Abstract
We intended to investigate whether muscle and adipose masses were associated with prognosis among patients with stage III non-small-cell lung cancer (NSCLC) who were undergoing chemoradiotherapy (CCRT). We retrospectively explored data of patients with stage III NSCLC who underwent definitive CCRT (≥ 60 Gy) between January 2004 and March 2018 at our hospital. We examined the relationship of overall survival (OS) with body mass index (BMI), skeletal muscle index (SMI), psoas muscle index (PMI), visceral adipose tissue index (VAI), subcutaneous adipose tissue index (SAI), and visceral-to-subcutaneous adipose tissue area ratio (VSR) using log-rank tests for the univariate analysis and Cox proportional hazard models for the multivariate analysis. Overall, 16, 32, and 12 patients had stage IIIA, IIIB, and IIIC NSCLC, respectively. The total radiotherapy dose ranged from 60 Gy/30 fractions to 66 Gy/33 fractions. In the univariate analysis, the performance status (PS), BMI, and SMI were associated with OS, whereas the PMI, VAI, SAI, and VSR were not. In the multivariate analysis, the PS and SMI were associated with OS. The hazard ratios and 95% confidence intervals were 2.91 and 1.28–6.64 for PS, and 2.36 and 1.15–4.85 for SMI, respectively. The 1, 3, and 5-year OS rates were 92.1%, 59.6%, and 51.0% in patients with high SMI, and 63.6%, 53.8%, and 17.9% in patients with low SMI, respectively. The SMI correlated with prognosis in our study population, whereas adipose mass did not. Therefore, sarcopenia should be considered while predicting the OS in such patients.
Subject terms: Medical research, Oncology
Introduction
The incidence of lung cancer is increasing worldwide, with 1.6 million cases reported in 2008 and 2.1 million in 20181,2. The age-standardized 5-year overall survival (OS) rate associated with lung cancer is as low as 10–20%, and the disease remains associated with poor prognosis3; therefore, there is an urgent need to develop effective prognostic and management strategies.
Sarcopenia, first defined by Rosenberg4, is the loss of muscle mass in association with aging, decreased activity levels, malnutrition, organ failure, and diseases such as malignancies5,6. An association between sarcopenia and adverse health effects, including falls, disability, hospitalization, long-term care placement, poor quality of life, and mortality has been confirmed6. Sarcopenia is also associated with poor prognosis after treatment for malignancies, including lung cancer7–13. Further, an association between sarcopenia and poor prognosis in surgical cases, especially those involving early-stage lung cancer, has been demonstrated in many studies12,13. In a study by Baracos et al., 46.8% of the patients with stage III–IV non-small-cell lung cancer (NSCLC) were diagnosed with sarcopenia14. Concurrent chemoradiotherapy (CCRT) is among the standard treatments for stage III lung cancer15,16. Few studies have focused on the association between prognosis and sarcopenia in patients with NSCLC who were treated with chemoradiotherapy. Bowden et al. demonstrated that muscle attenuation, and not muscle mass, was associated with long-term survival in patients with lung cancer, including those with small-cell lung cancer and NSCLC, who underwent chemoradiotherapy17. Kiss et al. did not identify an association between muscle mass or muscle attenuation and OS in patients with NSCLC who underwent CCRT18. Existing evidence on the association between sarcopenia and OS in patients treated with CCRT for stage III NSCLC is insufficient.
Body mass index (BMI)-determined obesity is a poor prognostic factor after treatment in most cancer types19,20. However, the relationship between obesity and OS after treatment in patients with lung cancer is controversial19,20. In a meta-analysis, Gupta et al. showed that patients with obesity and overweight had lower lung cancer mortality rates than those with normal BMI21. Dahlberg et al. showed that patients with advanced NSCLC and obesity who received chemotherapy had better OS than those without obesity in the early stages of their study22. In locally advanced NSCLC settings, Lam et al. showed that the OS of patients who were obese was better than that of those who were normal-weight23. In the aforementioned studies, obesity was assessed based on BMI alone without using computed tomography (CT). In recent years, sarcopenia and adiposity have been measured based on muscle and fat masses using CT, which is necessary for diagnosing and staging cancer7,11,17,18,24–26. Although BMI is not a poor prognostic factor, the visceral-to-subcutaneous adipose tissue mass, in addition to muscle mass, is an independent poor prognostic factor in patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma25,26. Because weight includes components besides muscles, the measurement of fat mass in addition to muscle mass using CT is desirable. However, no studies to date have examined the association between fat mass and OS in patients with NSCLC who were treated with CCRT.
The identification of the level of risk before treatment and provision of appropriate interventions for sarcopenia and obesity may improve patient prognosis. Therefore, we intended to investigate whether CT-determined muscle and fat masses were prognostic factors in patients with stage III NSCLC who have undergone CCRT.
Patients and methods
Patients and treatment
We retrospectively explored data of patients with stage III NSCLC who underwent definitive CCRT (≥ 60 Gy) between January 2004 and March 2018 at our hospital. This study included patients for whom pre-CCRT digital plain CT of the third lumbar vertebra (L3) was available in the picture archiving and communication systems. Patients who underwent treatment for NSCLC before CCRT and those who received preoperative CCRT with chest surgery before recurrence were excluded. Staging was determined using the tumor-node-metastasis classification, 8th edition. We examined the patients’ histology, smoking history, performance status (PS), location, laterality, and forced expiratory volume in 1 s. The indication of CCRT was determined by consensus of board-certified respiratory physicians and radiation oncologists. The chemotherapy regimen was determined by board-certified respiratory physicians, and details regarding radiotherapy were finally determined by board-certified radiation oncologists. Chemotherapy mainly involved the administration of cisplatin plus docetaxel; tegafur/gimeracil/oteracil was used for vulnerable patients. All patients were treated with three-dimensional conformal radiotherapy. Details regarding the three-dimensional radiotherapy which was employed have been described elsewhere27. The gross tumor included the primary tumor and the clinically diagnosed metastatic lymph node. The margin for the clinical target volume was 5–10 mm, and subcarinal and ipsilateral hilar nodal stations were included in cases of elective nodal irradiation. The internal margin was determined using fluoroscopic images, and the planning target volume margin was 5–10 mm. We used a 10 MV photon beam generated from a linear accelerator (Primus or ONCOR, or Mevatron, Canon Medical Systems, Tochigi, Japan).
All procedures were performed according to the ethical standards set out in the 1964 Declaration of Helsinki and subsequent amendments. This study was approved by our institutional review board of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences and Okayama University Hospital (approval number 1809-018). Written informed consent for CCRT was obtained from all patients before treatment. The choice to opt out was provided through notifications displayed on the hospital’s website and in the outpatient ward before starting the study.
Image analysis
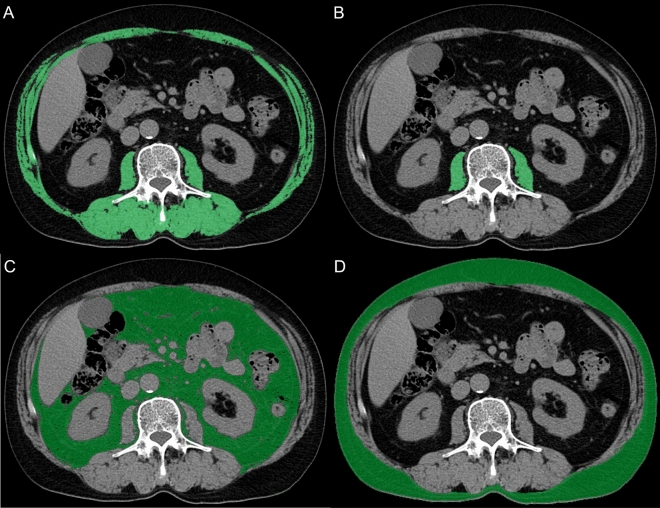
Abdominal CT data, which were obtained from the institution’s picture archiving and communication systems, were transferred to a workstation computer (SYNAPSE VINCENT version 5.5, FUJIFILM Medical Co., Ltd., Tokyo, Japan). Plain CT was used to examine the vertebra at the L3 level, and contours were extracted automatically by setting the Hounsfield unit (HU) thresholds within a certain range. According to previous studies, the HU thresholds were set from − 29 to + 150 for the skeletal and psoas muscles, − 150 to − 50 for visceral adipose tissues, and − 190 to − 30 for subcutaneous adipose tissues7,24. Skeletal muscles included all muscles at the L3 level. Areas that were not obviously targeted muscle or adipose tissues were deleted by manual manipulation. Figure 1A–D illustrate an example for each of the muscle and adipose tissues. These four areas (cm2) were divided by the square of height (m) to obtain the skeletal muscle index (SMI), psoas muscle index (PMI), visceral adipose index (VAI), and subcutaneous adipose index (SAI)25,28. Muscle attenuation (MA), as a measure of muscle quality, was defined as the mean of the HU value of the SMI; furthermore, the visceral-to-subcutaneous adipose ratio (VSR) was calculated7,25.
Figure 1.
Computed tomography images of the third lumbar vertebra for each area. (a) Green highlights indicate the skeletal muscle area for the skeletal muscle index. (b) Green highlights indicate the psoas muscle area for the psoas muscle index. (c) Green highlights indicate the visceral adipose tissue area for the visceral adipose index. (d) Green highlights indicate the subcutaneous adipose tissue area for the subcutaneous adipose index.
Evaluation and statistical analyses
Cutoff values for continuous variables were calculated based on individual time-dependent receiver operating characteristics29. According to previous reports26, we calculated the cutoff values for men and women and divided them into two groups. The survival curves were obtained using the Kaplan–Meier method. We examined the relationship between the aforementioned parameters and OS using log-rank tests for the univariate analysis and Cox proportional hazard models for the multivariate analysis. Statistical significance was determined at p-values < 0.05 (two-sided). R software version 3.5.1 and additional package of survival ROC version 1.0.3 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
Results
This study included 60 eligible patients. Table 1 shows the patients’ characteristics. Overall, 16 patients had stage IIIA NSCLC, 32 had stage IIIB, and 12 had stage IIIC. The follow-up period was 29.1 months (2.4–121.4 months) from the initiation of CCRT. The total radiotherapy doses were 60 Gy/30 fractions in 59 patients and 66 Gy/33 fractions in one patient. Details regarding the chemotherapy regimen are shown in Table 1. The median BMI, SMI, PMI, MA, VAI, SAI, and VSR were 21.7 kg/m2 (range: 14.2–34.1 kg/m2), 43.1 cm2/m2 (range: 23.5–64.8 cm2/m2), 5.1 cm2/m2 (range: 2.7–8.6 cm2/m2), 35.9 HU (range: 8.6–49.0 HU), 27.0 cm2/m2 (0.7–134.4 cm2/m2), 28.7 cm2/m2 (0.0–127.1 cm2/m2), and 1.0 (range: 0.1–25.8), respectively.
Table 1.
Patient characteristics.
| % | |||
|---|---|---|---|
| Sex | Male | 51 | 85 |
| Female | 9 | 15 | |
| Age (years) | Median (range) | 66 (35–87) | – |
| T stage | 1 | 6 | 10 |
| 2 | 12 | 20 | |
| 3 | 5 | 8 | |
| 4 | 33 | 55 | |
| X | 4 | 7 | |
| N stage | 0 | 3 | 5 |
| 1 | 5 | 8 | |
| 2 | 26 | 43 | |
| 3 | 26 | 43 | |
| Clinical stage | IIIA | 16 | 27 |
| IIIB | 32 | 53 | |
| IIIC | 12 | 20 | |
| Histology | Adenocarcinoma | 26 | 43 |
| Squamous cell carcinoma | 28 | 47 | |
| Non-small-cell carcinoma | 6 | 10 | |
| Smoking historya | Never | 5 | 8 |
| Former | 31 | 52 | |
| Current | 22 | 37 | |
| ECOG-PSa | 0 | 21 | 35 |
| 1 | 36 | 60 | |
| Lobea | Upper | 45 | 75 |
| Middle | 1 | 2 | |
| Lower | 11 | 18 | |
| Laterality | Right | 31 | 52 |
| Left | 26 | 43 | |
| FEV1 (l)a | Median (range) | 2.15 (0.86–4.11) | – |
| Chemotherapy | Standard | 49 | 82 |
| Cisplatin + Docetaxel | 40 | 67 | |
| Cisplatin + Vinorelbine + Nimotuzumab | 1 | 2 | |
| Cisplatin + Etoposide | 1 | 2 | |
| Cisplatin + Tegafur/Gimeracil/Oteracil | 5 | 8 | |
| Carboplatin + Paclitaxel | 2 | 3 | |
| Reduced | 11 | 18 | |
| Cisplatin | 1 | 2 | |
| Tegafur/Gimeracil/Oteracil | 10 | 17 | |
| Radiation dose (Gy) | Median (range) | 60 (60–66) | – |
| BMIa (kg/m2) | Median (range) | 21.7 (14.2–34.1) | – |
| SMI (cm2/m2) | Median (range) | 43.1 (23.5–64.8) | |
| PMI (cm2/m2) | Median (range) | 5.1 (2.7–8.6) | – |
| MA (HU) | Median (range) | 35.9 (8.6–49.0) | – |
| VAI (cm2/m2) | Median (range) | 27.0 (0.7–134.4) | – |
| SAI (cm2/m2) | Median (range) | 28.7 (0.0–127.1) | – |
| VSR | Median (range) | 1.0 (0.1–25.8) | – |
ECOG-PS Eastern Cooperative Oncology Group performance status, FEV1 forced expiratory volume in 1 s, BMI body mass index, MA mean muscle attenuation, SMI skeletal mass muscle index, PMI psoas muscle index, VAI visceral adiposity index, SAI subcutaneous adiposity index, VSR visceral-to-subcutaneous fat ratio.
aThese factors have missing values.
Table 2 shows the relationship between clinicopathologic factors and sarcopenia (low SMI). Sarcopenia was significantly associated with lower BMI (p = 0.017). Table 3 shows the cutoff values and the results of the univariate and multivariate analyses of factors associated with OS. The female/male cutoff BMI, SMI, PMI, MA, VAI, SAI, and VSR values determined from the time-dependent receiver operating characteristic curve were 17/25 kg/m2, 24/43 cm2/m2, 4.7/7.3 cm2/m2, 9/36 HU, 97/40 cm2/m2, 127/28 cm2/m2, and 0.8/0.6, respectively. The PS, SMI, and BMI were significant prognostic factors associated with OS in the univariate analysis (p = 0.004, 0.01, and 0.03), whereas the PMI, MA, VAI, SAI, and VSR were not (p = 0.4, > 0.99, 0.1, 0.09, and 0.1, respectively). In the multivariate analysis, the PS and SMI were significant prognostic factors associated with OS (p = 0.011 and 0.020, respectively). The hazard ratios and 95% confidence intervals (CIs) were 2.91 and 1.28–6.64 for the PS, 2.36 and 1.15–4.85 for the SMI, respectively. Since BMI and SMI are significantly correlated, we excluded BMI from the multivariate analysis according to previous reports30,31.
Table 2.
Relationship between clinicopathologic factors and sarcopenia.
| Factor | With sarcopenia (low SMI) | % | Without sarcopenia (high SMI) | % | p value | |
|---|---|---|---|---|---|---|
| Sex | Female | 1 | 5 | 8 | 21 | 0.14 |
| Male | 21 | 95 | 30 | 79 | ||
| Age (years) | < 66 | 8 | 36 | 20 | 53 | 0.29 |
| ≥ 66 | 14 | 64 | 18 | 47 | ||
| T stage | X, 1–2 | 5 | 23 | 17 | 45 | 0.1 |
| 3–4 | 17 | 77 | 21 | 55 | ||
| N stage | 0–1 | 6 | 27 | 2 | 5 | 0.042 |
| 2–3 | 16 | 73 | 36 | 95 | ||
| Clinical stage | IIIA | 6 | 27 | 10 | 26 | 0.52 |
| IIIB | 10 | 45 | 22 | 58 | ||
| IIIC | 6 | 27 | 6 | 16 | ||
| Histology | Adenocarcinoma | 8 | 36 | 18 | 47 | 0.43 |
| Others | 14 | 64 | 20 | 53 | ||
| Smoking historya | Never/former | 15 | 68 | 21 | 53 | 0.58 |
| Current | 7 | 32 | 15 | 47 | ||
| ECOG-PSa | 0 | 7 | 32 | 14 | 40 | 0.58 |
| 1 | 15 | 68 | 21 | 60 | ||
| Laterality | Right | 6 | 73 | 25 | 29 | 0.002 |
| Left | 16 | 27 | 10 | 71 | ||
| Lobe | Upper/middle | 18 | 82 | 31 | 82 | > 0.99 |
| Lower | 4 | 18 | 7 | 18 | ||
| FEV1 (l)a | < 2.2 | 8 | 42 | 21 | 66 | 0.15 |
| ≥ 2.2 | 11 | 58 | 11 | 34 | ||
| Chemotherapy | Standard | 17 | 77 | 32 | 84 | 0.51 |
| Reduced | 5 | 23 | 6 | 16 | ||
| BMIa | F: < 17, M: < 25 | 19 | 90 | 22 | 58 | 0.017 |
| F: ≥ 17, M: ≥ 25 | 2 | 10 | 16 | 42 | ||
| PMI | F: < 4.7, M: < 7.3 | 21 | 95 | 31 | 82 | 0.24 |
| F: ≥ 4.7, M: ≥ 7.3 | 1 | 5 | 7 | 18 | ||
| MA | F: < 9, M: < 36 | 11 | 50 | 13 | 34 | 0.28 |
| F: ≥ 9, M: ≥ 36 | 11 | 50 | 25 | 66 | ||
| VAI | F: < 97, M: < 40 | 18 | 82 | 24 | 63 | 0.15 |
| F: ≥ 97, M: ≥ 40 | 4 | 18 | 14 | 37 | ||
| SAI | F: < 127, M: < 28 | 15 | 68 | 19 | 50 | 0.19 |
| F: ≥ 127, M: ≥ 28 | 7 | 32 | 19 | 50 | ||
| VSR | F: < 0.8, M: < 0.6 | 8 | 36 | 11 | 29 | 0.58 |
| F: ≥ 0.8, M: ≥ 0.6 | 14 | 64 | 27 | 71 |
ECOG-PS Eastern Cooperative Oncology Group performance status, FEV1 forced expiratory volume in 1 s, BMI body mass index, MA mean muscle attenuation, SMI skeletal mass muscle index, PMI psoas muscle index, VAI visceral adiposity index, SAI subcutaneous adiposity index, VSR visceral-to-subcutaneous fat ratio, CI confidence interval.
aThese variables have missing values.
Table 3.
Univariate and multivariate analyses of factors associated with overall survival.
| Factor | Number of deaths | Univariate analysis | Multivariate analysis | Hazard ratio | |
|---|---|---|---|---|---|
| p-value | p-value | (95% CI) | |||
| Sex | F | 4/9 | 0.3 | NE | – |
| M | 32/51 | ||||
| Age (years) | < 66 | 16/28 | 0.6 | NE | – |
| ≥ 66 | 20/32 | ||||
| T Stage | X, 1–2 | 10/22 | 0.2 | NE | – |
| 3–4 | 26/38 | ||||
| N Stage | 0–1 | 3/8 | 0.4 | NE | – |
| 2–3 | 33/52 | ||||
| Clinical stage | IIIA | 6/16 | 0.3 | NE | – |
| IIIB | 22/32 | ||||
| IIIC | 8/12 | ||||
| Histology | Adenocarcinoma | 16/26 | 0.5 | NE | – |
| Others | 20/34 | ||||
| Smoking historya | Never/former | 21/36 | 0.9 | NE | – |
| Current | 13/22 | ||||
| ECOG-PSa | 0 | 8/21 | 0.004 | 0.011 | 2.91 |
| 1 | 25/36 | (1.28–6.64) | |||
| Laterality | Right | 17/31 | 0.5 | NE | – |
| Left | 18/26 | ||||
| Lobe | Upper/middle | 28/49 | 0.2 | NE | – |
| Lower | 8/11 | ||||
| FEV1 (l)a | < 2.2 | 17/29 | 0.2 | NE | – |
| ≥ 2.2 | 13/22 | ||||
| Chemotherapy | Standard | 29/49 | 0.5 | NE | – |
| Reduced | 7/11 | ||||
| BMIa | F: < 17, M: < 25 | 28/41 | 0.03 | NE | – |
| F: ≥ 17, M: ≥ 25 | 7/18 | ||||
| SMI | F: < 24, M: < 43 | 17/22 | 0.01 | 0.02 | 2.36 |
| F: ≥ 24, M: ≥ 43 | 19/38 | (1.15–4.85) | |||
| PMI | F: < 4.7, M: < 7.3 | 31/52 | 0.4 | NE | – |
| F: ≥ 4.7, M: ≥ 7.3 | 5/8 | ||||
| MA | F: < 9, M: < 36 | 15/24 | > 0.99 | NE | – |
| F: ≥ 9, M: ≥ 36 | 21/36 | ||||
| VAI | F: < 97, M: < 40 | 28/42 | 0.1 | NE | – |
| F: ≥ 97, M: ≥ 40 | 8/18 | ||||
| SAI | F: < 127, M: < 28 | 23/34 | 0.09 | NE | – |
| F: ≥ 127, M: ≥ 28 | 13/26 | ||||
| VSR | F: < 0.8, M: < 0.6 | 8/19 | 0.1 | NE | – |
| F: ≥ 0.8, M: ≥ 0.6 | 28/41 |
NE not entered, ECOG-PS Eastern Cooperative Oncology Group performance status, FEV1 forced expiratory volume in 1 s, BMI body mass index, MA mean muscle attenuation, SMI skeletal mass muscle index, PMI psoas muscle index, VAI visceral adiposity index, SAI subcutaneous adiposity index, VSR visceral-to-subcutaneous fat ratio, CI confidence interval.
aThese variables have missing values.
Figure 2 shows OS curve for all patients. The 1, 3, and 5-year OS rates were 81.7%, 57.9%, and 37.8%, respectively, for all patients. Further, the 1, 3, and 5-year OS rates were 92.1%, 59.6%, and 51.0% in patients with high SMI, and 63.6%, 53.8%, and 17.9% in patients with low SMI, respectively (Fig. 3). Details of the OS rates for sarcopenia are shown in Table 4. The 1, 3, and 5-year survival rates were 90.5%, 85.4%, and 64.3% for the groups with a PS of 0, and 75.0%, 44.7%, and 25.6% for the groups with a PS of 1, respectively (p = 0.004).
Figure 2.
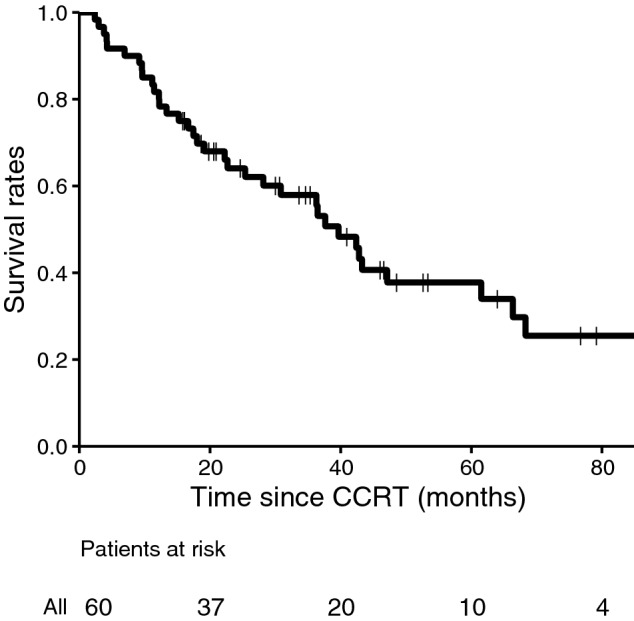
Overall survival curve. Proportion of surviving patients shown according to the number of months after radiotherapy. CCRT concurrent chemoradiotherapy.
Figure 3.
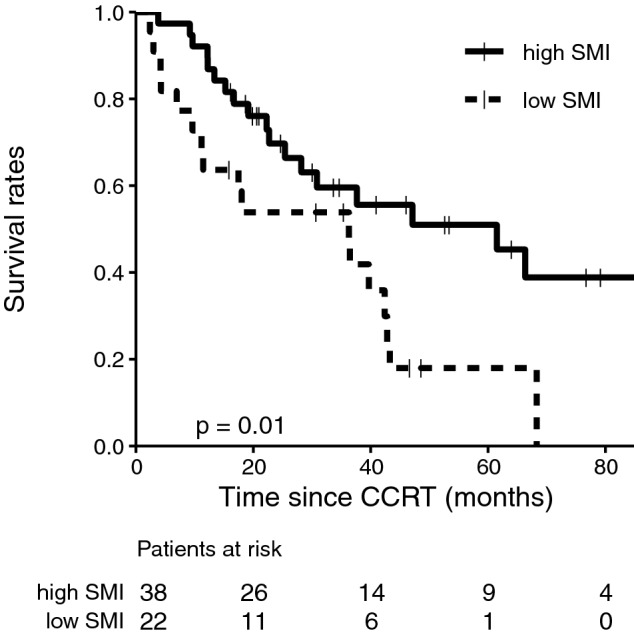
Subgroup analysis of overall survival stratified according to SMI. Proportion of surviving patients shown according to the number of months after radiotherapy, where the filled line represents a low SMI and the dotted line indicates a high SMI. SMI skeletal muscle index, CCRT concurrent chemoradiotherapy.
Table 4.
Overall survival rates for sarcopenia.
| year | OS (95% CI) for all patients | OS with sarcopenia (low SMI) (95% CI) | OS without sarcopenia (high SMI) (95% CI) |
|---|---|---|---|
| 1 | 81.7% (72.4–92.1%) | 63.6% (46.4–87.3%) | 92.1% (83.9–100%) |
| 2 | 64.1% (52.8–77.7%) | 53.8% (36.4–79.7%) | 69.7% (56.1–86.6%) |
| 3 | 57.9% (46.3–72.5%) | 53.8% (36.4–79.7%) | 59.6% (45.0–78.9%) |
| 4 | 37.8% (25.9–55.0%) | 17.9% (6.6–49.0%) | 51.0% (35.7–72.7%) |
| 5 | 37.8% (25.9–55.0%) | 17.9% (6.6–49.0%) | 51.0% (35.7–72.7%) |
CI confidence interval, OS overall survival, SMI skeletal mass muscle index.
Discussion
This was the first study to demonstrate that the SMI was associated with OS in patients with stage III NSCLC who underwent definitive CCRT. Studies focusing on the relationship between the SMI and OS in patients with cancer have predominantly been conducted in gastrointestinal cancer settings. Fujiwara et al. reported a significant association of the SMI and MA with mortality in patients with hepatocellular carcinoma, and Okumura et al. showed a relationship of the SMI and MA with OS in patients who underwent surgery for cholangiocarcinoma25,26. In terms of lung cancer, several systematic reviews and meta-analyses have focused on the association between sarcopenia and OS after treatment for lung cancer. Buentzel et al. conducted an analysis of 15 studies on patients with lung cancer (including all stages of NSCLC and small-cell lung cancer), and based on the findings of multivariate analysis, reported that patients with lung cancer and sarcopenia had a three-fold greater risk of death than those without sarcopenia8. Yang et al. who conducted an analysis of thirteen studies showed that sarcopenia was an independent prognostic factor for poor OS in patients with stage III–IV NSCLC9. Those reviews included several treatment modalities, including surgery, stereotactic radiotherapy10, chemoradiotherapy, and chemotherapy. Only two studies have considered SMI as an assessment tool for sarcopenia and examined its relationship with OS in patients who were treated with chemoradiotherapy. Kiss et al. reported that 41 patients with stage I–III NSCLC who received CCRT showed a significant decrease in the SMI and MA at 4 weeks after starting treatment and that low MA tended to be associated with poor OS (p = 0.13)18. However, the presence of sarcopenia before treatment is not associated with poor OS. Bowden et al. demonstrated that low MA was associated with significantly lower OS rates in patients with lung cancer, including those with NSCLC and small-cell lung cancer, who were treated with chemoradiotherapy17. The SMI was not associated with OS in Bowden et al.’s17 study as well. In our study, the SMI was a prognostic factor for OS, whereas MA was not. The study by Kiss et al. differs from our study with respect to the following aspects: the HU thresholds for SMI measurements ranged from − 19 to 150, and they included stage I–III cases. The study by Bowden et al. differs from our study with respect to the following aspects: it included sequential chemotherapy cases and CCRT cases, patients with small-cell lung cancer, and patients with all disease stages. In addition, the treatment strategies for stage III disease vary across institutions, with our institution prioritizing preoperative CCRT plus surgery. Therefore, we cannot rule out the possibility that the extent of progression and general condition in our patients may have significantly differed from those in patients in the aforementioned reports.
In our study, we included patients with stage III NSCLC who had CT data pertaining to the L3 level, with no restrictions on the chemotherapy regimen. Thus, our results may have differed from those of previous studies. In our study, the PMI was also not a prognostic factor. Hamaguchi et al. reported that in 541 adult liver donors (living donor liver transplantation), the PMI was positively correlated with the SMI28. Theoretically, if the SMI is significantly associated with OS, similar results should have been obtained for the PMI; however, it is not clear why this was not the case. Mitsuyoshi et al. reported that a high PMI was not correlated with high OS (p = 0.873) in 89 patients with stage III NSCLC with sex-specific cutoffs32; therefore, the PMI may not be an important parameter in lung cancer. Data on the association of the SMI and PMI with OS in patients with NSCLC are scarce in the setting of CCRT compared with surgery, and thus, further studies are warranted.
Muscle loss is a major symptom of cancer cachexia, along with anorexia, weight loss, anemia, and altered carbohydrate, lipid, and protein metabolism. Cancer cachexia is recognized as a paraneoplastic syndrome in 60–80% of the patients with advanced-stage disease. More than 30% of the patients with cancer die due to cachexia, and more than 50% of them die in the presence of cachexia, which is associated with adverse prognosis and shorter survival times33,34. Sarcopenia may be a marker to preemptively predict the development of cancer cachexia. Recently, skeletal muscle has been identified as a secretory organ, which secretes myokines that may affect the growth of cancer cells35. Hojman et al. reported that myokines, which are released from muscles during exercise inhibit the growth of breast cancer cells and induce apoptosis of these cells36. Therefore, as muscle mass decreases, myokine levels and responses may decrease, making it easier for cancer to progress.
In our study, a higher BMI was correlated with a higher OS rate in the univariate analysis. A high BMI is correlated to poor OS after cancer treatment19; however, there is some controversy regarding the same in patients with lung cancer. In their meta-analysis, Gupta et al. showed that patients who were obese in the pre-treatment stage had lower lung cancer mortality rates than those with normal weight; our results showed the same trend. In gastrointestinal cancers, high VAI, SAI, and VSR have been shown to be associated with poor OS rates25,26; however, no correlation was found in our study that examined data of patients with lung cancer following CCRT. The reason for this is that despite difficulties in the performance of simple comparisons, the median VAI and SAI values were 31.8 cm2/m2 and 41.4 cm2/m2, respectively, in the study by Fujiwara et al., which are higher than the values of 27.0 cm2/m2 and 28.7 cm2/m2, respectively, observed in our study. This may be largely attributed to differences in patients’ backgrounds. Regarding OS, the result observed in patients with lung cancer may differ from that in those with other carcinomas with respect to adipose mass, as with BMI. In patients undergoing CCRT for stage III NSCLC, adipose mass reductions are not given high priority, with efforts primarily driven toward increasing or maintaining patients’ muscle mass. To the best of our knowledge, our study was the first to examine the association between post-treatment OS rates for lung cancer and the VAI, SAI, and VSR, as assessed using CT. In our study, the BMI scores (median 21.7) of all patients were normal, unlike that in the study by Martin et al., in which the average BMI scores were 26.0 for men and 25.1 for women7. Among patients with lung cancer and normal BMI, adipose mass may not be related to prognosis; although, further studies on adipose mass are warranted.
In the study of Bowden et al., a PS ≥ 2 was associated with poor OS17. Similarly, in our study, the PS was a predictor of prognosis, which is a reasonable result.
Exercise is effective in counteracting the catabolic effects of muscle by increasing the protein synthesis rate and decreasing the proteolysis rate owing to its anti-inflammatory effects37,38. Exercise training can attenuate or reverse the process of muscle wasting through anti-inflammatory and antioxidant effects that can attenuate the signaling pathways of proteolysis and activate protein synthesis molecules38. Gould et al. stated in their review that exercise successfully improved muscle strength and physical function in patients with cachexia in non-cancer-related diseases and that exercise interventions provided at various stages of treatment may improve the protein synthesis rate and help patients recover from low body weight and reverse proteolysis37. Delrieu et al. reported that participation in a 6-month personalized walking program resulted in significant improvements in the 6-min walking distance test (+ 7%) and isometric quadriceps strength (+ 22%) among patients with metastatic breast cancer39. Exercise interventions may aid in increasing or maintaining the muscle strength in patients with lung cancer. Future clinical trials are warranted to determine whether improvements in muscle strength can lead to OS enhancements.
Our study has some limitations. Since this study was conducted at a single center with a short follow-up period, and CCRT is not the only treatment option for stage III NSCLC, the presence of an unmeasured bias cannot be ruled out. According to the European Working Group on Sarcopenia in Older People guidelines, the diagnosis of sarcopenia requires low muscle strength or low physical performance in addition to low muscle mass; however, because of the retrospective nature of the study, muscle strength measurements could not be performed.
In conclusion, we demonstrated that the SMI is correlated with prognosis in patients with stage III lung cancer undergoing CCRT, whereas adipose mass is not. The additional consideration of sarcopenia improved the predictability degree for OS in our settings, and therefore, could aid in early intervention to maintain muscle mass and improve prognosis.
Acknowledgements
We thank all participants whose data was used in this study. We are grateful to Dr. Keiji Matsuda and Dr. Kimiko Nishigaki (Department of Radiology, Okayama University Hospital), Dr. Toshio Kubo (Center for Clinical Oncology, Okayama University Hospital), and Dr. Nobuaki Ochi (Department of General Internal Medicine 4, Kawasaki Hospital, Kawasaki Medical School) for their assistance with the data collection. This research was funded by Tsuyama Chuo Hospital. The sponsors of this study were not involved in the conduct of this study, including the study design, data collection, data analysis, data interpretation, manuscript writing, or the decision to submit the manuscript for publication. We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
K.K. collected the data, performed data analysis, and drafted the manuscript. T.O. performed data analysis and statistical analysis. S.S. collected the data, performed data analysis, and drafted the manuscript. K.K., T.O., S.S., K.Y., M.K., T.H., K.K., Y.M., S.T., and S.K. participated in the design of the study and read and approved the final manuscript.
Data availability
The institution’s review board prohibits data sharing.
Competing interests
The authors declare no competing interests.
Footnotes
The original online version of this Article was revised: The original version of this Article contained errors in Table 3, where some values in Number of deaths were incorrectly given.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/12/2021
A Correction to this paper has been published: 10.1038/s41598-021-93955-6
References
- 1.Ferlay J, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg IH. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LK, et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Martin L, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 8.Buentzel J, et al. Sarcopenia as prognostic factor in lung cancer patients: A systematic review and meta-analysis. Anticancer Res. 2019;39:4603–4612. doi: 10.21873/anticanres.13640. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, et al. Prognostic value of sarcopenia in lung cancer: A systematic review and meta-analysis. Chest. 2019;156:101–111. doi: 10.1016/j.chest.2019.04.115. [DOI] [PubMed] [Google Scholar]
- 10.Matsuo Y, et al. Impact of low skeletal muscle mass on non-lung cancer mortality after stereotactic body radiotherapy for patients with stage I non-small cell lung cancer. J. Geriatr. Oncol. 2018;9:589–593. doi: 10.1016/j.jgo.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kim EY, et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J. Thorac. Oncol. 2015;10:1795–1799. doi: 10.1097/JTO.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 12.Deng HY, et al. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: A comprehensive systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019;45:728–735. doi: 10.1016/j.ejso.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Nishimura JM, et al. Computed tomography-assessed skeletal muscle mass as a predictor of outcomes in lung cancer surgery. Ann. Thorac. Surg. 2019;108:1555–1564. doi: 10.1016/j.athoracsur.2019.04.090. [DOI] [PubMed] [Google Scholar]
- 14.Baracos VE, et al. Body composition in patients with non-small cell lung cancer: A contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 2010;91:1133S–1137S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 15.Curran, W. J. et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J. Natl. Cancer Inst.103, 1452–1460 (2011). [DOI] [PMC free article] [PubMed]
- 16.Antonia SJ, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 17.Bowden, J.C.S. et al. Prediction of 90 day and overall survival after chemoradiotherapy for lung cancer: Role of performance status and body composition. Clin. Oncol. (R. Coll. Radiol.)29, 576–584 (2017). [DOI] [PubMed]
- 18.Kiss N, Beraldo J, Everitt S. Early skeletal muscle loss in non-small cell lung cancer patients receiving chemoradiation and relationship to survival. Support Care Cancer. 2019;27:2657–2664. doi: 10.1007/s00520-018-4563-9. [DOI] [PubMed] [Google Scholar]
- 19.Calle, E.E. et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med.348, 1625–1638 (2003). [DOI] [PubMed]
- 20.Lauby-Secretan B, et al. Body fatness and cancer–Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, et al. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer. 2016;102:49–59. doi: 10.1016/j.lungcan.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Dahlberg SE, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J. Thorac. Oncol. 2013;8:1121–1127. doi: 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam VK, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2017;104:52–57. doi: 10.1016/j.lungcan.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Prado CM, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara N, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 26.Okumura S, et al. Impact of skeletal muscle mass, muscle quality, and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2017;24:1037–1045. doi: 10.1245/s10434-016-5668-3. [DOI] [PubMed] [Google Scholar]
- 27.Katsui K, et al. Radiation pneumonitis after definitive concurrent chemoradiotherapy with cisplatin/docetaxel for non-small cell lung cancer: Analysis of dose-volume parameters. Cancer Med. 2020;9:4540–4549. doi: 10.1002/cam4.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamaguchi Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition. 2016;32:1200–1205. doi: 10.1016/j.nut.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341X.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura R, et al. Sarcopenia in resected NSCLC: Effect on postoperative outcomes. J. Thorac. Oncol. 2018;13:895–903. doi: 10.1016/j.jtho.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara S, et al. Impact of sarcopenia on surgical outcomes in non-small cell lung cancer. Ann. Surg. Oncol. 2020;27:2427–2435. doi: 10.1245/s10434-020-08224-z. [DOI] [PubMed] [Google Scholar]
- 32.Mitsuyoshi T, et al. Evaluation of a prognostic scoring system based on the systemic inflammatory and nutritional status of patients with locally advanced non-small-cell lung cancer treated with chemoradiotherapy. J. Radiat. Res. 2018;59:50–57. doi: 10.1093/jrr/rrx060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silvério R, et al. l-carnitine and cancer cachexia: Clinical and experimental aspects. J. Cachexia Sarcopenia Muscle. 2011;2:37–44. doi: 10.1007/s13539-011-0017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Haehling S, et al. Cachexia as a major underestimated and unmet medical need: Facts and numbers. J. Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen BK, et al. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 36.Hojman P, et al. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol. Endocrinol. Metab. 2011;301:E504–510. doi: 10.1152/ajpendo.00520.2010. [DOI] [PubMed] [Google Scholar]
- 37.Gould DW, et al. Cancer cachexia prevention via physical exercise: Molecular mechanisms. J. Cachexia Sarcopenia Muscle. 2013;4:111–124. doi: 10.1007/s13539-012-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle. 2015;6:197–207. doi: 10.1002/jcsm.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delrieu, L. et al. Feasibility and health benefits of an individualized physical activity intervention in women with metastatic breast cancer: Intervention study. JMIR. Mhealth. Uhealth.8, e12306 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The institution’s review board prohibits data sharing.