Abstract
Motivational deficits (e.g., apathy) and dysregulation (e.g., addiction) in HIV-1 seropositive individuals, despite treatment with combination antiretroviral therapy, necessitates the development of innovative adjunctive therapeutics. S-Equol (SE), a selective estrogen receptor β agonist, has been implicated as a neuroprotective and/or neurorestorative therapeutic for HIV-1 associated neurocognitive disorders (HAND); its therapeutic utility for motivational alterations, however, has yet to be systematically evaluated. Thus, HIV-1 transgenic (Tg) and control animals were treated with either a daily oral dose of SE (0.2 mg) or vehicle and assessed in a series of tasks to evaluate goal-directed and drug-seeking behavior. First, at the genotypic level, motivational deficits in HIV-1 Tg rats treated with vehicle were characterized by a diminished reinforcing efficacy of, and sensitivity to, sucrose. Motivational dysregulation was evidenced by enhanced drug-seeking for cocaine relative to control animals treated with vehicle. Second, treatment with SE ameliorated both motivational deficits and dysregulation in HIV-1 Tg rats. Following a history of cocaine self-administration, HIV-1 Tg animals treated with vehicle exhibited lower levels of dendritic branching and a shift towards longer dendritic spines with decreased head diameter. Treatment with SE, however, led to long-term enhancements in dendritic spine morphology in HIV-1 Tg animals supporting a potential underlying basis by which SE exerts its therapeutic effects. Taken together, SE restored motivated behavior in the HIV-1 Tg rat, expanding the potential clinical utility of SE to include both neurocognitive and affective alterations.
Subject terms: Motivation, HIV infections, Spine structure
Introduction
Motivation, a multi-faceted construct, is an adaptational system that utilizes internal and external conditions to regulate behavior. Mechanistically, motivation is regulated by the fronto-striatal circuit (for review1), which includes the ventral tegmental area (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC). Disruption of the fronto-striatal circuit manifests in motivational alterations (for review2), which fall along a continuum and can be broadly categorized as motivational deficits (e.g., apathy), dysregulation (e.g., addiction), and/or excess (e.g., bipolar disorder). Etiologies, including human immunodeficiency virus type 1 (HIV-1; for review3), that target the neural substrates of the fronto-striatal circuit are commonly associated with prominent motivational alterations.
Indeed, HIV-1 seropositive individuals exhibit an increased neurobehavioral burden, evidenced by a greater prevalence of apathy4–6, addiction7, and bipolar disorder7, relative to their seronegative counterparts. Motivational alterations are associated with profound functional consequences, including greater impairment in activities of daily living4,8, an increased number of cognitive complaints4,8 and neurocognitive impairments9, decreased medication adherence9–11, and decreased quality of life5,12. Thus, there remains a critical need to develop innovative adjunctive therapeutics to mitigate the prominent motivational alterations in HIV-1 seropositive individuals.
Gamma-aminobutyric acid (GABA) medium spiny neurons (MSNs) of the NAc, a key component of the fronto-striatal circuit, play a central role in regulating motivational behaviors (e.g.,13). Accounting for approximately 95% of the cells within the region14, MSNs are characterized by a centrifugal morphology and high densities of dendritic spines15. The morphological parameters of dendritic spines are tightly coupled with synaptic function (e.g.,16–19), affording a unique tool to infer the function of spine synapses (for review20). Indeed, long-term HIV-1 viral protein exposure induces a prominent distributional shift in the morphology of dendritic spines, characterized by decreased dendritic spine volume21,22 and increased dendritic spine length22; morphological parameters which support synaptic dysfunction. Alterations in the dendritic branching complexity21,22 and neuronal excitability23 of MSNs have also been observed following chronic HIV-1 viral protein exposure. Most critically, MSNs have been implicated as a key structural locus for the actions of HIV-1 viral proteins on goal-directed behaviors22 supporting a key target for the development of innovative therapeutics.
Equol, a phytoestrogen that is structurally similar to 17β-estradiol24, may serve as a novel therapeutic to target the prominent synaptic dysfunction observed in MSNs following chronic HIV-1 viral protein exposure. Following the ingestion of the soy derived phytoestrogen daidzein, Equol is produced by the gut microbiota25. S-Equol (SE), the only enantiomer produced by humans26, penetrates the central nervous system via the blood–brain barrier and exhibits selective affinity for estrogen receptor β (ERβ;26,27). ERβ is widely distributed in the fronto-striatal circuit, including in the PFC and VTA28. Critically, 17β-estradiol alters the structure of MSNs in the NAc29,30. With regards to HIV-1 viral proteins, pretreatment with SE precludes synapse loss resulting from exposure to Tat27; research which corroborates studies demonstrating the utility of daidzein, a precursor to SE25, to both protect and restore synaptodendritic damage due to HIV-1 viral proteins31. Furthermore, SE has been implicated as an efficacious therapeutic for the neurocognitive impairments, collectively termed HIV-1 associated neurocognitive disorders (HAND), associated with the disease32–34 heralding an investigation of its utility for motivational alterations associated with HIV-1.
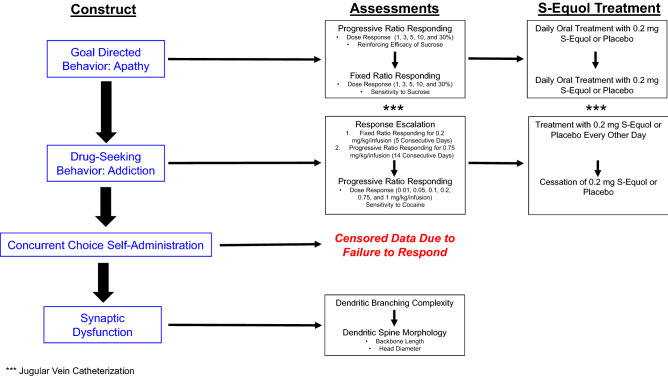
Thus, the goals of the present study were twofold. First, to systematically evaluate the therapeutic efficacy of SE for motivational alterations in the HIV-1 Tg rat. Goal-directed and drug-seeking behavior, indices of apathy and addiction, respectively, were investigated using operant conditioning. Second, to evaluate a potential underlying basis for the therapeutic effects of SE for motivational alterations. The morphology of MSNs of the NAc, and associated dendritic spines, was examined using a ballistic labeling technique after the completion of behavioral assessments. Given that pharmacological treatments for apathy35 and/or addiction36 are currently limited, the development of a novel therapeutic for motivational alterations associated with HIV-1 has the potential for broad clinical significance.
Methods
Animals
The efficacy of SE as an innovative therapeutic for motivational alterations associated with chronic HIV-1 viral protein exposure were evaluated in ovariectomized (OVX) female Fisher (F344/NHsd; Harlan Laboratories, Inc., Indianapolis, IN) HIV-1 Tg (n = 21) and control (n = 21) rats. The HIV-1 Tg rat, originally reported by Reid et al.37, expresses 7 of the 9 HIV-1 viral proteins (i.e., env, tat, rev, vif, vpr, vpu, nef) constitutively throughout development38,39. Although the HIV-1 Tg rat is rendered non-infectious by the deletion of gag and pol, the biological system expresses viral proteins relevant to central nervous system damage (e.g., gp12040, tat31,41). The HIV-1 Tg rat exhibits relatively good health throughout the functional lifespan, evidenced by similar growth rates relative to control animals21,38 and intact sensory and motor system function38,42.
Unrelated animals were requested to prevent the violation of the independent observation assumption inherent in many traditional statistical techniques. Furthermore, given the potential for sporadic transgene insertion elsewhere in the rat genome, age-matched F344/NHsd controls (rather than littermates) were purchased from Harlan Laboratories to assure the most developmentally appropriate and genetically stable baseline.
Animals were delivered to the animal vivarium, after being OVX at Harlan Laboratories, between six and eight months of age. SE, the therapeutic of interest, is a nonsteroidal estrogen that exhibits a selective affinity for ERβ26,27. Therefore, to preclude any potential confounding effects of endogenous hormones, female animals were OVX and fed a minimal phytoestrogen diet (≤ 20 ppm; Teklad 2020X Global Extruded Rodent Diet (Soy Protein-Free)). Animals had ad libitum access to rodent food and water, unless otherwise specified.
Animals were maintained in AAALAC-accredited facilities according to guidelines established by the National Institutes of Health and the ARRIVE guidelines. The animal colony was maintained at 21 ± 2 °C, 50 ± 10% relative humidity and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina (Federal Assurance #D16-00028) approved all experimental procedures. The study was carried out following all the relevant National Institutes of Health guidelines.
Apparatus
Assessments of goal-directed and drug-seeking behavior were conducted in operant chambers (ENV-008; Med-Associates, St. Albans, VT, USA) located within sound-attenuating enclosures and controlled by Med-PC computer interface software. The front panel of the operant chamber contained a magazine that allowed a recessed 0.01 cc dipper cup (ENV-202C) to deliver a solution through a 5 cm × 5 cm opening (ENV 202M-S), two retractable “active” metal levers (i.e., responding resulted in reinforcement; ENV-112BM), and two white cue lights (28 V). Head entries into the magazine were detected using an infrared sensor (ENV 254-CB). To control for side bias, an “active” lever retracted after five consecutive presses on a single lever. The rear panel of the operant chamber had one non-retractable “inactive” lever, whereby responding was recorded, but not reinforced, and a house light (28 V).
During the assessment of drug-seeking behavior, intravenous cocaine infusions were delivered through a water-tight swivel (Instech 375/22ps 22GA; Instech Laboratories, Inc., Plymouth Meeting, PA), which was connected to the backmount of the animal using Tygon tubing (ID, 0.020 IN; OD, 0.060 IN) enclosed by a stainless steel tether (Camcaths, Cambridgeshire, Great Britain), using a syringe pump (PHM-100). A Med-PC computer program was utilized to calculate pump infusion times based on an animal’s daily bodyweight.
Drugs
Cocaine hydrochloride (Sigma-Aldrich Pharmaceuticals, St. Louis, MO) was weighed as a salt and was dissolved in physiological saline (0.9%; Hospira, Inc., Lake Forest, IL). To preclude any significant hydrolysis of cocaine43, solutions were prepared immediately prior to the start of each testing session for every animal. Sucrose solutions were prepared at the beginning of each testing day.
After obtaining SE from Cayman Chemical Company (Ann Arbor, MI, USA), it was incorporated into 100 mg sucrose pellets (0.05 mg SE per sucrose pellet) by Bio-Serv (Flemington, NJ). Sucrose pellets (100 mg) were purchased from Bio-Serv for the vehicle group.
Experimental timeline
The experimental timeline for SE treatment, evaluation of goal-directed and drug-seeking behavior as well as neuroanatomical assessments, is illustrated in Fig. 1.
Figure 1.
Experimental design schematic.
Treatment
Animals were randomly assigned to receive either 0.2 mg SE (Control: n = 11; HIV-1 Tg: n = 11) or vehicle (Control: n = 10; HIV-1 Tg: n = 10). Between approximately 7 and 9 months of age, and one week prior to the start of operant training, HIV-1 Tg and control animals began receiving a daily treatment of either SE or vehicle. Given that each sucrose pellet contained 0.05 mg SE, animals receiving SE treatment received four pellets per day. Animals receiving vehicle treatment received four sucrose pellets per day. Daily treatment continued until jugular vein catheterization. Following jugular vein catheterization, animals were not treated for one week. When treatment resumed, it was given every other day until the end of the 14-day cocaine self-administration progressive ratio (PR) task. No treatment occurred during the cocaine self-administration PR dose–response task or concurrent choice self-administration. In total, HIV-1 Tg and control animals were treated for 84 days.
The 0.2 mg dose of SE was selected for two complementary reasons. First, using a dose–response experiment design, 0.2 mg SE was established as the most efficacious dose for the alleviation of sustained attention deficits in the HIV-1 Tg rat32. Second, the dose selected yielded a daily amount of 0.25–1.0 mg/kg SE (i.e., equivalent to a 2.5–10 mg dose in a 60 kg human); a dose well below the daily isoflavone intake of most elderly Japanese (i.e., 30–50 mg;44).
Preliminary training
Previously established research protocols were used to conduct dipper training and autoshaping45,46. During dipper training, animals were trained to approach the magazine and drink a 5% sucrose solution (w/v) from the dipper receptacle. Subsequently, during autoshaping, animals learned to lever press for the 5% sucrose solution (w/v) using a fixed-ratio (FR) 1 schedule of reinforcement. Water restriction (12–15 h prior to assessment) was implemented throughout preliminary training. Animals had ad libitum access to water for 9–12 h after the completion of testing.
To successfully acquire autoshaping, animals were required to achieve at least 60 reinforcers for 3 consecutive days. Ad libitum access to water was reinstated following the successful completion of preliminary training. Water was available ad libitum for all subsequent assessments.
Goal-directed behavior: apathy
Recognized as a multidimensional syndrome resulting in diminished motivation8, apathy is characterized by a quantitative reduction in voluntary and purposeful (goal-directed) behaviors47.
Progressive-ratio responding: dose response
Under a PR schedule of reinforcement, the response requirements are increased immediately following the delivery of a reinforcer48 affording a method to evaluate reinforcing efficacy49.
Following one maintenance session (i.e., 5% sucrose on an FR-1 schedule of reinforcement), animals were assessed using a PR schedule of reinforcement (Maximum Session Length: 120 Minutes). A dose–response experimental design was utilized, whereby the reinforcer was one of five sucrose concentrations (1, 3, 5, 10, and 30% w/v), presented using a Latin-Square experimental design, on test days, which occurred every other day. A maintenance session also occurred on intervening non-test days.
During PR tests, the ratio requirement was completed by responding across the two “active” levers. After successfully meeting the ratio requirement, the active levers were retracted and animals had 4 s of access to sucrose. The ratio requirement (rounded to the nearest integer) increased progressively according to the following exponential function: [5e(reinforcer number × 0.2)] − 549.
Fixed-ratio responding: dose response
Under an FR schedule of reinforcement, the animal receives a reinforcer following a set number of responses (e.g., 1). Varying the unit-dose of sucrose changes responding, affording an opportunity to evaluate sensitivity to sucrose.
Animals responded for the same dose–response sucrose concentrations (i.e., 1, 3, 5, 10, and 30% w/v) on an FR-1 schedule of reinforcement. Testing days occurred every other day and a maintenance session occurred on the intervening non-test days.
Jugular vein catheterization
Following the completion of assessments measuring goal-directed behavior, jugular vein catheterization was performed using the methods reported by Bertrand et al.46. In brief, HIV-1 Tg and control animals were anesthetized using 5% inhalant sevofluorane and maintained at 3.5–4% sevofluorane throughout the surgical procedure. After anesthesia induction, a sterile IV catheter, which extended dorsally and connected to an acrylic pedestal embedded with mesh, was implanted into the right jugular vein and secured with sterile sutures (4–0 Perma–Hand silk; EthiconEnd-Surgery, Inc.). The dorsal portion of the catheter/backmount was implanted subcutaneously above the right and left scapulae and stitched into place using sterile, absorbable sutures (4–0 Monoweb). Immediately following surgery, post-operative analgesia was provided by butorphenol (Dorolex; 0.8 mg/kg, SC; Merck Animal Health, Millsboro, DE) and the antibiotic gentamicin sulfate (0.2 ml 1%, IV; VEDCO, Saint Joseph, MO) was administered to prevent infection. Rats were monitored in a heat-regulated warm chamber following surgery and returned to the colony room after recovery from anesthesia. Two HIV-1 Tg animals died immediately following surgery; one of unknown causes and one suffered from a seizure after returning to the home cage, yielding Control Vehicle, n = 10; Control SE, n = 11; HIV-1 Tg Vehicle, n = 9; HIV-1 Tg SE, n = 10 for assessment of drug-seeking behavior.
For one week following surgery, catheters were “flushed” daily with a solution containing heparin (2.5%; APP Pharmaceuticals, Schamburg, IL) and the antibiotic gentamicin sulfate (1%) to prevent clotting and infections, respectively. Seven days after jugular vein catheterization, animals began assessments of drug-seeking behavior and resumed treatment with either SE or vehicle. Prior to operant testing each day, catheters were “flushed” with 0.9% saline solution (Baxter, Deerfield, IL). After the completion of daily operant testing, catheters were “flushed” with post-flush solution.
Drug-seeking behavior: addiction
Response escalation
A hallmark of addiction is the progressive increase in the frequency and amount of drug intake50. Two phases (i.e., FR-1 Responding and PR Responding), modified from Morgan et al.51 and previously employed in our laboratory by Bertrand et al.46, were utilized to produce an escalation of cocaine-maintained responding; an experimental paradigm that affords a critical opportunity to model a key aspect of drug addiction in humans.
First, rats responded for cocaine (0.2 mg/kg/inf) according to a FR-1 schedule of reinforcement for 5 consecutive days (Session Length: 1 h). Second, rats responded for cocaine (0.75 mg/kg/inf) on a PR schedule of reinforcement, whereby the ratio requirement was defined using the exponential function defined above for 14 consecutive days (Maximum Session Length: 120 Minutes). During both FR-1 and PR responding, a 20 s time-out (i.e., active levers were retracted and the house light was extinguished) following the completion of a response requirement.
Progressive-ratio responding: dose response
Under a PR schedule of reinforcement, the response requirements are increased immediately following the delivery of a reinforcer48 affording a method to evaluate reinforcing efficacy49.
Cocaine concentrations (0.01, 0.05, 0.1, 0.2, 0.75, and 1.0 mg/kg/inf) were presented in ascending order. A maintenance session (i.e., 0.2 mg/kg/inf on an FR-1 schedule of reinforcement) occurred every other day.
Concurrent choice self-administration
After establishing a history of sucrose and cocaine maintained-responding, choice behavior was evaluated using a concurrent choice self-administration experimental paradigm for seven consecutive days. Throughout the experimental procedure, animals were responding for 5% (w/v) sucrose or 0.2 mg/kg/inf of cocaine. On the eighth day, saline was substituted for cocaine. On the ninth day, water was substituted for sucrose. Sucrose-paired lever presentation (right or left) was balanced between groups.
Each session began with four forced-choice trials (i.e., only one “active” lever was available) where animals responded for two sucrose and two cocaine reinforcers. Following the forced choice trials, both “active” levers were concurrently available to allow the animals to freely choose between sucrose and cocaine. After a response was made, a 20 s time-out occurred.
Data for the concurrent choice self-administration were censored due to the absence of responding, and thus absence of a choice, independent of genotype group. Censoring reduced group sample sizes by approximately 50%. Statistical power estimates for a three-way interaction (Genotype × Reinforcer × Day) investigating the impact of the HIV-1 transgene on choice behavior was 0.31; an estimate which is significantly lower than appropriate statistical power levels (i.e., 0.8;52) and thus precluded drawing any reliable inferences about choice behavior.
Neuronal and dendritic spine morphology in medium spiny neurons of the nucleus accumbens
Preparation of tissue
Animals were transcardially perfused within 24 h of their last self-administration session. After animals were deeply anesthetized using sevoflurane (Abbot Laboratories, North Chicaco, IL), transcardial perfusion was conducted using the methodology reported by Roscoe et al.21 with one minor modification. Specifically, after the brains were dissected, they were post-fixed in 4% paraformaldehyde for 10 min.
DiOListic labeling and confocal imaging
MSNs from the NAc were visualized using a ballistic labeling technique, originally described by Seabold et al.53. Methodology for the preparation of DiOlistic cartridges, preparation of Tefzel tubing, and DiOlistic labeling was previously described in detail21. MSNs were analyzed from the NAc, located approximately 2.28 mm to 0.60 mm anterior to Bregma54. Z-stack images were obtained using methodology previously reported21.
Dendritic branching complexity
Dendritic branching was manually evaluated for images with a clear dendritic arbor, as assessed by the maximum intensity projection image. Analyses were conducted on one to four MSNs per animal, yielding Control Vehicle, n = 7; Control SE, n = 7; HIV-1 Tg Vehicle, n = 5; HIV-1 Tg SE, n = 10. Given the nested experimental design, cluster means were calculated for dendritic branching, whereby the sample size (n) reflects the number of animals in each group.
Dendritic spine morphology quantification
Dendritic spine morphological parameters, including dendritic spine length19 and dendritic spine head17,18, are strongly correlated with synaptic strength and area of the postsynaptic density, respectively. Assessing how the HIV-1 transgene and/or SE alter dendritic spine morphology following a history of cocaine self-administration, therefore, affords a critical opportunity to evaluate the functional features of spine synapses20.
Dendritic spine parameters were analyzed using the AutoNeuron and AutoSpine extension modules in Neurolucida360 (MicroBrightfield, Williston, VT, USA). Spine analyses were conducted for MSNs meeting the following selection criteria, including 1. Continuous staining beginning in the cell body extending throughout the dendrite; 2. Minimal DiI diffusion; and 3. Low background fluorescence. Analyses were conducted on one to eight MSNs per animal, yielding Control Vehicle, n = 10; Control SE, n = 11; HIV-1 Tg Vehicle, n = 6; HIV-1 Tg SE, n = 10. Given the nested experimental design, cluster means were calculated for morphological parameters, whereby the sample size (n) reflects the number of animals in each group.
Two dendritic spine morphological parameters, including dendritic spine backbone length (µm) and dendritic spine head diameter (µm), were assessed. Dendritic spines were included in the analysis if they met the definitional criteria established using previously published manuscripts (i.e., backbone length, 0.2 to 4.0 µm55; head diameter, 0.0 to 1.2 µm56; volume, 0.05 to 0.85 µm357).
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) and regression techniques (SAS/STAT Software 9.4, SAS Institute, Inc., Cary, NC; SPSS Statistics 27, IBM Corp., Somer, NY; GraphPad Software, Inc., La Jolla, CA). An alpha criterion of p ≤ 0.05 was utilized to establish statistical significance. Orthogonal decompositions and/or the Greenhouse–Geisser df correction factor58 were implemented to account for variables that may violate the compound symmetry assumption. Based on the a priori aims of the present study, planned comparisons were conducted to evaluate the impact of chronic HIV-1 viral protein exposure on goal-directed and drug-seeking behavior (i.e., Control Vehicle vs. HIV-1 Tg Vehicle), the presence of an SE effect (i.e., HIV-1 Tg Vehicle vs. HIV-1 Tg SE), and the magnitude of the SE effect (i.e., Control Vehicle vs. HIV-1 Tg SE).
For goal-directed behavior, the a priori planned comparisons were addressed using regression and/or ANOVA techniques. Specifically, regression analyses were conducted to evaluate the shape and parameters of the best-fit function for the acquisition of autoshaping, PR responding, and FR-1 responding. To further evaluate the impact of chronic HIV-1 viral protein exposure on PR responding, a mixed-model ANOVA was conducted using SPSS Statistics 27. Two dependent variables of interest, including the number of reinforcers and the number of “active” lever presses, were investigated. In cases where an animal was tested more than once at a single concentration, a cluster mean was calculated to account for the nested experimental design59,60. The mean series imputation method was used for occasionally censored data, which occurred at the 1% (HIV-1 Tg SE: n = 1) and 10% (Control Vehicle: n = 1) concentration. One HIV-1 Tg animal treated with vehicle failed to acquire autoshaping and therefore did not complete either the PR or FR assessment; the animal was not included in the statistical analyses for these tasks.
For drug-seeking behavior, regression and/or ANOVA techniques were also utilized to statistically evaluate the a priori planned comparisons. FR-1 responding for a 0.2 mg/kg/inf dose of cocaine was evaluated using regression analyses and a mixed-model ANOVA (PROC MIXED; SAS/STAT Software 9.4) with an unstructured covariance structure. The number of infusions earned and number of “active” lever presses during the cocaine PR task (0.75 mg/kg/inf dose) were evaluated using regression analyses. Additionally, a mixed model ANOVA (PROC MIXED; SAS/STAT Software 9.4) with a compound symmetry covariance structure was also conducted to assess the number of “active” lever presses during the cocaine PR task. Regression analyses were used to statistically analyze the number of cocaine infusions during the dose–response cocaine PR assessment.
Neuronal morphology and dendritic spine morphological parameters were analyzed using ANOVA techniques. Specifically, dendritic spine branch order was evaluated using a mixed-model ANOVA (SPSS Statistics 27) and dendritic spine backbone length and head diameter were analyzed using a generalized linear mixed effects model with a Poisson distribution and an unstructured covariance structure (PROC GLIMMIX; SAS/STAT Software 9.4).
Results
Presence of the HIV-1 transgene
Preliminary training
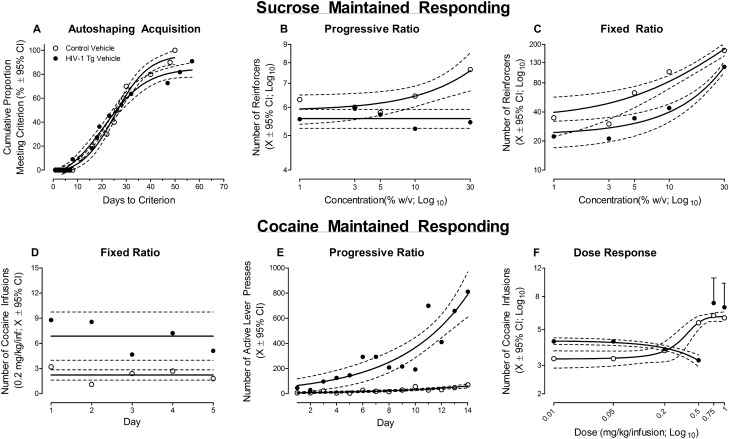
Acquisition of Autoshaping. At the genotypic level, HIV-1 Tg animals treated with vehicle acquired autoshaping, earning at least 60 reinforcers for three consecutive days, significantly slower than control animals treated with vehicle (Fig. 2A). After 66 days, 100% of the control and 90% of the HIV-1 Tg rats treated with vehicle met criteria. A sigmoidal-dose response curve (variable slope) afforded the best-fit for the number of days to criteria for both HIV-1 Tg (R2 = 0.98) and control (R2 = 0.98) animals treated with vehicle; albeit statistically significant differences in the parameters of the function [F(4, 27) = 4.6, p ≤ 0.006] were observed. Results support, therefore, a prominent deficit in stimulus-reinforcement learning in HIV-1 Tg rats treated with vehicle.
Figure 2.
The impact of the HIV-1 transgene on sucrose-maintained responding (A–C) and cocaine-maintained responding (D–F) is illustrated as a function of genotype (HIV-1 Tg Vehicle vs. Control Vehicle; ± 95% Confidence Intervals). HIV-1 Tg animals took significantly longer to successfully acquire autoshaping, supporting a profound deficit in stimulus-reinforcement learning (A). Under sucrose-maintained responding, apathetic behaviors in the HIV-1 Tg rat were characterized by a diminished sensitivity to (B), and reinforcing efficacy of (C), sucrose relative to control animals. Under cocaine-maintained responding, HIV-1 Tg animals exhibited an increased response vigor, independent of schedule of reinforcement (i.e., Fixed Ratio 1: D; Progressive Ratio: E and F), relative to control animals. Furthermore, an increased sensitivity to cocaine dose was observed in HIV-1 Tg animals relative to controls (F). Collectively, independent of differences in the unique response to either sucrose or cocaine self-administration, the HIV-1 Tg rat exhibits prominent alterations in goal-directed behaviors.
Goal-directed behavior: apathy
Progressive-ratio responding: dose-response
After successfully acquiring autoshaping, the reinforcing efficacy of sucrose was evaluated using a dose–response experimental design and PR schedule of reinforcement. HIV-1 Tg animals treated with vehicle exhibited a diminished reinforcing efficacy of sucrose relative to control animals treated with vehicle evidenced by a statistically significant genotype × concentration interaction for both the number of reinforcers (Fig. 2B; [F(4, 68) = 3.4, pGG ≤ 0.02, η2p = 0.165] with a prominent linear component [F(1,17) = 6.7, p ≤ 0.02, η2p = 0.282]) and “active” lever presses (Data Not Shown; [F(4, 68) = 2.9, pGG ≤ 0.05, η2p = 0.147] with a prominent linear component [F(1,17) = 6.0, p ≤ 0.03, η2p = 0.261]. Specifically, control animals treated with vehicle displayed a linear increase in the number of reinforcers (R2 = 0.87) and “active” lever presses (R2 = 0.93) as sucrose concentration increased. The number of reinforcers earned and “active” lever presses produced by HIV-1 Tg animals treated with vehicle, however, remained invariant across sucrose concentration, well-described by a horizontal line. HIV-1 Tg animals, therefore, exhibit a diminished reinforcing efficacy of sucrose.
Fixed-ratio responding: dose-response
The sensitivity to sucrose concentration was subsequently examined using an FR-1 schedule of reinforcement. HIV-1 Tg animals treated with vehicle exhibited a blunted sensitivity to sucrose concentration relative to control animals treated with vehicle (Fig. 2C), evidenced by a differential pattern of responding. Regression analyses revealed a dose–response function well-characterized by a one-phase association in control animals treated with vehicle (R2 = 0.95), whereas an exponential growth equation afforded a best-fit for HIV-1 Tg animals treated with vehicle (R2 = 0.98).
Collectively, results support prominent apathetic behaviors towards natural reinforcers in HIV-1 Tg rats; apathetic behaviors that are characterized by a diminished reinforcing efficacy of, and sensitivity to, sucrose.
Drug-seeking behavior: addiction
Response escalation: fixed-ratio responding
Following jugular vein catheterization, HIV-1 Tg and control animals responded for 0.2 mg/kg/inf of cocaine according to a FR-1 schedule of reinforcement for 5 consecutive days. HIV-1 Tg animals treated with vehicle displayed increased response vigor, independent of day, relative to control animals treated with vehicle (Fig. 2D; Main Effect: Genotype, [F(1,17) = 4.5, p ≤ 0.05]). Furthermore, the number of cocaine infusions across day was well-described by a horizontal line for both HIV-1 Tg and control animals treated with vehicle; albeit statistically significant differences in the mean of the function were observed [F(1,93) = 11.1, p ≤ 0.01]). Thus, HIV-1 Tg animals treated with vehicle exhibited increased response vigor for cocaine, supporting enhanced drug-seeking behavior, relative to control rats treated with vehicle.
Response escalation: progressive-ratio responding
Independent of genotype, both HIV-1 Tg and control animals treated with vehicle escalated their drug intake, evidenced by an increase in the number of “active” lever presses (Fig. 2E) and the number of cocaine infusions (Data Not Shown; 0.75 mg/kg/inf), across the 14 consecutive days of PR testing.
With regards to the number of “active” lever presses, HIV-1 Tg animals treated with vehicle escalated responding at a significantly faster rate relative to control animals treated with vehicle (Day × Genotype interaction, [F(1, 245) = 12.0, p ≤ 0.01]). An exponential growth equation afforded the best fit for the number of “active” lever presses for both HIV-1 Tg (R2 = 0.82) and control (R2 = 0.73) animals treated with vehicle; albeit statistically significant differences in the parameters of the function were observed [F(2,24) = 66.5, p ≤ 0.01].
Collectively, results support enhanced drug-seeking behavior in HIV-1 Tg animals, evidenced by an increased response vigor and faster escalation, relative to control animals treated with vehicle; an observation that is independent of schedule of reinforcement (i.e., FR vs. PR).
Progressive-ratio responding: dose-response
HIV-1 Tg animals treated with vehicle exhibited an increased reinforcing efficacy of cocaine dose (mg/kg/inf) relative to control animals treated with vehicle (Fig. 2F). Specifically, in HIV-1 Tg animals treated with vehicle, a linear (R2 = 0.98) decrease in the number of cocaine infusions was observed from the 0.01 to 0.5 mg/kg/inf dose; a dramatic increase in the number of infusions was observed at the 0.75 mg/kg/inf and 1 mg/kg/inf dose. In sharp contrast, in control animals treated with vehicle, the number of cocaine infusions across all doses was well-described by a sigmoidal-dose response curve (variable slope; R2 = 0.99). Collectively, results support enhanced drug-seeking behavior and an increased differential reinforcing efficacy of cocaine in HIV-1 Tg rats treated with vehicle relative to control rats treated with vehicle.
Neuronal and dendritic spine morphology in medium spiny neurons of the nucleus accumbens
Dendritic branching complexity
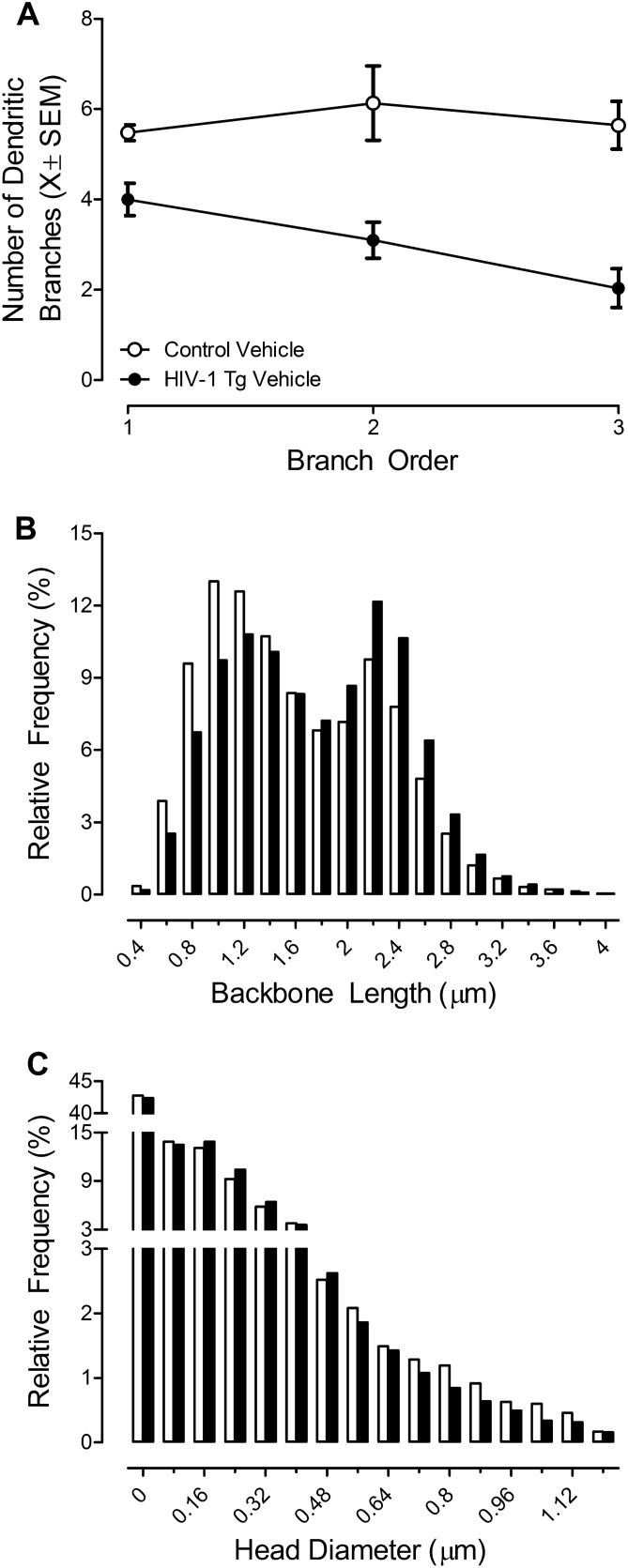
Dendritic branching was evaluated by counting the number of primary, secondary, and tertiary branches in MSNs of the NAc. Following chronic HIV-1 viral protein exposure, lower levels of dendritic branching were observed in HIV-1 Tg animals treated with vehicle relative to control animals treated with vehicle (Fig. 3A; Genotype × Branch Order interaction with a prominent linear component, [F(1,10) = 7.3, p ≤ 0.02, η2p = 0.422]). Thus, presence of the HIV-1 transgene leads to lower levels of dendritic branching in MSNs of the NAc.
Figure 3.
Following a history of cocaine self-administration, the impact of the HIV-1 transgene on dendritic branching (A; ± SEM) and dendritic spine morphology (B–C) in medium spiny neurons of the nucleus accumbens was examined and is illustrated as a function of genotype (HIV-1 Tg Vehicle vs. Control Vehicle). HIV-1 Tg animals exhibited a dramatic decrease in dendritic branching complexity (A) and a population shift towards longer dendritic spines (B) with decreased head diameter (C) relative to control animals.
Dendritic spine morphology
After a history of cocaine self-administration, dendritic spine morphology was examined in MSNs of the NAc. HIV-1 Tg animals treated with vehicle exhibited a prominent population shift towards longer dendritic spines (Fig. 3B; [F(1,1274) = 581.6, p ≤ 0.01]) with decreased head diameter (Fig. 3C; [F(1,1070) = 119.9, p ≤ 0.01]) relative to control animals treated with vehicle. Presence of the HIV-1 transgene, therefore, shifts the morphological parameters of dendritic spines in MSNs of the NAc to a more immature phenotype.
Therapeutic efficacy of S-Equol
Preliminary training
Acquisition of autoshaping
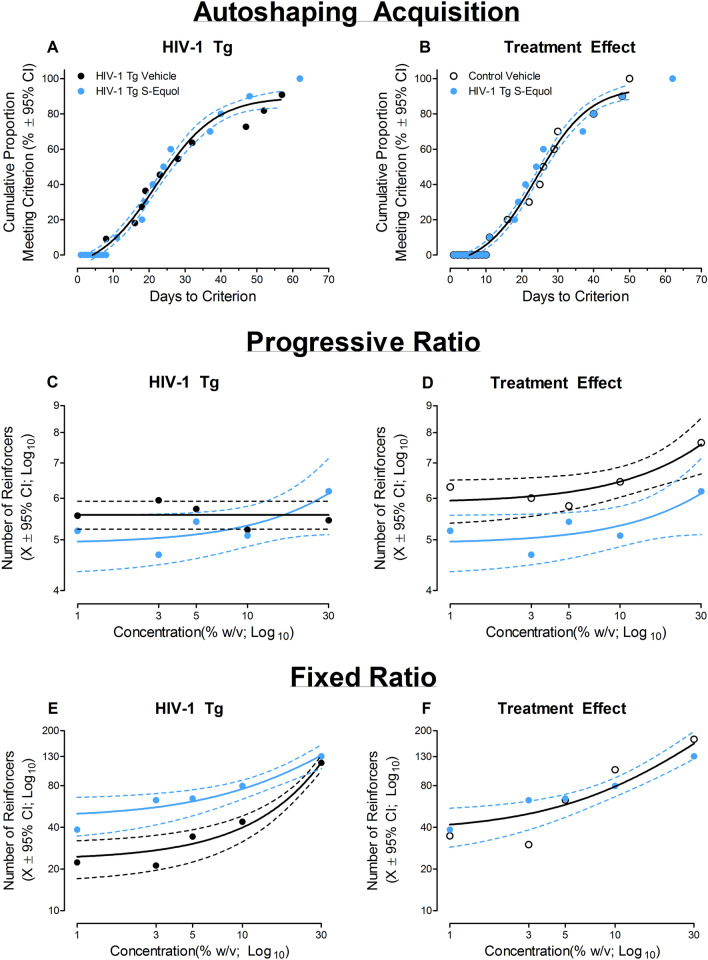
Treatment with SE ameliorated deficits in stimulus-reinforcement learning in HIV-1 Tg animals. All HIV-1 Tg animals treated with SE successfully acquired autoshaping. The best-fit function (i.e., global sigmoidal dose–response curve (variable slope)) for the number of days to criteria for HIV-1 Tg animals treated with SE was statistically indistinguishable from either HIV-1 Tg animals treated with vehicle (Fig. 4A; p > 0.05; R2 = 0.98) or control animals treated with vehicle (Fig. 4B; p > 0.05; R2 = 0.98). Thus, the marked impairment in stimulus-reinforcement learning observed in HIV-1 Tg animals treated with vehicle was mitigated by treatment with SE.
Figure 4.
The therapeutic efficacy of S-Equol (SE) as a novel therapeutic for apathetic behaviors under sucrose-maintained responding in the HIV-1 Tg rat is illustrated as a function of genotype (HIV-1 Tg vs. Control) and treatment (SE vs. Vehicle; ± 95% Confidence Intervals). The number of days required to successfully acquire autoshaping, an index of stimulus-reinforcement learning, for HIV-1 Tg animals treated with SE was statistically indistinguishable from either HIV-1 Tg animals treated with vehicle (A) or control animals treated with vehicle (B). Treatment with SE enhanced the reinforcing efficacy of sucrose (C–D) and ameliorated alterations in the sensitivity to sucrose (E–F) in HIV-1 Tg rats.
Goal-directed behavior: apathy
Progressive-ratio responding: dose response
The reinforcing efficacy of sucrose in HIV-1 Tg animals was enhanced by treatment with SE. First, comparison of HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle revealed a differential pattern of responding. Specifically, HIV-1 Tg animals treated with SE exhibited a linear increase in the number of reinforcers (Fig. 4C; R2 = 0.73) and “active” lever presses (Data Not Shown; R2 = 0.91) as sucrose concentration increased; a concentration-dependent effect not observed in HIV-1 Tg animals treated with vehicle (Best Fit: Horizontal Line).
Second, both HIV-1 Tg animals treated with SE and control animals treated with vehicle exhibited a linear increase in the number of reinforcers (Fig. 4D; R2 = 0.73 and 0.87, respectively) and “active” lever presses (Data Not Shown; R2 = 0.93 and 0.91, respectively) as sucrose concentration increased. Although HIV-1 Tg animals treated with SE exhibited decreased response vigor (i.e., Statistically Significant Difference in β0; Reinforcers: [F(1,6) = 12.4, p ≤ 0.01]; “Active” Lever Presses: [F(1,6) = 10.8, p ≤ 0.02]) relative to control animals treated with vehicle, the rate of increase (i.e., β1) across sucrose concentration was statistically indistinguishable between groups (p > 0.05). Treatment with SE, therefore, mitigates the diminished reinforcing efficacy of sucrose observed in older HIV-1 Tg animals.
Fixed-ratio responding: dose-response
Alterations in the sensitivity to sucrose were ameliorated by treatment with SE in HIV-1 Tg animals. Comparison of HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle (Fig. 4E) revealed differential patterns of responding (i.e., First-Order Polynomial (R2 = 0.95) and Exponential Growth Equation (R2 = 0.98), respectively), whereby HIV-1 Tg animals treated with SE exhibited greater sensitivity to varying sucrose concentrations. Furthermore, the number of sucrose reinforcers earned by HIV-1 Tg animals treated with SE and control animals treated with vehicle (Fig. 4F) was well-described by a global one-phase association (R2 = 0.91) supporting no statistically significant differences between groups in sucrose sensitivity. Taken together, treatment with SE mitigated deficits in stimulus-reinforcement learning and apathetic behaviors in older HIV-1 Tg rats by enhancing the reinforcing efficacy of, and sensitivity to, sucrose.
Drug-seeking behavior: addiction
Response escalation: fixed-ratio responding
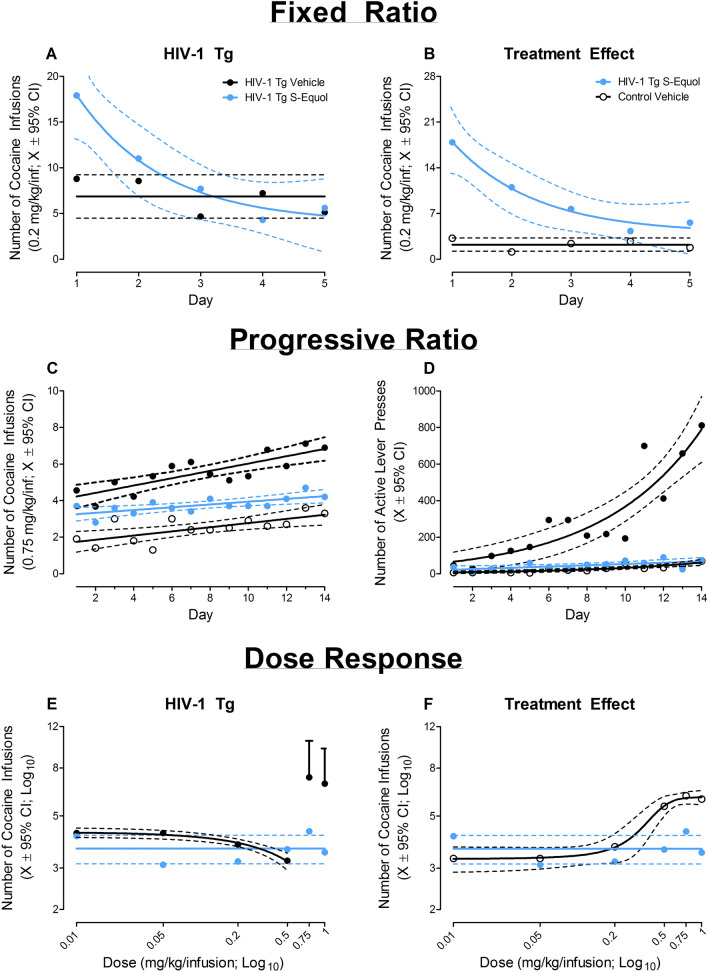
HIV-1 Tg animals treated with SE displayed an initial novelty response to cocaine self-administration followed by a rapid decay, to levels statistically indistinguishable from either HIV-1 Tg or control animals treated with vehicle, in the number of cocaine infusions earned across the five day testing period.
Comparison of HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle revealed a differential pattern of responding (Fig. 5A; Treatment × Day interaction, [F(1,74) = 4.42, p ≤ 0.04]). Specifically, a one-phase decay afforded the best-fit for the number of infusions earned by HIV-1 Tg animals treated with SE (R2 = 0.97), while a horizontal line was most appropriate for HIV-1 Tg animals treated with vehicle. However, the overlapping 95% confidence intervals observed from self-administration days two through five suggest that the differential pattern of responding is driven primarily by an initial novelty response in HIV-1 Tg animals treated with SE on the first day of cocaine self-administration.
Figure 5.
The therapeutic efficacy of S-Equol (SE) as a novel therapeutic for apathetic behaviors under cocaine-maintained responding in the HIV-1 Tg rat is illustrated as a function of genotype (HIV-1 Tg vs. Control) and treatment (SE vs. Vehicle; ± 95% Confidence Intervals). HIV-1 Tg animals treated with SE displayed an initial novelty response to cocaine self-administration followed by a rapid decay. At the end of the five day fixed ratio testing period, the number of cocaine infusions earned by HIV-1 Tg animals treated with SE were statistically indistinguishable from either HIV-1 Tg (A) or control animals (B) treated with vehicle. Treatment with SE reduced drug-seeking behavior in the HIV-1 Tg rat (C–D); an effect which generalized across cocaine dose (E–F).
A differential pattern of responding (i.e., HIV-1 Tg SE: One-Phase Decay, R2 = 0.97; Control Vehicle: Horizontal Line) was also observed when comparing HIV-1 Tg animals treated with SE and control animals treated with vehicle (Fig. 5B; Genotype × Day interaction, [F(1,78) = 12.4, p ≤ 0.01]). Again, however, the overlapping 95% confidence intervals observed at self-administration days four and five suggests that the number of cocaine infusions earned by HIV-1 Tg animals treated with SE at the end of FR-1 responding is statistically indistinguishable from control animals treated with vehicle.
Response escalation: progressive-ratio responding
Independent of genotype and/or treatment, an escalation of cocaine intake, evidenced by an increase in the number of cocaine infusions (Fig. 5C) and number of “active” lever presses (Fig. 5D), was observed across the 14 consecutive days of PR testing.
Examination of the number of cocaine infusions revealed a linear increase in the number of cocaine infusions for all three groups (Control Vehicle: R2 = 0.48, HIV-1 Tg Vehicle: R2 = 0.69, HIV-1 Tg SE: R2 = 0.49). Treatment with SE, however, mitigated response vigor and drug escalation relative to HIV-1 Tg animals treated with vehicle. Specifically, comparison of HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle revealed statistically significant differences in the intercept (i.e., β0; [F(1, 24) = 5.0, p ≤ 0.04]) and in the rate of escalation (i.e., β1; [F(1,24) = 7.8, p ≤ 0.01]) between groups. Albeit, response vigor in HIV-1 Tg animals treated with SE was still significantly greater than control animals treated with vehicle (β0: [F(1,24) = 30.0, p ≤ 0.01]; no statistically significant differences in the rate of escalation (i.e., β1) were observed (p > 0.05).
With regards to the number of “active” lever presses, HIV-1 Tg animals treated with SE escalated responding significantly slower than HIV-1 Tg animals treated with vehicle (Day × Treatment interaction, [F(1,245) = 12.2, p ≤ 0.01]). Specifically, HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle exhibited differential patterns of escalation, evidenced by different best-fit functions (i.e., First-Order Polynomial, R2 = 0.39 and Exponential Growth Equation, R2 = 0.82, respectively). Critically, the escalation of responding in HIV-1 Tg animals treated with SE was statistically indistinguishable from control animals treated with vehicle (Day × Treatment interaction, p > 0.05). Thus, treatment with SE reduced drug-seeking behavior in HIV-1 Tg animals.
Progressive-ratio responding: dose-response
Variations in the dose (mg/kg/inf) of cocaine revealed an alteration in the reinforcing efficacy of cocaine in HIV-1 Tg animals treated with SE. Comparison of HIV-1 Tg animals treated with SE and HIV-1 Tg animals treated with vehicle revealed a differential pattern of cocaine self-administration dependent upon dose (Fig. 5E)). Specifically, HIV-1 Tg animals treated with vehicle exhibited prominent dose-dependent changes in responding (i.e., a linear (R2 = 0.98) decrease in the number of cocaine infusions from the 0.01 to 0.5 mg/kg/inf dose followed by a dramatic increase in the number of cocaine infusions at the 0.75 and 1 mg/kg/inf dose); a dose-dependent effect not observed in HIV-1 Tg animals treated with SE (Best Fit: Horizontal Line). A differential pattern of responding (i.e., HIV-1 Tg SE: Horizontal Line; Control Vehicle: Sigmoidal Dose Response (Variable Slope); R2 = 0.99) was also observed when comparing HIV-1 Tg animals treated with SE and control animals treated with vehicle (Fig. 5F). Treatment with SE, therefore, precludes the increase in responding at higher cocaine doses supporting a diminished reinforcing efficacy of cocaine in HIV-1 Tg animals. Furthermore, it is notable that utilization of a dose–response experimental paradigm also supports the generalization of reduced drug-seeking behavior in HIV-1 Tg animals across dose.
Neuronal and dendritic spine morphology in medium spiny neurons of the nucleus accumbens
Branch order
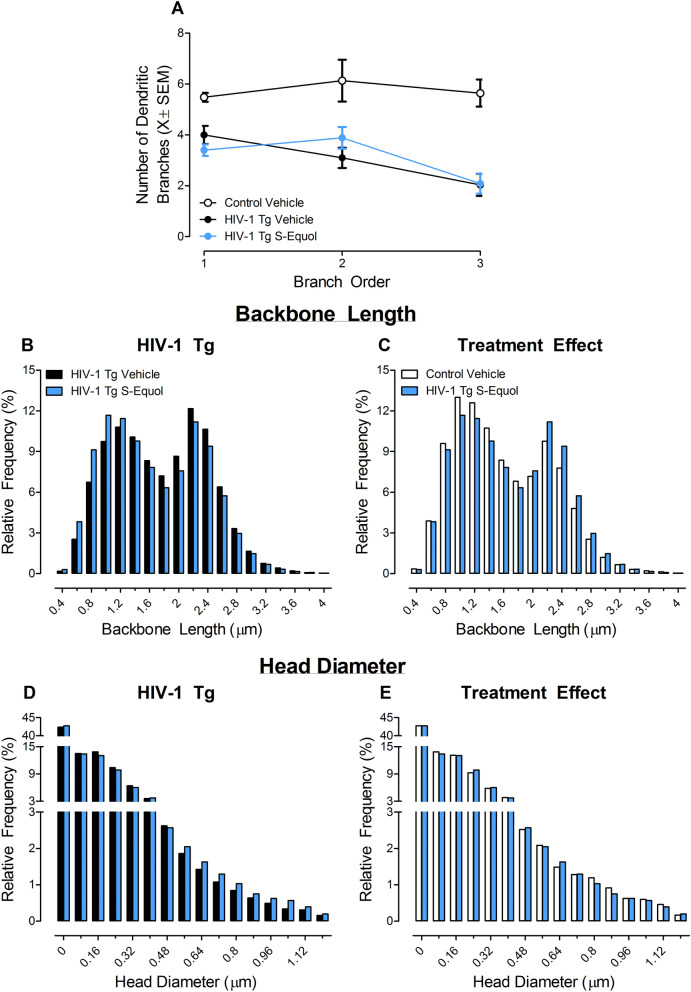
Treatment with SE failed to increase the level of dendritic branching complexity in HIV-1 Tg animals in MSNs of the NAc following a history of cocaine self-administration (Fig. 6A). Specifically, dendritic branching complexity in HIV-1 Tg animals treated with SE was indistinguishable from HIV-1 Tg animals treated with vehicle (p > 0.05) and remained significantly lower relative to control animals treated with vehicle (Genotype × Branch Order interaction with a prominent linear component, [F(1,15) = 5.3, p ≤ 0.04, η2p = 0.259]).
Figure 6.
The utility of S-Equol (SE) to modify neuronal (A; ± SEM) and dendritic spine morphology (B–E) in medium spiny neurons of the nucleus accumbens is illustrated as a function of genotype (HIV-1 Tg vs. Control) and treatment (SE vs. Vehicle; ± 95% Confidence Intervals). Although treatment with SE failed to alter dendritic branching (A), long-term modifications in dendritic spine morphology were observed in HIV-1 Tg animals, evidenced by a prominent population shift towards shorter dendritic spines (B) with increased head diameter (D) relative to HIV-1 Tg animals treated with vehicle. Treatment with SE shifted the morphological parameters of dendritic spines in MSNs of the NAc to a more mature phenotype relative to HIV-1 Tg animals treated with vehicle, evidenced by a prominent population shift towards shorter dendritic spines (Fig. 6B; [F(1,1538) = 79.6, p ≤ 0.01]) with increased head diameter (Fig. 6D; [F(1,1292) = 25.4, p ≤ 0.01]). Relative to control animals treated with vehicle, HIV-1 Tg animals treated with SE exhibited significantly longer dendritic spines (C), no statistically significant differences in head diameter (E) between the two groups were observed.
Dendritic spine morphology
Morphological parameters, including backbone length and head diameter, of dendritic spines in medium spiny neurons (MSNs) of the nucleus accumbens (NAc) were enhanced in HIV-1 Tg animals treated with SE. Treatment with SE shifted the morphological parameters of dendritic spines in MSNs of the NAc to a more mature phenotype relative to HIV-1 Tg animals treated with vehicle, evidenced by a prominent population shift towards shorter dendritic spines (Fig. 6B; [F(1,1388) = 216.2, p ≤ 0.01]) with increased head diameter (Fig. 6D; [F(1,1166) = 112.7, p ≤ 0.01]). Furthermore, although HIV-1 Tg animals treated with SE exhibited significantly longer dendritic spines (Fig. 6C; [F(1,1498) = 155.5, p ≤ 0.01]) relative to control animals treated with vehicle, no statistically significant differences in head diameter between the two groups were observed (Fig. 6E; p > 0.05). Thus, treatment with SE induced long-term modifications in dendritic spine morphology resulting in a more mature morphological phenotype.
Discussion
HIV-1 Tg rats treated with vehicle exhibited alterations in goal-directed and drug-seeking behavior relative to their control counterparts, supporting prominent motivational alterations; alterations that were mitigated by treatment with SE. At the genotypic level, apathetic behavior in HIV-1 Tg rats treated with vehicle was characterized by a diminished reinforcing efficacy of, and sensitivity to, sucrose. Motivational alterations in the HIV-1 Tg rat were further evidenced by enhanced drug-seeking for cocaine, supporting an addictive phenotype. Treatment with SE, however, ameliorated alterations in goal-directed and drug-seeking behaviors in HIV-1 Tg rats. The therapeutic benefits of SE may be due, at least in part, to the partial restoration of synaptic function, evidenced by a population shift towards a more mature dendritic spine phenotype in HIV-1 Tg animals treated with SE. Taken together, SE restored motivated behavior in HIV-1 Tg rats, expanding the potential clinical utility of SE to include both neurocognitive and affective alterations.
The assessment of goal-directed61 and drug-seeking62 behavior, as in the present study, relies upon Pavlovian conditioning and operant, or instrumental, conditioning. Pavlovian conditioning utilizes repeated associations of two stimuli to activate behavior. With reference to operant conditioning, the utilization of either “positive” (i.e., the addition of a stimulus following a response) or “negative” (i.e., the removal of a stimulus following a response) reinforcement increases the likelihood that the response will occur again63. Once conditioning processes were learned (e.g., via autoshaping acquisition), an aspect of reward expectation (e.g., unit-dose of the reward; schedule of reinforcement: FR vs. PR) was manipulated to elucidate changes in the motivated behavior resulting from either HIV-1 viral proteins and/or SE treatment.
Constitutive expression of HIV-1 viral proteins induced apathetic and addictive behaviors affording strong evidence for motivational alterations. Apathetic behaviors for a natural reward were evidenced by a diminished reinforcing efficacy of, and sensitivity to, sucrose. Notably, the behavioral presentation of apathy in the present study is distinct from the decreased response vigor previously reported in young (i.e., 2 months of age) HIV-1 Tg rats46. Given the positive correlation between apathy and age in HIV-1 seropositive individuals64, differences in the behavioral presentation of apathy may be due, at least in part, to age; albeit there were also notable differences in experimental design. Addictive behaviors in HIV-1 Tg rats treated with vehicle were characterized by an enhanced response vigor for cocaine. The enhanced response vigor for cocaine observed in the present study is again distinct from observations by Wayman et al. (65; i.e., no statistically significant difference in responding) or Bertrand et al. (46); i.e., reduced response vigor); inconsistencies which may reflect differences in age, the experimental protocol, and/or cocaine dose. However, the observed increased sensitivity to cocaine in older HIV-1 Tg rats is consistent with previous observations in relatively young (i.e., 3–4 months of age) HIV-1 Tg rats66. Therefore, the differences in sensitivity to cocaine between the Bertrand et al.46 manuscript and the present study more likely results from differences in cocaine dose. Collectively, independent of differences in the response to either sucrose or cocaine self-administration across studies, the HIV-1 Tg rat exhibits prominent motivational alterations supporting an advantageous biological system for the evaluation of therapeutics for apathy resulting from chronic HIV-1 viral protein exposure.
Motivational alterations resulting from chronic HIV-1 viral protein exposure were mitigated by treatment with SE; results which extend the therapeutic utility of SE. The therapeutic efficacy of SE, a selective ERβ agonist, for neurocognitive impairments associated with HAND has been critically tested across multiple ages (i.e., treatment beginning at PD 28, 2–3 months of age, and 6–8 months of age), neurocognitive domains (e.g., temporal processing, stimulus-reinforcement learning, sustained attention), and the factor of biological sex32–34. Results of the present study extend the therapeutic utility of SE to include the mitigation of apathetic behaviors, as evidenced by the enhanced reinforcing efficacy of, and sensitivity to, sucrose observed in HIV-1 Tg rats treated with SE. Furthermore, treatment with SE dramatically reduced drug-seeking behavior in the HIV-1 Tg rat; a finding that deserves further consideration given the well-recognized relationship between estrogens and drug abuse67.
Women are uniquely vulnerable to cocaine addiction, evidenced by a faster acquisition of cocaine self-administration68,69, greater willingness-to-work for cocaine (i.e., breakpoint;70) and a greater sensitivity to cocaine71,72; preclinical observations which are consistent with the clinical picture of addiction73. The enhanced behavioral responses to cocaine result, at least in part, from the activation of ERβ in the NAc74. Notably, when control animals are treated with SE, an agonist selective for ERβ26, an increased reinforcing efficacy of, and altered sensitivity to, cocaine is observed (Supplementary Fig. 1A–C). The structure and function of the NAc, however, is altered by chronic HIV-1 viral protein exposure [e.g.,11,22,75,76], which may preclude this untoward side effect (i.e., enhanced drug-seeking behavior) in HIV-1 Tg rats treated with SE.
Examination of dendritic spine morphology in MSNs of the NAc following a history of cocaine self-administration affords additional evidence for structural alterations in the NAc in the HIV-1 Tg rat. Dendritic spines, tiny specialized protrusions that emerge from dendritic shafts, are the primary postsynaptic target for excitatory synaptic transmission77. Morphologically, dendritic spines are classically divided into four primary categories, including thin, stubby, mushroom and filopodia78; albeit the classification system has significant limitations given that, in reality, spine morphology occurs along a continuum20. Strong correlations between dendritic spine morphology (e.g., head volume, neck length) and area of the post-synaptic density (PSD)16–18, presynaptic vesicles16,17, as well as synaptic strength19 support a tight coupling between morphological parameters and function. Assessments of dendritic spine morphology, therefore, may provide critical information on synaptic function20.
Following a history of cocaine self-administration, HIV-1 Tg animals treated with vehicle exhibited a population shift towards longer dendritic spines with decreased dendritic spine head diameter relative to control animals treated with vehicle; a population shift consistent with a ‘filopodia’-like morphology. Unstable, immature filopodia often lack a clear dendritic spine head and asymmetric synapse implying that these spines also lack synaptic contacts79. However, treatment with SE led to long-term modifications in dendritic spines in MSNs of the NAc in HIV-1 Tg animals. Specifically, HIV-1 Tg animals treated with SE exhibited a prominent population shift towards a more mature phenotype (i.e., ‘thin’) relative to HIV-1 Tg animals treated with vehicle. Flexible thin spines have a small synapse, but have structural flexibility to accommodate changes in inputs80. Given that motivation is mediated in part by the NAc13,81, the shift in dendritic spine morphology affords a potential mechanism by which SE exerts its therapeutic effects. Critically, the beneficial effects of SE on the morphological parameters of dendritic spines in the HIV-1 Tg rat may reflect the utility of SE to more broadly remodel neuronal circuitry.
Indeed, the ovarian steroid hormone 17β-estradiol acts on multiple brain regions (i.e., PFC, NAc) associated with the fronto-striatal circuit. In the PFC, 17β-estradiol increases dendritic spine density [e.g.,82,83] via the ERβ pathway83 and induces morphological alterations in dendritic spines82. Within the NAc, however, 17β-estradiol significantly reduces dendritic spine density in the NAc core and increases the prevalence of immature spine types (i.e., stubby, filopodia;29,30). In a consistent manner, in the present study, control animals treated with SE exhibited a population shift towards a less mature dendritic spine phenotype relative to control animals treated with vehicle (Supplementary Fig. 1D–F). To more comprehensively evaluate if and/or how SE remodels neuronal circuitry in the HIV-1 Tg rat, studies in the absence of cocaine and across additional brain regions involved in the fronto-striatal circuit are needed.
Assessments in the present study were limited to female OVX animals, a notable caveat that affords key opportunities for future research. First, prominent sex differences in cocaine addiction73 and HAND84 are well-recognized. To date, how biological sex influences HIV-1 associated motivational alterations remains understudied supporting the need for additional research. Second, there are notable sex differences in the expression levels of ER-β in the brain85. However, no statistically significant differences in the expression levels of ER-β have been observed in brain regions associated with the fronto-striatal circuit [PFC:85; Striatum:86]. Finally, there remains a critical need to evaluate the therapeutic efficacy of SE for motivational alterations in male and intact female HIV-1 Tg animals. Although the phytoestrogen SE exhibits strong affinity for ERβ26,27, it also binds 5α-dihydrotestosterone87 supporting its potential efficacy in both males and females. Indeed, with regards to HAND, SE serves as an efficacious neuroprotective therapeutic in both male and intact female animals33.
An additional caveat that merits further discussion is the utilization of sucrose as an exemplar of a natural reinforcer. The sweet taste test, which utilizes the random presentation of multiple sucrose concentrations88,89 or sweet stimuli90 to evaluate hedonic responses and sensitivity to sucrose, is commonly employed to assess anhedonia in clinical populations. Although anhedonia is a prominent diagnostic criterion of multiple neuropsychiatric disorders [e.g., depression91], hedonic responses for sucrose in these populations are not statistically different from those observed in healthy controls88–90. Therefore, sucrose may not be the most translationally relevant reinforcer for the evaluation of anhedonia. The assessment of goal-directed behavior in the present study, however, requires a motivational component (i.e., lever-pressing for reward) that is not necessary for the sweet taste test. Nevertheless, there remains a critical need to more comprehensively evaluate apathy following chronic HIV-1 viral protein exposure via novel reinforcers (e.g., voluntary wheel running).
In conclusion, following chronic HIV-1 viral protein exposure, prominent motivational deficits and dysregulation, as well as a population shift towards an immature dendritic spine phenotype were observed. In HIV-1 Tg animals, treatment with SE mitigated motivational alterations. Furthermore, morphological parameters of dendritic spines in MSNs of the NAc in HIV-1 Tg animals treated with SE were shifted towards a more mature phenotype, supporting a potential underlying basis for the therapeutic effects of SE for motivational alterations. Taken together, SE restored motivated behavior in the HIV-1 Tg rat, expanding the potential clinical utility of SE to include both neurocognitive and affective alterations.
Supplementary Information
Acknowledgements
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development HD043680; National Institute of Mental Health, MH106392; National Institute of Neurological Disorders and Stroke, NS100624) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Author contributions
R.M.B. and C.F.M. designed the study. S.J.B., C.F.M. and S.B.H. planned the study and S.J.B. and J.M.I. collected the behavioral and neuroanatomical data. K.A.M., S.J.B., J.M.I., and C.F.M. analyzed the data. S.J.B., J.M.I., S.B.H., R.M.B. and C.F.M., wrote the early drafts of the manuscript, and K.A.M., C.F.M., and R.M.B. updated and revised the final draft of the manuscript. All authors critically appraised and approved the final version of the manuscript.
Data availability
All relevant data are within the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91240-0.
References
- 1.Wise RA, McDevitt RA. Drive and reinforcement circuitry in the brain: Origins, neurotransmitters, and projection fields. Neuropsychopharmacology. 2018;43:680–689. doi: 10.1038/npp.2017.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein J, Silbersweig D. The neuropsychiatric spectrum of motivational disorders. J. Neuropsychiatry Clin. Neurosci. 2015;27:7–18. doi: 10.1176/appi.neuropsych.13120370. [DOI] [PubMed] [Google Scholar]
- 3.Illenberger JM, et al. HIV infection and neurocognitive disorders in the context of chronic drug abuse: Evidence for divergent findings dependent upon prior drug history. J. Neuroimmune Pharmacol. 2020;15:715–728. doi: 10.1007/s11481-020-09928-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamat R, et al. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch. Clin. Neuropsychol. 2012;27:520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamat R, Woods SP, Cameron MV, Iudicello JE, HIV Neurobehavioral Research Program (HNRP) Group Apathy is associated with lower mental and physical quality of life in persons infected with HIV. Psychol. Health Med. 2016;21:890–901. doi: 10.1080/13548506.2015.1131998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker KA, Brown GG. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: A systematic review and meta-analysis. J. Clin. Exp. Neuropsychol. 2018;40:357–376. doi: 10.1080/13803395.2017.1349879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jallow A, Ljunggren G, Wӓndell P, Wahlström L, Carlsson AC. HIV-infection and psychiatric illnesses-A double edged sword that threatens the vision of a contained epidemic: The Greater Stockholm HIV Cohort Study. J. Infect. 2017;74:22–28. doi: 10.1016/j.jinf.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Kamat R, et al. Implications of apathy and depression for everyday functioning in HIV/AIDS in Brazil. J. Affect. Disord. 2013;150:1069–1075. doi: 10.1016/j.jad.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. J. Behav. Med. 2011;34:128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DJ, et al. HIV-infected individuals with co-occurring bipolar disorder evidence poor antiretroviral and psychiatric medication adherence. AIDS Behav. 2012;16:2257–2266. doi: 10.1007/s10461-011-0072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babicz MA, Woods SP, Fazeli P, Morgan EE. Apathy is associated with critical psychological determinants of medication adherence in HIV disease. J. Clin. Psychol. Med. Settings. 2020 doi: 10.1007/s10880-020-09715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millar BM, Starks TJ, Gurung S, Parsons JT. The impact of comorbidities, depression, and substance use problems on quality of life among older adults living with HIV. AIDS Behav. 2017;21:1684–1690. doi: 10.1007/s10461-016-1613-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsutsui-Kimura I, et al. Dysfunction of ventrolateral striatal dopamine receptor type 2-expressing medium spiny neurons impairs instrumental motivation. Nat. Commun. 2017;8:14304. doi: 10.1038/ncomms14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp JM, Powell TP. The structure of the caudate nucleus of the cat: Light and electron microscopy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1971;262:383–401. doi: 10.1098/rstb.1971.0102. [DOI] [PubMed] [Google Scholar]
- 15.Cheng HW, et al. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentations: A Golgi study. Exp. Neurol. 1997;147:287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- 16.Harris KM, Stevens JK. Dendritic spines of rat cerebellar purkinje cells: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 1988;8:4455–4469. doi: 10.1523/JNEUROSCI.08-12-04455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 1989;9:2982–2997. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arellano JI, Benavides-Piccione R, DeFelipe J, Yuste R. Ultrastructure of dendritic spines: Correlation between synaptic and spine morphologies. Front. Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araya R, Vogels TP, Yuste R. Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proc. Natl. Acad. Sci. U.S.A. 2014;111:E2895–E2904. doi: 10.1073/pnas.1321869111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuste R. Dendritic Spines. MIT Press; 2010. [Google Scholar]
- 21.Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J. Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaurin KA, et al. Synaptic connectivity in medium spiny neurons of the nucleus accumbens: A sex-dependent mechanism underlying apathy in the HIV-1 transgenic rat. Front. Behav. Neurosci. 2018;12:285. doi: 10.3389/fnbeh.2018.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schier CJ, et al. Selective vulnerability of striatal D2 versus D1 dopamine receptor-expressing medium spiny neurons in HIV-1 tat transgenic male mice. J. Neurosci. 2017;37:5758–5769. doi: 10.1523/JNEUROSCI.0622-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Arch. Intern. Med. 2001;161:1161–1172. doi: 10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- 25.Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: Possible roles in hormone-dependent disease. Am. J. Clin. Nutr. 1984;40:569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- 26.Setchell KD, et al. S-Equol, a potent ligand for estrogen receptor β, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am. J. Clin. Nutr. 2005;81:1072–1079. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand SJ, Hu C, Aksenova MV, Mactutus CF, Booze RM. HIV-1 Tat and cocaine mediated synaptopathy in cortical and midbrain neurons is prevented by the isoflavone Equol. Front. Microbiol. 2015;6:894. doi: 10.3389/fmicb.2015.00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(SICI)1096-9861(19971201)388:4<507::AID-CNE1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct. Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct. Funct. 2015;220:2415–2422. doi: 10.1007/s00429-014-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertrand SJ, Mactutus CF, Aksenova MV, Espensen-Sturges TD, Booze RM. Synaptodendritic recovery following HIV Tat exposure: Neurorestoration by phytoestrogens. J. Neurochem. 2014;128:140–151. doi: 10.1111/jnc.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran LM, McLaurin KA, Booze RM, Mactutus CF. Neurorestoration of sustained attention in a model of HIV-1 associated neurocognitive disorders. Front. Behav. Neurosci. 2019;13:169. doi: 10.3389/fnbeh.2019.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLaurin KA, Li H, Cook AK, Booze RM, Mactutus CF. S-Equol: A neuroprotective therapeutic for chronic neurocognitive impairments in pediatric HIV. J. Neurovirol. 2020;26:704–718. doi: 10.1007/s13365-020-00886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLaurin KA, Moran LM, Booze RM, Mactutus CF. Selective estrogen receptor β agonists: A therapeutic approach for HIV-1 associated neurocognitive disorders. J. Neuroimmune Pharmacol. 2020;15:264–279. doi: 10.1007/s11481-019-09900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogdan A, Manera V, Koenig A, David R. Pharmacologic approaches for the management of apathy in neurodegenerative disorders. Front. Pharmacol. 2020;10:1581. doi: 10.3389/fphar.2019.01581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volkow ND. Personalizing the treatment of substance use disorders. Am. J. Psychiatry. 2020;177:113–116. doi: 10.1176/appi.ajp.2019.19121284. [DOI] [PubMed] [Google Scholar]
- 37.Reid W, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng J, et al. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J. Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Abbondanzo SJ, Chang SL. HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS ONE. 2014;9:e105256. doi: 10.1371/journal.pone.0105256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toggas SM, et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 41.Dickens AM, et al. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci. Rep. 2017;7:7748. doi: 10.1038/s41598-017-07570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLaurin KA, Booze RM, Mactutus CF. Evolution of the HIV-1 transgenic rat: Utility in assessing the progression of HIV-1 associated neurocognitive disorders. J. Neurovirol. 2018;24:229–245. doi: 10.1007/s13365-017-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta VD. Stability of cocaine hydrochloride solutions at various pH values as determined by high-pressure liquid chromatography. Int. J. Pharm. 1982;10:249–257. doi: 10.1016/0378-5173(82)90075-8. [DOI] [Google Scholar]
- 44.Akaza H. Prostate cancer chemoprevention by soy isoflavones: Role of intestinal bacteria as the “second human genome”. Cancer Sci. 2012;103:969–975. doi: 10.1111/j.1349-7006.2012.02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacy RT, Hord LL, Morgan AJ, Harrod SB. Intravenous gestational nicotine exposure results in increased motivation for sucrose reward in adult rat offspring. Drug Alcohol Depend. 2012;124:299–306. doi: 10.1016/j.drugalcdep.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertrand SJ, Mactutus CF, Harrod SB, Moran LM, Booze RM. HIV-1 proteins dysregulate motivational processes and dopamine circuitry. Sci. Rep. 2018;8:7869. doi: 10.1038/s41598-018-25109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb. Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 48.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 49.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 50.Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav. Pharmacol. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan D, Liu Y, Roberts DCS. Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology. 2006;31:121–128. doi: 10.1038/sj.npp.1300773. [DOI] [PubMed] [Google Scholar]
- 52.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 53.Seabold GK, Daunais JB, Rau A, Grant KA, Alvarez VA. DiOLISTIC labeling of neurons from rodent and non-human primate brain slices. J. Vis. Exp. 2010;41:2081. doi: 10.3791/2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. Elsevier Academic Press; 2014. [Google Scholar]
- 55.Ruszczycki B, et al. Sampling issues in quantitative analysis of dendritic spines morphology. BMC Bioinf. 2012;13:213. doi: 10.1186/1471-2105-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Konur S, Rabinowitz D, Fenstermaker VL, Yuste R. Systematic regulation of spine sizes and densities in pyramidal neurons. J. Neurobiol. 2003;56:95–112. doi: 10.1002/neu.10229. [DOI] [PubMed] [Google Scholar]
- 57.Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat. Rev. Neurosci. 2001;2:880–888. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 58.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. doi: 10.1007/BF02289823. [DOI] [Google Scholar]
- 59.Denenberg VH. Some statistical and experimental considerations in the use of the analysis-of-variance procedure. Am. J. Physiol. Reg. 1984;I(246):R403–R408. doi: 10.1152/ajpregu.1984.246.4.R403. [DOI] [PubMed] [Google Scholar]
- 60.Wears RL. Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data. Acad. Emerg. Med. 2002;9:330–341. doi: 10.1197/aemj.9.4.330. [DOI] [PubMed] [Google Scholar]
- 61.Rescorla R. A Pavlovian analysis of goal-directed behavior. Am. Psychol. 1987;42:119–129. doi: 10.1037/0003-066X.42.2.119. [DOI] [Google Scholar]
- 62.Lynch JJ, Fertziger AP, Teitelbaum HA, Cullen JW, Gantt WH. Pavlovian conditioning of drug reactions: Some implications for problems of drug addiction. Cond. Reflex. 1973;8:211–223. doi: 10.1007/BF03000677. [DOI] [PubMed] [Google Scholar]
- 63.Skinner B. The Behavior of Organisms: An Experimental Analysis. Appleton-Century; 1938. [Google Scholar]
- 64.Bryant VE, et al. Depression and apathy among people living with HIV: Implications for treatment of HIV associated neurocognitive disorders. AIDS Behav. 2015;19:1430–1437. doi: 10.1007/s10461-014-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wayman WN, Chen L, Hu XT, Napier TC. HIV-1 transgenic rat prefrontal cortex hyper-excitability is enhanced by cocaine self-administration. Neuropsychopharmacology. 2016;41:1965–1973. doi: 10.1038/npp.2015.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntosh S, Sexton T, Pattison LP, Childers SR, Hemby SE. Increased sensitivity to cocaine self-administration in HIV-1 transgenic rats is associated with changes in striatal dopamine transporter binding. J. Neuroimmune Pharmacol. 2015;10:493–505. doi: 10.1007/s11481-015-9594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol. Sci. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 68.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 69.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 70.Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 71.Russo SJ, et al. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/S0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- 72.Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol. Biochem. Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becker JB. Sex differences in addiction. Dialogues Clin. Neurosci. 2016;18:395–402. doi: 10.31887/DCNS.2016.18.4/jbecker. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Satta R, Certa B, He D, Lasek AW. Estrogen receptor β in the nucleus accumbens regulates the rewarding properties of cocaine in female mice. Int. J. Neuropsychopharmacol. 2018;21:382–392. doi: 10.1093/ijnp/pyx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuhn T, et al. The effects of HIV and aging on subcortical shape alterations: A 3D morphometric study. Hum. Brain Mapp. 2017;38:1025–1037. doi: 10.1002/hbm.23436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Denton AR, et al. Selective monoaminergic and histaminergic circuit dysregulation following long-term HIV-1 protein exposure. J. Neurovirol. 2019;25:540–550. doi: 10.1007/s13365-019-00754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uchizono K. Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature. 1965;207:642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- 78.Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am. J. Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- 79.Arellano JI, Espinosa A, Fairén A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Holtmaat AJGD, et al. Transient and persistent dendritic spines in the neocortex in vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Brown CA, et al. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Exp. Neurol. 2012;236:190–197. doi: 10.1016/j.expneurol.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hao J, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang S, Zhu J, Xu T. 17β-estradiol (E2) promotes growth and stability of new dendritic spines via estrogen receptor β pathway in intact mouse cortex. Brain Res. Bull. 2018;137:241–248. doi: 10.1016/j.brainresbull.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 84.Maki PM, et al. Differences in cognitive function between women and men with HIV. J. Acquir. Immune Defic. Syndr. 2018;79:101–107. doi: 10.1097/QAI.0000000000001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/S0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 86.Küppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci. Lett. 1999;276:95–98. doi: 10.1016/S0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- 87.Lund TD, et al. Equol is a novel anti-androgen that inhibitos prostate growth and hormone feedback. Biol. Reprod. 2004;70:1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 88.Berline I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur. Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 89.Dichter GS, Smoski M, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the sweet taste test. Depress Anxiety. 2010;27:859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCabe C, Cowen PJ, Harmer CJ. Neural representation of reward in recovered depressed patients. Psychopharmacology. 2009;205:667–677. doi: 10.1007/s00213-009-1573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th ed.) 10.1176/appi.books.9780890425596 (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper.