Abstract
This review will summarize clinical, genetic and pathophysiologic characteristics that are shared between children with enthesitis related arthritis (ERA) with axial involvement and adults with non-radiographic, and in some cases radiographic, axial spondyloarthritis (SpA); and between children with ERA and primarily peripheral disease manifestations and adults with peripheral SpA. Due to the differences in classification criteria for children with ERA and adults with axial and peripheral SpA, the FDA granted automatic full waivers of studies in children for new medications for “axial spondyloarthropathies including ankylosing spondylitis” up until July 2020. Thus, although current juvenile idiopathic arthritis (JIA) treatment guidelines recommend the use of biologic disease modifying anti-rheumatic drugs (DMARDs) as part of the early treatment for patients with ERA, none of the FDA-approved therapies for peripheral SpA or non-radiographic axial SpA (certolizumab pegol, ixekizumab, and secukinumab) have been studied or are labelled for use in children with ERA. Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis and clinical manifestations summarized in this review, medications approved for axial SpA or peripheral SpA should also be studied in children with active ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children. Considering the current lack of effective FDA-approved therapies for ERA, the FDA should also consider requiring pediatric studies for medications that have already been approved for the treatment of adults with SpA.
Keywords: Spondyloarthritis, juvenile spondyloarthritis, enthesitis-related arthritis, Juvenile idiopathic arthritis, medications, pediatrics
Introduction
Juvenile idiopathic arthritis (JIA) is a group of chronic pediatric rheumatic diseases of unknown etiology that present by the age of 16. JIA is classified into six mutually exclusive categories by the International League of Associations for Rheumatology (ILAR) criteria (1); a seventh category, “undifferentiated” is for children fulfilling criteria for more than one category. Patients categorized as extended oligoarticular JIA or polyarticular JIA are accepted as the pediatric extensions of rheumatoid arthritis for FDA drug approval, and juvenile psoriatic arthritis of psoriatic arthritis in adults, respectively. Enthesitis related arthritis (ERA) was the JIA category applied to children with spondyloarthritis (SpA), recognizing enthesitis as a defining characteristic. The prevalence of JIA is estimated at 20 to 45 per 100,000 children, of which 15–20% have ERA (2). The ILAR criteria for ERA are arthritis plus enthesitis; or arthritis or enthesitis plus at least 2 of the following: sacroiliac tenderness or inflammatory back pain, HLA-B27 positivity, first degree relative with HLA-B27 associated disease, acute anterior uveitis, and arthritis in a male older than 6 years (1). The ERA criteria do not specifically account for inflammatory bowel disease arthropathy, ankylosing spondylitis, or reactive arthritis, clinical conditions included with adult spondyloarthritis; children with these conditions may or may not meet the ERA criteria depending upon what disease features are present.
This review will summarize clinical, genetic and pathophysiologic characteristics shared between children with ERA with axial involvement and adults with non-radiographic, and in some cases radiographic, axial SpA; and between children with ERA and peripheral disease manifestations and adults with peripheral SpA. Further, insights into validated outcome measures and therapy of ERA and adult SpA are provided.
Evidence that ERA and SpA are similar diseases based on biology
Much of our understanding of ERA pathogenesis is derived from studies of HLA-B27, a risk allele for adult and juvenile SpA. HLA-B27 is linked to activation of the interleukin (IL)-23/IL-17 axis through non-canonical mechanisms not involving antigen presentation to CD8+ T cells (3). A population of CD4/CD8-negative T cells in the entheses was shown to mediate IL-23-driven SpA (5). These cells were first identified in mice, and an equivalent type 3 innate-like lymphocyte has been described in human entheses (6). Juvenile SpA, like its adult counterpart, may also have an extra-synovial basis of disease (8, 9). The overlap in genetic susceptibility to ERA and SpA also includes endoplasmic reticulum aminopeptidase (ERAP1)(10), a peptidase specialized to produce peptides presented on class I major histocompatibility complex molecules, and a major risk gene for ankylosing spondylitis (11).
Subsets of adults with SpA and children with ERA have bowel inflammation (12). This has been studied more in adults (13) as access to intestinal tissue from children with subclinical inflammation is limited by ethical concerns. A number of different cell types have been implicated and studies have emphasized the potential importance of bacterial dysbiosis, although cause and effect relationships remain unclear.
Similarity of Clinical Features
Spondyloarthritis develops on a continuum with a major peak of onset in young adulthood (14). Although sacroiliitis is well-documented in ERA (15), the ILAR classification criteria focus on the importance of extra-axial manifestations, i.e. peripheral arthritis and enthesitis. Conversely, SpA classification in adults considers the presence of axial disease and peripheral disease (1). For reasons that remain unclear, common presenting features of juvenile onset disease localize more to hips and peripheral joints (16), while adults experience predominantly inflammatory back pain (17).
Table 1 highlights the similarities and differences between the ERA classification criteria, the Assessment of SpondyloArthritis International Society (ASAS) criteria for non-radiographic axial SpA, and the ASAS criteria for peripheral SpA (18). The principal commonalities of children with ERA and axial arthritis, and adults with non-radiographic axial SpA, include enthesitis, arthritis, inflammatory back pain, anterior uveitis, HLA-B27 positivity, and family history of HLA-B27-associated disease. MRI is increasingly used to confirm the presence of subchondral bone marrow edema around the sacroiliac joints, many have elevated C-reactive protein, and the majority experience some response to nonsteroidal anti-inflammatory drugs (NSAIDs). One study reported 62% of ERA patients had axial disease at diagnosis and that 63% of patients with only peripheral arthritis at diagnosis, developed axial involvement within 5 years (19). Figure 1 demonstrates the inflammatory changes in the sacroiliac joints are indistinguishable between adults and children. In children, maturational changes may be mistaken for inflammatory changes by those with less experience evaluating the pediatric joint (Figure 2) (20). Unlike non-radiographic axial SpA, ERA is exclusive of psoriasis while inflammatory bowel disease and reactive arthritis are largely ignored. Taken together, despite common clinical, laboratory and radiographic features, differences in the classification between ERA and adult SpA can unduly complicate communication between providers, insurance carriers and regulatory agencies including the FDA,transition from pediatric to adult care, and access to medications.
Table 1:
Comparison of classification criteria used in children and adults
| ERA | Non-radiographic axial SpA | Peripheral SpA | ||
|---|---|---|---|---|
| Criteria set | ILAR | ASAS | ASAS | |
| Inclusion or entry criteria | Arthritis and enthesitis OR Arthritis or enthesitis plus ≥2 supporting features |
≥3 months of back pain starting before age 45 years AND Sacroiliitis on imaging plus ≥1 SpA feature OR ≥2 SpA features |
Arthritis* OR Enthesitis* OR Dactylitis* OR Plus ≥1 Group A feature OR ≥ 2 Group B features |
|
| Supporting features | Group A | Group B | ||
| Enthesitis | X | X | X | |
| Arthritis | X | X | X | |
| Dactylitis | X | X | ||
| Sacroiliac tenderness or IBP | X | X† | X† | |
| Anterior uveitis | X | X | X | |
| Psoriasis | X | X | ||
| IBD | X | |||
| Preceding infection^ | X | |||
| Imaging | X# | |||
| HLA-B27 positivity | X | |||
| Family history | HLA-B27-associated disease in 1st-degree relative | 1st or 2nd degree relative with SpA | 1st or 2nd degree relative with SpA | |
| Markers of inflammation/elevated C-reactive protein | X | |||
| Therapeutic response to NSAIDs | X | |||
ILAR: International League Against Rheumatism; ASAS: Assessment of SpondyloArthritis international Society; IBP: inflammatory back pain ; IBD: inflammatory bowel disease;
Inflammatory back pain only.
Must be present at the time of evaluation.
Urethritis/cervicitis or diarrhea within 1 month prior to onset of symptoms; SpA: spondyloarthritis.
Sacroiliitis on imaging (bilateral grade 2 to 4 or unilateral grade 3 to 4 on radiographs or active sacroiliitis on MRI.
Figure 1:
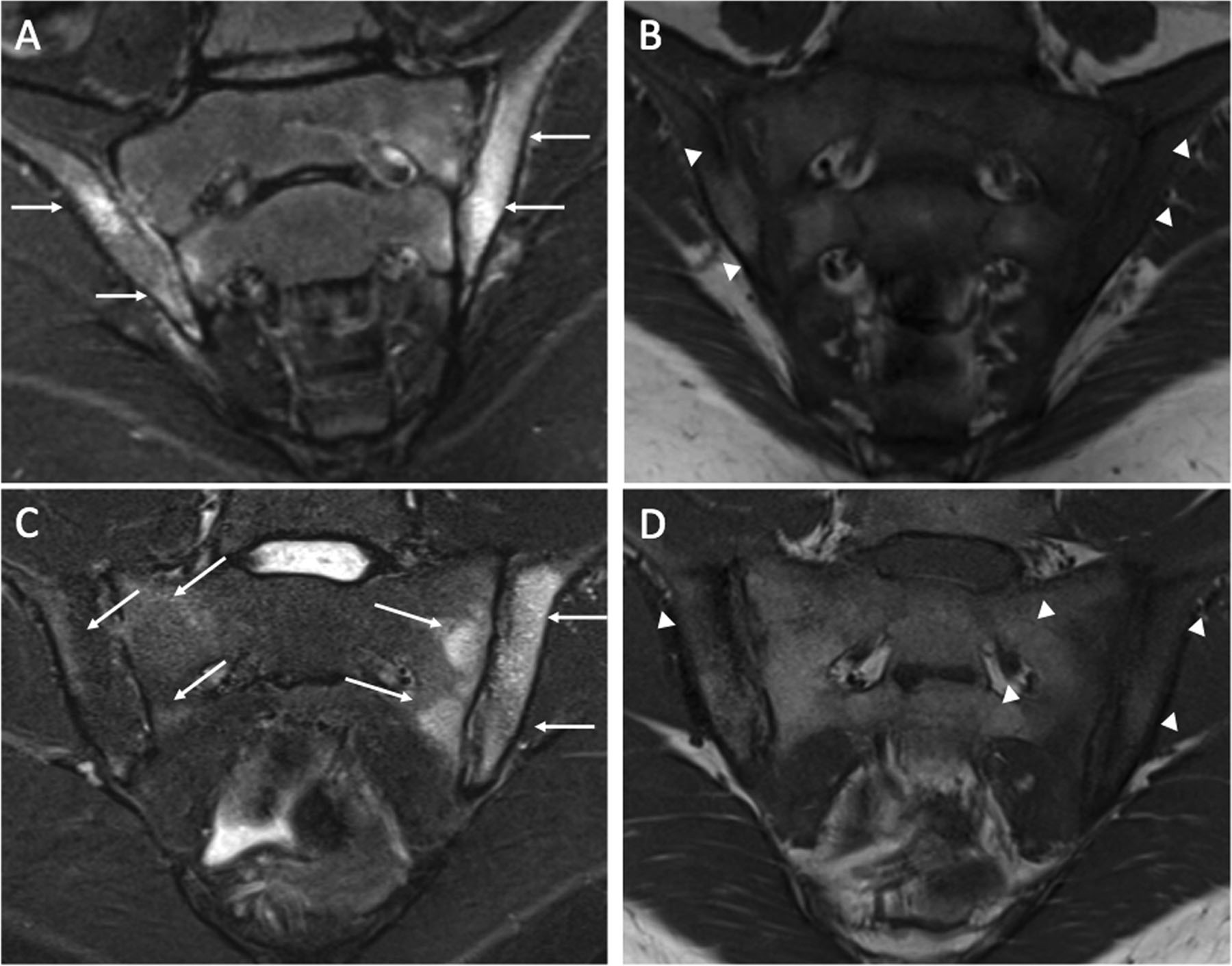
Coronal oblique short tau inversion recovery (STIR) (A and C) and coronal oblique T1-weighted (B) images of the sacroiliac joints of a 7-year old HLA-B27+ female (A and B) and a 20-year old HLA-B27+ male (C and D). There is active sacroiliitis with periarticular bone marrow edema within the iliac aspect of both joints as demonstrated by increased signal intensity on STIR (A) imaging (arrows) and decreased signal intensity on T-1 weighted (B) imaging (arrowhead). There is active sacroiliitis with periarticular bone marrow edema within the sacral and iliac bones, much more intense on the left than the right, as demonstrated by increased signal intensity on STIR (C) imaging (arrows) and decreased signal intensity on T-1 weighted (D) imaging (arrowheads).
Figure 2.

Coronal oblique short tau inversion recovery (STIR) image of the sacroiliac joints of a 15-year old female demonstrating metaphyseal-equivalent hyperintense signal (arrows), a normal variant, that could be mistaken for subchondral inflammation by less experienced reviewers.
Imaging outcome measures in pediatric and adult disease are similar
For children and adults, evaluation for axial disease often includes MRI. Pediatric studies (15, 21) utilize the ASAS MRI lesion definitions (22). Further, there are validated tools for assessment of axial joint inflammation and damage in adults and children. The Spondyloarthritis Research Consortium of Canada (SPARCC) sacroiliac joint inflammation score (SIS, range 0–72) considers site, extent and severity of sacroiliac joint inflammation and has been validated for use in adults and children to capture response to therapy (23, 24). A change in SIS score of 2.5 is considered clinically relevant in both populations (23). Damage in the sacroiliac joint can be quantified by the SPARCC sacroiliac structural score (SSS) which features four domains, including erosion (0–40), fat metaplasia (0–40), backfill (0–20), and ankylosis (0–20); there is no total score. The SPARCC structural score (SSS) is validated in children and adults (24, 25).
Similarity of response to therapy in children and adults
Algorithms to treat ERA with axial arthritis and non-radiographic axial SpA are similar as is evidenced by published American College of Rheumatology (ACR) treatment recommendations for both conditions (26, 27).
The recommended initial treatment of both is NSAIDs, followed by TNF inhibitors if NSAIDs are not tolerated or ineffective. Numerous trials in adults have shown that conventional disease modifying anti-rheumatic drugs (cDMARDs) do not improve axial disease (28). Although similar trials have not been conducted in ERA, ACR pediatric treatment recommendations strongly advise against methotrexate monotherapy and moving directly to anti-TNF therapy, based on extrapolation from the adult studies and clinical experience (26).
Treatment algorithms for children with ERA and adults with SpA and peripheral disease depend upon the number of affected joints and risk factors present. For peripheral disease affecting fewer than 5 joints, intraarticular joint injections with or without NSAIDS are considered first-line therapy (26). For peripheral disease affecting 5 or more joints, cDMARDs including methotrexate are first-line therapy and may be used with TNF inhibitors, if joint damage is present or if there is involvement of high-risk joints (cervical spine, wrist, hip) (26). While there are no formal guidelines for treatment of adults with peripheral SpA, treatment algorithms are analogous to those used in children with ERA and inflammation of peripheral joints.
Response to therapies is also similar in ERA and adults with SpA. Randomized placebo-controlled clinical trials in adults (30, 31) and data from children (32, 33) show the efficacy of TNF inhibitors for peripheral arthritis, enthesitis, and axial arthritis. However, as many as half of adults with axial disease are unable to achieve remission with TNF inhibitors, with 15% of adults with axial SpA failing to show any improvement with TNF inhibitors (34). Similarly, 33% of children with ERA treated with TNF inhibitors and NSAIDs lack response to therapy (19). In one study only 24% of children with ERA achieved inactive disease during the initial 12 months of treatment (35), and fewer than 20% achieved remission within five years (36). Additionally, physical function limitations and moderate chronic pain are more prevalent with ERA than with other JIA categories (37). Thus, achieving inactive disease status or clinical remission is difficult for children with ERA and many continue to have disease activity despite off-label use of existing therapies.
Regulatory environment for medication approval
In the United States, the Food and Drug Administration (FDA) is the federal agency charged with overseeing drug manufacturing, labelling, advertisement, and safety of medications and biological products. The Best Pharmaceuticals for Children Act (BPCA) (39) and the Pediatric Research Equity Act (PREA) (40) govern medication approval for children in the United States. While the BPCA encourages drug companies to test their products in children, PREA necessitates the study of new drugs and biological DMARDs in children if there is a pediatric disease similar to the non-orphan adult disease, and if it is likely that the new agent will be used in children (41).
The FDA gives automatic full waivers from conducting studies in children under PREA if the pediatric equivalent of the adult disease “rarely or never occur in pediatrics“. This is because studies in children would be highly impractical. ERA is common, comprising 15–20% of JIA in the United States. Indeed ERA is at least as common as systemic JIA for which clinical trials have been successfully completed (42). However, due to the differences in classification criteria outlined above, the FDA has granted automatic full waivers of studies in children for new medications for “axial spondyloarthropathies including ankylosing spondylitis” up until July 2020. Thus, although current JIA treatment guidelines recommend the use of biologic DMARDs as part of the early treatment for patients with ERA (43), none of the FDA-approved therapies for peripheral SpA or non-radiographic axial SpA [certolizumab pegol (2019), ixekizumab (2020), and secukinumab (2020)] have been studied or are labelled for use in children with ERA.
Recommendations to improve treatment options for children with ERA
Evidence of uncontrolled disease despite a trial of NSAIDs could identify children with ERA who require advanced therapies and may participate in clinical trials, irrespective of the presence of axial or peripheral involvement. Clinical trials in ERA should capture and evaluate response of axial and peripheral disease separately. This may be done via sub-analysis of axial and peripheral disease response. Similar to trials of non-radiographic axial SpA (44), eligibility criteria for children with ERA and axial features could include the presence of some of the following disease features: active inflammatory sacroiliitis based on typical MRI changes according to ASA/OMERACT criteria; elevated CRP; and inadequate response or intolerance to NSAIDs. Because axial disease does not respond to treatment with cDMARDs and approximately 40% of children are HLA-B27 negative (45), absence of these features should not be exclusionary. Presence of acute uveitis should also not be exclusionary as this is generally treatable with topical medications. The FDA grants partial waivers for study conduct in certain pediatric age groups. With respect to ERA, a partial waiver for studies of children younger than 6 years seems sensible as disease onset prior to this age is unusual.
Similar to trials of adults with peripheral SpA (46), active disease in children with ERA and peripheral disease can be defined by a combination of the following: persistence of active arthritis in one or more joints, active enthesitis and/or dactylitis despite NSAID exposure; evidence of systemic inflammation; physician global assessment of disease activity reflective of active disease; and patient global assessment of pain indicating ongoing ERA-related pain. Efficacy could be assessed using clinically meaningful change in validated composite disease activity scores or patient reported outcomes. Given the challenges of entheseal assessment in children (47) and the lack of a validated pediatric enthesitis index we caution against the use of enthesitis as a primary outcome.
The FDA encourages extrapolation of effectiveness from adult to pediatric populations when appropriate. With regard to ERA, extrapolation of effectiveness of a medication to control signs and symptoms should assume that an appropriate pediatric dose can be established either through achieving a similar exposure in children as the proven therapeutic exposure in adults, or by using an appropriate pharmacodynamic or clinical endpoint to achieve the targeted effect (48). Conversely, the ability to extrapolate safety from adults with SpA to children with ERA is limited and special consideration should be made to utilize trial designs that allow for the assessment of unique pediatric toxicities including the potential impact of the drug on growth and development (48).
To ensure the most appropriate dosing and confirm anticipated efficacy of a medication to be used in children with ERA, sufficient data need to be available. As is detailed in the Center for Drug Evaluation and Research document (49) the types of studies needed will depend on what is already known about pediatric dosing (PK) and whether there are differences between pediatric and adult pharmacodynamics, hence potential differences in efficacy. Study needs will have to be determined on a case-by-case basis. Depending on the available knowledge base, no additional studies may be required or a randomized double-blinded study might be needed.
In summary, despite FDA-approved treatments for adult axial and peripheral SpA, there remains an unmet need for effective medications for children with spondyloarthropathies. Considering the similarities between adult spondyloarthritis and ERA in terms of etiology, genetics, pathogenesis and clinical manifestations (50), it is evident that medications approved for axial or peripheral SpA should be studied in children with ERA involving axial or peripheral joints, respectively, with the intent to achieve labeling for use in children. Considering the current lack of effective therapies for ERA, the FDA should consider requiring pediatric studies for medications that have already been approved for the treatment of adults with SpA. The design of trials in ERA will depend on the amount of prior knowledge about a given drug and could entail full and partial extrapolation strategies in support of achieving an indication for the treatment of ERA.
Grant Support:
Pamela F. Weiss is supported by NIAMS NIH grant R01 AR074098. Robert A. Colbert is supported by the NIAMS Intramural Research Program Z01 AR041184.
Financial Disclosures: PF Weiss: Consultancies/honoraria (<$10,000 each) from Pfizer and Lilly. DJ Lovell: Consultancies/honoraria (<$10,000 each) from AstraZeneca, Wyeth, Amgen, Abbott, Pfizer, Hoffmann-LaRoche, Novartis, UBC, Takeda, Janssen, GlaxoSmithKline, Boehringer Ingelheim, Celgene, Bristol-Myers Squibb, AbbVie, and Forest Research. HI Brunner: Speaking fees for Novartis and Roche (both >$10,000) and GlaxoSmithKline (<$10,000); Consultancies/honoraria (<$10,000): Ablynx, AbbVie, Astra Zeneca-Medimmune, Biogen, Boehringer, Bristol-Myers Squibb, Celgene, Eli Lilly, EMD Serono, Genzyme, GlaxoSmithKline, F. Hoffmann-La Roche, Merck, Novartis, R-Pharm, Sanofi. The Cincinnati Children’s Hospital, where HBR works as a full-time public employee, has received contributions (>$10,000 each) from the following industries in the past 3 years: Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, F. Hoffmann-La Roche, Janssen, Novartis, and Pfizer. This funding has been reinvested for the research activities of the hospital in a fully independent manner, without any commitment to third parties.
Contributor Information
Pamela F. Weiss, Children’s Hospital of Philadelphia, Perlman School of Medicine at the University of Pennsylvania School of Medicine; USA..
Robert C. Fuhlbrigge, Children’s Hospital Colorado, University of Colorado School of Medicine, USA;
Emily von Scheven, University of California, San Francisco. California, USA..
Daniel J Lovell, Cincinnati Children’s Hospital Medical Center, University of Cincinnati; USA;.
Robert A Colbert, National Institute of Arthritis, Musculoskeletal, and Skin Diseases, National Institutes of Health, Bethesda, MD;.
Hermine I Brunner, Cincinnati Children’s Hospital Medical Center, University of Cincinnati; USA;.
REFERENCES
- 1.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 2.Petty RE, R.M. L, L.R. W Juvenile Idiopathic Arthritis. In: Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, editors. Textbook of Pediatric Rheumatology. 7th ed. Philadelphia: Elsevier; 2016. [Google Scholar]
- 3.Taurog JD, Chhabra A, Colbert RA. Ankylosing Spondylitis and Axial Spondyloarthritis. N Engl J Med. 2016;374(26):2563–74. [DOI] [PubMed] [Google Scholar]
- 4.Colbert RA, Navid F, Gill T. The role of HLA-B*27 in spondyloarthritis. Best Pract Res Clin Rheumatol. 2017;31(6):797–815. [DOI] [PubMed] [Google Scholar]
- 5.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–76. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbert RJ, Fragkakis EM, Dunsmuir R, Li Z, Coles M, Marzo-Ortega H, et al. Brief Report: Group 3 Innate Lymphoid Cells in Human Enthesis. Arthritis Rheumatol. 2017;69(9):1816–22. [DOI] [PubMed] [Google Scholar]
- 7.Jacques P, McGonagle D. The role of mechanical stress in the pathogenesis of spondyloarthritis and how to combat it. Best Pract Res Clin Rheumatol. 2014;28(5):703–10. [DOI] [PubMed] [Google Scholar]
- 8.Lee EY, Sundel RP, Kim S, Zurakowski D, Kleinman PK. MRI findings of juvenile psoriatic arthritis. Skeletal Radiol. 2008;37(11):987–96. [DOI] [PubMed] [Google Scholar]
- 9.Tuttle KS, Vargas SO, Callahan MJ, Bae DS, Nigrovic PA. Enthesitis as a component of dactylitis in psoriatic juvenile idiopathic arthritis: histology of an established clinical entity. Pediatr Rheumatol Online J. 2015;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinks A, Martin P, Flynn E, Eyre S, Packham J, Childhood Arthritis Prospective Study C, et al. Subtype specific genetic associations for juvenile idiopathic arthritis: ERAP1 with the enthesitis related arthritis subtype and IL23R with juvenile psoriatic arthritis. Arthritis Res Ther. 2011;13(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans DM, Spencer CC, Pointon JJ, Su Z, Harvey D, Kochan G, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43(8):761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielants H, Veys EM, Cuvelier C, De Vos M, Goemaere S, Maertens M, et al. Gut inflammation in children with late onset pauciarticular juvenile chronic arthritis and evolution to adult spondyloarthropathy--a prospective study. J Rheumatol. 1993;20(9):1567–72. [PubMed] [Google Scholar]
- 13.Rizzo A, Ferrante A, Guggino G, Ciccia F. Gut inflammation in spondyloarthritis. Best Pract Res Clin Rheumatol. 2017;31(6):863–76. [DOI] [PubMed] [Google Scholar]
- 14.Colbert RA. Classification of juvenile spondyloarthritis: Enthesitis-related arthritis and beyond. Nat Rev Rheumatol. 2010;6(8):477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss PF, Xiao R, Biko DM, Chauvin NA. Assessment of Sacroiliitis at Diagnosis of Juvenile Spondyloarthritis by Radiography, Magnetic Resonance Imaging, and Clinical Examination. Arthritis Care Res (Hoboken). 2016;68(2):187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos-Vargas R, Vazquez-Mellado J. The early clinical recognition of juvenile-onset ankylosing spondylitis and its differentiation from juvenile rheumatoid arthritis. Arthritis Rheum. 1995;38(6):835–44. [DOI] [PubMed] [Google Scholar]
- 17.Riley MJ, Ansell BM, Bywaters EG. Radiological manifestations of ankylosing spondylitis according to age at onset. Ann Rheum Dis. 1971;30(2):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777–83. [DOI] [PubMed] [Google Scholar]
- 19.Goirand M, Breton S, Chevallier F, Duong NP, Uettwiller F, Melki I, et al. Clinical features of children with enthesitis-related juvenile idiopathic arthritis / juvenile spondyloarthritis followed in a French tertiary care pediatric rheumatology centre. Pediatr Rheumatol Online J. 2018;16(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss PF, Brandon TG, Bohnsack J, Heshin-Bekenstein M, Francavilla ML, Jaremko JL, et al. Variability in magnetic resonance imaging interpretation of the pediatric sacroiliac joint. Arthritis Care Res (Hoboken). 2020. [DOI] [PubMed] [Google Scholar]
- 21.Bray TJP, Lopes A, Fisher C, Ciurtin C, Sen D, Hall-Craggs MA. Sacroiliac Joint Ankylosis in Young Spondyloarthritis Patients Receiving Biologic Therapy: Observation of Serial Magnetic Resonance Imaging Scans. Arthritis Rheumatol. 2019;71(4):594–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudwaleit M, Haibel H, Baraliakos X, Listing J, Marker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009;60(3):717–27. [DOI] [PubMed] [Google Scholar]
- 23.Maksymowych WP, Lambert RG, Brown LS, Pangan AL. Defining the Minimally Important Change for the SpondyloArthritis Research Consortium of Canada Spine and Sacroiliac Joint Magnetic Resonance Imaging Indices for Ankylosing Spondylitis. J Rheumatol. 2012;39(8):1666–74. [DOI] [PubMed] [Google Scholar]
- 24.Weiss PF, Maksymowych WP, Xiao R, Biko DM, Francavilla ML, Lambert RG, et al. Spondyloarthritis Research Consortium of Canada sacroiliac joint inflammation and structural scores: change score reliability and recalibration utility in children. Arthritis Res Ther. 2020;22(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss PF, Maksymowych WP, Lambert RG, Jaremko JL, Biko DM, Paschke J, et al. Feasibility and Reliability of the Spondyloarthritis Research Consortium of Canada Sacroiliac Joint Structural Score in Children. J Rheumatol. 2018;45(10):1411–7. [DOI] [PubMed] [Google Scholar]
- 26.Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res (Hoboken). 2019;71(6):717–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward MM, Deodhar A, Gensler LS, Dubreuil M, Yu D, Khan MA, et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Care Res (Hoboken). 2019;71(10):1285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haibel H, Brandt HC, Song IH, Brandt A, Listing J, Rudwaleit M, et al. No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis. 2007;66(3):419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molto A, Sieper J. Peripheral spondyloarthritis: Concept, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2018;32(3):357–68. [DOI] [PubMed] [Google Scholar]
- 30.Paramarta JE, De Rycke L, Heijda TF, Ambarus CA, Vos K, Dinant HJ, et al. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without ankylosing spondylitis or psoriatic arthritis. Ann Rheum Dis. 2013;72(11):1793–9. [DOI] [PubMed] [Google Scholar]
- 31.Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, et al. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: results of a randomised placebo-controlled trial (ABILITY-1). Ann Rheum Dis. 2013;72(6):815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgos-Vargas R, Tse SM, Horneff G, Pangan AL, Kalabic J, Goss S, et al. A Randomized, Double-Blind, Placebo-Controlled Multicenter Study of Adalimumab in Pediatric Patients With Enthesitis-Related Arthritis. Arthritis Care Res (Hoboken). 2015;67(11):1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, et al. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): significant short term improvement. Arthritis Res Ther. 2012;14(5):R230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deodhar A, Strand V, Conaghan PG, Sullivan E, Blackburn S, Tian H, et al. Unmet needs in ankylosing spondylitis patients receiving tumour necrosis factor inhibitor therapy; results from a large multinational real-world study. BMC Rheumatol. 2020;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-alpha inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. J Rheumatol. 2011;38(12):2675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flato B, Aasland A, Vinje O, Forre O. Outcome and predictive factors in juvenile rheumatoid arthritis and juvenile spondyloarthropathy. J Rheumatol. 1998;25(2):366–75. [PubMed] [Google Scholar]
- 37.Taxter AJ, Wileyto EP, Behrens EM, Weiss PF. Patient-reported outcomes across categories of juvenile idiopathic arthritis. The Journal of rheumatology. 2015;42(10):1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumsey DG, Guzman J, Rosenberg AM, Huber AM, Scuccimarri R, Shiff NJ, et al. Characteristics and Course of Enthesitis in a Juvenile Idiopathic Arthritis Inception Cohort. Arthritis Care Res (Hoboken). 2018;70(2):303–8. [DOI] [PubMed] [Google Scholar]
- 39.Best pharmaceuticals for children act of 2007. Food and Drug Administration Amendments Act (FDAAA), Title V [Google Scholar]
- 40.Pediatric Research Equity Act. 2003:Pub L No. 108–55 [Google Scholar]
- 41.Field MJ, Boat TF, Institute of Medicine (U.S.), National Research Council (U.S.). Safe and effective medicines for children : pediatric studies conducted under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. Washington, D.C.: The National Academies Press; 2012. xxiii, 408.pages p. [PubMed] [Google Scholar]
- 42.Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406. [DOI] [PubMed] [Google Scholar]
- 43.Ringold S, Angeles-Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Treatment of Juvenile Idiopathic Arthritis: Therapeutic Approaches for Non-Systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Rheumatol. 2019;71(6):846–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deodhar A, van der Heijde D, Gensler LS, Kim TH, Maksymowych WP, Ostergaard M, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet. 2020;395(10217):53–64. [DOI] [PubMed] [Google Scholar]
- 45.Gmuca S, Xiao R, Brandon TG, Pagnini I, Wright TB, Beukelman T, et al. Multicenter inception cohort of enthesitis-related arthritis: variation in disease characteristics and treatment approaches. Arthritis Res Ther. 2017;19(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carron P, Varkas G, Cypers H, Van Praet L, Elewaut D, Van den Bosch F, et al. Anti-TNF-induced remission in very early peripheral spondyloarthritis: the CRESPA study. Ann Rheum Dis. 2017;76(8):1389–95. [DOI] [PubMed] [Google Scholar]
- 47.Weiss PF, Chauvin NA, Klink AJ, Localio R, Feudtner C, Jaramillo D, et al. Detection of enthesitis in children with enthesitis-related arthritis: dolorimetry compared to ultrasonograph. Arthritis Rheumatol. 2014;66(1):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Food U, Administration D. Pediatric study plans: content of and process for submitting initial pediatric study plans and amended initial pediatric study plans: guidance for industry. Draft Guidance Revision. 2016;1. [Google Scholar]
- 49.Programs affecting safety and innovation in pediatric therapies. In: research. USDoHaHSFaDACfdea, editor. 2007. [Google Scholar]
- 50.Brunner HI, Schanberg LE, Kimura Y, Dennos A, Co DO, Colbert RA, et al. New medications are needed for children with juvenile idiopathic arthritis. Arthritis & Rheumatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


