Abstract
Innate Lymphoid Cells (ILC) are involved in homeostasis and immunity. Their dynamic differentiation and characterization depend on their tissue of residency and is adapted to their role within these tissues. Lymphoid Tissue inducer (LTi) cells are an ILC member and essential for embryonic lymph node (LN) formation. LNs are formed at pre-defined and strategic positions throughout the body and how LTi cells are initially attracted towards these areas is under debate. Besides their role in LN formation, LTi-like and the closely related ILC type 3 (ILC3) cells have been observed within the embryonic gut. New studies have now shown more information on their origin and differentiation within the embryo.
This review will evaluate the embryonic LTi cell origin from a specific embryonic hemogenic wave, which has recently been described in mouse. Moreover, I will discuss their differentiation and similarities with the closely related ILC3 cells in embryo and adult.
Keywords: ILC3, ILC, Hemogenic endothelium, Bone-marrow, Hematopoietic stem cells (HSC), Lymph node
The enigmatic Innate Lymphoid Cell (ILC) family consists of 3 main family members in which transcription factors for development, marker expression and functionality are shared [[1], [2], [3]]. These are the 1) ILC1 and natural killer cell (NK), 2) ILC2 and 3) ILC3 and Lymphoid Tissue Inducer (LTi) cell members. Their role in defense and repair is versatile, as are their cues for differentiation in situ determining their action. NK cells are considered cytotoxic ILCs, while generally other ILCs are considered ILC-helper cells. The LTi cells were previously considered part of the ILC3 ontogeny, based on retinoic acid orphan receptor isoform γt (RORγt) expression plus overlap in marker and cytokine expression. However, in both human and mice, ILC3 progenitors cells require the transcription factor promyelocytic leukemia zinc finger (Plzf) (encoded by gene Zinc finger and BTB domain containing 16 -Zbtb16) for differentiation [4,5], while in the mouse Zbtb16 knock-out LTi cells were still observed [6]. Therefore, LTi cells are now considered to be separate from ILC3 cells [7]. Additionally, evolutionary the LTi cells emerged later when compared to the other ILCs, and their function in LN formation was mainly associated with placentation [8,9].
LTi cells are essential for the formation of the secondary lymphoid organs during embryogenesis. They interact with the Lymphoid Tissue organizer (LTo) cells at specific locations within the embryo to first form aggregates which are named LN anlagen. There is some discussion on which cells attract the first LTi cells involved in the initiation of LN formation when using different mouse models [[10], [11], [12]]. ILC3s, which consists of Natural cytotoxicity triggering receptor 1 (NKp46)+ and NKp46– (LTi-like) cells, have immune modulatory and homeostatic roles within the gut. LTi cells have a unique function during embryogenesis and are the first of the ILCs to be observed during embryogenesis [11]. In later embryonic stages other ILCs were reported, mainly in gut and lung [[13], [14], [15]], but their role during embryogenesis is unclear. ILCs were shown to be derived from the HSC lineage within the bone-marrow in adult [[16], [17], [18], [19]]. However, since there is no contribution from bone-marrow within the mouse embryo, the embryonic ILCs, like embryonic LTi cells, are likely to be derived from another hematopoietic lineage than from the HSC derived lineage. During adulthood, these embryonic LTi cells are replaced by bone-marrow derived LTi cells. The role of replaced LTi cells in adult remains obscure.
In this review, I will discuss the role of the LTi cells within LN formation and evaluate the newest insights on initiation of LN formation. Furthermore, I will discuss the new developments in LTi cell ontogeny and functioning, also in relation to the closely related ILC3s.
ILC ontogeny
Hemogenic endothelium progenitors
During early embryogenesis, few endothelial cells undergo Endothelial to Hematopoietic cell Transition (EHT) to become hematopoietic progenitor cells [[20], [21], [22]]. Small clusters of hematopoietic progenitors appear within the yolk-sac and embryo, from which cells bud off and are transported through the vascular system. Recent studies using single cell sequencing have contributed to the understanding of the earliest genes to facilitate EHT, such as CD44 and RUNX family transcription factor 1 (Runx1) [[23], [24], [25]]. The first wave of hematopoietic progenitors originates from the yolk-sac and occurs between embryonic gestation day 7.5 (E7.5) until around E9 in mouse. This wave includes progenitors for microglia, erythroid-myeloid progenitors, neutrophils, and mast cells [[26], [27], [28]]. Later lineages and progenitors for hematopoietic stem cell precursors (pre-HSCs) appear from embryonic arterial walls between E8.5-E11.5 [20,21]. The most studied embryonic hemogenic site in different organisms is the aorta-gonad-mesonephros (AGM) [29], although other hemogenic endothelial sites to generate hematopoietic progenitors such as the vitelline artery, heart endocardium, head somitic region and umbilical cord were described in mouse [[30], [31], [32], [33]]. Macrophages located in the heart-valves in mouse were shown to be derived from the heart endocardium [33]. However, it is not clear which other specific hematopoietic lineages appear from these embryonic sites.
In vitro cultured mouse yolk-sac cells isolated at E8.5 had a potential to become NK cells [15]. To directly show a relation between the yolk-sac and NK cells fate mapping models are required. Simic et al. [19] used the Cxcr4-CreErt2 fate mapping model to exclude a yolk-sac contribution as chemokine receptor Cxcr4 is not expressed within the yolk-sac [19,34,35]. Consequently, progenitors from the yolk-sac don't express Cxcr4 [35]. Fate mapping in the Cxcr4-CreErt2 model only labelled embryonic derived progenitors [35] and it was shown that LTi cell progenitors originated around E8.5 from an embryonic hemogenic source [19]. Since the first hemogenic clusters in the AGM are observed after E8.5 [20,23], the LTi hemogenic source could either be the embryonic vitelline artery or the AGM [32]. Described as active in later stages, other embryonic regions were less likely to be the source for LTi cells [30,31,33]. The appearance of the embryonic LTi progenitors preceded the appearance of the pre-HSCs, making it unlikely that embryonic LTi cells are derived from HSCs. On the contrary, embryonic LTi cells involved in LN formation were replaced in the adult mice by bone-marrow HSC derived LTi cells, as was also shown for ILC2s and some ILC3s [14,36]. In human, ILCs were observed in the fetal gut but it has not been shown if they are HSC derived, nor if they will be replaced in the neonate by ILCs from HSCs in the bone-marrow [37]. Interestingly, thymic LTi cells in the mouse embryo around E18 were described to originate from HSC and could indicate the first LTi cells to originate from a HSC origin [38]. How the HSC derived ILCs functionally relate to the embryonic derived ILC they replace is not yet known.
Fetal liver
Hematopoietic progenitors migrate from the hemogenic endothelial sites towards the fetal liver (FL). Intriguingly, in mouse embryos the FL is mostly colonized around E11.5, so it is unclear where earliest hematopoietic progenitors reside between E7.5-E11. Within the FL, progenitors expand and differentiate toward precursors stages of their respective lineages, except for the microglial precursors which differentiate within the brain [39]. The FL plays an important role in the expansion of the progenitors as it was estimated that the progenitors multiply up to 33 times within this environment [40].
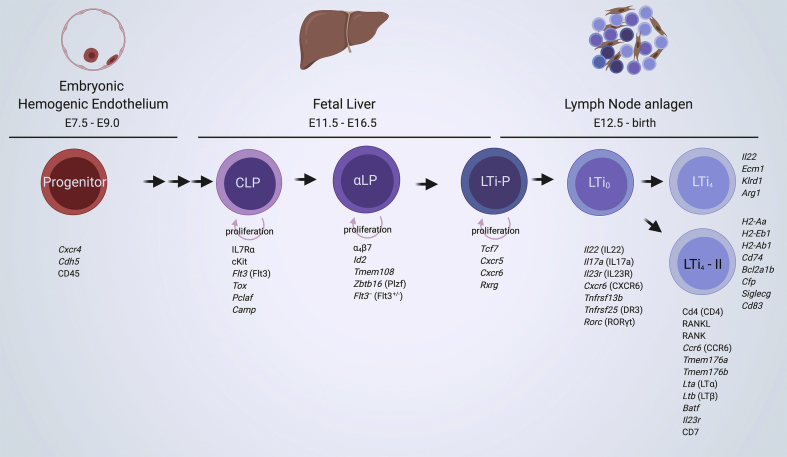
Common Lymphoid Progenitors (CLP) are present within the FL and express Fms related receptor tyrosine kinase 3 (Flt3) [7,19,41]. They are driven towards an ILC lineage by the expression of the Inhibitor of DNA binding 2 (Id2), which inhibits Single-stranded DNA-binding protein (E2A) functioning and thereby excludes commitment to a B-cell lineage [42]. At this stage, they are named α4β7-Integrin expressing Lymphoid Precursor (αLP) cells. Flt3 RNA transcripts were not observed in this stage, and protein expression was detected on a αLP subset [7,19,43]. An αLP Flt3– subset is sensitive to Notch signaling and affects its proliferation [43] (please see the review by Golub in this issue for the role of Notch in ILC differentiation). Within the αLP Flt3– stage, transcription factor Plzf marks differentiation into the ILC-Precursor (ILCP), which will generate ILC1, 2 and 3 cells in both human and mouse [[4], [5], [6]]. In adult mouse Plzf expression was reported to be lowered, while it remained high in human ILCs [4]. LTi-precursors (LTiP) in mouse, on the other hand, do not require Plzf, express chemokine receptors CXCR5 and CXCR6, and appear to segregate from the other ILC populations [6,7,44,45](reviewed in Ref. [46]) [Fig. 1]. These chemokine receptors expressed during the LTiP stage could aid their migration to- and retention within the LN anlagen, essential for the next phase in LTi differentiation.
Fig. 1.
Embryonic LTi cell ontogeny. LTi ontogeny and associated markers from the origin in the hemogenic endothelium to their presence within the LN anlagen. Progenitors originate from the embryonic hemogenic endothelium between E7.5-9 as observed by the use of Cxcr4 and Cdh5 fate mapping models [19]. Subsequent hematopoietic precursors reside, proliferate and differentiate within the fetal liver towards the ILC lineage [7,19,43,106]. The αLP/LTiP population migrate towards the LN anlagen, where these cells differentiate into LTi cells, marked by the expression of Rorc. Two clusters of LTi cell populations were observed within the LN anlagen enriched tissue, mainly segregated based on MHCII related genes and hence named LTi4 and LTi4-II cells. Genes (in italics) and proteins upregulated at a specific stage are shown below the populations, while genes enriched in the two LTi4 cell populations are shown to their right [7,19,43,44,106,107].
The role of mature ILC3 and LTi cells within the mouse FL is unclear. Several studies reported on the RORγt expressing (mature) ILC3 and LTi cells within the mouse FL [7,36,47]. On the other hand, the number of LTi0 and LTi4 cells within the FL was very low compared to LN anlagen enriched tissue [19,48]. Single cell sequencing of the FL and LN anlagen enriched tissue revealed that there was no direct connection in ontogeny between LTiP and LTi cells within the FL, while this was evident within the peripheral studied LTi cells [19]. Therefore, the final maturation would be most likely to occur within the LN anlagen, and in this scenario LTi cells present within the FL migrated back from the embryo.
ILC3s and LTi cells in LN anlagen and embryonic gut
αLP-LTiP cells migrate out from the FL towards the LN anlagen to differentiate into LTi0 and finally the mature LTi4 cells. During this transition, RORγt expression is induced by retinoic acid which leads to LTi lineage commitment. Lack of retinoic acid signaling within these cells halts LTi differentiation [49]. RORγt drives the transcriptional program for LTi cells in both human [50] and mice [51], although there have been RORγt deficient LTi cells observed [51,52]. However, these are not sufficient for continuation of LN formation, as Rorc (RORγt) knock-out mice are devoid of LN [51,53] and RORc deficient humans did not have palpable LNs [50]. Notch was shown to be involved in proliferation of the αLP population [43], but Notch signaling is not necessary for LTi commitment nor differentiation [44](please see the review by Golub in this issue for the role of Notch in ILC differentiation). The LTi0 cells do not yet express the molecules necessary for interaction with mesenchymal cell within the lymph node anlagen niche [49,54]. Only after differentiation into the mature LTi4 stage, these cells interact and amplify the synthesis of cytokines and adhesion factors [Fig. 2]. This leads to the attraction and retention of more LTiP and LTi cells.
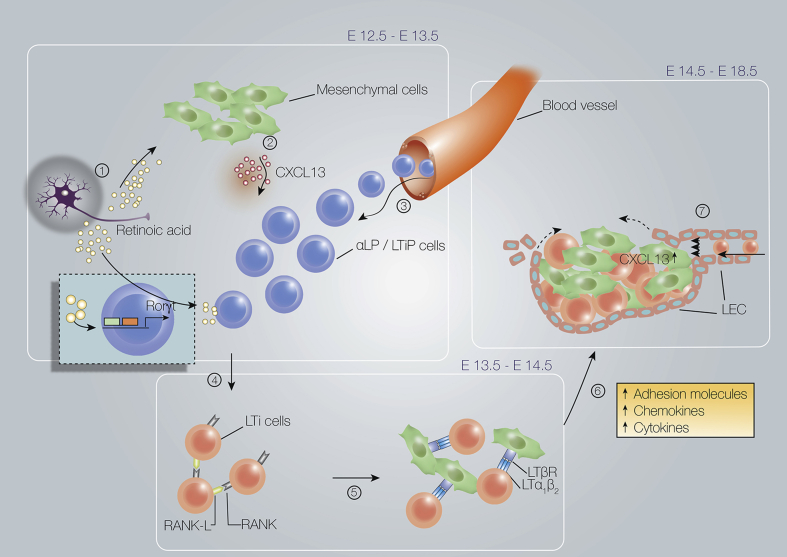
Fig. 2.
Overview of embryonic lymph node formation. Earliest phases in lymph node formation. 1) Retinoic acid, possibly produced by nerve fibers, induces the expression of (2) CXCL13 by mesenchymal cells in specific areas. This attracts LTiP and LTi cells from the blood to form the first clusters. Retinoic acid also induces expression of RORγt (encoded by Rorc) in LTiP cells, which will differentiate them into LTi0 cells. (4) Subsequently, when the LTi aggregation is dense enough, RANK-L expressed on LTi cells and mesenchymal cells interacts with RANK on the LTi cells which leads finally to (5) Lymphotoxin-α1ß2 (LTα1ß2) expression on LTi4 cells. (6) Interaction of LTα1β2-expressing LTi4 cells with lymphotoxin-β receptor (LTβR)-expressing stromal cells results in differentiation into stromal organizer cells, which are induced to express more and different chemokines, adhesion molecules and cytokines. These factors support the attraction and retention of more LTi and/or LTiP cells.(7) After lymphotoxin signaling, lymphatic endothelial cells migrate to the LN anlagen and circumvent it to eventually form the subcapsular sinus. LTi cells from the skin are drained into the LN anlagen by newly formed lymphatic vessels. The flow of the lymphatic vessel is also important for the maintenance of Cxcl13 expression within the LN anlagen. The attraction, retention and migration of new LTi- and LTiP cells towards the LN anlagen leads to an exponential increase of the lymph node anlagen.
It is unclear whether there is only one LTi4 cell population. Mosaic CD11b and MHCII expressing LTi cells were observed within the embryo [48,51,55]. Using single cell sequencing of the LN anlagen enriched tissue, two separate LTi4 clusters were observed. The first LTi4 population expressed Il22, a cytokine involved in gut immunity. Also, Arg1 is expressed within this LTi4 population, which was also associated with fetal gut ILC precursors (ftILCP) and ILC2s [13]. Specifically, in the same study, the fetal gut contained Arginase 1(Arg1)YFP+ LTi fate mapped cells and a smaller Arg1YFP– population [13], confirming two separate LTi4 populations also within the intestines. The ftILCPs could generate ILC1, 2 and 3 and were not fate mapped by the RORγt-Cre reporter, indicating that they are likely analogous to αLP and ILCP cells. Arg1 transcripts were not observed within fetal liver progenitors but only in the LN anlagen enriched tissue [19], indicating that Arg1 could be expressed after leaving the FL and entering peripheral LN anlagen or fetal gut. The second LTi4 population observed in the single cell sequencing study was segregated based on genes associated with Mayor histocompatibility complex II (MHCII), and hence named LTi4-II. Within the LTi4-II population, Cfp (Complement Factor Properdin) was observed, a ligand for binding to NKp46 [56]. This could indicate that the LTi4 –II population produce the ligand for NKp46+ NK, ILC1, ILC3 cells, but this has not yet been shown within the adult. A relation between the two embryonic LTi populations in the LN anlagen enriched tissue and the Arg1+ or MHCII+ ILC3 and LTi-like cells observed in the gut remains to be established.
Origin of ILCs and LTi cells in adult
Hematopoietic lineages within the adult are almost all derived from HSC residing within the bone-marrow. Bone-marrow derived ILC precursors in the mouse do not express Arg1, while embryonic ILCPs express Arg1 [13]. This is an indication that the differentiation pathway towards embryonic derived ILCs is likely different than those derived from HSCs in the adult bone-marrow. The mouse embryonic LTi cells, ILC3s and ILC2s were shown to be replaced by bone-marrow HSC derived ILCs [14,19,36]. Also, it was shown that ILCs can be generated from the bone-marrow using elegant reporter mouse models and in vitro differentiation [13,[16], [17], [18]], although only 1 study reported the generation of LTi cells from bone marrow, while the other reporter mouse models could not follow LTi cells in their fate-mapping models [41]. The discrepancy between these studies can be explained by the differentiation stage that is used as source for the in vitro- or in vivo differentiation studies. Similar as their differentiation within the mouse embryo, LTi cells follow a slightly different pathway for their formation as other ILCs, and the precursors used in the studies could have lost their LTi cell potential. In immunodeficient patients who received bone-marrow transfer lymphocytes were regained, but not ILCs [57]. This observation could reflect the inability of some studies to observe LTi cell differentiation within the adult from HSCs. Similar as for LTi cells, it is important to understand from which hematopoietic precursor exactly ILCs originate in (human) fetus. Possibly, other human HSCs or progenitors need to be isolated which still have the potential to become ILC after transfer, as was shown for circulating and tissue resident ILCp cells [58]. The presence of different types of ILC1, 2, 3 or LTi cells in different tissues could also reflect their difference in origin, i.e. embryonic resident ILCs vs. bone-marrow derived ILCs, and thus affect their functioning. More studies are required to understand the precise ILC progenitors and their differentiation pathways and what the functional difference is between the embryonic vs. adult ILCs.
LTi cells in embryonic lymph node formation
Secondary lymphoid organs, like mesenteric and peripheral lymph nodes (LN) and Peyer's patches, are formed during embryogenesis in which the LTi cells are critical. Consequently, deleting or mutation of RORc in both mice [51] and human [50] results in loss of LTi cells and concomitantly loss of LNs (reviewed in Refs. [11]). RORc deficient humans lacked palpable LNs but still had tonsils, but are surprisingly only more sensitive to Mycobacteria and Candida due to defective γδ-T cells and CD4+CCR6+CXCR3+-αβ T cells [50]. The presence of the tonsils, and other lymphoid structures like spleen which do not rely on LTi cells could be sufficient to drive the adaptive immune response. In mouse, initiation of most lymph nodes occurs between E12.5 until E15.5 [Fig. 2, Fig. 3]. The aggregates of LTi- and mesenchymal cells, called lymph node anlagen, are present until birth in mouse. Final organization of the LN due to differentiation of the stromal subsets and attraction of the specific lymphocytes to their respective regions take place in the first weeks after birth. On the contrary, in human, LN organogenesis takes place around the 12–17th week of pregnancy and humans are born with a fully organized LN at birth [59].
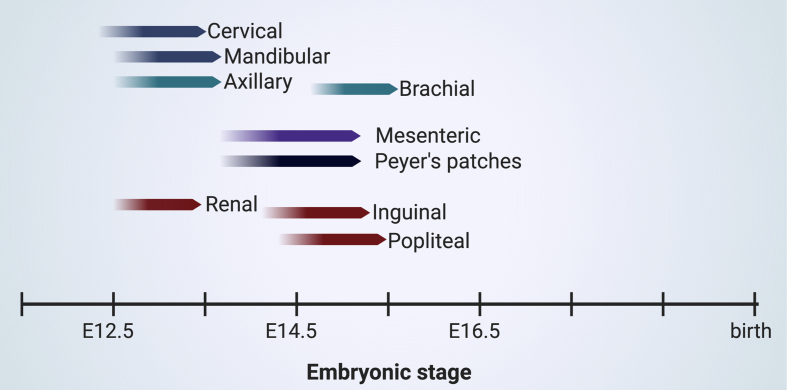
Fig. 3.
Overview of the timing of initiation for LTi cell dependent lymphoid organs including Peyer's patches (PP) at specific locations within the embryo. The table is based on the earliest observation of LTi cell clusters within the specific regions [19,51,68].
The first event which can be visualized in embryonic LN formation is the aggregation of the LTi cells at fixed positions within the embryo [Fig. 2]. The chemokine CXCL13 is essential for the aggregation of the LTi cells in most LN anlagen, except the mesenteric LN [60,61]. CXCR5+ LTiP cells are present within the LN anlagen, to be retained by local CXCL13 expression within the niches where LN are formed [7,19,43]. The role of CXCR6 [43,44] on these cells remains unknown in the attraction toward the LN niche. Only CCR7 was shown to be very potent in attracting LTi cells, but only when lymphatic endothelial cells secrete the ligand CCL21 [12,52,61]. In later LN formation stages, after lymphotoxin signaling and when the mesenchymal cells have differentiated into lymphoid tissue inducer (LTo) cells, the other CCR7 ligand CCL19 is expressed by LTo cells within the LN anlagen. This occurs around the time when the lymphatic endothelial cells have circumvented the LN anlagen. Some rescue could be observed by the lymphatic endothelial cell (LEC) expressed chemokine CCL21 [61], although these are likely to be aggregations within lymphatic vessels [12]. The presence of LECs and thus expression of CCL21 occurred after initial LTi cell aggregation and initial CXCL13 expression within the LN anlagen [12,52]. Moreover, lack of all lymphatic endothelial cells in the Prox1 knock-out mouse model did not affect the initial aggregation of the LTi cell clusters [62]. Initial Cxcl13 expression, but not other chemokines like Ccl21 and Ccl19, is induced by retinoic acid. Cxcl13 expression is required to attract the first precursor LTi cells towards the LN anlagen niche. It was proposed that nearby neurons synthesize the retinoic acid, thus inferring to a neuronal influence on LN formation [52]. However, it is unclear which type of neurons are involved in LN formation. After the initial LTi cell differentiation, Receptor activator of nuclear factor κ B Ligand (RANK-L), also known as TNFSF11A, either on LTi cells [54,63] or mesenchymal cells [64] activates RANK on LTi cells. RANK signaling induced expression of Lymphotoxin-α1ß2 ligand on the LTi cells, which is the latest maturation step of the LTi cell. Thus, loss of this signaling resulted in the loss of LTi4 cells and consequently loss of lymph nodes [54,64]. Lymphotoxin- α1ß2 will subsequently interact with its LTβ-receptor on the mesenchymal cells. This interaction triggers the expression of cytokines like IL7, chemokines like Cxc13 and adhesion molecules like Madcam-1 [63,65], which attract and retain more (precursor) LTi cells. These increasing interactions results in an amplification of the process and hence a strong increase in the size of the aggregate.
There is currently a discussion on the definition of the initial phase of LN formation. It was suggested that embryonic lymphatic vessels interacted with LTi cells through lymphotoxin signaling and that this delineated the initial event of LN formation [10]. Indeed, lymphatic vessels play an important role in lymph node formation but notably during the expansion phase. During the earliest clustering of LTi cells, well before E14.5, lymphatic vessels were not present nor required [12,52,62]. Starting at E14.5 in the anterior lymph node anlagen and later in the posterior located anlagen, LECs circumvent the LN anlagen and connect the lymphatic vasculature to the LN anlagen. This allows the migration of LTi cells from the skin towards the LN anlagen [12,62,66]. Notably, an elegant study by the Petrova lab showed that flow from the lymphatic vasculature was important for Cxcl13 expression within the inguinal lymph node anlagen. Blocking lymphangiogenesis by administering vascular endothelial growth factor receptor 3 (VEGFR3)-blocking antibodies resulted in an arrest of the small, initial, LTi cell aggregate and significant fewer LTi4 cells within the inguinal LN anlagen [12].
The first visible LN anlagen during human fetal development are found after week 12 [67], although the initial clustering of LTi cells occurs most likely earlier. In the mouse, the first LTi cell aggregate was observed at E12.5, either in hCD2GFP embryos [68] or analysis by flow-cytometry and (3D) immunofluorescence of RORytGFP embryos [19,51] [Fig. 3]. In time, other lymph node anlagen appear, notably in an anterior to posterior chronological manner, with the popliteal lymph nodes appearing the latest after E14.5 [19,69]. During initiation at E12.5, the anterior situated mandibular and cervical LTi cell clusters are very closely situated near each other within the cervical region. Separation of the cervical and mandibular LN anlagen occur clearly at E13.5. The brachial lymph node anlagen is present after E14.5 and located near the axillary LN anlagen. In the posterior region, initially at E12.5-E13.5, a large LTi cell cluster was observed next to mesonephros. The renal, inguinal and popliteal LN anlagen could be separately identified after E14.5 [19]. Since in the adult renal LNs are not bigger than the inguinal LNs and LTi aggregation in the inguinal LN anlagen dramatically increases in size within a day while the number of LTi cells within the renal LN anlagen uncharacteristically plateau at the same stage [19], it could be that the large aggregation of the initial renal LN anlagen in the mesonephros region could aid formation of other LN anlagen nearby by ‘donating’ LTi cells to the new niche nearby.
ILC3 and LTi-like in adult lymph nodes and gut
In the adult mouse, RORγt+CCR6+CD4+ LTi4 cells are mainly observed in lymph nodes and in gut. Lymph nodes are important for the functioning of the adaptive immune system, as they organize and facilitate the interaction between antigen presenting cells and the lymphocytes. The antigens are transported towards the lymph nodes by the afferent lymphatic vessels either unbound or bound to antigen presenting cells. The lymphatic vessels are connected to the lymphatic subcapsular sinus circumventing the lymph node. The antigen and cells are spread over the subcapsular sinus and migrate into the B-cell follicles or into the T-cell areas, depending on the antigen size or type of antigen presenting cell [[70], [71], [72]](reviewed in Ref. [73]). Each immune reaction is taking place in a specialized region within the lymph node, thus compartmentalizing the immune response. These regions are meticulously organized and maintained by specialized stromal cells. Initially, only T-cell zone stromal cells (fibroblastic reticular cells) in the paracortex, B-cell stromal zone cells (follicular dendritic cells) and endothelial cells were described (reviewed in Ref. [74]). This was later amended by the marginal reticular cells (MRC), positioned in the paracortex, adjacent to the B-cell follicles and just below the subcapsular sinus [75]. However, the use of single cell sequencing has allowed the identification of nine stromal cell clusters within the lymph node [76], each with specific expression profiles and functions (reviewed in Ref. [74]). These stromal cells differentiate from mesenchymal precursor cells, or LTo cells, in the first weeks after birth in the mouse [[77], [78], [79]]. Although there are indications that lymphotoxin signaling, retinoic acid or microbiota mediate mesenchymal differentiation [52,78,80], the specific cues for differentiation towards a specific stromal cell subset are yet unclear [81].
Embryonic LTi cells, which are involved in embryonic LN formation, are replaced in the adult by LTi cells derived from the bone-marrow [19,36]. Thus, since their origin is different, the LTi cells in adult are likely to be functionally different compared to the embryonic LTi cells. Within the lymph node, LTi cells were found adjacent to the MRC without an apparent function. Since the MRCs were assumed being a precursor for other stromal cells during expansion of the lymph node during inflammation [77], the MRC-LTi cell communication might reflect the interaction between embryonic LTi cells with LTo cells occurring during embryonic lymph node formation. Besides the obvious role for LTi cells during embryonic lymph node formation, there is not much known on their role in the adult. There is phenotypically not much difference, although adult LTi cells endogenously express OX40 and CD30 ligands, involved in clonal expansion and memory of lymphocytes, while the fetal LTi cells need DR3 stimulation to express these ligands [82]. It was described that adult LTi cells were involved in the formation of tertiary lymphoid structures during cancer [83]. They have been described in the restoration of lymphoid tissues, as they were involved in spleen restoration after viral infection [84] and rescue of mucosal associated lymphoid tissue (MALT) in an Id2-knock out model [85].
A close related LTi cell subset within the ILC3 branch is the LTi-like population, present in high numbers within the adult gut mucosa. These cells are different than the closely related NKp46+CCR6–RORγt+ ILC3 cells, which originate from NKp46–CCR6– progenitors driven by T-Bet which upregulates IFN-y and NKp46 expression [86,87]. LTi-like cells are characterized as NKp46–CCR6+ (CD4+/−) ILC3 cells, and found to be involved in antigen presentation and immune modulation and secrete IL22 [88,89]. They are guided by CCR7 expression [90] and play an important role in interaction between follicular Th-cells and IgA producing B-cells [91]. An embryonic LTi cell subset (LTi4 –II) expressed MHCII related genes [19], and it is interesting to establish whether there is a lineage and functional relationship between embryonic LTi4-II and LTi-like cells in the adult gut.
There are numerous reports stating the importance of ILC3s in the immune defense. They are involved in immune cells interactions to induce IgA production on B-cells [90,91]. They secrete IL17 and notably IL22 secretion upon IL23R activation, shown in mice and human and in different disease settings [88,[92], [93], [94]]. Besides their role in immune reactions, IL22 secreted by ILC3s was shown to be also important for maintenance of epithelial integrity. IL22 from ILC3/LTi-like cells induced the fucosyltransferase 2 (Fut2) expression in gut epithelial cells, which is required for fucosylation and resistance to Salmonella typhimurium infection [95].
There is discussion on the possible redundancy of NKp46+ ILC3s in the gut-immune response. A Ncr1 (NKp46)- Cre model was used to delete ILC3 cells or specifically knock-out Il22 within NKp46+ ILC3s during gut infection. In these models, the NKp46+ ILC3 population was shown to be redundant during an infection with Citrobacter rodentium and ILC3 deletion only affected cecal homeostasis [87]. However, Il22 expression in the NKp46–CCR6+ LTi-like cell population was not affected in these models [87] and it remains unclear whether this population could (partially) rescue the loss of IL22 from the NKp46+ ILC3 population. Indeed, it was shown that the ILC3 are partially redundant but play an essential role in T-cell deficient mice [96]. In another example of possible redundancy it was observed that in patients with severe combined immunodeficiency (SCID), the restoration of the hematopoietic compartment did not restore the ILC family without any obvious susceptibility to a disease [57]. Lastly, in human patients with a RORc loss of function mutation, lacking RORγt functionality and thus all ILC3 and LTi-like cells, were only more susceptible to Mycobacterium and Candida infections. This effect was attributed to defective γδ- and αβ- T-cells but not ILC3's [50]. Therefore, it remains unclear what the unique roles of ILC3 and LTi-like cells are within the gut. Is there indeed redundancy, as was proposed before [57,87], or is there a rescue mechanism which arises during fetal development and neonatal stages? Better mouse models, in which specifically ILC3 and/or LTi like cells can be deleted by induction at a specific time point to prevent a rescue mechanism during development, are required to answer this question. Additionally, the use of a unique gene for each population, but not genes such as Ncr1 or Rorc as drivers, are required to establish the specific effect of a specific ILC3 cell during disease.
Neuropilin-1 (NRP-1) was found to be expressed by ILC3 and LTi cells in mouse and human and involved in the formation of ectopic lymphoid organs, especially in the lungs of smokers and COPD patients [97]. Recently, Neuropilin-1 was also associated as host factor for Sars-Cov2 entry and the virus was associated with NRP-1 positive cells within the nasal cavity [98,99]. If Sars-Cov2 specifically transfects ILC3 and LTi cells is still unknown. It will be important to establish whether during this infection LTi cells could mediate ectopic lymphoid formation in lungs, which possibly exacerbates the immune reaction.
In general, ILC differentiation in embryo and adult is very flexible in order to adapt to the niche they reside in. Also, ILCs can be derived from different progenitors (reviewed in Ref. [100,101]). Therefore, marker expression for the ILC family members can vary and depend on their origin and differentiation program. This complicates the determination of the ILC members and induces discussion on their nomenclature. Especially for the characterization of enigmatic ILC1 subset this has been problematic [102] and based on CyTOF analysis it was even suggested that there is no specific human ILC1 population [103]. Other testimonies on ILC dynamicity are that the ILC3 cells within the gut could differentiate into ILC1 cells [87], fetal human intermediate-ILC1 were observed to become NK and ILC3s [37] progenitors in the gut differentiate to ILC2 cells which migrate to the lung [104](reviewed in Ref. [100]). Since the tissue residency and its immune status is of importance to the phenotype of the ILC, it is therefore of importance to isolate different tissues to characterize the ILC in question. The use of tonsil alone will skew characterization or provide a partial marker expression for human ILCs [105]. The origin of the ILCs, being embryonic or adult, is important to understand their eventual functioning. Therefore, it remains of much interest to pinpoint the embryonic hemogenic source of the ILCs and when they are replaced in the neonate by bone-marrow derived ILCs. This knowledge will aid our understanding of their miscellaneous roles in immunity and how to possibly dampen them in unwanted immunological reactions.
Conflicts of interest
The author declares no conflict of interest.
Acknowledgements
The group was supported by the FRM Amorçage de jeunes équipes (AJE20150633331), ANR ACHN (ANR-16-ACHN-0011), ANR PRCI (ANR-17-CE13-0029-01) and institutional grants from Inserm, CNRS and Aix-Marseille University to the CIML. I would like to thank Ana Cumano for the discussions on the hematopoietic progenitors, Lionel Spinelli and Julie Bavais for their help on the transcriptional data. I am grateful to Milesa Simic, Shuaiwei Wang and especially Rachel Golub for critically reading the manuscript.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Eberl G., Di Santo J.P., Vivier E. The brave new world of innate lymphoid cells. Nat Immunol. 2015;16:1–5. doi: 10.1038/ni.3059. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G. Innate lymphoid cells: 10 Years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa M., Germar K., Blom B., Spits H. Human CD5+ innate lymphoid cells are functionally immature and their development from CD34+ progenitor cells is regulated by Id2. Front Immunol. 2017;8:1047. doi: 10.3389/fimmu.2017.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Constantinides M.G., Gudjonson H., McDonald B.D., Ishizuka I.E., Verhoef P.A., Dinner A.R. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantinides M.G., McDonald B.D., Verhoef P.A., Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishizuka I.E., Chea S., Gudjonson H., Constantinides M.G., Dinner A.R., Bendelac A. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17:269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vivier E., van de Pavert S.A., Cooper M.D., Belz G.T. The evolution of innate lymphoid cells. Nat Immunol. 2016;17:790–794. doi: 10.1038/ni.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lane P.J.L., Gaspal F.M., McConnell F.M., Withers D.R., Anderson G. Lymphoid tissue inducer cells: pivotal cells in the evolution of CD4 immunity and tolerance? Front Immunol. 2012;3:24. doi: 10.3389/fimmu.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onder L., Mörbe U., Pikor N., Novkovic M., Cheng H.W., Hehlgans T. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity. 2017;47:80–92. doi: 10.1016/j.immuni.2017.05.008. e4. [DOI] [PubMed] [Google Scholar]
- 11.van de Pavert S.A., Mebius R.E. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 12.Bovay E., Sabine A., Prat-Luri B., Kim S., Son K., Willrodt A.H. Multiple roles of lymphatic vessels in peripheral lymph node development. J Exp Med. 2018;215:2760–2777. doi: 10.1084/jem.20180217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bando J.K., Liang H.E., Locksley R.M. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2014;16:153–160. doi: 10.1038/ni.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider C., Lee J., Koga S., Ricardo-Gonzalez R.R., Nussbaum J.C., Smith L.K. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity. 2019;50:1425–1438. doi: 10.1016/j.immuni.2019.04.019. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dege C., Fegan K.H., Creamer J.P., Berrien-Elliott M.M., Luff S.A., Kim D. Potently cytotoxic natural killer cells initially emerge from erythro-myeloid progenitors during mammalian development. Dev Cell. 2020;53:229–239. doi: 10.1016/j.devcel.2020.02.016. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Cherrier D.E., Chea S., Vosshenrich C., Serafini N., Petit M. An Id2RFP-reporter mouse redefines innate lymphoid cell precursor potentials. Immunity. 2019;50:1054–1068. doi: 10.1016/j.immuni.2019.02.022. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker J.A., Clark P.A., Crisp A., Barlow J.L., Szeto A., Ferreira A.C.F. Polychromic reporter mice reveal unappreciated innate lymphoid cell progenitor heterogeneity and elusive ILC3 progenitors in bone marrow. Immunity. 2019;51:104–118. doi: 10.1016/j.immuni.2019.05.002. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seillet C., Mielke L.A., Amann-Zalcenstein D.B., Su S., Gao J., Almeida F.F. Deciphering the innate lymphoid cell transcriptional program. Cell Rep. 2016;17:436–447. doi: 10.1016/j.celrep.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Simic M., Manosalva I., Spinelli L., Gentek R., Shayan R.R., Siret C. Distinct waves from the hemogenic endothelium give rise to layered lymphoid tissue inducer cell ontogeny. Cell Rep. 2020;32:108004. doi: 10.1016/j.celrep.2020.108004. [DOI] [PubMed] [Google Scholar]
- 20.Boisset J.-C., van Cappellen W., Andrieu-Soler C., Galjart N., Dzierzak E., Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 21.Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golub R., Cumano A. Embryonic hematopoiesis. Blood Cells Mol Dis. 2013;51:226–231. doi: 10.1016/j.bcmd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Baron C.S., Kester L., Klaus A., Boisset J.C., Thambyrajah R., Yvernogeau L. Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nat Commun. 2018;9:2517. doi: 10.1038/s41467-018-04893-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lis R., Karrasch C.C., Poulos M.G., Kunar B., Redmond D., Duran J.G.B. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545:439–445. doi: 10.1038/nature22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Q., Gao P., Tober J., Bennett L., Chen C., Uzun Y. Developmental trajectory of pre-hematopoietic stem cell formation from endothelium. Blood. 2020;136:845–856. doi: 10.1182/blood.2020004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGrath K.E., Frame J.M., Fegan K.H., Bowen J.R., Conway S.J., Catherman S.C. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mass E., Ballesteros I., Farlik M., Halbritter F., Günther P., Crozet L. Specification of tissue-resident macrophages during organogenesis. Science. 2016;353:aaf4238. doi: 10.1126/science.aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gentek R., Ghigo C., Hoeffel G., Bulle M.J., Msallam R., Gautier G. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. 2018;48:1160–1171. doi: 10.1016/j.immuni.2018.04.025. e5. [DOI] [PubMed] [Google Scholar]
- 29.Yvernogeau L., Klaus A., Maas J., Morin-Poulard I., Weijts B., Schulte-Merker S. Multispecies RNA tomography reveals regulators of hematopoietic stem cell birth in the embryonic aorta. Blood. 2020;136:831–844. doi: 10.1182/blood.2019004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yzaguirre A.D., Speck N.A. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev Dynam. 2016;245:1011–1028. doi: 10.1002/dvdy.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon-Keylock S., Sobiesiak M., Rybtsov S., Moore K., Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122:2338–2345. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zovein A.C., Turlo K.A., Ponec R.M., Lynch M.R., Chen K.C., Hofmann J.J. Vascular remodeling of the vitelline artery initiates extravascular emergence of hematopoietic clusters. Blood. 2010;116:3435–3444. doi: 10.1182/blood-2010-04-279497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigeta A., Huang V., Zuo J., Zhou B., Shigeta A., Huang V. Endocardially derived macrophages are essential for valvular remodeling article endocardially derived macrophages are essential for valvular remodeling. Dev Cell. 2019;48:1–14. doi: 10.1016/j.devcel.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGrath K.E., Koniski A.D., Maltby K.M., McGann J.K., Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. doi: 10.1006/dbio.1999.9405. [DOI] [PubMed] [Google Scholar]
- 35.Werner Y., Mass E., Ashok Kumar P., Ulas T., Händler K., Horne A. Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nat Neurosci. 2020;23:351–362. doi: 10.1038/s41593-020-0585-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawa S., Cherrier M., Lochner M., Satoh-Takayama N., Fehling H.J., Langa F. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 37.Li N., van Unen V., Höllt T., Thompson A., van Bergen J., Pezzotti N. Mass cytometry reveals innate lymphoid cell differentiation pathways in the human fetal intestine. J Exp Med. 2018;215:1383–1396. doi: 10.1084/jem.20171934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elsaid R., Meunier S., Burlen-Defranoux O., Soares-da-Silva F., Perchet T., Iturri L. A wave of bipotent T/ILC-restricted progenitors shapes the embryonic thymus microenvironment in a time-dependent manner. Blood. 2021;137:1024–1036. doi: 10.1182/blood.2020006779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoeffel G., Chen J., Lavin Y., Low D., Almeida F.F., See P. C-Myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ema H., Nakauchi H. Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood. 2000;95:2284–2288. [PubMed] [Google Scholar]
- 41.Klose C.S.N., Flach M., Möhle L., Rogell L., Hoyler T., Ebert K. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Boos M.D., Yokota Y., Eberl G., Kee B.L. Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J Exp Med. 2007;204:1119–1130. doi: 10.1084/jem.20061959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chea S., Schmutz S., Berthault C., Perchet T., Petit M., Burlen-Defranoux O. Single-cell gene expression analyses reveal heterogeneous responsiveness of fetal innate lymphoid progenitors to Notch signaling. Cell Rep. 2016;14:1500–1516. doi: 10.1016/j.celrep.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Possot C., Schmutz S., Chea S., Boucontet L., Louise A., Cumano A. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 45.Chea S., Perchet T., Petit M., Verrier T., Guy-Grand D., Banchi E.G. Notch signaling in group 3 innate lymphoid cells modulates their plasticity. Sci Signal. 2016;9:ra45. doi: 10.1126/scisignal.aaf2223. [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka I.E., Constantinides M.G., Gudjonson H., Bendelac A. The innate lymphoid cell precursor. Annu Rev Immunol. 2016;34:299–316. doi: 10.1146/annurev-immunol-041015-055549. [DOI] [PubMed] [Google Scholar]
- 47.Cherrier M., Sawa S., Eberl G. Notch, Id2, and RORγt sequentially orchestrate the fetal development of lymphoid tissue inducer cells. J Exp Med. 2012;209:729–740. doi: 10.1084/jem.20111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mebius R.E., Rennert P., Weissman I.L. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. doi: 10.1016/s1074-7613(00)80371-4. [DOI] [PubMed] [Google Scholar]
- 49.van de Pavert S.A., Ferreira M., Domingues R.G., Ribeiro H., Molenaar R., Moreira-Santos L. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature. 2014;508:123–127. doi: 10.1038/nature13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okada S., Markle J.G., Deenick E.K., Mele F., Averbuch D., Lagos M. Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science. 2015;349:606–613. doi: 10.1126/science.aaa4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 52.van de Pavert S.A., Olivier B.J., Goverse G., Vondenhoff M.F., Greuter M., Beke P. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Z., Unutmaz D., Zou Y.R., Sunshine M.J., Pierani A., Brenner-Morton S. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 54.Kim D., Mebius R.E., MacMicking J.D., Jung S., Cupedo T., Castellanos Y. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mebius R.E., Streeter P.R., Michie S., Butcher E.C., Weissman I.L. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narni-Mancinelli E., Gauthier L., Baratin M., Guia S., Fenis A., Deghmane A.E. Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aam9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vély F., Barlogis V., Vallentin B., Neven B., Piperoglou C., Ebbo M. Evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2016;17:1291–1299. doi: 10.1038/ni.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim A.I., Li Y., Lopez-Lastra S., Stadhouders R., Paul F., Casrouge A. Systemic human ILC precursors provide a substrate for tissue ILC differentiation. Cell. 2017;168:1086–1100. doi: 10.1016/j.cell.2017.02.021. e10. [DOI] [PubMed] [Google Scholar]
- 59.Hoorweg K., Cupedo T. Development of human lymph nodes and Peyer's patches. Semin Immunol. 2008;20:164–170. doi: 10.1016/j.smim.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Förster R., Sedgwick J.D. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 61.Luther S a, Ansel K.M., Cyster J.G. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vondenhoff M.F., van de Pavert S.A., Dillard M.E., Greuter M., Goverse G., Oliver G. Lymph sacs are not required for the initiation of lymph node formation. Development. 2009;136:29–34. doi: 10.1242/dev.028456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida H., Naito A., Inoue J.I., Satoh M., Santee-Cooper S.M., Ware C.F. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 64.Sugiyama M., Nakato G., Jinnohara T., Akiba H., Okumura K., Ohno H. Expression pattern changes and function of RANKL during mouse lymph node microarchitecture development. Int Immunol. 2012;24:369–378. doi: 10.1093/intimm/dxs002. [DOI] [PubMed] [Google Scholar]
- 65.Vondenhoff M.F., Greuter M., Goverse G., Elewaut D., Dewint P., Ware C.F. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439–5445. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z., Chai Q., Zhu M. Differential roles of LTβR in endothelial cell subsets for lymph node organogenesis and maturation. J Immunol. 2018;201:69–76. doi: 10.4049/jimmunol.1701080. [DOI] [PubMed] [Google Scholar]
- 67.Cupedo T. Human lymph node development: an inflammatory interaction. Immunol Lett. 2011;138:4–6. doi: 10.1016/j.imlet.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Veiga-Fernandes H., Coles M.C., Foster K.E., Patel A., Williams A., Natarajan D. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446:547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 69.Rennert P.D., James D., Mackay F., Browning J.L., Hochman P.S. Lymph node genesis is induced by signaling through the lymphotoxin β receptor. Immunity. 1998;9:71–79. doi: 10.1016/s1074-7613(00)80589-0. [DOI] [PubMed] [Google Scholar]
- 70.Roozendaal R., Mempel T.R., Pitcher L a, Gonzalez S.F., Verschoor A., Mebius R.E. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Braun A., Worbs T., Moschovakis G.L., Halle S., Hoffmann K., Bölter J. Afferent lymph–derived T cells and DCs use different chemokine receptor CCR7–dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12:879–887. doi: 10.1038/ni.2085. [DOI] [PubMed] [Google Scholar]
- 72.Martens R., Permanyer M., Werth K., Yu K., Braun A., Halle O. Efficient homing of T cells via afferent lymphatics requires mechanical arrest and integrin-supported chemokine guidance. Nat Commun. 2020;11:1114. doi: 10.1038/s41467-020-14921-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jalkanen S., Salmi M. Lymphatic endothelial cells of the lymph node. Nat Rev Immunol. 2020;20:566–578. doi: 10.1038/s41577-020-0281-x. [DOI] [PubMed] [Google Scholar]
- 74.Gentek R., Bajénoff M. Lymph node stroma dynamics and approaches for their visualization. Trends Immunol. 2017;38:236–247. doi: 10.1016/j.it.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Katakai T., Suto H., Sugai M., Gonda H., Togawa A., Suematsu S. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 76.Rodda L.B., Lu E., Bennett M.L., Sokol C.L., Wang X., Luther S.A. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity. 2018;48:1014–1028. doi: 10.1016/j.immuni.2018.04.006. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarjour M., Jorquera A., Mondor I., Wienert S., Narang P., Coles M.C. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med. 2014;211:1109–1122. doi: 10.1084/jem.20132409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koning JJ, Rajaraman A, Reijmers RM, Konijn T, Pan J, Ware CF, et al. Development of follicular dendritic cells in lymph nodes depends on retinoic acid mediated signaling. BioRxiv 2020.05.20.106385 [Preprint]. Available from: 10.1101/2020.05.20.106385. [DOI] [PMC free article] [PubMed]
- 79.Denton A.E., Carr E.J., Magiera L.P., Watts A.J.B., Fearon D.T. Embryonic FAP + lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J Exp Med. 2019;216:2242–2252. doi: 10.1084/jem.20181705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pezoldt J., Pasztoi M., Zou M., Wiechers C., Beckstette M., Thierry G.R. Neonatally imprinted stromal cell subsets induce tolerogenic dendritic cells in mesenteric lymph nodes. Nat Commun. 2018;9:3903. doi: 10.1038/s41467-018-06423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cupedo T., Mebius R.E. Cellular interactions in lymph node development. J Immunol. 2005;174:21–25. doi: 10.4049/jimmunol.174.1.21. [DOI] [PubMed] [Google Scholar]
- 82.Kim M., Toellner K., White A., McConnell F.M., Gaspal F.M.C., Parnell S.M. Neonatal and adult CD4+ CD3- cells share similar gene expression profile, and neonatal cells up-regulate OX40 ligand in response to TL1A (TNFSF15) J Immunol. 2006;177:3074–3081. doi: 10.4049/jimmunol.177.5.3074. [DOI] [PubMed] [Google Scholar]
- 83.Shields J.D., Kourtis I.C., Tomei A a, Roberts J.M., Swartz M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- 84.Scandella E., Bolinger B., Lattmann E., Miller S., Favre S., Littman D.R. Restoration of lymphoid organ integrity through the interaction of lymphoid tissue-inducer cells with stroma of the T cell zone. Nat Immunol. 2008;9:667–675. doi: 10.1038/ni.1605. [DOI] [PubMed] [Google Scholar]
- 85.Fukuyama S., Hiroi T., Yokota Y., Rennert P.D., Yanagita M., Kinoshita N. Initiation of NALT organogenesis is independent of the IL-7R, LTβR, and NIK signaling pathways but requires the Id2 gene and CD3−CD4+CD45+ cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- 86.Klose C.S.N., Kiss E.A., Schwierzeck V., Ebert K., Hoyler T., D'Hargues Y. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 2013;494:261–265. doi: 10.1038/nature11813. [DOI] [PubMed] [Google Scholar]
- 87.Rankin L.C., Girard-Madoux M.J.H., Seillet C., Mielke L.A., Kerdiles Y., Fenis A. Complementarity and redundancy of IL-22-producing innate lymphoid cells. Nat Immunol. 2015;17:179–186. doi: 10.1038/ni.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L., He Z., Slinger E., Bongers G., Lapenda T.L., Pacer M.E. IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology. Mucosal Immunol. 2015;8:390–402. doi: 10.1038/mi.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marchesi F., Martin A.P., Thirunarayanan N., Devany E., Mayer L., Grisotto M.G. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2:486–494. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 90.Mackley E.C., Houston S., Marriott C.L., Halford E.E., Lucas B., Cerovic V. CCR7-dependent trafficking of RORγ+ ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Melo-Gonzalez F., Kammoun H., Evren E., Dutton E.E., Papadopoulou M., Bradford B.M. Antigen-presenting ILC3 regulate T cell–dependent IgA responses to colonic mucosal bacteria. J Exp Med. 2019;216:728–742. doi: 10.1084/jem.20180871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J.S., Cella M., McDonald K.G., Garlanda C., Kennedy G.D., Nukaya M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ciccia F., Guggino G., Rizzo A., Saieva L., Peralta S., Giardina A. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015;74:1739–1747. doi: 10.1136/annrheumdis-2014-206323. [DOI] [PubMed] [Google Scholar]
- 94.Bernink J.H., Krabbendam L., Germar K., de Jong E., Gronke K., Kofoed-Nielsen M. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 95.Goto Y., Obata T., Kunisawa J., Sato S. Ivanov II, Lamichhane A, et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science. 2014;345:1254009. doi: 10.1126/science.1254009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Song C., Lee J.S., Gilfillan S., Robinette M.L., Newberry R.D., Stappenbeck T.S. Unique and redundant functions of NKp46+ ILC3s in models of intestinal inflammation. J Exp Med. 2015;212:1869–1882. doi: 10.1084/jem.20151403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shikhagaie M.M., Björklund Å.K., Mjösberg J., Erjefält J.S., Cornelissen A.S., Ros X.R. Neuropilin-1 is expressed on lymphoid tissue residing LTi-like group 3 innate lymphoid cells and associated with ectopic lymphoid aggregates. Cell Rep. 2017;18:1761–1773. doi: 10.1016/j.celrep.2017.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daly J.L., Simonetti B., Klein K., Chen K.-E., Williamson M.K., Antón-Plágaro C. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020;370:861–865. doi: 10.1126/science.abd3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bal S.M., Golebski K., Spits H. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20:552–565. doi: 10.1038/s41577-020-0282-9. [DOI] [PubMed] [Google Scholar]
- 101.Gronke K., Kofoed-Nielsen M., Diefenbach A. Innate lymphoid cells, precursors and plasticity. Immunol Lett. 2016;179:9–18. doi: 10.1016/j.imlet.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 102.Björklund A.K., Forkel M., Picelli S., Konya V., Theorell J., Friberg D. The heterogeneity of human CD127+ innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol. 2016;17:451–460. doi: 10.1038/ni.3368. [DOI] [PubMed] [Google Scholar]
- 103.Simoni Y., Fehlings M., Kløverpris H.N., McGovern N., Koo S.L., Loh C.Y. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017;46:148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang Y., Mao K., Chen X., Sun M., Kawabe T., Li W. S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science. 2018;359:114–119. doi: 10.1126/science.aam5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bar-Ephraim Y.E., Cornelissen F., Papazian N., Konijn T., Hoogenboezem R.M., Sanders M.A. Cross-tissue transcriptomic analysis of human secondary lymphoid organ-residing ILC3s reveals a quiescent state in the absence of inflammation. Cell Rep. 2017;21:823–833. doi: 10.1016/j.celrep.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 106.Berthault C., Ramond C., Burlen-Defranoux O., Soubigou G., Chea S., Golub R. Asynchronous lineage priming determines commitment to T cell and B cell lineages in fetal liver. Nat Immunol. 2017;18:1139–1149. doi: 10.1038/ni.3820. [DOI] [PubMed] [Google Scholar]
- 107.Bennion B.G., Croft C.A., Ai T.L., Qian W., Menos A.M., Miner C.A. STING gain-of-function disrupts lymph node organogenesis and innate lymphoid cell development in mice. Cell Rep. 2020;31:107771. doi: 10.1016/j.celrep.2020.107771. [DOI] [PMC free article] [PubMed] [Google Scholar]