Abstract
The nucleus reuniens (RE) of the ventral midline thalamus is strongly reciprocally connected with the hippocampus (HF) and medial prefrontal cortex (PFC), serving a critical role in affective and cognitive functioning. While midline thalamic nuclei have been implicated in the modulation of states of arousal and consciousness, few studies have addressed RE’s role in behavioral state control. Accordingly, as a first line of investigation, we examined the discharge properties of RE neurons in behaving rats throughout the sleep-wake cycle. We analyzed 153 units in RE which demonstrated heterogeneity in discharge rates and pattern of activity across sleep wake states. Using a rate ratio of activity in wake vs. REM, we found that the majority of cells displayed state-related changes and were classified into distinct cell types, exhibiting their highest discharge rates during active waking (AW), REM sleep, or maintaining equivalent activity across AW/REM. We further distinguished cells as either slow firing (SF = < 10 Hz) or fast firing (FF = >10 Hz) cells. The majority of cells, independent of state-related preference, were SF. FF RE cells were primarily wake active and wake/REM cell types. This diverse set of RE neurons are likely modulated by key brainstem and hypothalamic nuclei, which in turn, drive RE to exert strong effects on its cortical targets during waking and REM sleep. RE may not only act as a node in HF-PFC circuitry, but also as a critical thalamic link in ascending arousal and attentional networks.
Keywords: hippocampus, medial prefrontal cortex, arousal, attention, paraventricular nucleus of thalamus, cognition
The nucleus reuniens (RE) of the ventral midline thalamus is the largest of the midline nuclei in the rat, spanning approximately the anterior two-thirds of the thalamus. As now well established, RE is strongly reciprocally connected with the hippocampal formation (HF) and the medial prefrontal cortex (mPFC), representing a major interface between these structures. Accordingly, several reports have described a direct involvement of RE in functions associated with the HF, the mPFC or their interactions -- such as cognition, working memory, and executive behaviors (for review [1–4]).
While RE’s role in cognition has been extensively examined, much less attention has been devoted to its involvement in processes of arousal or attention. This is perhaps surprising since the midline and intralaminar nuclei of the thalamus have long been recognized as an integral part of an ascending brainstem-thalamo-cortical “activating” system [1, 5]. Despite this, very few reports have examined the activity of midline/intralaminar cells in awake animals across sleep-waking states. In one such early study, however, Glenn and Steriade [6] described the discharge properties of a subset of cells of the central lateral (CL) intralaminar nucleus in behaving cats which were activated with midbrain reticular formation stimulation and antidromically driven with cortical stimulation. These cells fired at high rates of activity during both waking (W) and REM (or desynchronized, D) sleep, and very low rates during non-REM or slow wave sleep (SWS) ‐‐ leading them to conclude that this pattern of discharge fits well “with a role in the tonic activation processes that characterize the W and D states” [6].
With respect to RE, previous studies described differential c-fos expression of RE/midline thalamic nuclei across sleep/wake states [7–9]. For instance, Cirelli and Tononi [8] demonstrated significantly greater c-fos expression in nuclei of the midline thalamus (PV, RH, RE) during spontaneous or forced waking than during sleep, while Sastre et al. [9] described significantly more c-fos immunopositive cells in RE in REM sleep compared to waking or NREM sleep.
While no previous report has examined the activity of RE neurons in behaving animals across states of vigilance, a few studies have described the discharge properties of RE cells in behaving rats during certain tasks or conditions such as spatially tuned [10] and “trajectory-dependent” cells [11]. To provide a fundamental understanding of the discharge characteristics of RE cells across naturally occurring states, we examined the activity of single RE neurons during sleep-wake states in behaving rats. We expected that some RE neurons might exhibit properties similar to CL cells [6], that is, firing at enhanced rates during states of cortical EEG arousal. We found that the majority of RE neurons discharged at high rates of activity during both waking and REM sleep compared to significantly reduced rates in slow-wave sleep. RE cells were further subdivided into distinct classes based on differing patterns of activity across active waking and REM sleep. We discuss the functional implications of these patterns of discharge of RE neurons.
Methods
Animals
Sprague-Dawley male rats (n=13; Charles River, Wilmington, MA; Harlan/Envigo, Dublin, VA) weighing 275-325 grams at the time of arrival were housed individually in a climate-controlled environment on a 12/12hr off light cycle. Access to food, water, and enrichment was available ad libitum. These experiments were approved by the Florida Atlantic University IACUC and conform to all federal regulations and NIH guidelines for the care and use of laboratory animals.
Surgical Procedures
Rats were anesthetized with isoflurane (4-5% induction, 1.5-3% maintenance), placed in a stereotaxic frame and anesthetized locally with an injection of 0.5% Marcaine along the incision site. Two burr holes were drilled over the midline thalamus with ~1.0 mm anterior/posterior separation (stereotaxic coordinates: −1.6/−1.7 and −2.6/−2.7 mm AP; +0.4 mm L; 6.0 – 7.0 mm DV) for implantation of a custom-built microelectrode assembly (modified from Stackman et al. [12]) containing two tetrodes targeting anterior and mid-levels of RE. A third burr hole was positioned over the dorsal hippocampus (coordinates: −3.5 mm AP; −3.0 mm L; 3.0 mm DV) for implanting a twisted bipolar electrode (125 um, MedWire, Mt. Vernon, NY) to record hippocampal LFP. A stainless steel screw was fastened to the skull over the frontal cortex to record cortical EEG and a ground screw was attached to the skull over the parietal cortex. Bone screws and SS clips anchored the microelectrode which was secured with dental cement. The incision site was sealed with tissue glue and monofilament sutures. A single s.c. injection of Buprenorphine SR (0.6mg/kg) was given to rats for postsurgical analgesia. Rats were allowed at least 1 week of postsurgical recovery before experimental procedures began.
Recording sessions
All recording sessions were conducted in a 26” W x 30” L x 12” H faraday enclosure. Rats were acclimated to the testing apparatus and procedures prior to the start of daily (4-5x weekly) recording sessions. During each session, the rats’ electrode assembly was connected to a tethered cable where rats could freely move in the enclosure and retrieve chocolate pellets (20 mg, Bioserve, Flemington, NJ) during wake periods. If no units were observed on any channel, the tetrode was lowered ~1/8 of turn (~60 um) until cells were detected. When single cells were identified, rats were sleep deprived overnight using the flowerpot technique [13] to augment REM sleep [14]. Rats were recorded for 3-4 hours on the following day as they cycled through sleep/wake cycles.
Acute Sleep Deprivation
Rats were sleep deprived for a period of 12-18 hours using the inverted flowerpot technique [13,14] prior to sleep recording sessions. Rats were placed on an inverted flowerpot (6” diameter) in the center of a cylindrical container (12” diameter x 15” h) which was filled with 2” of water and rats had access to both food and water throughout the duration of sleep deprivation. Once rats were removed from the chamber, they were placed in their home cage with access to food/water for 30 minutes before recordings began. There was a minimum of 3 days between each sleep deprivation session and deprivations were limited to a maximum of 3 per rat.
Electrophysiological recordings
Extracellular signals (RE units, hippocampal LFP and cortical EEG) were relayed from the assembly to a 16-channel headstage (Plexon, Dallas, TX), mounted onto a shielded cable connected to a commutator, and from there to amplifiers for pre-amplification (1000x). Signals were filtered (RE units, .03 – 10 kHz; hippocampus, 3-100 Hz; cortex, .01-300 Hz), digitized, (sampling rates, 200 – 22 kHz), stored and analyzed using Spike 2 of Cambridge Electronic Design (CED, Cambridge, UK) data acquisition and analysis system. The behavior of rats was recorded using an overhead video camera (c920, Logitech). Spike waveforms, sampled at 22 kHz, were sorted based on spike amplitude and shape using the Spike 2 software template matching algorithm. When sorting multi-unit signals on a channel, the clustering of waveform templates was verified through principal component analysis, which calculates the distance between each spike waveform cluster centroid emphasizing the differences between spike classes (K means), to ensure that detected spikes were distinct from other waveforms within each of the channels.
Sleep-wake states
Sleep-wake stages were first presorted and identified using a custom-made script in Spike2 [15]). Stages were then visually inspected and manually verified by a trained coder using hippocampal LFP and cortical EEG, with respective power spectra (FFT, Spike 2 software) -- coupled to the video recordings (Fig. 1A). Active wake (AW) was identified by: (1) desynchronized low-amplitude cortical EEG activity; (2) a predominant theta band (6-10 Hz) component in the hippocampal LFP; and (3) active exploratory or consummatory behaviors. Slow-wave sleep (SWS) was characterized by: (1) high-amplitude slow waves (1.5–4 Hz; delta) in the cortical EEG; (2) mixed, non-synchronous (irregular) activity in the hippocampal LFP; and (3) immobility with a sleep posture with rat’s eyes closed. Rapid-eye-movement (REM) sleep was identified by: (1) low-voltage cortical EEG activity; (2) continuous theta band activity (6-10 Hz) of the hippocampal LFP; and (3) immobility with a sleep posture and eyes closed of the rat. Mean discharge rates were calculated using 10 s bins for AW and SWS and 5 or 10 s bins for REM sleep.
Figure 1.
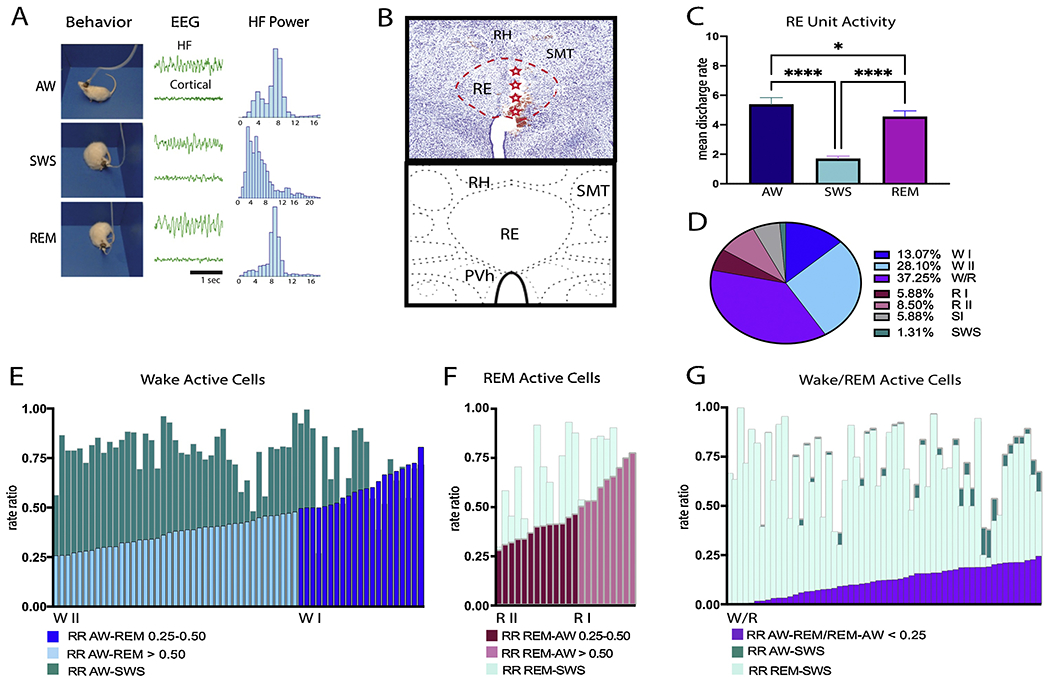
A: Awake-sleep state periods were identified and confirmed using the following parameters: cortical EEG, hippocampal (HF) LFP and recorded behavior. Left: Video snapshots demonstrating an example of a rat’s behavior during active wake, slow wave sleep and REM states during one of the recording sessions. Middle: HF LFP (top) and cortical EEG activity corresponding to the snapshot on the left. Right: HF LFP spectral analysis for each state showing a prominent theta rhythm in waking and REM sleep. B. High magnification photomicrograph (top) of a coronal section through the nucleus reuniens (RE) (red dashed lines) showing the electrode track and recording sites along it (red stars) for a representative case at a mid-level of the thalamus, depicted in the schematic coronal section (bottom). Adapted from Swanson (2018). C. Bar graph illustrating the mean discharge rates across sleep-wake states for all recorded units of nucleus reuniens (RE). D. Pie chart showing relative percentages of RE cells identified in RE based on their patterns of discharge profile across sleep-wake states using rate ratios (RR): Wake active I (WI) and II (WII), REM active I (RI) and II (RII), Wake/REM active (W/R), state independent (SI), and SWS cells. E-G: Detailed stacked histograms of the RR for wake active (E), REM active (F) and W/R cells. The RR was calculated as (AW-REM)/REM and (AW-SWS)/SWS for wake active neurons (E). WI cells (dark blue) were defined as having a RR AW-REM > 0.50 while WII cells (light blue) had a RR AW-REM > 0.25 but < 0.50. The majority of wake active neurons exhibited RR AW-SWS (teal) of > 0.50%. The RR of REM active neurons was calculated using (REM-AW)/REM and (REM-SWS)/REM (F). RI cells had a RR REM-AW > 0.50 (light pink). RII cells had a RR AW-REM > 0.25 but < 0.50 (dark pink). All REM active cells had RR REM-SWS that were > 0.25 (light teal). Wake/REM (W/R) cells were defined by having a RR for AW-SWS (dark teal) and REM-SWS (light teal) of > 0.25 and exhibiting a RR value between AW and REM (AW-REM)/AW or (REM-AW)/REM (purple) of < 0.25 (G). Most W/R cells had RR for AW-SWS (dark teal) and REM-SWS (light teal) > 0.50. Each bar in the histograms (E-G) represents a single recorded unit. Significance value: * p < 0.05; **** is p < 0.0001. Error bars represent SEM. Abbreviations: PVh, paraventricular nucleus of hypothalamus; RH, rhomboid nucleus of thalamus; SMT, submedial nucleus of thalamus.
Histological analysis
Upon completion of the experiment, rats were anesthetized with isoflurane and an electrolytic lesion was made by passing a small DC current (10-20 μA, 3-10s) [16] to mark the location of tetrode tips, either at the end of the tract or at the dorsoventral level at which units were identified (Fig. 1B). Rats were then given an i.p. injection of Euthasol (250 mg/kg) and transcardially perfused with 100ml heparinized saline followed by 400 ml of 4% paraformaldehyde. Brains were preserved in 4% paraformaldehyde for 24 hours and cryoprotected in 30% sucrose until coronally sectioned (50 um) using a freezing sliding microtome, mounted onto chrome–alum gelatin-coated slides, and processed with a cresyl violet Nissl stain. Photomicrographs through the thalamus were captured using a Nikon FI-3 camera mounted on a Nikon Eclipse E600 microscope to confirm electrode locations.
Statistical analysis
If data was normally distributed, repeated measures ANOVAS using post hoc Bonferroni pairwise comparisons were performed. When data was not normally distributed, non-parametric analyses using the Friedman’s repeated measures test followed by Dunn’s pairwise comparisons examined differences across sleep stages. 2 x 3 mixed ANOVAS using the Greenhouse-Geisser correction were computed to examine differences across cell types and states. Pearson’s correlation coefficient (R) was used to determine the linear dependency of unit firing across sleep-wake states. All statistical analyses were computed using Prism 8.0 (GraphPad) with a significance level of p < 0.05. All data in text are reported as mean ± SD.
Results
RE neurons: discharge rates and state related activity
We analyzed unit activity from 5 rats and in each case the tetrodes were confined to RE, mainly localized to the anterior sector of nucleus reuniens (RE) (Fig. 1B). Cases in which the electrode tip fell outside of RE or tracts that could not be reconstructed (n=8) were excluded from analysis. A total of 153 units in RE were analyzed across 41 recording sessions as behaving rats cycled through sleep-wake states. The numbers and percentage of cells analyzed from each rat are as follows: EP28= 22 cells (14.38% of all units sampled); EP31= 32 cells (20.92% of all units sampled); EP35= 31 cells (20.26% of all units sampled); EP37= 18 cells (11.76% of all units sampled); and EP39= 50 cells (32.68% of all units sampled). The mean discharge rates of RE cells varied widely across active waking (AW), slow-wave sleep (SWS) and REM sleep (AW-SWS – p < 0.0001; AW-REM – p < 0.05; REM-SWS – p < 0.0001; Fig. 1C), with significant differences across each sleep-wake state (X2= 182.38, p < 0.0001). Overall, RE cells discharged at highest rates in AW (M= 5.36 Hz ± 5.70), at slightly reduced rates in REM sleep from AW (M= 4.55 Hz ± 4.80) and at low rates during SWS (M= 1.69 Hz ± 2.20).
To examine RE neurons for state-related discharge patterns, we calculated the mean difference of the rate ratio (RR) of activity in AW compared to REM and SWS as previously done by others [17,18], to thereby classify RE units with respect to preferential state related firing patterns (Fig. 1D). The RR was calculated using the following equation: RR AW-REM= (AW-REM)/AW and RR AW-SWS= (AW-SWS)/AW. Positive values signified higher rates of discharge in AW than REM (or SWS). We identified two types of cells with activity greatest in AW (Fig. 1E) denoted as wake active cells. Wake active I (WI) cells (n=20, 13.07%) discharged at mean rates which were ≥50% (RR ≥ 0.5) higher in AW compared to both REM (mean RR AW-REM= 0.61± 0.09) and SWS (mean RR AW-SWS = 0.79± 0.10) (Fig. 2A, B). Wake active II (WII) cells (n=43, 28.10%) discharged at mean rates which were ≥ 25 but < 50% (RR= 0.25-0.50) in AW vs. REM (mean RR AW-REM= 0.37± 0.07) (Fig. 2A, C). The majority of W II cells fired > 50% in AW vs. SWS (mean RR AW-SWS= 0.74± 0.16).
Figure 2.
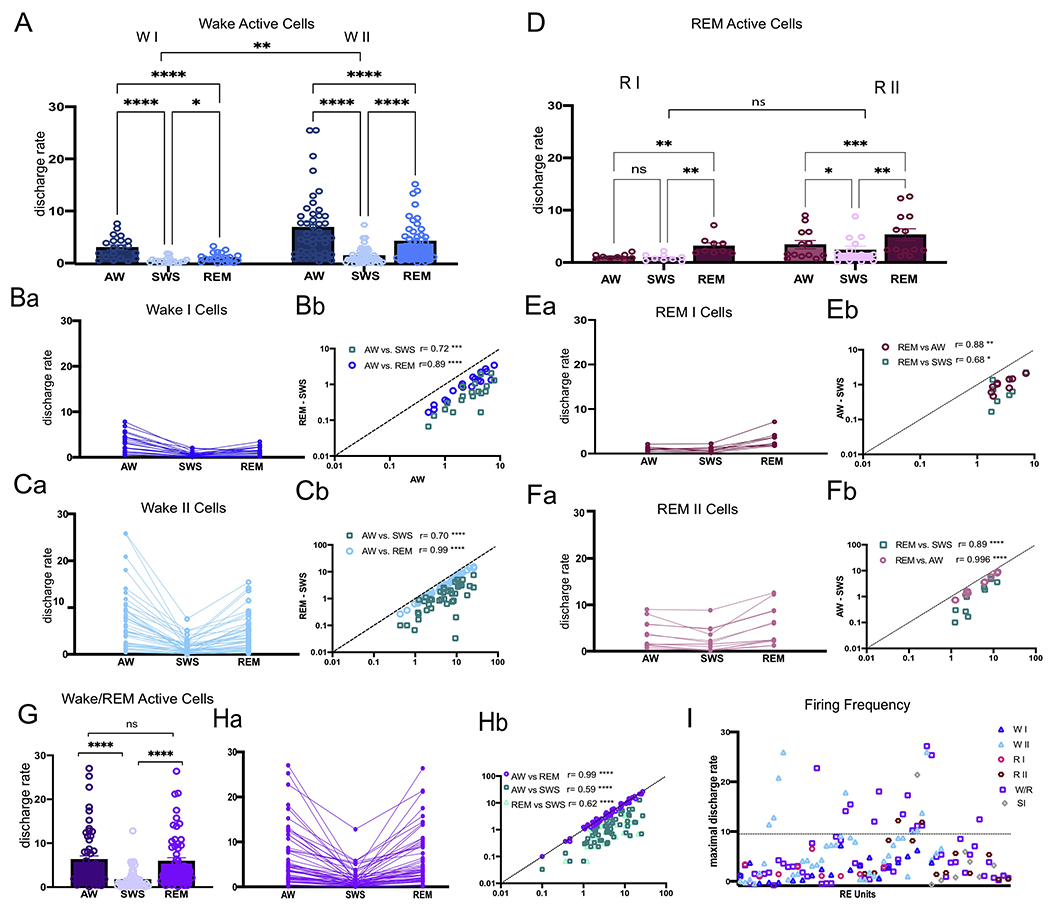
Mean (A) and individual (Ba,Ca) discharge rates for wake active (WI and II) cells in AW, SWS, and REM sleep. Pearson’s correlation coefficient showing the relationship of individual WI (Bb) and WII (Cb) unit firing rates during AW-REM and AW-SWS, demonstrating that the discharge properties of W cells were highly correlated across states. Mean (D) and individual (Ea, Fa) discharge rates for REM active (RI and RII) cells across AW, SWS, and REM. Pearson’s correlation coefficient showing the relationship between individual RI (Eb) and RII (Fb) unit firing rates during REM-AW and REM-SWS (H), demonstrating that the discharge properties of RI and RII cells were highly correlated across states. Mean (G) and individual (Ha) discharge rates for wake/REM cells in AW, SWS, and REM. Pearson’s correlation coefficient showing the relationship of individual wake/REM (Hb) unit firing rates during AW-REM, AW-SWS, and REM-SWS demonstrating that the discharge properties of W/R cells were highly correlated across states. I: Plots of the maximal discharge rate of individual cells of nucleus reuniens (RE) showing the distribution of slow firing (SF, < 10Hz) and fast firing (FF, > 10Hz) neurons by cell type. Significance value: * p < 0.05; ** p < 0.01; *** p < 0.001 **** is p < 0.0001. Error bars represent SEM. Abbreviations; SI, state independent cells.
Negative values for the RR AW-REM indicated discharge rates that were higher in REM vs. AW. We then used the following equation to calculate the REM RR: REM-AW= (REM-AW)/REM and REM-SWS= (RE-SWS)/REM. We also identified two types of RE cells whose activity was greatest during REM sleep (Fig. 1F). REM I (RI) cells (n= 9, 5.88%) discharged at mean rates of ≥ 50% in REM compared to AW (mean RR= 0.64± 0.10) and SWS (mean RR=0.69± 0.21) (Fig. 2D, E). REM II (RII) cells (n= 13, 8.50%) discharged at mean rates that were ≥25 but < 50% in REM vs. AW (mean RR AW= 0.38± 0.06) and SWS (mean RR SWS= 0.62± 0.22) (Fig. 2D, F).
The most common type of RE neuron of the various cell types, however, was wake/REM (W/R) cells (n= 57, 37.25%); that is, cells which maintained high rates of discharge in both AW and REM -- with < 25% difference between AW and REM (mean RR= 0.12± 0.07). The majority of these cells fired > 50% in AW/REM vs. SWS (mean RR AW-SWS= 0.70± 0.19; RR REM-SWS= 0.69± 0.19) (Fig. 2G, H). The remaining cells fell into one of two categories: (1) cells in which the activity differed by less than 25% across states (AW, REM, and SWS, mean RR < 0.25) and did not significantly differ from one another (X2= 0.81, p > 0.05) which were defined as state independent (SI) cells (9 cells, 5.88%), and (2) cells which showed maximal firing rates that were ≥ 25 but < 50% in SWS (2 units, 1.31%).
Each cell type discharged at variable frequencies and were further classified by their maximal firing rate in their preferred firing state (AW or REM). Units were classified as slow firing (SF) or fast firing (FF) units, using a breakpoint of 10 Hz similar to a previous report [19]. SF and FF RE units are plotted by cell type in Fig. 2I The vast majority of RE cells (independent of state) were SF units (n= 123, 83.66%) exhibiting discharge rates of < 10 Hz (mean AW= 3.31± 2.52; SWS= 1.18± 1.21; REM= 2.80± 2.14) and overall had significantly higher discharge rates in AW and REM than SWS (X2= 140, p < 0.0001). FF cells made up 16.34% (n= 25) of all RE units (mean AW= 15.99± 5.81; SWS= 4.30± 3.95; REM= 13.52± 4.923) and also exhibited discharge rates that were significantly higher in AW and REM vs. SWS (X2 = 39.92, p < 0.0001). Supplementary Table 1 depicts the number and proportion of RE cells by their cell type and discharge rates. The ratio and proportion of cell types across rats was similar such all three major cell types (W, R, and W/REM) were present in 4 of 5 rats, with the larger proportion being W and W/REM neurons. No “REM selective” cells were found in one rat (EP28), and interestingly the sparse number of neurons discharging at highest rates in SWS were only identified in one rat (EP39). SF and FF units were present in all, with greater numbers of SF than FF neurons in each of them.
Wake active cells
RE units that fired maximally during AW (WI and WII cells) collectively made up the largest proportion of RE neurons (41.20%). A (cell type X state) ANOVA yielded a highly significant effect of state (F1.07.65.23= 42.10, p < 0.0001) for WI and WII cell types (F1,61= 9.67, p < 0.01) (Fig. 2A). Both WI and WII cells had significantly higher discharge rates in AW (WI= 3.23 Hz ± 2.16; WII= 7.12 Hz ± 6.92) than in SWS (WI= 0.65 Hz ± 0.57; WII= 1.63 Hz ± 1.57) and in REM sleep (WI= 1.27 Hz ± 0.91; WII= 4.45 Hz ± 3.87, p < 0.0001). Both WI and WII cells also had higher discharge rates in REM as compared to SWS (WI p < 0.05; WII p < 0.0001). Overall, the mean discharge rate of WII cells (4.40 Hz ± 3.91) was significantly higher than WI cells (M= 1.72 Hz ± 1.21, p < 0.01) and a cell type x state interaction (F2,122= 5.940, p < 0.01) revealed that WII cells maintained higher discharge rates across AW, SWS and REM compared to WI cells. All WI units were SF cells (range 0.5-7.8Hz in AW), and similar to “arousal-related” cells of the brainstem and hypothalamus (see Discussion), discharged at highest rate in AW, with the majority falling to near silence in SWS (range 0.07-2.07 Hz) and REM sleep (0.17-3.43 Hz; Fig. 2Ba). While the discharge rates of WI neurons varied across AW, activity was highly correlated across states (AW-REM: r20= 0.89, p < 0.0001 and AW-SWS: r20= 0.72, p < 0.001) (Fig. 2Bb).
WII cells showed a wider distribution of maximal discharge rates across AW (Fig 2Ca) (0.43-25.87 Hz), but like WI cells, activity highly correlated across states (AW-REM – r43= 0.99, p < 0.0001; AW-SWS – r43= 0.70, p < 0.0001; Fig. 2Cb). The majority (~79%; n=34) of WII units were SF cells (0.43-9.67Hz) – with drastically reduced firing in SWS (0.03-4.20Hz). Interestingly, more than 1/3 of WI and WII cells fired at very low tonic rates during AW (< 3Hz). WII FF cells (~21%, n=9) discharged at high rates during AW (10.47-25.87Hz) which significantly slowed during SWS (1.33-7.57 Hz). All WII (SF and FF) units exhibited rebound activity in REM (SF: 0.27-6.73Hz; FF: 6.73-15.43Hz) but not to the levels of AW.
REM active cells
REM active neurons made up 14.38% (n=22) of the population of RE neurons. A cell type X state ANOVA yielded highly significant effect across state (F1.25,25.08= 37.14, p < 0.0001) such that RI and RII cells had significantly higher discharge rates in REM (RI= 3.17 Hz ± 1.74; RII= 5.31 Hz ± 4.11) compared to AW (RI= 1.10 Hz ± 0.51; RII= 3.42 Hz ± 2.87) and to SWS (RI= 0.93 Hz ± 0.65; RII= 2.39 Hz ± 2.54), p < 0.01 (Fig. 2D). While RI cells were nearly quiescent in both waking and SWS, RII cells fired maximally in REM, and at progressively lower rates in AW and SWS (AW-SWS p < 0.01). While RII cells exhibited faster overall discharge rates (4.40 Hz ± 3.91) than RI cells (1.73 Hz ± 3.12; Fig. 2Ea, Fa), this was not significant (F1,20= 3.379, p > 0.05, nor was there a cell type by state interaction (F1,20= 1.06, p > 0.05; Fig 2D). Finally, RI and the majority of RII cells were SF units with discharge rates in REM highly correlated with those in AW (RI – r9= 0.88,p < 0.01; RII – r13= 0.99, p < 0.0001) and in SWS (RI – r9= 0.68, p < 0.05; r13= 0.89, p < 0.0001; Fig. 2Eb, Fb) across cells.
Wake/REM (W/R) cells
W/R cells, the most abundant unit of the cell groupings, fired tonically at high rates in AW (M= 6.28 Hz ± 6.36) and REM (M= 5.92 Hz ± 5.945), and significantly reduced rates in SWS (M=1.71Hz± 2.15), X2= 88.54, p < 0.0001. Importantly, unique to this cell type, there was no difference in discharge rates between AW and REM (p > 0.05; Fig. 2G). While the discharge rates of W/R cells varied widely in both AW (Fig. 2Ha) (range 0.10-27.03Hz) and REM (0.10-26.40 Hz), activity was highly correlated between AW and REM (r57= 0.99, p < 0.0001) and between AW/REM and SWS (AW-SWS - r57= 0.59, p < 0.0001; REM-SWS - r57= 0.62, p < 0.0001; Fig. 2Hb) across cells. While the majority of W/R cells were SF (n=44, ~77%), W/R cells made up the largest number of FF cells (n= 13).
Figures 3 and 4 depict the activity of 6 RE cells across states (AW, SWS, REM), along with the corresponding hippocampal LFP and cortical EEG, comparing the activity of the most common subtypes identified in RE: WII (units 1 and 6) and W/R (units 2, 3, 4 and 5) taken from a recording session from two representative rats (EP37 units 1-4 and EP31 units 5-6). As depicted, unit 1 is a FF WII cell, which fired at high mean tonic rates in AW (19.6 Hz ± 8.87), irregularly and at significantly lower rates in SWS (mean discharge unit 1 = 5.2 Hz ± 2.90) and rebounded in REM with rates significantly greater than in SWS but less than that of AW (RR AW-REM= 0.33; mean discharge rate in REM, unit 1= 13.2 Hz ± 6.75). Unit 6 illustrates the activity a SF WII cell which was virtually silent during SWS. Neurons of this type (nearly quiescent in SWS) consisted about ~30% of AW cells (WI n=9; WII n=10). Units 2 and 4 exemplify SF W/R cells. While there was no difference in the firing rate of units 2 and 4 across AW and REM (unit 2 – AW-REM= 6.2 vs. 4.8; unit 4 AW-REM – 6.8 vs. 7.5Hz), both cells showed drastically reduced activity in SWS (unit 2= 2.6 Hz± 2.12; unit 4= 1.5 Hz± 0.85). Units 3 and 5 depict FF W/R cells, with contrasting rates and patterns of activity in AW, SWS and REM. For instance, unit 3 discharged at high tonic rates in AW (M= 10.00Hz± 3.95) and REM (M= 10.40± 5.46) at significantly reduced rates in SWS (M= 4.40Hz± 3.01), while unit 5 maintained very high phasic rates of activity in AW (M=21.8 Hz± 5.79) and REM (M=22.40 Hz± 6.00) with periods of near quiescence in SWS.
Figure 3.
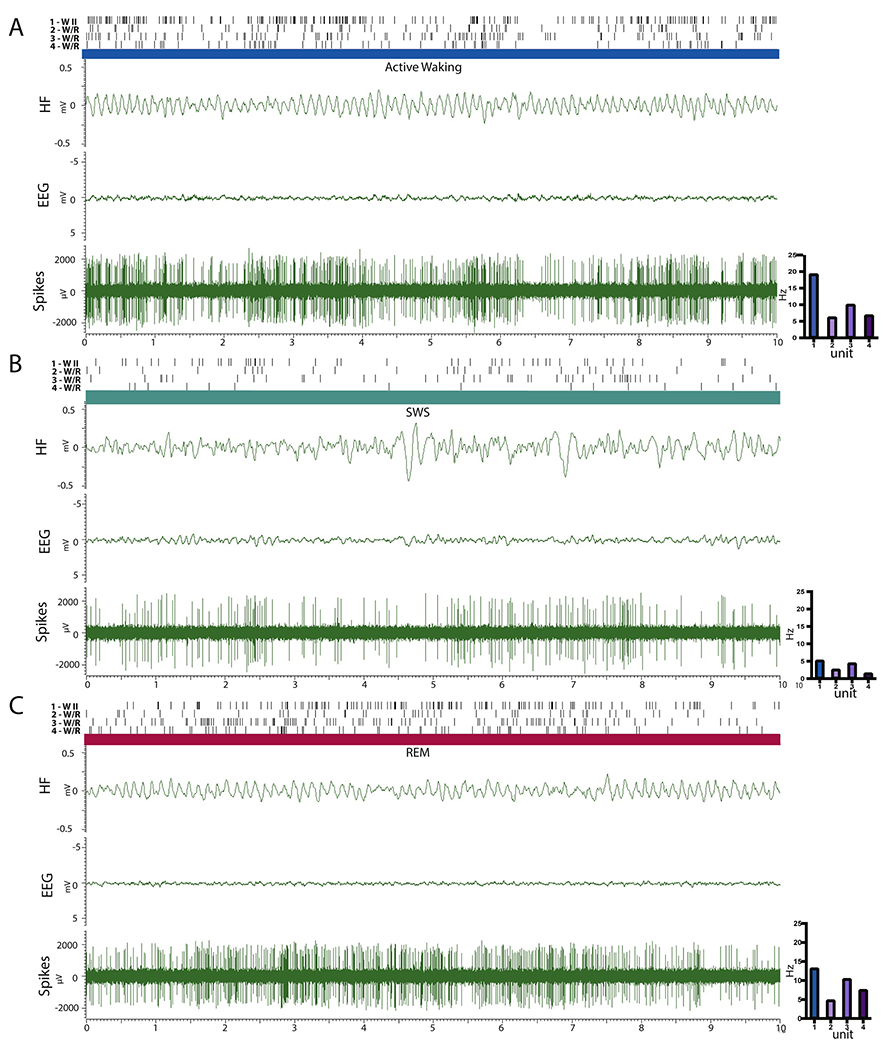
Polygraphic recordings of hippocampal LFP (HF) and cortical EEG (EEG) and RE multiunit activity before sorting (Spikes) for one W II cell (unit 1) and three wake/REM (W/R) cells (units 2-4) depicting patterns of discharge in a representative subject (EP37) across active waking (AW) (A), slow-wave sleep (SWS) (B), and REM sleep (C). For each unit, the lines on the top represent the discharge activity of each cell ‐‐ as shown below. The bar graphs (on the right) depict the mean firing rate of each unit across the recorded periods. Unit 1 is an example of a fast firing (FF) W II cell which maintained high rates of discharge in AW, dramatically decreased in rate in SWS, and resumed tonic activity in REM sleep – but at lower rates than in AW. Units 2 and 4 are slow firing wake/REM (W/R) cells that discharged at equivalent rates in AW and REM but with significantly reduced activity during SWS. Unit 3 is a FF W/R cell with tonic activity in AW that did not significantly differ from that of REM sleep but showed reduced irregular activity in SWS.
Figure 4:
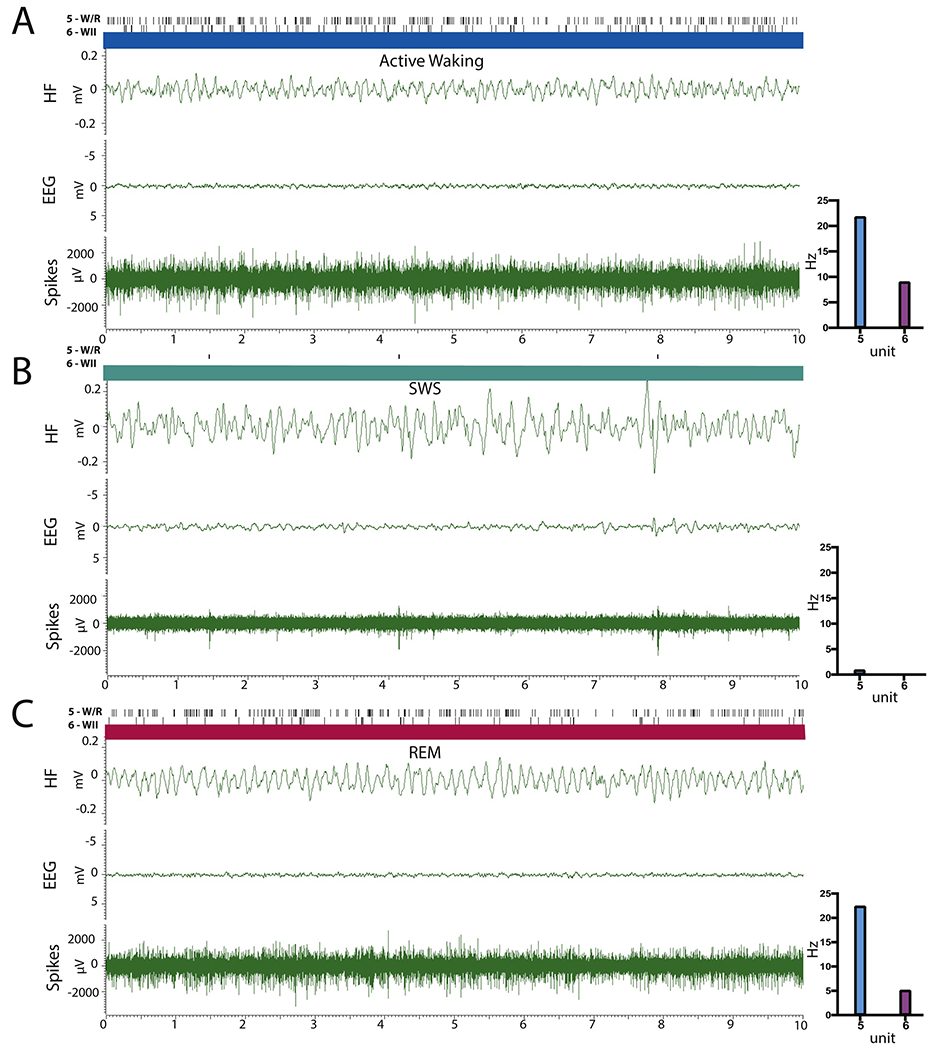
Polygraphic recordings of hippocampal LFP(HF) and cortical EEG (EEG), and RE multiunit activity before sorting (Spikes) for one wake/REM (W/R) (unit 5) and one W II cell (unit 6) in a representative subject (EP31) during active waking (AW) (A), slow-wave sleep (SWS) (B), and REM sleep (C). For each unit, the lines on the top represent the discharge activity of each cell ‐‐ as shown below. The bar graphs (on the right) depict the mean firing rate of each unit across the recorded periods. Unit 5 is an example of a fast firing W/R cell, which maintained very high rates of activity in AW and REM sleep (> 20 Hz) and discharged at significantly reduced rates in SWS (< 1 Hz). Unit 6 is an example of a slow firing W II which showed sustained activity in AW, was virtually silent in SWS and resumed tonic firing in REM ‐‐ but less than that of AW.
Discussion
We analyzed the activity of single cells of nucleus reuniens (RE) in freely moving rats as they naturally cycled through sleep and wake states. Of 153 recorded RE cells, 140 (> 90%) fired at high tonic rates (1) in active waking (AW), (2) in REM sleep or (3) during both states (AW/REM) and at significantly reduced rates during slow-wave sleep (SWS). When separated using a rate ratio (RR) of state-related activity, we identified five classes of RE neurons with state related activity: wake type I (WI) and wake type II (WII) cells, which were more active in AW than the other states; REM type I (RI) and REM type II (RII) cells, which were more active in REM sleep than other states; and wake/REM (W/R) cells, discharging at almost equal rates in AW and REM, and significantly more than SWS. Of these categories, W/R were the most abundant. Comparing ratios of activity, WI cells showed greater differential rates of activity (AW to REM; RR > 50%) than did WII cells (RR 25-50%); whereas RI cells exhibited a greater differential rates of discharge (REM to AW; RR > 50%) than did RII cells (RR: 25-50%).
RE cells were further classified by maximal frequency of discharge during AW or REM yielding two population of neurons: slow firing (SF < 10 Hz) and fast firing (FF > 10 Hz) neurons. The majority of RE cells, independent of cell type, were SF neurons (n= 123, 83.66%) while FF neurons (n= 25; 16.34%) were fewer in number and mainly WII and W/R cells.
Comparisons of state-related activity of neurons in other midline IL nuclei of the thalamus
Very few reports have described the discharge properties of midline/intralaminar neurons across sleep/wake states, most of which have focused on the paraventricular nucleus (PV) and intralaminar nuclei. In an early report in awake cats, Glenn and Steriade [6] showed that cells of the central lateral (CL) intralaminar nucleus fired at high rates of activity in waking and REM sleep and at significantly lower rates in SWS. Specifically, they reported the following mean rates of activity for 26 CL neurons: waking, 7.86Hz, SWS, 3.66Hz and REM sleep 8.40Hz. The rates and discharge profiles of these CL cells are very comparable to our slow firing (SF) W/R neurons – or more specifically a subset of W/R cells that maintained a slightly higher rate of discharge in REM than in AW.
Redingbaugh et al. [20] recently described a population of CL cells in monkeys that fired at high rates in waking and at significantly reduced rates in NREM sleep. They further showed that CL stimulation restored waking consciousness in sleeping or anaesthetized monkeys, or as stated “wake-like neural processing”. Comparably, Gent et al. [21] reported that cells of the central medial intralaminar nucleus (CM) in behaving mice discharged at significantly higher rates in waking and REM sleep than in SWS, and interestingly at much higher rates in REM (25.9 Hz ± 1.6) than in waking (6.3Hz ± 0.5). While these cells are similar to the present REM active (RI and RII) cells, no RI/RII cells fired at such high rates in REM, with comparably reduced firing in AW.
Ren et al. [22] recently showed in behaving mice that glutamatergic cells of PV fired at much higher rates in waking than in NREM sleep, with characteristic increases or decreases, respectively, in transitions from sleep to waking or waking to sleep. In addition, most PV cells fired at higher rates in waking than in REM sleep, and at mean rates of ~ 7 Hz in waking, with 25% of them discharging at > 10 Hz in waking. This discharge profile is very comparable to our W neurons (WI and WII) which fire “selectively” in waking, and 21% of WII discharge at rates > 10 Hz. Finally, Mátyás et al. [23] demonstrated that graded optogenetic stimulation of calretinin-containing (CR) cells (primarily in PV) of the dorsomedial thalamus produced corresponding graded effects on arousal/wakefulness; that is, brief simulation elicited “microarousals” in NREM (or REM) sleep, whereas longer stimulation (~10 s) resulted in prolonged periods of wakefulness, accompanied by active locomotion.
Whereas a limited number of reports have examined the discharge properties of midline/intralaminar cells in behaving animals across sleep/wake states, the findings are consistent with the present results for RE, showing a heterogeneous population of central thalamic neurons, with the dominant types being cells that fire maximally in waking or waking and REM sleep. RE receives a vast array of input from the brainstem and hypothalamus involved in behavioral state control [1–3, 24]. For instance, RE receives projections from cells of the pontomesencephalic reticular formation, which have been shown to discharge maximally in waking and REM sleep and could be the source of excitatory drive to a subset of reuniens W/R cells, thereby contributing to the cortical EEG activation of waking and REM sleep – part of the classic ascending reticular activating system (for review see [25–27]).
RE also receives substantial input from brainstem/diencephalic structures in which most (or large percentages) of cells have been shown to discharge maximally in the waking state. These would include aminergic nuclei (dorsal and median raphe, locus coeruleus and tuberomammillary nucleus) and prominently orexin-containing cells of the lateral hypothalamus [1–3, 24, 28, 29], These selective “wake active” cells have been variously linked to the control of wakefulness (per se), as well as, to certain process of waking such as arousal, attention or awareness. Whereas brainstem “arousal-like” actions on RE are shared by other midline/IL nuclei [24, 28,29], RE appears unique in that it almost exclusively projects to “cognitive-related” structures of the forebrain [1–3] ‐‐ and thus may be a critical source of arousal (or attention-like) actions on these forebrain structures.
The increased rate of discharge of populations of RE cells in active waking (AW) or waking and REM sleep (AW/REM) could suggest that these RE neurons may play a role in modulating the theta rhythm of waking and REM [30–32], or perhaps could serve to synchronize theta activity between the hippocampus (HF) and the mPFC during these states. With respect to the latter, Griffin and colleagues [33] reported that theta oscillations between the HF and mPFC became synchronized during successful performance on a spatial working memory task, and further that the inactivation of RE disrupted synchronous hippocampal-mPFC oscillations as well as performance on the working memory task. While the present report did not specifically analyze the relationship between RE unit activity and theta, this will be examined in greater detail in future studies.
RE afferents to the hippocampus (or other forebrain targets) may also provide attentional drive to them, important for cognitive processing. In this regard, Dolleman-van der Weel et al. [34] reported that the paired (or synchronous) stimulation of RE and the entorhinal cortex produced much greater (supralinear) effects at CA1 of HF than did RE or entorhinal stimulation alone. In effect, the co-activation of RE and entorhinal inputs to the hippocampus enhanced the effectiveness of EC actions on the HF. Accordingly, RE might serve as an “attentional signal,” gating the flow information to the HF from the entorhinal ‐‐ or other sources.
Conclusion
In summary, the present report is the first to describe the activity of nucleus reuniens neurons in freely moving animals across sleep/wake states. Based on rates and patterns of discharge, we identified five distinct classes of neurons in RE, with the most prominent being cells that fired selectively in active waking and REM sleep (W/R neurons) and those that discharged selectively in the waking state (W neurons). The heterogeneous nature of RE cells may indicate that they participate in several distinct functions, a common feature of midline thalamic nuclei. The W/R cells would appear to participate in functions common in waking and REM sleep such as cortical EEG activation of waking/REM states, whereas the W cells may be the source of arousing (or attentive) actions on RE target forebrain structures during the waking state. Specifically, RE may be an important source of excitatory drive to the hippocampus and the mPFC in their well-documented role in cognitive and executive functions.
Supplementary Material
Highlights.
Nucleus reuniens (RE) cells were recorded in behaving rats across sleep/wake states.
RE has a heterogenous population of cells exhibiting distinct state related activity.
The majority of RE cells discharged preferentially in waking and REM sleep.
RE is a critical thalamic link in ascending arousal/attentional networks.
Acknowledgment
The authors thank Alexandria Athanason, Mary Gorora and Jonathan Nakhla for their assistance in data and histological analyses. This was supported by NIH grant: NS108259
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cassel JC, Pereira de Vasconcelos A, Loureiro M, Cholvin T, Dalrymple-Alford JC, Vertes RP (2013) The reuniens and rhomboid nuclei: Neuroanatomy, electrophysiological characteristics and behavioral implications. Prog Neurobiol 111:34–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Griffin AL (2015) Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vertes RP, Linley SB, Hoover WB (2015a) Limbic circuitry of the midline thalamus. Neurosci Biobehav Rev 54:89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dolleman-van der Weel MJ, Griffin AL, Ito HT, Shapiro ML, Witter MP, Vertes RP, Allen TA (2019) The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn Mem 26:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Van Der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev 39:107–140. [DOI] [PubMed] [Google Scholar]
- [6].Glenn LL, Steriade M (1982) Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci 2:1387–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cirelli C, Pompeiano M, & Tononi G (1995) Sleep deprivation and c-fos expression in the rat brain. J Sleep Res 4: 92–106. [DOI] [PubMed] [Google Scholar]
- [8].Cirelli C, Tononi G (2000) On the functional significance of c-fos induction during the sleep-waking cycle. Sleep 23: 9–25. [PubMed] [Google Scholar]
- [9].Sastre JP, Buda C, Lin JS, Jouvet M (2000) Differential c-fos expression in the rhinencephalon and striatum after enhanced sleep–wake states in the cat. European J Neurosci, 12:1397–1410. [DOI] [PubMed] [Google Scholar]
- [10].Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, O’Mara SM (2014) Nucleus reuniens of the thalamus contains head direction cells. Elife. 3:e03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ito HT, Zhang SJ, Witter MP, Moser EI, Moser MB (2015) A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature 522:50–55. [DOI] [PubMed] [Google Scholar]
- [12].Stackman RW, Clark AS, Taube JS (2002) Hippocampal spatial representations require vestibular input. Hippocampus 12:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vertes RP (1977) Selective firing of rat pontine gigantocellular neurons during movement and REM sleep. Brain Res 128:146–152. [DOI] [PubMed] [Google Scholar]
- [14].Mehta R, Khan S, Mallick BN (2018) Relevance of deprivation studies in understanding rapid eye movement sleep. Nat Sci Sleep. 10:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Costa-Miserachs D, Portell-Cortés I, Torras-Garcia M, Morgado-Bernal I (2003) Automated sleep staging in rat with a standard spreadsheet. J Neurosci Methods 130:93–101. [DOI] [PubMed] [Google Scholar]
- [16].Viana Di Prisco G, Albo Z, Vertes RP, Kocsis B (2002) Discharge properties of neurons of the median raphe nucleus during hippocampal theta rhythm in the rat. Exp Brain Res 145:383–394. [DOI] [PubMed] [Google Scholar]
- [17].Datta S, Siwek DF (2002) Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. J Neurosci Res 70: 611–621. [DOI] [PubMed] [Google Scholar]
- [18].Alam MA, Kostin A, Siegel J, McGinty D, Szymusiak R, Alam MN (2018) Characteristics of sleep-active neurons in the medullary parafacial zone in rats. Sleep, 41: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kocsis B, Vertes RP (1992) Dorsal raphe neurons: synchronous discharge with the theta rhythm of the hippocampus in the freely behaving rat. J Neurophys 68: 1463–1467. [DOI] [PubMed] [Google Scholar]
- [20].Redinbaugh MJ, Phillips JM, Kambi NA, Mohanta S, Andryk S, Dooley GL, Afrasiabi M, Raz A, Saalmann YB (2020) Thalamus modulates consciousness via layer-specific control of cortex. Neuron 106:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gent TC, Bandarabadi M, Herrera CG, Adamantidis AR (2018) Thalamic dual control of sleep and wakefulness. Nat Neurosci 21:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ren S, Wang Y, Yue F, Cheng X, Dang R, Qiao Q, Sun X, Li X, Jiang Q, Yao J, Qin H, Wang G, Liao X, Gao D, Xia J, Zhang J, Hu B, Yan J, Wang Y, Xu M, Han Y, Tang X, Chen X, He C, Hu Z (2018) The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362:429–434. [DOI] [PubMed] [Google Scholar]
- [23].Mátyás F, Komlósi G, Babiczky Á, Kocsis K, Barthó P, Barsy B, Dávid C, Kanti V, Porrero C, Magyar A, Szűucs I, Clasca F, Acsády L (2018) A highly collateralized thalamic cell type with arousal-predicting activity serves as a key hub for graded state transitions in the forebrain. Nat Neurosci 21:1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peyron C, Tighe DK, Van Den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Datta S, MacLean RR (2007) Neurobiological mechanisms for the regulation of mammalian sleep–wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci & Biobehav Rev 31: 775–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jones BE (2017) Principal cell types of sleep-wake regulatory circuits. Curr Opin Neurobiol. 44:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92: 1087–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McKenna JT, Vertes RP (2004) Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480:115–142. [DOI] [PubMed] [Google Scholar]
- [29].Krout KE, Belzer RE, Loewy AD (2002) Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol 448:53–101. [DOI] [PubMed] [Google Scholar]
- [30].Bland BH, Colom LV (1993) Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Progress Neurobiol 41: 157–208. [DOI] [PubMed] [Google Scholar]
- [31].Vertes RP, Kocsis B (1997) Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neurosci 81: 893–926. [DOI] [PubMed] [Google Scholar]
- [32].Vertes RP, Hoover WB, Di Prisco GV (2004) Theta rhythm of the hippocampus: subcortical control and functional significance. Behav Cog Neurosci Rev 3: 173–200. [DOI] [PubMed] [Google Scholar]
- [33].Hallock HL, Wang A, Griffin AL (2016) Ventral midline thalamus is critical for hippocampal–prefrontal synchrony and spatial working memory. J Neurosci 36: 8372–8389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dolleman-van der Weel MJ, Lopes da Silva FH, Witter MP (2017) Interaction of nucleus reuniens and entorhinal cortex projections in hippocampal field CA1 of the rat. Brain Struct Funct 222:2421–2438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


