Abstract
This study explored systemic immune changes in 11 subjects with X-linked retinoschisis (XLRS) in a phase I/IIa adeno-associated virus 8 (AAV8)-RS1 gene therapy trial (ClinicalTrials.gov: NCT02317887). Immune cell proportions and serum analytes were compared to 12 healthy male controls. At pre-dosing baseline the mean CD4/CD8 ratio of XLRS subjects was elevated. CD11c+ myeloid dendritic cells (DCs) and the serum epidermal growth factor (EGF) level were decreased, while CD123+ plasmacytoid DCs and serum interferon (IFN)-γ and tumor necrosis factor (TNF)-α were increased, indicating that the XLRS baseline immune status differs from that of controls. XLRS samples 14 days after AAV8-RS1 administration were compared with the XLRS baseline. Frequency of CD11b+CD11c+ DCc was decreased in 8 of 11 XLRS subjects across all vector doses (1e9–3e11 vector genomes [vg]/eye). CD8+human leukocyte antigen-DR isotype (HLA-DR)+ cytotoxic T cells and CD68+CD80+ macrophages were upregulated in 10 of 11 XLRS subjects, along with increased serum granzyme B in 8 of 11 XLRS subjects and elevated IFN-γ in 9 of 11 XLRS subjects. The six XLRS subjects with ocular inflammation after vector application gave a modestly positive correlation of inflammation score to their respective baseline CD4/CD8 ratios. This exploratory study indicates that XLRS subjects may exhibit a proinflammatory, baseline immune phenotype, and that intravitreal dosing with AAV8-RS1 leads to systemic immune activation with an increase of activated lymphocytes, macrophages, and proinflammatory cytokines.
Keywords: X-linked retinoschisis, gene therapy clinical trial, ocular inflammation, immune function, T cells, AAV8 vector, retinoschisin, cytokines
Graphical abstract
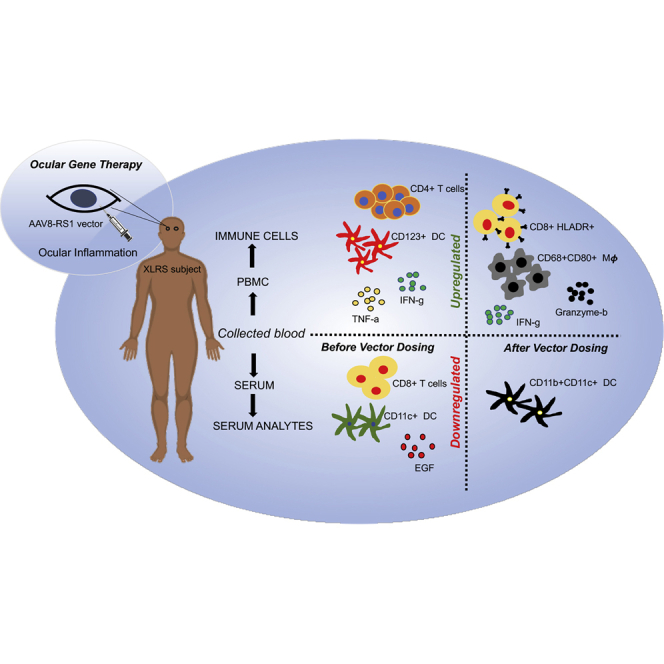
Systemic immune status was evaluated in X-linked retinoschisis subjects in an ocular AAV8-RS1 gene therapy trial. Baseline changes before dosing showed altered CD4/CD8 T cell ratios, dendritic cell subsets, and pro-inflammatory cytokine levels. Vector application caused systemic immune activation with an increase of activated cytotoxic lymphocytes, macrophages, and pro-inflammatory cytokines.
Introduction
Adeno-associated virus (AAV) vectors are a frequent choice for therapeutic gene delivery,1 including the safe and efficacious therapy for Leber congenital amaurosis (LCA) with voretigene neparvovec-rzyl (Luxterna).2,3 However, at higher doses AAV vectors may elicit inflammatory immune responses4, 5, 6 that can present a therapeutic challenge. Upon administeration into the eye, the vector encounters local and circulating immune cells that trigger innate and adaptive immune responses,6 whether administered by intravitreal or subretinal application. This immune response is potentially injurious to ocular tissues and can lead to focal retinal inflammation and disrupted retinal architecture,7 and the immune activity may interfere with effective delivery to target cells. Both intravitreal and subretinal dosing with AAV2-7m8 and AAV8-BP2 caused retinal glial activation and lymphocytic infiltration in non-human primates (NHPs) at the highest injected dose of 1 × 1012 vector genomes (vg)/eye in this study.8 Seitz et al.9 also demonstrated a dose-dependent AAV distribution and shedding in blood and draining lymphatic tissue in both intravitreal and subretinal AAV8 vector delivery at 1e11 and 1e12 vg/eye, with the vector genome being more abundant and persistent in systemic circulation upon intravitreal delivery.
Although clinical ocular trials for several retina conditions have shown suitable safety profiles for AAV vectors,2,10, 11, 12 two retinal gene therapy trials showed a decline of therapeutic efficacy over time (ClinicalTrials.gov: NCT00481546 and NCT00643747).13, 14, 15 Clearance of transduced cells by immune mechanisms is a possible etiology,16 suggesting that further understanding of immune responses to the vector would be beneficial for managing AAV gene therapy. As neutralizing antibodies (NABs) against AAV8 capsid proteins and interferon (IFN)-γ-producing T cell responses to AAV8 capsid were observed in our AAV8-RS1 trial (ClinicalTrials.gov: NCT02317887),17 both adaptive immunity and vector load likely contribute to ocular inflammation.
The present study reports the peripheral blood immune cell profiles and serum analytes in X-linked retinoschisis (XLRS) subjects treated in the AAV8-RS1 gene therapy trial we are conducting. Although the number of subjects in this trial is small, it brings new information to light on the immune consequences of human ocular AAV gene therapy. While the clinical features of the first nine subjects enrolled in the trial are already published,17 the present study provides a view into the immune status of these XLRS trial subjects and two additional subjects, before and after vector application. The statistical statements are limited by the small number of subjects in this trial, particularly at the higher levels of 1e11 and 3e11 vg/eye for which clinical ocular inflammation was observed. While definitive features are not yet known, the information gleaned by a systematic analysis of the immune status may be useful in guiding future studies.
Results
Baseline immune cell changes in XLRS
To assess whether the AAV8-RS1 vector dose led to a systemic immune response in XLRS trial subjects, we analyzed the immune cell populations by flow cytometry and serum cytokine/chemokine analysis, at pre-dosing baseline and at day 14 after vector application. Nine of eleven XLRS subjects showed higher CD4/CD8 ratios than those of normal controls, which typically range from 1.5 to 2.5.18 The XLRS mean CD4/CD8 ratio at baseline was considerably elevated compared with the 12 healthy controls (control, 2.2 ± 0.7; XLRS baseline, 3.5 ± 0.9; p < 0.001; Figures 1A and 1B; Table S1). The elevated ratio resulted from increased CD4+ T cells and a decrease of CD8+ T cells for many of the XLRS subjects.
Figure 1.
CD4/CD8 ratio in XLRS at baseline and correlation to ocular inflammation
(A) Representative flow cytometric plots for CD4 and CD8 populations gated on CD45+CD3+ cells for all groups. (B) The baseline CD4/CD8 ratio was calculated for XLRS subjects (n = 11) and compared to healthy controls (n = 12). Bar shows standard deviation. (C) Linear regression analysis for the baseline CD4/CD8 ratio in subjects manifesting ocular inflammation after vector dosing showed positive association to ocular inflammation scores.
Effect of duration of cryostorage
Due to trial logistics, the XLRS samples were stored in liquid nitrogen (LN) for up to 1 year before analysis, whereas the 12 control blood samples were processed a month after collection. To evaluate whether storage duration affected the outcome, we repeated the CD4/CD8 analysis of the 12 healthy controls using their peripheral blood mononuclear cell (PBMC) samples stored in LN for 12 months, comparable to the XLRS samples. Analysis showed that 12-month LN cryostorage decreased the CD4+ T cell counts, which in turn decreased their CD4/CD8 ratio minimally compared with analysis of 1-month samples (Figures S1A and S1B). As a consequence, the CD4/CD8 ratios for XLRS at baseline remained significantly elevated above controls for both 1-month and 12-month LN storage.
Subject S9 (1e11 vg/eye) had the highest baseline CD4/CD8 ratio and also had the most ocular inflammation after vector application. A total of six subjects in the AAV8-RS1 trial exhibited clinical ocular inflammation after dosing, and we considered whether the degree of inflammation after dosing correlated with their pre-dosing baseline CD4/CD8 ratios. Of the six individuals who exhibited inflammation, five received high doses (1e11 or 3e11 vg/eye) and one received the medium dose (1e10 vg/eye). We excluded five subjects at lower doses who had no inflammation as uninformative for the question (three at a low dose, 1e9 vg/eye, and two at a medium dose, 1e10 vg/eye). Testing the six with inflammation for the simplest, linear relationship of baseline CD4/CD8 ratios to their respective post-dosing inflammation grade gave a modest positive association (R2 = 0.70, p = 0.036; Figure 1C). For completeness, performing this analysis with all 11 subjects, including the 5 with no ocular inflammation at the lower doses (1e9 and 1e10 vg/eye), gave no association (R2 = 0.12; p = 0.3; Figure S2).
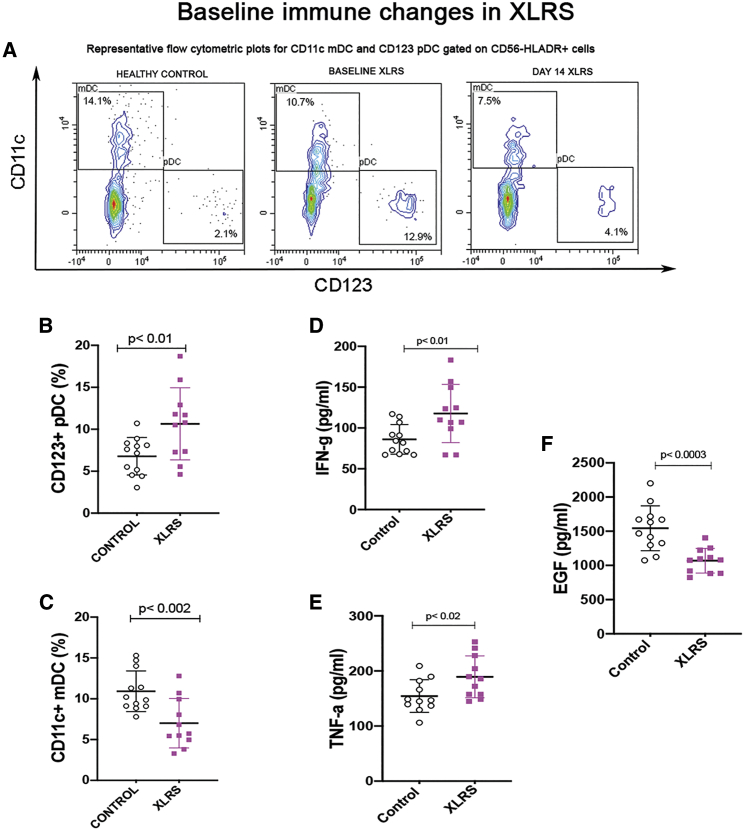
The dendritic cell (DC) subsets of XLRS subjects were more variable than those of healthy controls (Figure 2A), which may obviate a prognostic value. CD123+CD11c− plasmacytoid DCs (pDCs, Figure 2B) were increased (healthy controls, 6.8 ± 2.2; XLRS baseline, 10.6 ± 4.2; p = 0.01) but CD11c+ myeloid DCs (mDCs, Figure 2C) were decreased compared to healthy controls (controls, 10.9 ± 2.5; XLRS baseline, 7.0 ± 3.0; p = 0.002; mean ± SD values are indicated as percentages) (tabulated in Table S2). Both pDCs and mDCs have broad antiviral activity and type I IFN secretion, and they bridge the innate and adaptive systems by regulating early immune activation toward a cellular-based response.19 pDCs are also responsible for proinflammatory activation of effector T cells.20 pDCs are recruited to human cerebrospinal fluid under neuroinflammatory conditions and also for some non-inflammatory neurological conditions.21 The increased proportion of pDCs for XLRS at the pre-dosing baseline may reflect an underlying retinal neuroinflammatory state. pDCs that are actively recruited to the site of neurodegeneration would mount a T helper 1 (Th1) response,19 which is generally injurious to ocular tissue.
Figure 2.
Cell-mediated immune and serum cytokine changes in XLRS at baseline
Cell-mediated immune cell populations were analyzed by flow cytometry, and serum analytes were analyzed by cytometric bead array in 11 XLRS subjects and 12 healthy controls. Bars represent standard deviation. Significant frequency changes are identified in DCs. (A) Representative flow cytrometric plots for CD11c+ mDCs and CD123+ pDCs gated on CD56−HLA-DR+ cells. (B and C) CD123+ pDCs had an elevated frequency (B), while CD11c+ mDCs showed decreased proportions compared to healthy controls (C). Baseline values for serum cytokines/chemokines of XLRS subjects were evaluated compared with controls. (D–F) Serum levels of both IFN-γ and TNF-α were elevated (D and E), and EGF was downregulated (F). p < 0.05 was considered significant.
Serum cytokine/chemokine levels in XLRS at baseline
To look further at the baseline immune status of the XLRS subjects, we analyzed their cytokine levels and found significant changes in serum concentration (pg/mL) of several pro-inflammatory factors. Levels of IFN-γ (XLRS baseline, 117.7 ± 35.6; healthy controls, 86.1 ± 18.2) and tumor necrosis factor (TNF)-α (XLRS baseline, 183.8 ± 35.5; healthy controls, 154.5 ± 29.7) were elevated (36% higher for IFN-γ, p = 0.01; 19% higher for TNF-α, p = 0.02; Figures 2D and 2E), while epidermal growth factor (EGF) levels were 30% lower than those of controls (XLRS baseline, 1,069.2 ± 180.4; controls, 1,544.5 ± 328.3; p = 0.0003, Figure 2F) (tabulated in Table S2).
Cell and cytokine changes after vector injection implicate Th1-mediated activity
AAV8-RS1 dosing led to a marginal increase in the CD4/CD8 ratio in some XLRS subjects, but this was not significant in aggregate (Table S1). The proportions of CD68+CD80+ macrophages (XLRS baseline, 10.9 ± 5.6; XLRS day 14, 17.0 ± 6.5; p = 0.02; Figure 3A) and CD8+human leukocyte antigen-DR isotype (HLA-DR)+ activated cytotoxic T cells (Tc cells) (XLRS baseline, 15.5 ± 9.2; XLRS day 14, 24.9 ± 10.2; p = 0.04; Figure 3B) were elevated above baseline in 10 of 11 subjects to various degrees. CD11b+CD11c+ immunoregulatory DCs were decreased (XLRS baseline, 3.1 ± 2.6; XLRS day 14, 1.1 ± 0.9; p = 0.02; Figure 3C) after vector administration in 8 of 11 subjects.
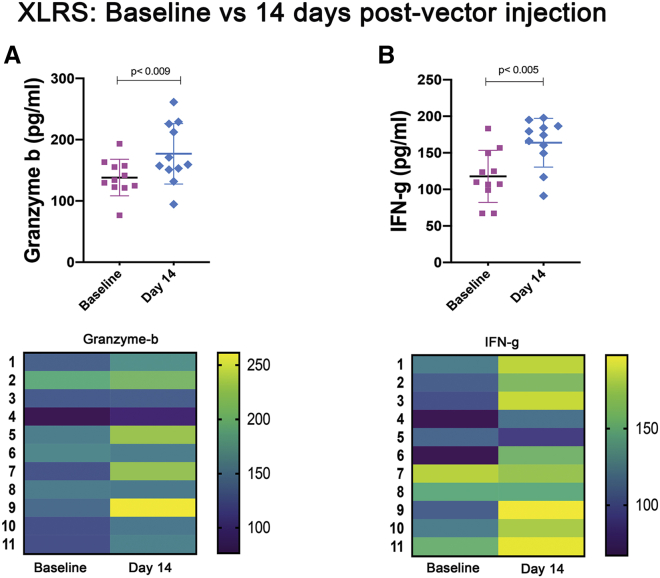
Figure 3.
Systemic immune cell alteration after AAV8-RS1 vector dosing
Relative immune cell proportions in peripheral blood were evaluated for each XLRS subject before and after vector dosage. Representative flow cytometric plots and heatmaps were constructed for evaluating changes in each subject. (A and B) CD68+CD80+ macrophages (A) and CD8+HLA-DR+ activated cytotoxic T cells (B) were upregulated in 10 XLRS subjects 14 days after vector injection. (C) CD11b+CD11c+ regulatory DCs were downregulated in 8 of 11 XLRS subjects after vector injection.
Two cytokine/chemokine analytes were substantially increased on day 14 after vector administration: 8 of 11 XLRS subjects showed an increase of granzyme B by 1.2- to 1.8-fold (XLRS baseline, 138.1 ± 29.9; XLRS day 14, 177.0 ± 49.2; p = 0.009, Figure 4A), and 9 of the 11 subjects had elevated IFN-γ by 1.2- to 2.4-fold (XLRS baseline, 117.7 ± 35.6; XLRS day 14, 163.8 ± 33.3; p = 0.005; Figure 4B). Granzyme B is a serine protease in granules of cytotoxic T cells and natural killer (NK) cells and can eliminate virus-infected cells by programmed cell death.22 IFN-γ is upregulated during a Th1/Th2 imbalance and indicates activation of the TNF/mitogen-activated protein kinase (MAPK)/Toll-like receptor (TLR) major inflammatory signaling pathways.23,24
Figure 4.
Serum cytokine changes after AAV8-RS1 vector dosing
(A and B) Pro-inflammatory cytokine granzyme B (A) and IFN-γ (B) levels were elevated on day 14 after vector injection. Heatmap analysis of each subject showed that granzyme B was upregulated in five of six subjects that had ocular inflammation at day 14 after vector dosing. IFN-γ did not show any particular trend with vector dose and was generally upregulated.
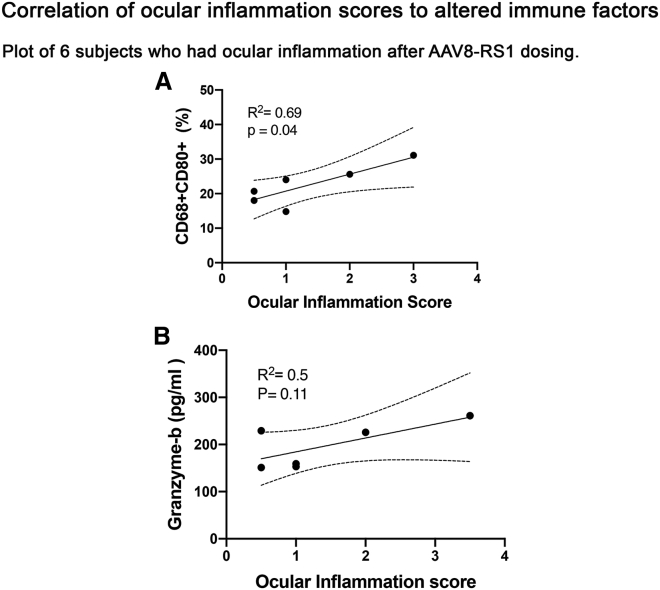
We considered whether any of these peripheral inflammatory parameters correlated with the degree of inflammation and found positive correlation with CD68+CD80+ activated macrophages (R2 = 0.69, p = 0.04) (Figure 5A). Granzyme B levels were elevated in 8 of 11 subjects, including 5 of 6 who had clinical ocular inflammation (subjects 5, 7, 9, 10, and 11) but also in some without clinical inflammation (subjects 1, 2, and 4). Granzyme B levels did not show significant association with ocular inflammation scores (R2 = 0.5, p = 0.1; Figure 5B), but the significantly elevated granzyme B levels in subjects with ocular inflammation (1.2- to 1.8-fold) after vector dosing may suggest a role in vector-induced inflammation. The cell proportions/serum levels of immune factors elevated after vector dosing are tabulated in Table S3. Other major immune cell subsets, including B cells, monocytes, regulatory T cells (Tregs), NK cells, and NK T (NKT) cells were analyzed at baseline and 14 days after vector injection, but they did not show a significant change between XLRS patients and controls.
Figure 5.
Association of pro-inflammatory systemic immune analytes to ocular inflammation after vector dosing
Linear regression analysis identified two immune analytes associated with the ocular inflammation scores in XLRS subjects after vector dosing. (A and B) CD68+CD80+ macrophages (R2 = 0.69; p = 0.04) (A), and granzyme B (R2 = 0.50; p = 0.11) (B) are both pro-inflammatory immune factors that play a major role in cytotoxic immune reaction.
Discussion
Observations from this study led to several hypotheses that warrant further analysis. First, the CD4/CD8 ratios of XLRS subjects at pre-dosing baseline were elevated compared to age-similar and sex-matched healthy controls. Second, at day 14 after vector dosing, the XLRS peripheral immune system showed activation, as evidenced by activated T cells, macrophages, and proinflammatory cytokines. Third, the degree of ocular inflammation after vector administration was roughly correlated to the XLRS subject’s baseline CD4/CD8 status for the six individuals who exhibited inflammation after vector dosing. Recognizing that the number of subjects treated in this study is small, these observations remain initial results that need further clarification, including whether an XLRS subject’s inflammatory response to the vector may relate to baseline immune status, in addition to the AAV8 vector load. A larger dataset is required to gain further insight into this possibility.
XLRS immune changes at baseline
The present study suggests that these XLRS subjects have an abnormal immune phenotype even at baseline. They exhibit altered systemic immune cell profiles and serum cytokines even before vector administration, and the results point toward these baseline changes as a potential factor in inflammation after vector administration. If an immune suppression regimen could be tailored to the immune activation after vector dosing, one would expect better immune control and improved therapeutic efficacy.
For the analysis, we focused only on those six subjects who showed clinical inflammation, as the absence of inflammation provides an ambiguous signal. The five subjects without clinical ocular inflammation (subjects 1–4 and 6) had received the lower doses (1e9 and 1e10 vg/eye), and they provide no information on possible responses to higher doses. When we further limited the group to only the five dosed at 1e11 or 3e11 vg/eye (subjects 7–11), the correlation continued to indicate a relationship of ocular inflammation to the baseline CD4/CD8 ratio (R2 = 0.64, p = 0.10), albeit less strongly than including inflammation subject 5 and significant only at p = 0.10. This relationship between CD4/CD8 and post-dosing inflammation is lost (R2 = 0.13; p = 0.3) when the low dose-treated subjects without inflammation are included in the analysis.
Of course one possibility to explain the relationship to baseline CD4/CD8 values of XLRS before dosing would be random variation (i.e., chance). The statistical likelihood of such an event can be calculated given that an elevated CD4/CD8 ratio was observed in 9 of 11 cases, and using a binary outcome of immune normal versus elevated states. The cutoff point used is the upper limit of the 95% confidence interval (CI) for CD4/CD8 values of the 12 healthy controls, which is a CD4/CD8 ratio ≥2.53. Poisson statistics for small case numbers (P[x = 9]) gives a probability of 0.052 (5.2%), indicating that this occurrence is unlikely to be a random observation, but that it is still within the realm of possibility. This suggests that the XLRS condition itself is the cause of the systemic immune status deviation from normal at baseline before dosing.
The literature reports that CD4/CD8 ratios generally range from 1.5 to 2.5.18 Some reports indicate that a subject’s race may regulate certain immune cell subsets,25 but the immune cell proportions for the 12 healthy controls in this study were within the expected normal range of 1.5–2.5 despite the racial mix, indicating that race differences do not negate the finding of a difference in CD4/CD8 status and other immune subsets of the XLRS subjects at the pre-dosing baseline.
Increased CD4/CD8 ratios generally are associated with conditions of chronic autoimmunity and inflammation.26, 27, 28 Dysregulation of the T cell ratio is reported for multiple sclerosis, sarcoidosis, and Alzheimer’s disease, and also in elderly subjects.29, 30, 31 Females generally have higher CD4/CD8 ratios than do males, which may predispose them toward autoimmune diseases.32,33 Higher CD4/CD8 ratios were found in the vitreous of subjects with proliferative diabetic retinopathy (PDR) and interpreted as a local inflammatory response.34 Similarly, Dave et al.35 also found increased CD4/CD8 ratios in aqueous humor for sarcoid uveitis. We note that the present XLRS analysis utilizes systemic CD4/CD8 ratios, but this shows a pattern of CD4/CD8 increase for XLRS subjects at baseline similar to that observed in ocular samples in the aforementioned studies. The elevated baseline CD4/CD8 ratio for XLRS found in this study in 9 of 11 subjects is not itself sufficient to predict ocular inflammation after gene therapy. However, given the correlation of the CD4/CD8 ratio to the ocular inflammation observed, attention to the baseline immune status may help tailor future immune modulation regimens for XLRS subjects receiving ocular gene therapy. The trial protocol queried the XLRS subjects about recent infections or inflammatory conditions, but not in great detail, and while their responses were negative, this may have influenced the baseline CD4/CD8 ratio. The controls were sex matched and approximately age matched. A larger cohort of treated XLRS subjects will be required to probe this correlation further.
The considerable deviation from normal in the DC axis of XLRS at baseline is a further indication of the altered immune status of these trial subjects. Flow cytometry pointed toward downregulation of CD11c+ myeloid DCs and an elevation of CD123+ pDCs. pDCs produce type I IFNs in response to viruses and drive anti-viral immunity, and the balance between mDCs and pDCs is an important factor for immunological tolerance.36,37 This elevated frequency of pro-inflammatory cells in XLRS at baseline would be consistent with a chronic inflammatory state. Cytokine analysis at baseline also showed elevated levels of Th1 pro-inflammatory cytokines IFN-γ and TNF-α. Th1 responses generally are injurious to ocular tissues, as Th1 cells secrete IFN-γ, which promotes phagocytosis and immunoglobulin (Ig)G2a secretion, a complement-fixing class of antibodies.38,39 Others have noted a synergistic effect of IFN-γ and TNF-α that contributes to corticosteroid resistance,40 which may diminish the effects of systemic immune suppression. This may apply to subject 9 for whom pre-dosing with oral corticosteroid was minimally effective in curbing ocular inflammation after dosing.
Reduced serum EGF at XLRS baseline is consistent with an imbalance in immune modulation activity. Dermatitis studies have linked elevated EGF with reduced inflammatory signals41 and with improving immune defects in EGF receptor inhibitor (EGFRI)-related skin toxicities.42 EGF supplementation enhanced the immune system in rats,43 and injecting EGF caused T cell-dependent immune suppression against sheep red blood cell (RBC) antigens.44 EGF also suppresses Th cell responses,45,46 and, conversely, it is possible that Th cells show expansion and activation when EGF levels are downregulated in XLRS, thus affecting the CD4/CD8 ratio in XLRS.
The cytokine/chemokine alterations suggest an altered Th1-Th2 balance in XLRS at baseline that would impact signaling pathways, including the MAPK, TNF receptor (TNFR), and TLR pathways.23,24 If substantiated in a larger study, this may prove useful in devising an immune suppression strategy for XLRS and other gene therapy trials. Although systemic immune changes may not manifest clinically in XLRS at baseline, such changes may play a role in modulating the immune response after vector dosing of XLRS subjects. Further studies are needed to evaluate the pathways and mechanisms underlying the baseline immune alteration we observed in XLRS subjects. Immune homeostasis requires multiple and complex regulatory mechanisms that are still not well understood. Whether the immune imbalance in XLRS is influenced by a lack of redistribution from secondary lymphoid tissues, by greater retinal antigen leakage into systemic circulation, or by other mechanisms affecting systemic immune cell proportions remains unknown at this time.
Reports indicate that retinal degenerative diseases may manifest observable ocular immune activation. Cell-mediated immunity is reported against sequestered antigens in geographic atrophy macular degeneration47 and retinitis pigmentosa (RP).48 Yoshida et al.49 reported that one-third of adult RP subjects have clinically observable cellular reaction in the anterior vitreous. Sudharsan et al.50 showed involvement of innate immune system pathways in rcd1 and xlpra2 dog models of RP at late stages. These reports implicate involvement of the cell-mediated immunity in retinal degeneration, and that peripheral immune changes can reflect the ocular immune status in retinal disorders according to Verhagen et al.,51 who noted elevated levels of pro-inflammatory immune mediators in baseline serum of a CRB1 RP patient. One possible contributor to elevated baseline CD4/CD8 ratios in XLRS is the slow loss of photoreceptors in the posterior retina.52 Retinal proteins reaching systemic circulation could activate the immune system, consistent with the activation described for other inherited retinal disorders.47,53
The altered baseline immune status in XLRS affected individuals is consistent with a proinflammatory state observed in XLRS mouse models as described in our recent study.54 Analysis of differential gene expression profiles in Rs1-knockout (KO) (Rs1−/Y) retina showed major dysregulation of immune response genes at an early age, indicating an initial cell injury response to structural schisis. The study also demonstrated that the retina lacking Rs1 can transition back to immune quiescence upon expressing AAV8-RS1 vector, implying that the lack of Rs1 destabilizes retinal immune regulation.
Immune profile changes after dosing
Several interesting observations stand out after AAV8-RS1 administration. Circulating CD11b+CD11c+ immunoregulatory cells were substantially decreased in 8 of the 11 XLRS subjects at day 14. Four of five subjects who received the higher doses of 1e11 or 3e11 vg/eye had a decrease of circulating CD11b+CD11c+ cells. CD11b+CD11c+ cells are known to maintain, or are implicated in maintaining, immune tolerance in the peripheral circulation.55 A reduced frequency in subjects after dosing may increase susceptibility to immune activation, particularly for the higher vector doses.
The vector appears to elicit a cytotoxic T cell immune response, as CD8+HLA-DR+ cytotoxic T cells were upregulated in 10 of the 11 subjects after vector administration. Activated CD8+ killer T cells have been shown to play a role in response to the vector capsid.56 CD68+CD80+ macrophages were also elevated after vector dosing. These macrophages are known to produce pro-inflammatory cytokines and activate other antigen-presenting cells.57 The correlation of ocular inflammation scores to the level of CD68+CD80+ macrophages in this study of XLRS trial subjects indicates that vector administration may aggravate cytotoxic immune reactions associated with ocular inflammation. In the autoimmune diseases of atherosclerosis and rheumatoid arthritis, macrophages expressing granzyme B mediate a cytotoxic immune response causing degradation of extracellular matrix remodelling.58 This is interesting, as one hallmark of XLRS disease is the disruption of the structural extracellular matrix integrity leading to schisis cavities, and we found increased serum levels of granzyme B in five of six subjects who showed ocular inflammation.
Immune changes after vector dosing may indicate that AAV8 vector capsid activates or aggravates systemic effector and antigen-presenting cells. However, it is not known whether the ocular inflammatory response is directed against the capsid protein or the transgene, or as others have found, against cis-regulatory sequences.5 The published interim report of the AAV8-RS1 trial17 showed induction of NABs against AAV8 capsid for subjects 4–9 dosed at medium and higher doses of 1e10 and 1e11 vg/eye, but there was no indication of antibodies against the therapeutic RS1 transgene at these doses.
Cellular immune responses were also noted in AAV delivery of human coagulation factor IX (FIX) in hemophilia B patients,59, 60, 61 including CD8 T cell responses for high vector doses.62 Similarly, AAV1-AAT (α-1 antitrypsin) elicited a cellular immune response against capsid proteins by day 14 in all treated subjects with AAT deficiency.63 Ocular trials have reported cellular responses, including five of eight adult participants who received subretinal AAV2-RPE65 at 1e11 or 1e12 vg/eye and who exhibited immune-related events with circulating anti-AAV2 NABs and IFN-γ responses to the capsid by enzyme-linked immunospot (ELISPOT) at 4 weeks post-injection.13 These reports implicate IFN-γ-secreting T cells as contributors to the AAV capsid immune response for either systemic or local administration. Our study was concurrent to previous reports and found a systemic activation of CD8+HLA-DR+ T cells in AAV8-RS1-injected subjects at day 14 after vector injection, implicating a greater role of cytotoxic T cells in vector-evoked immune response.
Gene therapy in this trial was applied via intravitreal injection, while most trials perform subretinal injections. These routes of application have a very different biodistribution profile, as indicated in NHP studies.9 Inflammation is reported more frequently following intravitreal vector delivery64,65 but is also encountered following subretinal application,13 albeit milder and less frequently. The mechanisms of ocular immune responses may not be identical for these two routes of delivery; for example, cellular infiltrates were observed with intravitreal injection66 but not for subretinal delivery in which a Th2-mediated immune deviant response is mounted.67 The best medical coverage may require immune suppression regimens that match the immune activation pathway and severity.
Few comparisons have been made as to the intraocular versus systemic immune profiles following ocular vector delivery, and these give discordant outcomes: one showing induction of both ocular and systemic cellular responses,7,17,64 a second that shows the absence of systemic immune reponse upon ocular AAV delivery,68 while a third shows neither an ocular or systemic immune response.69,70 Evaluation of vector biodistribution following AAV high vector dosing has indicated substantial vector shedding in peripheral and systemic circulation, including tears and urine after both subretinal and intravitreal administration,9 which could lead to either or both the humoral and cellular adaptive immune responses.71 This indicates value in monitoring systemic immune profiles in gene therapy trials. Ultimately the question of concordance between intraocular vitreous or aqueous samples versus systemic immune response levels in humans can only be resolved by studies performing direct comparison. Until then, the present study shows that some information can derive from monitoring systemic immune activity in ocular gene therapy trials.
Materials and methods
Study design
The XLRS subjects in the present study derive from an ongoing clinical gene therapy trial conducted by the National Eye Institute (NIH protocol 15-EI-0038) as a single center, open-label phase I/IIa study at the Clinical Center of the National Institutes of Health (ClinicalTrials.gov: NCT02317887). The trial adheres to the tenets of the Declarations of Helsinki, and the protocol was reviewed and approved by the NIH Institutional Review Board and the Recombinant DNA Advisory Committee. Study oversight is provided by an independent Data and Safety Monitoring Committee. Each subject provided informed consent prior to enrollment and consented to blood collection for purposes of studying systemic factors affected by vector dosing.
Analysis of immune responses was performed for the first 11 subjects dosed in this trial who received doses of 1e9–3e11 vg/eye. Each subject received a single dose of AAV8-RS1 vector in one eye. All doses were administered by intravitreal injection using a ½-inch 28G needle. Three subjects each were dosed at 1e9 and 1e10 vg/eye, four at 1e11 vg/eye, and one at 3e11 vg/eye. The sample collection protocol was determined prior to study initiation, and blood was collected for PBMCs and serum at baseline prior to vector administration and at day 14 after injection. Immune cell profiles and cytokine/chemokine levels were analyzed for these time points. Twelve healthy individuals were analyzed as controls, with de-identified blood and serum samples provided by the NIH Blood Bank (Bethesda, MD, USA), anonymized except for age, race, and sex (all male, as were the XLRS trial subjects). The following cell immune profile changes were analyzed: (1) XLRS subjects at baseline versus healthy controls, and (2) XLRS subjects at baseline versus day 14 after AAV8-RS1 vector application.
The vector dose, ocular inflammation, and immune suppression regimen for each subject are summarized in Table S4. Tables S5 and S6 present information on the uveitis observed in the subjects. The ocular inflammation after vector application was graded according to the Society of Uveitis Nomenclature (SUN) scale,72 by the presence of vitreous cells (VCs) and by inflammatory cells in the anterior chamber (AC). The higher values of the AC and VC grades were used for correlation studies with immune factors. The blood samples in XLRS at baseline and day 14 were drawn before steroid treatment in subjects 1–8. Subject 9 was pretreated with oral prednisone and topical steroid at 2 days before dosing and continued beyond day 14. Subjects 10 and 11 were treated with cyclosporine at 175 mg twice daily beginning 3 weeks before dosing, and myclophenolate mofetil (MMF) at 500 mg twice daily at 3 weeks and 1,000 mg twice daily at 2 weeks before dosing plus prednisone at 60 mg 2 days before vector dosing. Therefore, the baseline and day 14 blood samples for subjects 9–11 were drawn while the immune suppression regimen was ongoing.
Management of clinical ocular inflammation in the AAV8-RS1 trial
The immune status is presented for the first 11 subjects in the dose-ranging phase I/IIa XLRS gene therapy trial, including the 9 initial trial subjects for whom clinical details were published previously.17 The AAV8-RS1 trial is a dose-ranging safety study, and the medical protocol for handling ocular inflammation evolved and changed during the trial. Immune suppression evolved during the trial as follows: six subjects (1–6) received the lower doses (1e9 and 1e10 vg/eye) and had no immune modulation treatment at baseline or for day 14 samples. Only subject 5 (1e10 vg/eye) showed mild ocular inflammation with delayed onset at day 32, and he then received oral prednisone briefly with rapid resolution of the inflammation. At the higher doses (1e11 and 3e11 vg/eye), all five subjects developed ocular inflammation, four had moderate or greater inflammation by day 14, while subject 10 had mild inflammation with delayed onset at month 3, when the systemic immune drug regimen had been stopped. For immune modulation, subjects 7 and 8 (1e11 vg/eye) received no prophylactic treatment for baseline or day 14 samples but then were treated medically with oral and topical steroids after ocular inflammation occurred. With this as a guide, subject 9 (1e11 vg/eye) was pre-treated prophylactically with oral prednisone beginning 2 days before vector administration, but he exhibited the greatest ocular inflammation nevertheless. Prophylactic immune suppression was augmented for subject 10 (1e11 vg/eye) and subject 11 (3e11 vg/eye), using cyclosporine and MMF beginning 2–4 weeks before vector dosing plus oral prednisone started 2 days before dosing. Subject 10 (1e11 vg/eye) had no inflammation until 3 months after dosing when the AC showed trace to 1+ cell. With this apparent success of systemic suppression, the dose was increased for subject 11 (3e11 vg/eye); he had modest inflammation by 14 days post-dosing, but the inflammation was less than for subject 9 (1e11 vg/eye), who was pre-treated only with oral prednisone before dosing.
Control subjects
Anonymized blood and serum samples of 12 healthy male subjects were provided by the NIH Blood Bank (Bethesda, MD, USA). The 11 XLRS subjects ranged in age from 23 to 72 years (mean, 49 ± 12 years); the 12 subjects for PBMC controls were 21–73 years of age (mean, 53 ± 17 years); and the 12 serum controls were 25–65 years of age (mean, 50 ± 13 years). The racial mix was not fully identical: 10 of the 11 XLRS subjects were white and 1 was Hispanic; the PBMC controls were 6 white, 5 black, and 1 Asian; and the serum controls were 7 white, 4 black, and 1 Asian.
Blood collection and PBMC isolation
Blood was collected from each XLRS subject at baseline and day 14 in heparinized tubes. Samples were transported the same day to Advanced Biosciences Laboratory (ABL, Rockville, MD, USA) for PBMC isolation. The PBMCs were maintained frozen in LN until use. These samples were subsequently retrieved, batched, and run together about 1 year after obtaining the final sample from subject 11. Anonymized control buffy coat samples were obtained from 12 healthy donors by the NIH Blood Bank of the NIH Clinical Center (Bethesda, MD, USA), and samples were processed immediately in our laboratory following protocols as used by ABL for XLRS samples by Ficoll density centrifugation73 to isolate PBMCs. Cells were collected and washed three times with phosphate-buffered saline (PBS) after RBC lysis using ACK lysis buffer (Thermo Fisher Scientific, Waltham, MA, USA), cryopreserved in recovery cryomedia (recovery cell culture freezing medium, Gibco, Gaithersburg, MD, USA), and stored frozen in LN until use.
Serum isolation
Blood was collected in serum separator tubes from XLRS subjects, and serum was isolated in the clinical laboratories of the Ophthalmic Genetics and Visual Function Branch of the National Eye Institute. The NIH Blood Bank (Bethesda, MD, USA) provided fresh blood samples from 12 healthy donors who were the volunteers for that day. Samples were left undisturbed at room temperature (RT) for 30 min to allow for clot formation. The clot was removed by refrigerated centrifugation at 1,200 × g for 10 min. Samples from XLRS and controls were handled the same way. Serum samples were stored at −80°C until retrieved for use at a later time for cytokine/chemokine profiling.
Panel design and optimization for immunophenotyping
A panel of antibodies was designed to analyze and enumerate various peripheral blood immune cell populations and subsets. The spillover compensation and fluorochrome compatibility was determined by a spillover matrix and antibody stain index preparation. Live and dead cells were differentiated using Live/Dead fixable aqua dye and DAPI (4′,6-diamidino-2-phenylindole) as markers. Table S7 provides information on the clones for the antibodies, stain index, vendors, and catalog numbers. Fluorescence minus one (FMO) controls were prepared to gate for the required cell subsets and to minimize false staining.
Sample preparation
An antibody cocktail for surface markers was prepared with optimized dilution for each antibody. 106 cells/50 μL of buffer were added to 50 μL of antibody cocktail and incubated for 30 min at RT, followed by two washes with fluorescence-activated cell sorting (FACS) buffer (PBS + 1% fetal bovine serum [FBS]). For staining with intracellular markers, the cells were first stained with cell surface markers, permeabilized, and washed with Perm/Wash buffer (BD Biosciences, San Jose, CA, USA) for 20 min at RT, and then intracellular antibody was added to the cells and incubated at RT for a further 30 min. Finally, the cells were washed twice and resuspended in 300 μ of FACS buffer. The data were acquired within an hour on a CytoFLEX flow cytometer (Beckman Coulter Life Sciences, USA).
Data analysis and gating strategy
The data were analyzed using CytExpert v2.3 and FlowJo v10.1 (BD Biosciences, San Jose, CA, USA) using the schematic analysis summarized in Figure S3. The panel selects the live cells based on exclusion of dead cells by DAPI and VivoTag staining. The whole live cells were then gated (forward scatter [FSC]-area versus FSC-height) to exclude the doublets. The CD45+CD3+ population was then gated to identify Th cells (CD3+CD4+), cytotoxic T cells (CD3+CD8+), activated T cells (CD4+HLA-DR+ and CD8+HLA-DR+), and B cells (CD3-CD19+). The Tregs (CD4+CD25+FOXP3+) were gated on the CD45+CD4+ cells. Lymphocytes were gated for CD45+CD3+CD19−/CD45+CD3−CD19− cells. The CD3−CD19− cells were gated for CD56+ cells and were then analyzed for CD56bright and CD16+CD56− NK cells. The CD3+CD19− cells were gated for CD56+ cells and analyzed for total NKT cells. The CD3−CD19− population was also plotted for classical, intermediate, and non-classical monocytes (CD14+CD16−, CD14+CD16+, and CD14−CD16+). The CD14−CD16− population was then gated for CD56 and HLA-DR. The CD56−HLA-DR+ population was further gated for CD123+CD11c− pDCs, CD11c− mDCs, and CD11b+CD11c+ DCs. The CD11b+ cells from a whole-singlet population were plotted for CD68+CD80+ and CD68+CD163+ macrophages.
Multiplex bead analysis of cytokines/chemokines
The serum concentrations of cytokines/chemokines were analyzed with Luminex multiplex bead immunoassay technology with fluorescent multiplex beads (Luminex performance human XL cytokine discovery magnetic panel, R&D Systems, Minneapolis, MN, USA) performed according to the manufacturer’s instructions. The panel was designed for simultaneous quantitation of 45 cytokines and chemokines that are produced in innate immune responses to promote inflammation and recruitment of T cells that trigger an adaptive immune response. The serum analytes were cytokines/chemokines that are secreted by major immune cell subsets such as T cells, B cells, DCs, NK cells, and monocytes/macrophages. All samples were run in duplicates, and the mean of two measurements was used for any statistical analysis. Positive and negative controls were included to determine cytokine recovery, and blank values were subtracted from all readings. Standard curves for each cytokine were generated by using the reference cytokine concentrations supplied by the manufacturer, and values for the upper and lower limits of quantification were determined using Luminex 100 software. Cytokines with values out of range (OOR), either above ORR or below ORR, reflect saturated or undetectable levels of the cytokine, respectively. Analytes for which 25% or more subjects/healthy controls were OOR were excluded in comparative analyses, thus bringing down the number of analyzed parameters to 29. Significance was considered at p ≤0.05. The immune cells and serum analytes that exhibited significant difference from normal at baseline or after vector dosing have been tabulated in the Tables S2 and S3, respectively. The non-adjusted p values for all comparisons made in the study have been tabulated in Tables S8 and S9 for immune cells and serum analytes, respectively.
Statistical analysis
An unpaired t test was used to compare PBMC populations and cytokine/chemokine levels between the baseline XLRS and 12 healthy controls. A paired t test was performed to compare XLRS at pre-dosing baseline and day 14 after vector injection. p values were not corrected for multiple comparisons, as this study cannot be definitive due to the small numbers of subjects in the AAV8-RS1 trial. Immune cell subsets or cytokine/chemokines that exhibited significant difference from normal (p ≤ 0.05) at pre-dosing baseline or day 14 after dosing were evaluated further by simple linear regression to explore relationships between XLRS clinical ocular inflammation after dosing (using the inflammation scores for each subject) with baseline values of cells and cytokines. To analyze whether individual XLRS subjects showed a significant change in the CD4/CD8 ratio, immune cell subsets/serum cytokines, or chemokines, a 95% CI of the sample distribution was determined for the healthy controls. CD4/CD8 values of XLRS individuals higher than the upper CI of healthy controls was considered elevated, while those below the lower CI were considered downregulated. Significance was considered at p ≤0.05. Statistical tests were performed using GraphPad Prism 8 (GraphPad, La Jolla, CA, USA).
Statistically significant outcomes for this study are limited because of the small number of individuals who have been dosed in this trial, including at the higher dose levels of 1e11 and 3e11 vg/eye, which elicited ocular inflammation. As the number of XLRS subjects in the trial is small, values were not corrected for multiple comparisons.
Acknowledgments
We thank Rafael Villasmil and Julie Laux of the NEI Flow Cytometry Core for their help in flow cytometry sample acquisition and analysis. Ankit Agrawal of the NHLBI Flow Cytometry Core helped with Luminex multiplex bead assays. The NIH Blood Bank provided deidentified human blood samples for donor buffy coat for PBMCs and whole blood for serum from healthy control volunteers. This study was supported by the NIH Intramural Research Program through the National Eye Institute (NEI) (DC000065), and by the UC Davis Medical School Dean’s Fund support to P.A.S. The content is the responsibility of the authors and does not necessarily represent an official view of the NEI or NIH.
Author contributions
A.M. designed and implemented the studies, prepared samples, acquired data, performed first-pass analysis, and wrote the original manuscript draft and revised the manuscript. C.V. assisted with study conception, design, sample handling, and manuscript editing. C.A.C. performed trial medical care and clinical observation, subject consenting, and manuscript editing. H.E.W. assisted with trial patient care, determination of clinical inflammation, administration of the clinical vector, and manuscript editing. H.N.S. gave oversight of immune data analysis and manuscript editing. Y.Z. assisted with sample preparation and handling and manuscript editing. L.L.W. assisted with study conception and manuscript editing. P.A.S. assisted in study conception and conduct, writing, editing, and revision of the original manuscript and final drafts, and supervised the research activity.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.02.013.
Supplemental information
References
- 1.Dalkara D., Sahel J.A. Gene therapy for inherited retinal degenerations. C. R. Biol. 2014;337:185–192. doi: 10.1016/j.crvi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennet J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang X., Yu C., Tzekov R.T., Zhu Y., Li W. The effect of human gene therapy for RPE65-associated Leber’s congenital amaurosis on visual function: a systematic review and meta-analysis. Orphanet J. Rare Dis. 2020;15:49. doi: 10.1186/s13023-020-1304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dimopoulos I.S., Hoang S.C., Radziwon A., Binczyk N.M., Seabra M.C., MacLaren R.E., Somani R., Tennant M.T.S., MacDonald I.M. Two-year results after AAV2-mediated gene therapy for choroideremia: the Alberta experience. Am. J. Ophthalmol. 2018;193:130–142. doi: 10.1016/j.ajo.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Xiong W., Wu D.M., Xue Y., Wang S.K., Chung M.J., Ji X., Rana P., Zhao S.R., Mai S., Cepko C.L. AAV cis-regulatory sequences are correlated with ocular toxicity. Proc. Natl. Acad. Sci. USA. 2019;116:5785–5794. doi: 10.1073/pnas.1821000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdera H.C., Kuranda K., Mingozzi F. AAV vector immunogenicity in humans: a long journey to successful gene transfer. Mol. Ther. 2020;28:723–746. doi: 10.1016/j.ymthe.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vandenberghe L.H., Bell P., Maguire A.M., Cearley C.N., Xiao R., Calcedo R., Wang L., Castle M.J., Maguire A.C., Grant R. Dosage thresholds for AAV2 and AAV8 photoreceptor gene therapy in monkey. Sci. Transl. Med. 2011;3:88ra54. doi: 10.1126/scitranslmed.3002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramachandran P.S., Lee V., Wei Z., Song J.Y., Casal G., Cronin T., Willett K., Huckfeldt R., Morgan J.I., Aleman T.S. Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 2017;28:154–167. doi: 10.1089/hum.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seitz I.P., Michalakis S., Wilhelm B., Reichel F.F., Ochakovski G.A., Zrenner E., Ueffing M., Biel M., Wissinger B., Bartz-Schmidt K.U., RD-CURE Consortium Superior retinal gene transfer and biodistribution profile of subretinal versus intravitreal delivery of AAV8 in nonhuman primates. Invest. Ophthalmol. Vis. Sci. 2017;58:5792–5801. doi: 10.1167/iovs.17-22473. [DOI] [PubMed] [Google Scholar]
- 10.Flotte T.R. Recombinant adeno-associated virus vectors for cystic fibrosis gene therapy. Curr. Opin. Mol. Ther. 2001;3:497–502. [PubMed] [Google Scholar]
- 11.Conlon T.J., Deng W.T., Erger K., Cossette T., Pang J.J., Ryals R., Clément N., Cleaver B., McDoom I., Boye S.E. Preclinical potency and safety studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum. Gene Ther. Clin. Dev. 2013;24:23–28. doi: 10.1089/humc.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabatino D.E., Lange A.M., Altynova E.S., Sarkar R., Zhou S., Merricks E.P., Franck H.G., Nichols T.C., Arruda V.R., Kazazian H.H., Jr. Efficacy and safety of long-term prophylaxis in severe hemophilia A dogs following liver gene therapy using AAV vectors. Mol. Ther. 2011;19:442–449. doi: 10.1038/mt.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge J.W., Mehat M.S., Sundaram V., Robbie S.J., Barker S.E., Ripamonti C., Georgiadis A., Mowat F.M., Beattie S.G., Gardner P.J. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cideciyan A.V., Jacobson S.G., Beltran W.A., Sumaroka A., Swider M., Iwabe S., Roman A.J., Olivares M.B., Schwartz S.B., Komáromy A.M. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc. Natl. Acad. Sci. USA. 2013;110:E517–E525. doi: 10.1073/pnas.1218933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson S.G., Cideciyan A.V., Roman A.J., Sumaroka A., Schwartz S.B., Heon E., Hauswirth W.W. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breous E., Somanathan S., Bell P., Wilson J.M. Inflammation promotes the loss of adeno-associated virus-mediated transgene expression in mouse liver. Gastroenterology. 2011;141:348–357. doi: 10.1053/j.gastro.2011.04.002. 357.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cukras C., Wiley H.E., Jeffrey B.G., Sen H.N., Turriff A., Zeng Y., Vijayasarathy C., Marangoni D., Ziccardi L., Kjellstrom S. Retinal AAV8-RS1 gene therapy for X-linked retinoschisis: initial findings from a phase I/IIa trial by intravitreal delivery. Mol. Ther. 2018;26:2282–2294. doi: 10.1016/j.ymthe.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBride J.A., Striker R. Imbalance in the game of T cells: what can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13:e1006624. doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKenna K., Beignon A.S., Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J. Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swiecki M., Gilfillan S., Vermi W., Wang Y., Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8+ T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pashenkov M., Huang Y.M., Kostulas V., Haglund M., Söderström M., Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- 22.Mellor-Heineke S., Villanueva J., Jordan M.B., Marsh R., Zhang K., Bleesing J.J., Filipovich A.H., Risma K.A. Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front. Immunol. 2013;4:72. doi: 10.3389/fimmu.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenborn J.R., Wilson C.B. Regulation of interferon-γ during innate and adaptive immune responses. Adv. Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 24.Stenvinkel P., Ketteler M., Johnson R.J., Lindholm B., Pecoits-Filho R., Riella M., Heimbürger O., Cederholm T., Girndt M. IL-10, IL-6, and TNF-α: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 25.Noren Hooten N., Longo D.L., Evans M.K. Age- and race-related changes in subpopulations of peripheral blood lymphocytes in humans. In: Fulop T., Franceschi C., Hirokawa K., Pawelec G., editors. Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Springer International; 2018. pp. 1–30. [Google Scholar]
- 26.D’Amelio R., Frati C., Fattorossi A., Aiuti F. Peripheral T-cell subset imbalance in patients with vitiligo and in their apparently healthy first-degree relatives. Ann. Allergy. 1990;65:143–145. [PubMed] [Google Scholar]
- 27.Jabs D.A., Arnett F.C., Bias W.B., Beale M.G. Familial abnormalities of lymphocyte function in a large Sjögren’s syndrome kindred. J. Rheumatol. 1986;13:320–326. [PubMed] [Google Scholar]
- 28.Kaaba S.A., Al-Harbi S.A. Abnormal lymphocyte subsets in Kuwaiti patients with type-1 insulin-dependent diabetes mellitus and their first-degree relatives. Immunol. Lett. 1995;47:209–213. doi: 10.1016/0165-2478(95)00088-5. [DOI] [PubMed] [Google Scholar]
- 29.Shalit F., Sredni B., Brodie C., Kott E., Huberman M. T lymphocyte subpopulations and activation markers correlate with severity of Alzheimer’s disease. Clin. Immunol. Immunopathol. 1995;75:246–250. doi: 10.1006/clin.1995.1078. [DOI] [PubMed] [Google Scholar]
- 30.Crucian B., Dunne P., Friedman H., Ragsdale R., Pross S., Widen R. Alterations in levels of CD28−/CD8+ suppressor cell precursor and CD45RO+/CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin. Diagn. Lab. Immunol. 1995;2:249–252. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazuardi L., Jenewein B., Wolf A.M., Pfister G., Tzankov A., Grubeck-Loebenstein B. Age-related loss of naïve T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amadori A., Zamarchi R., De Silvestro G., Forza G., Cavatton G., Danieli G.A., Clementi M., Chieco-Bianchi L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1995;1:1279–1283. doi: 10.1038/nm1295-1279. [DOI] [PubMed] [Google Scholar]
- 33.Jentsch-Ullrich K., Koenigsmann M., Mohren M., Franke A. Lymphocyte subsets’ reference ranges in an age- and gender-balanced population of 100 healthy adults—a monocentric German study. Clin. Immunol. 2005;116:192–197. doi: 10.1016/j.clim.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Urbančič M., Kloboves Prevodnik V., Petrovič D., Globočnik Petrovič M. A flow cytometric analysis of vitreous inflammatory cells in patients with proliferative diabetic retinopathy. BioMed Res. Int. 2013;2013:251528. doi: 10.1155/2013/251528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dave N., Chevour P., Mahendradas P., Venkatesh A., Kawali A., Shetty R., Ghosh A., Sethu S. Increased aqueous humor CD4+/CD8+ lymphocyte ratio in sarcoid uveitis. Ocul. Immunol. Inflamm. 2019;27:1033–1040. doi: 10.1080/09273948.2017.1421232. [DOI] [PubMed] [Google Scholar]
- 36.Fang W.N., Shi M., Meng C.Y., Li D.D., Peng J.P. The balance between conventional DCs and plasmacytoid DCs is pivotal for immunological tolerance during pregnancy in the mouse. Sci. Rep. 2016;6:26984. doi: 10.1038/srep26984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadowaki N. The divergence and interplay between pDC and mDC in humans. Front. Biosci. 2009;14:808–817. doi: 10.2741/3279. [DOI] [PubMed] [Google Scholar]
- 38.Tau G., Rothman P. Biologic functions of the IFN-γ receptors. Allergy. 1999;54:1233–1251. doi: 10.1034/j.1398-9995.1999.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willett K., Bennett J. Immunology of AAV-mediated gene transfer in the eye. Front. Immunol. 2013;4:261. doi: 10.3389/fimmu.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Britt R.D., Jr., Thompson M.A., Sasse S., Pabelick C.M., Gerber A.N., Prakash Y.S. Th1 cytokines TNF-α and IFN-γ promote corticosteroid resistance in developing human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2019;316:L71–L81. doi: 10.1152/ajplung.00547.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi S.Y., Lee Y.J., Kim J.M., Kang H.J., Cho S.H., Chang S.E. Epidermal growth factor relieves inflammatory signals in Staphylococcus aureus-treated human epidermal keratinocytes and atopic dermatitis-like skin lesions in Nc/Nga mice. BioMed Res. Int. 2018;2018:9439182. doi: 10.1155/2018/9439182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.M., Ji J.H., Kim Y.S., Lee S., Oh S.Y., Huh S.J., Son C.H., Kang J.H., Ahn S.Y., Choo J.E. rhEGF treatment improves EGFR inhibitor-induced skin barrier and immune defects. Cancers (Basel) 2020;12:E3120. doi: 10.3390/cancers12113120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grases-Pintó B., Torres-Castro P., Marín-Morote L., Abril-Gil M., Castell M., Rodríguez-Lagunas M.J., Pérez-Cano F.J., Franch À. Leptin and EGF supplementation enhance the immune system maturation in preterm suckling rats. Nutrients. 2019;11:E2380. doi: 10.3390/nu11102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koch J.H., Rowe J. Immunosuppressive activity of submaxillary gland extracts of the mouse. I. Effect on antibody formation in response to sheep red blood cells. Eur. J. Immunol. 1976;6:583–588. doi: 10.1002/eji.1830060811. [DOI] [PubMed] [Google Scholar]
- 45.Acres R.B., Lamb J.R., Feldman M. Effects of platelet-derived growth factor and epidermal growth factor on antigen-induced proliferation of human T-cell lines. Immunology. 1985;54:9–16. [PMC free article] [PubMed] [Google Scholar]
- 46.Koch J.H., Fifis T., Bender V.J., Moss B.A. Molecular species of epidermal growth factor carrying immunosuppressive activity. J. Cell. Biochem. 1984;25:45–59. doi: 10.1002/jcb.240250105. [DOI] [PubMed] [Google Scholar]
- 47.Tamm S.A., Whitcup S.M., Gery I., Wiggert B., Nussenblatt R.B., Kaiser-Kupfer M.I. Immune response to retinal antigens in patients with gyrate atrophy and other hereditary retinal dystrophies. Ocul. Immunol. Inflamm. 2001;9:75–84. doi: 10.1076/ocii.9.2.75.3972. [DOI] [PubMed] [Google Scholar]
- 48.Heckenlively J.R., Jordan B.L., Aptsiauri N. Association of antiretinal antibodies and cystoid macular edema in patients with retinitis pigmentosa. Am. J. Ophthalmol. 1999;127:565–573. doi: 10.1016/s0002-9394(98)00446-2. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida N., Ikeda Y., Notomi S., Ishikawa K., Murakami Y., Hisatomi T., Enaida H., Ishibashi T. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120:100–105. doi: 10.1016/j.ophtha.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Sudharsan R., Beiting D.P., Aguirre G.D., Beltran W.A. Involvement of innate immune system in late stages of inherited photoreceptor degeneration. Sci. Rep. 2017;7:17897. doi: 10.1038/s41598-017-18236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verhagen F., Kuiper J., Nierkens S., Imhof S.M., Radstake T., de Boer J. Systemic inflammatory immune signatures in a patient with CRB1 linked retinal dystrophy. Expert Rev. Clin. Immunol. 2016;12:1359–1362. doi: 10.1080/1744666X.2016.1241709. [DOI] [PubMed] [Google Scholar]
- 52.Kjellstrom S., Bush R.A., Zeng Y., Takada Y., Sieving P.A. Retinoschisin gene therapy and natural history in the Rs1h-KO mouse: long-term rescue from retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:3837–3845. doi: 10.1167/iovs.07-0203. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto J.H., Okajima O., Mochizuki M., Shinohara T., Wiggert B., Chader G.J., Gery I., Nussenblatt R.B. Cellular immune responses to retinal antigens in retinitis pigmentosa. Graefes Arch. Clin. Exp. Ophthalmol. 1992;230:119–123. doi: 10.1007/BF00164648. [DOI] [PubMed] [Google Scholar]
- 54.Vijayasarathy C., Zeng Y., Brooks M.J., Fariss R.N., Sieving P.A. Genetic rescue of X-linked retinoschisis mouse (Rs1−/y) retina induces quiescence of the retinal microglial inflammatory state following AAV8-RS1 gene transfer and identifies gene networks underlying retinal recovery. Hum. Gene Ther. 2020 doi: 10.1089/hum.2020.213. Published online December 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H., Zhang G.X., Chen Y., Xu H., Fitzgerald D.C., Zhao Z., Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng Q., Luo Y., Chang C., Wu H., Ding Y., Xiao R. The emerging epigenetic role of CD8+T cells in autoimmune diseases: a systematic review. Front. Immunol. 2019;10:856. doi: 10.3389/fimmu.2019.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chu F., Shi M., Zheng C., Shen D., Zhu J., Zheng X., Cui L. The roles of macrophages and microglia in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2018;318:1–7. doi: 10.1016/j.jneuroim.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Kim W.J., Kim H., Suk K., Lee W.H. Macrophages express granzyme B in the lesion areas of atherosclerosis and rheumatoid arthritis. Immunol. Lett. 2007;111:57–65. doi: 10.1016/j.imlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Mueller C., Flotte T.R. Clinical gene therapy using recombinant adeno-associated virus vectors. Gene Ther. 2008;15:858–863. doi: 10.1038/gt.2008.68. [DOI] [PubMed] [Google Scholar]
- 60.Mingozzi F., Maus M.V., Hui D.J., Sabatino D.E., Murphy S.L., Rasko J.E., Ragni M.V., Manno C.S., Sommer J., Jiang H. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 61.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 62.Markusic D.M., Herzog R.W. Liver-directed adeno-associated viral gene therapy for hemophilia. J. Genet. Syndr. Gene Ther. 2012;1:1–9. doi: 10.4172/2157-7412.S1-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nathwani A.C., Tuddenham E.G., Rangarajan S., Rosales C., McIntosh J., Linch D.C., Chowdary P., Riddell A., Pie A.J., Harrington C. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bouquet C., Vignal Clermont C., Galy A., Fitoussi S., Blouin L., Munk M.R., Valero S., Meunier S., Katz B., Sahel J.A., Thomasson N. Immune response and intraocular inflammation in patients with Leber hereditary optic neuropathy treated with intravitreal injection of recombinant adeno-associated virus 2 carrying the ND4 gene: a secondary analysis of a phase 1/2 clinical trial. JAMA Ophthalmol. 2019;137:399–406. doi: 10.1001/jamaophthalmol.2018.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Timmers A.M., Newmark J.A., Turunen H.T., Farivar T., Liu J., Song C., Ye G.J., Pennock S., Gaskin C., Knop D.R., Shearman M.S. Ocular inflammatory response to intravitreal injection of adeno-associated virus vector: relative contribution of genome and capsid. Hum. Gene Ther. 2020;31:80–89. doi: 10.1089/hum.2019.144. [DOI] [PubMed] [Google Scholar]
- 66.Reichel F.F., Dauletbekov D.L., Klein R., Peters T., Ochakovski G.A., Seitz I.P., Wilhelm B., Ueffing M., Biel M., Wissinger B., RD-CURE Consortium AAV8 can induce innate and adaptive immune response in the primate eye. Mol. Ther. 2017;25:2648–2660. doi: 10.1016/j.ymthe.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett J., Pakola S., Zeng Y., Maguire A. Humoral response after administration of E1-deleted adenoviruses: immune privilege of the subretinal space. Hum. Gene Ther. 1996;7:1763–1769. doi: 10.1089/hum.1996.7.14-1763. [DOI] [PubMed] [Google Scholar]
- 68.Ye G.J., Komáromy A.M., Zeiss C., Calcedo R., Harman C.D., Koehl K.L., Stewart G.A., Iwabe S., Chiodo V.A., Hauswirth W.W. Safety and efficacy of AAV5 vectors expressing human or canine CNGB3 in CNGB3-mutant dogs. Hum. Gene Ther. Clin. Dev. 2017;28:197–207. doi: 10.1089/humc.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ghazi N.G., Abboud E.B., Nowilaty S.R., Alkuraya H., Alhommadi A., Cai H., Hou R., Deng W.T., Boye S.L., Almaghamsi A. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector: results of a phase I trial. Hum. Genet. 2016;135:327–343. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 70.Wan X., Pei H., Zhao M.J., Yang S., Hu W.K., He H., Ma S.Q., Zhang G., Dong X.Y., Chen C. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci. Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bucher K., Rodríguez-Bocanegra E., Dauletbekov D., Fischer M.D. Immune responses to retinal gene therapy using adeno-associated viral vectors—implications for treatment success and safety. Prog. Retin. Eye Res. 2020 doi: 10.1016/j.preteyeres.2020.100915. Published online October 15, 2020. [DOI] [PubMed] [Google Scholar]
- 72.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T., Standardization of Uveitis Nomenclature (SUN) Working Group Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005;140:509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kleiveland C.R. Peripheral blood mononuclear cells. In The Impact of Food Bioactives on Health. In: Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. Vitro and Ex Vivo Models. Springer; 2015. pp. 161–167. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.