripotent Stem Cells into Fallopian Tube Epithelium, Sci. Rep 7 (2017) 1–9. 10.1038/s41598-017-05519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, Ferrante M, Vankelecom H, Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability, Dev. 144 (2017) 1775–1786. 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- [157].M.Y. T, L. G, J. H, T. CD, M.J. G, L. F, M. H, S.G.E. M, J.J. B, H.O. C, B.D. S, M. H, B.-K. K, A. M, G.J. B, Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium, Nat. Cell Biol 19 (2017) 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J, Mendjan S, Latos PA, Knöfler M, Self-Renewing Trophoblast Organoids Recapitulate the Developmental Program of the Early Human Placenta, Stem Cell Reports. 11 (2018) 537–551. 10.1016/j.stemcr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, McWhinnie A, Esposito L, Fernando R, Skelton H, Reimann F, Gribble FM, Sharkey A, Marsh SGE, O’rahilly S, Hemberger M, Burton GJ, Moffett A, Trophoblast organoids as a model for maternal–fetal interactions during human placentation, Nature. 564 (2018) 263–281. 10.1038/s41586-018-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, van Wijk LM, Revilla SA, Theeuwsen R, van de Ven M, van Roosmalen MJ, Ponsioen B, Ho VWH, Neel BG, Bosse T, Gaarenstroom KN, Vrieling H, Vreeswijk MPG, van Diest PJ, Witteveen PO, Jonges T, Bos JL, van Oudenaarden A, Zweemer RP, Snippert HJG, Kloosterman WP, Clevers H, An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity, Nat. Med 25 (2019) 838–849. 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- [161].Maenhoudt N, Defraye C, Boretto M, Jan Z, Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen E, Vergote I, Van Rompuy AS, Lambrechts D, Timmerman D, Vankelecom H, Developing Organoids from Ovarian Cancer as Experimental and Preclinical Models, Stem Cell Reports. 14 (2020) 717–729. 10.1016/j.stemcr.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Maru Y, Tanaka N, Ebisawa K, Odaka A, Sugiyama T, Itami M, Hippo Y, Establishment and characterization of patient-derived organoids from a young patient with cervical clear cell carcinoma, Cancer Sci. 110 (2019) 2992–3005. 10.1111/cas.14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Komeya M, Kimura H, Nakamura H, Yokonishi T, Sato T, Kojima K, Hayashi K, Katagiri K, Yamanaka H, Sanjo H, Yao M, Kamimura S, Inoue K, Ogonuki N, Ogura A, Fujii T, Ogawa T, Long-term ex vivo maintenance of testis tissues producing fertile sperm in a microfluidic device, Sci. Rep 6 (2016) 1–10. 10.1038/srep21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Komeya M, Hayashi K, Nakamura H, Yamanaka H, Sanjo H, Kojima K, Sato T, Yao M, Kimura H, Fujii T, Ogawa T, Pumpless microfluidic system driven by hydrostatic pressure induces and maintains mouse spermatogenesis in vitro, Sci. Rep 7 (2017) 1–8. 10.1038/s41598-017-15799-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Anawalt BD, Approach to male infertility and induction of spermatogenesis, J. Clin. Endocrinol. Metab 98 (2013) 3532–3542. 10.1210/jc.2012-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Song H-W, Wilkinson M, In vitro spermatogenesis: A long journey to get tails, Spermatogenesis. 2 (2012) 238–244. 10.4161/spmg.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T, In vitro production of functional sperm in cultured neonatal mouse testes, Nature. 471 (2011) 504–508. 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- [168].Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T, In vitro production of fertile sperm from murine spermatogonial stem cell lines, Nat. Commun 2 (2011). 10.1038/ncomms1478. [DOI] [PubMed] [Google Scholar]
- [169].Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, Rizzo R, Bortolotti D, Candiani M, Viganò P, Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side, Sci. Rep 7 (2017) 1–13. 10.1038/s41598-017-05549-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ortega MA, Fernández-Garibay X, Castaño AG, De Chiara F, Hernández-Albors A, Balaguer-Trias J, Ramón-Azcón J, Muscle-on-a-chip with an on-site multiplexed biosensing system for: In situ monitoring of secreted IL-6 and TNF-α, Lab Chip. 19 (2019) 2568–2580. 10.1039/c9lc00285e. [DOI] [PubMed] [Google Scholar]
- [171].Henry OYF, Villenave R, Cronce MJ, Leineweber WD, Benz MA, Ingber DE, Organs-on-chips with integrated electrodes for trans-epithelial electrical resistance (TEER) measurements of human epithelial barrier function, Lab Chip. 17 (2017) 2264–2271. 10.1039/c7lc00155j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Maoz BM, Herland A, Henry OYF, Leineweber WD, Yadid M, Doyle J, Mannix R, Kujala VJ, Fitzgerald EA, Parker KK, Ingber DE, Organs-on-Chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities, Lab Chip. 17 (2017) 2294–2302. 10.1039/c7lc00412e. [DOI] [PubMed] [Google Scholar]


