Abstract
Zebra bodies in kidney biopsy specimens are widely accepted as a specific feature of Fabry disease but they can also be present in a drug-induced mimic of Fabry disease, phospholipidosis. Chloroquine and hydroxychloroquine may both induce zebra body formation and kidney phospholipidosis. However, the frequency and clinical significance of such changes remain unknown. We report 5 serial kidney biopsy cases diagnosed as lupus nephritis during hydroxychloroquine administration. All 5 patients exhibited a few, but varying amounts, of zebra bodies in glomerular intrinsic cells, that is, podocytes, parietal epithelial cells, mesangial cells, and endothelial cells. Most of the zebra bodies detected were subtle, though certainly recognizable; these zebra bodies were much smaller than those observed in Fabry disease. Zebra bodies were not observed in patients with lupus nephritis in the absence of chloroquine or hydroxychloroquine administration. All patients with lupus nephritis who received hydroxychloroquine achieved complete remission during continuous use of hydroxychloroquine, though kidney toxicity of drug-induced phospholipidosis might be masked by immunosuppression. Based on this small series of cases, we speculate that the hydroxychloroquine-associated manifestation of zebra bodies and phospholipidosis in the kidney may be frequent phenomena and may have only a subclinical influence on kidney function, at least in the short term.
Index Words: Chloroquine, Fabry disease, hydroxychloroquine, lupus nephritis, phospholipidosis, zebra body
Introduction
Chloroquine and hydroxychloroquine, which were first developed as antimalarial agents, are now used widely for treating systemic lupus erythematosus because of their numerous immunomodulatory functions.1,2 Chloroquine and hydroxychloroquine ameliorate systemic lupus erythematosus by suppressing the activation of Toll-like receptors that are expressed on the surface of endosomes.1,2 At the same time, chloroquine and hydroxychloroquine accumulate in lysosomes, resulting in inhibition of the endosome-lysosome pathway and autophagic flux.2,3 Chloroquine and hydroxychloroquine are effective for systemic lupus erythematosus treatment; however, some serious adverse effects, such as retinopathy, myopathy, and cardiomyopathy, have been reported.1,2,4,5 The mechanism underlying the adverse effects of chloroquine and hydroxychloroquine is associated with their accumulation in lysosomes, which increases their intravesicular pH, resulting in lysosomal enzyme dysfunction and metabolite accumulation, namely phospholipids.1,2,3,6 Experiments investigating chloroquine-associated phospholipidosis have demonstrated the accumulation of zebra bodies and phospholipids in systemic organs.4 Biopsy tissues of patients exhibiting myopathy and cardiomyopathy contain zebra bodies.5 Therefore, the emergence of zebra bodies is assumed to be a sign of chloroquine- and hydroxychloroquine-induced lysosomal dysfunction, which may result in metabolite accumulation and organ damage.
Kidneys are one of the main target organs of systemic lupus erythematosus. Chloroquine and hydroxychloroquine protect against kidney damage in lupus nephritis.1,2,7 Although rare, zebra body accumulation in the kidney has been reported in patients treated with chloroquine and hydroxychloroquine6 (Table S18, 9, 10, 11, 12, 13, 14, 15). Detection of such unusual intracellular structures requires differential diagnosis from Fabry disease, a condition that is typically characterized by zebra bodies. After the exclusion of Fabry disease, potentially useful chloroquine and hydroxychloroquine treatment should be discontinued to avoid organ damage caused by phospholipidosis. However, the prevalence and clinical significance of chloroquine- and hydroxychloroquine-associated zebra bodies in the kidney remain unknown.
We report 5 serial kidney biopsy cases diagnosed as lupus nephritis during hydroxychloroquine treatment. All biopsies contained varying amounts of zebra bodies. These results suggested that zebra body formation and kidney phospholipidosis may be frequently associated with hydroxychloroquine administration.
Case report
Clinical characteristics of the patients at the time of the kidney biopsies and their clinical courses are summarized in Tables 1 and S2. None of the patients had a family history or manifested symptoms associated with Fabry disease (Table S2). In detail, none of the patients had a history of transient ischemic attack or stroke or were aware of neuropathic pain or hearing loss. Electrocardiograms were normal in all patients, and echocardiogram testing in 3 patients did not show apparent thickening of the intraventricular septum and posterior left ventricular wall. Dermatologic assessment indicated no angiokeratoma, and ophthalmic assessment indicated no cornea verticillata. The indications of the biopsies were nephritis (3 patients) and nephrotic syndrome (2 patients). Durations of hydroxychloroquine treatment were 10 days to 4 years. Daily and cumulative dosages of hydroxychloroquine were 4.3 to 7.2 mg/kg of body weight and 3 to 576 g, respectively. Immunosuppressants selected for induction therapy were prednisolone and mycophenolate mofetil in 4 patients and prednisolone, mycophenolate mofetil, and tacrolimus in 1 patient. All patients continued hydroxychloroquine treatment after the kidney biopsy. Urinary protein levels improved in all 5 patients, reaching <0.3 g/g creatinine in 4 patients and <0.5 g/g creatinine in 1 patient.
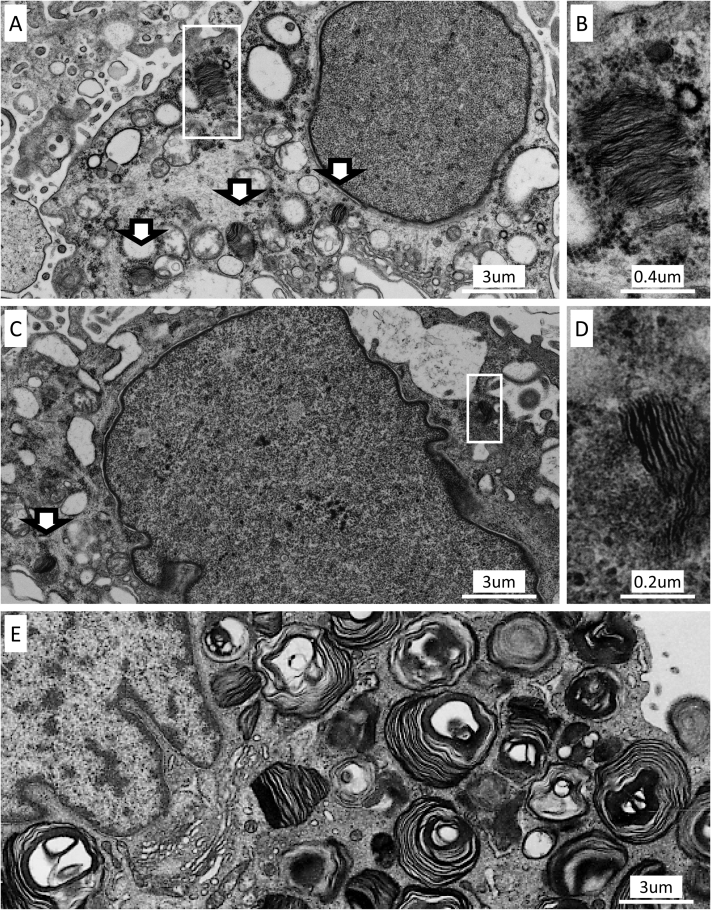
The following diagnoses were made based on biopsies: lupus nephritis class III (2two patients), class IV-S (2 patients), and class IV-S + V (1 patient; Table 1). Zebra bodies were apparent in all 5 patients; 4 in podocytes, 1 in parietal epithelial cells, 3 in mesangial cells, and 1 in endothelial cells. Zebra bodies were not detected in 14 patients with lupus nephritis who were not treated with chloroquine and hydroxychloroquine diagnosed in the same period. Patient 3 exhibited the most prominent zebra bodies (Fig 1A and B). Up to 6 zebra bodies per single cell were observed in multiple podocytes. However, most of the zebra bodies detected were subtle, although certainly recognizable (patient 2, Fig 1C and D). The number of zebra bodies was much lower in hydroxychloroquine-associated patients than in 4 male patients with Fabry disease diagnosed at our institute (Fig 1E). The maximum number of zebra bodies per single cell was 2 to 6 (average, 3.6) in hydroxychloroquine-associated cases, but 59 to 141 (average, 89.5) in Fabry disease cases. In addition, the “size” of zebra bodies was small in hydroxychloroquine-associated cases (Fig 1A and C) compared with Fabry disease cases (Fig 1E).
Table 1.
Clinical Characteristics and Kidney Biopsy Findings of Present Cases
| Case | Age, y/Sex | Diagnosis | HCQ Doses per BW, mg/kg | Duration of HCQ Treatment at Biopsy, mo | Cumulative HCQ Dose at Biopsy, g | Urinary Protein at Biopsy, g/g Cr | Scr at Biopsy, mg/dL | Diagnosis of Kidney Biopsy | Treatment | Observation Period After Biopsy, mo | Urinary Protein After Biopsy, g/g Cr | Scr After Biopsy, mg/dL | Maximum No. of Zebra Bodies per Cell; Cell Type; No. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34/F | SLE | 5.9 | 8 | 63 | 0.35 | 0.54 | III(A/C) | PSL, MMF, HCQ | 8 | 0.17 | 0.78 | Mes; 2 |
| 2 | 51/F | SLE | 5.5 | 28 | 243 | 2.9 | 0.77 | IV-S(A/C) | PSL, MMF, HCQ | 8 | 0.25 | 0.74 | Pod; 3, Endo; 1 |
| 3 | 20/F | SLE, SjS | 4.3 | 4 | 27 | 1.2 | 0.49 | IV-S(A/C) | PSL, MMF, HCQ | 5 | 0 | 0.41 | Pod; 6, Mes; 1 |
| 4 | 34/F | SLE | 7.2 | 48 | 576 | 9.1 | 0.52 | IV-G(A/C)+V | PSL, MMF, Tac, HCQ | 13 | 0.49 | 0.69 | Pod; 5, PEC; 1, Endo 1 |
| 5 | 42/F | SLE, SjS, APS | 5.0 | 0.3 | 3 | 0.45 | 0.71 | III(A/C) | PSL, MMF, HCQ | 11 | 0 | 0.73 | Mes; 2, Pod; 1 |
Abbreviations: APS, antiphospholipid syndrome; BW, body weight; Endo, endothelial cell; F, female; HCQ, hydroxychloroquine; Mes, mesangial cell; MMF, mycophenolate mofetil’ PEC, parietal epithelial cell; Pod, podocyte; PSL, prednisolone; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus; Tac, tacrolimus.
Figure 1.
Ultrastructural findings of the kidney biopsies. (A, B) Patient 3. (A) Podocyte with several zebra bodies (arrowed and framed box around). The maximum number of zebra bodies in a single podocyte was 6 in this patient. (B) High-magnification image of the boxed zebra body. (C, D) Patient 2. (C) Podocyte with subtle zebra bodies (arrowed and framed box around). Most zebra bodies in hydroxychloroquine-treated patients were like these and difficult to detect, though certainly recognizable in a high-magnification image. (D) High-magnification image of the boxed zebra body. (E) Podocyte from a male patient with Fabry disease shows numerous zebra bodies. (A, C, E) Presented in the same magnification, though the size and number of zebra bodies differ between (A, C) hydroxychloroquine-treated patients and (E) patient with Fabry disease.
Vesicles with osmiophilic granules were observed in the fragmented podocytes in 2 cases (Fig S1A) and in proximal tubular epithelial cells in all cases (Fig S1B). These osmiophilic granules were surrounded by a single-unit membrane, which indicated their presence in lysosome-associated pathways. Curvilinear body, another unusual intracellular structure that emerges in phospholipidosis,8,9,12 was not observed.
Discussion
We report 5 serial kidney biopsies of patients with lupus nephritis during hydroxychloroquine treatment. All patients exhibited zebra bodies, and to our knowledge, this is the first report of their high prevalence. However, the number and size of zebra bodies formed during hydroxychloroquine treatment were far smaller than those in patients with Fabry disease. These findings might be consistent with a previous review,6, p. 242 which specified that “podocyte inclusions are more numerous in Fabry disease.” Clinically, all patients continued hydroxychloroquine treatment and achieved complete remission. This makes it difficult to determine the clinical significance of hydroxychloroquine-associated zebra bodies in lupus nephritis.
Chloroquine and hydroxychloroquine are cationic amphiphilic drugs that have an affinity for components of the endosome-lysosome pathway and autophagic flux because of their relatively low electron potential.1, 2, 3 They accumulate in lysosomes, thereby increasing their intravesicular pH, and inhibit various lysosomal enzymes, including α-galactosidase and phospholipases A and C.1, 2, 3 Alpha-galactosidase is the causative enzyme for Fabry disease. Absence or reduced α-galactosidase activity results in the accumulation of glycosphingolipids, a subtype of glycolipids, which ultrastructurally form zebra bodies.6,8, 9, 10, 11, 12, 13, 14, 15 Phospholipases A and C metabolize phospholipids, and their reduced activity results in an accumulation of osmiophilic phospholipids.8,10,13,16 Therefore, inhibition of these enzymes by chloroquine and hydroxychloroquine is presumed to result in the formation of zebra bodies and osmiophilic granules.
In previous studies investigating zebra bodies during chloroquine or hydroxychloroquine treatment by kidney biopsy, enzyme activity or genetic analyses were performed in 7 of the 8 patients 8, 9, 10, 11, 12, 13, 14, 15 (Table S1). All tested patients did not have Fabry disease.8, 9, 10, 11, 12,14,15 In 1 patient who did not undergo enzyme activity or genetic analyses, a repeat kidney biopsy was performed after the cession of hydroxychloroquine treatment, which indicated the disappearance of zebra bodies.13 In our cases, the patients lacked family histories and symptoms suggestive of Fabry disease, including cornea verticillata, which is reported to be observed in most women with Fabry disease.17 Further, based on the small number and size of the zebra bodies, a likely diagnosis of hydroxychloroquine-associated phospholipidosis was made.6 However, because genetic analysis is the only method to confirm the diagnosis of female Fabry disease and Fabry disease shows various phenotypic variants, genetic analysis should be considered if possible.
Histologic differences between hydroxychloroquine-associated kidney phospholipidosis and Fabry disease remain to be clarified. The existence of curvilinear bodies has been reported to be a distinguishing factor.5,8,9,12 However, not all previously reported cases10,11,13, 14, 15 and none of the present cases exhibited curvilinear bodies. Another histologic finding was the presence of intracellular vesicles with osmiophilic granules in the cytoplasm of some podocytes (Fig S1A) and all proximal tubular epithelial cells (Fig S1B). In our cases, these vesicles containing osmiophilic granules were detected in only hydroxychloroquine-associated cases, but not in lupus nephritis without chloroquine and hydroxychloroquine use or Fabry disease. We speculate that these osmiophilic granules represent phospholipids accumulated due to drug-induced lysosomal enzyme disfunction.8,9,16 Moreover, another cationic amphiphilic drug, amiodarone, is reported to cause zebra bodies and vesicles containing osmiophilic granules.18,19 Because tubular secretion eliminates 40% to 70% of orally administered chloroquine and hydroxychloroquine,1,20 it is likely that these drugs accumulate in proximal tubular epithelial cells, resulting in further lysosomal dysfunction. In contrast to rare and small zebra bodies in glomeruli, vesicles containing osmiophilic granules in proximal tubular epithelial cells might easily distinguish hydroxychloroquine-associated phospholipidosis from Fabry disease.
The clinical significance of hydroxychloroquine-associated zebra bodies and kidney phospholipidosis is another unclarified issue. Of the 8 patients—in previous reports—who exhibited zebra bodies during chloroquine and hydroxychloroquine treatment8, 9, 10, 11, 12, 13, 14, 15 (Table S1), only 1 showed apparent improvement in kidney function on suspension of chloroquine treatment alone.8 Three patients with suspended chloroquine or hydroxychloroquine therapy did not show apparent improvement.10, 11, 12 In 1 patient, in whom improvement of proteinuria might have been associated with angiotensin-converting enzyme inhibitor administration, the zebra bodies disappeared after ceasing hydroxychloroquine treatment.13
These previous reports suggest that the zebra bodies—a sign of phospholipid accumulation—do not necessarily decrease kidney function. In our cases, the formation of a small number of tiny zebra bodies might be a subclinical phenomenon, at least in the short term, though its kidney toxicity might be masked by immunosuppression. Because chloroquine and hydroxychloroquine can effectively prevent systemic lupus erythematosus flare-ups and prevent kidney damage in lupus nephritis,1,2,7 continuous administration of these drugs might benefit patients even if the kidney biopsy reveals zebra body formation and phospholipidosis.
In summary, we report 5 patients with lupus nephritis treated with hydroxychloroquine who exhibited a small number of tiny zebra bodies and kidney phospholipidosis. Manifestation of hydroxychloroquine-associated zebra bodies might be a frequent and subclinical phenomenon. This suggests that it is not necessary to cease potentially beneficial hydroxychloroquine therapy. Further studies are required to determine the true prevalence and clinical significance of hydroxychloroquine-associated zebra bodies and kidney phospholipidosis.
Article Information
Authors’ Full Names and Academic Degrees
Shun Manabe, MD, Toshio Mochizuki, MD, PhD, Masayo Sato, MD, PhD, Hiroshi Kataoka, MD, PhD, Sekiko Taneda, MD, PhD, Kazuho Honda, MD, PhD, Keiko Uchida, MD, PhD, and Kosaku Nitta, MD, PhD.
Support
None.
Financial Disclosure
Dr Mochizuki received travel fees and honoraria for lectures from Otsuka Pharmaceutical. Drs Mochizuki and Kataoka belong to an endowed department sponsored by Otsuka Pharmaceutical, Chugai Pharmaceutical, Kyowa Hakko Kirin, and JMS.
Acknowledgements
We gratefully acknowledge the technical assistance of Mr Shigeru Horita, Mr Hideki Nakayama, and Ms Mayumi Ohno.
Patient Protections
The authors declare that they have obtained consent from each patient reported in this article for publication of the information about him/her that appears within this Case Report and any associated supplementary material.
Peer Review
Received September 1, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form January 17, 2021.
Footnotes
Complete author and article information provided before references.
Figure S1. Ultrastructural findings of the kidney biopsy
Table S1. Clinical characteristics and kidney biopsy findings for previously reported cases
Table S2. Clinical characteristics, kidney biopsy findings, and assessment for Fabry disease for the present cases
Supplementary Material
Figure S1; Tables S1-S2.
References
- 1.Shippey E.A., Wagler V.D., Collamer A.N. Hydroxychloroquine: an old drug with new relevance. Cleve Clin J Med. 2018;85(6):459–467. doi: 10.3949/ccjm.85a.17034. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G., Ramos-Casals M., Brito-Zeron P., Khamashta M.A. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 3.Galluzzi L., Bravo-San Pedro J.M., Levine B., Green D.R., Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16(7):487–511. doi: 10.1038/nrd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahon G.J., Anderson H.R., Gardiner T.A. Chloroquine causes lysosomal dysfunction in neural retina and RPE: implications for retinopathy. Curr Eye Res. 2004;28(4):277–284. doi: 10.1076/ceyr.28.4.277.27835. [DOI] [PubMed] [Google Scholar]
- 5.Tönnesmann E., Stroehmann I., Kandolf R. Cardiomyopathy caused by longterm treatment with chloroquine: a rare disease, or a rare diagnosis? J Rheumatol. 2012;39(5):1099–1103. doi: 10.3899/jrheum.110959. [DOI] [PubMed] [Google Scholar]
- 6.Loh A.H., Cohen A.H. Drug-induced kidney disease – pathology and current concepts. Ann Acad Med Singapore. 2009;38(3):240–250. [PubMed] [Google Scholar]
- 7.Pons-Estel G.J., Alarcón G.S., McGwin G., Jr. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: LXV, data from a multiethnic US cohort. Arthritis Rheum. 2009;61(6):830–839. doi: 10.1002/art.24538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller-Höcker J., Schmid H., Weiss M., Dendorfer U., Braun G.S. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry's disease: case report and review of the literature. Hum Pathol. 2003;34(3):285–289. doi: 10.1053/hupa.2003.36. [DOI] [PubMed] [Google Scholar]
- 9.Albay D., Adler S.G., Philipose J. Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol. 2005;18(5):733–738. doi: 10.1038/modpathol.3800344. [DOI] [PubMed] [Google Scholar]
- 10.Bracamonte E.R., Kowalewska J., Starr J., Gitomer J., Alpers C.E. Iatrogenic phospholipidosis mimicking Fabry disease. Am J Kidney Dis. 2006;48(5):844–850. doi: 10.1053/j.ajkd.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 11.Woywodt A., Hellweg S., Schwarz A., Schaefer R.M., Mengel M. A wild zebra chase. Nephrol Dial Transplant. 2007;22(10):3074–3077. doi: 10.1093/ndt/gfm462. [DOI] [PubMed] [Google Scholar]
- 12.Costa R.M., Martul E.V., Reboredo J.M., Cigarrán S. Curvilinear bodies in hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease. Clin Kidney J. 2013;6(5):533–536. doi: 10.1093/ckj/sft089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Menezes Neves P.D.M., Machado J.R., Custódio F.B. Ultrastructural deposits appearing as “zebra bodies” in renal biopsy: Fabry disease?– comparative case reports. BMC Nephrol. 2017;18(1):157. doi: 10.1186/s12882-017-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bojic M., Kozakowski N., Bécède M., Kerschbaumer A., Bobacz K. Myeloid bodies in the kidney biopsy of a patient with systemic lupus erythematosus. Kidney Int. 2017;92(1):271–272. doi: 10.1016/j.kint.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 15.Sperati C.J., Rosenberg A.Z. Hydroxychloroquine-induced mimic of renal Fabry disease. Kidney Int. 2018;94(3):634. doi: 10.1016/j.kint.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzawa Y., Hostetler K. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4’-bis alpha, beta-diethyldiphenylethane. J Biol Chem. 1980;255(11):5190–5194. [PubMed] [Google Scholar]
- 17.Sodi A., Ioannidis A.S., Mehta A. Ocular manifestations of Fabry’s disease: data from the Fabry Outcome Survey. Br J Ophthalmol. 2007;91(2):210–2114. doi: 10.1136/bjo.2006.100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guigui B., Perrot S., Berry J.P. Amiodarone-induced hepatic phospholipidosis: a morphological alteration independent of pseudoalcoholic liver disease. Hepatology. 1998;8(5):1063–1068. doi: 10.1002/hep.1840080514. [DOI] [PubMed] [Google Scholar]
- 19.Delage C., Lagace R., Huard J. Pseudocyanotic pigmentation of the skin induced by amiodarone: a light and electron microscopic study. Can Med Assoc J. 1975;112(10):1205–1208. [PMC free article] [PubMed] [Google Scholar]
- 20.Gustafsson L.L., Walker O., Alvan G. Disposition of chloroquine in man after single intravenous and oral doses. Br J Clin Pharmacol. 1983;15(4):471–479. doi: 10.1111/j.1365-2125.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Tables S1-S2.