Abstract
Objectives
This case–control study was aimed to investigate associations between HBV infection and extrahepatic digestive system cancers.
Methods
The patients of gastric, small intestinal, colonic, rectal, anal, biliary tract, and pancreatic cancers were retrospectively collected between 2016.5 and 2017.12. Simultaneously, the healthy controls were collected from the health check‐up registry, and cancer‐free status was confirmed based on medical records. Propensity score matching was performed to reduce bias. Multinomial logit model and conditional logistic regression model were used to assess the risk of individual cancer according to HBV serological markers and classifications.
Results
Totally, 4748 patients involving seven cancers, and 57,499 controls were included. After matching, HBsAg was associated with increased risk of gastric cancer (aOR = 1.39, 95% CI: 1.05–1.85), and anti‐HBs served as a protective factor for gastric (aOR = 0.72, 95% CI: 0.61–0.85), colonic (aOR = 0.73, 95% CI: 0.60–0.89), rectal (aOR = 0.73, 95% CI: 0.63–0.85), and pancreatic (aOR = 0.58, 95% CI: 0.42–0.82) cancers. Compared to subgroups with non‐infection and vaccination status, inactive HBsAg carriers and active HBV infection subgroup were correlated with gastric carcinogenesis (aOR = 1.41, 95% CI: 1.03–1.93). However, no clear association was found between HBV infection and other cancers.
Conclusions
HBV infection was potentially associated with an increased risk of gastric cancer. The development mechanism of HBV‐associated gastric cancer needs to investigate further.
Keywords: case–control study, digestive system cancer, hepatitis B virus, infection, oncovirus
This case–control study was aimed to investigate associations between HBV infection and extrahepatic digestive system cancers. Our study revealed that HBV infection was potentially associated with an increased risk of gastric cancer. However, because of the limited ability to establish a causal relationship for case–control study, large‐scale prospective cohort studies are urgencies, and the latent mechanism about HBV extrahepatic carcinogenesis needs to be investigated as well.
1. INTRODUCTION
It has been well known that several infectious oncoviruses are carcinogenic for specific human cancers. 1 , 2 , 3 As one of eleven established carcinogenic agents (group 1) by the International Agency for Research on Cancer, hepatitis B virus (HBV) infection accounts for around 45% of cases of primary hepatocellular carcinoma (HCC). 4 , 5 , 6 Its causal link to HCC has been investigated thoroughly and the natural history has been recognized. HBV carriers may progress to cirrhosis and HCC with chances. 7 Since the existence of HBV was detected in some extrahepatic tissues (including pancreas, bile duct, stomach, etc.), several studies were conducted to illuminate a potential role in the development of extrahepatic cancers. 8 , 9 , 10 , 11 , 12 Due to the blood vessels and bile ducts shared with the liver, the pancreas is vulnerable to viral hepatitis and serves as a potential reservoir of hepatitis viruses. 13 Meanwhile, cholangiocarcinoma may share similar processes for HBV‐related carcinogenesis which originates from hepatic progenitor cells. 14 Likewise, associations between HBV infection and various extrahepatic cancers were observed in several epidemiological studies which could be explained by the mobility of HBV through the bloodstream. 15 , 16 , 17
As one of the HBV‐endemic areas, China is under a substantial disease burden resulted from HBV infection. Particularly, several studies implicated the HBV involvement in the onset of digestive system cancers including cholangiocarcinoma, pancreatic cancer, gastric cancer, small intestinal adenocarcinoma, etc. 18 , 19 However, the associations between HBV infection and the development of extrahepatic digestive system cancers were rarely described, and inconsistency was found because of the differences in study design, populations investigated, and the incidences of cancers or hepatitis. 20 , 21 , 22 Compared to the well‐established association between HBV infection and HCC, the relationship between HBV infection and extrahepatic cancers in the abdominal digestive system was not systematically observed. Thus, we conducted this hospital‐based case–control study to investigate whether there were associations between HBV infection and extrahepatic digestive system cancers.
2. METHODS
2.1. Study population and eligibility
This hospital‐based case–control study was performed in the West China Hospital, Sichuan University, a joint with the Sichuan Gastric Cancer Early Detection and Screening (SIGES) research project. 23 The patients with gastric, small intestinal, colonic, rectal, anal, biliary tract, and pancreatic cancers (n = 5105) were retrospectively searched according to the International Classification of Diseases‐10 (ICD‐10) codes from the electronic inpatient registry between 2016.5 and 2017.12. For patients with multiple cancers, only the first reported cancer type was included as the primary cancer. Included patient whose different cancers were recognized simultaneously was treated as different cases. For the same patients hospitalized repeatedly for the same cancer, only data of newly diagnosed were collected. The panel of HBV serology included hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti‐HBs), hepatitis B e antigen (HBeAg), antibody to HBeAg (anti‐HBe), and antibody to hepatitis B core antigen (anti‐HBc). After excluding patients without pathological diagnosis and test results of the panel of HBV serology (n = 67), medical records were manually retrospective collected for consecutive hospitalized adult patients (≥18 years old) newly diagnosed with selected extrahepatic digestive system cancers (n = 5038). Anatomic locations of extrahepatic digestive system cancers were confirmed by ICD‐10 codes, imaging examination results, and/or surgical operation records. All patients of cancers were newly diagnosed with digestive system carcinoma according to pathologic examination, whereas patients with other histologic types, such as melanoma, sarcoma, lymphoma, gastrointestinal stromal tumor, and neuroendocrine neoplasm were excluded (n = 144).
During the same period, consecutive adult controls (≥18 years old, n = 59,496) who undergone HBV serological tests were collected from the health check‐up registry in the West China Hospital, Sichuan University. Their cancer‐free status was confirmed manually by the medical records including present and past medical history, and individuals with histories of cancer were excluded (n = 200).
The information including sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, family history of cancers, and results of HBV serology was collected in all observations. Any observation missing any medical record mentioned above was excluded in cancers (n = 146) and controls (n = 1797). Finally, 4748 inpatients of extrahepatic digestive system cancers including gastric (n = 1356), small intestinal (n = 111), colonic (n = 977), rectal (n = 1523), anal (n = 89), biliary tract (n = 352), and pancreatic cancer (n = 340), and 57,499 cancer‐free outpatients were included in this study (Figure 1).
FIGURE 1.
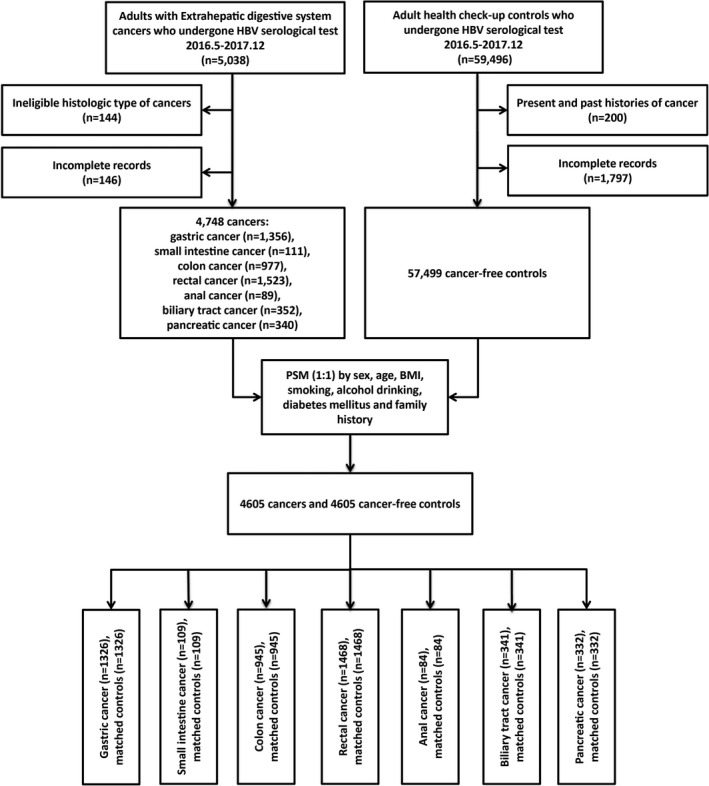
The flowchart of this hospital‐based case–control study
2.2. Ethics
This hospital‐based case–control study retrospectively collected the information of baselines and serologic results of HBV markers. The SIGES study was approved by the Biomedical Ethical Committee of West China Hospital, Sichuan University (id: 2015–151‐V2, 2018–215‐V1). The informed consent was waived by the approval of the Biomedical Ethical Committee because of the retrospective nature. The private information was anonymized when analyzing and reporting data.
2.3. Laboratory tests
The presence of HBsAg in the blood indicates HBV infection. Following seroclearance of HBsAg, the appearance of anti‐HBs confers protection from HBV infection. Patients immunized after vaccination could be characterized by the presence of anti‐HBs as well. Seroprevalence of HBeAg reflects high levels of viral DNA replication and infectivity, and the presence of anti‐HBe represents host immune activation response to HBeAg and usually indicates decreasing HBV DNA and infectivity. Detection of anti‐HBc indicates previous HBV exposure. 24 , 25 , 26 In our study, Electrochemiluminescence immunoassay (ECLIA) was used to test for the presence of five HBV‐related antibodies and antigens mentioned above. Meanwhile, tumor marker tests (including CA125, CA19‐9, CEA, and AFP) were performed for partial participants using ECLIA.
According to the WHO guideline and the clinical significance on the basis of HBV serology status, the observations were classified into three subgroups as follows: (a) Group A characterized by HBsAg–, HBeAg–, anti‐HBe– and anti‐HBc–, without or with anti‐HBs+ (non‐infection and vaccination); (b) Group B referred to HBsAg–/HBeAg–, and at least one of anti‐HBe+or anti‐HBc+, regardless of anti‐HBs+or not (resolved HBV infection); (c) Group C featured with HBsAg+/HBeAg±, regardless of the status of anti‐HBs, anti‐HBe, and anti‐HBc (inactive HBsAg carriers and active HBV infection). 24 , 25 , 26
2.4. Statistical analysis
Three categories were classified on the basis of BMI: <23.0 kg/m2, 23.0–29.9 kg/m2, and ≥30.0 kg/m2. 27 The Student's t‐test was used to compare continuous variables, and Fisher's exact test or Chi‐square test was used to assess categorical variables, respectively. 4748 patients in the case group were randomly matched with 57499 cancer‐free controls, and traditional propensity score matching was conducted to balance covariates (including sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers) and reduce bias, using a nearest‐neighbor algorithm by 1:1 matching with a caliper width of 0.25 (Figure 1). 28 Standardized mean differences (SMDs) of covariates were estimated to evaluate the pre‐match imbalance and post‐match balance, and Love plots were presented and absolute SMDs<10% were considered inconsequential. 29 Univariate or multivariate multinomial logit models were used to assess the risk associations between HBV serological markers or classifications and each cancer before matching. Re‐analyses based on matched datasets were conducted using a conditional logistic regression model for each cancer. Five HBV serological markers and three classifications were included for multivariate analyses adjusted by sex, age, BMI, smoking, alcohol drinking, diabetes mellitus, and family history of cancers. Adjusted odds ratios (aOR) and 95% confidence intervals (CIs) were estimated.
Data analyses were performed using the software R, version 3.6.0 (R Project for Statistical Computing), the multinomial logit model, propensity score matching, Love plots, conditional logistic model, and the forest plots were conducted with the packages of “mlogit,” “MatchIt,” “cobalt,” “survival,” and “forestplot.” 18 , 30 , 31 , 32 , 33 , 34 In all analyses, a two‐tailed p values <0.05 was considered as statistically significant.
3. RESULTS
3.1. Characteristics of study population
Basic characteristics of extrahepatic digestive system cancers and cancer‐free controls are shown in Table 1. Significant differences were observed between cancers and control populations in terms of sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers (p < 0.05). The seroprevalence of HBsAg was 8.4% in cancer cases and 8.3% in non‐cancer controls. As for individual cancer, serum positive rates of HBsAg in gastric, small intestinal, colonic, rectal, anal, biliary tract, and pancreatic cancers were 10.0%, 9.9%, 8.0%, 7.8%, 7.9%, 6.8%, and 6.5%, respectively, and the seroprevalence of anti‐HBc hold the largest proportion in all seven cancers. Similarly, most subjects both in each cancer case and controls were Group B (resolved HBV infection) (Table S1 and Figure 2).
TABLE 1.
Basic characteristics of extrahepatic digestive system cancers and cancer‐free controls before and after matching
| Variables | Unmatched dataset | Matched dataset | ||||
|---|---|---|---|---|---|---|
|
Cancers (n = 4748) No. (%) |
Controls (n = 57499) No. (%) |
p |
Cancers (n = 4605) No. (%) |
Controls (n = 4605) No. (%) |
p | |
| Sex | <0.001 | 0.949 | ||||
| Female | 1824 (38.4) | 27141 (47.2) | 1805 (39.2) | 1801 (39.1) | ||
| Male | 2924 (61.6) | 30358 (52.8) | 2800 (60.8) | 2804 (60.9) | ||
| Age (mean ± SD), years | 59.99±12.32 | 45.01±12.16 | <0.001 | 59.40±12.00 | 58.83±12.96 | 0.026 |
| BMI (kg/m2) | <0.001 | 0.772 | ||||
| <23.0 | 2689 (56.6) | 25408 (44.2) | 2548 (55.3) | 2516 (54.6) | ||
| 23.0–29.9 | 1972 (41.5) | 30232 (52.6) | 1971 (42.8) | 2005 (43.5) | ||
| ≥30.0 | 87 (1.8) | 1859 (3.2) | 86 (1.9) | 84 (1.8) | ||
| Smoker | <0.001 | 0.135 | ||||
| Never | 2916 (61.4) | 43951 (76.4) | 2888 (62.7) | 2958 (64.2) | ||
| Previous/Current | 1832 (38.6) | 13548 (23.6) | 1717 (37.3) | 1647 (35.8) | ||
| Alcohol drinker | <0.001 | 0.535 | ||||
| Never | 3969 (83.6) | 51028 (88.7) | 3842 (83.4) | 3865 (83.9) | ||
| Previous/Current | 779 (16.4) | 6471 (11.3) | 763 (16.6) | 740 (16.1) | ||
| Diabetes mellitus (yes) | 167 (3.5) | 1490 (2.6) | <0.001 | 166 (3.6) | 158 (3.4) | 0.692 |
| Family history of cancers (yes) | 667 (14.0) | 7419 (12.9) | 0.026 | 651 (14.1) | 615 (13.4) | 0.290 |
| HBV markers | ||||||
| HBsAg positive | 397 (8.4) | 4752 (8.3) | 0.837 | 389 (8.4) | 336 (7.3) | 0.044 |
| Anti‐HBs positive | 2754 (58.0) | 37727 (65.6) | <0.001 | 2667 (57.9) | 3022 (65.6) | <0.001 |
| HBeAg positive | 23 (0.5) | 333 (0.6) | 0.464 | 23 (0.5) | 11 (0.2) | 0.059 |
| Anti‐HBe positive | 1488 (31.3) | 16968 (29.5) | 0.008 | 1439 (31.2) | 1461 (31.7) | 0.638 |
| Anti‐HBc positive | 3437 (72.4) | 33763 (58.7) | <0.001 | 3318 (72.1) | 3255 (70.7) | 0.153 |
| Classifications a | <0.001 | 0.057 | ||||
| Group A | 1300 (27.4) | 23669 (41.2) | 1276 (27.7) | 1345 (29.2) | ||
| Group B | 3051 (64.3) | 29078 (50.6) | 2940 (63.8) | 2924 (63.5) | ||
| Group C | 397 (8.4) | 4752 (8.3) | 389 (8.4) | 336 (7.3) | ||
(1) Group A characterized by HBsAg–, HBeAg–, anti‐HBe– and anti‐HBc–, without or with anti‐HBs+ (non‐infection and vaccination); (2) Group B referred to HBsAg–/HBeAg–, and at least one of anti‐HBe+or anti‐HBc+, regardless of anti‐HBs+or not (resolved HBV infection); (3) Group C featured with HBsAg+/HBeAg±, regardless of the status of anti‐HBs, anti‐HBe and anti‐HBc (inactive HBsAg carriers and active HBV infection).
FIGURE 2.
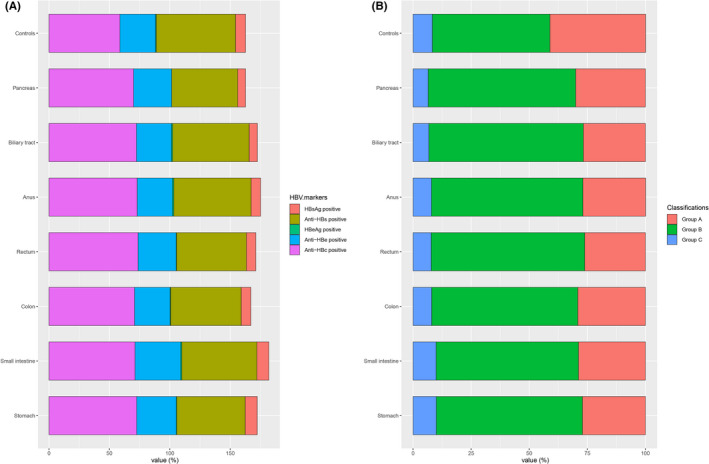
Positive rate of five HBV serological markers and three HBV serologic classifications by tumor site among cancer patients and non‐cancer subjects before matching. (A) Positive rate of five HBV serological markers; (B) Positive rate of three HBV serologic classifications
After matching, there was no substantial difference between cancer cases and controls in terms of sex, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers (p > 0.05) (Table 1 and Figure 3). As for individual cancer, the seroprevalence of HBsAg was higher only in gastric cancer compared with matched controls (p = 0.017). A significant difference between gastric cancer and controls was also observed in terms of HBV serologic classifications (p = 0.036). In addition, the serum positive rates of anti‐HBs were lower in gastric, colonic, rectal, and pancreatic cancers than those in matched controls (p < 0.05) (Table S2).
FIGURE 3.

Standardized mean differences of covariates before and after matching
3.2. Associations between HBV serology status and each extrahepatic digestive system cancer before matching
In univariate analysis, HBsAg (OR = 1.24, 95% CI: 1.03–1.48) and anti‐HBe (OR = 1.16, 95% CI: 1.04–1.30) were associated with increased risk of gastric cancer only. Anti‐HBc was the risk factor for all seven cancers. On the contrary, anti‐HBs was statistically associated with decreased risk of gastric (OR = 0.68, 95% CI: 0.61–0.76), colonic (OR = 0.74, 95% CI: 0.65–0.84), rectal (OR = 0.72, 95% CI: 0.65–0.80), and pancreatic (OR = 0.63, 95% CI: 0.51–0.78) cancers. In terms of HBV serologic classifications, the prevalence of group B in all seven cancers and group C in gastric, colonic, rectal cancers were significantly higher compared with group A (Table S3).
In multivariable analysis, there was a significant association between HBsAg positivity and gastric cancer compared with cancer‐free controls (aOR = 1.30, 95% CI: 1.08–1.57), and HBeAg was the risk factor for colonic (aOR = 2.49, 95% CI: 1.01–6.18) and biliary tract (aOR = 4.25, 95% CI: 1.33–13.60) cancers. Similarly, anti‐HBs was statistically associated with decreased risk of gastric (aOR = 0.70, 95% CI: 0.62–0.78), colonic (aOR = 0.74, 95% CI: 0.65–0.84), rectal (aOR = 0.73, 95% CI: 0.65–0.81), and pancreatic (aOR = 0.63, 95% CI: 0.51–0.79) cancers. In terms of HBV serologic classifications, the prevalence of group C in gastric cancer was significantly higher compared with group A (aOR = 1.35, 95% CI: 1.10–1.66) (Table 2, Figure 4A and Figure 5A).
TABLE 2.
Associations between HBV serology status and extrahepatic digestive system cancers: multivariate analyses adjusted by age, sex, BMI, smoking, alcohol drinking, diabetes mellitus, and family history of cancers according to tumor location before and after matching
| Variables | Unmatched datasets | Matched datasets | ||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p | aOR | 95% CI | p | |
| Gastric cancer | ||||||
| HBsAg (+/−) | 1.30 | 1.08–1.57 | 0.005 | 1.39 | 1.05–1.85 | 0.024 |
| Anti‐HBs (+/−) | 0.70 | 0.62–0.78 | <0.001 | 0.72 | 0.61–0.85 | <0.001 |
| HBeAg (+/−) | 2.03 | 0.94–4.37 | 0.072 | 1.72 | 0.41–7.28 | 0.461 |
| Anti‐HBe (+/−) | 1.02 | 0.90–1.15 | 0.770 | 0.94 | 0.79–1.12 | 0.496 |
| Anti‐HBc (+/−) | 1.09 | 0.96–1.23 | 0.190 | 1.04 | 0.87–1.25 | 0.660 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 1.06 | 0.93–1.21 | 0.376 | 1.02 | 0.85–1.23 | 0.846 |
| Group C | 1.35 | 1.10–1.66 | 0.004 | 1.41 | 1.03–1.93 | 0.034 |
| Small intestinal cancer | ||||||
| HBsAg (+/−) | 1.34 | 0.72–2.51 | 0.355 | 1.03 | 0.35–2.98 | 0.960 |
| Anti‐HBs (+/−) | 0.86 | 0.59–1.27 | 0.458 | 0.71 | 0.38–1.33 | 0.284 |
| HBeAg (+/−) | 3.46 | 0.48–25.19 | 0.220 | — | — | — |
| Anti‐HBe (+/−) | 1.32 | 0.90–1.94 | 0.156 | 1.10 | 0.57–2.12 | 0.786 |
| Anti‐HBc (+/−) | 1.14 | 0.75–1.74 | 0.543 | 0.66 | 0.33–1.31 | 0.230 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 1.09 | 0.71–1.68 | 0.692 | 0.63 | 0.31–1.29 | 0.207 |
| Group C | 1.42 | 0.71–2.84 | 0.316 | 0.79 | 0.25–2.50 | 0.690 |
| Colonic cancer | ||||||
| HBsAg (+/−) | 1.08 | 0.85–1.37 | 0.524 | 1.03 | 0.72–1.46 | 0.878 |
| Anti‐HBs (+/−) | 0.74 | 0.65–0.84 | <0.001 | 0.73 | 0.60–0.89 | 0.002 |
| HBeAg (+/−) | 2.49 | 1.01–6.18 | 0.048 | 1.59 | 0.39–6.44 | 0.518 |
| Anti‐HBe (+/−) | 0.88 | 0.77–1.02 | 0.088 | 0.91 | 0.74–1.11 | 0.352 |
| Anti‐HBc (+/−) | 0.95 | 0.82–1.10 | 0.471 | 0.93 | 0.76–1.15 | 0.520 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 0.93 | 0.81–1.08 | 0.360 | 0.92 | 0.75–1.14 | 0.457 |
| Group C | 1.03 | 0.80–1.33 | 0.820 | 0.97 | 0.67–1.42 | 0.892 |
| Rectal cancer | ||||||
| HBsAg (+/−) | 1.03 | 0.85–1.25 | 0.764 | 1.09 | 0.81–1.46 | 0.563 |
| Anti‐HBs (+/−) | 0.73 | 0.65–0.81 | <0.001 | 0.73 | 0.63–0.85 | <0.001 |
| HBeAg (+/−) | 1.77 | 0.77–4.06 | 0.176 | 8.30 | 0.70–98.18 | 0.093 |
| Anti‐HBe (+/−) | 0.97 | 0.87–1.09 | 0.600 | 0.98 | 0.83–1.15 | 0.801 |
| Anti‐HBc (+/−) | 1.10 | 0.98–1.25 | 0.105 | 1.06 | 0.90–1.26 | 0.487 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 1.11 | 0.98–1.25 | 0.106 | 1.06 | 0.89–1.25 | 0.541 |
| Group C | 1.11 | 0.89–1.37 | 0.358 | 1.13 | 0.83–1.55 | 0.444 |
| Anal cancer | ||||||
| HBsAg (+/−) | 1.04 | 0.48–2.26 | 0.918 | 0.71 | 0.15–3.38 | 0.662 |
| Anti‐HBs (+/−) | 0.95 | 0.61–1.46 | 0.807 | 0.69 | 0.31–1.55 | 0.371 |
| HBeAg (+/−) | 5.13 | 0.70–37.63 | 0.108 | — | — | — |
| Anti‐HBe (+/−) | 0.87 | 0.55–1.38 | 0.558 | 1.58 | 0.65–3.86 | 0.318 |
| Anti‐HBc (+/−) | 1.07 | 0.66–1.73 | 0.780 | 1.10 | 0.49–2.49 | 0.816 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 1.06 | 0.65–1.72 | 0.821 | 1.15 | 0.50–2.65 | 0.738 |
| Group C | 1.08 | 0.46–2.52 | 0.855 | 0.77 | 0.15–3.95 | 0.750 |
| Biliary tract cancer | ||||||
| HBsAg (+/−) | 0.93 | 0.61–1.41 | 0.721 | 1.36 | 0.70–2.63 | 0.362 |
| Anti‐HBs (+/−) | 0.91 | 0.73–1.13 | 0.379 | 0.80 | 0.57–1.13 | 0.204 |
| HBeAg (+/−) | 4.25 | 1.33–13.60 | 0.015 | 12.57 | 0.60–262.07 | 0.102 |
| Anti‐HBe (+/−) | 0.87 | 0.69–1.10 | 0.247 | 1.03 | 0.72–1.46 | 0.885 |
| Anti‐HBc (+/−) | 1.06 | 0.83–1.35 | 0.644 | 1.18 | 0.82–1.70 | 0.360 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 1.11 | 0.87–1.42 | 0.414 | 1.23 | 0.85–1.78 | 0.278 |
| Group C | 0.99 | 0.63–1.57 | 0.980 | 1.59 | 0.77–3.25 | 0.208 |
| Pancreatic cancer | ||||||
| HBsAg (+/−) | 0.83 | 0.54–1.29 | 0.418 | 0.75 | 0.41–1.37 | 0.344 |
| Anti‐HBs (+/−) | 0.63 | 0.51–0.79 | <0.001 | 0.58 | 0.42–0.82 | 0.002 |
| HBeAg (+/−) | — | — | — | — | — | — |
| Anti‐HBe (+/−) | 0.97 | 0.77–1.22 | 0.781 | 0.99 | 0.70–1.41 | 0.964 |
| Anti‐HBc | 0.96 | 0.76–1.22 | 0.739 | 0.78 | 0.55–1.10 | 0.155 |
| Classifications a | ||||||
| Group A | 1.00 | Reference | 1.00 | Reference | ||
| Group B | 0.97 | 0.76–1.23 | 0.795 | 0.80 | 0.56–1.13 | 0.202 |
| Group C | 0.82 | 0.51–1.30 | 0.394 | 0.64 | 0.33–1.23 | 0.176 |
(1) Group A characterized by HBsAg–, HBeAg–, anti‐HBe– and anti‐HBc–, without or with anti‐HBs+ (non‐infection and vaccination); (2) Group B referred to HBsAg–/HBeAg–, and at least one of anti‐HBe+or anti‐HBc+, regardless of anti‐HBs+or not (resolved HBV infection); (3) Group C featured with HBsAg+/HBeAg±, regardless of the status of anti‐HBs, anti‐HBe and anti‐HBc (inactive HBsAg carriers and active HBV infection).
FIGURE 4.

The subgroup analyses on cancer risk by five HBV serological markers (multivariate analyses adjusted sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers) (A) before matching; (B) after matching
FIGURE 5.
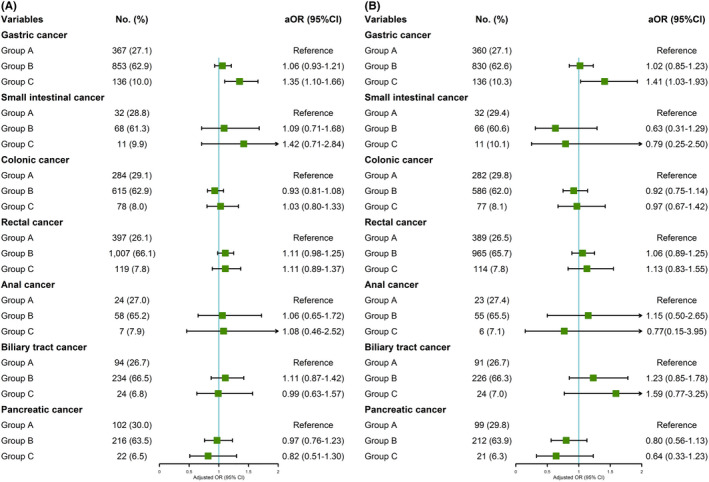
The subgroup analyses on cancer risk by three HBV serologic classifications (multivariate analyses adjusted sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers) (A) before matching; (B) after matching
3.3. Re‐analyses after matching
Re‐analyses based on matched datasets were conducted. In univariate analysis, HBsAg remained the positive association with gastric cancer (OR = 1.41, 95% CI: 1.07–1.85). Similarly, anti‐HBs still was a protective factor for gastric (OR = 0.73, 95% CI: 0.62–0.85), colonic (OR = 0.72, 95% CI: 0.60–0.88), rectal (OR = 0.71, 95% CI: 0.62–0.83), and pancreatic (OR = 0.58, 95% CI: 0.42–0.80) cancers. In terms of HBV serologic classifications, compared with group A, group C was associated with an increased risk of gastric cancer (OR = 1.49, 95% CI: 1.10–2.02) (Table S3).
In multivariable analysis, HBsAg was still associated with increased risk of gastric cancer (aOR = 1.39, 95% CI: 1.05–1.85), anti‐HBs was also associated with decreased risk of gastric (aOR = 0.72, 95% CI: 0.61–0.85), colonic (aOR = 0.73, 95% CI: 0.60–0.89), rectal (aOR = 0.73, 95% CI: 0.63–0.85), and pancreatic (aOR = 0.58, 95% CI: 0.42–0.82) cancers, group C (aOR = 1.41, 95% CI: 1.03–1.93) was associated with an increased risk of gastric cancer compared with group A in terms of HBV serologic classifications. Results mentioned above were consistent with results before matching (Table 2, Figure 4B and Figure 5B).
3.4. Associations between risk factors and HBsAg positive in gastric cancer
We have observed a positive correlation trend between HBsAg and gastric cancer before and after matching, associations between risk factors and HBsAg positive in gastric cancer were analyzed based on the above conclusions. Four serum tumor markers including CA125, CA19‐9, CEA, and AFP were tested in 1161 of 1356 gastric cancer patients. Significant differences were observed between gastric cancer patients with and without HBsAg positive in terms of sex, age, family history of cancers, and AFP level (p < 0.05). Both in univariate and multivariable unconditional logistic regression model, sex, age, and AFP level were correlated with HBsAg in gastric cancer. Compared to HBsAg– patients, HBsAg+patients were younger which had a higher prevalence of abnormal AFP level (≥8 ng/ml) and a higher proportion of males (Table 3).
TABLE 3.
Basic characteristics of patients with and without HBsAg positive, associations between risk factors and HBsAg positive in gastric cancer
| Variables |
HBsAg positive (n = 115) No. (%) |
HBsAg negative (n = 1046) No. (%) |
p | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | ||||
| Sex | 0.021 | ||||||||
| Female | 28 (24.3) | 372 (35.6) | 1.00 | Reference | 1.00 | Reference | |||
| Male | 87 (75.7) | 674 (64.4) | 1.71 | 1.10–2.67 | 0.017 | 2.00 | 1.14–3.52 | 0.016 | |
| Age (mean ±SD), years | 55.82±10.63 | 58.60±11.61 | 0.014 | 0.98 | 0.96–1.00 | 0.014 | 0.97 | 0.95–0.99 | 0.001 |
| BMI (kg/m2) | 0.190 | ||||||||
| <23.0 | 59 (51.3) | 627 (59.9) | 1.00 | Reference | 1.00 | Reference | |||
| 23.0–29.9 | 54 (47.0) | 400 (38.2) | 1.43 | 0.97–2.12 | 0.070 | 1.43 | 0.95–2.14 | 0.083 | |
| ≥30.0 | 2 (1.7) | 19 (1.8) | 1.12 | 0.25–4.92 | 0.882 | 1.07 | 0.24–4.79 | 0.933 | |
| Smoker | 0.229 | ||||||||
| Never | 57 (49.6) | 585 (55.9) | 1.00 | Reference | 1.00 | Reference | |||
| Previous/Current | 58 (50.4) | 461 (44.1) | 1.29 | 0.88–1.90 | 0.194 | 0.87 | 0.53–1.42 | 0.579 | |
| Alcohol drinker | 0.217 | ||||||||
| Never | 88 (76.5) | 855 (81.7) | 1.00 | Reference | 1.00 | Reference | |||
| Previous/Current | 27 (23.5) | 191 (18.3) | 1.37 | 0.87–2.17 | 0.175 | 1.32 | 0.79–2.20 | 0.296 | |
| Diabetes mellitus (yes) | 4 (3.5) | 30 (2.9) | 0.939 | 1.22 | 0.42–3.53 | 0.713 | 1.10 | 0.37–3.29 | 0.859 |
| Family history of cancers (yes) | 25 (21.7) | 150 (14.3) | 0.049 | 1.66 | 1.03–2.67 | 0.037 | 1.63 | 0.99–2.67 | 0.053 |
| CA125 (≥35 U/ml) | 8 (7.0) | 69 (6.6) | 1.000 | 1.06 | 0.50–2.26 | 0.883 | 0.99 | 0.45–2.19 | 0.981 |
| CA19‐9 (≥22 U/ml) | 32 (27.8) | 231 (22.1) | 0.201 | 1.36 | 0.88–2.10 | 0.164 | 1.52 | 0.96–2.41 | 0.076 |
| CEA (≥3.4 ng/ml) | 33 (28.7) | 288 (27.5) | 0.877 | 1.06 | 0.69–1.62 | 0.791 | 0.96 | 0.61–1.51 | 0.854 |
| AFP (≥8 ng/ml) | 14 (12.2) | 54 (5.2) | 0.005 | 2.55 | 1.37–4.75 | 0.003 | 2.28 | 1.20–4.34 | 0.012 |
4. DISCUSSION
As one of HBV serological markers, the presence of detectable HBsAg indicated HBV infection. Following seroclearance of HBsAg, the appearance of anti‐HBs confers protection from HBV infection. 24 Our study revealed that HBsAg was associated with an increased risk of gastric cancer and anti‐HBs served as a protective factor for gastric, colonic, rectal, and pancreatic cancers. Compared to subgroups with non‐infection and vaccination status, inactive HBsAg carriers and active HBV infection subgroup were correlated with gastric carcinogenesis.
China is one of the HBV‐endemic areas and the prevalence of HBsAg carriers experienced a decline from 9.8% to 7.2% during 1992–2006 due to national immunization. 35 , 36 , 37 However, the majority of HBV infection develops into persistent and chronic viral infection more easily by reason of early onset age and low spontaneous clearance rate of HBsAg. 36 , 37 In our study, the serum positive rates of HBsAg was 8.4% (6.5%‐10.0%) in cancer cases and 8.3% in non‐cancer controls, respectively, slightly above national average rate (around 8%). 38 , 39
Since overexpression of HBsAg and HBcAg was detected in gastric epithelial cells, several previous studies sought to investigate the association between HBV infection and gastric cancer with conflicting results. 11 , 15 , 16 , 18 , 22 , 40 , 41 , 42 As precancerosis to gastric cancer, gastric premalignant changes was found to be correlated with HBV infection. 22 Meta‐analyses also found the association between HBV infection and gastric cancer. 43 , 44 In our study, the seroprevalence of HBsAg was much higher in gastric cancer compared with matched controls (p = 0.017). We verified that HBsAg was significantly associated with increased risk of gastric cancer independently. 18 , 41 , 42 Meanwhile, for patients with gastric cancer, HBsAg+patients were younger and had a higher proportion of male compared with HBsAg– patients. 45
In addition to gastric cancer, a higher incidence of colorectal adenoma was also associated with HBV infection. 46 As precancerosis to colorectal cancer, colorectal adenoma can progress to colorectal carcinoma with chances. For pancreatic cancer, its association with HBV infection was controversial. 13 , 20 , 21 , 47 , 48 , 49 Statistically significant association between patients with past exposure to HBV and the risk of pancreatic cancer was observed in Hassan's study not in Tang's. 13 , 50 In our study, 340 patients with pancreatic cancer were included and the serum‐positive rates of HBsAg were 6.5% (22/340) which was lower than Tian's (7.2%, 146/2039). 49 Non‐significant association between HBsAg positivity and pancreatic cancer was observed that may be due to the small sample size in our study. Furthermore, the different prevalence of HBV and pancreatic cancer by different geographic regions investigated, year of pancreatic cancer diagnosis, and treatment of confounding factors might explain this discrepancy in results. 49 It was also found that HBV was an independent factor in the risk for cholangiocarcinoma. 51 , 52 , 53 , 54 , 55 , 56 However, no clear association was found between HBV infection and other cancers except gastric cancer in our study. Interestingly, our study revealed that anti‐HBs were statistically associated with decreased risk of gastric, colonic, rectal, and pancreatic cancers. As a protecting antibody arising after HBsAg, anti‐HBs was associated with decreased risk of cancers mentioned above perhaps signified the positive associations between HBsAg and cancers mentioned above. 42
HBV has been recognized as a causative pathogen for HCC which had high incidence and mortality in China. It has been suggested that HBV trigger the host's immune responses to create a hypoxic environment for supporting virus persistent replication and prolonging chronic inflammation without virus clearance, which causes the integration of viral encoded proteins into human chromosomes and the mutation of host gene expression and cellular phenotypes that confer the pathogenesis of HCC. 57
As a transactivating protein encoded by HBV, hepatitis B X (HBX) protein is associated with initiating the development of HCC. The expression of HBX and anti‐HBc was detected in gastric cancer which indicated persistent and chronic viral infection. 11 , 18 , 41 , 42 Chronic inflammation triggered by HBV caused immunosuppression which might play a role in gastric carcinogenesis. Cui et al found cellular atypia and lymphocytes’ infiltration induced by HBX in gastric epithelial cells. 44 As the oncogenic protein, HBX might also involve in the development of gastric cancer. For patients with gastric cancer, the proportion of AFP abnormal elevation was much higher in HBsAg+patients compared with HBsAg– patients which indicated a similar mechanism of HBV‐related HCC exists in gastric carcinogenesis.
It has been reported that HBX competitively binds adenomatous polyposis coli (APC) to activate Wnt/b‐catenin signaling, and then induces hallmark changes of cancer further. 58 Wnt/b‐catenin signaling was also identified as a related signaling pathway to colorectal carcinogenesis. For this reason, it was speculated that a similar mechanism may exist in colorectal cancer. 46 Iloeje et al considered that HBV persistent replication might exist in the pancreas which is vulnerable to viral hepatitis and serves as a potential reservoir of hepatitis viruses. Cell injury, immunoreaction, and inflammation caused by chronic HBV infection might play an important role in pancreatic carcinogenesis. 47 , 59 Like the pancreas adjacent to the liver, cholangiocarcinoma may share similar processes for HBV‐related carcinogenesis which originate from hepatic progenitor cells, and chronic inflammatory process might involve in the development of cholangiocarcinoma as well. 14 , 60 , 61
Compared with previous studies, the relationship between HBV infection and the risk of extrahepatic digestive system cancers was systematically examined in our study. Unlike several previous studies which only defined HBV infection by HBsAg status leaving the other four HBV serological markers out of consideration, only patients with results of all five HBV serological markers were included in our study and the association between prior exposure to HBV (resolved HBV infection) and the development of extrahepatic cancer was investigated as well. 15 , 16 , 18 , 45 , 51 , 62 Therefore, more comprehensive and accurate information of the status of HBV infection could be provided and more credible results could be obtained in our study compared with others. As a case–control study, we chose individuals without malignant tumors who received routine medical checkups in the same hospital as non‐cancer controls to eliminate selection bias. Meanwhile, non‐cancer controls could more represent the general population than controls selected from hospitalized patients. Traditional propensity score matching was also conducted to balance covariates (including sex, age, BMI, smoking status, alcohol drinking status, diabetes mellitus, and family history of cancers) and reduce bias.
However, limitations still remained in our study. First, occult HBV infection which was defined as the absence of detectable HBsAg in serum and presence of HBV DNA in the liver (anti‐HBc, anti‐HBs, and anti‐HBe, are detected frequently in serum) might exist accompanied by HCV infection. As a possible confounding factor that may affect the role of HBV infection, we did not rule out the possibility of occult HBV infection. However, the prevalence of HCV was low in China (0.43%), and low prevalence of occult HBV infection has been reported as well. 63 , 64 As a limitation, participants with HIV or HCV infection were not excluded in our study due to information absence from cancer‐free controls. However, the prevalence of HIV and HCV was very low for included patients with cancers in our study (0.3% and 0.5%, respectively) and it was hard to determine the causal relationship between infection of viruses (HIV and HCV) and risk of gastric cancer, results of our study were still credible. 65 , 66 , 67 Second, the number of non‐cancer controls was much larger than that of the cancer group before matching. Association was not observed between HBV infection and other cancers such as bile duct, small intestinal, and anal cancers which might be attributed to the small sample size and it was difficult to conclude cancers with low incidence. As esophageal squamous cell carcinoma is the predominant histological type, esophageal cancer was not included in our study. Thus, more cases were needed to be concerned with different types of cancers with low morbidity 68 Third, as a well‐known infectious agent, H.pylori coinfection was not taken into consideration when analyzing the relationship between HBV infection and gastric cancer. However, evidence supporting the interaction between HBV and H. pylori was insufficient and a significant difference in H. pylori prevalence between the gastric cancer and any controls was not found in many previous studies. 11 , 40 , 42 , 67 , 69 , 70 , 71 In addition, potential effects of confounding factors (included subjects’ nutritional status, dietary intakes, environmental exposure, socioeconomic status, access to health, educational level, etc.) could not be eliminated in our study by the absence of information which might cause decreased statistical power. Finally, as a more direct and accurate measure of active HBV infection compared with HBV serological markers, HBV DNA was not tested in our study. However, as an effective means to evaluate the status of HBV infection, HBV serological markers are still irreplaceable currently.
In summary, our study revealed that HBV infection was potentially associated with an increased risk of gastric cancer. However, because of the limited ability to establish a causal relationship for case–control study, large‐scale prospective cohort studies are urgent, and the latent mechanism about HBV extrahepatic carcinogenesis needs to be investigated as well.
CONFLICTS OF INTEREST
None declared.
Supporting information
Table S1‐S3
ACKNOWLEDGMENTS
The work is a joint with the Sichuan Gastric Cancer Early Detection and Screening (SIGES) research project. The authors thank the substantial work of the Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China.
Hui Wang, Xin‐Zu Chen and Xiao‐Long Chen contributed equally as co‐first authors.
Funding information
(1) National Key R&D Program of China (No.2017YFC0907504); (2) National Natural Science Foundation of China (No.81702366, No.81372344); (3) Sichuan Science and Technology Program (No.2019YFS0255, No.2018SZS0261); (4) 1‧3‧5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No.ZY2017304); (5) Wu Jieping Medical Foundation (No.320.2710.1815, No.320.2710.1865).
Contributor Information
Hui Wang, Email: 1779690040@qq.com.
Huai‐Rong Tang, Email: hujkwch@126.com, Email: 1651682099@qq.com.
Jian‐Kun Hu, Email: hujkwch@126.com, Email: 1651682099@qq.com.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Chen XZ, Chen H, Castro FA, Hu JK, Brenner H. Epstein‐Barr virus infection and gastric cancer: a systematic review. Medicine. 2015;94(20):e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang R, Liu K, Chen XZ, SIGES research group . Associations between gastric cancer risk and virus infection other than Epstein‐Barr virus: the protocol of a systematic review and meta‐analysis based on epidemiological studies. Medicine. 2019;98(32):e16708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Chen XZ, Waterboer T, Castro FA, Brenner H. Viral infections and colorectal cancer: a systematic review of epidemiological studies. Int J Cancer. 2015;137(1):12‐24. [DOI] [PubMed] [Google Scholar]
- 4. Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4(9):e609‐e616. [DOI] [PubMed] [Google Scholar]
- 5. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45(4):529‐538. [DOI] [PubMed] [Google Scholar]
- 6. Wang R, Chen XZ. High mortality from hepatic, gastric and esophageal cancers in mainland China: 40 years of experience and development. Clin Res Hepatol Gastroenterol. 2014;38(6):751‐756. [DOI] [PubMed] [Google Scholar]
- 7. McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49(5 Suppl):S45‐S55. [DOI] [PubMed] [Google Scholar]
- 8. Dejean A, Lugassy C, Zafrani S, Tiollais P, Brechot C. Detection of hepatitis B virus DNA in pancreas, kidney and skin of two human carriers of the virus. J Gen Virol. 1984;65(3):651‐655. [DOI] [PubMed] [Google Scholar]
- 9. Hoefs JC, Renner IG, Askhcavai M, Redeker AG. Hepatitis B surface antigen in pancreatic and biliary secretions. Gastroenterology. 1980;79(2):191‐194. [PubMed] [Google Scholar]
- 10. Wang WL, Gu GY, Hu M. Expression and significance of HBV genes and their antigens in human primary intrahepatic cholangiocarcinoma. World J Gastroenterol. 1998;4(5):392‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen NL, Bai L, Deng T, Zhang C, Kong QY, Chen H. Expression of hepatitis B virus antigen and Helicobacter pylori infection in gastric mucosa of patients with chronic liver disease. Hepatobiliary Pancreat Dis Int. 2004;3(2):223‐225. [PubMed] [Google Scholar]
- 12. Chen XZ, Wang R, Hu JK. Hepatitis B virus infection and gastric cancer risk: pitfalls in the potential association. Br J Cancer. 2015;112(11):1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassan MM, Li D, El‐Deeb AS, et al. Association between hepatitis B virus and pancreatic cancer. J Clin Oncol. 2008;26(28):4557‐4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsing AW, Zhang M, Rashid A, et al. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population‐based study in China. Int J Cancer. 2008;122(8):1849‐1853. [DOI] [PubMed] [Google Scholar]
- 15. Mahale P, Engels EA, Koshiol J. Hepatitis B virus infection and the risk of cancer in the elderly US population. Int J Cancer. 2019;144(3):431‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. An J, Kim JW, Shim JH, et al. Chronic hepatitis B infection and non‐hepatocellular cancers: a hospital registry‐based, case‐control study. PLoS One. 2018;13(3):e0193232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamiza AB, Su FH, Wang WC, Sung FC, Chang SN, Yeh CC. Chronic hepatitis infection is associated with extrahepatic cancer development: a nationwide population‐based study in Taiwan. BMC Cancer. 2016;16(1):861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wei X‐L, Luo H‐Y, Li C‐F, et al. Hepatitis B virus infection is associated with younger median age at diagnosis and death in cancers. Int J Cancer. 2017;141(1):152‐159. [DOI] [PubMed] [Google Scholar]
- 19. DeFilippis EM, Mehta M, Ludwig E. A potential association between exposure to hepatitis B virus and small bowel adenocarcinoma. J Gastrointest Oncol. 2016;7(3):495‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krull Abe SIM, Sawada N, Iwasaki M, et al. JPHC Study, Group . Hepatitis B and C virus infection and risk of pancreatic cancer: a population‐based cohort study. Cancer Epidemiol Biomarkers Prev. 2016;25(3):555‐557. [DOI] [PubMed] [Google Scholar]
- 21. Chang MC, Chen CH, Liang JD, et al. Hepatitis B and C viruses are not risks for pancreatic adenocarcinoma. World J Gastroenterol. 2014;20(17):5060‐5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baghbanian M, Hoseini Mousa SA, Doosti M, Moghimi M. Association between gastric pathology and hepatitis B virus infection in patients with or without helicobacter pylori. Asian Pac J Cancer Prev. 2019;20(7):2177‐2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu JK, Chen XZ. Early Detection and Screening Strategy of Gastric Cancer in Sichuan Province‐A Prospective Cohort Study (WCH‐GC‐SIGES‐01) . ClinicalTrialsgov. 2018 (ClinicalTrials.gov Identifier: NCT03597672): https://www.clinicaltrials.gov/ct2/show/NCT03597672 Assessed by Dec 22, 2019.
- 24. WHO Guidelines Approved by the Guidelines Review Committee . WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization Copyright (c) World Health Organization. 2017. [Google Scholar]
- 25. Seto WK, Lo YR, Pawlotsky JM, Yuen MF. Chronic hepatitis B virus infection. Lancet. 2018;392(10161):2313‐2324. [DOI] [PubMed] [Google Scholar]
- 26. Ganem D, Prince AM. Hepatitis B virus infection—natural history and clinical consequences. N Engl J Med. 2004;350(11):1118‐1129. [DOI] [PubMed] [Google Scholar]
- 27. Organization WH . The Asia‐Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 28. Lee J, Little TD. A practical guide to propensity score analysis for applied clinical research. Behav Res Ther. 2017;98:76‐90. [DOI] [PubMed] [Google Scholar]
- 29. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28(25):3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Croissant Y, mlogit: Multinomial Logit Models 2020. Available from: https://cran.r‐project.org/web/packages/mlogit/
- 31. Stuart DEHaKIaGKaEA . MatchIt: Nonparametric Preprocessing for Parametric Causal Inference 2011. Available from: https://cran.r‐project.org/web/packages/MatchIt/
- 32. Greifer N, cobalt: Covariate Balance Tables and Plots 2020. Available from: https://cran.r‐project.org/web/packages/cobalt/
- 33. Therneau TM. survival: Survival Analysis 2020. Available from: https://cran.r‐project.org/web/packages/survival/
- 34. Lumley MGaT . forestplot: Advanced Forest Plot Using ‘grid’ Graphics 2019. Available from: https://cran.r‐project.org/web/packages/forestplot/
- 35. Liu Z, Yang Q, Shi O, Ye W, Chen X, Zhang T. The epidemiology of hepatitis B and hepatitis C infections in China from 2004 to 2014: an observational population‐based study. J Viral Hepat. 2018;25(12):1543‐1554. [DOI] [PubMed] [Google Scholar]
- 36. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099‐2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liaw Y‐F, Chu C‐M. Hepatitis B virus infection. Lancet. 2009;373(9663):582‐592. [DOI] [PubMed] [Google Scholar]
- 38. Wang FZ, Zhang GM, Shen LP, et al. Comparative analyze on hepatitis B seroepidemiological surveys among population aged 1–29 years in different epidemic regions of China in 1992 and 2014. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51(6):462‐468. [DOI] [PubMed] [Google Scholar]
- 39. Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China–declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27(47):6550‐6557. [DOI] [PubMed] [Google Scholar]
- 40. Fattahi S, Nikbakhsh N, Taheri H, et al. Prevalence of multiple infections and the risk of gastric adenocarcinoma development at earlier age. Diagn Microbiol Infect Dis. 2018;92(1):62‐68. [DOI] [PubMed] [Google Scholar]
- 41. Song CI, Lv J, Liu Y, et al. Associations between hepatitis B virus infection and risk of all cancer types. JAMA Netw Open. 2019;2(6):e195718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei X‐L, Qiu M‐Z, Jin Y, et al. Hepatitis B virus infection is associated with gastric cancer in China: an endemic area of both diseases. Br J Cancer. 2015;112(7):1283‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang H, Chen X‐L, Liu K, et al. Associations between gastric cancer risk and virus infection other than epstein‐barr virus: a systematic review and meta‐analysis based on epidemiological studies. Clini Transl Gastroenterol. 2020;11(7):e00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cui H, Jin Y, Chen F, et al. Clinicopathological evidence of hepatitis B virus infection in the development of gastric adenocarcinoma. J Med Virol. 2020;92(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 45. Lu T, Yang Q, Li M, et al. HBV infection and extra‐hepatic cancers in adolescents and 20s: a retrospective study in China. Cancer Epidemiol. 2018;55:149‐155. [DOI] [PubMed] [Google Scholar]
- 46. Patel BB, Lipka S, Shen H, Davis‐Yadley AH, Viswanathan P. Establishing the link between hepatitis B virus infection and colorectal adenoma. J Gastrointest Oncol. 2015;6(5):492‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xu JH, Fu JJ, Wang XL, Zhu JY, Ye XH, Chen SD. Hepatitis B or C viral infection and risk of pancreatic cancer: a meta‐analysis of observational studies. World J Gastroenterol. 2013;19(26):4234‐4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Yang S, Song F, et al. Hepatitis B virus status and the risk of pancreatic cancer: a meta‐analysis. Eur J Cancer Prev. 2013;22(4):328‐334. [DOI] [PubMed] [Google Scholar]
- 49. Tian T, Song CI, Jiang L, et al. Hepatitis B virus infection and the risk of cancer among the Chinese population. Int J Cancer. 2020;147(11):3075‐3084. [DOI] [PubMed] [Google Scholar]
- 50. Tang J, Sharma R, Lamerato L, Sheehan M, Krajenta R, Gordon SC. Is previous exposure to hepatitis B a risk factor for pancreatic cancer or hepatocellular carcinoma? J Clin Gastroenterol. 2014;48(8):729‐733. [DOI] [PubMed] [Google Scholar]
- 51. Lee BS, Park EC, Park SW, Nam CM, Roh J. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case‐control study in Korea. World J Gastroenterol. 2015;21(2):502‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case‐control study. Am J Gastroenterol. 2008;103(7):1716‐1720. [DOI] [PubMed] [Google Scholar]
- 53. Zhou YU, Zhou Q, Lin Q, et al. Evaluation of risk factors for extrahepatic cholangiocarcinoma: ABO blood group, hepatitis B virus and their synergism. Int J Cancer. 2013;133(8):1867‐1875. [DOI] [PubMed] [Google Scholar]
- 54. Zhou Y, Zhao Y, Li B, et al. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: evidence from a meta‐analysis. BMC Cancer. 2012;12(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li M, Li J, Li P, et al. Hepatitis B virus infection increases the risk of cholangiocarcinoma: a meta‐analysis and systematic review. J Gastroenterol Hepatol. 2012;27(10):1561‐1568. [DOI] [PubMed] [Google Scholar]
- 56. Tanaka M, Tanaka H, Tsukuma H, Ioka A, Oshima A, Nakahara T. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat. 2010;17(10):742‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arzumanyan A, Reis HM, Feitelson MA. Pathogenic mechanisms in HBV‐ and HCV‐associated hepatocellular carcinoma. Nat Rev Cancer. 2013;13(2):123‐135. [DOI] [PubMed] [Google Scholar]
- 58. Hsieh A, Kim HS, Lim SO, Yu DY, Jung G. Hepatitis B viral X protein interacts with tumor suppressor adenomatous polyposis coli to activate Wnt/beta‐catenin signaling. Cancer Lett. 2011;300(2):162‐172. [DOI] [PubMed] [Google Scholar]
- 59. Iloeje UH, Yang H‐I, Jen C‐L, et al. Risk of pancreatic cancer in chronic hepatitis B virus infection: data from the REVEAL‐HBV cohort study. Liver Int. 2010;30(3):423‐429. [DOI] [PubMed] [Google Scholar]
- 60. Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37(9):1211‐1216. [DOI] [PubMed] [Google Scholar]
- 61. Gatselis NK, Tepetes K, Loukopoulos A, et al. Hepatitis B virus and intrahepatic cholangiocarcinoma. Cancer Invest. 2007;25(1):55‐58. [DOI] [PubMed] [Google Scholar]
- 62. Andersen ES, Omland LH, Jepsen P, et al. Risk of all‐type cancer, hepatocellular carcinoma, non‐Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J Viral Hepat. 2015;22(10):828‐834. [DOI] [PubMed] [Google Scholar]
- 63. Cui Y, Jia J. Update on epidemiology of hepatitis B and C in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):7‐10. [DOI] [PubMed] [Google Scholar]
- 64. Samal J, Kandpal M, Vivekanandan P. Molecular mechanisms underlying occult hepatitis B virus infection. Clin Microbiol Rev. 2012;25(1):142‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Allison RD, Tong X, Moorman AC, et al. Increased incidence of cancer and cancer‐related mortality among persons with chronic hepatitis C infection, 2006–2010. J Hepatol. 2015;63(4):822‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amin J, Dore GJ, O’Connell DL, et al. Cancer incidence in people with hepatitis B or C infection: a large community‐based linkage study. J Hepatol. 2006;45(2):197‐203. [DOI] [PubMed] [Google Scholar]
- 67. Kayamba V, Asombang AW, Mudenda V, et al. Gastric adenocarcinoma in Zambia: a case‐control study of HIV, lifestyle risk factors, and biomarkers of pathogenesis. S Afr Med J. 2013;103(4):256‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rustgi AK, El‐Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499‐2509. [DOI] [PubMed] [Google Scholar]
- 69. Estevens J, Fidalgo P, Tendeiro T, et al. Anti‐Helicobacter pylori antibodies prevalence and gastric adenocarcinoma in Portugal: report of a case‐control study. Eur J Cancer Prev: the official journal of the European Cancer Prevention Organisation (ECP). 1993;2(5):377‐380. [DOI] [PubMed] [Google Scholar]
- 70. Peleteiro B, Lunet N, Barros R, La Vecchia C, Barros H. Factors contributing to the underestimation of Helicobacter pylori‐associated gastric cancer risk in a high‐prevalence population. Cancer Causes & Control: CCC. 2010;21(8):1257‐1264. [DOI] [PubMed] [Google Scholar]
- 71. Kirchner GI, Beil W, Bleck JS, Manns MP, Wagner S. Prevalence of Helicobacter pylori and occurrence of gastroduodenal lesions in patients with liver cirrhosis. Int J Clin Exp Med. 2011;4(1):26‐31. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
Data are available upon reasonable request.


