Abstract
Objective:
Ovarian cancer is characterized by poor prognosis, high levels of distress, disturbed sleep, and compromised quality of life (QOL). Although life stressors have been shown to significantly impact physical and psychological health in cancer populations, no studies have used a high-resolution stress assessment to differentiate effects of acute versus chronic stressors among women with ovarian cancer. We addressed this issue in the present prospective longitudinal study by examining how acute and chronic stress exposure in the year pre-diagnosis relate to depressive symptoms, sleep quality, and QOL over the first year post-diagnosis in women with ovarian cancer.
Methods:
One hundred thirty-seven women completed the Life Events and Difficulties Schedule within a month of initial treatment for suspected ovarian cancer. Depressive symptoms, sleep, and QOL were measured pre-treatment, at six months, and one-year post-diagnosis. Mixed models were used to examine associations of acute and chronic stress pre-diagnosis with (a) change in psychosocial outcomes over the first year post-diagnosis and (b) levels of psychosocial outcomes across all time points.
Results:
Both the number and severity of chronic difficulties (but not acute life events) were related to significantly greater depression, and poorer sleep quality and QOL, across all time-points. In contrast, these stress indices were unrelated to changes in psychosocial functioning over time.
Conclusions:
Chronic but not acute stress exposure predicted average levels of depression, sleep, and QOL in the first year post-diagnosis among women with ovarian cancer. Assessing stressors and designing interventions for reducing stress may thus be beneficial for ovarian cancer patients.
Keywords: cancer, depression, oncology, ovarian neoplasms, psychological, psychosocial functioning, psycho-oncology, quality of life, sleep, stress
1 |. BACKGROUND
Ovarian cancer is the second most common gynecologic cancer and is responsible for the most gynecologic cancer deaths per year.1 Factors such as poor prognosis, rigorous treatment regimens, and treatment side effects contribute to sustained sleep impairments, depression, and compromised quality of life (QOL) in women with ovarian cancer.2,3 We have previously reported that more than 58% of patients report disturbed sleep one-year postdiagnosis and that clinical levels of depression are evident in many patients at one-year postdiagnosis.3
Life stress is known to have substantial effects on health4 and has been considered a key factor that may shape QOL and psychosocial outcomes of cancer patients.5,6 Recent life stress has well-established effects on sleep7,8 and depression9,10 in non-cancer populations, and has been related to depression and QOL in women with breast cancer11 and those with cancer of heterogeneous primary sites.12 Severe life events, as measured by the Life Events and Difficulties Schedule (LEDS), have been shown to predict poorer immune response to tumor in patients with basal cell carcinoma.13 To date, however, no studies have used the LEDS to differentiate the effects of acute versus chronic stressors on psychological and QOL outcomes in cancer patients.
Life stress has been examined from a variety of perspectives.14 Acute life events are stressors that occur over 1–2 days, with longer-term implications unfolding over 10–14 days.15 They include stressors happening both to the participant (e.g., job loss) and close others (e.g., spouse’s job loss). Chronic difficulties are stressors that persist for a minimum of 4 weeks (e.g., persistent financial or marital problems15). Stressors have been measured in various ways, including using self-report checklist measures and interview-based systems.16 Semi-structured interviews provide the most detailed accounts of the nature, impact, and overall meaning of stressors by obtaining contextual details of each stressor experienced, as well as relevant biographical details that might shed light on the individual meaning and impact of a stressor.14 One such semi-structured interview, the LEDS,17 is considered the gold standard for stress measurement due to the operationalized definitions that help investigators comprehensively characterize stressor exposures that may impact health.
We previously reported that among women with ovarian cancer, non-cancer stressors (assessed by self-report) in the year pre-surgery were not related to psychosocial factors assessed around the time of surgery; however, a greater number and severity of stressors at the time of surgery were prospectively associated with poorer QOL 1-year post-diagnosis.18 Early-stage ovarian cancer survivors reporting fewer recent stressful life events (assessed using self-report) reported better mental health than those experiencing more stressful life events.19 However, the differential impact of stressors of varying durations and severity has not been investigated in this population using the LEDS or a similar high-resolution instrument.
To address these issues, we examined how recent acute and chronic life stress exposure (measured by the LEDS) was related to changes in depressive symptoms, sleep quality, and QOL in women with ovarian cancer over time. We hypothesized that women who experienced a greater number and severity of acute life events and chronic difficulties during the year prior to their diagnosis would report worse depression, sleep quality, and QOL. Furthermore, we hypothesized that women with greater life stress exposure would exhibit less improvement in depression, sleep, and QOL during the first-year post-diagnosis.
2 |. METHODS
2.1 |. Participants
Women were recruited as part of a larger biobehavioral study in patients with epithelial ovarian cancer. Potential participants presented at gynecologic oncology clinics at the University of Iowa Hospitals and Clinics (UIHC) and Washington University for assessment of a pelvic mass suspicious for ovarian cancer. Women were eligible for inclusion if they were ≥18 years old and had a tumor that was ultimately histologically diagnosed as epithelial cancer of the ovaries, fallopian tubes, or peritoneum. Exclusion criteria included severe cognitive impairment, use of systemic corticosteroid medications within the last month, current pregnancy, medical conditions with known effects on the immune system, and cancer of another primary site within the last 5 years. Women receiving either upfront surgery or neoadjuvant chemotherapy prior to interval debulking were eligible. Participants were contacted to complete the life stress interview approximately a month post-surgery or study enrollment (if receiving neoadjuvant chemotherapy). Surveys were completed prior to upfront surgery or neoadjuvant chemotherapy and approximately 6 months and 1-year post-diagnosis. This study was approved by IRBs at both study sites (Washington University IRB #201104242; UIHC IRB #200308061). All participants provided written informed consent.
2.2 |. Measures
2.2.1 |. Recent life stress
The LEDS17 assesses stressors occurring across 10 life domains (e.g., education, work, deaths). Trained raters independently judge each stressor’s specific characteristics as specified by a 500-page manual containing more than 5000 pre-rated case vignettes.14,16 These procedures minimize the influence of selective recall and reporting biases on stress assessment.20
For this study, questions were cued to the year prior to diagnosis to capture the stress exposure of women coming into their cancer diagnosis. The interview, lasting 1.5–2.0 hours per participant, obtains extensive factual information about each stressor and its unique biographical context. Acute life events and chronic difficulties were rated separately. Interviews were conducted by L. Garvin and two other graduate-level interviewers who were trained and supervised by G. M. Slavich. Interviews were conducted and summarized at both clinical sites and presented to two to four expert LEDS raters at UCLA, who were “blind” to the clinical status of participants. Each rater provided an independent contextual threat (i.e., severity) rating on a scale from 1 (little-to-no-threat) to 4 (severe threat) for events and 1 (low some) to 6 (high marked) for difficulties, with greater threat indicating more significant implications for the participant’s goals, plans, and aspirations for the future given her unique biographical history and the contextual features of the stressor.15 Rating inconsistences were discussed and resolved using consensus discussions. Interrater agreement for the severity ratings was very good (κ = 0.89). For analyses, we used the four main LEDS indices of recent life stress exposure number and severity of acute life events and chronic difficulties during the year prior to diagnosis.
2.2.2 |. Depression
Depressive symptoms were assessed using the Center for Epidemiological Studies-Depression Scale,21 a 20-item self-report measure assessing mood symptoms over the prior week. Higher scores indicate greater depressive symptoms, with a cut-off score of ≥16 indicating clinical depression.21
2.2.3 |. Sleep
The Pittsburgh Sleep Quality Index (PSQI) includes 19 items assessing type and frequency of sleep disturbances over the prior month. A global sleep score is obtained by summing seven component scores; a cut-off score of greater than five discriminates those with disturbed sleep. The PSQI has high internal consistency (α = 0.83) and correlates strongly with objective sleep measures, such as polysomnography.22 Sleep data were collected for women at UIHC but not Washington University.
2.2.4 |. QOL
The Functional Assessment of Cancer Therapies (FACT) provides a global assessment of QOL over the past week, derived from component scores reflecting physical, emotional, functional, and social domains of life.23 Higher scores indicate better QOL. The FACT demonstrates good reliability and adequate validity.24
2.2.5 |. Demographic and clinical variables
Sociodemographic information including age, race, ethnicity, education, employment, marital status, and income were completed by self-report. Clinical information was obtained from medical records.
2.3 |. Data analysis
Data analyses were conducted using SPSS (V25.0). Distributions were examined for outliers and violations of assumptions of normality. All longitudinal models included stage, age, site, antidepressant use, and chemotherapy at one year (receiving vs. not receiving chemotherapy) as a priori covariates given their potential impact and clinical significance in cancer. Because grade and stage were highly correlated (p < 0.001), only stage was included in final models. Potential covariates (education, employment status, body mass index [BMI], racial and ethnic status, income, and relationship status) were tested for associations with outcome variables and included in final models if significant. As sleep outcomes were only examined at UIHC, site was not included in models testing sleep.
Longitudinal analyses used the SPSS MIXED procedure for mixed effect models. This procedure allows for estimation of parameters missing data using maximum likelihood methods, which produces more reliable estimates than list-wise deletion. This analytic approach takes baseline values into account to estimate change over time and main effects. The life stress predictor variables (i.e., acute events or chronic difficulties) were entered into models as continuous variables. For longitudinal models, predictor variables were those representing the life stress × time interaction and the main effect of life stress across all time points (categorical baseline, 6 months, 12 months). The life stress × ← time interaction tested whether trajectories in psychosocial outcomes differed by stress exposure. First, interaction effects were examined; if interaction effects were not significant, they were eliminated from models and main effects were examined. Main effect terms tested whether life stress was associated with psychosocial outcomes averaged across the three time points. A diagonal covariance structure was used in models as indicated by best fit.25
3 |. RESULTS
3.1 |. Participant characteristics
Of 196 eligible women, 137 (76 from UIHC, 61 from Washington University) enrolled and completed the LEDS (Figure 1). Incomplete LEDS interviews (n = 59) were primarily due to declined interviews. There were no significant differences between those who did and did not complete the LEDS with regard to cancer stage, grade, histopathology, race or ethnic category, age, relationship status (p > 0.38), depressive symptoms (p > 0.23), or sleep (p > 0.06) at any time point. Women who completed the LEDS reported significantly higher income (p < 0.001) and pretreatment QOL (p = 0.04) but did not differ in their QOL at 6 months or 1 year (p > 0.32).
FIGURE 1.
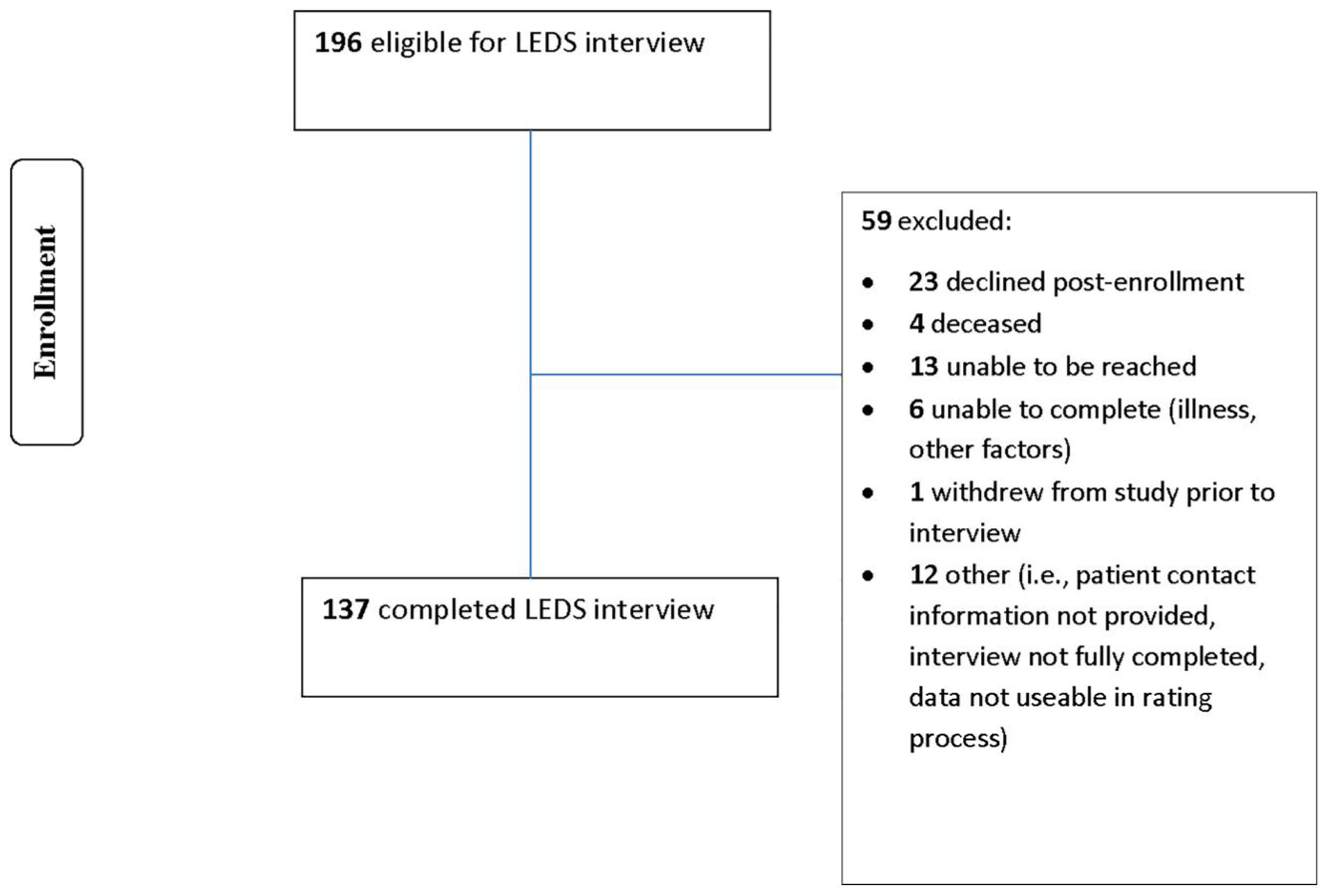
LEDS interview consort chart. LEDS, Life Events and Difficulties Schedule
As described in Table 1, the majority of women had advanced stage (73.8%) and high grade (89.1%) ovarian cancer. Participants were primarily non-Hispanic Caucasian and average age was 59.1 (±0.9) years. Over one-third (37.1%) had completed college; women were most commonly married or cohabitating (67.9%). Of potential covariates tested (listed above), only BMI had significant associations with outcomes (p < 0.05) and was therefore included in analyses along with a priori covariates.
TABLE 1.
Patient characteristics
| Characteristic | M (SD) or % |
|---|---|
| Age, years | |
| Mean (SD) | 59.12 (10.86) |
| Ethnicity | |
| Non-Hispanic | 99.20% |
| Race | |
| Asian | 0.70% |
| Black/African American | 3.00% |
| White | 96.30% |
| Education | |
| Less than high school graduate | 2.90% |
| High school graduate | 31.90% |
| Trade school/some college | 28.20% |
| College graduate | 25.20% |
| Postgraduate | 11.90% |
| Income | |
| <10,000 | 6.10% |
| 10,001–30,000 | 28.90% |
| 30,001–50,000 | 21.00% |
| 50,001–80,000 | 22.80% |
| >80,000 | 21.10% |
| Marital status | |
| Single | 13.10% |
| Divorced/separated | 13.10% |
| Widowed | 5.80% |
| Married/living with partner | 67.90% |
| Cancer stage | |
| I | 18.40% |
| II | 7.40% |
| III | 65.40% |
| IV | 8.80% |
| Grade | |
| High grade | 89.10% |
| Tumor histology | |
| Serous | 69.10% |
| Nonserous | 30.90% |
| Neoadjuvant status | |
| Neoadjuvant | 5.80% |
| Primary surgical therapy | 94.20% |
| Surgical debulking | |
| Optimal | 74.20% |
| Chemotherapy status—1 year | |
| Receiving chemotherapy | 16.70% |
Women recruited from Washington University reported more recent acute life events (F1,135 = 6.51, p = 0.01), and chronic difficulties (F1,135 = 27.75, p < 0.001), and had greater total severity of chronic difficulties (F1,135 = 20.82, p < 0.001) than those from UIHC. Furthermore, they reported a slightly lower annual household income (F1,112 = 3.91, p = 0.05). There were no significant differences between study sites regarding depressive symptoms (p > 0.14) or QOL (p > 0.07) at any time point. Income was marginally related to the severity of chronic difficulties (r = −0.18, p = 0.06), but not to any other LEDS indices of recent life stress (p > 0.16).
3.2 |. Life stress exposure and psychosocial outcomes
Descriptive information regarding stress exposure at baseline and psychosocial outcomes at each time point is provided in Table 2. Participants experienced an average of 5.10 (±2.18; range 2–13) acute life events and 3.00 (±1.80; range 0–7) chronic difficulties during the year prior to diagnosis. At baseline, 39.8% of women reported depressive symptoms that were at or above the CES-D cut-off for clinical depression.21 These rates decreased to 21.1% and 21.0%, respectively, at 6 months and 1 year. Approximately half of the sample reported consistently disturbed sleep (PSQI > 5) during the first-year postdiagnosis, including 56.9% of women at baseline and 48.1% at 1 year. Mean QOL improved over time but remained within a compromised range.26
TABLE 2.
Means and standard deviations of psychosocial outcomes at three timepoints
| Pre-surgery | Six Months | One Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure | N | M | SD | N | M | SD | N | M | SD |
| Number of acute life events | 137 | 5.10 | 2.18 | ||||||
| Severity of acute life events | 137 | 26.51 | 9.67 | ||||||
| Number of chronic difficulties | 137 | 3.01 | 1.80 | ||||||
| Severity of chronic difficulties | 137 | 8.28 | 5.79 | ||||||
| Depressive symptoms (CES-D) | 133 | 16.11 | 10.47 | 114 | 11.01 | 9.03 | 100 | 9.49 | 9.06 |
| Sleep quality (PSQI) | 72 | 7.36 | 3.98 | 61 | 6.40 | 3.50 | 52 | 6.79 | 4.63 |
| Quality of life (FACT) | 131 | 74.43 | 16.68 | 109 | 80.11 | 12.47 | 92 | 83.95 | 14.53 |
Abbreviations CES-D, Center for Epidemiological Depression Scale; FACT, Functional Assessment of Cancer Therapies; PSQI, Pittsburgh Sleep Quality Index.
3.3 |. Acute life events and psychosocial outcomes
The interaction effect for acute life events and time was not significant for any psychosocial outcome (number ps > 0.26; severity ps > 0.58). Because there was no significant interaction, we examined the main effect of acute life events on each psychosocial outcome. There was no main effect of acute events on levels of depression, sleep, or QOL (number of events ps > 0.10; severity of events ps > 0.09).
3.4 |. Ongoing difficulties and psychosocial outcomes over time
The interaction effect for number of chronic difficulties and time on psychosocial outcome variables was not significant (ps > 0.43). Similar findings were found for severity of chronic difficulties (ps > 0.20). The non-significant interaction indicates that participants with more difficulties showed roughly the same relative level of change in the year following surgery as did those with fewer chronic difficulties.
In the absence of a significant interaction, we tested the main effect of chronic difficulties and found a significant main effect for both number and severity of chronic difficulties on key symptom outcomes across all time points. Patients with a greater number of chronic difficulties in the year prior to surgery reported significantly worse depressive symptoms (F1,98 = 9.18, p = 0.003), sleep quality (F1,47 = 4.18, p < 0.05), and QOL (F1,99 = 16.41, p < 0.001) over the one-year study period. Similarly, greater severity of chronic difficulties was associated with significantly worse depressive symptoms (F1,100 = 10.89, p = 0.001) and QOL (F1,100 = 18.44, p < 0.001), and a trend for poorer sleep quality (F1,46 = 4.00, p = 0.05) during this one-year period. These findings indicate that patients experiencing more chronic stress during the year prior to treatment had worse psychosocial functioning averaged across all time points. To illustrate impact on key symptoms, Figure 2A,B shows mean at each time point for a hypothetical (i.e., estimated) patient experiencing zero versus two chronic difficulties. For full final statistical models (see Tables S1 and S2).
FIGURE 2.
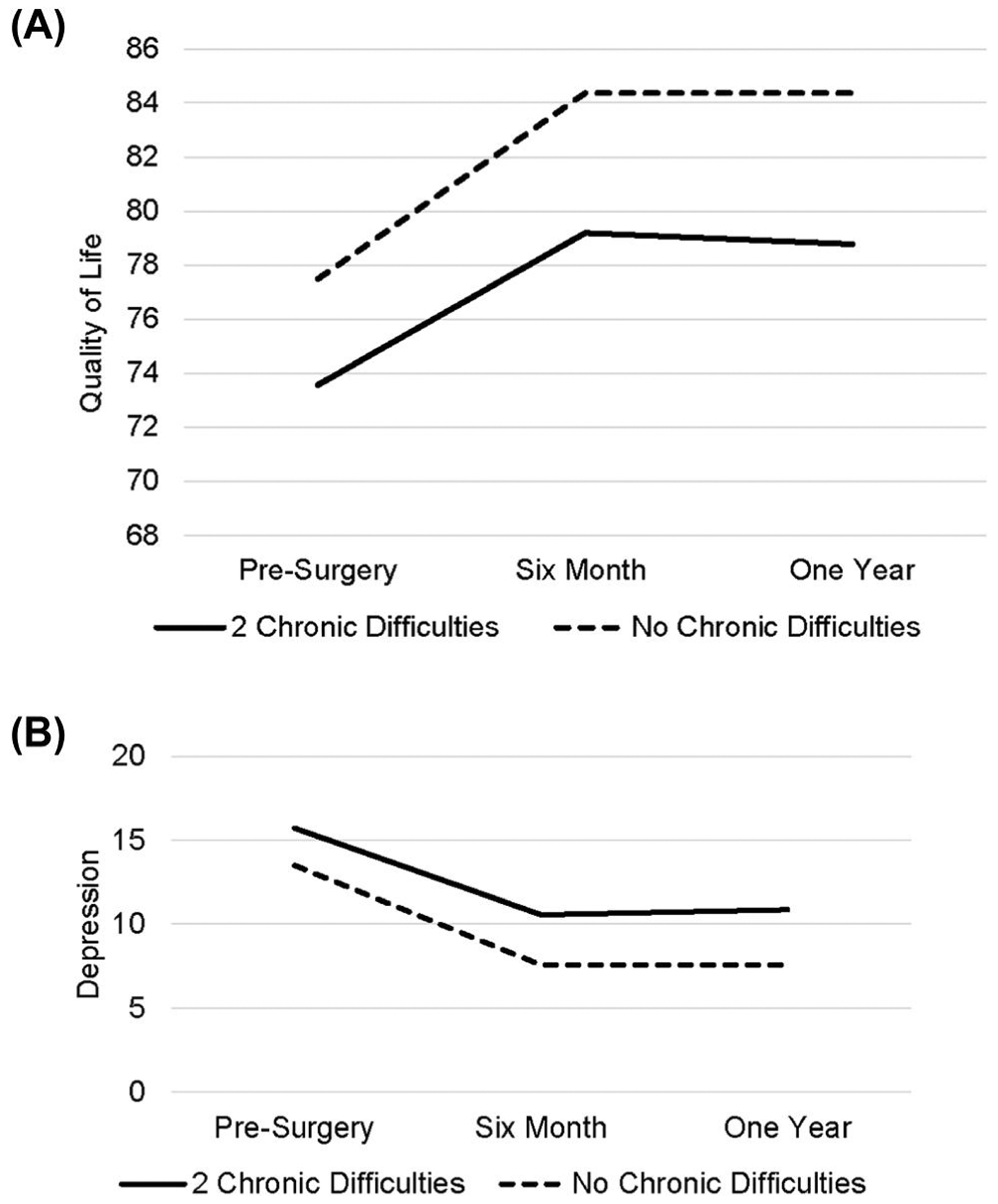
Illustration of estimated (A) QOL and (B) depression for a hypothetical 50-year-old patient, with stage III disease, not receiving antidepressants or chemotherapy at 1 year. Values are shown at each time point for such a patient who experienced two versus no chronic difficulties. QOL, quality of life
4 |. DISCUSSION
Although prior research has examined the role that life stress plays in cancer, few studies have included a longitudinal component or employed instruments for assessing life stress exposure that provide high-resolution stress data that are not confounded with the outcomes under study (e.g., depressive symptoms). We addressed these issues in the present investigation by utilizing the LEDS for assessing life stress in the context of a one-year prospective study examining how recent stress exposure influences depressive symptoms, sleep quality, and QOL in women with ovarian cancer during the first-year post-diagnosis. These women experienced an average of five acute life events and three chronic difficulties. To contextualize these results, a non-cancer, clinical sample of women with major depression reported an average of 2.83 life events, as assessed by the LEDS, in the 6 months prior to diagnosis.27 Life stress was strongly associated with these key outcomes; however, effects differed substantially by the specific types of stress experienced. Specifically, we found that chronic difficulties (but not acute life events) were related to greater depressed mood and worse sleep quality and QOL across all time points, but were not related to trajectories of change in these outcomes. In this study, a consistent pattern suggested that chronic stress may be more relevant than acute stress for shaping psychosocial functioning in women with ovarian cancer during the first-year postdiagnosis.
It should be noted that acute life events and chronic difficulties may be interrelated. For example, acute life events can initiate chronic difficulties (e.g., getting fired can start an ongoing financial difficulty) and chronic difficulties can, in turn, give rise to acute life events (e.g., ongoing marital difficulties can initiate loss of income). However, the LEDS enables a more nuanced examination of differential effects. Furthermore, these findings are consistent with a broader literature indicating that chronic stressors can have a greater impact on depression than acute life events.14,28 In the present study, the effects of ongoing difficulties may eclipse those of acute events in the context of cancer diagnosis and treatment, which itself serves as an ongoing chronic stressor. This interpretation is consistent with the concept of a “saturation effect” in which individuals managing high levels of chronic stress may be less impacted by the effects of acute events.29
Chronic difficulties may uniquely influence health via multiple pathways. For example, such difficulties may lead to repeated activation of physiological stress response systems; this has been described as one mechanism contributing to “allostatic load,” or the resulting “wear and tear” on the body or brain resulting from repeated adaptation to challenges.30 Chronic difficulties may also divert time, energy, and resources and leave women more vulnerable to greater depression, poorer sleep, and poorer QOL when navigating their cancer diagnosis. Furthermore, chronic stress is strongly associated with biological processes, including HPA-axis dysregulation and inflammation, which may in turn impact the outcomes studied.10,31,32 It is also possible that chronic stressors may be a marker of cumulative life stress burden.33 For example, individuals may be “locked into” highly stressful family environments characterized by discord, or socioeconomically disadvantaged environments, which confer risk for greater lifetime chronic stress.34
4.1 |. Study limitations
Study limitations include a sample predominantly composed of Caucasian, non-Hispanic, and well-educated women with advanced cancer; therefore, future studies incorporating greater diversity with respect to demographic and disease variables would help increase the generalizability of the results. Second, we did not examine additional aspects of the stress experience (e.g., controllability, nature of the stressor) that may have had differential associations with the outcomes studied. Third, although the LEDS is specifically designed to limit the influence of self-reporting biases, such characteristics still could have affected the results. Finally, we assessed stressors occurring only over the prior year, and stressor duration was examined independent of initiating or perpetuating factors. Future studies could thus focus on a wider timeframe of pre-diagnosis stressors or indices of post-diagnosis stress exposure.
4.2 |. Clinical implications
The present findings suggest that assessment and intervention in patients experiencing chronic difficulties could impact major psychosocial outcomes for women navigating the experience of ovarian cancer diagnosis and treatment. In cancer centers, conducting needs assessment using screening tools is an appropriate step to facilitate referrals for distress and unmet needs.35 Assessing chronic difficulties may enable providers to better screen and coordinate services to provide concrete assistance to help ease underlying chronic stress. Stress management interventions such as mindfulness-based stress reduction, cognitive behavioral stress management, and yoga have shown positive effects on psychological outcomes including sleep, depression, QOL, and stress relief36–38 in cancer populations.
Although the LEDS is an ideal instrument for assessing life stress,14 the time burden involved in interviewing and rating makes it unpractical for clinical settings. Other more time efficient and scalable approaches for assessing stress are available. One such instrument, the Stress and Adversity Inventory (STRAIN39), uses a 20-minute online interview to assess lifespan exposure to both acute and chronic stressors. Moreover, the STRAIN has been validated in both cancer10,40 and non-cancer samples.4,39
5 |. CONCLUSIONS
These data are the first to show that greater number and severity of chronic difficulties prospectively predicted worse depression, sleep quality, and QOL in the year post-diagnosis in ovarian cancer patients. Several unique strengths (including use of a high-resolution interview to assess stress exposure; employing independent raters to help ensure that the stress assessment was not confounded with the outcomes assessed; and the prospective, longitudinal nature of the study that enabled us to examine temporal associations that cannot be investigated using a cross-sectional design) characterized this study, which highlights the importance of evaluating stress using high-quality instruments that can differentiate acute versus chronic stressors. Identifying women experiencing chronic difficulties in particular may help clinicians provide targeted and timely practical support and psychological interventions to enhance their health and well-being.
Supplementary Material
ACKNOW EDGMENTS
This work was supported by National Cancer Institute (Grant no. CA193249, CA246540; S. L.); a Society in Science-Branco Weiss Fellowship, NARSAD (Grant no. 23958) from the Brain & Behavior Research Foundation, and NIH (Grant no. K08MH103443; G. M. S.); and by grant P30CA086862 (Weiner). The authors thank Jen Bayer, Katie Collins, Annie White, and Keely Muscatell for assisting with the LEDS, and women who participated in this study.
Funding information
Brain and Behavior Research Foundation; U.S. Department of Health and Human Services; National Institutes of Health; Branco Weiss Fellowship – Society in Science
Footnotes
CONFLICT OF INTERESTS
Dr. Thaker has done consulting and/or speaking for Stryker, Iovance Biotherapeutics, Abbvie/Stemcentrx, Clovis Oncology, Unleash Immunolytics, Celsion, Merck, Glaxo Smith Kline and Astra Zeneca, has research funding from Merck and Glaxo Smith Kline, and is a Celsion shareholder; Dr. Sood has done consulting for Merck and Kiyatec, has had research funding from M-Trap, and is a Biopath shareholder. Other authors declare no confict of interests.
DATA AVAILABI ITY STATEMENT
Data supporting the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA A Cancer J Clin. 2020;70 7–30. [DOI] [PubMed] [Google Scholar]
- 2.McCorkle R, Pasacreta J, Tang ST. The silent killer psychological issues in ovarian cancer. Holist Nurs Pract. 2003;17 300–308. [DOI] [PubMed] [Google Scholar]
- 3.Clevenger L, Schrepf A, DeGeest K, et al. Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer. 2013;119 3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toussaint L, Shields GS, Dorn G, Slavich GM. Effects of lifetime stress exposure on mental and physical health in young adulthood how stress degrades and forgiveness protects health. J Health Psychol. 2016;21 1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu D, Andrae B, Valdimarsdóttir U, et al. Psychologic distress is associated with cancer-specific mortality among patients with cervical cancer. Cancer Res. 2019;79 3965–3972. [DOI] [PubMed] [Google Scholar]
- 6.Lutgendorf SK, Slavich GM, DeGeest K, et al. Non-cancer life stressors contribute to impaired quality of life in ovarian cancer patients. Gynecol Oncol. 2013;131 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall M, Buysse DJ, Nowell PD, et al. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosom Med. 2000;62 227–230. [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65 259–267. [DOI] [PubMed] [Google Scholar]
- 9.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156 837–841. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Crosswell AD, Slavich GM. Childhood adversity and cumulative life stress. Clin Psychol Sci. 2014;2 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden-Kreutz DM, Andersen BL. Depressive symptoms after breast cancer surgery relationships with global, cancer-related, and life event stress. Psychooncology. 2004;13 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lehto U-S, Ojanen M, Väkevä A, Aromaa A, Kellokumpu-Lehtinen P. Noncancer life stresses in newly diagnosed cancer. Support Care Cancer. 2008;16 1231–1241. [DOI] [PubMed] [Google Scholar]
- 13.Fagundes CP, Glaser R, Johnson SL, et al. Basal cell carcinoma stressful life events and the tumor environment. Arch Gen Psychiatry. 2012;69 618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annu Rev Clin Psychol. 2008;4 33–52. [DOI] [PubMed] [Google Scholar]
- 15.Brown GW, Harris TO. Life Events and Illness. New York, NY: Guilford Press; 1989. [Google Scholar]
- 16.Slavich GM. Stressnology the primitive (and problematic) study of life stress exposure and pressing need for better measurement. Brain Behav Immun. 2019;75 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GW, Harris T. Social origins of depression a reply. Psychol Med. 1978;8 577–588. [DOI] [PubMed] [Google Scholar]
- 18.Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30 S19–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matulonis UA, Kornblith A, Lee H, et al. Long-term adjustment of early-stage ovarian cancer survivors. Int J Gynecol Cancer. 2008;18 1183–1193. [DOI] [PubMed] [Google Scholar]
- 20.Monroe SM, Slavich GM. Major life events a review of conceptual, definitional, measurement issues, and practices. In Harkness KL, Hayden EP, ed. The xford Handbook of Stress and Mental Health. Oxford Oxford University Press; 2020. [Google Scholar]
- 21.Radloff LS. The CES-D scale. Appl Psychol Meas. 1977;1 385–401. [Google Scholar]
- 22.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28 193–213. [DOI] [PubMed] [Google Scholar]
- 23.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale development and validation of the general measure. J Clin Oncol. 1993;11 570–579. [DOI] [PubMed] [Google Scholar]
- 24.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy- ovarian. J Clin Oncol. 2001;19 1809–1817. [DOI] [PubMed] [Google Scholar]
- 25.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19 1793–1819. [DOI] [PubMed] [Google Scholar]
- 26.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harkness KL, Alavi N, Monroe SM, Slavich GM, Gotlib IH, Bagby RM. Gender differences in life events prior to onset of major depressive disorder the moderating effect of age. J Abnorm Psychol. 2010;119 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennant C Life events, stress and depression a review of recent findings. Aust N Z J Psychiatry. 2002;36 173–182. [DOI] [PubMed] [Google Scholar]
- 29.Cairney J, Boyle M, Offord DR, Racine Y. Stress, social support and depression in single and married mothers. Soc Psychiatry Psychiatr Epidemiol. 2003;38 442–449. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Protection and damage from acute and chronic stress allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004; 1032 1–7. [DOI] [PubMed] [Google Scholar]
- 31.Ferrell B, Smith SL, Cullinane CA, Melancon C. Psychological well being and quality of life in ovarian cancer survivors. Cancer. 2003;98 1061–1071. [DOI] [PubMed] [Google Scholar]
- 32.Heim C, Binder EB. Current research trends in early life stress and depression review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233 102–111. [DOI] [PubMed] [Google Scholar]
- 33.Epel ES, Crosswell AD, Mayer SE, et al. More than a feeling a unified view of stress measurement for population science. Front Neuroendocrinol. 2018;49 146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammen C Stress and depression. Annu Rev Clin Psychol. 2005;1 293–319. [DOI] [PubMed] [Google Scholar]
- 35.Carlson LE, Waller A, Mitchell AJ. Screening for distress and unmet needs in patients with cancer review and recommendations. J Clin Oncol. 2012;30 1160–1177. [DOI] [PubMed] [Google Scholar]
- 36.Carlson LE, Garland SN. Impact of mindfulness-based stress reduction (MBSR) on sleep, mood, stress and fatigue symptoms in cancer outpatients. Int J Behav Med. 2005;12 278–285. [DOI] [PubMed] [Google Scholar]
- 37.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors. Cancer. 2012;118 3766–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitive-behavioral stress management for women with breast cancer 11-year follow-up of a randomized controlled trial. Cancer. 2015;121 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slavich GM, Shields GS. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN) an overview and initial validation. Psychosom Med. 2018;80 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuneo MG, Schrepf A, Slavich GM, et al. Diurnal cortisol rhythms, fatigue and psychosocial factors in five-year survivors of ovarian cancer. Psychoneuroendocrinology. 2017;84 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


