Abstract
Neuron-glial interactions shape neural circuit establishment, refinement and function. One of the key neuron-glial interactions takes place between axons and oligodendroglial precursor cells. Interactions between neurons and oligodendrocyte precursor cells (OPCs) promote OPC proliferation, generation of new oligodendrocytes and myelination, shaping myelin development and ongoing adaptive myelin plasticity in the brain. Communication between neurons and OPCs can be broadly divided into paracrine and synaptic mechanisms. Following the Nobel mini-symposium “The Dark Side of the Brain” in late 2019 at the Karolinska Institutet, this mini-review will focus on the bright and dark sides of neuron-glial interactions and discuss paracrine and synaptic interactions between neurons and OPCs and their malignant counterparts.
The bright side of myelin plasticity: neuron-glial interactions and myelination
The discovery twenty years ago that OPCs form functional synapses with neurons in the hippocampus1 (Figure 1A) led to a paradigm shift in our understanding of the brain, refuting the idea that only neurons can form synapses with each other. The axon-OPC synapse has since been found during development and throughout the mature central nervous system (CNS), in both gray1–3 and white matter4–7. It appears that OPCs receive synaptic inputs predominantly from unmyelinated axons in both white and gray matter4,5,8. The axon-OPC synapse enables OPCs to sense and decode neuronal activity, thus providing a possible mechanism for neuronal activity to regulate OPC proliferation and differentiation. OPCs have been shown to receive both glutamatergic and GABAergic synaptic inputs, in both grey matter (e.g., hippocampus, cortex, and cerebellum4–7) and white matter (e.g., corpus callosum and cerebellar white matter8,9), but the relative contributions of each may differ depending on the brain region. Similar to neuron – neuron synapses, rabies-virus tracing of presynaptic neuronal input to OPCs has shown that OPCs receive brain-wide input from multiple neurons and neuronal subtypes within a given circuit, and form both glutamate and GABAergic inputs13, demonstrating that OPCs are positioned to integrate circuit activity with a complexity similar to that of neurons. Thus, axon-OPC synapses may provide a cellular mechanism through which OPCs can lead to myelin changes, by differentiating into myelinating oligodendrocytes in response to neuronal activity. The synaptic inputs, in particular the miniature inputs, detected in OPCs are similar in kinetics to those detected in some postsynaptic neurons, and OPCs express many of the molecules needed for postsynaptic development and function. Importantly they express both inotropic and metabotropic neurotransmitter receptors for the two main neurotransmitters in the CNS, glutamate and GABA, in addition to having receptors to neuromodulators. OPCs express all the ionotropic glutamate receptors e.g. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), kainate receptors (KAR) and N-methyl-D-aspartate receptors (NMDARs), as well as metabotropic (G protein-coupled) glutamate receptors such as mGluR5, which has been found to regulate the expression of AMPAR10. Similarly, OPCs express ionotropic GABA receptors, GABAA receptors, and the metabotropic GABAB receptors6,15–17, and as it is in early developing neurons, GABA is excitatory, like glutamate, in OPCs3,16. Therefore, OPCs and neurons are similarly equipped to monitor neuronal activity via synaptic inputs. However, unlike neurons, OPCs may potentially respond to these inputs by proliferating, or differentiating.
Figure 1.
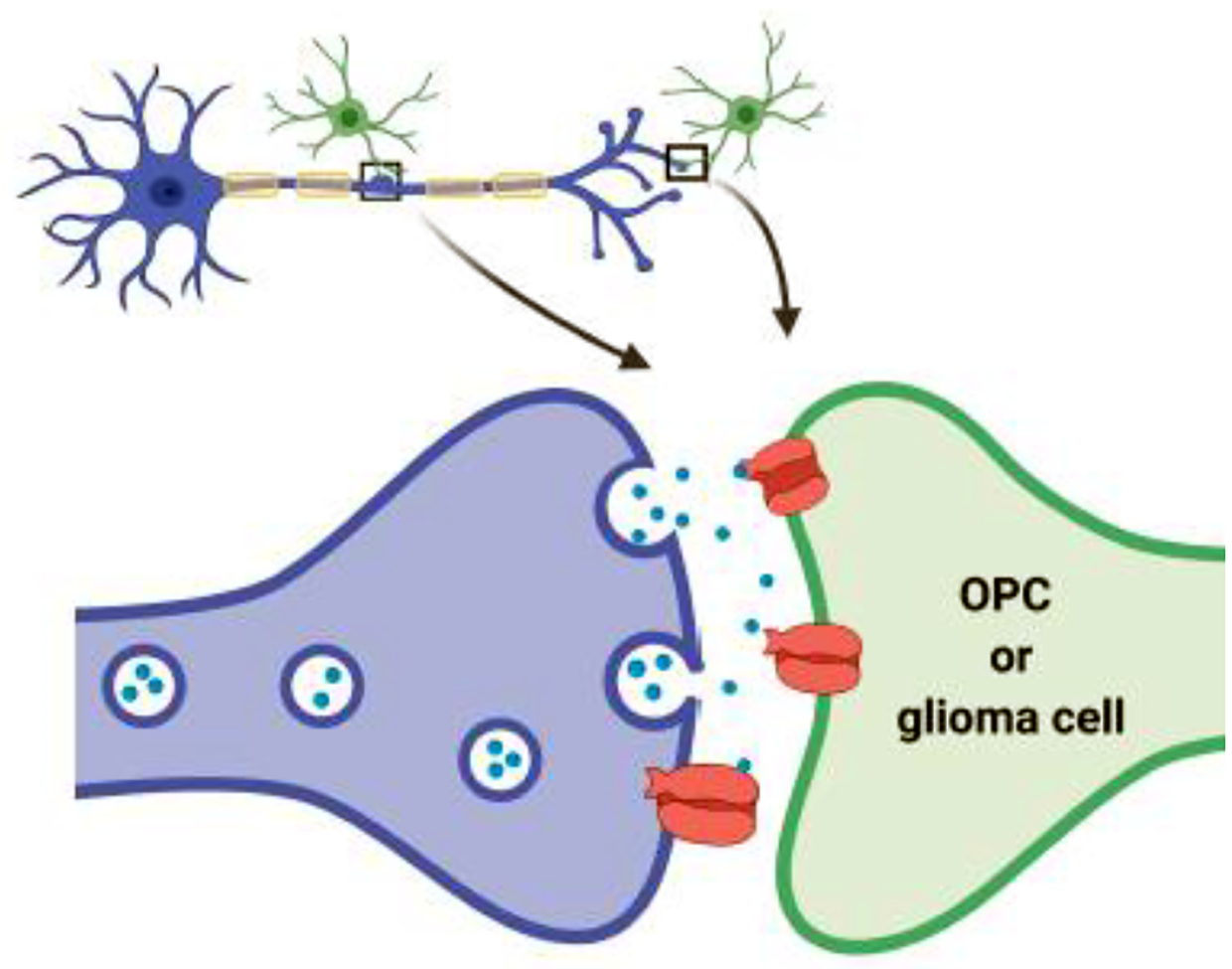
Axon-glial and axon-glioma synapses. A) In the healthy brain, synapses form between presynaptic neurons (blue) and post-synaptic oligodendrocyte precursor cells (green), in both white matter (via ‘en passage’ synapse8), and grey matter (where OPCs often share synapses with neurons1). B) Similar synapses form between presynaptic neurons and post-synaptic malignant glioma cells (green) in brain cancer, as between neurons and OPCs in gray matter.
Emerging evidence clearly shows that neuronal activity promotes myelination. Increasing neuronal firing rate in vivo using optogenetics, chemogenetics, receptor agonists/antagonists, or physiological manipulations promotes OPC proliferation, differentiation18,19, and enhances myelination19–22. Conversely, decreasing neuronal activity using pharmacological manipulations23, physiological manipulations (whisker removal or raising mice in social isolation or with reduced sensory inputs24–27) or reducing activity directly with chemogenetics28, impedes OPC differentiation and myelination in mice. However, the role that neuron-OPC synapses and neurotransmitter signaling may play in regulating OPC proliferation, differentiation, and subsequent myelination is not fully clear. Conceivably, the neuron-OPC synapse could mediate much of the effects of neuronal activity on OPCs. Rodent in vitro data indicate that neurotransmitters can modulate OPC proliferation, differentiation, or myelination29–32 and in vivo data in the developing zebrafish indicate that vesicular release modulates myelination21. Hence, neuronal activity, via the release of neurotransmitters, is likely an important mechanism for regulating myelination.
It is important to note that myelination can also occur in the absence of neuronal activity33–35. Studies using similar approaches, including sensory deprivation or physiological manipulations, to alter neuronal activity have failed to show an effect on developmental myelination36–38. Likewise, it has become clear that oligodendrocytes can ensheath and make myelin like wraps, around inert nanofibers33–35. Studies aimed at elucidating the role of neurotransmitter signaling by knocking out neurotransmitter receptors in OPCs or vesicular release of neurotransmitter from axons have similarly failed to find support for neurotransmitter-dependent myelination during developmental myelination in the regions studied. These studies have shown that when vesicular release of glutamate from axons is reduced (by knocking out VGlut2 in retinal ganglion cell axons) or when the AMPAR subunits GluR2, 3, and 4 (GluR1 is not expressed) or the NMDAR subunits GluN1 or GluN3 are knocked out in OPCs, there is little to no effect on OPC proliferation or myelination39–42.
A potential explanation for these apparently conflicting findings, whether neuronal activity regulates myelination43,30,44–49,21 or not33–35,50–53, is that perhaps there are two distinct modes of myelination, one that is independent of neuronal activity and another that depends on activity-regulated signaling to OPCs30. In fact, different neuronal subtypes in the same brain regions, are either myelinated independent of activity or must be active to become myelinated43,54. For instance neuronal activity modulates myelination in cortico-callosal projection neurons, but not cortico-fugal projection neurons43, and myelination of the reticulospinal, but not the commissural primary ascending neurons of the developing spinal cord depends on vesicular release, presumably of neurotransmitter54. When levels of the growth factors neuregulin 1 (NRG1) or brain derived neurotrophic factor (BDNF) are elevated, presumably by release from active neurons55,56, the density of NMDARs in OPCs increases, and OPCs switch from an activity-independent mechanism of myelination to a faster activity-dependent mechanism30. Intriguingly, deleting ErbB357, a receptor for NRG1, in oligodendrocyte lineage cells has no effect on developmental myelination, but disrupts experience-dependent myelination57, and blocking activity-dependent BDNF release or deleting the BDNF receptor TrkB in OPCs blocks activity-dependent myelination58 in young adult animals. Similarly, neuronal regulation of myelination is perhaps a bit more nuanced; an orchestra of paracrine and synaptic (temporal) communications that need to co-exist in order to initiate activity-dependent myelination. Indeed, when AMPAR subunits are genetically modified postnatally at the peak of the myelination period, as opposed to being knocked out embryonically, OPC proliferation and differentiation are affected59, suggesting that modifying receptor properties at specific timepoints can alter OPC dynamics and potentially activity-dependent myelination. This temporal dependence on receptors may be explained by the fact that OPCs differ between ages and brain regions60–64. One significant difference between OPCs with both age and region is their ion channel and neurotransmitter densities, and therefore the difference in their capacity to monitor and respond to neuronal activity63. Potentially, the paracrine signals in the environment around the OPCs may alter the ‘state’ of the OPCs and therefore their response to neuronal activity65,66. Conceivably, the activity-dependent myelination may have evolved in order to speed up and target myelination to ‘correctly’ firing axons during specific periods of circuit refinement or learning, and thus it may be important to fine-tune neuronal circuits.
The bright side of myelin plasticity: neuron-glial interactions and remyelination
Myelin regeneration is an exceptional regenerative process within the CNS. Several lines of evidence suggest that remyelination and myelin plasticity are two sides of the same process. OPCs that enter demyelinating lesions that are undergoing regeneration recapitulate postnatal OPCs, as identified by both electrophysiological and transcriptional studies67–69. In lesions, as at the peak of myelination, OPCs are equipped to monitor the firing pattern of neurons, as they express voltage-gated ion channels and glutamate receptors, and receive synaptic inputs from demyelinated neurons29,70. Blocking vesicular release, AMPARs or NMDARs prevents remyelination in ethidium bromide-induced white matter lesions29,71. Similarly, as during myelination, blocking neuronal activity during remyelination prevents myelin regeneration29, while enhancing activity72 and stimulating BDNF signaling58 improves remyelination. This suggests that adult de novo myelination (or myelin plasticity) and remyelination share a similar mechanism. Therefore, the neuron-OPC synapse might be an important signal through which neuronal activity regulates both myelin plasticity and remyelination. Understanding this common mechanism is important to identify therapeutic strategies to promote myelin regeneration after demyelinating injury.
The dark side of myelin plasticity: neuron-glial interactions and brain cancer
Neuron-glioma interactions mirror neuron-OPC interactions and regulate brain cancer growth
Malignant gliomas are a family of primary brain cancers that include adult glioblastoma, anaplastic astrocytoma, anaplastic oligodendroglioma, pediatric glioblastoma, diffuse intrinsic pontine glioma (DIPG) and other H3K27M+ diffuse midline gliomas. Collectively, these high-grade glial malignancies represent the leading cause of primary brain cancer-related death in both children and adults73. Precursor cells in the oligodendroglial lineage are thought to represent the cellular origins of many forms of malignant glioma74–79, and prominent subpopulations of glioma cells in a given tumor molecularly resemble OPCs80–82. Given these similarities between OPCs and malignant glioma, it stands to reason that malignant gliomas may respond to the same environmental cues as healthy OPCs. Glutamatergic cortico-callosal projection neuronal activity robustly promotes the proliferation of healthy OPCs43,58. Activity-regulated secretion of BDNF is a required component of the mechanism regulating neuron-OPC interactions58,71, and may prime OPCs to respond to additional activity-regulated cues30. Similarly, glutamatergic neuronal activity promotes the proliferation and growth of malignant glioma83. Activity-regulated, secreted factors contribute to the effect of cortical neuronal activity on glioma proliferation, an effect that is conserved across the various clinically and molecularly distinct subtypes of malignant glioma described above83.
Paracrine mechanisms mediating neuron-glioma interactions: BDNF and Neuroligin-3
How do glutamatergic neurons influence glioma growth? Like the role BDNF plays in normal neuron-OPC interactions30,58, BDNF is one mediator of neuronal activity-regulated glioma proliferation83. Unexpectedly, another key activity-regulated mechanism that mediates glioma proliferation involves activity-dependent shedding of neuroligin-3 (NLGN3)83, a synaptic adhesion molecule84. Shedding NLGN3 robustly promotes the proliferation of each major subtype of high-grade glioma83. Not only is NLGN3 a powerful mitogen in glioma, but expression of NLGN3 in the brain microenvironment is required for tumor growth in preclinical models85. High-grade glioma xenografts fail to progress in the environment of the NLGN3 knock out mouse brain, while other cancer types, such as breast cancer brain metastases, can grow without impediment in the absence of NLGN385.
The surprisingly important role that NLGN3 appears to play in glioma pathophysiology demands a detailed understanding of NLGN3 release into the tumor microenvironment and subsequent actions in glioma cells. NLGN3 is present on the post-synaptic cell chiefly at excitatory synapses and contributes to synaptic maturation and function86,87. Neuroligins contain a large n-terminal ectodomain, with a transmembrane domain and a smaller c-terminal endodomain anchoring it to the post-synaptic membrane. The N-terminal ectodomain of NLGN3 is shed in an activity-dependent manner through the enzymatic activity of the metalloprotease ADAM-1085. While neurons are one source of shed NLGN3, OPCs also express robust levels of NLGN315,88 and represent a major source of shed NLGN3 in the brain85. Conditional genetic mouse modeling illustrates that while OPCs are the major source of activity-regulated NLGN3 shedding in the cerebrum, neurons are the source of activity-regulated ADAM10 secretion85. Since ADAM10 can be released in synaptic vesicles89, these findings suggest that secretion of ADAM10 by presynaptic neurons at the axon-glial synapse may result in NLGN3 shedding by post-synaptic OPCs, although a non-synaptic mechanism of activity-regulated NLGN3 shedding by OPCs may also occur. Inhibition of NLGN3 shedding with pharmacological ADAM10 inhibitors blocks glioma progression in preclinical models, and this therapeutic strategy is presently in clinical trial for children with high-grade gliomas (NCT04295759).
How does NLGN3 induce proliferation of glioma cells? While the binding partner of NLGN3 on glioma cells remains to be defined, it is clear that upon binding, NLGN3 causes early upstream activation of focal adhesion kinase (FAK) and downstream activation of PI3K-mTOR, RAS and SRC signaling pathways83,85. While this helps to explain the role of NLGN3 in promoting glioma growth, it does not explain the unexpected dependency. The failure of glioma progression observed in the absence of microenvironmental NLGN3, as discussed above, suggests that NLGN3 contributes to a process fundamental to glioma pathophysiology. NLGN3 induces prominent changes in gene expression, including upregulation of numerous synapse-related genes85, which raises the possibility of axon-glioma synapses, a malignant version of the axon-glial synapses observed between neurons and OPCs in the healthy brain.
Axon-glioma synapses mediate activity-dependent brain cancer growth
Examination of single cell transcriptomic data from each major subtype of malignant glioma revealed prominent expression of synapse-related genes, especially AMPAR subunit genes and synapse-related structural proteins90,91. Synapse-related gene expression is particularly enriched in the OPC-like tumor cells within a given patient tumor92. Electron microscopy shows structural evidence of synapses between presynaptic neurons and postsynaptic glioma cells in primary patient tumor tissue and patient-derived glioma xenografts90,91. Co-culture of patient-derived glioma cells with neurons isolated from NLGN3 knockout mice or wildtype mice supports a role for NLGN3 in glioma synaptogenes90. Whole cell patch clamp electrophysiology demonstrates calcium-permeable AMPAR-mediated synapses in a subset of glioma cells within each patient-derived xenograft model examined90,91, as well as in acutely resected primary tumor tissue91. The calcium-permeable AMPAR-mediated axon-glioma synapses, which exhibit multiple electrophysiological synaptic characteristics such as miniature EPSCS and paired pulse facilitation, are reminiscent of similar calcium-permeable AMPAR-mediated axon-glial synapses on OPCs1 (Figure 1B). Genetic or pharmacological blockade of AMPAR signaling in glioma xenograft models robustly decreases tumor growth, indicating an important functional role for glutamatergic neurotransmission in glioma90. Membrane depolarization appears to be a key aspect of neuron-glioma synaptic signaling for glioma growth, as optogenetically inducing glioma cell membrane depolarization alone promotes glioma proliferation in vivo90. While the voltage-dependent mechanisms through which membrane depolarization promotes proliferation of malignant glioma cells remains to be determined, this observation parallels the roles played by electrical signaling in neural precursor cell populations during brain development92.
Other neurotransmitter-mediated effects in glioma
While it remains to be determined if other synapses that use different neurotransmitters or neuromodulators exist in gliomas, signaling roles for a range of neurotransmitters are coming to light. Non-synaptic, autocrine/paracrine glutamate signaling can promote the proliferation and migration of adult glioblastoma cells93,94. Underscoring the heterogeneity between among various forms of gliomas, non-synaptic glutamate signaling promotes migration but not proliferation in pediatric glioma90. Roles are also emerging for other neurotransmitters. Like the effects of glutamate signaling, dopaminergic signaling may be growth-promoting in adult glioblastoma95. Conversely, GABAergic signaling appears to inhibit tumor progression in both patient-derived xenograft and murine models of adult glioblastoma96,97. However, the role of GABA signaling in pediatric gliomas remains to be fully determined. It is presently unknown whether other neurotransmitters such as acetylcholine and serotonin influence glioma progression.
Conclusions
The parallel paracrine and synaptic mechanisms that mediate normal plasticity, regeneration and malignant neuron-glial interactions underscores the extent to which effective regeneration depends on and glial malignancies subvert normal mechanisms of neurodevelopment and neural plasticity. This heightens the importance to fully understand the mechanisms of myelin plasticity for regeneration and calls for a neuroscience-based approach to understanding brain cancers. These shared mechanisms at play in normal circuit plasticity in health, circuit functional recovery after injury or malignant circuit establishment in brain cancer underscores the need for future work to leverage these mechanistic similarities for improved therapies. Myelin biology thus elucidates both “the bright and dark sides of the brain” in brain regeneration and glial malignancies, respectively.
Acknowledgements:
NIH Director’s Pioneer Award (M.M.), US National Institutes of Neurological Disorders and Stroke (M.M), Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation (M.M.), Cancer Research UK (M.M.). ERC consolidator grant Award (No 771411; R.T.K,); Allen Distinguished Investigator Award (#12076; R.T.K); and the Lister Institute Research Prize (R.T.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Bergles D, Roberts J, Somogyi P & Jahr C Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Müller J et al. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J. Gen. Physiol 134, 115–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin SC & Bergles DE Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat.Neurosci 7, 24–32 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Kukley M, Capetillo-Zarate E & Dietrich D Vesicular glutamate release from axons in white matter. Nat. Neurosci 10, 311–320 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Ziskin JL, Nishiyama A, Rubio M, Fukaya M & Bergles DE Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci 10, 321–330 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karadottir R, Hamilton NB, Bakiri Y & Attwell D Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci 11, 450–6 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karadottir R, Cavelier P, Bergersen LH & Attwell D NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomassy GS et al. Distinct Profiles of Myelin Distribution Along Single Axons of Pyramidal Neurons in the Neocortex. Science 344, 319–324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jabs R et al. Synaptic transmission onto hippocampal glial cells with hGFAP promoter activity. JCell Sci 118, 3791–3803 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Passlick S et al. Expression of the γ2-Subunit Distinguishes Synaptic and Extrasynaptic GABAA Receptors in NG2 Cells of the Hippocampus. J. Neurosci 33, 12030–12040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velez-Fort M, Maldonado PP, Butt AM, Audinat E & Angulo MC Postnatal Switch from Synaptic to Extrasynaptic Transmission between Interneurons and NG2 Cells. J. Neurosci 30, 6921–6929 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zonouzi M, Renzi M, Farrant M & Cull-Candy SG Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells. Nat. Neurosci 14, 1430–1438 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mount CW, Yalçın B, Cunliffe-Koehler K, Sundaresh S & Monje M Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer S, Volbracht K, Lundgaard I & Káradóttir RT Glutamate signalling: A multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 110, 574–585 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci 34, 11929–11947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton NB et al. Endogenous GABA controls oligodendrocyte lineage cell number, myelination, and CNS internode length. Glia 65, 309–321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luyt K et al. Developing oligodendrocytes express functional GABA(B) receptors that stimulate cell proliferation and migration. J. Neurochem 100, 822–840 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Gibson EM et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitew S et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demerens C et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. U. S. A (1996) doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mensch S et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat Neurosci 18, 628–630 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauber H, Waehneldt TV & Neuhoff V Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett (1980) doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- 23.Barres BA & Raff MC Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Liu J et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat Neurosci 15, 1621–1623 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makinodan M, Rosen KM, Ito S & Corfas G A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill RA, Patel KD, Goncalves CM, Grutzendler J & Nishiyama A Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat Neurosci 17, 1518–1527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swire M, Kotelevtsev Y, Webb DJ, Lyons DA & ffrench-Constant C Endothelin signalling mediates experience-dependent myelination in the CNS. eLife 8, e49493 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitew S et al. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun 9, 306 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautier HOB et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun 6, 8518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgaard I et al. Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biol 11, e1001743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baraban M, Koudelka S & Lyons DA Ca 2+ activity signatures of myelin sheath formation and growth in vivo. Nat. Neurosci 21, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasnow AM & Attwell D NMDA Receptors: Power Switches for Oligodendrocytes. Neuron 91, 3–5 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Bechler ME, Byrne L & ffrench-Constant C CNS Myelin Sheath Lengths Are an Intrinsic Property of Oligodendrocytes. Curr. Biol 25, 2411–2416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg SS, Kelland EE, Tokar E, Asia R & Chan JR The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci 105, 14662–14667 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colello RJ, Devey LR, Imperato E & Pott U The chronology of oligodendrocyte differentiation in the rat optic nerve: Evidence for a signaling step initiating myelination in the CNS. J. Neurosci (1995) doi: 10.1523/jneurosci.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukui Y, Hayasaka S, Bedi KS, Ozaki HS & Takeuchi Y Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. J. Anat (1991). [PMC free article] [PubMed] [Google Scholar]
- 38.Shrager P & Novakovic SD Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Dev. Brain Res (1995) doi: 10.1016/0165-3806(95)00081-N. [DOI] [PubMed] [Google Scholar]
- 39.Etxeberria A et al. Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity. J. Neurosci 36, 6937–6948 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kougioumtzidou E et al. Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6, e28080 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saab AS et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91, 119–132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Biase LM et al. NMDA Receptor Signaling in Oligodendrocyte Progenitors Is Not Required for Oligodendrogenesis and Myelination. J. Neurosci 31, 12650–12662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibson EM et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 344, 1252304 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demerens C et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci 93, 9887–9892 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hines JH, Ravanelli AM, Schwindt R, Scott EK & Appel B Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci 18, 683–689 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wake H, Lee PR & Fields RD Control of Local Protein Synthesis and Initial Events in Myelination by Action Potentials. Science 333, 1647–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gyllensten L & Malmfors T Myelinization of the Optic Nerve and its Dependence on Visual Function— A Quantitative Investigation in Mice. J. Embryol. Exp. Morphol 11, 255–266 (1963). [PubMed] [Google Scholar]
- 48.Stevens B, Tanner S & Fields R Control of myelination by specific patterns of neural impulses. J. Neurosci 18, 9303–9311 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tauber H, Waehneldt TV & Neuhoff V Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett 16, 235–238 (1980). [DOI] [PubMed] [Google Scholar]
- 50.Fukui Y, Hayasaka S, Bedi KS, Ozaki HS & Takeuchi Y Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. J. Anat 174, 37–47 (1991). [PMC free article] [PubMed] [Google Scholar]
- 51.Colello RJ, Devey LR, Imperato E & Pott U The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J. Neurosci 15, 7665–7672 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrager P & Novakovic SD Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Dev. Brain Res 88, 68–78 (1995). [DOI] [PubMed] [Google Scholar]
- 53.Colello RJ & Pott U Signals that initiate myelination in the developing mammalian nervous system. Mol. Neurobiol 15, 83–100 (1997). [DOI] [PubMed] [Google Scholar]
- 54.Koudelka S et al. Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release. Curr. Biol 26, 1447–1455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ozaki M, Itoh K, Miyakawa Y, Kishida H & Hashikawa T Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J.Neurochem 91, 176–188 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Balkowiec A & Katz DM Cellular Mechanisms Regulating Activity-Dependent Release of Native Brain-Derived Neurotrophic Factor from Hippocampal Neurons. J. Neurosci 22, 10399–10407 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Makinodan M, Rosen KM, Ito S & Corfas G A Critical Period for Social Experience–Dependent Oligodendrocyte Maturation and Myelination. Science 337, 1357–1360 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geraghty AC et al. Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment. Neuron 103, 250–265.e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen TJ et al. In Vivo Regulation of Oligodendrocyte Precursor Cell Proliferation and Differentiation by the AMPA-Receptor Subunit GluA2. Cell Rep. (2018) doi: 10.1016/j.celrep.2018.09.066. [DOI] [PubMed] [Google Scholar]
- 60.Rivers LE et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11, 1392–1401 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vigano F, Mobius W, Gotz M & Dimou L Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci 16, 1370–1372 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Moshrefi-Ravasdjani B et al. Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct. Funct 222, 831–847 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Spitzer SO et al. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 101, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young KM et al. Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonetto G, Kamen Y, Evans KA & Káradóttir RT Unraveling Myelin Plasticity. Front. Cell. Neurosci 14, 156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spitzer SO et al. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 101, 459–471.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moyon S et al. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J. Neurosci 35, 4–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Falcão AM et al. Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nature Medicine vol. 24 1837–1844 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lundgaard I, et al. , Neuregulin and BDNF induce a switch to NMDA receptor- dependent myelination by oligodendrocytes, PLoS Biol. 11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sahel A et al. Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination. Front. Cell. Neurosci 9, 77 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundgaard I et al. Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. 11, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ortiz FC et al. Neuronal activity in vivo enhances functional myelin repair. JCI Insight 5, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ostrom QT et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-Oncol. 18, v1–v75 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu C et al. Mosaic Analysis with Double Markers Reveals Tumor Cell of Origin in Glioma. Cell 146, 209–221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monje M et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc. Natl. Acad. Sci. U. S. A 108, 4453–4458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galvao RP et al. Transformation of quiescent adult oligodendrocyte precursor cells into malignant glioma through a multistep reactivation process. Proc. Natl. Acad. Sci. U. S. A 111, E4214–4223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagaraja S et al. Histone Variant and Cell Context Determine H3K27M Reprogramming of the Enhancer Landscape and Oncogenic State. Mol. Cell 76, 965–980.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alcantara Llaguno SR et al. Adult Lineage-Restricted CNS Progenitors Specify Distinct Glioblastoma Subtypes. Cancer Cell 28, 429–440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugiarto S et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell 20, 328–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nagaraja S et al. Transcriptional Dependencies in Diffuse Intrinsic Pontine Glioma. Cancer Cell 31, 635–652.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Filbin MG et al. Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science 360, 331–335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neftel C et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178, 835–849.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Venkatesh HS et al. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell 161, 803–816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ichtchenko K, Nguyen T & Südhof TC Structures, alternative splicing, and neurexin binding of multiple neuroligins. J. Biol. Chem 271, 2676–2682 (1996). [DOI] [PubMed] [Google Scholar]
- 85.Venkatesh HS et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varoqueaux F et al. Neuroligins determine synapse maturation and function. Neuron 51, 741–754 (2006). [DOI] [PubMed] [Google Scholar]
- 87.Südhof TC Neuroligins and Neurexins Link Synaptic Function to Cognitive Disease. Nature 455, 903–911 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Proctor DT et al. Axo-glial communication through neurexin-neuroligin signaling regulates myelination and oligodendrocyte differentiation. Glia 63, 2023–2039 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Lundgren JL et al. ADAM10 and BACE1 are localized to synaptic vesicles. J. Neurochem 135, 606–615 (2015). [DOI] [PubMed] [Google Scholar]
- 90.Venkatesh HS et al. Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Venkataramani V et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 573, 532–538 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Smith RS & Walsh CA Ion Channel Functions in Early Brain Development. Trends Neurosci. 43, 103–114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishiuchi S et al. Ca2+-Permeable AMPA Receptors Regulate Growth of Human Glioblastoma via Akt Activation. J. Neurosci 27, 7987–8001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T & Sontheimer H Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 67, 9463–9471 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dolma S et al. Inhibition of Dopamine Receptor D4 Impedes Autophagic Flux, Proliferation, and Survival of Glioblastoma Stem Cells. Cancer Cell 29, 859–873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blanchart A et al. Endogenous GABAA receptor activity suppresses glioma growth. Oncogene 36, 777–786 (2017). [DOI] [PubMed] [Google Scholar]
- 97.Tantillo E et al. Differential roles of pyramidal and fast-spiking, GABAergic neurons in the control of glioma cell proliferation. Neurobiol. Dis 141, 104942 (2020). [DOI] [PubMed] [Google Scholar]


