Abstract
Smart scaffolds based on shape memory polymer (SMPs) have been increasingly studied in tissue engineering. The unique shape actuating ability of SMP scaffolds has been utilized to improve delivery and/or tissue defect filling. In this regard, these scaffolds may be self-deploying, self-expanding, or self-fitting. Smart scaffolds are generally thermoresponsive or hydroresponsive wherein shape recovery is driven by an increase in temperature or by hydration, respectively. Most smart scaffolds have been directed towards regenerating bone, cartilage, and cardiovascular tissues. A vast variety of smart scaffolds can be prepared with properties targeted for a specific tissue application. This breadth of smart scaffolds stems from the variety of compositions employed as well as the numerous methods used to fabricated scaffolds with the desired morphology. Smart scaffold compositions span across several distinct classes of SMPs, affording further tunability of properties using numerous approaches. Specifically, these SMPs include those based on physically cross-linked and chemically cross-linked networks and include widely studied shape memory polyurethanes (SMPUs). Various additives, ranging from nanoparticles to biologicals, have also been included to impart unique functionality to smart scaffolds. Thus, given their unique functionality and breadth of tunable properties, smart scaffolds have tremendous potential in tissue engineering.
Graphical Abstract
Introduction
Shape memory polymers (SMPs) are a class of smart materials capable of responding to external stimuli with a shape change. This response encompasses shape fixity (deformation followed by fixation into a temporary shape), and shape recovery (a return to the original, permanent shape). “Netpoints” are chemical or physical crosslinks that set the permanent shape whereas “switching segments” allow for fixation into a temporary shape and for recovery back to the permanent shape. Thermoresponsive SMPs, whose shape is modulated by application of heat, have been widely studied. Thermal transition temperatures (Ttrans) associated with SMP switching segments may either be a glass transition temperature (Tg) or a melting transition temperature (Tm). Thus, a temporary shape formed by deformation at T > Ttrans can be fixed by cooling to T < Ttrans and can also be subsequently recovered by heating to T > Ttrans again. For SMPs, the shape memory effect is entropically driven.1–3
The unique shape shifting capabilities of SMPs have been used toward advancing numerous biomedical applications.4–11 In the 1940s, thermoplastic polymer resins with “elastic memory” were developed as dental fillings that could be thermally triggered to expand into tooth cavities.12 Today, several FDA-approved SMP devices exist, including DYNACORD™ (a self-tightening suture),13 Eclipse™ (a soft tissue anchor),14 and Morphix® (an orthopedic suture anchor).15 More recently, IMPEDE-FX, based on a shape memory polyurethane (SMPU) foam, was approved as an embolization plug. Crimped for catheter delivery, the foam plug undergoes shape recovery (i.e. expansion) within the vasculature as it is hydrated and warmed to body temperature.16, 17 Bioresorbable SMPs have also been explored extensively, beginning with efforts to develop self-tightening sutures.4 However, the interest in tissue engineering (TE) has prompted the exploration of biodegradable SMPs as smart scaffolds.7, 18 Scaffolds play a critical role to regenerate healthy tissues lost to injury, disease, or congenital defects (Figure 1). Exogenous growth factors and/or pre-seeded cells are frequently incorporated into the scaffold to better promote neotissue formation. Scaffold chemical and physical properties have also been shown to potently direct cellular regeneration. Additionally, scaffolds with tailored mechanical properties and degradation profiles are sought to afford the necessary mechanical support and to match the rate of neotissue formation, respectively.19–21 As smart scaffolds, SMPs offer unique and differentiating characteristics. Namely, this is related to their shape shifting ability, allowing them to fill tissue defects of varying and sometimes irregular geometries with fidelity. Most typically, the shape change is triggered by heat (“thermoresponsive”). Electrically conductive and magnetic scaffolds permit thermally-induced shape actuation via resistive heating and application of a magnetic field, respectively.22, 23 Some SMP scaffolds that have appreciable hydrophilicity and water-absorbing abilities (e.g. hydrogels) undergo shape change in the form of swelling upon hydration (“hydroresponsive”).24 In some cases, the absorption of water acts as a plasticizer to reduce the scaffold’s Ttrans (Tg), resulting in shape recovery.25, 26 Herein, we highlight recent advances in the use of smart scaffolds with translational potential in TE.
Figure 1.
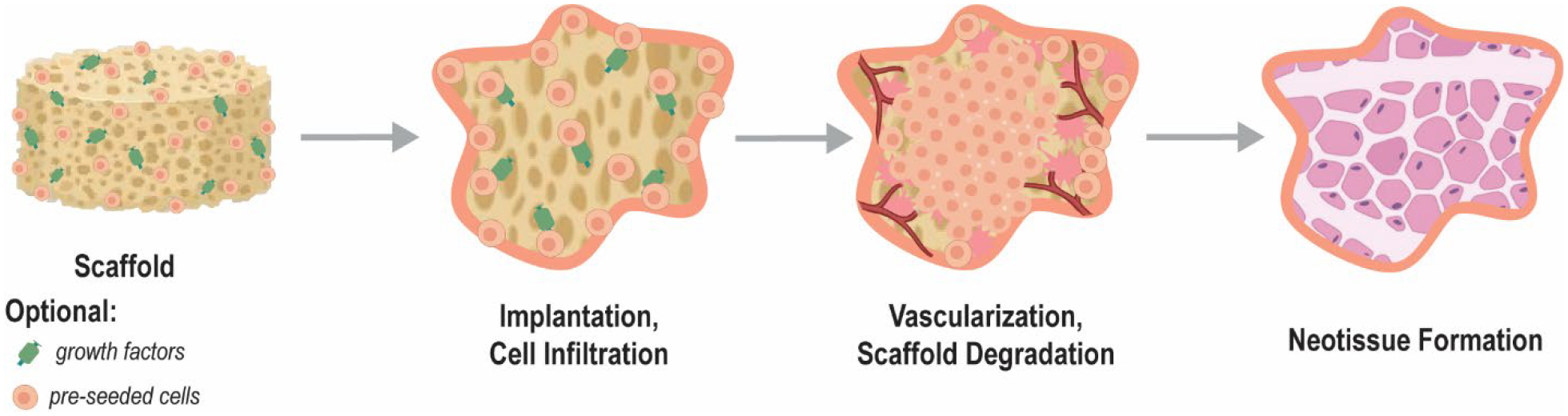
Schematic showing the general stages of tissue engineering (TE) whereby a highly porous scaffold, optionally loaded with growth factors and/or pre-seeded with cells, promotes tissue healing. Eventually the scaffold construct degrades and is replaced with healthy neotissue.
Smart scaffold functionality
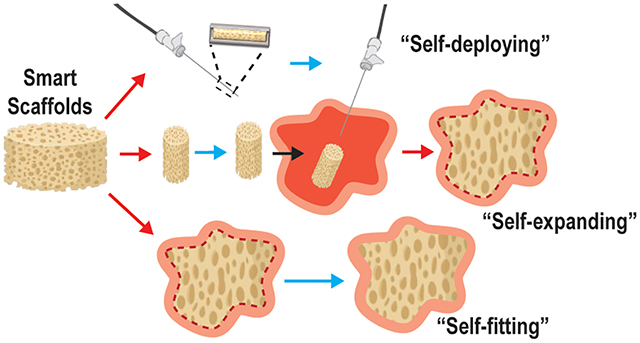
Smart SMP scaffolds may be classified according to the nature of functional delivery into tissue defects afforded by shape memory behavior, including self-deploying, self-expanding, and/or self-fitting (Figure 2). These scaffolds leverage their shape fixity and recovery for their minimally invasive delivery, triggered volumetric filling, and/or conformal fitting within irregularly shaped spaces. SMP scaffolds afford the opportunity to achieve excellent contact with adjacent tissue, an integral aspect of tissue integration and healing. Filling of irregular spaces is a feature usually associated with in situ forming materials (e.g. bone cements, injectable hydrogels, etc.). However, these are associated with various limitations including exothermic cures, slow setting times, low pore interconnectivity, and shrinkage resulting in loss of contact with adjacent tissues.27–34 In some cases, the permanent shape and size of the SMP scaffolds is designed to match that of the tissue defect. Alternatively, the SMP scaffold is of a generic geometry but is used to fill various and even irregularly shaped defects.
Figure 2.
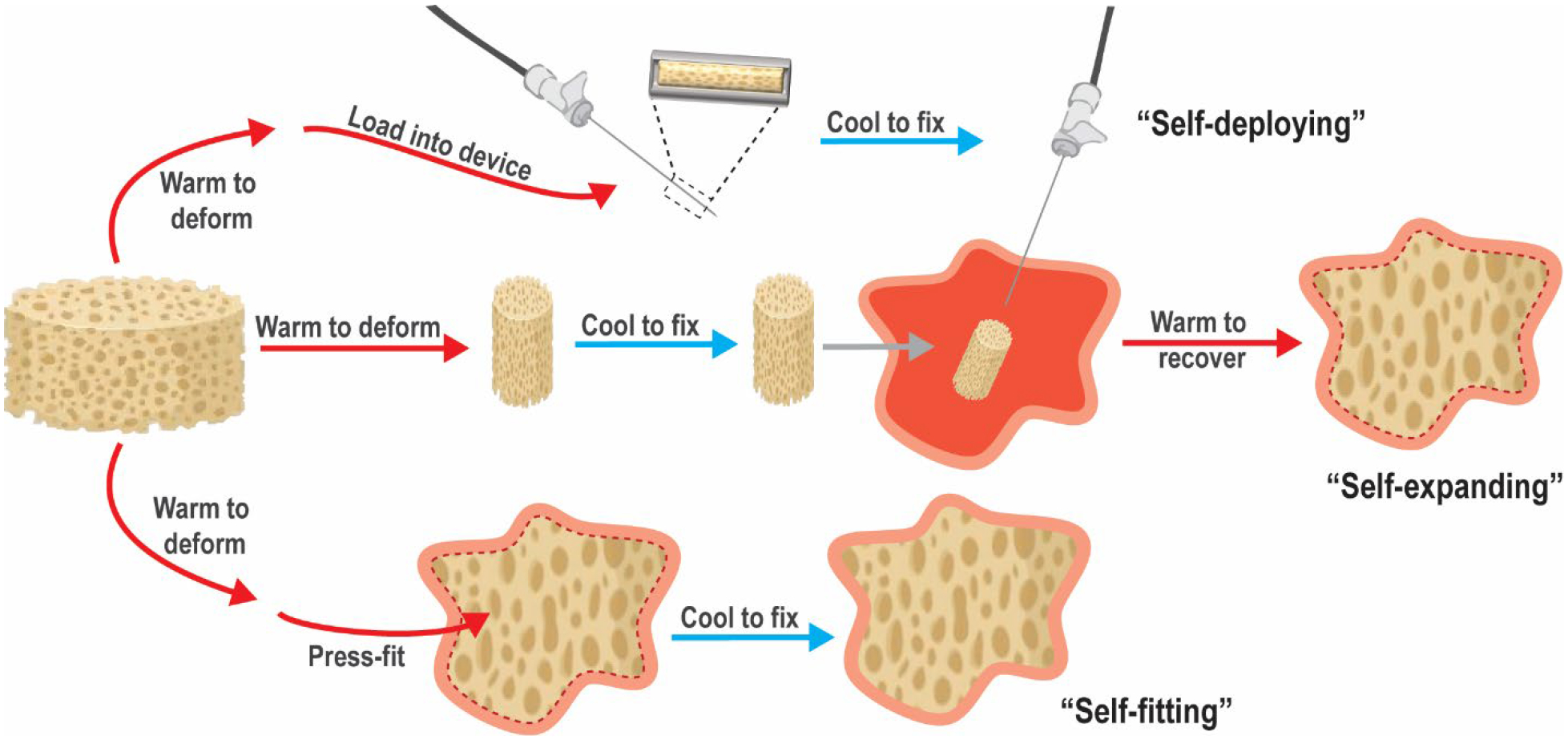
Classes of SMP scaffolds based on mode of delivery into tissue defect.
Self-deploying scaffolds.
Smart SMP scaffolds that self-deploy are typically warmed to their Ttrans to permit crimping into a compact geometry and subsequently cooled to fix this shape, affording loading into a catheter or needle. Thus, these shape fixed scaffolds can be delivered in a minimally invasive fashion. Self-deploying scaffolds typically have a Ttrans near body temperature (Tbody ~37 °C), such that upon delivery, the scaffold is triggered to shape recover (i.e. expand). Hydration that occurs after delivery can also trigger shape recovery.
Self-expanding scaffolds.
While not delivered via minimally invasive techniques, self-expanding scaffolds also undergo shape recovery in tissue defects or voids. The scaffold, previously fixed in a relatively compressed shape, is triggered to expand within the tissue space via shape recovery. Likewise, shape recovery can be initiated by warming to Tbody and/or hydration upon implantation.
Self-fitting scaffolds.
Self-fitting scaffolds are often based on SMPs having a Ttrans slightly above Tbody. These may be warmed (e.g. with saline; T > Ttrans) to cause softening, allowing the scaffold to be press-fitted into the tissue defect. Shape recovery then drives expansion of the scaffold within the defect, including those with irregular geometries. As the scaffold cools to Tbody ~37 °C, it becomes shape fixed in this new geometry. If cooling occurs too rapidly to permit expansion to defect edges, irrigation with warm saline, if at an acceptably tissue-safe temperature, could be used to promote continued shape recovery. Hydration can also drive self-fitting of a SMP scaffolds into a defect, pending non-brittle mechanical properties permit press-fitting.
Smart scaffolds for targeted tissue regeneration
The advantages associated with delivery, conformal fitting, and/or integration with surrounding host tissue make smart scaffolds excellent candidates for engineering a large variety of tissues (Figure 3 and Table 1). Most particularly, smart scaffolds have been evaluated for bone, cartilage, and cardiovascular tissue regeneration. Herein, we primarily highlight smart scaffolds that have been shown to support the differentiation and proliferation of human mesenchymal stem cell (hMSCs) and/or have advanced to in vivo studies.
Figure 3.
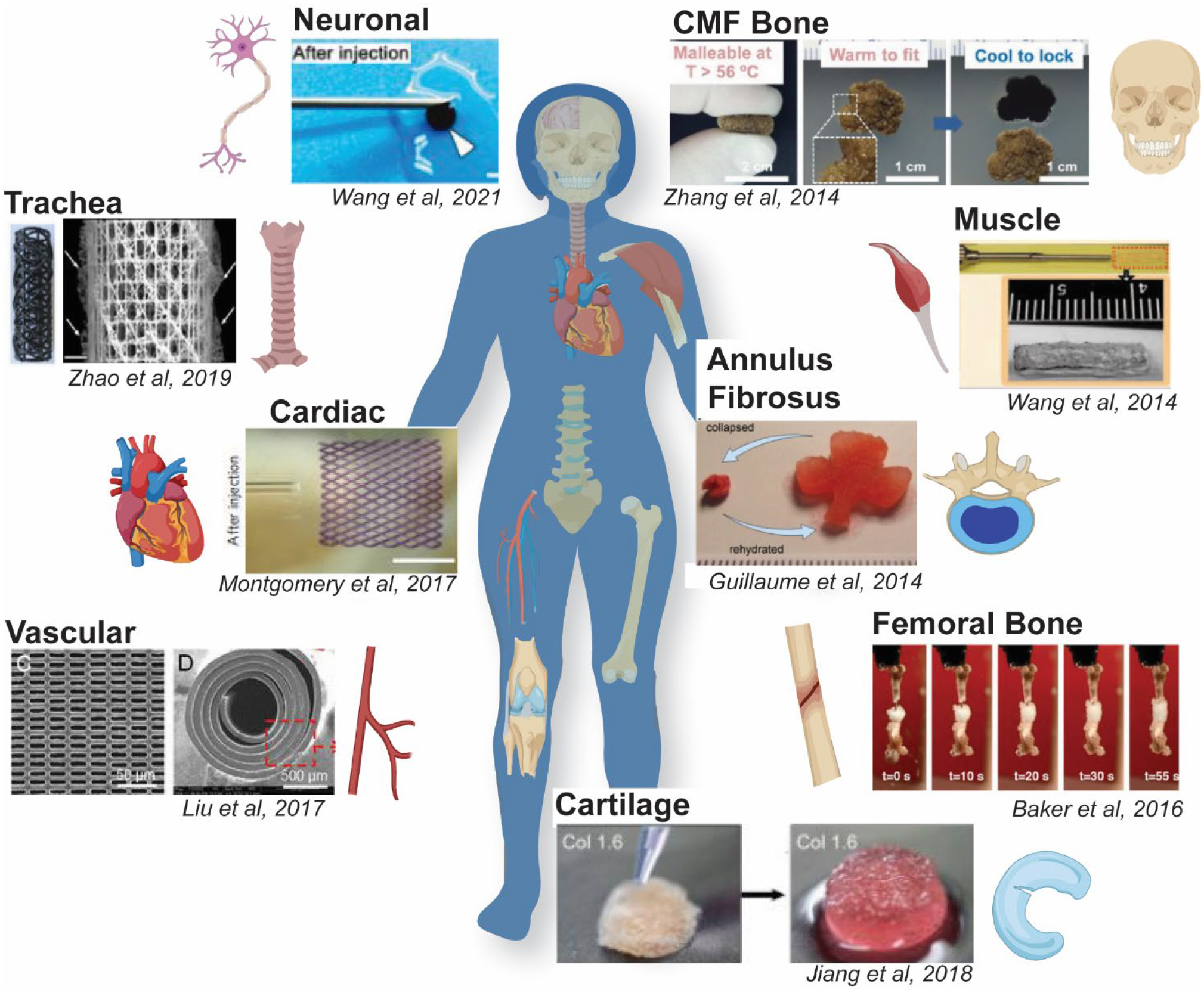
Utility of smart SMP scaffolds in the regeneration of various tissues.
Table 1.
Select recent smart scaffolds for targeted tissue regeneration and their key properties.
| Tissue | SMP Material | Additives | Intended Functionality | Transition Temperature (Ttrans) | Fabrication | Year | Ref |
|---|---|---|---|---|---|---|---|
| Bone | SMPU based on PCL or PLLA segments + PEG or gelatin | SPIO NPs | Self-expanding | Tuned to ~ Tbody via plasticization | 3D printing (i.e. FDM) | 2018 | 35 |
| PCL/dextran carbodiimide linker | HAp coating | Self-expanding | Tuned to ~ Tbody via PCL/dextran ratio | SCPL | 2020 | 38 | |
| PCL-DA | Polydopamine coating Cell adhesion peptide (RGD) | Self-fitting | Tm,PCL ~ 55 °C | SCPL | 2015 | 40 | |
| tBA/BA tEG-DMA linker | Self-expanding | 45 °C tuned via tBA/BA ratio | SCPL | 2016 | 41 | ||
| SMPU based on PCL segments | HAp | Self-expanding | Tm,PCL ~ 40 °C | Gas foaming | 2018 | 42 | |
| Cartilage | Alginate carbodiimide linker | Type II collagen coating | Self-expanding | Hydroresponsive | Freeze drying | 2017 | 43 |
| Collagen carbodiimide linker | Pre-seeded chondrocytes | Self-expanding | Hydroresponsive | Freeze drying | 2018 | 44 | |
| PGS/PPS | Kartogenin | Self-deploying | Tm,PGS ~ 35–45 °C | SCPL | 2020 | 45 | |
| PLLA | Cartilaginous particles from iPS cells | Self-fitting | Tg,PLLA ~ 60 °C | Threads woven into mesh tubes | 2020 | 46 | |
| Cardiovascular | Soybean oil epoxidized acrylate | Self-expanding | Ttrans ~ Tbody | UV cure thin film + Stereolithography | 2016 | 49 | |
| Poly(octamethylene maleate (anhydride) citrate)) | Rat or human CMs | Self-deploying | Ttrans ~ Tbody | Microfabrication | 2017 | 50 | |
| PEG-PCL-Ac | Self-deploying | Ttrans ~ Tbody | Melt cast + UV cure | 2017 | 51 | ||
| PGD-Ac | Self-expanding | 20–37 °C tuned via cure time | 4D printing | 2021 | 52 | ||
| PCL-co-ACPCL | Self-wrapping | Ttrans ~ Tbody | Laser ablation | 2021 | 53 | ||
| Skeletal Muscle | Alginate carbodiimide linker | Myoblasts, IGF-1 and VEGF | Self-deploying | Hydroresponsive | Freeze drying | 2014 | 54 |
| Neuronal | CNT-doped sericin | Pre-seeded BMSCs | Self-deploying | Hydroresponsive | Freeze drying | 2021 | 55 |
Bone tissues.
Several smart scaffolds have been developed for bone TE. SMPU scaffolds containing PCL and poly(L-lactic acid) (PLLA) soft segments were blended with poly(ethylene glycol) (PEG) or gelatin to adjust viscosity for fused deposition modeling (FDM) fabrication. Due to water uptake by the PEG or gelatin, the Ttrans (Tg, PLLA ~50 °C) was lowered via plasticization to Tbody, affording a self-expanding scaffold. These scaffolds supported osteogenesis of hMSCs, which was further enhanced via incorporation of superparamagnetic iron oxide (SPIO) nanoparticles (NPs).35 Another SMPU, prepared using 6-arm star PLLA with an aniline trimer incorporated for electroactivity, showed improved osteogenic differentiation of myoblasts versus those prepared without aniline.36 SMP films based on UV-curable PCL-dimethacrylate (MA) (PCL-MA) (Ttrans = Tm, PCL ~54 °C) supported hMSC differentiation into osteoblasts, even following thermally-triggered shape recovery.37 Using solvent casting particulate leaching (SCPL), PCL-based scaffolds were prepared with diol-terminated PCL, dextran, and a carbodiimide linker, and then coated with bioactive hydroxyapatite (HAp) via solution precipitation. By adjusting the PCL/dextran ratio, the Ttrans (Tm, PCL/dextran) was adjusted to ~ Tbody to support self-expansion, and the scaffolds were shown to support bone MSC (BMSC) osteogenesis. In vivo degradation was also monitored in a rat subcutaneous model, and scaffolds were shown to degrade fully within ~6 months.38
Other bone TE smart scaffolds have been developed for more targeted scenarios. We have reported self-fitting scaffolds for treatment of irregularly-shaped craniomaxillofacial (CMF) bone defects. PCL-diacrylate (PCL-DA) scaffolds (Ttrans = Tm, PCL ~55 °C) were prepared via SCPL with a fused salt template for pore interconnectivity, and subsequently coated with a bioactive polydopamine coating.39 Scaffolds exhibited HAp mineralization in vitro, and, when modified with a cell adhesive peptide, were shown to support osteogenic hMSC differentiation.40 Intended to treat femoral segmental bone defects, self-expanding scaffolds were prepared from acrylate monomers and a crosslinker (tetraethylene glycol dimethacrylate, tEG-DMA) using SCPL. The ratio of tert-butyl acrylate (tBA) and butyl acrylate (BA) (92:8 wt%) was tuned to achieve a Ttrans ~ Tbody. These scaffolds were fixed into a compressed shape, and subsequent irrigation with 45 °C saline triggered scaffold expansion into a mouse femoral defect. Overall, these SMP grafts showed integration with native bone after 12 weeks, and torsional mechanical properties comparable to an allograft.41 To treat a confined femoral bone defect, a PCL-HAp SMP scaffold (Ttrans = Tm, PCL ~40 °C) was prepared via gas foaming. A shape fixed, compressed scaffold was implanted into a rabbit femoral defect, where irrigation with warm saline prompted self-expansion. After 12 weeks, bone ingrowth at the periphery and neovascularization was observed.42
Cartilage tissues.
Several smart scaffolds have been designed to repair cartilage, including the various types found in joints, ears, intervertebral discs, and trachea. Many of these are hydrogels and so are hydroresponsive. Targeted for articular cartilage repair, a self-expanding alginate gel, cross-linked via carbodiimide chemistry, was prepared with aligned pores via directional freezing. These scaffolds exhibited robust mechanical properties and were capable of reversible compression. Aligned pores allowed for improved collagen deposition by cultured hMSCs and was further improved with a Type II collagen coating.43 Another self-expanding hydrogel scaffold, prepared with collagen or denatured collagen and a carbodiimide cross-linker, was used to treat full thickness defects in the knee joints of NZ white rabbits. These smart scaffolds, optionally pre-seeded with chondrocytes, promoted cartilage and subchondral bone repair.44 Smart scaffolds were prepared via SCPL using poly(glycerol sebacate) (PGS) and poly(1,3-propylene sebacate) (PPS) as well as bioactive kartogenin (KGN). These acellular scaffolds could be prepared with a broad Ttrans (Tm, PGS ~35–45 °C), and so exhibited excellent shape recovery at Tbody as well as supported chondrogenic differentiation of BMSCs. Furthermore, acellular scaffolds were self-deployed into full-thickness defects of the rat femoropatellar groove where they supported chrondrogenic differentiation and formation of neocartilage.45 A smart scaffold was developed to mimic the complexly shaped auricular cartilage of human ears. PLLA threads were woven into mesh tubes that, upon heating above the Ttrans (Tg, PLLA ~60 °C), could be molded into helical shapes like those of human ears. These scaffolds were seeded with cartilaginous particles derived from human pluripotent stem (iPS) cells and implanted subcutaneously into a mouse model where the shape and cartilage features were maintained for one year.46 For tracheal repair, a smart scaffold was prepared from a PLLA iron-oxide (Fe3O4) nanocomposite (Ttrans = Tg, PLLA ~ 65 °C) using FDM. The Fe3O4 NPs were shown to permit magnetically-induced thermal actuation when exposed to a 30 kHz alternating magnetic field.47 Targeted for the repair of annulus fibrosus (AF) tissue in herniated intervertebral discs, a hydroresponsive, alginate/carbodiimide-linked self-expanding hydrogel scaffold was evaluated. These were shown to support AF cell proliferation and adhesion with extracellular matrix (ECM) secretion after 21 days in culture with transforming growth factor beta 3 (TGF-β3) supplementation.48
Cardiovascular tissues.
Smart scaffolds have been frequently directed towards cardiovascular TE, including in the form of cardiac patches, vascular grafts, and vascular wraps. Smart cardiac patches were prepared from a soybean oil epoxidized acrylate network as thin film with complex porous micropatterns using stereolithography. These patches were designed to be self-expanding (Ttrans ~ Tbody) and were shown to support hMSC cardiomyogenic differentiation.49 To permit minimally invasive delivery, a smart scaffold was prepared as an injectable cardiac patch from elastic poly(octamethylene maleate (anhydride) citrate)) microfabricated with a diamond-shaped lattice which drives shape recovery. These smart patches were seeded with rat cardiomyocytes (CMs) and were self-deployed (i.e. injected) into a myocardial infarction rat model where they were shown to increase wall thickness. The same type of cardiac patches, but seeded with human stem cell derived CMs and scaled up in size, were also successfully delivered via minimally invasive surgical techniques into a porcine model.50
Smart scaffolds have also been utilized to prepare vascular grafts. A 6-armed poly(ethylene glycol)-PCL-acrylate (PEG-PCL-Ac) was melt casted and UV-cured over a mold to afford micropatterned pores of different geometries on each side. To permit minimally invasive delivery, the scaffold (Ttrans ~ Tbody) was shape fixed into a tightly rolled conformation and expansion (i.e. shape recovery) triggered by body temperature. These were successfully implanted into the cervical artery of NZ white rabbits and supported endothelial cells (ECs) on the surface with vascular smooth muscle cells (VSMCs) on the inner surface.51 A smart vascular scaffold based on 4D-printed biodegradable poly(glycerol dodecanoate)-Ac (PGD-Ac) exhibited a tunable Ttrans (20–37 °C), depending on the duration of thermal curing. In this way, the scaffold was successfully implanted as a self-expanding vascular graft into a mouse aorta and supported endothelial and smooth muscle cell proliferation.52 A perivascular smart wrap based on PCL-co-(α-allyl carboxylate ε-caprolactone) (PCL-co-ACPCL) was UV-cured into a film and then porated via laser ablation. Following implantation into mouse subcutaneous tissue, the microporous scaffolds showed upregulated neovascularization, fibrogenesis, and angiogenesis. Having a Ttrans ~ Tbody, these scaffolds are expected to actuate upon implantation to afford self-wrapping.53
Other tissues.
For skeletal muscle, a freeze-dried alginate/carbodiimide-linked self-deploying hydroresponsive scaffold was delivered via minimally invasive techniques in a severe skeletal muscle mouse injury model. The dehydrated scaffold was loaded into a needle and the syringe then filled with myoblasts and growth factors (e.g. insulin-like growth factor 1 (IGF-1) and vascular endothelial growth factor (VEGF)) to improve cell engraftment and muscle contraction.54 To treat neuronal tissue damage caused by severe ischemic stroke, hydroresponsive scaffolds based on carbon nanotube (CNT)-doped silkworm sericin were prepared with geometries to match that of an irregularly-shaped cavity in an ischemic stroke mouse model. Following pre-seeding with BMSCs, these scaffolds were delivered via minimally invasive procedures and were shown to support neuronal differentiation.55
SMP materials, scaffold fabrication, and property tunability
As evidenced by the many smart scaffolds evaluated for various TE applications, a variety of SMP materials have been utilized (Figure 4). All SMPs must include two functional design elements: netpoints (responsible for the memorized shape or “shape recovery”) and switching segments (responsible for the temporary programmed shape or “shape fixity”). These elements can be achieved via differing network designs, including physically cross-linked, chemically cross-linked, or PUs, which may rely on physical and/or chemical cross-linking. Variations of each of these affords additional tunability of shape memory behavior, and important material properties (e.g. modulus, and degradation rate). Moreover, fabrication methods can be used to further enhance SMP scaffold efficacy.
Figure 4.
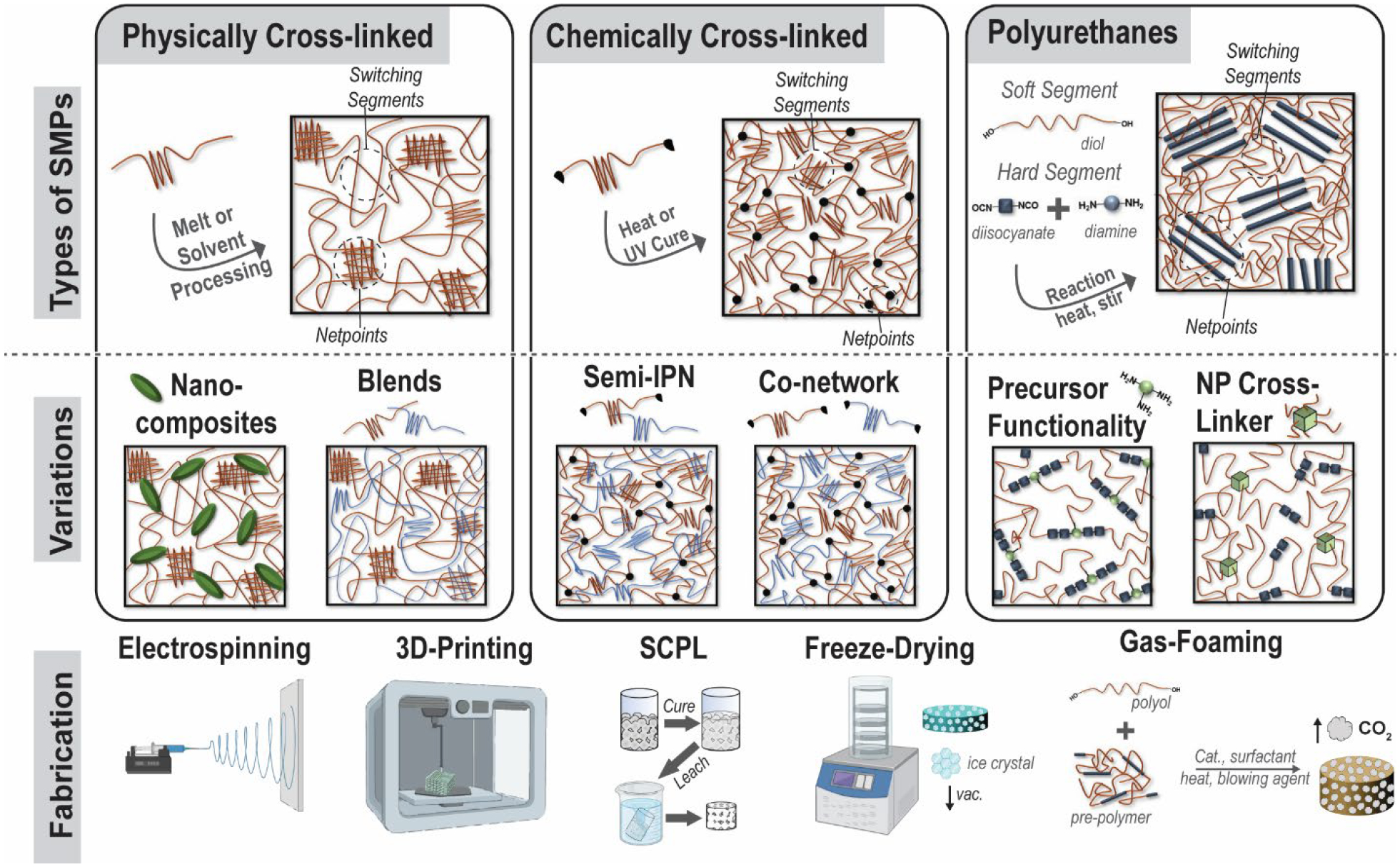
(top) Classes of SMPs used to prepare smart scaffolds: physically cross-linked, chemically cross-linked, and PU systems. (middle) Variations to these main SMP classes that afford property tunability. (bottom) Different fabrication methods used to achieve smart scaffolds with targeted morphological features.
Physically cross-linked.
As noted above, two examples of PLLA-based physically cross-linked smart scaffolds were developed toward the regeneration of ear cartilage and tracheal tissue. For thermoplastic PLLA, where crystalline lamellae serve as netpoints and amorphous chain segments act as switching segments. Typically, Tg of PLLA (Tg ~ 50–65 °C, depending on molecular weight)56 serves as the Ttrans, and so body temperature cannot trigger shape recovery. However, this Ttrans range is ideal for maintaining a fixed shape upon implantation, as for Uto’s ear regeneration study.46 Alternatively, incorporating magnetic nanoparticles, as in the tracheal scaffold study, can provide remote shape actuation of PLLA-based smart scaffolds.47
Physically cross-linked SMPs may be refined via copolymerization, formation of nanocomposites, and by blending. Poly(D,L-lactide)-co-trimethylene carbonate (PDLLA-TMC) and poly(lactide-glycolide)-TMC (PLGA-TMC) copolymers were electrospun into scaffolds for bone and vascular TE, respectively. The TMC was used to tune the Tg to ~ Tbody and physical cross-links were afforded by chain entanglements.57, 58 Likewise, biodegradable amorphous poly(propylene carbonate) (PPC) exhibits shape memory (Ttrans ~ Tg ~ 60 °C) that was shown to be enhanced following the addition of microfibrillated cellulose as a composite.59 PLA-based composites have also been prepared with chitosan for potential antimicrobial activity60 as well as with graphene for electrical SMP activation.61 Blending PLA with PCL has been commonly used as a strategy to improve PLA toughness, and depending on the PLA/PCL weight percent, has also been shown to exhibit shape memory with PCL crystalline lamellae as the switching segment (Ttrans ~ Tm,PCL ~55 °C).62 PLA/PCL blends have also been combined with nanohydroxyapatite (nHA) as bioactive nanocomposites.63 Physically immiscible blends, like the PLA/PCL constructs, attain shape memory behavior due to phase separation, which allows one phase (PLA) to behave as the netpoints, while the other phase (PCL) behaves as the switching segment. PLLA has also been blended, but in a miscible system, with poly(3-hydroxybutarate-co-hydroxyvalerate) (PHBV) to lower Ttrans toward Tbody and PLLA/PHBV blends prepared as scaffolds via electrospinning were shown to support osteogenesis of BMSCs.64, 65 Miscible PLLA/poly(methyl-methacrylate) (PMMA) blends have been shown to have a broad Tg that allows for triple shape memory.66 PLLA/poly(vinyl acetate) (PVAc) blends were also shown to be miscible but the incorporation of nanohydroxyapatite (nHA) or graphene can lead to phase separation and induce triple shape memory via two distinct thermal transitions.61, 67 The addition of nHA may also be advantageous to bone TE in providing an osteoconductive environment for mineralization, while nanocarbons like graphene may allow for electrical trigger of shape memory via resistive heating.
Chemically cross-linked.
Various chemically cross-linked SMPs have been used to prepare smart scaffolds for TE, as noted above. In these systems, chemical cross-links act as netpoints while polymer segments between cross-links act as switching segment. Like physically cross-linked systems, shape memory can be actuated thermally and/or via hydration but chemically cross-linked systems tend to have more robust shape recovery. Acrylate (Ac)- or methacrylate (MA)-based networks, prepared from macromers with these terminal crosslinkable groups, have been commonly used toward both bone and cardiovascular TE.37, 39, 40, 49, 53 These form networks comprised of crosslinks (i.e. netpoints) consisting of hydrolysable esters. Semi-crystalline PCL has been particularly utilized, since the Tm (~43–60°C) generally decreases with decreased molecular weight (Mn), the Ttrans (Tm) may be tuned.68 Additional modifications to PCL-network scaffolds can afford properties useful to regeneration. For instance, we have made numerous modifications to smart scaffolds for CMF defects formed from PCL networks using SCPL fabrication.39, 69 To impart bioactivity, a polydopamine coating was applied to PCL-DA scaffolds.40 In another approach, SMP scaffolds were likewise prepared as co-networks with macromers comprised of PCL and polydimethylsiloxane (PDMS) segments.70 Attributed to the silicon-based and hydrophobic nature of the PDMS, these PCL-PDMS scaffolds exhibited HAp mineralization in vitro. Using a semi-interpenetrating network (semi-IPN) design based on PCL-DA and thermoplastic PLLA scaffolds were also prepared with accelerated degradation and increased modulus.71 Lastly, using a star-PCL architecture, we showed that the Ttrans could be reduced to ~45 °C (Tm,PCL) for improved tissue safety during thermally driven self-fitting. Cardiovascular TE scaffolds based on PGD-Ac networks represent another type of acrylate network with PGD switching segments having a Ttrans (Tg, PGD) that can be readily tuned to ~Tbody via cure time.72 Acrylate-based networks have also been used to form other chemically crosslinked SMP scaffolds. These include bone TE scaffolds based on butyl-based monomers as well as soybean epoxidize acrylate. As noted above, the Ttrans (Tg) can be tuned to ~ Tbody with monomer ratio41 and could also be formed with acrylated PEG to afford plasticization (i.e. Tg lowering) due to hydration.73 Similarly, methacrylate (MA) macromers were used to form PCL/PEG (“CLEG”) co-network hydrogels with shape memory properties.74 PTMC-MA macromers have also been used to form chemically cross-linked SMPs, and have been combined as nanocomposites with PLA fibers,75 plasticized with PEG co-networks, and copolymerized with PDLLA to tune properties.76 Chemical cross-linking moieties based on radical crosslinking can also be introduced into polymer repeat unit pendant groups or backbone. For instance, PCL-based copolymer having crosslinkable pendant allyl groups were used to prepare SMP scaffolds for vascular TE and whose Ttrans (Tm) could be adjusted to ~ Tbody based on the ratio of ε-caprolactone (CL) to α-allyl carboxylate-CL.53, 77 Co-polyesters containing fumaric acid (FA) segments, bearing crosslinkable double bonds in the backbone, have been prepared as hydroresponsive scaffolds via SCPL.78 SMP scaffolds for bone TE were recently prepared by 4D printing of poly(propylene fumarate) (PPF). These PFF scaffolds exhibited a tunable Ttrans (Tg ~30–40 °C) depending on cure time.79
Chemically cross-linked networks have been prepared with other cross-linking chemistries as well. Examples include, PTMC cross-linked with a bis(cyclic carbonate) via ring opening polymerization (ROP),80 PCL/PEG co-networks cross-linked via thiol-ene chemistry,81 and alginate or collagen hydrogels cross-linked with via carbodiimide chemistry.43, 44, 48, 54, 82, 83 Several other carbodiimide cross-linked hydrogel scaffolds were described earlier, often prepared via freeze-drying or lyophilization.43, 48, 54 Smart hydrogel scaffolds have also been prepared as interpenetrating networks (IPN) cryogels consisting of covalently and permanently crosslinked polyacrylamide (PAAm) and covalently and dynamically crosslinked oligoethylene glycol (OEG)-based dendronized polymers. The Schiff-base cross-linking allow for dynamic, pH-modulated control over shape memory.84
Polyurethanes.
SMPUs are a distinct class of widely studies SMPs, relying on physical cross-linking (thermoplastics) or chemical cross-linking (thermosets). SMPUs are generally characterized by thermodynamically immiscible soft segments (switching segments) and hard segments (netpoints). These are connected via urethane linkages formed through reactions of alcohol and isocyanate moieties. Chain extenders, low molecular weight diols (or diamines), may be reacted with diisocyanates to increase the length of the hard segments.85 Thermoplastic PUs (TPUs) are formed into physically cross-linked PU scaffolds frequently via electrospinning or 3D-printing, where the molecular weight of these types of chains yields conducive rheological properties. TPUs have been modified in many common ways that have been previously described for other SMP systems, including: PCL-based co-polymers,86, 87 PLA-based blends,88, 89 bio-based (i.e. castor oil) precursors,7 combining with hydrophilic polymers,35 and nanocomposites.88 CNT,90 HAp,91 and iron-based NPs seen in previous systems have been employed in TPUs. One nanocomposite SMP not previously discussed is a PLA-based TPU with nanosilicates, shown to induce HAp mineralization for bone TE.92
Chemically cross-linked SMPU porous scaffolds are frequently formed via gas foaming. In this process, pre-polymers with isocyanate moieties react with polyols to produce PU linkages, and carbon dioxide (CO2) generation occurs as a by-product of the reaction between excess isocyanates and water. This process also usually employs a combination of catalysts, surfactants, heat, and blowing agents.93, 94 Maitland et al. utilized gas foaming to create smart SMPU embolization devices,25, 95, 96 including those that are modified with tungsten NPs for radio-opacity.97 Other chemically cross-linked SMPUs have used NPs that are functionalized to act as chemical cross-linkers. For instance, oxidized CNTs98 and star-shaped polyester having a polyhedral oligomeric silsesquioxane (POSS)core99 have been employed as multifunctional cross-linkers. Polymers typically used to construct smart scaffolds and methods to achieve tunability of properties are summarized in Figure 5. In addition to thermoresponsive and hydroresponsive SMP scaffolds, another class of SMPs are elastomers that can be fabricated into mesh lattices (typically via 3D printing) whose geometries support SMP abilities, as previously noted for the cardiac patch example.50, 100 To participate in cell-like signaling, other SMP scaffolds may leverage dynamic linkages, including those based on Schiff bases18 and ionic chemistries. One major class of these are chitosan-based SMP systems as chitosan can crosslink via link via physical, chemical, or ionic linkages.101, 102 The ionic linkages have the potential to be controlled by pH,103 and so could be utilized to produce shape memory behavior.
Figure 5.
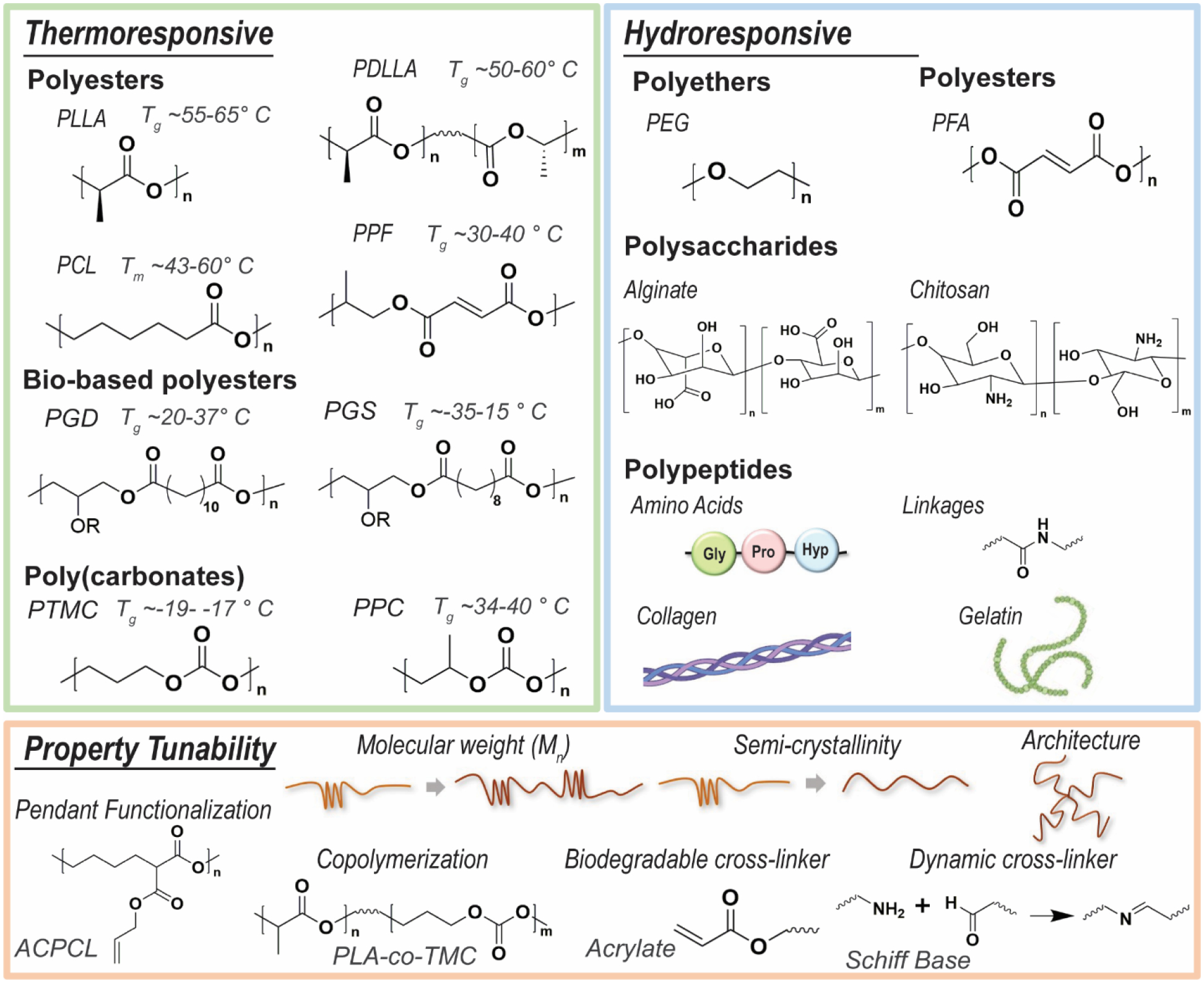
SMPs typically used to achieve smart scaffolds with thermoresponsive and/or hydroresponsive behaviors, and additional ways that SMP scaffold properties may be tuned.
Conclusions
Smart scaffolds based on thermoresponsive and hydroresponsive SMPs hold vast potential to advance the regeneration of numerous tissues, particularly bone, cartilage, and cardiovascular tissues. The shape memory ability of smart scaffolds has largely been leveraged to achieve tissue defect filling, either via self-deploying, self-expanding, or self-fitting. This unique feature affords convenient routes for implantation as well as superior scaffold-tissue contact to promote healing. Collectively, SMP scaffolds possess a breadth of useful properties that afford further tunability through a variety of strategies. SMP materials used to prepare scaffolds can be classified as physically cross-linked, chemically cross-linked, or SMPUs. Within each of these classes of SMPs, a variety of fabrication methods can be used to prepare smart scaffolds with specific morphological features. More recently, the shape shifting ability of SMPs has been utilized to respond to cellular triggers104 or for on/off switching of cell behavior (e.g. alignment and differentiation).105–107 Such SMPs have the potential to create smart scaffolds with greater control over directing tissue regeneration.
Increasing evidence of the utility of smart scaffolds is supported by a growing body of work with cultured stem cells as well as with animal models. However, a remaining challenge is clinical translation, evident in the lack of smart scaffolds currently available on the market. The first SMP device to receive FDA approval as a permanent implant was the WedgeLoc suture anchor, now referred to as Morphix®, as noted herein in the Introduction.15, 108 Also noted in the Introduction, the Impede-FX SMP device recently gained FDA 510 (k) clearance in 2019 as an embolization plug. Significantly, this is the first porous and biodegradable SMP device to achieve such clearance.16, 17 A regulatory hurdle to consider is that 510 (k) clearance relies on comparisons to a “substantially equivalent” approved device. This may make the pathway to approval more feasible for smart scaffolds that can act as substitutes for existing devices such as bone void fillers.109 Moreover, the incorporation of functional additives, and/or pre-seeded cells may present further regulatory hurdles as they convolute device classification. But the FDA recently released a new guidance on evaluation of regenerative devices to “simplify and streamline” approval of such combinatory therapies.110 Thus, the development of smart scaffolds is making promising headway towards clinical translation in regenerative engineering.
Acknowledgements
Funding from NIH NIDCR 1R01DE025886-01A1 and the Texas A&M Engineering Experiment Station is gratefully acknowledged.
Footnotes
Conflicts of interest
The authors declare no competing financial interest.
References
- 1.Behl M and Lendlein A, Mater. Today Commun, 2007, 10, 20–28. [Google Scholar]
- 2.Behl M and Lendlein A, Soft Matter, 2007, 3, 58–67. [DOI] [PubMed] [Google Scholar]
- 3.Lendlein A, J. Mater. Chem, 2010, 20, 3332–3334. [Google Scholar]
- 4.Lendlein A and Langer R, Science, 2002, 296, 1673. [DOI] [PubMed] [Google Scholar]
- 5.Delaey J, Dubruel P and Van Vlierberghe S, Adv. Funct. Mater, 2020, 30, 1909047. [Google Scholar]
- 6.Lendlein A, Behl M, Hiebl B and Wischke C, Expert Rev. Med. Devices, 2010, 7, 357–379. [DOI] [PubMed] [Google Scholar]
- 7.Hasan SM, Nash LD and Maitland DJ, J. Polym. Sci., Part B: Polym. Phys, 2016, 54, 1300–1318. [Google Scholar]
- 8.Small IVW, Singhal P, Wilson TS and Maitland DJ, J. Mater. Chem, 2010, 20, 3356–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakacki CM and Gall K, in Shape-Memory Polymers, ed. Lendlein A, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 147–175. [Google Scholar]
- 10.Wang K, Strandman S and Zhu XX, Front. Chem. Sci. Eng, 2017, 11, 143–153. [Google Scholar]
- 11.Chen H-M, Wang L and Zhou S-B, Chin. J. Polym. Sci, 2018, 36, 905–917. [Google Scholar]
- 12.Vernon L and Vernon H, US patent, 1941, 2234993. [Google Scholar]
- 13.Metz RM and Kaar SG, Oper. Tech. Orthop, 2020, 30, 100818. [Google Scholar]
- 14.Christensen J, Fischer B, Nute M and Rizza R, J. Foot Ankle Surg, 2018, 57, 60–64. [DOI] [PubMed] [Google Scholar]
- 15.Berg-Johansen B, Lovald S, Altiok E and Kurtz SM, in PEEK Biomaterials Handbook (Second Edition), ed. Kurtz SM, William Andrew Publishing, 2019, pp. 291–300. [Google Scholar]
- 16.Morgan RA, Loftus I, Ratnam L, Das R, Mailli L, Hamady M and Lobotesis K, CVIR Endovasc, 2021, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen A-JS, van Schaik PM, Martens JM and Reijnen MM, CVIR Endovasc, 2020, 3, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang K, Wang S, Zhou C, Cheng L, Gao X, Xie X, Sun J, Wang H, Weir MD, Reynolds MA, Zhang N, Bai Y and Xu HHK, Bone Res, 2018, 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhandayuthapani B, Yoshida Y, Maekawa T and Kumar DS, Int. J. Polym. Sci, 2011, 2011, 290602. [Google Scholar]
- 20.Qu H, Fu H, Han Z and Sun Y, RSC Adv, 2019, 9, 26252–26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollister SJ, Nat. Mater, 2005, 4, 518–524. [DOI] [PubMed] [Google Scholar]
- 22.Leng J, Lan X, Liu Y and Du S, Prog. Mater. Sci, 2011, 56, 1077–1135. [Google Scholar]
- 23.Liu Y, Lv H, Lan X, Leng J and Du S, Compos. Sci. Technol, 2009, 69, 2064–2068. [Google Scholar]
- 24.Shang J, Le X, Zhang J, Chen T and Theato P, Polym. Chem, 2019, 10, 1036–1055. [Google Scholar]
- 25.Jang LK, Fletcher GK, Monroe MBB and Maitland DJ, J. Biomed. Mater. Res. A, 2020, 108, 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai S, Sun Y-C, Ren J and Naguib HE, J. Mater. Chem. B, 2017, 5, 8845–8853. [DOI] [PubMed] [Google Scholar]
- 27.Lennon AB and Prendergast PJ, J. Biomech, 2002, 35, 311–321. [DOI] [PubMed] [Google Scholar]
- 28.Lobb DC, DeGeorge BR and Chhabra AB, J. Hand Surg, 2019, 44, 497–505. e492. [DOI] [PubMed] [Google Scholar]
- 29.Orr JF, Dunne NJ and Quinn JC, Biomaterials, 2003, 24, 2933–2940. [DOI] [PubMed] [Google Scholar]
- 30.Hou Q, De Bank PA and Shakesheff KM, J. Mater. Chem, 2004, 14, 1915–1923. [Google Scholar]
- 31.Migliaresi C, Motta A and DiBenedetto AT, in Engineering of Functional Skeletal Tissues, eds. Bronner F, Farach-Carson MC and Mikos AG, Springer London, London, 2007, pp. 95–109. [Google Scholar]
- 32.Van der Stok J, Van Lieshout EM, El-Massoudi Y, Van Kralingen GH and Patka P, Acta Biomater, 2011, 7, 739–750. [DOI] [PubMed] [Google Scholar]
- 33.Low KL, Tan SH, Zein SH, Roether JA, Mouriño V and Boccaccini AR, J. Biomed. Mater. Res. B Appl. Biomater, 2010, 94, 273–286. [DOI] [PubMed] [Google Scholar]
- 34.Serbetci K, Korkusuz F and Hasirci N, Polym. Test, 2004, 23, 145–155. [Google Scholar]
- 35.Wang Y-J, Jeng US and Hsu S.-h., ACS Biomater. Sci. Eng, 2018, 4, 1397–1406. [DOI] [PubMed] [Google Scholar]
- 36.Xie M, Wang L, Ge J, Guo B and Ma PX, ACS Appl. Mater. Interfaces, 2015, 7, 6772–6781. [DOI] [PubMed] [Google Scholar]
- 37.Neuss S, Blomenkamp I, Stainforth R, Boltersdorf D, Jansen M, Butz N, Perez-Bouza A and Knüchel R, Biomaterials, 2009, 30, 1697–1705. [DOI] [PubMed] [Google Scholar]
- 38.Huang K, Yang M.-s., Tang Y.-j., Ling S-Y, Pan F, Liu X.-d. and Chen J, J. Biomater. Appl, 2020, 35, 823–837. [DOI] [PubMed] [Google Scholar]
- 39.Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS and Grunlan MA, Acta Biomater, 2014, 10, 4597–4605. [DOI] [PubMed] [Google Scholar]
- 40.Erndt-Marino JD, Munoz-Pinto DJ, Samavedi S, Jimenez-Vergara AC, Diaz-Rodriguez P, Woodard L, Zhang D, Grunlan MA and Hahn MS, ACS Biomater. Sci. Eng, 2015, 1, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker RM, Tseng L-F, Iannolo MT, Oest ME and Henderson JH, Biomaterials, 2016, 76, 388–398. [DOI] [PubMed] [Google Scholar]
- 42.Xie R, Hu J, Hoffmann O, Zhang Y, Ng F, Qin T and Guo X, Biochim. Biophys. Acta, 2018, 1862, 936–945. [DOI] [PubMed] [Google Scholar]
- 43.Almeida HV, Sathy BN, Dudurych I, Buckley CT, O’Brien FJ and Kelly DJ, Tissue Eng. Part A, 2016, 23, 55–68. [DOI] [PubMed] [Google Scholar]
- 44.Jiang LB, Su DH, Liu P, Ma YQ, Shao ZZ and Dong J, Osteoarthr. Cartil, 2018, 26, 1389–1399. [DOI] [PubMed] [Google Scholar]
- 45.Xuan H, Hu H, Geng C, Song J, Shen Y, Lei D, Guan Q, Zhao S and You Z, Acta Biomater, 2020, 105, 97–110. [DOI] [PubMed] [Google Scholar]
- 46.Uto S, Hikita A, Sakamoto T, Mori D, Yano F, Ohba S, Saito T, Takato T and Hoshi K, Tissue Eng. Part A, 2020. [DOI] [PubMed]
- 47.Zhao W, Zhang F, Leng J and Liu Y, Compos. Sci. Technol, 2019, 184, 107866. [Google Scholar]
- 48.Guillaume O, Daly A, Lennon K, Gansau J, Buckley SF and Buckley CT, Acta Biomater, 2014, 10, 1985–1995. [DOI] [PubMed] [Google Scholar]
- 49.Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, Fisher JP and Zhang LG, Sci. Rep, 2016, 6, 27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montgomery M, Ahadian S, Davenport Huyer L, Lo Rito M, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li R-K, Caldarone CA and Radisic M, Nat. Mater, 2017, 16, 1038–1046. [DOI] [PubMed] [Google Scholar]
- 51.Liu D, Xiang T, Gong T, Tian T, Liu X and Zhou S, ACS Appl. Mater. Interfaces, 2017, 9, 19725–19735. [DOI] [PubMed] [Google Scholar]
- 52.Zhang C, Cai D, Liao P, Su J-W, Deng H, Vardhanabhuti B, Ulery BD, Chen S-Y and Lin J, Acta Biomater, 2021, 122, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boire TC, Himmel LE, Yu F, Guth CM, Dollinger BR, Werfel TA, Balikov DA and Duvall CL, J. Biomed. Mater. Res. A, 2021, 109, 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Cao L, Shansky J, Wang Z, Mooney D and Vandenburgh H, Mol. Ther, 2014, 22, 1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Li X, Song Y, Su Q, Xiaohalati X, Yang W, Xu L, Cai B, Wang G, Wang Z and Wang L, Bioact. Mater, 2021, 6, 1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed J and Varshney SK, Int. J. Food Prop, 2011, 14, 37–58. [Google Scholar]
- 57.Bao M, Lou X, Zhou Q, Dong W, Yuan H and Zhang Y, ACS Appl. Mater. Interfaces, 2014, 6, 2611–2621. [DOI] [PubMed] [Google Scholar]
- 58.Chen M, Li L, Xia L, Zhang F, Jiang S, Hu H, Li X and Wang H, Macromol. Biosci, 2020, 20, 1900312. [DOI] [PubMed] [Google Scholar]
- 59.Qi X, Yang G, Jing M, Fu Q and Chiu F-C, Journal of Materials Chemistry A, 2014, 2, 20393–20401. [Google Scholar]
- 60.Pandey A, Singh G, Singh S, Jha K and Prakash C, J. Mech. Behav. Biomed. Mater, 2020, 108, 103781. [DOI] [PubMed] [Google Scholar]
- 61.Sabzi M, Babaahmadi M and Rahnama M, ACS Appl. Mater. Interfaces, 2017, 9, 24061–24070. [DOI] [PubMed] [Google Scholar]
- 62.Navarro-Baena I, Sessini V, Dominici F, Torre L, Kenny JM and Peponi L, Polym. Degrad. Stab, 2016, 132, 97–108. [Google Scholar]
- 63.Peponi L, Sessini V, Arrieta MP, Navarro-Baena I, Sonseca A, Dominici F, Gimenez E, Torre L, Tercjak A, López D and Kenny JM, Polym. Degrad. Stab, 2018, 151, 36–51. [Google Scholar]
- 64.Wang X, Yan H, Zhou Y, Lou X and Zhang Y, J. Control. Release, 2017, 259, e144–e145. [Google Scholar]
- 65.Wang X, Yan H, Shen Y, Tang H, Yi B, Qin C and Zhang Y, Tissue Eng. Part A, 2020, 27, 142–152. [DOI] [PubMed] [Google Scholar]
- 66.Samuel C, Barrau S, Lefebvre J-M, Raquez J-M and Dubois P, Macromolecules, 2014, 47, 6791–6803. [Google Scholar]
- 67.Amini M and Wu S, Comp. Comm, 2021, 23, 100564. [Google Scholar]
- 68.Wang S, Lu L, Gruetzmacher JA, Currier BL and Yaszemski MJ, Biomaterials, 2006, 27, 832–841. [DOI] [PubMed] [Google Scholar]
- 69.Nail LN, Zhang D, Reinhard JL and Grunlan MA, JoVE, 2015, e52981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beltran FO, Houk CJ and Grunlan MA, ACS Biomater. Sci. Eng, 2021, 7, 1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woodard LN, Kmetz KT, Roth AA, Page VM and Grunlan MA, Biomacromolecules, 2017, 18, 4075–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang C, Cai D, Liao P, Su J-W, Deng H, Vardhanabhuti B, Ulery BD, Chen S-Y and Lin J, Acta Biomater, 2021, 122, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antony GJM, Jarali CS, Aruna ST and Raja S, J. Mech. Behav. Biomed. Mater, 2017, 65, 857–865. [DOI] [PubMed] [Google Scholar]
- 74.Nöchel U, Reddy CS, Uttamchand NK, Kratz K, Behl M and Lendlein A, Eur. Polym. J, 2013, 49, 2457–2466. [Google Scholar]
- 75.Zhang X, Geven MA, Grijpma DW, Peijs T and Gautrot JE, Polymer, 2017, 122, 323–331. [Google Scholar]
- 76.Sharifi S, van Kooten TG, Kranenburg H-JC, Meij BP, Behl M, Lendlein A and Grijpma DW, Biomaterials, 2013, 34, 8105–8113. [DOI] [PubMed] [Google Scholar]
- 77.Boire TC, Gupta MK, Zachman AL, Lee SH, Balikov DA, Kim K, Bellan LM and Sung H-J, Acta Biomater, 2016, 34, 73–83. [DOI] [PubMed] [Google Scholar]
- 78.Xie Y, Lei D, Wang S, Liu Z, Sun L, Zhang J, Qing F-L, He C and You Z, ACS Biomater. Sci. Eng, 2019, 5, 1668–1676. [Google Scholar]
- 79.Le Fer G and Becker ML, ACS Appl. Mater. Interfaces, 2020, 12, 22444–22452. [DOI] [PubMed] [Google Scholar]
- 80.Yang L-Q, He B, Meng S, Zhang J-Z, Li M, Guo J, Guan Y-M, Li J-X and Gu Z-W, Polymer, 2013, 54, 2668–2675. [Google Scholar]
- 81.Baker RM, Henderson JH and Mather PT, J. Mater. Chem. B, 2013, 1, 4916–4920. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Shansky J, Borselli C, Mooney D and Vandenburgh H, Tissue Eng. Part A, 2012, 18, 2000–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang K, Yang M.-s., Tang Y.-j., Ling S-Y, Pan F, Liu X.-d. and Chen J, J. Biomater. Appl, 2020, 0885328220950062. [DOI] [PubMed]
- 84.Zhang X, Liu K, Liu J, Ding Y, Li W and Zhang A, Eur. Polym. J, 2020, 141, 110092. [Google Scholar]
- 85.Touchet TJ and Cosgriff-Hernandez EM, in Advances in Polyurethane Biomaterials, eds. Cooper SL and Guan J, Woodhead Publishing, 2016, pp. 3–22. [Google Scholar]
- 86.Matsumoto H, Ishiguro T, Konosu Y, Minagawa M, Tanioka A, Richau K, Kratz K and Lendlein A, Eur. Polym. J, 2012, 48, 1866–1874. [Google Scholar]
- 87.Kai D, Prabhakaran MP, Chan BQY, Liow SS, Ramakrishna S, Xu F and Loh XJ, Biomed. Mater, 2016, 11, 015007. [DOI] [PubMed] [Google Scholar]
- 88.Song JJ, Chang HH and Naguib HE, Eur. Polym. J, 2015, 67, 186–198. [Google Scholar]
- 89.Gu S-Y, Liu L-L and Gao X-F, Polym. Int, 2015, 64, 1155–1162. [Google Scholar]
- 90.Shao L.-n., Dai J, Zhang Z.-x., Yang J.-h., Zhang N, Huang T and Wang Y, RSC Adv., 2015, 5, 101455–101465. [Google Scholar]
- 91.Yu J, Xia H, Teramoto A and Ni Q-Q, J. Biomed. Mater. Res. A, 2018, 106, 244–254. [DOI] [PubMed] [Google Scholar]
- 92.Yan B, Gu S and Zhang Y, Eur. Polym. J, 2013, 49, 366–378. [Google Scholar]
- 93.Sambasivam M, White R and Cutting K, in Wound Healing Biomaterials, ed. Ågren MS, Woodhead Publishing, 2016, pp. 251–260. [Google Scholar]
- 94.Ashida K and Iwasaki K, in Handbook of Plastic Foams, ed. Landrock AH, William Andrew Publishing, Park Ridge, NJ, 1995, pp. 11–220. [Google Scholar]
- 95.Singhal P, Rodriguez JN, Small W, Eagleston S, Van de Water J, Maitland DJ and Wilson TS, J. Polym. Sci., Part B: Polym. Phys, 2012, 50, 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singhal P, Small W, Cosgriff-Hernandez E, Maitland DJ and Wilson TS, Acta Biomater, 2014, 10, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hasan SM, Harmon G, Zhou F, Raymond JE, Gustafson TP, Wilson TS and Maitland DJ, Polym. Adv. Technol, 2016, 27, 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Y, Jiang J-S, Liu Z-W, Zeng Y and Zhang W-G, Compos. Part A Appl. Sci. Manuf, 2013, 53, 16–23. [Google Scholar]
- 99.Bothe M, Mya KY, Jie Lin EM, Yeo CC, Lu X, He C and Pretsch T, Soft Matter, 2012, 8, 965–972. [Google Scholar]
- 100.Montgomery M, Davenport Huyer L, Bannerman D, Mohammadi MH, Conant G and Radisic M, ACS Biomater. Sci. Eng, 2018, 4, 3691–3703. [DOI] [PubMed] [Google Scholar]
- 101.Liu Y, Fang N, Liu B, Song L, Wen B and Yang D, Mater. Lett, 2018, 233, 78–81. [Google Scholar]
- 102.Gao H-L, Lu Y, Mao L-B, An D, Xu L, Gu J-T, Long F and Yu S-H, Mater. Horiz, 2014, 1, 69–73. [Google Scholar]
- 103.Yan K, Xu F, Li S, Li Y, Chen Y and Wang D, Colloids Surf. B: Biointerfaces, 2020, 190, 110907. [DOI] [PubMed] [Google Scholar]
- 104.Lendlein A, Balk M, Tarazona NA and Gould OEC, Biomacromolecules, 2019, 20, 3627–3640. [DOI] [PubMed] [Google Scholar]
- 105.Wang J, Quach A, Brasch ME, Turner CE and Henderson JH, Biomaterials, 2017, 140, 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tseng L-F, Mather PT and Henderson JH, Acta Biomater, 2013, 9, 8790–8801. [DOI] [PubMed] [Google Scholar]
- 107.Yang P, Baker RM, Henderson JH and Mather PT, Soft Matter, 2013, 9, 4705–4714. [Google Scholar]
- 108.Safranski DL and Griffis JC, in Shape-Memory Polymer Device Design, eds. Safranski DL and Griffis JC, William Andrew Publishing, 2017, pp. 189–222. [Google Scholar]
- 109.Webber MJ, Khan OF, Sydlik SA, Tang BC and Langer R, Ann. Biomed. Eng, 2015, 43, 641–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belouin T, Journal, 2019, 84, 4821–4823. [Google Scholar]


