Abstract
Temporomandibular joint (TMJ) osteoarthritis is a common chronic degenerative disease of the TMJ. In order to explore its aetiology and pathological mechanism, many animal models and cell models have been constructed to simulate the pathological process of TMJ osteoarthritis. The main pathological features of TMJ osteoarthritis include chondrocyte death, extracellular matrix (ECM) degradation and subchondral bone remodelling. Chondrocyte apoptosis accelerates the destruction of cartilage. However, autophagy has a protective effect on condylar chondrocytes. Degradation of ECM not only changes the properties of cartilage but also affects the phenotype of chondrocytes. The loss of subchondral bone in the early stages of TMJ osteoarthritis plays an aetiological role in the onset of osteoarthritis. In recent years, increasing evidence has suggested that chondrocyte hypertrophy and endochondral angiogenesis promote TMJ osteoarthritis. Hypertrophic chondrocytes secrete many factors that promote cartilage degeneration. These chondrocytes can further differentiate into osteoblasts and osteocytes and accelerate cartilage ossification. Intrachondral angiogenesis and neoneurogenesis are considered to be important triggers of arthralgia in TMJ osteoarthritis. Many molecular signalling pathways in endochondral osteogenesis are responsible for TMJ osteoarthritis. These latest discoveries in TMJ osteoarthritis have further enhanced the understanding of this disease and contributed to the development of molecular therapies. This paper summarizes recent cognition on the pathogenesis of TMJ osteoarthritis, focusing on the role of chondrocyte hypertrophy degeneration and cartilage angiogenesis.
Keywords: angiogenesis, bone remodelling, cartilage degeneration, chondrocyte hypertrophy, osteoarthritis, temporomandibular joint
1. INTRODUCTION
Temporomandibular joint (TMJ) osteoarthritis is a progressive degenerative cartilage disease that affects cartilage and subchondral bone. 1 , 2 Chondrocyte death, extracellular matrix (ECM) degradation and subchondral bone remodelling are considered to be the main characteristics of TMJ osteoarthritis. 3 , 4 The characteristic progressive breakdown of cartilage results from the abnormal regulation of chondrocytes and the imbalance between the degradation and formation of tissue.
Chondrocytes maintain balance between synthesis and degradation of the ECM. 5 However, this homeostasis can be disrupted by the uncoupled catabolic and synthetic activities. 6 Moreover, the number of apoptotic chondrocytes increases significantly in the osteoarthritis, which is closely related to the endoplasmic reticulum and death receptor pathways. 7 , 8 The quality of condylar bone is closely related to the development of TMJ osteoarthritis. When TMJ osteoarthritis occurs, the density of subchondral bone is reduced. 4 , 9
The mechanism and role of chondrocyte hypertrophy and cartilage angiogenesis in TMJ osteoarthritis have attracted the attention of researchers. Hypertrophic chondrocytes promote ECM degradation and cartilage calcification. This denatured cartilage shows less mechanical adaption to adverse stimuli such as trauma. 10 Apart from hypertrophic chondrocytes, angiogenesis has been reported to participate in TMJ osteoarthritis. 11 Neo‐angiogenesis has been shown to exacerbate chronic pain and promote cartilage ossification. 12 , 13 However, the exact mechanism is unclear. The possible pathogenesis of TMJ osteoarthritis is summarized in Figure 1.
FIGURE 1.
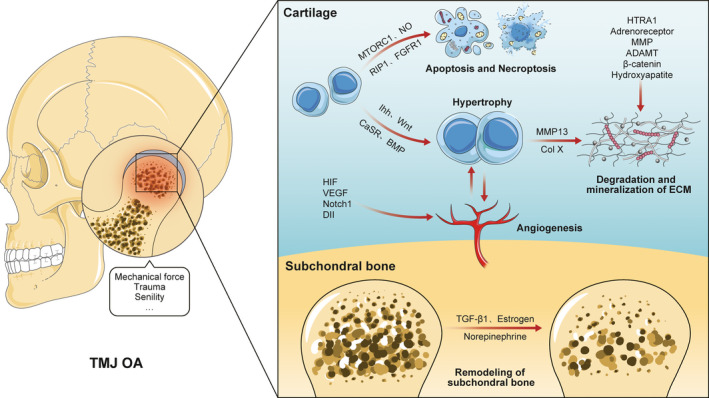
The main pathological changes in TMJ osteoarthritis
In this review, we summarize the latest studies on the pathogenesis of TMJ osteoarthritis and analyse the pathological changes with an emphasis on chondrocyte hypertrophy and angiogenesis.
2. IN VIVO ANIMAL MODELS AND IN VITRO CELL MODELS OF TMJ OSTEOARTHRITIS
Animal studies of the development of TMJ osteoarthritis used techniques including modifying the occlusal state, gene editing, injecting inflammatory mediators and surgery (Table 1).
TABLE 1.
Animal models and cell models used to mimic TMJ osteoarthritis in recent years
| Intervention | Animals/Cells | Methods | Year | Authors |
|---|---|---|---|---|
| In vivo | ||||
| Mechanical modification | Rats | Unilateral anterior crossbite | 2019 | Yang, H., et al |
| 2018 | Ye, T., et al | |||
| Bite‐raising | 2019 | Long, HQ, et al | ||
| Steady mouth‐opening | 2017 | Ge, X., R., et al | ||
| External compressive mechanical force | 2017 | Zhang, C., et al | ||
| 2016 | Zhu, M., et al | |||
| Rabbits | Unilateral dental splints | 2015 | Henderson., et al | |
| Medicine | Rats | Monosodium iodoacetic acid | 2019 | Zhang, S., et al |
| Freund's complete adjuvant | 2016 | Xu, L., et al | ||
| Rabbits | Type II collagenase | 2020 | Yi, X., et al | |
| Surgery | Mice | Unilateral partial discectomy | 2019 | Chen, PH et al |
| Goats | Total disc removal | 2017 | Lan, L., et al | |
| Cartilage removal of condyle | 2017 | Wang, F., et al | ||
| Gene | Mice | Collagen type XI haploinsufficient mice | 2019 | Chen, PH, et al |
| Camurati–Engelmann disease mice | 2018 | Zheng, L., et al | ||
| β‐catenin conditional activation mice | 2018 | Hui, T., et al | ||
| Expressing human SHOX | 2016 | Liang, W., et al | ||
| Conditional deletion of FGFR3 | 2016 | Zhou, S., et al | ||
| SMAD3 deficiency mice | 2015 | Mori, H., et al | ||
| Guinea pig | Dunkin‐Hartley strain guinea pig | 2016 | Wu, M., et al | |
| Senescence | Mice | 2018 | Wang, Z., et al | |
| In vitro | ||||
| Mechanical force | ATDC5 | Flow fluid shear stress | 2019 | Yang, H., et al |
| Pig condylar chondrocytes | 2017 | Zhang, M., et al | ||
| Cytokines | Rat condylar chondrocytes | IL‐1β | 2019 | Zhang, S., et al |
| 2016 | Chen, H., et al | |||
| TNF‐α+cycloheximide | 2017 | Zhang, C., et al | ||
Mechanical modification of the occlusal state is the most commonly used method to induce TMJ osteoarthritis‐like changes. The TMJ receives direct occlusal loading, and disruption of occlusal harmony may traumatize the TMJ during functional loading. 14 Intraoral occlusal devices such as unilateral anterior crossbite (UAC) and bite‐raising plates have been used to induce TMJ osteoarthritis‐like lesions. 7 , 15 , 16 In addition, an extraoral compression mechanical force device that directly exerts compressive pressure on the TMJ has also been used. 8 , 17
Similar to other osteoarthritis animal models, gene editing such as overexpression of short stature homeobox 2 (SHOX), transforming growth factor‐β1 (TGF‐β1), and β‐catenin, 18 , 19 , 20 , 21 and inhibiting or knocking out genes such as Discoidin domain receptor 1 (DDR1), small mother against decapentaplegic 3 (SMAD3), and fibroblast growth factor receptor (FGFR3), could lead to the formation of TMJ osteoarthritis. 1 , 22 , 23 Intra‐articular injection of chemical mediators such as monosodium iodoacetic acid (MIA) and complete Freund's adjuvant (CFA) could mimic the inflammatory response of the TMJ over a short period of time. 24 , 25 , 26 Surgical methods have been used to destroy part or all of the disc or cartilage of the condyle. 27 , 28 , 29 In addition, since the occurrence of osteoarthritis is closely related to age, 30 aged mice were used in some studies to mimic spontaneous TMJ osteoarthritis. 31
In vitro (Table 1), primary chondrocytes could be directly extracted from TMJ osteoarthritis patients or model animal and used as cell models. Chondrocyte inflammation can also be induced by inflammatory chemical mediators such as IL‐1β, 32 , 33 and mechanical devices such as Flexcell. 34 , 35
3. THE CANONICAL PATHOLOGICAL CHANGES DURING THE TMJ OSTEOARTHRITIS
3.1. The death of chondrocytes
Several studies have demonstrated that increased turnover of chondrocytes initiates the degeneration of condylar cartilage. 36 Apoptosis, autophagy, and necroptosis, play crucial roles in chondrocyte death. Apoptosis and necroptosis accelerate the destruction of articular cartilage. 37 , 38 Chondrocyte apoptosis provides space for neovascularization, and the apoptotic bodies produced by this process are thought to be the source of cartilage mineralization. 10 , 39 Autophagy is considered a self‐protective mechanism, by which chondrocytes recycle or reuse large biomolecules. 40 In general, abnormal death of chondrocytes not only reduces the number of chondrocytes, but also initiates the degeneration of cartilage and the destruction of subchondral bone. 41 The pathways associated with chondrocyte death in TMJ osteoarthritis are illustrated in Figure 2.
FIGURE 2.
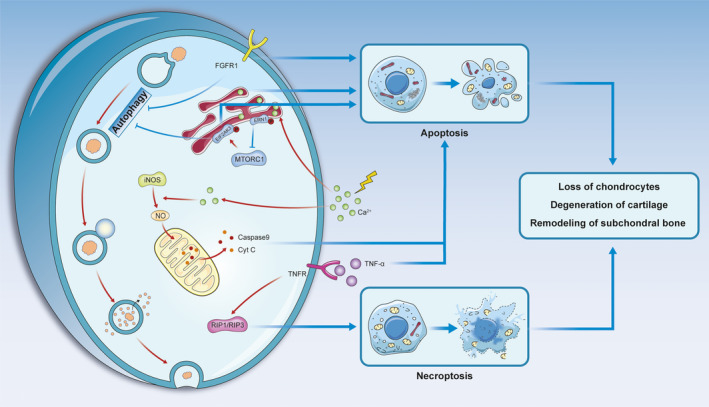
Pathways associated with chondrocyte death in TMJ osteoarthritis. Apoptosis and necroptosis are promoted in TMJ osteoarthritis, but the general trend of autophagy activity is inhibited
3.1.1. Apoptosis and necroptosis in TMJ osteoarthritis
Calcium plays an essential role in chondrocyte apoptosis and calcium concentration in chondrocytes can be increased under mechanical overload. 42 Endoplasmic reticulum stress (ERS) caused by calcium influx can cause chondrocyte apoptosis, which is known as ERS‐mediated apoptosis. 17 , 35 , 42 A high intracellular calcium concentration can activate inducible nitric oxide synthase (iNOS). Nitric oxide (NO) induced by iNOS inhibits mitochondrial respiration and leads to chondrocyte apoptosis through the release of cytochrome C (Cyt C) and caspase‐9. 43 Furthermore, tumour necrosis factor (TNF) and FGFR1 are crucial cytokines that can facilitate chondrocyte apoptosis by acting on the death receptor pathway. 44 , 45
Necroptosis is mediated by oxidative stress and plays an important role in osteoarthritis. 8 TNF and receptor‐interacting protein 1 and 3 (RIP1/RIP3)‐mediated necroptosis exacerbates the cartilage destruction. Studies have shown that the necroptosis pathway is enhanced when apoptosis is inhibited. 8 , 46
3.1.2. Autophagy in TMJ osteoarthritis
Autophagy is an important mechanism of chondrocyte survival in osteoarthritis. 40 The main process of autophagy involves the formation of autophagosomes, which sequester discarded organelles or macromolecules. Autophagosomes then fuse with lysosomes to form autolysosomes, which eventually degrade the contained materials and release small molecules that can be reused. 47 The markers of autophagy, beclin 1 and light chain 3 beta (LC3B) increase in the early stage of TMJ osteoarthritis but decrease significantly in the late stage. 7 The initial enhancement in autophagy protects chondrocytes from various environmental changes. With the destruction of cartilage, inhibition of autophagy is associated with cell death. 48 In models of abnormal dental occlusion and age‐associated spontaneous osteoarthritis, the loss of FGFR1 inhibits the development of osteoarthritis by promoting autophagic activity. 49 In addition, the endoplasmic reticulum‐associated proteins endoplasmic reticulum to nucleus signalling 1 (ERN1), mechanistic target of rapamycin kinase complex 1 (MTORC1) and eukaryotic translation initiation factor 2 alpha kinase3 (EIF2AK3) not only induce apoptosis but also act as a regulatory valve to inhibit autophagy. This pathway is known as the ERN1‐MTORC1‐EIF2AK3 signalling axis. 7 Therefore, regulating autophagy may be an effective mechanism for the treatment of TMJ osteoarthritis.
3.2. Degenerative changes in the ECM
The ECM of cartilage is mainly composed of collagen fibres and large proteoglycans. It not only acts as a protective scaffold against elastic and shear forces for cartilage, but also regulates the behaviour of chondrocytes through matrix‐cell interactions. 5 , 50
In TMJ osteoarthritis, ECM degradation starts with expression of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) in the cell. 51 , 52 The destruction of type II collagen (Col2A1) promotes the hypertrophy of chondrocytes through the bone morphogenetic protein (BMP) pathway, thus exacerbating the progression of TMJ osteoarthritis. 53 In addition, mineralization of cartilage has been found to be involved in the development of TMJ osteoarthritis. 10 The signalling molecules involved in the regulation of ECM degeneration are summarized below.
The role of β‐catenin, which regulates MMP13 and ADAMT5, is controversial in TMJ osteoarthritis. Compressive mechanical force reduces endogenous β‐catenin and leads to ECM degradation. These pathological changes can be recovered by restoring the β‐catenin signalling. 54 However, other authors found that mice with conditional β‐catenin activation showed TMJ osteoarthritis‐like phenotypes and up‐regulation of MMP13 and ADAMT5 expression. 21 , 55
The Notch cascade consists of Notch and Notch ligands, which are involved in regulating the proliferation, differentiation, maturation and apoptosis of chondrocytes. 56 Regulation of this cascade (Notch1/Jagged1/Hes5) promotes the expression of MMPs and reduces the expression of tissue inhibitor of metalloproteinase‐1 (TIMP‐1) in the cell. 28 , 32 , 57
The activation of α2A‐adrenergic receptor signals via the extracellular regulated protein kinases 1 and 2 (ERK1/2) and protein kinase A (PKA) pathways stimulates the production of matrix degradation associated enzymes, including MMP‐3 and MMP‐13. 58 Osteopontin, an inflammatory factor, induces the expression of MMPs via the NF‐κB signalling pathway. 59
The HTRA1‐DDR2‐MMP‐13 axis plays an important role in ECM degradation. 27 This process starts with the overexpression of high–temperature requirement A1 (HTRA1) and the degradation of the pericellular matrix components, such as type VI collagen. The disappearance of pericellular matrix can cause the transmembrane protein DDR2 to be activated by Col2A1. Ultimately, DDR2 activates MMP13 and accelerates TMJ osteoarthritis. 27 , 60
3.3. Remodelling of subchondral bone in TMJ osteoarthritis
Abnormal subchondral bone remodelling is one of the first pathological features and clinical signs of TMJ osteoarthritis. 3 In the initial stage of TMJ osteoarthritis, the subchondral bone predominantly exhibits bone loss, while slow repair activities increase bone mass at the subchondral plate in the late stage. 61 , 62 The loss of subchondral bone in the early stages of TMJ osteoarthritis contributes to cartilage degeneration and the onset of osteoarthritis. 4 Increases in the stiffness and thickness of the condylar osteochondral interface (the region that covers the calcified cartilage and subchondral cortical bone) at the late stage of TMJ osteoarthritis are caused by the calcification of cartilage and the formation of subchondral cortical bone. 62 , 63
It is worth noting that abnormal subchondral bone remodelling in TMJ osteoarthritis is induced by decreased osteoblast and increased osteoclast activities. 4 , 62 The WNT5A/receptor tyrosine kinase‐like orphan receptor 2 (Ror2) pathway was found to promote the migration and differentiation of osteoclast precursors via the Ca2+/nuclear factor of activated T‐cells (NFAT) pathway. 64 In addition, overexpression of transforming growth factor‐β1 (TGF‐β1) increased the uncoupling of osteoclastic and osteoblastic activity and led to abnormal changes in subchondral bone. 19 , 20 The activation of α2A‐adrenergic and β2‐adrenergic receptors via neurotransmitters could destroy the subchondral bone by inducing osteoclast maturation via the RANKL pathway. 58 , 65
The role of sex hormones, such as oestrogen and progesterone, in TMJ osteoarthritis is still unclear. 66 , 67 oestrogen is involved in the formation of condylar fibrocartilage and subsequently maintains cartilage stability by inhibiting the Wnt pathway through oestrogen receptor‐α. 68 The level of oestrogen is associated with the severity of TMJ osteoarthritis. In the early stage of TMJ osteoarthritis, a high level of oestrogen exerts protective effects by inhibiting osteoclast activity and reversing the abnormal absorption of subchondral bone. 66 In contrast, a low level of oestrogen may increase the severity of TMJ osteoarthritis. 69 However, the results of different studies are inconsistent. Some studies have suggested that oestrogen promotes MIA‐induced subchondral bone erosion by activating oestrogen receptor‐β. 70 Interestingly, a high level of progesterone exerts a protective effect by reducing the degeneration of subchondral bone by inhibiting NF‐κB activity. 67
4. THE PERSPECTIVES ON HYPERTROPHY AND ANGIOGENESIS IN TMJ OSTEOARTHRITIS
Endochondral osteogenesis‐like changes have been reported in both human osteoarthritis and experimental models of the osteoarthritic process. 71 Chondrocyte hypertrophy and cartilage angiogenesis are important features of endochondral osteogenesis. 71 , 72 Interestingly, these two processes have also been shown to play an important role in TMJ osteoarthritis. Cytokines secreted by hypertrophic chondrocytes can direct and attract endothelial cells into cartilage. 39 Increasing evidence has suggested that most hypertrophic chondrocytes have the potential to transform into osteoblasts and osteocytes, which are then involved in bone formation. 73
4.1. Chondrocyte hypertrophy in TMJ osteoarthritis
Hypertrophic chondrocytes are characterized by cellular expression of Col X, MMP13 and alkaline phosphatase (ALP). 74 , 75 Van der Kraan et al reported that chondrocyte hypertrophy‐like changes play an essential role in the early and late stages of osteoarthritis. 76 Hypertrophic chondrocytes highly express MMPs to degrade their surroundings, as observed in TMJ osteoarthritis. The process of hydroxyapatite deposition and the mineralization of cartilage are accelerated when Col X is present in the cartilage matrix. 77 The matrix vesicles secreted by hypertrophic chondrocytes contain phosphatidylserine that aggregates calcium and phosphate to form mineralized nodules. 10 In addition, the process of angiogenesis, which includes the migration and adhesion of endothelial cells, is further enhanced by hypertrophic chondrocytes in TMJ osteoarthritis. 78
Chondrocyte hypertrophy is regulated by different molecular signalling pathways, such as the Indian hedgehog (Ihh) pathway. 15 Calcium/calmodulin‐dependent protein kinase II (CaMKII), which up‐regulates Ihh expression, can be activated by increases in the intracellular calcium concentration. Activation of CaMK II not only promotes the expression of Ihh but also alleviates the inhibitory effect of parathyroid hormone receptor (PTH1R) on chondrocyte hypertrophy. 79 Activation of PTH1R inhibits chondrocyte hypertrophy via an Ihh‐PTHrP negative feedback pathway, thereby maintaining the balance between chondrocyte proliferation and hypertrophy. 80 , 81 The expression of FGFR3 inhibits Ihh, but this effect is diminished in TMJ osteoarthritis. 23 Ihh transmits information to the nucleus through the Ihh‐Smo‐Gli signalling pathway. 15 , 77 The pathway ultimately induces chondrocyte hypertrophy via Runt‐related transcription factor 2 (Runx2), a transcription factor that directly regulates the expression of Col X and MMP13. 76 , 77 , 82 , 83 In addition, an increased intracellular calcium concentration can promote chondrocyte hypertrophy through calcium‐sensing receptor (CaSR) in the endoplasmic reticulum. 84
Col2A1, the main component of the ECM, can be degraded by hypertrophic chondrocytes in the context of TMJ osteoarthritis. A reduction in collagen in the ECM, in turn, induces chondrocyte hypertrophy. 53 In a disease‐free model, Col2A1 activated integrin β1 (ITGB1). In addition to their interaction with Col2A1, ITGB1 receptors compete with BMP for SMAD1 binding and then inhibit SMAD1 activation and nuclear transport. The loss of Col2A1 promotes TMJ osteoarthritis by activating the BMP‐SMAD1 signalling pathway and increasing the expression of Runx2 and Col X. 53
The Wnt family is another critical signalling pathway that regulates chondrocyte hypertrophy in TMJ osteoarthritis. The canonical Wnt pathway promotes Runx2 and Col X synthesis via β‐catenin. This pathway can be activated during TMJ osteoarthritis by the down‐regulation of DNA methyltransferase 3B (Dnmt3b). 21 , 55 , 85 In addition to the canonical Wnt pathway, the non‐canonical Wnt pathway induces chondrocyte hypertrophy and migration via the c‐Jun N‐terminal kinase (JNK) signalling pathway. 86
Figure 3 summarizes some of the related signalling pathways that are critical to chondrocyte hypertrophy.
FIGURE 3.
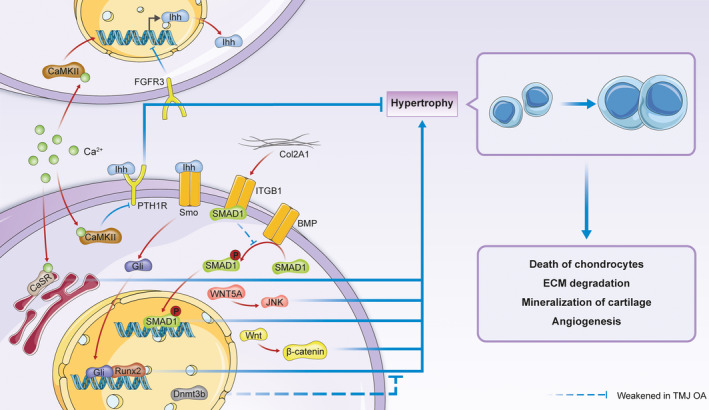
Molecular mechanism of chondrocyte hypertrophy in TMJ osteoarthritis
4.2. Angiogenesis promotes the development of TMJ osteoarthritis
Studies have revealed that angiogenesis can promote the development of osteoarthritis. 87 , 88 Wang et al found that the number of newly formed blood vessels at the osteochondral junction was increased in mice with TMJ osteoarthritis‐like changes. 89 These new blood vessels can transport inflammatory mediators and sustain inflammation in TMJ osteoarthritis. 90 When new blood vessels invade the cartilage, they promote both chondrocyte hypertrophy and mineral deposition in the matrix. 88 Osteophytes can then incorporate with the newly formed vessels on the surface of the joint to facilitate hard tissue formation through the process of endochondral osteogenesis. 88 Neurogenesis follows angiogenesis. An increase in the formation of blood vessels and nerves between the subchondral bone and articular cartilage may mediate the pathological process of osteoarthritis and contribute to the pain associated with osteoarthritis. 12
Vascular endothelial growth factor (VEGF) is crucial for angiogenesis and is an important mediator of TMJ osteoarthritis. Injection of VEGF led to TMJ osteoarthritis‐like changes in mice by stimulating endothelial cell proliferation and migration and stabilizing newly formed blood vessels. 91 The expression of VEGF is up‐regulated by several transcription factors, such as hypoxia‐inducible factor‐1 (HIF‐1). 11 , 92 High levels of the dickkopf‐related protein‐1 (DKK‐1) protein were found in the synovial fluid of TMJ osteoarthritis patients. It has been suggested that DKK‐1 and high‐mobility group box 1 (HMGB1) direct the nuclear localization of HIF‐1 and thus promote the synthesis of VEGF. 92 , 93 The inflammatory factors IL‐6 and IL‐1β increase the level of VEGF by inducing VEGF transcription in the nucleus. IL‐6 activates oestrogen‐related receptor γ (ERRγ) via extracellular signal‐regulated kinase (ERK1/2). In contrast, IL‐1β directly activates NF‐κB. 94 , 95
Vascular endothelial growth factor stimulates cartilage neovascularization through several different downstream pathways. The Notch signalling pathway is another important pathway associated with angiogenesis, and VEGF is a positive regulatory factor of Notch. A study showed that the HIF‐1‐VEGF‐Notch1 signalling pathway mediated angiogenesis in TMJ osteoarthritis. 11 Delta‐like ligand 4 (Dll4) also plays a key role and contributes to vascular development. VEGF can up‐regulate the expression of Dll4 by activating p‐ERK1/2 signalling, thus facilitating angiogenesis in TMJ osteoarthritis. 96 In addition to VEGF, angiopoietins (Angs), such as Ang‐1 and Ang‐2, are overexpressed in the injured TMJ. Studies have shown that overexpression of Ang‐1 is caused by IL‐1β‐mediated activation of the MAPK pathway. 94 , 97
Neurovascular interactions are involved in the progression of TMJ osteoarthritis. Proangiogenic factors such as VEGF and molecules secreted by endothelial cells were found to stimulate nerve growth. 98 The growth of nerve endings also plays a role in angiogenesis. Nerve growth factor (NGF), which regulates nerve growth, survival and repair, can stimulate angiogenesis in TMJ osteoarthritis. 99
The regulatory mechanism and role of angiogenesis in TMJ osteoarthritis are shown in Figure 4.
FIGURE 4.
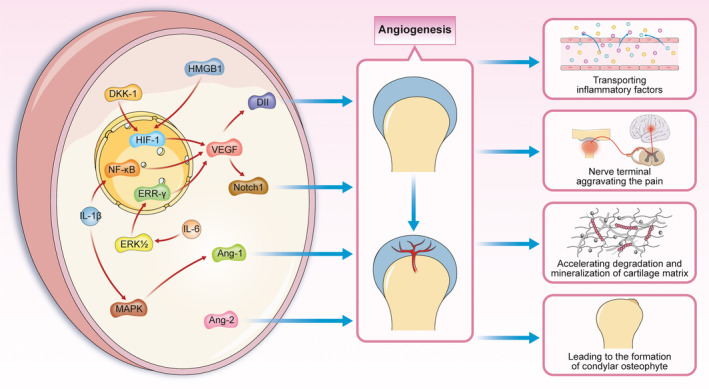
Molecular mechanism of angiogenesis in TMJ osteoarthritis
5. CONCLUSION
The TMJ in animals has been successfully constructed in a variety of ways to stimulate the structural and organizational changes in osteoarthritis. Chondrocyte death, ECM degradation and subchondral bone remodelling play essential roles in TMJ osteoarthritis. Moreover, the endochondral osteogenesis‐like changes such as chondrocyte hypertrophy and endochondral angiogenesis suggest that the pathological changes of TMJ osteoarthritis are closely related to the process of osteogenesis. The molecular pathways that regulate TMJ osteoarthritis help us to better understand the pathological mechanisms of TMJ osteoarthritis. More importantly, these signalling molecules may serve as potential therapeutic targets to help find effective disease‐modifying strategies for TMJ osteoarthritis.
AUTHOR CONTRIBUTIONS
Baochao Li: Conceptualization (lead); Visualization (lead); Writing‐original draft (lead). Guangzhao Guan: Formal analysis (equal); Project administration (equal); Writing‐original draft (supporting). Li Mei: Data curation (equal); Investigation (equal); Writing‐original draft (supporting). Kai Jiao: Project administration (equal); Validation (supporting); Writing‐review & editing (equal). Huang Li: Conceptualization (lead); Funding acquisition (lead); Project administration (lead); Resources (lead); Supervision (lead); Writing‐review & editing (lead).
ACKNOWLEDGEMENTS
The work was supported by the National Natural Science Foundation of China (grants 81470712 and 81670960), the Natural Science Foundation of Jiangsu Province (grant BK20171123), Jiangsu Provincial Medical Youth Talent, and Jiangsu Provincial 333 Talent (the second level). The authors declare no potential conflicts of interest with respect to authorship and the publication of this article.
Li B, Guan G, Mei L, Jiao K, Li H. Pathological mechanism of chondrocytes and the surrounding environment during osteoarthritis of temporomandibular joint. J Cell Mol Med. 2021;25:4902–4911. 10.1111/jcmm.16514
Contributor Information
Baochao Li, Email: lihuang76@nju.edu.cn.
Huang Li, Email: lihuang76@nju.edu.cn.
DATA AVAILABILITY STATEMENT
This article does not contain available data.
REFERENCES
- 1. Schminke B, Muhammad H, Bode C, et al. A discoidin domain receptor 1 knock‐out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2014;71(6):1081‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen DI, Shen J, Zhao W, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang XD, Zhang JN, Gan YH, Zhou YH. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J Dent Res. 2015;94(5):666‐673. [DOI] [PubMed] [Google Scholar]
- 4. Embree M, Ono M, Kilts T, et al. Role of subchondral bone during early‐stage experimental TMJ osteoarthritis. J Dent Res. 2011;90(11):1331‐1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Y, Hu X, Cheng J, et al. A small molecule promotes cartilage extracellular matrix generation and inhibits osteoarthritis development. Nat Commun. 2019;10(1):1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Utreja A, Dyment NA, Yadav S, et al. Cell and matrix response of temporomandibular cartilage to mechanical loading. Osteoarthr Cartil. 2016;24(2):335‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang H, Wen YI, Zhang M, et al. MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy. 2020;16(2):271‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang C, Lin S, Li T, et al. Mechanical force‐mediated pathological cartilage thinning is regulated by necroptosis and apoptosis. Osteoarthr Cartil. 2017;25(8):1324‐1334. [DOI] [PubMed] [Google Scholar]
- 9. Shi J, Lee S, Pan HC, et al. Association of condylar bone quality with TMJ osteoarthritis. J Dent Res. 2017;96(8):888‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan JF, Qin WP, Xiao BC, et al. Pathological calcification in osteoarthritis: an outcome or a disease initiator? Biol Rev Camb Philos Soc. 2020;95(4):960‐985. [DOI] [PubMed] [Google Scholar]
- 11. Chen Y, Zhao B, Zhu Y, Zhao H, Ma C. HIF‐1‐VEGF‐Notch mediates angiogenesis in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11(5):2969‐2982. [PMC free article] [PubMed] [Google Scholar]
- 12. Ashraf S, Wibberley H, Mapp PI, Hill R, Wilson D, Walsh DA. Increased vascular penetration and nerve growth in the meniscus: a potential source of pain in osteoarthritis. Ann Rheum Dis. 2011;70(3):523‐529. [DOI] [PubMed] [Google Scholar]
- 13. Babarina AV, Möllers U, Bittner K, Vischer P, Bruckner P. Role of the subchondral vascular system in endochondral ossification: endothelial cell‐derived proteinases derepress late cartilage differentiation in vitro. Matrix Biol. 2001;20(3):205‐213. [DOI] [PubMed] [Google Scholar]
- 14. Zhou P, Zhang J, Zhang M, et al. Effects of occlusion modification on the remodelling of degenerative mandibular condylar processes. Oral Dis. 2020;26(3):597‐608. [DOI] [PubMed] [Google Scholar]
- 15. Long HQ, Tian PF, Guan YX, Liu LX, Wu XP, Li B. Expression of Ihh signaling pathway in condylar cartilage after bite‐raising in adult rats. J Mol Histol. 2019;50(5):459‐470. [DOI] [PubMed] [Google Scholar]
- 16. Henderson SE, Tudares MA, Tashman S, Almarza AJ. Decreased temporomandibular joint range of motion in a model of early osteoarthritis in the rabbit. J Oral Maxillofac Surg. 2015;73(9):1695‐1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu M, Zhou S, Huang Z, Wen J, Li H. Ca2+‐dependent endoplasmic reticulum stress regulates mechanical stress‐mediated cartilage thinning. J Dent Res. 2016;95(8):889‐896. [DOI] [PubMed] [Google Scholar]
- 18. Liang W, Li X, Chen H, et al. Expressing human SHOX in Shox2SHOX KI/KI mice leads to congenital osteoarthritis‐like disease of the temporomandibular joint in postnatal mice. Mol Med Rep. 2016;14(4):3676‐3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiao K, Zhang M, Niu L, et al. Overexpressed TGF‐β in subchondral bone leads to mandibular condyle degradation. J Dent Res. 2014;93(2):140‐147. [DOI] [PubMed] [Google Scholar]
- 20. Zheng L, Pi C, Zhang J, et al. Aberrant activation of latent transforming growth factor‐β initiates the onset of temporomandibular joint osteoarthritis. Bone Res. 2018;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hui T, Zhou Y, Wang T, et al. Activation of β‐catenin signaling in aggrecan‐expressing cells in temporomandibular joint causes osteoarthritis‐like defects. Int J Oral Sci. 2018;10(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mori H, Izawa T, Tanaka E. Smad3 deficiency leads to mandibular condyle degradation via the sphingosine 1‐phosphate (S1P)/S1P3 signaling axis. Am J Pathol. 2015;185(10):2742‐2756. [DOI] [PubMed] [Google Scholar]
- 23. Zhou S, Xie Y, Li W, et al. Conditional deletion of Fgfr3 in chondrocytes leads to osteoarthritis‐like defects in temporomandibular joint of adult mice. Sci Rep. 2016;6:24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu L, Guo H, Li C, Xu J, Fang W, Long X. A time‐dependent degeneration manner of condyle in rat CFA‐induced inflamed TMJ. Am J Transl Res. 2016;8(2):556‐567. [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S, Teo KYW, Chuah SJ, Lai RC, Lim SK, Toh WS. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35‐47. [DOI] [PubMed] [Google Scholar]
- 26. Yi X, Wu L, Liu J, Qin YX, Li B, Zhou Q. Low‐intensity pulsed ultrasound protects subchondral bone in rabbit temporomandibular joint osteoarthritis by suppressing TGF‐β1/Smad3 pathway. J Orthop Res. 2020;38(11):2505‐2512. [DOI] [PubMed] [Google Scholar]
- 27. Chen PH, Tang T, Liu C, et al. High‐temperature requirement A1 protease as a rate‐limiting factor in the development of osteoarthritis. Am J Pathol. 2019;189(7):1423‐1434. [DOI] [PubMed] [Google Scholar]
- 28. Lan L, Jiang Y, Zhang W, Li T, Ying B, Zhu S. Expression of Notch signaling pathway during osteoarthritis in the temporomandibular joint. J Craniomaxillofac Surg. 2017;45(8):1338‐1348. [DOI] [PubMed] [Google Scholar]
- 29. Wang F, Sun Y, He D, Wang L. Effect of concentrated growth factors on the repair of the goat temporomandibular joint. J Oral Maxillofac Surg. 2017;75(3):498‐507. [DOI] [PubMed] [Google Scholar]
- 30. Madej W, van Caam A, Davidson EN, Hannink G, Buma P, van der Kraan PM. Ageing is associated with reduction of mechanically‐induced activation of Smad2/3P signaling in articular cartilage. Osteoarthr Cartil. 2016;24(1):146‐157. [DOI] [PubMed] [Google Scholar]
- 31. Li K, Zhang Y, Zhang Y, et al. Tyrosine kinase Fyn promotes osteoarthritis by activating the β‐catenin pathway. Ann Rheum Dis. 2018;77(6):935‐943. [DOI] [PubMed] [Google Scholar]
- 32. Ying W, Yuan F, He P, Ji P. Inhibition of Notch1 protects against IL‐1β‐induced inflammation and cartilage destruction in temporomandibular chondrocytes. Mol Med Rep. 2017;15(6):4391‐4397. [DOI] [PubMed] [Google Scholar]
- 33. Tabeian H, Betti BF, dos Santos Cirqueira C, et al. IL‐1β damages fibrocartilage and upregulates MMP‐13 expression in fibrochondrocytes in the condyle of the temporomandibular joint. Int J Mol Sci. 2019;20(9):2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang M, Yang H, Lu L, et al. Matrix replenishing by BMSCs is beneficial for osteoarthritic temporomandibular joint cartilage. Osteoarthr Cartil. 2017;25(9):1551‐1562. [DOI] [PubMed] [Google Scholar]
- 35. Huang Z, Zhou M, Wang Q, Zhu M, Chen S, Li H. Mechanical and hypoxia stress can cause chondrocytes apoptosis through over‐activation of endoplasmic reticulum stress. Arch Oral Biol. 2017;84:125‐132. [DOI] [PubMed] [Google Scholar]
- 36. Charlier E, Relic B, Deroyer C, et al. Insights on molecular mechanisms of chondrocytes death in osteoarthritis. Int J Mol Sci. 2016;17(12):2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370(5):455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Musumeci G, Castrogiovanni P, Trovato F, et al. Biomarkers of chondrocyte apoptosis and autophagy in osteoarthritis. Int J Mol Sci. 2015;16(9):20560‐20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332‐336. [DOI] [PubMed] [Google Scholar]
- 40. Cheng NT, Guo A, Meng H. The protective role of autophagy in experimental osteoarthritis, and the therapeutic effects of Torin 1 on osteoarthritis by activating autophagy. BMC Musculoskelet Disord. 2016;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim HA, Blanco FJ. Cell death and apoptosis in osteoarthritic cartilage. Curr Drug Targets. 2007;8(2):333‐345. [DOI] [PubMed] [Google Scholar]
- 42. Li H, Zhang X‐Y, Wu T‐J, et al. Endoplasmic reticulum stress regulates rat mandibular cartilage thinning under compressive mechanical stress. J Biol Chem. 2013;288(25):18172‐18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ren H, Yang H, Xie M, et al. Chondrocyte apoptosis in rat mandibular condyles induced by dental occlusion due to mitochondrial damage caused by nitric oxide. Arch Oral Biol. 2019;101:108‐121. [DOI] [PubMed] [Google Scholar]
- 44. Dobsak T, Heimel P, Tangl S, Schwarze UY, Schett G, Gruber R. Impaired periodontium and temporomandibular joints in tumour necrosis factor‐α transgenic mice. J Clin Periodontol. 2017;44(12):1226‐1235. [DOI] [PubMed] [Google Scholar]
- 45. Xu W, Xie Y, Wang Q, et al. A novel fibroblast growth factor receptor 1 inhibitor protects against cartilage degradation in a murine model of osteoarthritis. Sci Rep. 2016;6:24042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14(11):727‐736. [DOI] [PubMed] [Google Scholar]
- 47. Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14(2):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y‐S, Zhang F‐J, Zeng C, et al. Autophagy in osteoarthritis. Joint Bone Spine. 2016;83(2):143‐148. [DOI] [PubMed] [Google Scholar]
- 49. Wang Z, Huang J, Zhou S, et al. Loss of Fgfr1 in chondrocytes inhibits osteoarthritis by promoting autophagic activity in temporomandibular joint. J Biol Chem. 2018;293(23):8761‐8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gao Y, Liu S, Huang J, et al. The ECM‐cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu J, Mursu E, Typpö M, et al. MMP‐3 and MMP‐8 in rat mandibular condylar cartilage associated with dietary loading, estrogen level, and aging. Arch Oral Biol. 2019;97:238‐244. [DOI] [PubMed] [Google Scholar]
- 52. Wu M, Lu H, Yu F, Zhou Y. Trend of Cadherin‐11 expression and its impact on cartilage degradation in the temporomandibular joints of guinea pigs with spontaneous osteoarthritis. J Oral Pathol Med. 2016;45(7):534‐538. [DOI] [PubMed] [Google Scholar]
- 53. Lian C, Wang X, Qiu X, et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1‐SMAD1 interaction. Bone Res. 2019;7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang YY, Wen J, Gong C, et al. BIO alleviated compressive mechanical force‐mediated mandibular cartilage pathological changes through Wnt/β‐catenin signaling activation. J Ortho Res. 2018;36(4):1228‐1237. [DOI] [PubMed] [Google Scholar]
- 55. Wang M, Li S, Xie W, et al. Activation of β‐catenin signalling leads to temporomandibular joint defects. Eur Cells Mater. 2014;28:223‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Karlsson C, Brantsing C, Egell S, Lindahl A. Notch1, Jagged1, and HES5 are abundantly expressed in osteoarthritis. Cells Tissues Organs. 2008;188(3):287‐298. [DOI] [PubMed] [Google Scholar]
- 57. Luo X, Jiang Y, Bi R, Jiang N, Zhu S. Inhibition of notch signaling pathway temporally postpones the cartilage degradation progress of temporomandibular joint arthritis in mice. J Craniomaxillofac Surg. 2018;46(7):1132‐1138. [DOI] [PubMed] [Google Scholar]
- 58. Jiao K, Zeng G, Niu L‐N, et al. Activation of α2A‐adrenergic signal transduction in chondrocytes promotes degenerative remodelling of temporomandibular joint. Sci Rep. 2016;6:30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ding F, Wang J, Zhu G, Zhao H, Wu G, Chen L. Osteopontin stimulates matrix metalloproteinase expression through the nuclear factor‐κB signaling pathway in rat temporomandibular joint and condylar chondrocytes. Am J Transl Res. 2017;9(2):316‐329. [PMC free article] [PubMed] [Google Scholar]
- 60. Ge C, Mohamed F, Binrayes A, Kapila S, Franceschi RT. Selective role of discoidin domain receptor 2 in murine temporomandibular joint development and aging. J Dent Res. 2018;97(3):321‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang J, Jiao K, Zhang M, et al. Occlusal effects on longitudinal bone alterations of the temporomandibular joint. J Dent Res. 2013;92(3):253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665‐673. [DOI] [PubMed] [Google Scholar]
- 63. Zhang J, Liao L, Zhu J, et al. Osteochondral interface stiffening in mandibular condylar osteoarthritis. J Dent Res. 2018;97(5):563‐570. [DOI] [PubMed] [Google Scholar]
- 64. Yang T, Zhang J, Cao Y, et al. Wnt5a/Ror2 mediates temporomandibular joint subchondral bone remodeling. J Dent Res. 2015;94(6):803‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jiao K, Niu L, Xu X, et al. Norepinephrine regulates condylar bone loss via comorbid factors. J Dent Res. 2015;94(6):813‐820. [DOI] [PubMed] [Google Scholar]
- 66. Ye T, Sun D, Mu T, et al. Differential effects of high‐physiological oestrogen on the degeneration of mandibular condylar cartilage and subchondral bone. Bone. 2018;111:9‐22. [DOI] [PubMed] [Google Scholar]
- 67. Xue X‐T, Kou X‐X, Li C‐S, et al. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF‐κB pathway in ovariectomized rats. Sci Rep. 2017;7(1):15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robinson JL, Soria P, Xu M, et al. Estrogen promotes mandibular condylar fibrocartilage chondrogenesis and inhibits degeneration via estrogen receptor alpha in female mice. Sci Rep. 2018;8(1):8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu Y, Kadota‐Watanabe C, Ogawa T, Moriyama K. Combination of estrogen deficiency and excessive mechanical stress aggravates temporomandibular joint osteoarthritis in vivo. Arch Oral Biol. 2019;102:39‐46. [DOI] [PubMed] [Google Scholar]
- 70. Wang XD, Kou XX, Meng Z, et al. Estrogen aggravates iodoacetate‐induced temporomandibular joint osteoarthritis. J Dent Res. 2013;92(10):918‐924. [DOI] [PubMed] [Google Scholar]
- 71. Xiao ZF, Su GY, Hou Y, Chen SD, Lin DK. Cartilage degradation in osteoarthritis: a process of osteochondral remodeling resembles the endochondral ossification in growth plate? Med Hypotheses. 2018;121:183‐187. [DOI] [PubMed] [Google Scholar]
- 72. Staines KA, Pollard AS, McGonnell IM, Farquharson C, Pitsillides AA. Cartilage to bone transitions in health and disease. J Endocrinol. 2013;219(1):R1‐R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci USA. 2014;111(33):12097‐12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gu J, Lu Y, Li F, et al. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014;5(10):e1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kozhemyakina E, Lassar AB, Zelzer E. A pathway to bone: signaling molecules and transcription factors involved in chondrocyte development and maturation. Development. 2015;142(5):817‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthr Cartil. 2012;20(3):223‐232. [DOI] [PubMed] [Google Scholar]
- 77. Amano K, Densmore M, Nishimura R, Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. J Biol Chem. 2014;289(36):24898‐24910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pesesse L, Sanchez C, Delcour J‐P, et al. Consequences of chondrocyte hypertrophy on osteoarthritic cartilage: potential effect on angiogenesis. Osteoarthr Cartil. 2013;21(12):1913‐1923. [DOI] [PubMed] [Google Scholar]
- 79. Liu Q, Yang H‐X, Wan X‐H, et al. Calcium‐/calmodulin‐dependent protein kinase II in occlusion‐induced degenerative cartilage of rat mandibular condyle. J Oral Rehabil. 2018;45(6):442‐451. [DOI] [PubMed] [Google Scholar]
- 80. Wang D, Taboas JM, Tuan RS. PTHrP overexpression partially inhibits a mechanical strain‐induced arthritic phenotype in chondrocytes. Osteoarthr Cartil. 2011;19(2):213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang H, Zhang M, Liu Q, et al. Inhibition of Ihh reverses temporomandibular joint osteoarthritis via a PTH1R signaling dependent mechanism. Int J Mol Sci. 2019;20(15):3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li F, Lu Y, Ding M, et al. Runx2 contributes to murine Col10a1 gene regulation through direct interaction with its cis‐enhancer. J Bone Miner Res. 2011;26(12):2899‐2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP‐13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr Cartil. 2004;12(12):963‐973. [DOI] [PubMed] [Google Scholar]
- 84. Zhang M, Yang H, Wan X, et al. Prevention of injury‐induced osteoarthritis in rodent temporomandibular joint by targeting chondrocyte CaSR. J Bone Miner Res. 2019;34(4):726‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zhou Y, Chen M, O'Keefe RJ, et al. Epigenetic and therapeutic implications of dnmt3b in temporomandibular joint osteoarthritis. Am J Transl Res. 2019;11(3):1736‐1747. [PMC free article] [PubMed] [Google Scholar]
- 86. Ge X, Shi R, Ma X. The secreted protein WNT5A regulates condylar chondrocyte proliferation, hypertrophy and migration. Arch Oral Biol. 2017;82:171‐179. [DOI] [PubMed] [Google Scholar]
- 87. Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20(5):573‐580. [DOI] [PubMed] [Google Scholar]
- 88. Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 89. Wang Q‐Y, Dai J, Kuang B, et al. Osteochondral angiogenesis in rat mandibular condyles with osteoarthritis‐like changes. Arch Oral Biol. 2012;57(6):620‐629. [DOI] [PubMed] [Google Scholar]
- 90. Pesesse L, Sanchez C, Henrotin Y. Osteochondral plate angiogenesis: a new treatment target in osteoarthritis. Joint bone spine. 2011;78(2):144‐149. [DOI] [PubMed] [Google Scholar]
- 91. Shen P, Jiao ZiXian, Zheng JS, et al. Injecting vascular endothelial growth factor into the temporomandibular joint induces osteoarthritis in mice. Sci Rep. 2015;5:16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Feng Y, Ke J, Cao P, et al. HMGB1‐induced angiogenesis in perforated disc cells of human temporomandibular joint. J Cell Mol Med. 2018;22(2):1283‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang SJ, Li W, Li YJ, Fang W, Long X. Dickkopf‐related protein 1 induces angiogenesis by upregulating vascular endothelial growth factor in the synovial fibroblasts of patients with temporomandibular joint disorders. Mol Med Rep. 2015;12(4):4959‐4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xu J, Cai H, Meng Q, et al. IL‐1β‐regulating angiogenic factors expression in perforated temporomandibular disk cells via NF‐κB pathway. J Oral Pathol Med. 2016;45(8):605‐612. [DOI] [PubMed] [Google Scholar]
- 95. Zhao H, Liu S, Ma C, et al. Estrogen‐related receptor γ induces angiogenesis and extracellular matrix degradation of temporomandibular joint osteoarthritis in rats. Front Pharmacol. 2019;10:1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dong Y, Wu G, Zhu T, et al. VEGF promotes cartilage angiogenesis by phospho‐ERK1/2 activation of Dll4 signaling in temporomandibular joint osteoarthritis caused by chronic sleep disturbance in Wistar rats. Oncotarget. 2017;8(11):17849‐17861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jing Z, Gu Z, Feng J. Forward mandibular positioning enhances the expression of Ang‐1 and Ang‐2 in rabbit condylar chondrocytes. Mol Med Rep. 2013;8(4):1094‐1098. [DOI] [PubMed] [Google Scholar]
- 98. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390‐398. [DOI] [PubMed] [Google Scholar]
- 99. Yu X, Qi Y, Zhao T, et al. NGF increases FGF2 expression and promotes endothelial cell migration and tube formation through PI3K/Akt and ERK/MAPK pathways in human chondrocytes. Osteoarthr Cartil. 2019;27(3):526‐534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain available data.


