Abstract
Objective:
Due to concerns of infection and medication disruptions during the COVID-19 pandemic, rheumatology patients at the pandemic epicenter were at risk of distress and poor health outcomes. We sought to investigate medication disruptions and COVID-related distress shortly after the peak of the pandemic in the Bronx, NY, and determine whether factors related to the pandemic were associated with flares, disease activity, and overall health.
Methods:
We surveyed adult patients and parents of pediatric patients from rheumatology clinics in the Bronx, in the month following the epidemic peak regarding medication access, medication interruptions, COVID-19 infection, COVID-19 hospitalization, and COVID-related distress. We examined which factors associated with patient-reported flares, disease activity, and overall health scores in regression models accounting for socio-demographics and rheumatologic disease type.
Results:
Of 1,692 patients and parents contacted, 361 (21%) responded; 16% reported medication access difficulty, 14% medication interruptions, and 41% flare. In a multivariable logistic regression model, medication access difficulty was associated with increased odds of flare (OR 4.0, 95% CI 1.5–10.4, p=0.005), as was high COVID-related distress (OR 2.4, 95% CI 1.2–4.6, p=0.01). In multivariable linear regression models, medication access difficulty and high distress were associated with worse disease activity scores and high distress was associated with worse health scores.
Conclusion:
Medication access difficulties and flares were common among rheumatology patients from the Bronx, NY in the month following the epidemic peak. Medication access difficulty and COVID-related distress were highly associated with flare and disease activity. COVID-related distress was associated with overall health scores.
INTRODUCTION
As of October 2020, approximately 8 million cases of Coronavirus disease (COVID-19) and over 218,000 related deaths have been reported in the United States (1). The first major peak in the pandemic in the US occurred in New York City (NYC) in mid-April 2020 (2, 3). Early in the pandemic, the Centers for Disease Control and Prevention widely publicized guidance that immunocompromised individuals were at higher risk for COVID-19 (4). In addition to the stress of being high risk for infection, people with rheumatologic conditions have been subjected to the stress of medication shortages while medications used to treat rheumatologic diseases, such as hydroxychloroquine, were being used for COVID-19 (5, 6). A recent national survey showed that the COVID-19 pandemic led to disruption in medication therapy and care, as well as increased anxiety among rheumatology patients (7). Some individuals may be especially vulnerable to the challenges brought upon by the pandemic, including those with low-income and from racial and ethnic minorities (8).
The Bronx, which has the highest density of cases per capita in NYC, has been the most affected borough, with greater than 45,000 cases by June 2020 (9). Demographically, the Bronx is more than 90% Black or Hispanic/Latinx (10), populations disproportionately affected by COVID-19 in NYC (11). The Bronx includes the poorest congressional district in the US and has consistently scored lowest on every health indicator among all 62 counties in New York (12).
Data are lacking on those most vulnerable to the pandemic’s impact on resources, stress, and health.
To this end, we surveyed rheumatology patients seen at Montefiore Medical Center (MMC) in the Bronx in the month following the COVID-19 epidemic peak in NYC. Our goals were (a) to identify challenges related to medication disruptions during the pandemic and (b) to determine whether factors related to the pandemic were associated with flares, disease activity, and overall health, specifically, medication access difficulty, medication interruptions, COVID infection, and COVID-related distress.
METHODS
Population.
The population surveyed was rheumatology patients treated at MMC rheumatology clinics. MMC is the largest medical center within the Bronx, providing care to over 2 million people and is associated with Albert Einstein College of Medicine. Potential participants were identified through Clinical Looking Glass (CLG), a web-based application developed at MMC to enable clinician researchers to extract information from the electronic medical record (EMR) (13). Participants were identified based on (a) the presence of two ICD-10 billing diagnosis codes related to rheumatologic diseases (Appendix 1) and (b) two visits within 6 months of each other to an adult or pediatric rheumatology clinic at MMC between 3/1/2018–3/1/2020 (beginning 2 years prior to the onset of the COVID-19 epidemic in NY), and (c) a prescription for an immunomodulator (Appendix 2) within 180 days from the most recent rheumatology visit. This search strategy was based upon validated algorithms to identify Rheumatoid Arthritis (RA) patients from administrative data (14). Patients were additionally identified from the Einstein Lupus Cohort Registry, a large cohort of over 500 patients with systemic lupus erythematosus (SLE), as well as registries of dermatomyositis and polymyositis patients seen at MMC clinics. To be eligible, patients had to be ≥18 years of age to respond for themselves; parents of patients <18 years of age were eligible for the study.
Recruitment.
Potential participants were contacted by email or phone if no email was included in their EMR. Phone recruitment was done by 8 members of the research staff, including 3 native Spanish speakers who called patients with Spanish language preference in their EMR. A script guided recruitment; those interested in participating could either provide an email address for a web link to the consent and survey or could consent and take the survey over the phone. Data was collected and managed using REDCap (Research Electronic Data Capture)(15, 16). The study was approved by the Institutional Review Board of Albert Einstein College of Medicine (IRB 2020–11330) and all participants consented to participate.
The survey was reviewed by a patient and parent from the Rheumatology Patient Advisory Group for content and language. Surveys were conducted between 5/8/2020 and 6/1/2020 in English and Spanish. Surveys took 10–15 minutes to complete online and 20–30 minutes by phone. Surveys included mental health and physical health questionnaires not included in this baseline analysis.
Study variables
Demographics and clinical factors.
Race/ethnicity was determined from patient-reported categorization in the EMR. Socio-economic status (SES) was characterized by an SES index, a census-derived combined z-score reflecting the deviation of a patient’s neighborhood SES from the mean of the New York State population (17). For the analyses, rheumatologic disease type was classified as SLE, RA, or other.
The following patient-reported data were assessed over the prior 30 days:
COVID-19 symptoms, diagnoses, and care.
Participants were asked if they experienced any symptoms not related to their rheumatologic disease (fever, cough, sore throat, diarrhea, muscle aches, headache, fatigue, difficulty smelling/tasting, redness/swelling in toes and/or fingers or any new rash). We asked if participants were tested for COVID-19, the results of the test (positive, negative, unknown), emergency room or urgent care visits for COVID-19, hospitalizations, and in those who reported hospitalization, whether they required ventilatory support.
Prescription medication access and/or interruptions.
If participants indicated access difficulties and/or experienced interruptions, they were asked to select from a list of reasons (see survey for details, Appendix 3). If participants indicated medication shortages, or interruptions in their medication therapy, they were asked to select which medication(s) from a list including hydroxychloroquine, chloroquine, IL-6 inhibitors, IL-1 inhibitors, JAK inhibitors or other. These medications were queried because, at the time of survey development, they were being used or considered for the treatment of COVID-19. We also included medications suggested in public discussions to be harmful in COVID (steroids and NSAIDs).
Medication disruption was defined as either medication access difficulty or medication interruption.
COVID-19 related distress.
Adult participants were asked to rate their distress related to COVID-19 on a scale of 0–10. Parent participants were asked to rate the distress of their children from 1–10. Child distress questions were part of a questionnaire used in the COVID-19 Exposure and Family Impact Survey (CEFIS) (Center for Pediatric Traumatic Stress, 2020). To standardize results on COVID-related distress, we used a dichotomized variable for a patient’s (child or adult) with reported distress based on whether they had scores in the top quartile for either child or adult patient groups. A free text question to collect qualitative data on the impact of the pandemic asked: “Is there another way that the coronavirus pandemic has affected your ability to care for your (or your child’s) rheumatologic or autoimmune condition?” All responses were tabulated and read in their original language (Spanish or English) by DM and TR and examined for repeating themes and illustrative quotations for each theme were selected.
Outcomes.
Respondents were asked whether they had a flare in their rheumatologic condition and the intensity (mild, moderate, severe). We assessed disease activity and overall health with a 0–10 scale (0 least active/most well to 10 most active/least well).
Statistical Analysis.
Statistical analysis was performed using Stata 14 (StataCorp, College Station, Texas). To assess for participation bias in our study sample, we examined differences in socio-demographics and patient characteristics between patients who did and did not respond to the survey. We used Pearson chi-squared tests for categorical variables and Mann-Whitney U tests to compare continuous non-parametric variables. Multivariable linear regression models were built to determine which COVID-related exposures independently associated with scores for disease activity and for overall health. Multivariable logistic regression models were used to determine which COVID-related exposures associated with flare. Multivariable models included respondents with complete data. Socio-demographic covariates (age, sex, race/ethnicity and SES) and disease category (SLE, RA/JIA, or other) were included in all multivariable models. COVID-related exposure covariates were included if they met a p≤0.2 threshold in univariable regression models. Variables were tested for collinearity with the Spearman rank test. We performed a subgroup analysis of pediatric patients. Due to the small sample size, we did not conduct regression analyses of the pediatric sub-group.
There were 15%, 16%, and 23% missing values on scales of overall health, disease activity and distress scores respectively, among respondents who completed those questionnaires. Because the REDCap visual analog scale by default had the marker set at 5, we performed a sensitivity analysis, where we assigned 5 for these missing values.
RESULTS
Survey response and participant demographics.
Of 1,692 identified patients, 1,129 (67%) were sent an email invitation, and 563 (33%) were contacted exclusively by phone. We received responses from 361 study participants of whom 245 (68%) responded by email link. Response rates were higher among women (23%) vs. men (15%), p = 0.001. Response rates were the highest among Hispanics patients (26%) and the lowest among Black non-Hispanic patients (16%). Responses were higher in Spanish versus English speakers (31% vs. 21%, p=0.001), and patients with SLE (25%) versus RA/JIA (19%) or another disease (17%), p = 0.002. Age, socioeconomic status, and the proportion of pediatric parents versus adult patients were similar in the respondent and non-respondent groups.
Demographics and clinical characteristics of participants are shown in Table 1. Most (n=307, 85%) were adult patients and 53 (15%) were parents responding for pediatric patients. The majority of respondents were female (317; 88%); 175 (49%) were Hispanic, 93 (26%) were non-Hispanic Black, and 33 (9%) were other races; 230 (64%) of participants had SLE. Over 90% of the survey respondents were from the Bronx, NY (Figure 1).
Table 1.
Demographic and clinical characteristics of survey participants
| Montefiore Medical Center Rheumatology survey participants (n= 361) | |
| Respondent | |
| Patient (≥18 years old) | 307 (85%) |
| Parent (for patients <18 years old) | 54 (15%) |
| Sex | |
| Female | 317 (88%) |
| Male | 44 (12%) |
| Age | |
| Median, IQR | 42 (23, 58) |
| Race/ ethnicity | |
| Black, non-Hispanic | 93 (26%) |
| Hispanic | 175 (49%) |
| White | 22 (6%) |
| Other | 33 (9%) |
| Unknown/ Declined | 38 (11%) |
| Language | |
| English | 297 (82%) |
| Spanish | 64 (18%) |
| Primary rheumatic disease | |
| Systemic lupus erythematosus | 230 (64%) |
| Rheumatoid arthritis/ JIA | 70 (19%) |
| Dermatomyositis/polymyositis | 35 (10%) |
| Sarcoidosis | 5 (1%) |
| Other | 16 (4%) |
| Rheumatic disease activity | |
| Flared in the past month* | 147 (41%) |
| Mild | 51 (14%) |
| Moderate | 61 (17%) |
| Severe | 34 (10%) |
| Disease activity VAS score 0–10 (median, IQR)** | 3.5 (0.5, 6.5) |
| Overall health VAS score 0–10 (median, IQR)*** | 3.5 (0.6, 6.2) |
| COVID-19 reported symptom | |
| ≥ 1 COVID symptom | 115 (32%) |
| ≥3 COVID symptoms | 43 (5%) |
| COVID-19 related care | |
| Total reported tested | 70 (19%) |
| Positive test | 21 (6%) |
| COVID-19 urgent care/ ER visits | 14 (4%) |
| COVID-19 hospitalizations | 7 (2%) |
| COVID-19 required mechanical ventilator | 2 (0.6%) |
4 missing
87 missing
90 missing
Figure 1. Geographic distribution of Rheumatology COVID-19 Survey respondents in NYC vs. Geographic distribution of confirmed positive COVID-19 case rates in NYC.
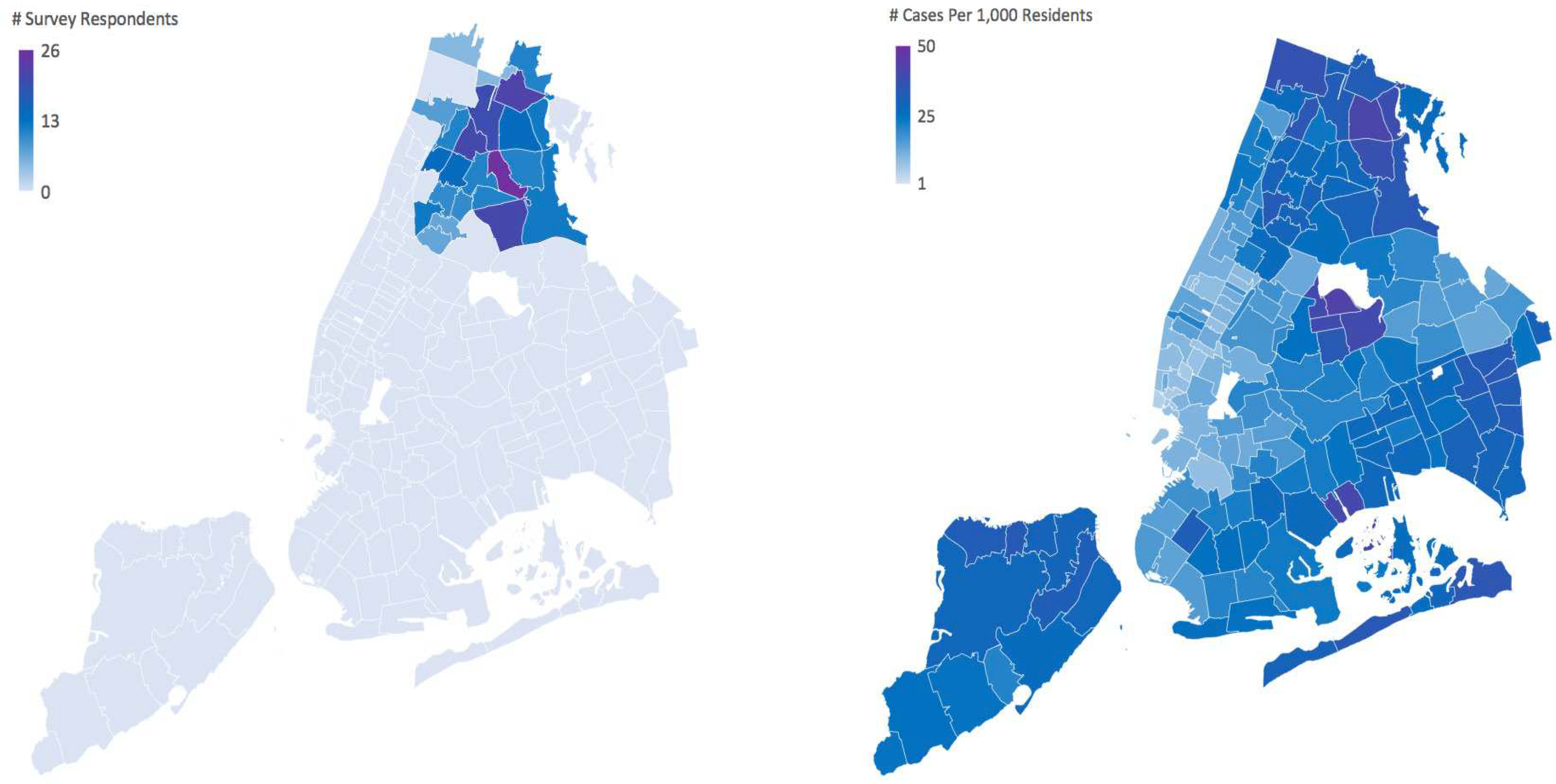
The figure to the left shows the geographic distribution of survey respondents, of surveys collected between May 8th 2020 to June 1st 2020 and areas of highest density of responses. To protect the privacy of survey respondents, all zip codes with less than 5 responses were not shaded in the figure. The figure to the right shows the geographic distribution of confirmed positive COVID-19 case rates in NYC as of May 30th, 2020.
COVID-19 symptoms, testing, and care.
Of the 361 respondents, 23 (6%) tested positive for COVID-19 (2 pediatric): 14 were seen in an emergency room or urgent care center for COVID-19 (1 pediatric), 7 were hospitalized (none were pediatric), and 2 required ventilatory support.
Of the 115 respondents who reported >1 symptom of COVID-19 that they thought were unrelated to their rheumatologic condition, 37 were tested (16 positive). Among the 43 patients who reported ≥3 symptoms, 16 were tested (10 positive). The most common symptoms were fatigue and muscle aches, both found in 15% of respondents. Of the 246 who did not report any symptoms, 33 were tested (5 positive, 4 unknown).
Medication access difficulty was reported in 56 (16%) of respondents. Among those reporting difficulties, 27 (48%) had difficulty due to shortages, 15 (27%) had difficulty reaching a prescriber, 9 (16%) had difficulty physically getting to a pharmacy, 6 (11%) had difficulty paying for medication, 5 (9%) had loss of insurance, and 10 (18%) reported “other” reason. In free-texts describing other reasons 3 mentioned difficulties obtaining the medication from pharmacies, 2 because of long lines, and 1 because a prior authorization was needed.
Medication interruptions were reported in 50 respondents (14%). Among those, 13 (26%) reported interruptions <1 week, 11 (22%) from 1–2 weeks, 8 (16%) 2–4 weeks, and 17 (34%) ≥4 weeks. The 50 respondents who reported interruptions, selected the following reasons: 18 (36%) medication shortages, 10 (20%) difficulty reaching a prescriber, 9 (18%) difficulty physically getting to a pharmacy, 5 (10%) feeling unsafe about the medication, 5 (10%) loss of insurance, 3 (6%) not making medications a priority, 3 (6%) difficulty paying for the medication, 3 (10%) unable to obtain infusion.
Seventy-eight (22%) respondents reported a medication disruption, among which 56 had access difficulty, 50 had a medication interruption, and 28 had both. Medication shortage was the most frequently reported reason for medication disruption, affecting 30 (8%) of respondents. The most frequent medication disrupted was hydroxychloroquine; 23/26 respondents who reported hydroxychloroquine disruptions had SLE. Medication interruptions were associated with medication access difficulty; 59% of respondents reporting medications interruptions experienced medication access difficulty, compared to 9% of those who did not have medication interruptions (p< 0.001, chi-square test). Neither overall medication disruptions, difficulty obtaining medication, nor interruptions in medications were associated with race/ethnicity, or SES.
COVID-related distress.
The median level of COVID-related distress for adult participants was 6 (IQR 3–8), 0–10 scale. For child distress reported by parents the median range was 5 (IQR 3–7), 1–10 scale. Based on top quartiles, adult high COVID distress scores were defined as >8, and child as >7. High COVID distress was not associated with either access difficulty or medication interruptions.
Factors associated with disease flare.
In univariable analyses, flare was associated with female sex, high COVID distress, medication access difficulty, and medication interruption. In the multivariable analysis, flare was associated with female sex, lower SES, high COVID distress, and medication access difficulty (Table 2). The strongest association was seen with medication difficulty: people with access difficulty were 4 times as likely to report flares compared to those without difficulty.
Table 2.
Demographic, clinical, and COVID-related factors associated with flare*
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age | 1.0 | 1.0–1.0 | 0.3 | 1.0 | 1.0–1.0 | 0.1 |
| Sex (male vs female) | 0.23 | 0.10–0.54 | 0.001 | 0.15 | 0.042–0.55 | 0.004 |
| Race/ethnicity | ||||||
| Hispanic vs Black | 1.6 | 0.95–2.7 | 0.08 | 1.4 | 0.68–2.8 | 0.3 |
| White vs Black | 1.1 | 0.41–2.9 | 0.9 | 2.2 | 0.59–8.2 | 0.2 |
| Other vs Black | 1.4 | 0.62–3.2 | 0.4 | 1.6 | 0.57–4.5 | 0.4 |
| Unknown vs Black | 1.2 | 0.55–2.7 | 0.6 | 0.78 | 0.26–2.4 | 0.7 |
| SES | 1.0 | 0.9–1.0 | 0.3 | 0.89 | 0.80–1.0 | 0.05 |
| Rheumatologic disease | ||||||
| RA vs SLE | 0.79 | 0.45–1.4 | 0.4 | 0.89 | 0.39–2.0 | 0.8 |
| Other vs SLE | 1.1 | 0.62–2.0 | 0.6 | 1.4 | 0.59–3.1 | 0.5 |
| COVID-positive | 2.2 | 0.89–5.6 | 0.1 | -- | -- | -- |
| COVID-hospitalized | 3.7 | 0.70–19 | 0.1 | 2.3 | 0.21–27 | 0.5 |
| High COVID distress | 2.4 | 1.4–4.2 | 0.002 | 2.4 | 1.2–4.6 | 0.01 |
| Medication access difficulty | 4.2 | 2.2–7.9 | <0.001 | 4.0 | 1.5–10.4 | 0.005 |
| Medication interruption | 2.3 | 1.3–4.3 | 0.007 | 1.1 | 0.39–2.9 | 0.9 |
Among n=357 with complete flare data.
High COVID distress was defined as scoring in the upper quartile of the COVID-related distress question. Medication access difficulty and medication interruption were defined as difficulty obtaining a prescribed medication and an interruption in prescribed medication therapy, respectively.
In a subgroup analysis of pediatric respondents, flares were associated with medication access difficulty. All 3 of the respondents who reported access difficulty for their children reported flares versus 22% in those who did not report access difficulty (p=0.02). Flares significantly correlated to COVID-related distress scores in children (rho=0.3, p=0.02).
Factors associated with disease activity scores.
In univariable analyses, worse disease activity was associated with increasing age, high COVID distress, medication access difficulty, and medication interruption (Table 3). In the multivariable model, disease activity was associated with increasing age, female sex, having RA/JIA vs. SLE, high COVID distress, and medication access difficulty. The strongest association was seen with medication access difficulty, which was associated with an increase of 1.5 units on a scale from 0–10 of worsening disease activity scores.
Table 3.
Demographic, clinical, and COVID-related factors associated with disease activity scores*
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p value | β | 95% CI | p value | |
| Age | 0.027 | 0.0097–0.045 | 0.003 | 0.023 | 0.0029–0.043 | 0.03 |
| Sex | −0.65 | −1.8–0.50 | 0.3 | −1.6 | −3.0– −0.25 | 0.02 |
| Race/ethnicity | ||||||
| Hispanic vs Black | 0.86 | −0.030–1.8 | 0.06 | 0.48 | −0.50–1.5 | 0.3 |
| White vs Black | −0.72 | −2.4–0.99 | 0.4 | −0.53 | −2.4–1.4 | 0.6 |
| Other vs Black | 0.71 | −0.67–2.1 | 0.3 | 0.28 | −1.2–1.8 | 0.7 |
| Unknown vs Black | −0.24 | −1.5–1.0 | 0.7 | −0.84 | −2.3–0.63 | 0.3 |
| SES | −0.11 | −0.25–0.036 | 0.1 | −0.075 | −0.23–0.083 | 0.4 |
| Rheumatologic disease | ||||||
| RA vs SLE | 0.55 | −0.42–1.5 | 0.3 | 1.2 | 0.13–2.4 | 0.03 |
| Other vs SLE | −0.065 | −1.1–0.93 | 0.9 | 0.56 | −0.61–1.7 | 0.4 |
| COVID-positive | 0.93 | −0.64–2.5 | 0.2 | -- | -- | -- |
| COVID-hospitalized | 2.1 | −0.69–4.8 | 0.1 | 1.2 | −1.7–4.2 | 0.4 |
| High COVID distress | 1.6 | 0.68–2.5 | 0.001 | 1.2 | 0.24–2.1 | 0.01 |
| Medication access difficulty | 2.3 | 1.3–3.3 | <0.001 | 1.5 | 0.31–2.8 | 0.02 |
| Medication interruption | 1.6 | 0.53–2.7 | 0.004 | 0.85 | −0.47–2.2 | 0.2 |
N=271 with complete disease activity scores.
High COVID distress was defined as scoring in the upper quartile of the COVID-related distress question. Medication access difficulty and medication interruption were defined as difficulty obtaining a prescribed medication and an interruption in prescribed medication therapy, respectively. Disease activity scores were rated by participants on a scale of 0–10 where 0 was no activity and 10 was the most activity.
In the pediatric subgroup, disease activity was not significantly associated with difficulties in medication access, interruptions in medications, or COVID-related distress.
Factors associated with overall health scores.
In univariable analyses, worse health scores were associated with increasing age, Hispanic versus non-Hispanic Black race/ethnicity, lower SES, COVID positive status, having been hospitalized for COVID, high COVID distress, and medication difficulty (Table 4). In the multivariable model, worse health scores were associated with increasing age and high COVID distress. High COVID distress was associated with an increase of 1.8 units on the scale from 0–10 of worsening health scores.
Table 4.
Demographic, clinical, and COVID-related factors associated with overall health scores*
| Univariable model | Multivariable model | |||||
|---|---|---|---|---|---|---|
| β | 95% CI | p value | β | 95% CI | p value | |
| Age | 0.026 | 0.010–0.042 | 0.001 | 0.024 | 0.006–0.042 | 0.008 |
| Sex (male vs female) | −0.26 | −1.3–0.75 | 0.6 | −0.007 | −1.2–1.2 | 1.0 |
| Race/ethnicity | ||||||
| Hispanic vs Black | 1.0 | 0.25–1.8 | 0.01 | 0.60 | −0.29–1.5 | 0.2 |
| White vs Black | −0.63 | −2.2–0.92 | 0.42 | −0.36 | −2.1–1.4 | 0.7 |
| Other vs Black | 0.71 | −0.60–2.01 | 0.29 | 1.2 | −0.20–2.6 | 0.09 |
| Unknown vs Black | −0.26 | −1.4–0.85 | 0.64 | −0.61 | −1.9–0.71 | 0.4 |
| SES | −0.14 | −0.27–0.009 | 0.04 | −0.092 | −0.24–0.050 | 0.2 |
| Rheumatologic disease | ||||||
| RA vs SLE | −0.26 | −1.1–0.60 | 0.55 | −0.050 | −1.07–0.97 | 0.9 |
| Other vs SLE | −0.020 | −0.93–0.89 | 0.97 | 0.28 | −0.79–1.3 | 0.6 |
| COVID-positive | 2.1 | 0.66–3.5 | 0.004 | 1.3 | −0.32–2.9 | 0.1 |
| COVID-hospitalized | 3.1 | 0.63–5.6 | 0.01 | -- | -- | -- |
| High COVID distress | 1.8 | 1.0–2.6 | <0.001 | 1.8 | 0.98–2.7 | <0.001 |
| Medication access difficulty | 1.2 | 0.22–2.2 | 0.02 | 0.45 | −0.67–1.6 | 0.4 |
| Medication interruption | 0.85 | −0.15–1.8 | 0.1 | 0.44 | −0.76–1.6 | 0.5 |
N=275 with complete data on health scores.
High COVID distress was defined as scoring in the upper quartile of the COVID-related distress question. Medication access difficulty and medication interruption were defined as difficulty obtaining a prescribed medication and an interruption in prescribed medication therapy, respectively. Overall health scores were rated by participants on a scale of 0–10 (0 = very well, 10 = very poorly).
In the pediatric subgroup, worse health scores were significantly associated with COVID-related distress scores (rho 0.5, p=0.001).
Sensitivity analyses with 5 assigned to missing values on visual analog scales for overall health, disease activity and COVID-related distress in submitted surveys did not yield significantly different results, except in the case of imputed disease activity and distress scores. In multivariable models of disease activity, high COVID-related distress was no longer a significant predictor (β 0.6, 95%CI −0.2–1.3, p = 0.1).
Qualitative responses on the impact of COVID-19 on rheumatologic disease care.
Themes in the qualitative responses included those of stress and anxiety over the pandemic, avoiding medical care for fear of exposure to COVID-19, and relative inactivity due to avoidance of leaving the home. Illustrative quotations are presented in Table 5.
Table 5.
Illustrative free-text answers on the impact of the COVID-19 pandemic on rheumatologic care from survey participants
| Stress & Anxiety | “Stress and worry about the pandemic has cause in my opinion my flare ups...” |
| “I been getting really anxious...” | |
| “I find myself getting panic attacks if I have to go outside.” | |
| “Psicológico, el miedo a salir y saber que soy débil del sistema immunológico” [Psychological, fear of going out and knowing I have a weak immune system] | |
| Avoiding medical care for fear of exposure | “Scared of visiting my doctor when I had a flare” |
| “I HAVE BEEN FORCED TO STAY HOME AND CRY MYSELF TO SLEEP FROM MORNING TO NIGHT FROM THE PAINS DUE TO SEVERAL SEVERE FLARES, INSTEAD OF GOING TO THE ER” | |
| “I am less likely to go to the doctor right now out of fear of catching COVID19” | |
| “I have very important appointments... I am petrified of going.” | |
| Avoiding outdoors/being inactive | “Limited ability to go out for light exercise and fresh air due to concerns about exposure.” |
| “I am more inactive daily” | |
| “El no poder salir y caminar para fortalecer mis huesos, porque en la casa no es lo mismo y respirar aire, no a sido fácil, te da depresión y ansiedad” [Not being able to go out and walk to strengthen my bones, because at the house it’s not the same, breathing air, it has not been easy, it gives you depression and anxiety] |
Free-text answers to the question “Is there another way that the coronavirus pandemic has affected your ability to care for your (your child’s) rheumatologic or autoimmune condition?”
DISCUSSION
In this survey of rheumatology patients in the month following the peak of the COVID-19 pandemic from the Bronx, NY, we identified common challenges that our rheumatology patients faced. As residents of the hardest hit borough our patients were truly at the epicenter of the pandemic in the spring of 2020. We found that approximately 1 in 5 respondents experienced medication disruptions and respondents reported high levels of COVID-related distress.
The peak of the COVID-19 pandemic in New York was associated with state shut downs termed “NY on PAUSE”, that began in March 2020 ordered by Governor Andrew Cuomo, followed by additional city restrictions put into place by Mayor Bill de Blasio which affected transportation and led to business closures (18). Pharmacies were considered essential and allowed to remain open, but the responses from our study indicate that a proportion of patients had difficulties obtaining medications related to physical access to pharmacies, access to their medications from pharmacies, and long lines at pharmacies.
The most common barrier related to medication disruptions was medication shortages, and this was by far most frequently reported with hydroxychloroquine. During the peak of COVID-19 hospitalizations in March, hydroxychloroquine was being used widely across all major NYC hospitals to treat COVID-19. Supplies of hydroxychloroquine were diverted to hospitals from community pharmacies and restrictions were placed on rheumatology patients’ access to the medication including requirements of confirmation of diagnosis and prior use, and even prior authorizations.
Our findings agree with a recent national survey of rheumatology patients during the earlier weeks of the pandemic, which also found challenges to medication access and care: 10% of patients were unable to obtain medications and 4% of patients were not able to contact their rheumatologist (7). Respondents of this national study were more likely to have higher education and to be white, and less likely to be from the Northeast US, which during the time of this study, had been the hardest hit region from the COVID-19 pandemic. Thus, our study provides insight into a population with demographics not well-represented previously, being largely low-income, minority race/ethnicity, and from a highly affected area – a population that is likely more vulnerable to the impact of the pandemic. Indeed, the proportion of respondents affected by difficulties with medication access appears somewhat higher in our study than that reported by Michaud et al. (16% versus 10%). However, this may have been driven by the higher proportion of SLE patients in our study, considering that the most frequently affected medication that we found was hydroxychloroquine – used in the majority of SLE patients.
We found that medication access difficulty and COVID-related distress were highly associated with flares and disease activity scores. In particular, we found that in multivariable models accounting for other sociodemographic factors and disease type, medication access difficulty was associated with a 4-fold increase in odds and high COVID-related distress was associated with a 2 times increase in odds of reporting flare. COVID-related distress was also highly associated with overall health scores. The direction of the associations between COVID-related distress or medication access difficulty and health outcomes is not revealed in this study. While distress and challenges with medication access may have helped precipitate flares, it is possible that the association between medication access difficulty and flare was driven by patients with flares being more likely to need or seek out medications and therefore more likely to experience medication access difficulties. Likewise, patients with flares may be more vulnerable to experiencing stress and thus be more likely to report high levels of COVID-related distress. Finally, these associations may not reflect causal links in either direction, but may be associated with other markers of mental and physical health. Longitudinal studies in specific disease populations, examining detailed mental and physical health data, are needed to understand these relationships more completely.
Our study found that 6% of rheumatology patients who completed the survey had a COVID-19 positive test and 2% were hospitalized. During a similar time range in the Bronx, the reported case rate is 3% and the hospitalization rate 0.8% (19). Collaborative global studies are currently underway to better define the COVID-19 disease burden in the rheumatology population (20).
Despite the fact that few pediatric patients were found to be COVID-positive or hospitalized for COVID, a relationship between COVID-related distress was seen with flares and with overall health scores in the sub-group analysis of parents responding on behalf of pediatric patients. This may have represented parents’ distress and further studies are planned to investigate the relationship between COVID-related parental distress, child distress, and disease outcomes.
Qualitative results from the survey illustrated that patients were anxious about pursuing care and avoided care for their rheumatologic conditions because of fears of infection. These themes were like those found by Michaud et al. in their survey (11). These findings, along with the high levels of COVID-related distress which we found in Bronx rheumatology patients underscores the importance of further investigation into the impact of the pandemic on the mental health of rheumatology patients and how this may impact health behaviors and future disease outcomes.
In addition to medication access difficulty, challenges contacting prescribers was identified as a problem for some patients and connected to medication interruptions. During the peak of the pandemic, many rheumatologists were deployed to care for COVID-19 inpatients either as hospitalists or as consultants to help manage cytokine storm and multisystem inflammatory syndrome associated with COVID-19 in children. Outreach programs to rheumatology patients and increased access with telehealth with allied health professionals may help if another wave of escalating COVID-19 cases occurs and rheumatologists are again deployed to inpatient care. Of concern, are patients who will be unreachable due to lack or loss of phone or internet service. Deploying mobile health teams to particularly low income and hard-hit areas may help provide access to care.
Important limitations to our study were the low response rate, the bias in respondent sample toward relatively higher SES and non-Black patients. Our survey strategy utilized both phone and email, in an effort to reach a population that has been underrepresented in other studies to date. However, several patients were still unreachable and phone numbers were noted to be disconnected. Our data may underrepresent those who were the most affected by the financial repercussions of the COVID-19 pandemic.
Notably this study only examined patient reported outcomes. This was intentional because of the recognized decrease in non-COVID-related patient encounters during the surge of the pandemic that made measuring flare and disease activity through other traditional means unreliable. Though these outcomes were obtained using questions from validated measures that correspond to physician-derived disease activity measures commonly used in rheumatology (21, 22), future studies during the pandemic investigating serologic markers of disease activity, healthcare utilization, and other important health outcomes are needed.
Higher rates of missing responses than expected were seen in distress, disease activity, and health scales. This may be due to the presentation of the visual analog scale through REDCap. Survey directions did not specify that respondents needed to click the marker to record a response in agreement with the default “5.” We recognize that granularity was lost in the crude grouping of diseases because of small numbers (SLE, RA/JIA, and other) and future studies assessing the impact in specific disease populations are warranted. Finally, in the complex relationship between distress and health outcomes there are likely additional confounders, including the presence of psychiatric disease and other factors regarding psychosocial health, that were not accounted for in our analyses.
In conclusion, we documented a link between both medication access difficulty and COVID-related distress with disease control in rheumatology patients. Medication access to vulnerable patients during the pandemic should be an advocacy priority in the rheumatology community. Future longitudinal studies are needed to understand the long-term impact of challenges related to the COVID-19 pandemic on Bronx rheumatology patients and will aid us in developing strategies to mitigate the adverse effects of the pandemic. Examining the long-term effects of psychological distress related to COVID-19 on disease outcomes will help us better understand the role that psychological stress may play in rheumatologic diseases, in general.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
We characterized medication access difficulty, medication interruptions, and flares among minority and low-income rheumatology patients from the epicenter of the COVID-19 pandemic in the month following the epidemic peak; 22% of patients experienced either medication access difficulty or medication interruptions and 41% experienced flares.
Medication access difficulty and COVID-related distress were highly associated with patient-reported flares and disease activity and COVID-related distress was highly associated with worse patient-reported health scores.
The findings of this survey underscore the importance of advocating for and providing medication access resources to rheumatology patients.
Future longitudinal studies of rheumatology patients during the pandemic should investigate the impact of the pandemic on physical and mental health.
ACKNOWLEDGEMENTS
We would like to thank Dr. Anand Khumthekar for his contribution to design, Vindhya Rao and Dr. Alisha Akinsete for their contributions to data collection, and Pancy Brown and Casha Parker for their assistance in reviewing survey language. We would like to thank all the participants who made this research possible.
The authors have no financial disclosures. Einstein-Montefiore REDCap is supported through the Clinical and Translational Science Award (CTSA) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) to the Muriel Block Institute for Clinical and Translational Research at Einstein and Montefiore (UL1TR002556).
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
REFERENCES
- 1.Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) Cases in the United States. 2020. [cited 2020 5/24/2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 2.Centers for Disease Control and Prevention. CDC COVID Data Tracker. 2020. [cited 2020 10/15/2020]; Available from: https://covid.cdc.gov/covid-data-tracker/#cases_casesinlast7days
- 3.Prevention CfDCa. CDC Covid Data Tracker. 2020. [cited 10/15/2020]; Available from: https://covid.cdc.gov/covid-data-tracker/#cases_casesinlast7days
- 4.Centers for Disease Control and Prevention. People who are at Higher Risk. 2020. [cited 6/7/2020]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/immunocompromised.html
- 5.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis 2020;20(4):400–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M. Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet. 2020;395(10224):e35–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud K, Wipfler K, Shaw Y, Simon TA, Cornish A, England BR, et al. Experiences of Patients With Rheumatic Diseases in the United States During Early Days of the COVID-19 Pandemic. ACR Open Rheumatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman CH, Ramsey-Goldman R. Widening Disparities Among Patients with Rheumatic Diseases in the COVID-19 Era: An Urgent Call to Action. Arthritis Rheumatol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NYSDOH. Persons Tested Positive by County. 2020. [cited 2020 5/24/2020]; Available from: https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Map?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n
- 10.U.S. Census Bureau. 2018 American Community Survey 1-year Estimates. 2018. [Google Scholar]
- 11.NYCDOH. COVID-19 Data Summary. Case, Hospitalization and Death Rates 2020. [cited 6/7/2020]; Available from: https://www1.nyc.gov/site/doh/covid/covid-19-data.page
- 12.University of Wisconsin Population Health Institute. County Health Rankings State Report 2020. 2020. [cited 5/26/2020]; Available from: www.countyhealthrankings.org
- 13.Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N. Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med 2010;85(8):1362–8. [DOI] [PubMed] [Google Scholar]
- 14.Chung CP, Rohan P, Krishnaswami S, McPheeters ML. A systematic review of validated methods for identifying patients with rheumatoid arthritis using administrative or claims data. Vaccine. 2013;31 Suppl 10:K41–61. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 18.NYSDOH. New York State on PAUSE. 2020. [cited 6/7/2020]; Available from: https://coronavirus.health.ny.gov/new-york-state-pause
- 19.NYCDOH. COVID-19 Data By Borough. 2020. [cited 2020 06/07/2020]; Available from: https://www1.nyc.gov/site/doh/covid/covid-19-data-boroughs.page
- 20.Wallace ZS, Bhana S, Hausmann JS, Robinson PC, Sufka P, Sirotich E, et al. The Rheumatology Community responds to the COVID-19 pandemic: the establishment of the COVID-19 global rheumatology alliance. Rheumatology (Oxford). 2020;59(6):1204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Askanase AD, Castrejon I, Pincus T. Quantitative data for care of patients with systemic lupus erythematosus in usual clinical settings: a patient Multidimensional Health Assessment Questionnaire and physician estimate of noninflammatory symptoms. J Rheumatol 2011;38(7):1309–16. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Yazici Y, Bergman MJ. RAPID3, an index to assess and monitor patients with rheumatoid arthritis, without formal joint counts: similar results to DAS28 and CDAI in clinical trials and clinical care. Rheum Dis Clin North Am 2009;35(4):773–8, viii. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


