Abstract
The outbreak of COVID-19 from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread all over the world with tremendous morbidity and mortality in the elderly. In-hospital treatment addresses the multifaceted nature of the illness including initial viral replication, cytokine storm, and endothelial injury with thrombosis. We identified nine reports of early treatment outcomes in COVID-19 nursing home patients. Multi-drug therapy including hydroxychloroquine with one or more anti-infectives, corticosteroids, and antithrombotic anti-blood clotting agents can be extended to seniors in the nursing home setting without hospitalization. Data from nine studies found hydroxychloroquine-based multidrug regimens were associated with a statistically significant > 60% reduction in mortality. Going forward, we conclude that early empiric treatment for the elderly with COVID-19 in the nursing home setting (or similar congregated settings with elderly residents/patients e.g. LTF or ALF) has a reasonable probability of success and acceptable safety. This group remains our highest at-risk group and warrants acute treatment focus prior to symptoms worsening. Given the rapidity and severity of SARS-CoV-2 outbreaks in nursing homes, in-center treatment of acute COVID-19 patients is a reasonable strategy to reduce the risks of hospitalization and death. If elderly high-risk patients in such congregated nursing home type settings are allowed to worsen with no early treatment, they may be too sick and fragile to benefit from in-hospital therapeutics and are at risk for pulmonary failure, life-ending micro-thrombi of the lungs, kidneys etc. The issue is timing of therapeutics, and we argue that early treatment before hospitalization, is the right time and can potentially save lives, especially among our higher-risk elderly populations hit hardest by severe illness and death from COVID-19. We must reiterate, we are talking about ‘early’ treatment before the disease is far along in the disease sequelae where the patient then needs hospitalization and aggressive interventions. We are referring to the initial days e.g. day one, post infection when symptoms emerge or there is strong clinical suspicion. This early therapeutic option deserves serious and urgent consideration by the medical establishment and respective decision-makers. Doctors must be allowed their clinical discretion in how they optimally treat their patients. Doctors must be brave and trust their skilled judgements and do all to save the lives of their patients.
We therefore hypothesize that early outpatient ambulatory treatment, once initiated as soon as symptoms begin in high-risk positive persons, would significantly reduce hospitalizations and prevent deaths. Specifically, the provision of early multi-drug sequenced therapy with repurposed drugs will reduce hospitalization and death in elderly patients being cared for in long-term-care facilities.
The most important implications of our hypothesis are: 1) hospitalizations and deaths would be reduced 2) transmission would be reduced due to the mitigation of symptoms and 3) recovery following infection and treatment provides for natural exposure immunity that is broad based, durable, and robust (helping towards natural immunity within the population). The end result is reduced strain on hospitals and systems that would allow for other non-COVID illnesses to receive care.
Keywords: SARS-CoV-2, COVID-19, Nursing home, Elderly, Hospitalization, Mortality, Ambulatory treatment, Anti-infective, Anti-inflammatory, Antiviral, Corticosteroid, Antiplatelet agent, Anticoagulant
Background
The pandemic of SARS-CoV-2 virus and the resulting clinical disease known as COVID-19 disease, has spread across the world in a relentless manner and it appears now that it is becoming endemic. This pathogen demonstrates high infectivity while the pathogenicity at present continues generally to be low, though it causes devastating consequences to a proportion of high-risk persons and principally the elderly among us with underlying medical conditions, as well as younger persons with risk or obese persons. Make no mistake, COVID-19 is a devastating illness for elderly persons with underlying medical conditions who are infected. COVID-19 very early on showed us that it was amenable to risk stratification, and that your baseline risk was prognostic on mortality. Case fatality (mortality) rates are elevated and have been based thus far on laboratory confirmed infections and have not yet included an accurate reflection of infection e.g. mild or asymptomatic cases that have recovered. Ideally, the infection fatality rate should be reported as comprehensive data become available which would provide a more accurate reflection of lethality.
In the previous year, we have found that SARS-CoV-2 virus may lead to a spectrum of COVID-19 syndromes, from asymptomatic exposure, to common cold symptoms, to influenza-like symptoms, to fulminant multiorgan system failure. The most important variable for risk stratification for both hospitalization and death is advanced age and obesity has emerged as a super-loaded prognostic factor. Nursing home patients have accounted for a large fraction of deaths in most developed countries.
A policy brief published by the American Geriatrics Society (AGS) has outlined clearly the impact of COVID-19 pathophysiology and severity of illness in the nursing home, highlighting the grave challenge in treating and preventing death in this patient population. The AGS reported that over 15,000 nursing homes and long-term care facilities provide care for the oldest Americans who are at greatest risk for COVID-19 and complications (pulmonary failure and death) especially as they have multiple chronic medical conditions [1], [2]. The AGS suggests consideration of hospital ‘at-home’ care models as a practical path forward.
Unfortunately, there have been no large, well-funded, high-quality randomized trials of single or multi-drug regimens for COVID-19 in nursing home residents. Given the enormous public health importance of the issue, we sought to assemble the available information concerning treatments that have been attempted in senior homes (nursing homes, old-aged homes, care homes, long-term care homes, assisted-living facilities etc.) and their associated outcomes. In most circumstances, the comparator or approach was watchful waiting and then hospitalization for severe symptoms e.g. when the patient had difficulty breathing. We found this to be an unacceptable approach when therapeutic options were already available.
We hypothesize that early outpatient treatment initiated as soon as symptoms develop in high-risk persons e.g. elderly with co-morbid conditions, obese etc. who test positive (or there is strong clinical suspicion), would significantly reduce hospitalizations and prevent deaths. This would result in: 1) hospitalizations and deaths being reduced 2) transmission being reduced due to the mitigation of symptoms and 3) broad, durable, and robust natural immunity following recovery with treatment. The good news is that recovery with then 'natural' immunity would help in the development of population level herd immunity. While the final result is lives being saved when high-risk and infected, this would also translate into less demands on hospitals which would allow hospitals and healthcare systems to respond to other non-COVID demands.
Methods
We searched PubMed/MEDLINE database for pragmatic reasons. We performed a literature search to December 2020 using relevant search terms including COVID-19, SARS-CoV-2, nursing homes, long-term care, nursing home residents, high-risk, elderly residents, mortality, death, treatment, and early treatment, to identify reports of attempts to treat COVID-19 in nursing homes with the primary outcome of study being mortality. We sought to access as complete a body of evidence as appropriate to inform this manuscript. Thus, study reference lists were also hand searched for any potentially eligible reports. Evidence was also considered where available, from additional sources such as online preprint publications not yet having completed the peer-review process (e.g. clinical medicine preprint repository, medRxiv.org etc.).
Relevant reports were examined in duplicate and independently for full agreement on final eligibility to inform the review. We were prepared to discuss in consensus debate in instances of potential eligibility disagreement and use of adjudication if necessary. From the reports, we extracted data on mortality among those treated with one or more drugs against COVID-19 and also for those in comparator groups. This step was also conducted in duplicate and checked for accuracy. MedCalc (https://www.softpedia.com/get/Science-CAD/MedCalc.shtml) was used to calculate odds ratios/relative risks and their 95% confidence intervals and p-values for mortality. For the purposes of this review, we defined ‘nursing home’ as any nursing home, long-term care facility, care home, assisted-living facility etc.
In sum, we were parsimonious in our approach while being as systematic and methodologically rigorous as feasible. We sought to alert the clinical and medical research community as well as policy makers, with the accumulated benefits of early treatment in a high-risk elderly population that remains at greatest risk of hospitalization and death from COVID-19. Our cardinal aim therefore given the accumulating evidence, is a summons to the medical research community for urgent high-quality, trustworthy, and robust comparative effectiveness research on early treatment in high-risk and symptomatic SARS-CoV-2 positive patients/residents (especially in the nursing home environment). This research must involve comparative assessment of multi-drug approaches.
Results
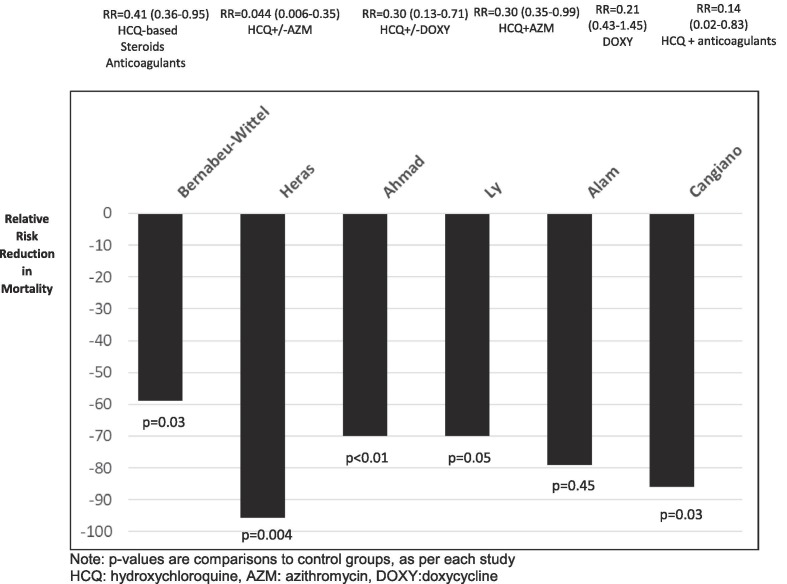
Our evidence searching uncovered 336 initial reports that were potentially eligible for our review. Following examination of the emerging reports, we made a judgement that nine (9) studies [3], [4], [5], [6], [7], [8], [9], [10], [11] were eligible for inclusion, these shedding light on early ambulatory outpatient sequential multidrug treatment (SMDT [12]) in nursing home residents (Table 1 ) (please refer to Reviews in Cardiovascular Medicine for complete details on the suggested early outpatient ambulatory SMDT dosing and treatment algorithm [12]). We also present the relative reduction in mortality risk (Fig. 1 ) where data allows in selected studies described below.
Table 1.
Reports of early prehospital sequenced multi-drug treatment (SMDT) in nursing home residents.
| First author’s surname | Treatment | Outcome |
|---|---|---|
| Bernabeu-Wittel [3] | HCQ/HCQ + lopinavir/ritonavir/HCQ + AZM | Survival in active treatment group independently associated with use of any antiviral treatment; survival in the antiviral treatment group was independently associated with receiving any of the antiviral treatments (OR = 28 [5–160]). |
| Heras [4] | HCQ/HCQ + AZM | HCQ + AZ correlated significantly with decreased mortality, HCQ alone also correlated but not significantly; 83 received pharmacological treatment, total survival was 76%, 7 (8%) patients were hospitalized, of which 4 died. |
| De Spiegeleer [5] | Effect of medication for preexisting conditions: Angiotensin-converting enzyme inhibitors (ACEi), angiotensin II receptor blockers (ARBs), or HMB-CoA reductase inhibitors (statins) | Statins significantly correlated with decreased mortality, ACEi and ARBs also correlated but not significantly; a statistically significant relationship between statin intake and the absence of symptoms during COVID-19 (OR 2.91; 95% CI 1.27 to 6.71). |
| Brouns [6] | Effect of medication for preexisting conditions: Oral antithrombotic therapy (vitamin K antagonists, direct oral anticoagulants, anti-platelet therapy) | Association of antithrombotics with diminished mortality but not significant and further reduced when controlled for age, sex, hypertension, and comorbidity; univariate analysis (OR 0.89, 95% CI 0.41 to 1.95, p = 0.776). |
| Ahmad [7] | HCQ + DOXY | Smaller percentage of deaths and hospitalizations compared to a demographically similar nursing home where residents were not treated; of 54, 9 did not complete treatment, and those completing the course of treatment were afebrile by at least 5 days after completion, and either had no more shortness of breath or had returned to previous oxygenation levels (for patients who had been on ventilation before contracting COVID-19). |
| Ly [8] | HCQ + AZM | Treated residents had significantly lower mortality. The overall mortality was 20.8%, and hospitalization including ICU admission was 20.8%. Mortality was lower in patients treated with HQC + AZM for at least 3 days (15.5%) than in those not so treated (26.4%), OR 0.37, p = 0.02. |
| Alam [9] | DOXY + standard of care | Researchers concluded that early treatment with DOXY for high-risk patients with moderate to severe COVID-19 infections in non-hospital settings, such as LTCFs, is associated with early clinical recovery, decreased hospitalization, and decreased mortality; 85% of patients (n = 76) revealed clinical recovery, 3% hospitalized |
| Cangiano [10] | HCQ/heparin/corticosteroid/anti-platelet | Mortality was significantly lower for patients who had previously been on chronic vitamin D supplementation, and was significantly reduced by COVID-19 treatment with HCQ (p = 0.03, 28 survived vs 5 who died). |
| Leriger [11] | HCQ/non-HCQ | After 2 weeks, 76 (72%) of residents receiving HCQ showed no symptoms, while 9% had symptoms, 2% continued in hospitalized, and 17% had died; control group had 58% (66) who were asymptomatic, 13% who had mild symptoms, 1% with moderate symptoms, 3% hospitalized, and 25% had died; the benefits of HCQ treatment in positive nursing home residents seem to outweigh the harms in terms of symptom severity and mortality, and particularly so in residents over 80 years of age and when HCQ was administered before symptoms began (started early). |
Fig. 1.
Relative risk reduction in mortality risk in nursing home COVID patients using early prehospital combined and sequenced multi-drug treatment (SMDT).
Bernabeu-Wittel et al. reported from four nursing homes in Spain [3] that 272 of 457 (59.5%) residents contracted SARS-CoV-2 of whom 189 (69.5%) were given ‘active standard care’ and the rest were given advanced palliative care. For patients assigned to active standard care (i.e. medicalization, see Table 1), survival was measured as both dichotomous and time-dependent outcomes. The findings demonstrated that 139 (73.5%) of these patients received antiviral treatment: 114 (60%) received hydroxychloroquine (HCQ), 18 (10%) HCQ + lopinavir/ritonavir, 7 (5%) HCQ + azithromycin (AZM). The investigators reported that survival in the ‘antiviral’ treatment group was associated independently with receiving any of the antiviral treatments (OR = 28 [5–160]). In addition, 119 (44%) received low molecular weight heparin, 62 (23%) received antimicrobials (e.g. “mild/moderate: oral amoxicillin/clavulanic acid, levofloxacin, or AZM; severe: parenteral ceftriaxone plus levofloxacin (or AZM; possibility of aspirative origin: oral amoxicillin/clavulanic acid, or parenteral ertapenem”), and 57 (21%) received systemic corticosteroids. Fewer patients who received active standard care died (24 (13%)). The researchers also reported that the ‘medicalization’ program (as opposed to watchful observation) led to increased survival in this group, 82% before MP, 96% during MP (p = 0.004). Survival time in actively treated patients was independently associated with use of any antiviral treatment. Hospital referrals were also recorded, but for all patients, including those who did not receive active care; hospitalization significantly decreased with introduction of medicalization program (Table 1).
Heras et al. reported on COVID-19 patients from a single nursing home in Spain [4]. This was designed as a retrospective cohort case-controlled analysis. There were 100 confirmed cases and a medicalization approach to treatment had been carried out in 83 subjects. It was reported that mostly, 70%, received HCQ and AZM while others received variously HCQ alone and in some cases beta-lactam or quinolone antibiotics. Total survival was 76%, 7 patients were hospitalized, of which 4 died. No statistically significant improvement in survival was observed for patients who started treatment within the first 24 h. Significantly greater risk of mortality was observed for lack of pharmacological treatment and type of treatment. Of survivors, 81.6% had received HCQ + A, whereas only 33.3% of those who died had received it. Of survivors, 7.9% had received HCQ alone, while 12.5% of those who died had received it. Of survivors, 10.5% had received ‘no treatment’, which also included beta-lactam or quinolone antibiotics, while 54.2% of those who died received it. No indication of the treatment for the 7 hospitalized patients were provided. In adjusted models, HCQ + AZM conveyed a 22.6-fold lower mortality risk, p = 0.004 (Fig. 1) compared to use beta-lactam or quinolone antibiotics only.
De Spiegeleer and coworkers published outcomes from two Belgian nursing homes in 2020 [5] to analyze the association between angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor antagonist (ARB) and/or statin use with the clinical outcome of COVID-19 (n = 154 COVID-19-positive subjects). Twenty percent were taking ACEi/ARBs and 20% were taking a statin, while 5% were taking both. Researchers reported a statistically significant relationship between statin intake and the absence of symptoms during COVID-19 (OR 2.91, 95% CI 1.27 to 6.71), retaining significance when adjusted for covariates. Forty-seven percent of 154 SARS-CoV-2 positive patients remained asymptomatic and 24% had severe disease. The conclusion was that statin use in such nursing home residents (older and more vulnerable adults) is potentially associated with a significant positive effect (better outcome) on COVID-19 clinical symptoms and course. The authors also reported that “The fact that statin intake is more strongly associated with asymptomatic status than serious COVID-19 suggests that the potential therapeutic effects of statins are more outspoken (sic) in the initial stages of COVID-19” [5].
Broun et al. reported a retrospective case series involving 14 nursing homes in the Netherlands (n = 101 residents) [6], concerning oral anticoagulants and mortality in nursing home residents with COVID-19. The overall mortality was 47.5% (48 deaths). Researchers found that anticoagulation was related to a non-significant reduced mortality via univariate analysis (OR 0.89, 95% CI 0.41 to 1.95, p = 0.78) with adjustment for gender, age, hypertension, and comorbidities.
Ahmad et al. published a report of 52 confirmed SARS-CoV-2 positive COVID-19 patients [7] from three nursing homes in New York. Upon diagnosis, residents were treated with doxycycline (DOXY) and HCQ. DOXY reportedly acts as a broad-spectrum inhibitor of MMPs (potentially having a direct action on metalloproteinase) and also acts as an antioxidant [13]. Additionally, researchers indicate that tetracyclines such as DOXY can impact viral infections via their direct antibacterial properties as well as via direct antiviral activity, thus capable of slowing/inhibiting COVID-19 disease progression [14]. Patients were followed for 11 days with treatment course of 7 days. Nine patients did not complete the 7-day therapy, 6 due to hospitalization, 2 due to death in the nursing home, and 1 due to adverse reaction (seizure). At the time of publication, of these 9, 3 had died. The remaining patients completing the course of treatment, were afebrile by at least 5 days after completion, and either had no more shortness of breath or had returned to previous oxygenation levels (for patients who had been on oxygen supplementation before contracting COVID-19). Researchers concluded that treatment with DOXY-HCQ in high-risk COVID-19 patients was associated with a benefit in clinical recovery, decreased transfer to hospital and decreased mortality [7]. For comparison, the authors also reported on a nursing home with similar demographics in Washington whose residence did not receive pharmacological treatment and had 57% hospitalization and 22% mortality, as compared to 11% and 6% in the study group, respectively.
Ly et al. [8] reported on the results of SARS-CoV-2 PCR-based screening campaigns conducted on dependent elderly residents (compared with staff members) in long-term care facilities (LTCFs) in Marseille, France, with follow-up of positive cases. This was a retrospective study of positive residents in 24 nursing homes whereby 226 of 1,691 residents (13.4%) tested positive. In total, 116 (51.4%) patients received courses of oral HCQ and AZM for ≥ 3 days and these patients otherwise differed little in comparison to patients who did not receive the treatment. The overall mortality was 20.8%. Mortality was reportedly lower in patients treated with HCQ + AZM for at least 3 days (15.5%) than in those not so treated (26.4%), adjusted OR = 0.37, p = 0.02. Researchers concluded that the elevated proportion of asymptomatic COVID-19 patients and independent factors for mortality “suggest that early diagnosis and treatment of COVID-19 patients in LTCFs may be effective in saving lives” [8].
Alam and coworkers [9] conducted a retrospective case-series study in New York to assess and document clinical outcomes of high-risk COVID-19 LTCF patients (n = 89 persons diagnosed March to May 2020). These patients had early intervention with doxycycline (DOXY) after presenting with moderate to severe symptoms. All 89 had developed sudden onset of fever, cough, shortness of breath (SOB), and hypoxia. Treatment with DOXY began within 12 h of symptom onset, 100 mg bid PO or intravenous (IV) for seven days and regular standard of care (11 additionally received broad-spectrum antibiotics). Researchers reported that 85% of patients (n = 76) had clinical recovery, “defined as resolution of fever (average 3.7 days, Coeff = -0.96, p = 0.0001), resolution of SOB (average 4.2 days), and improvement of POX: average 84% before treatment and average 95% after treatment (84.7 ± 7% vs. 95 ± 2.6%, p = 0.0001). Higher pre- and post-treatment POX is associated with lower mortality (oxygen saturation (Spo2) vs. Death, Coeff = -0.01, p = 0.023; post-Spo2 vs. Death, Coeff = -0.05, p = 0.0002)” [9]. Ten patients died (11%) within 10 days of symptom onset and 3% were transferred to hospital. Researchers concluded that “early treatment with DOXY for high-risk patients with moderate to severe COVID-19 infections in non-hospital settings, such as LTCFs, is associated with early clinical recovery, decreased hospitalization, and decreased mortality”. The data from Alam et al. [9] was compared to similar age groups in New York (Yang et al. 2020 [12]) during the same period of the pandemic in order to calculate the relative risk reduction with DOXY.
Cangiano et al. [10] studied mortality of nursing-home COVID-19 patients in Milan, Italy using an observational study design. These patients, average age 90 years, were treated in the facility with a standard-of-care multidrug therapy including HCQ, corticosteroids, and antithrombotics. Of the 98 patients followed, 56 survived and 42 died over the two months of the study. The authors found that mortality was significantly lower for patients who had previously been on chronic vitamin D supplementation, and was significantly reduced by COVID-19 treatment with HCQ (p = 0.03).
Finally, Leriger et al. (2020) [11] followed 233 residents in 11 skilled nursing homes in Indiana who had tested positive for COVID-19 (113 residents who acted as controls were selected from the same nursing homes and also followed for 14 days (all testing positive). Hydroxychloroquine (HCQ) was administered to 105 nursing home residents (45%). After 2 weeks, 76 (72%) of residents receiving HCQ showed no symptoms, while 9% had symptoms, 2% continued in hospitalized, and 17% had died. The control group had 58% (66) who were asymptomatic, 13% who had mild symptoms, 1% with moderate symptoms, 3% hospitalized, and 25% had died. The 2 residents who were hospitalized in the HCQ group recovered (initially hospitalized for chest pain/tachycardia) and asymptomatic after 2 weeks. Researchers concluded that the benefits of HCQ treatment in nursing home residents who are COVID-19 positive, seem to outweigh the harms in terms of symptom severity and mortality, and particularly so in residents over 80 years of age and when HCQ was administered before symptoms began (started early).
Discussion
We found nine reports [3], [4], [5], [6], [7], [8], [9], [10], [11] supporting the concept of early multidrug pharmacological intervention in order to improve clinical outcomes (the most important being reduction in death) in elderly nursing home residents suffering from COVID-19. We were focused on a high-risk group that typically worsens before treatment is administered within an in-patient hospital setting. While our focus here is on nursing home type residents, one could reasonably extrapolate these findings or hypothesis to similar high-risk persons residing in their private residences. In essence, the reports from nursing homes showed that early multidrug interventions including most commonly two or more intracellular anti-infectives, and before hospitalization, was associated with an overall reduction in mortality > 60%. These findings, although derived from independent investigations, were externally consistent and in most instances, clinical improvements as well as reduced rates of mortality were statistically significant and clinically significant. While the reports focused on HCQ and anti-infectives, as indicted in a prior early outpatient SMDT study [12], early treatment can potentially include other anti-virals/ anti-infectives, corticosteroids, and anti-thrombotic/platelet drugs based on clinician decision-making, availability etc. Our data imply that early medical therapy in addition to several other interventions in nursing homes when combined, could dramatically reduce hospitalization rates and improve survival. The elderly in nursing homes have been ravaged by COVID-19 and this is indeed very good news that merits serious consideration!
There can be no doubt that the COVID-19 crisis has markedly increased nursing home annualized death rates. For example, an Italian observational study found a two-month mortality of 40%, compared to 6.4% in the prior year (COVID-19 positive residents (43% increase) and negative residents (24% increase) [10]. Greater mortality was associated with being male, older, no previous vitamin D supplementation and lower “activities of daily living (ADL)” scores [10], leaving researchers to conclude that there is a greater elderly mortality due to COVID-19.
Similarly, Panagiotou et al. (2020) [15] sought to identify risk factors for 30-day all-cause mortality among US nursing home residents with COVID-19. The study was conducted in 351 US nursing homes involving 5256 nursing home residents with COVID-19–related symptoms who had severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection that was confirmed by RT-PCR testing (March to September). The median age was 79 years (IQR, 69–88 years) and researchers reported that increased age, being male, and weakened cognitive and physical function among these US nursing home residents with COVID-19 were independently associated with all-cause 30-day mortality.
The nursing home staff play a critical role in spreading and potentially containing the SARS-CoV-2 outbreak. A report from France [16] involved 17 nursing homes with 794 staff members confined to the facility along with 1,250 residents compared with a national sample of 9,513 facilities with 385,290 staff members and 695,060 residents. Lower rates of COVID-19 were found among facilities that had staff members live in the facility as compared to those who lived at home and traveled to the nursing home for work each day. These findings suggest SARS-CoV-2 is spread from home families of nursing home workers to the nursing home residents who are in lockdown. Similarly, Davidson and Szanton (2020) [17] called for better management of nursing home residents in the era of COVID-19 with reductions in overcrowding and congregate settings. Additionally, researchers [18] reported that outbreaks of COVID-19 are associated with the size of the nursing home type facility whereby institutions with > 150 beds are at much greater likelihood of transmission of the virus.
Surveillance for SARS-CoV-2 is an essential strategy for evaluating SARS-CoV-2 transmission within nursing homes. Along these lines of greater risk in nursing home settings, Graham et al. (2020) [19] conducted an outbreak investigation of 394 residents and 70 staff in 4 nursing homes impacted by COVID-19 outbreaks in central London. The investigation found that 26% of residents died over the two-month period and all-cause mortality increased by 203% compared to the same period in prior years. Testing identified 40% of residents as positive for SARS-CoV-2 (43% of them asymptomatic and 18% had only atypical symptoms with 4% of asymptomatic staff also testing positive). Similarly, researchers (2020) [20] reported very elevated death (33% of residents (34/101)) from COVID-19 in a long-term care facility in King County, Washington. After detecting one infected resident late February, by mid March, there were 167 confirmed cases of COVID-19 involving 101 residents, 50 health care personnel, and 16 visitors. Researchers reported that hospitalization rates for residents, visitors, and staff were 54.5%, 50%, and 6%, respectively. Similarly, by this time in March, 30 long-term care facilities were reporting at least one confirmed case of COVID-19.
Likewise, a recent report on Singapore’s handling of COVID-19 [21] showed that residents in nursing homes and long-term care facilities are at greater risk of transmission and severe illness and deaths. While Singapore’s nursing homes and long-term care facilities accounted for < 0.001% of total COVID-19 cases in April 2020, they accounted for 14% of all deaths from COVID-19. Arons et al. (2020) [22] sought to assess transmission risk after one case of COVID-19 emerged in a nursing facility in Washington. They found elevated transmission and death from that initial one case whereby at 23 days from the initial positive case, 64% of the 89 residents tested positive, and 11 were hospitalized (19%) and 15 died (26%). Approximately 99% of studied residents had at least one co-existing condition. A report on the COVID-19 situation in nursing homes in Hungary [23] echoed very similar findings on the significantly elevated risk of transmission in such settings, pointing to estimates of 42 to 57% of all COVID-19 deaths occurring in nursing homes in European nations such as Italy, Spain, France, Ireland, and Belgium.
The SARS-CoV-2 virus can spread quickly in nursing homes. A report on the COVID-19 in England and Wales [24] discussed the rapidity at which the infection spreads in such settings, and the finding that when the first resident shows symptoms, approximately 50% of the residents are already infected. The UK Office for National Statistics (ONS) reported that in April, 72% of deaths in care homes involved COVID-19. These findings unlike the SARS-CoV-1 coronavirus epidemic in 2003 [25], indicate that contagion-control methods alone are inadequate for COVID-19 and immediate treatment is needed to reduce mortality.
Reports from multiple sites in the world indicate that therapeutic nihilism is practiced with seniors who do not have access to healthcare outside of their facilities. This is indeed very distressing if there are potential treatment options. It has been reported that in Quebec, Canada, as of December 2, 2020, 63% of deaths due to COVID-19 occurred in private and public nursing homes (4,493 of 7,097) [26], [27]. Importantly, residents who are ill with COVID-19 are not provided any form of existing safe, simple, and inexpensive therapeutic drugs [26].
The Centers for Disease Control and Prevention (CDC) [28] recently highlighted the COVID-19 vulnerability among residents and staff members in long-term care facilities (LTCFs), and focused specifically on assisted living facilities (ALFs). LTCFs include ALFs and similar residential facilities, skilled nursing facilities (SNFs) as well as other nursing homes, and also include residential facilities for residents with varying degrees of intellectual and developmental disabilities. As of early November 2020, approximately 91,500 deaths were reported among residents and staff in LTCFs within the United States, representing 39% of total state COVID-19 deaths. By mid October 2020 and based on available ALFs data from 39 US states, among the 28,623 ALFs, 22% reported at least one COVID-19 case in their staff or residents. In addition, the cases that died comprised 21.2% of the residents and 0.3% of staff. This report made no mention of treatment provided to staff or residents. A print media report [29] stated that almost 50% of US COVID-19 deaths were linked to nursing homes. In addition, while the nursing home death toll was elevated significantly in New York, the deaths in New York’s nursing homes have been much higher than the state data reported [30]. None of these reports indicate that nursing home patients have been offered any forms of prehospital therapy.
We also wish to add some more discussion on how HCQ compares to other potential drugs such as remdesivir and ivermectin (IVM). Remdesivir has been authorized for use as inpatient treatment and not outpatient treatment, thus geared towards patients who are far along in the disease sequelae. If we look at treatment for pre-exposure prophylaxis (PrEP): HCQ = IVM, both work well as PrEP; if we look at early ambulatory outpatient treatment: HCQ is excellent, IVM is fairly good, and our position based on clinical experience is that both can be used together; in terms of in-hospital treatment: IVM is excellent, HCQ is unclear. Both HCQ and IVM have emerged as strong anti-virals when used early in the disease sequelae, to arrest viral replication. If used late e.g. past the viral replication phase of the illness, then there will be limited if any effectiveness. Dosing and timing are very critical considerations and therapeutics must be used under doctor/expert care.
With the emergence of the new vaccines, there is reason for hope that there might be increases in the level of protection for the elderly as well as others for SARS CoV-2. This said, it should be noted that several variants of SARS CoV-2 have emerged and some of these evidently evade immunity (immune escape) created by vaccination that has focused on a narrow 'spike-specific' immunity. It is noteworthy, however, that the multidrug prophylactic and therapeutic approaches described herein will not be affected by the mutations in any meaningful way. The effectiveness of the early outpatient multidrug treatment regime we are advocating is virtually completely insensitive to the mutations unlike the vaccines which rely on maintenance of very narrow specific viral epitopes. Specifically, with early treatment, we are targeting the host to interfere with the cytokine storm/ARDS, which will not be altered by the viral mutations. Similarly, we are interfering with the host ‘machinery’ that allows SARS CoV-2 to replicate.
This puts multidrug therapy in an extremely important position at this stage of the pandemic. As such, our position is that with known Brazilian, UK and South African (and other) virus variants of apparently increased transmissibility and possible greater aggressiveness spreading (should they get past the immunity), and unknown variants in the future, the medication regimens may need to be comparably aggressive in using more medications simultaneously, such as HCQ, IVM, doxycycline, steroids, zinc, vitamin D, anti-thrombotics etc. right from the outset of infection and symptom emergence (strong clinical suspicion). Recent anecdotal observations from clinicians in these countries have suggested that this approach is successful in early outpatient treatment. Going in hard and fast is optimal, for COVID-19 is a lethal illness and lethal viral illnesses often respond optimally to a 'cocktail' multi-drug approach.
Our report has all the limitations of reviews that extract data from multiple heterogeneous studies. We did focus on nursing home residents who are usually weaker and more infirmed than persons living independently in the community, making our results not applicable to community-dwelling adults. However, one can extrapolate that our findings are applicable given that persons who reside in the community and are high-risk and infected, would similarly benefit from early treatment. The reporting of early treatment in COVID-19 patients can be considered nascent and thus the number of available studies is limited as is their sample sizes. We relied on data presented in study reports, which in non-randomized studies may be subject to variable degrees of adjustment for potentially confounding factors. We assumed that the various studies and their study subjects were sufficiently similar to draw conclusions across them, and that the care facilities examined in the study reports are typical of such facilities in general and thus that the treatment benefits that we observed apply to nursing home and similar care facilities quite generally. We are proponents of the use of all evidence besides randomized controlled trial evidence in informing clinical and policy decision-making, and especially recognize the utility of 'real-world' evidence that is often non-randomized. We therefore judged that our report has retained the quality type research to add favourably to this discussion on early treatment.
Conclusion
In conclusion, nursing home residents are at the highest risk of death from SARS-CoV-2 infection (COVID-19) illness and they appear to be the victims of spread from staff who live in the community. This issue with staff is very vexing and must be acutely focused upon. The available reports indicate there is a large > 60% mortality risk reduction associated with multidrug treatment regimens that utilize two or more intracellular anti-infectives (HCQ and either AZM or DOXY) combined with other agents including corticosteroids, anti-thrombotics (anti-platelets), and nutraceutals [12]. We recognize that large randomized, placebo-controlled, multidrug clinical trials will provide conclusive evidence in the future. However, in the meantime, given the present emergency crisis, the observational non-randomized data we reviewed which we judged as acceptable to informing this report (and in line with expert arguments for observational study evidence use especially when RCT evidence is not available or of poor methodological quality) [31], suggest a treatment mandate is present and should replace therapeutic nihilism for nursing home residents with acute COVID-19.
We believe that it is not possible to overstate the philosophy that since early in-center treatment with already available medications (repurposed) in nursing homes is associated with a large reduction in mortality among nursing home residents, there can be no scientifically sound reasons, nor moral rationale for not utilizing these forms of treatment. We are trying to prevent hospitalizations and save lives and strongly believe that this approach can be impactful and merits strong consideration. The accumulating early treatment evidence is compelling and deserving of very serious consideration and study as a therapeutic option, given this emergency. To do otherwise is to fail our patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.American Geriatrics Society. American Geriatrics Society Policy Brief: COVID-19 and Nursing Homes. J Am Geriatr Soc. 2020 May;68(5):908-91doi: 10.1111/jgs.16477. Epub 2020 Apr 29. PMID: 32267538; PMCID: PMC7262210. [DOI] [PMC free article] [PubMed]
- 2.Centers for Disease Control and Prevention (CDC). People who are at higher risk for severe illness. url: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. (Accessed on December 25th 2020).
- 3.Bernabeu-Wittel M, Ternero-Vega JE, Nieto-Martín MD, Moreno-Gaviño L, Conde-Guzmán C, Delgado-Cuesta J, Rincón-Gómez M, Díaz-Jiménez P, Giménez-Miranda L, Lomas-Cabezas JM, Muñoz-García MM, Calzón-Fernández S, Ollero-Baturone M. Effectiveness of an On-Site Medicalization Program for Nursing Homes with COVID-19 Outbreaks. J Gerontol A Biol Sci Med Sci. 2020 Aug 1:glaa192. doi: 10.1093/gerona/glaa192. Epub ahead of print. PMID: 32738140; PMCID: PMC7454360. [DOI] [PMC free article] [PubMed]
- 4.Heras E, Garibaldi P, Boix M, Valero O, Castillo J, Curbelo Y, Gonzalez E, Mendoza O, Anglada M, Miralles JC, Llull P, Llovera R, Piqué JM. COVID-19 mortality risk factors in older people in a long-term care center. Eur Geriatr Med. 2020 Nov 27:1–7. doi: 10.1007/s41999-020-00432-w. Epub ahead of print. PMID: 33245505; PMCID: PMC7693854. [DOI] [PMC free article] [PubMed]
- 5.De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, Dobson R, Wynendaele E, Van De Wiele C, Vandaele F, Van Dijck D, Bean D, Fedson D, De Spiegeleer B. The Effects of ARBs, ACEis, and Statins on Clinical Outcomes of COVID-19 Infection Among Nursing Home Residents. J Am Med Dir Assoc. 2020 Jul;21(7):909–14.e2. doi: 10.1016/j.jamda.2020.06.018. Epub 2020 Jun 1PMID: 32674818; PMCID: PMC7294267. [DOI] [PMC free article] [PubMed]
- 6.Brouns SH, Brüggemann R, Linkens AEMJH, Magdelijns FJ, Joosten H, Heijnen R, Ten Cate-Hoek AJ, Schols JMGA, Ten Cate H, Spaetgens B. Mortality and the Use of Antithrombotic Therapies Among Nursing Home Residents with COVID-19. J Am Geriatr Soc. 2020 Aug;68(8):1647-1652. doi: 10.1111/jgs.16664. Epub 2020 Jul 21. PMID: 32633418; PMCID: PMC7361386. [DOI] [PMC free article] [PubMed]
- 7.Ahmad et al. 2020. Pre-print. Doxycycline and Hydroxychloroquine as Treatment for High-Risk COVID-19 Patients: Experience from Case Series of 54 Patients in Long-Term Care Facilities. url: https://www.medrxiv.org/content/10.1101/2020.05.18.20066902v1.
- 8.Ly TDA, Zanini D, Laforge V, Arlotto S, Gentile S, Mendizabal H, Finaud M, Morel D, Quenette O, Malfuson-Clot-Faybesse P, Midejean A, Le-Dinh P, Daher G, Labarriere B, Morel-Roux AM, Coquet A, Augier P, Parola P, Chabriere E, Raoult D, Gautret P. Pattern of SARS-CoV-2 infection among dependant elderly residents living in long-term care facilities in Marseille, France, March-June 2020. Int J Antimicrob Agents. 2020 Dec;56(6):106219. doi: 10.1016/j.ijantimicag.2020.106219. Epub 2020 Nov 13. PMID: 33189890; PMCID: PMC7661959. [DOI] [PMC free article] [PubMed]
- 9.Alam MM, Mahmud S, Rahman MM, Simpson J, Aggarwal S, Ahmed Z. Clinical Outcomes of Early Treatment With Doxycycline for 89 High-Risk COVID-19 Patients in Long-Term Care Facilities in New York. Cureus. 2020;12(8):e9658. Published 2020 Aug 11. doi:10.7759/cureus.9658. [DOI] [PMC free article] [PubMed]
- 10.Cangiano B, Fatti LM, Danesi L, Gazzano G, Croci M, Vitale G, Gilardini L, Bonadonna S, Chiodini I, Caparello CF, Conti A, Persani L, Stramba-Badiale M, Bonomi M. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging (Albany NY). 2020 Dec 22;12. doi: 10.18632/aging.202307. Epub ahead of print. PMID: 33353888. [DOI] [PMC free article] [PubMed]
- 11.Monica Leriger et al. (2020), American Senior Communities, Indianapolis, IN, personal communication, 2020. A Novel Study on the Use of Hydroxychloroquine in COVID‐19 Positive Residents in a Nursing Home Setting.
- 12.McCullough, et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19) Rev Cardiovasc Med. 2020;21(4):517–530. doi: 10.31083/j.rcm.2020.04.264. [DOI] [PubMed] [Google Scholar]
- 13.Sodhi M, Etminan M. Therapeutic Potential for Tetracyclines in the Treatment of COVID-19. Pharmacotherapy. 2020 May;40(5):487–8. doi: 10.1002/phar.2395. Epub 2020 May 4. PMID: 32267566; PMCID: PMC7262278. [DOI] [PMC free article] [PubMed]
- 14.Mosquera-Sulbaran JA, Hernández-Fonseca H. Tetracycline and viruses: a possible treatment for COVID-19? Arch Virol. 2020 Nov 2:1–7. doi: 10.1007/s00705-020-04860-8. Epub ahead of print. PMID: 33136210; PMCID: PMC7604546. [DOI] [PMC free article] [PubMed]
- 15.Panagiotou OA, Kosar CM, White EM, Bantis LE, Yang X, Santostefano CM, Feifer RA, Blackman C, Rudolph JL, Gravenstein S, Mor V. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents With COVID-19. JAMA Intern Med. 2021 Jan 4. doi: 10.1001/jamainternmed.2020.7968. Epub ahead of print. PMID: 33394006. [DOI] [PMC free article] [PubMed]
- 16.Belmin J, Um-Din N, Donadio C, et al. Coronavirus Disease 2019 Outcomes in French Nursing Homes That Implemented Staff Confinement With Residents. JAMA Netw Open. 2020;3(8):e2017533. Published 2020 Aug 3. doi:10.1001/jamanetworkopen.2020.17533. [DOI] [PMC free article] [PubMed]
- 17.Davidson P.M., Szanton S.L. Nursing homes and COVID-19: we can and should do better. J Clin Nurs. 2020;29(15–16):2758–2759. doi: 10.1111/jocn.15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrams H.R., Loomer L., Gandhi A., Grabowski D.C. Characteristics of U.S. Nursing homes with COVID-19 cases. J Am Geriatr Soc. 2020;68(1653–56) doi: 10.1111/jgs.16661. PMID:32484912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham N.S.N., Junghans C., Downes R., Sendall C., Lai H., McKirdy A., et al. SARS-CoV-2 infection, clinical features and outcome of COVID-19 in United Kingdom nursing homes. J Infect. 2020 Sep;81(3):411–419. doi: 10.1016/j.jinf.2020.05.073. Epub 2020 Jun 3 PMID: 32504743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMichael TM, Currie DW, Clark S, Pogosjans S, Kay M, Schwartz NG, Lewis J, Baer A, Kawakami V, Lukoff MD, Ferro J, Brostrom-Smith C, Rea TD, Sayre MR, Riedo FX, Russell D, Hiatt B, Montgomery P, Rao AK, Chow EJ, Tobolowsky F, Hughes MJ, Bardossy AC, Oakley LP, Jacobs JR, Stone ND, Reddy SC, Jernigan JA, Honein MA, Clark TA, Duchin JS; Public Health–Seattle and King County, Evergreen Health, and CDC COVID-19 Investigation Team. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020 May 21;382(21):2005–11. doi: 10.1056/NEJMoa2005412. Epub 2020 Mar 27. PMID: 32220208; PMCID: PMC7121761. [DOI] [PMC free article] [PubMed]
- 21.Tan LF, Seetharaman SK. COVID-19 Outbreak in Nursing Homes in Singapore. J Microbiol Immunol Infect. 2020 May 13. doi: 10.1016/j.jmii.2020.04.018. Epub ahead of print. PMID: 32405290; PMCID: PMC7219412. [DOI] [PMC free article] [PubMed]
- 22.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, Taylor J, Spicer K, Bardossy AC, Oakley LP, Tanwar S, Dyal JW, Harney J, Chisty Z, Bell JM, Methner M, Paul P, Carlson CM, McLaughlin HP, Thornburg N, Tong S, Tamin A, Tao Y, Uehara A, Harcourt J, Clark S, Brostrom-Smith C, Page LC, Kay M, Lewis J, Montgomery P, Stone ND, Clark TA, Honein MA, Duchin JS, Jernigan JA; Public Health–Seattle and King County and CDC COVID-19 Investigation Team. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. N Engl J Med. 2020 May 28;382(22):2081–90. doi: 10.1056/NEJMoa2008457. Epub 2020 Apr 24. PMID: 32329971; PMCID: PMC7200056. [DOI] [PMC free article] [PubMed]
- 23.Kemenesi G, Kornya L, Tóth GE, Kurucz K, Zeghbib S, Somogyi BA, Zöldi V, Urbán P, Herczeg R, Jakab F. Nursing homes and the elderly regarding the COVID-19 pandemic: situation report from Hungary. Geroscience. 2020 May 18;42(4):1–7. doi: 10.1007/s11357-020-00195-z. Epub ahead of print. PMID: 32426693; PMCID: PMC7232926. [DOI] [PMC free article] [PubMed]
- 24.Burki T. England and Wales see 20 000 excess deaths in care homes. Lancet. 2020 May 23;395(10237):1602. doi: 10.1016/S0140-6736(20)31199-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tse M.M., Pun S.P., Benzie I.F. Experiencing SARS: perspectives of the elderly residents and health care professionals in a Hong Kong nursing home. Geriatr Nurss. 2003;24:266–269. doi: 10.1016/s0197-4572(03)00251-9. PMID:14571239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-19 data in Quebec. url: https://www.inspq.qc.ca/covid-19/donnees (Accessed on December 24th 2020).
- 27.COVEXIT. url: https://covexit.com/covid-19-in-nursing-homes-a-way-forward-to-end-the-tragedy/ (Accessed on December 25th 2020).
- 28.CDC. Characterization of COVID-19 in Assisted Living Facilities — 39 States, October 2020. MMWR. url: https://www.cdc.gov/mmwr/volumes/69/wr/mm6946a3.htm (Accessed December 25th 2020). [DOI] [PMC free article] [PubMed]
- 29.Almost half of US COVID-19 deaths are linked to nursing homes. url: https://nypost.com/2020/06/27/almost-half-of-us-covid-19-deaths-are-linked-to-nursing-homes/ (Accessed on December 24th 2020).
- 30.Coronavirus deaths in NY nursing homes higher than state data shows, feds say. url: https://nypost.com/2020/08/11/covid-deaths-in-ny-nursing-homes-higher-than-data-shows-feds/ (Accessed on December 25th 2020).
- 31.Frieden T.R. Evidence for health decision making - beyond randomized, controlled trials. N Engl J Med. 2017 Aug 3;377(5):465–475. doi: 10.1056/NEJMra1614394. PMID: 28767357. [DOI] [PubMed] [Google Scholar]