Abstract
Innate lymphoid cell (ILC) subsets at barrier surfaces contribute to maintain tissue homeostasis and appropriate responses to infection. ILCs respond to environmental factors produced by non-hematopoietic cells within tissues, but also circulating cytokines or dietary compounds which allow them to adapt to organ milieu. Among these extrinsic signals, evidence is emerging that sex steroid hormones may act in a cell-intrinsic manner to regulate the development, maintenance in tissues and effector functions of specific subsets of ILCs. Understanding the nature and molecular mechanisms of sex steroid hormone actions on ILCs is important to unravel the cause of sexual disparity in human diseases and could lead to new drug development for the treatment of chronic inflammatory diseases or cancers. This review discusses the recent development in our understanding of the cell-intrinsic actions of sex steroid hormones on ILCs and their consequences on tissue-specific immunity with a particular focus on group 2 innate lymphoid cells and NK cells.
Keywords: Sex differences, Estrogen receptors, Androgen receptor, Allergic asthma, Group 2 innate lymphoid cells, NK cells
Innate immune cells differentially express sex-steroid hormone receptors, including ERs (Esr1, Esr2) and AR, to various degrees depending on their microenvironment [1,2]. For instance, group 2 innate lymphoid cells (ILC2) express Esr-1 when they are isolated from the uterus [3], whereas Esr-1 is barely detected in bone marrow ILC2 progenitors and tissue-resident ILC2 populations, which selectively express high level of Ar transcripts [4] (Fig. 2). Of note, emerging evidence support the implication of sex hormone receptor signaling in the direct regulation of innate and adaptive components of the immune systems in various pathophysiological contexts in vivo [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]].
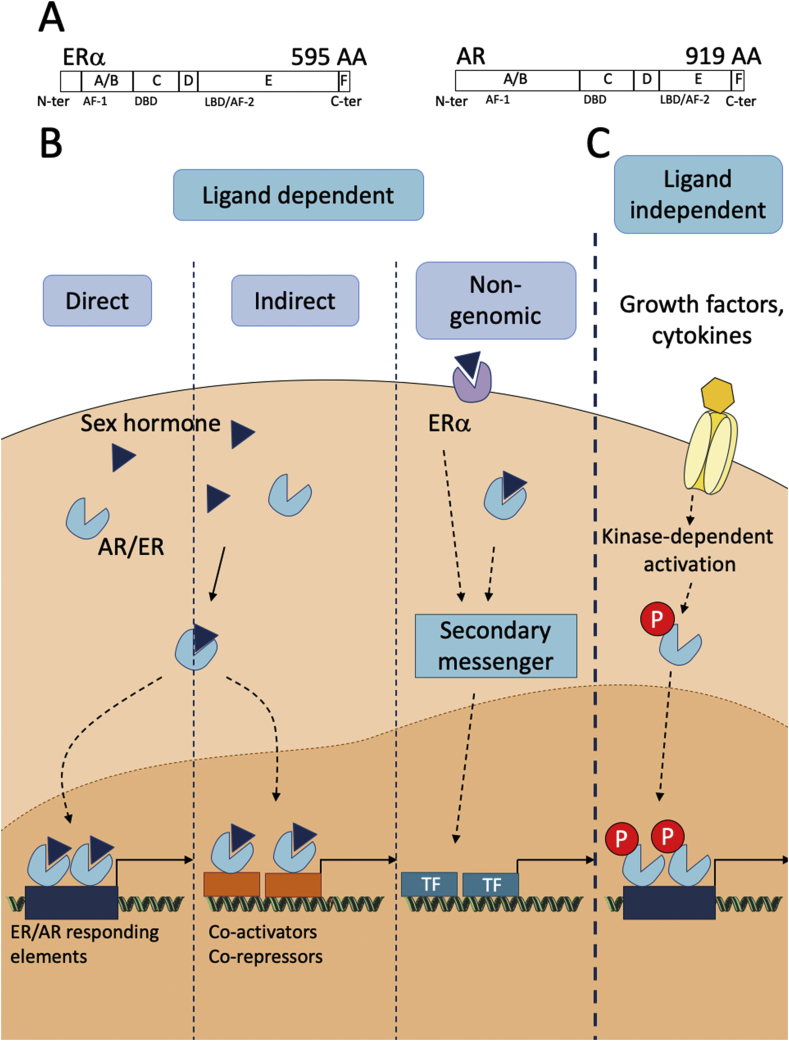
Fig. 2.
Sex steroid hormone receptor signaling pathway. (A) Schematic molecular structure of ERα and AR. Their functional domains are represented: the DNA Binding Domain (DBD), the Ligand Binding Domain (LBD) and the two transcriptional activation functions 1 and 2 (AF-1 and AF-2). (B, C) ERα and AR mode of action. (B) Hormone receptors can be activated in a ligand dependent manner either through direct binding of the receptor to hormone responsive element (HRE) on DNA or indirectly through association with transcription factors. Hormone can also trigger rapid non genomic activation of membrane-bound receptor generating second messengers. (C) Receptors can be also activated in a ligand-independent manner through kinase-dependent activation downstream of cytokine or growth factors receptors.
In this review, we will cover the current knowledge on sex differences in ILC development and functions, supporting evidence for a role of sex hormone receptor expression and signaling in ILCs. Because not much is known for most ILCs, we will focus on the contribution of sex steroid hormones on NK cell and ILC2 functions and development.
Developmental pathway and functions of ILCs
ILC development
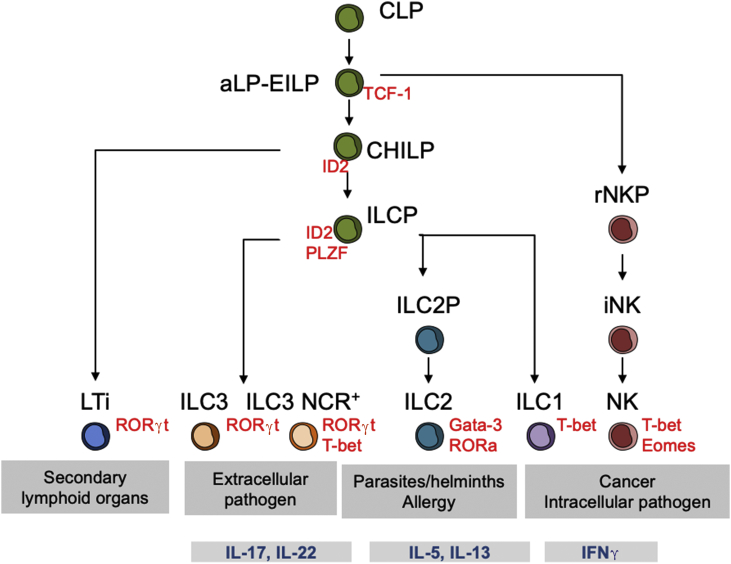
CLPs isolated from fetal liver or bone marrow have been shown to generate, together with adaptive lymphocytes, all types of ILCs both in vitro and in vivo [16]. Downstream of the CLP, the development of adaptive and innate cells diverges, and committed ILC precursors have been identified [17].
Two ILC progenitors, downstream of CLP but phenotypically linked to it, have been described: the α-lymphoid progenitor (αLP) and the early innate lymphoid progenitor (EILP). They both have the potential to differentiate into all ILC subtypes but have lost the ability to differentiate into T or B cells [16]. Restriction of the EILP downstream precursors to differentiate into all ILC subtypes appears to be a gradual process, with cells first losing the potential to develop into NK cells, followed by the loss of differentiation potential into LTi cells and, ultimately, restriction to ILC1, ILC2 or subsets of ILC3 [16] (Fig. 1).
Fig. 1.
Model of ILC development and function. All lymphoid cells can arise from the common lymphoid progenitor (CLP). The αLP (α-lymphoid progenitor) and EILP (early innate lymphoid progenitor) give rise to all ILC (NK, helper ILC and LTi). The CHILP (common helper ILC progenitor) has lost the ability to differentiate into NK cells. ILCP (helper ILC progenitor) has further lost the ability to differentiate into LTi (lymphoid tissue inducer). The main function and cytokine produced upon activation is indicated for each ILC. Key transcription factors requires for cell development are also specified.
Downstream EILP, several multipotent progenitors have been identified. A common helper ILC progenitor (CHILP) was described in 2014 [18]. This progenitor neither expresses Flt3 nor CD25 but expresses CD127 and α4β7. Unlike the EILP or the αLP, the CHILP has lost the ability to differentiate into NK cell but can give rise to all helper ILCs, including LTi cells. The CHILP population is heterogeneous and includes a subset of cells expressing PLZF, a transcription factor that has been associated with NKT cells [19]. This progenitor has lost the ability to generate LTi cells, but is able to differentiate into all helper ILC, hence its designation as ILC helper progenitor (ILCP). The unicellular gene expression profile of fetal ILCPs revealed the co-expression of mRNA encoding for transcription factors and cytokines associated with ILC1, ILC2 and ILC3. This progenitor therefore appears to be in a process called “lineage priming”, which is the first step in lineage commitment for a multipotent progenitor [20].
A progenitor restricted to the ILC2 cell line (ILC2P) has been identified in the bone marrow on the basis of Gata-3 and Id2 transcription factor, as well as numerous markers of ILC2 such as ICOS, CD25 and IL-1RL1 (IL-33 or ST2 receptor) [21].
Regarding NK cell development, the earliest progenitor already committed to the NK cell line (NKP) was discovered almost 20 years ago and described based on the expression of CD122, the absence of NK1.1, CD49b and receptors from the Ly49 family [22]. The NKP population is heterogeneous, however, 10 years later a revised NK progenitor (rNKP) was described. rNKP has a surface phenotype similar to that of CLP but without Flt3 and additionally expresses CD122 and CD127. In the process of differentiation, NKs go through an immature stage, iNK, before becoming mNK [22]. The iNK population closely resembles the ILC1s phenotypically, as it expresses NK1.1 but has not acquired DX5. A cartographic study even attributed up to 80% of iNKs to the ILC1 cell line [23].
Functions of ILCs in health and diseases
When NK cells encounter a potential target cell and are activated, a synapse is formed and lytic granules, carried on microtubules, converge to the synapse. Granules are organelles linked to lysosomes that contain the main effectors of cytotoxicity: perforin, which inserts into the plasma membrane of target cells and forms pores leading to osmotic lysis, and granzymes, which transfer through pores and activate caspases, causing apoptosis of target cells [24].
Besides their cytotoxic capacity, NK cells can secrete several cytokines (IFNγ, IL-13, TNF), chemokines (CCL3, CCL4, CCL5, XCL1) and growth factors (Flt3L) and can therefore influence the immune response. For example, the secretion of CCL5 and XCL1 allows the attraction of dendritic cells, whereas Flt3L increases the number of dendritic cells present within the tumor microenvironment. Through IFNγ secretion, NK cells promote Th1 polarization, induce MHCII expression on antigen presenting cells and activate macrophages, making them the ideal target for immunotherapy in cancer [25].
For a long time, ILC1s were considered as immature or developing NK. However, ILC1s are a lineage of lymphoid cells whose development is different from that of NKs [18]. ILC1s are tissue resident cells, and are present before birth. NK cells and ILC1s have several characteristics in common such as IFNγ production and Tbet requirement for this function. However, ILC1s produce IFNγ and GM-CSF (Granulocyte Macrophage Colony-Stimulating Factor) at much higher levels than NK cells, and depend on IL-7 and Gata-3 for their differentiation. ILC1s have generally no- or weak cytotoxic functions but can act as a first line of defense against infections with viruses and certain intracellular pathogens, such as Toxoplasma gondii or Clostridium difficile [26].
ILC2s have been discovered in gastrointestinal nematode infection models. Following helminth infection, these cells are the earliest and predominant source of IL-13 and IL-5 which induce eosinophilia, goblet cell hyperplasia, mucus production, and hyper-contractility smooth muscles therefore promoting worm elimination [27]. In the past ten years, several research groups have characterized the role of ILC2s in the development and chronicity of asthma in humans. More than 50% of asthmatic patients display allergic eosinophilic inflammation of the airways caused by an excessive type 2 immune response to inhaled allergens and/or BAL of asthma patients. In addition, ILC2s were found to produce more IL-5 and IL-13 in asthmatic patients [28].
ILC3s are abundant at mucosal membranes and are involved in the innate immune response against extracellular bacteria and in the containment of intestinal commensal bacteria. There are two distinct subsets of ILC3, positive or negative for NCRs, both depending on RORγt for their development and function. In mice, NCR negative ILC3s mainly produce IL-22, whereas NCR positive ILC3s produce both IL-22 and IL-17A. The IL-22 mediated-epithelial regeneration is one of the mechanisms by which the immune system support intestinal epithelium homeostasis and maintain its integrity [29].
IL-10-producing ILC2s have been reported under certain conditions [30,31]. It has been suggested that IL-10+ ILC2s could emerge from exhausted ILC2s or through the conversion of tissue-resident ILC2s under the influence of environmental cues such as retinoic acid [32]. Alternatively, It has been proposed that IL-10-producing ILCs could represent a separate regulatory ILC lineage (ILCregs) [33]. Murine ILCregs help to resolve intestinal inflammation by producing IL-10 and TGFβ. A similar population has also been found in humans. ILCregs are mainly located in the intestinal tract and express various ILC phenotypic markers, such as CD25, Sca-1 and CD90 but do not express Foxp3. ILCregs are derived from CHILP and strongly express the Id3 and Sox4 transcription factors, but no other transcription factors that are essential for the development of ILC, such as Nfil3, RORγt, Gata-3 or AHR [33]. However, the role of these cells in a physiological context, with a functional adaptive system, has yet to be explored.
LTi cells or Lymphoid Tissue Inducer cells play a special role in the ILC family. One of their major functions is to induce the development of lymphoid tissues among them lymph nodes and Peyer's patches early during embryonic development [27].
Sex hormones receptor signaling and expression pattern in ILCs
General principle of estrogen and androgen receptor signaling
Molecular structure of ERα and AR (Fig. 2)
ERs and AR are members of the large family of nuclear receptors which act essentially as regulators of transcription. Their molecular structures share five functional domains: the N-terminal transcriptional regulation domain (or A/B domain), the DNA binding domain (DBD or C domain), the hinge region (D domain), the ligand binding domain (LBD) and the C-terminal region (F domain). The A/B region is implicated in protein/protein interactions and in transcriptional regulation of targeted genes. The A/B domain contains the transactivation function 1 domain (AF-1) that can influence transcription through recruitment of co-activators or co-repressors. The A/B domain also contains phosphorylation sites, targets of several kinases involved in signaling pathways downstream of growth factors or cytokines. The C domain constitutes the DBD which is the most conserved domain among the different nuclear receptor members. The DBD selectively binds to hormone responsive element (HRE) within target genes to modulate their transcription allowing their specificity of action. The D domain is a flexible hinge region, poorly conserved, joining the DBD and the LBD. The C-term E/F region includes the LBD, the transcriptional activation function 2 (AF-2) and domain interacting with chaperons. Binding of the hormone to the LBD induces conformational changes allowing stabilization of the receptor dimers. It also includes a nuclear localization domain signal (NLS) which is required for receptor translocation to the nucleus after its dimerization [34,35].
Mode of action of ERα and AR
Hormones can trigger receptor-mediated signaling through different pathways (Fig. 2). Upon interaction of the hormone with its receptor in the cytoplasm, dimers translocate to the nucleus where they modulate target gene transcription either through direct DNA binding to their specific HRE and further recruitment of transcription co-repressors or co-activators or through indirect DNA binding via association with transcription factors such as AP-1 and SP-1 for ERα or ETS for AR. In addition to these classical genomic modes of action of the receptors that classically depend on AF-1 and/or AF-2 domains, rapid, non-genomic activity mediated by membrane-bound receptors in rafts or caveolae have been described. Theses Membrane Initiated Steroid signaling effects (MISS) trigger activation of several kinases (MAPK, PKC, PI3K) or phosphatases generating second messengers ultimately able to influence transcription [34,35]. AR signaling can also occur via non-classical membrane AR which are unrelated to nuclear steroid receptors (ZIP9, GPRC6A, OXER1), which engage G-protein coupled signaling mechanisms [36]. Besides these ligand-dependent pathways, ERα and AR can be regulated by others signals such as cytokines like IL-6 or growth factors like EGF (Epidermal growth factors) [34,37].
Interestingly, glucocorticoid receptor signaling has been shown to exert sex-mediated effects in malignant or non-malignant tissues. Indeed, studies have shown that androgens can directly regulate either positively or negatively glucocorticoid receptor activity in a tissue dependent manner [38].
Analysis of sex steroid hormones expression in ILCs: focusing on ILC2
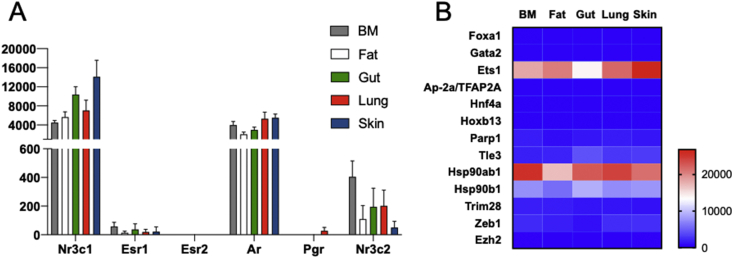
The expression pattern of steroid hormone receptor family members in ILC2, ILC3 and NK cells have been recently investigated using data extracted from the Immunological Genome Project database [2]. NK cells were reported to express high levels of ERα/Esr-1 gene in most populations examined, and also the progesterone receptor (Pgr) in some. They were negative for AR, GR (Nr3c1) and ERβ (Esr-2). ILC3s barely express sex steroid hormone receptors, whereas ILC2 were strongly positive for Ar gene expression [2]. This was in agreement with previous observations describing Ar as a prototypical signature gene of tissue-resident ILC2 [39]. We therefore investigated steroid hormone receptor expression from available NGS data obtained from steady state ILC2 purified from various tissues of mice based on the expression of the Il5 reporter allele Red5 (R5) [40].
ILC2 from all tissues tested highly express the glucocorticoid receptor (GR, Nr3c1) which upon activation by corticosteroids can efficiently inhibit IL-33 dependent proliferation and type-2 cytokine production by ILC2s [41]. Conversely, ILC2 express a narrow panel of sex steroid receptors. While androgen receptor is expressed at high level in tissue ILC2, similar to that of GR, they barely express ERα (Esr1) and the progesterone receptor while ERβ (Esr2) cannot be detected (Fig. 3A). These profiles of sex steroid hormone receptor expression are in agreement with recent papers demonstrated the key role of AR played on ILC2 biology [4].
Fig. 3.
Tissue-resident ILC2 selectively express the androgen receptor and the AR-related pioneer factor Ets1. Data were obtained from Ricardo-Gonzalez et al., 2018. ILC2 from steady state mice (n = 3–6) were sorted from BM (Lin−CD45+Yarg+), lung, fat, gut and skin (Lin−CD45+Red5+ cells) were sorted and analyzed by single-cell RNA sequencing as shown [40]. (A). Histograms show read counts obtained for the indicated sex hormone receptors Nr3c1 (glucocorticoid receptor), Esr-1 (estrogen receptor α), Esr-2 (estrogen receptor β), Ar (androgen receptor), Pgr (progesterone receptor) and Nr3c2 (mineralocorticoid receptor) from BM and tissue ILC2. (B). Heatmap showing expression Z-scores of the indicated androgen receptor co-regulators in ILC2 from BM and tissue ILC2s.
We then investigated the expression of known co-regulators of AR like chaperone proteins or pioneer factors which have been shown to facilitate AR action through interaction with AR at the DBD such as forkead box 1 (FOXA1), ETS1 (Fig. 3B). In prostate cancer, FOXA1-binding sites are typically found in close proximity to AR-binding sites, with a large amount of overlap between their respective cistromes in prostate cancer cells [35]. Pioneer or licensing factors guide the receptors to open chromatin sites and determine the distribution of the AR to appropriate gene loci in specific tissues and therefore play essential role in liganded AR selective actions [38]. Whereas ILC2 lack expression of FOXA1 and many other pioneer factors examined (Fig. 3B), they were strongly positive for ETS1 (Fig. 3B). Interestingly, ETS1 is a very early regulator in the transcriptional network controlling the emergence and function of ILC2s [42]. ETS1 functions to promote the up-regulation of Id2 mRNA that is observed in CHILP and ILCP during ILC2 development (Fig. 1). ETS1-deficiency compromised the fitness of CHILP in competition assay and was required for stable and high Id2 expression in CHILP as they differentiate into ILC2s. ETS1 was also found to positively regulate mature ILC2 expansion and cytokine production [42]. Whether and how liganded AR and ETS1 interact to promote a tissue-specific transcription programs in ILC2 remains to be investigated. In favor of a interaction between both transcription factors, it was reported that Klrg1 ranked first after Ets1 in the downregulated genes in in vitro expanded ETS1−/− ILC2 [42]. As described below, up-regulation of KLRG1 is a strong marker of androgen-sensitization of murine ILC2 (Fig. 4). Whether this is through direct AR-binding to regulatory elements of the Klrg1 gene is not known as consensus ARE were not found in this genomic region (D. Metzger, IGBMC, personal communication). ILC2 also strongly express mRNA for the classical chaperon HSP90b (Hsp90ab1) and to a lesser extent for the HSP90b1 (Hsp90b1). These proteins have the ability to stabilize the AR in the cytoplasm when not associated with androgen and to protect them from degradation [35].
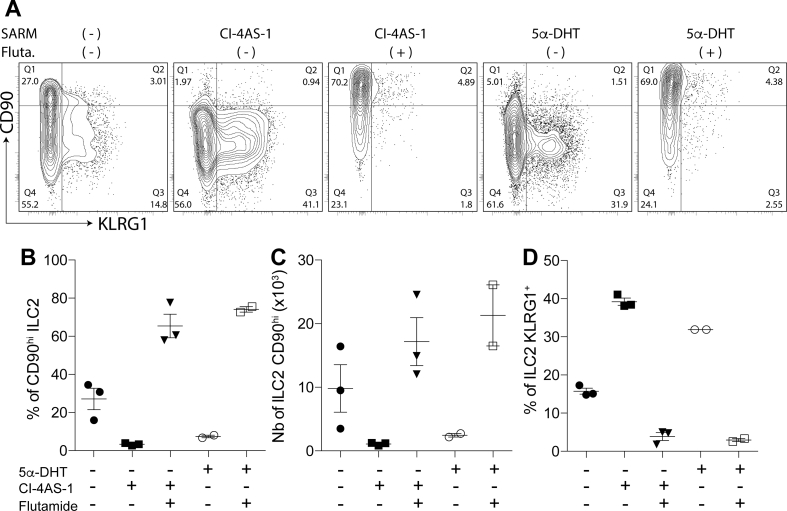
Fig. 4.
The SARM Cl-4AS-1 blocks ILC2P to ILC2 transition as efficiently as 5a-DHT.In vitro differentiation of ILC2p on OP9-DL1 feeder cells in complete medium supplemented with mouse IL-33 (1 ng/ml), IL-7 (5 ng/ml) and SCF (15 ng/ml) in presence or absence of AR-agonist 5α-DHT (1 nM) or CI-4AS-1 (10 nM) and the AR-antagonist Flutamide (100 nM). Flow cytometric analysis of ILC2 were done after 7 days differentiation. (A) Representative dot plot and proportion of CD90 and KLRG1 expression on ILC2. ILC2 were gated as live, Sca1+ and Gata-3+ cells. Representative histograms of (B) frequency and (C) numbers of CD90+ ILC2. (D) Frequency of KLRG1 expression on ILC2 in triplicates culture. Error bars indicate the mean ± SEM.
Biological impact of sex-hormone signaling on ILCs
NK cells a potential target for estrogens?
The expression pattern of steroid receptors among the ILC family is narrow and is often specific to an ILC group. While NK cells do not express Ar mRNA as opposed to ILC2, they are the only ILC members to express significant levels of the Esr1 mRNA (ERα) [2]. Interestingly, Esr1 mRNA expression by NKs is conserved between mouse and Human [43]. Despite evidence for Esr-1 gene expression in NK cells in mouse and human, studies aiming at identifying whether NK cells can be directly regulated by estrogens are limited and often contradictory both in mouse and humans.
Earlier works in mouse have shown that long term supplementation during several weeks with pharmacological dose of estradiol reduced in vivo NK cell cytotoxicity against tumor cell line [44]. Similarly, administration of high dose of E2 in post-menopausal women is associated with decreased NK cell cytotoxic activity [45]. On the opposite, other studies designed to evaluate the role of endogenous estradiol on NK cells, without exogenous administration of the hormone, have proposed that E2 is a positive regulator of NK cell activity. Hrushesky et al. have indeed shown that the strongest NK cytotoxicity activity in mouse is observed during the phases of the estrous cycle when estradiol is highest [46,47]. In the same lines, in vitro studies have shown that human NK cell line treated with E2 harbored enhanced cytotoxic activities against tumor cell. Addition of tamoxifen blocked the E2-mediated increase strongly suggesting that E2 potentiates NK cell killing function through ERα activation [48]. More recently, estrogen has been shown to stimulate the secretion of CCL2 by uterine NK cells which is critical to mediate endometrial NK cell-mediated angiogenesis [49].
Lack of concordant results between studies probably lies in the fact that the source of estrogen (endogenous versus exogenous administration), the dose (physiological versus pharmacological) and the duration of administration (physiological cycle versus chronic for weeks) are different. As ERα expression is shared by several immune cells (DCs, T, NK …), the final demonstration of an in vivo modulation of NKs by endogenous hormones will come from the use of mice selectively devoid of Esr-1 gene in NKs.
Impact of sex hormones on ILC2s
Regulation of ILC2 by estrogens
As opposed to NK cells, which express Esr1 mRNA at high levels, most tissue resident ILC2s lack expression of estrogen receptors Esr-1 and Esr-2 mRNA (Fig. 3), excepted uterine ILC2s [3]. Regarding uterine ILC2, it was reported that castration of female mice strongly reduced their numbers [3]. However, such results need to be interpretated with caution as uterine growth and epithelial cell proliferation is reduced by 80%–90% in ovariectomized (Ovx) mice. Thus, the reduction in uterine ILC2 numbers could be explain by the uterine hypotrophy in Ovx mice caused by the absence of ERα-signaling in the uterus epithelial cells and stromal cells resulting in a reduction in tissue resident ILC2s. Although uterine ILC2s incubated with 17β-estradiol (E2) exhibited changes in gene expression pattern, the role of ERα-signaling in steady-state accumulation of ILC2 in the uterus and its biological significance remains to be established [3]. Lung ILC2s, as many other tissue-resident ILC2s lack significant expression of ER (Fig. 3). Likewise, ovariectomy, E2-supplementation, and Esr1gene ablation in the hematopoietic or lymphoid compartment failed to demonstrate any effect of estrogens on lung ILC2s [3,4,50,51] (Fig. 5). This contrasted with the observation that ILC2 numbers and function were up-regulated in mice constitutively lacking ERα [50,51]. This was however explained by the elevated seric testosterone levels produced by the ovaries in ERα−/- female [51]. Indeed, ERα-deficient mice display elevated levels of Testosterone, estradiol and luteinizing hormones (LH) [52]. This is due to an excess production of LH as a consequence of the loss of ERα-signaling in the negative-feedback in the hypothalamic–pituitary axis resulting in hypergonadism and in a 10-fold raise in E2 and Testosterone [52]. As expected, castration of ERα−/- female mice abolished the difference in lung ILC2 as compared to intact female controls [51]. Moreover, ERα-deficient mice have increased body weights and total body fat and caution should be taken while interpretating results using fully ER-deficient animals. Thus, all current observations and the unbiased profiling of sex steroid hormone expression (Fig. 3) point towards a limited action of female sex hormones estrogens or progesterone on ILC2 biology, with the possible exception of the uterine ILC2 (Fig. 6).
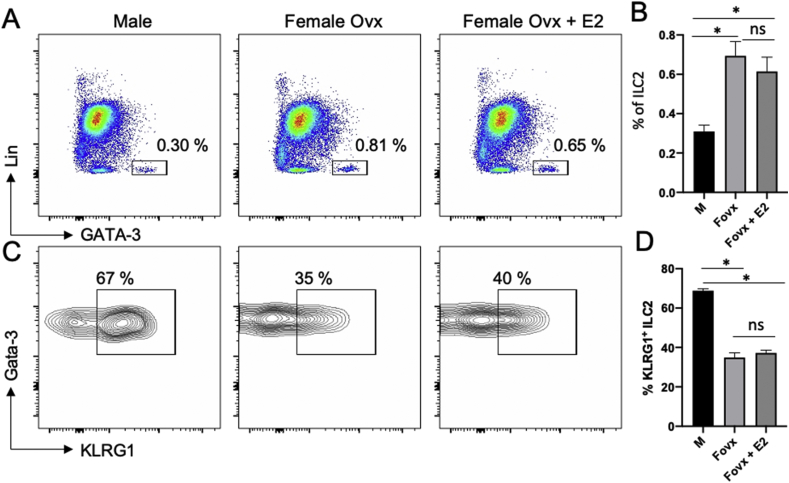
Fig. 5.
Estrogen treatment in females neither affects lung ILC2 frequency nor their KLRG1 expression. (A). Flow cytometry analysis of lung ILCs defined as Lin−CD45+CD90+GATA-3+ in male, ovariectomized (Ovx) females treated or not with 17ß-œstradiol (E2) for 7 days. (B). Histograms show the frequency of lung ILC2s. (C). Flow cytometry analysis of KLRG1 expression on lung ILC2. (D). Histograms show the frequency of lung KLRG1-expression ILC2s. Bar graphs show data as means ± SEM (n = 5 mice/group).
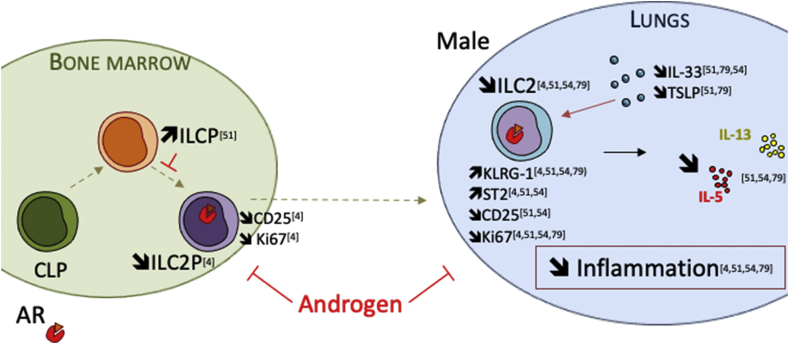
Fig. 6.
Mechanisms and hypothesis of ILC2 regulation by androgens in males mice. It has been shown that ILC2P in the bone marrow are reduced in males compared to females C57BL/6 mice. This was associated with a reduction of CD25 expression on male ILC2s which exhibited a reduction in spontaneous proliferation [4]. Accumulation of KLRG1+ ILC2 in the bone marrow of male mice was also reported [51]. PLZF+ ILC precursors accumulated in male bone marrow, suggesting that androgen may inhibit ILC2 development in the bone marrow by limiting the transition from ILCP to ILC2P [51]. Cephus et al. [54] showed that 5α-DHT treatment limited lung ILC2 numbers. In male mice, ILC2 had an increased expression of KLRG1 and ST2, whereas CD25 expression was diminished. This phenotype was associated with a reduction of lung inflammation upon allergen challenge, and a reduction of cytokine production by ILC2 in male mice, indicating that androgens may not only affect ILC2 development and maintenance in tissues, but also their function [54,82]. It has been suggested that IL-33 expression could be differentially regulated between female and male mice, with estrogens-dependent upregulation in female [50,54]. However, the sex bias in lung ILC2 numbers and phenotypic changes are not affected by IL-33 deficiency [82].
Regulation of ILC2 by androgen receptor signaling
Based on the central role of ILC2 in asthma pathophysiology and the evidence of sex bias in the disease, ILC2s have been recently suspected to be differently regulated between males and females [4]. We performed a careful examination of ILC2 numbers in mouse tissues based on the expression of CD45, CD90, GATA-3 and the lack of lineage-specific markers. This staining strategy has been reported to define all ILC2 subsets in the lung [53]. We showed that males exhibit reduced susceptibility to allergic airway inflammation in response to the environmental allergen HDM (house dust mite extracts), and less severe IL-33-driven lung inflammation. At steady state, the frequency and absolute number of ILC2s were increased in the lungs of female compared to male mice, as well as in adipose tissue and mesenteric lymph nodes. Importantly, orchiectomy, but not ovariectomy, abolished the sex differences in ILC2 development and IL-33-mediated lung inflammation.
Indeed, like ILC2P [4,40], lung ILC2 selectively expressed the Ar gene among other sexual hormone receptors (Fig. 3), in agreement with previous observations describing Ar as a prototypical signature gene of tissue-resident ILC2 [39]. The absence of estrogen receptor gene expression in lung ILC2s (Fig. 3) is consistent with the lack of effect of ovariectomy, estrogen administration or Esr-1 gene deletion in lung ILC2 numbers as shown in Fig. 4 [4,54]. The frequency of ILC2P was also augmented in female mice, suggesting an inhibitory effect of male sex hormones on the development of ILC2 immediate progenitors. Like ILC2 (Fig. 3), ILC2P also expressed AR, and 5α-dihydrotestosterone (DHT) inhibited the in vitro development of ILC2 from ILC2P cultured in the presence of IL-33 [4]. By contrast, Flutamide, a pure AR antagonist, had the opposite effect, demonstrating that ligand-inducible AR activation in developing ILC2 is amenable to pharmacological manipulation. Indeed, cell-intrinsic AR-signaling in the hematopoietic compartment was required to limit IL-33-driven ILC2 expansion in vivo in males [4].
A key role of AR-signaling on ILC2 development is further exemplified in Fig. 4 using an in vitro model of IL-33-driven ILC2 differentiation. We set-up optimal conditions to promote ILC2 development from ILC2P. In this model, we provided additional evidence that ILC2 are amenable to pharmacological manipulation through AR activation (Fig. 4). Supplementation of the cultures with 5α-DHT, or with the selective AR modulators (SARM) Cl-4AS-1 [55], induced a developmental switch in ILC2P to ILC2 transition in vitro, by favoring the differentiation of CD90dull ILC2, from which 40% express high level of KLRG1 (Fig. 4). This effect was inhibited by the AR antagonist Flutamide, promoting the development of KLRG1neg CD90hi ILC2 which resemble the dominant tissue-resident lung ILC2 population preferentially found in female mice [4,51]. These results provide the first evidence that the SARM Cl-4AS-1 can fully substitute to the pure agonist ligand 5α-DHT to inhibit the transition of ILC2P to KLRG1neg CD90hi ILC2. However, we cannot exclude that AR agonist might have limited impact on developing ILC2 regarding other parameters than up-regulation of KLRG1 expression. Indeed, due to the absence of E-cadherin in this in vitro system, no inhibitory signals into KLRG1+ ILC2s are anticipated. A detailed analysis of ILC2 subcategories that develop in the presence or absence of AR-activation will deserve further investigations. SARMs represent interesting alternative to Testosterone for in vivo therapy as they can maintain tissue–selective properties with greatly reduced unwanted side-effects on reproductive tissues and lower androgenic properties [55,56]. Whether such SARMs may substitute to Testosterone to inhibit ILC2-mediated lung inflammation in vivo will warrant further investigation.
Besides regulating lung ILC2 numbers, it was also shown that male ILC2s exhibited reduced capacities to produce IL-5 and IL-13 upon stimulation with the cytokines IL-2/IL-33 [54]. Of note, differences in effector functions between male and female ILC2 were not observed upon stimulation with PMA/ionomycin, suggesting a selective deficiency in IL2R- or ST-2-signaling pathways imprinted by androgen-signaling [54]. However, androgen-mediated developmental differences cannot be excluded as male lung ILC2s are enriched in cells expressing the lectin inhibitory receptors KLRG1 as compared to female [4,51,53]. Pulmonary ILC2s from female mice have a prominent population of functional KLRG1- ILC2s at steady state [4,51]. This robust phenotypic difference between male and female pulmonary ILC2s is context specific and vanishes upon allergen challenge or IL-33-mediated lung inflammation, resulting in upregulation of KLRG1 to similar extent in both sexes [4]. KLRG1 interacts with E-cadherins on epithelial cells [57], and could therefore transduce inhibitory signals upon ligand binding [[57], [58], [59]]. However, although the inhibitory effect of KLRG1/E-cadherin interactions were reported in vitro with human skin ILC2 in atopic dermatitis [60], the in vivo relevance of this pathway remains to be established. Of note, Klrg1-deficiency was not associated with functional changes in NK and T cells in response to viral infection [61]. KLRG1 was however recently reported to limit gut Treg cell accumulation in competitive settings [62]. The first in vivo relevance of KLRG1 on disease control has been established in an infection model [63]. KLRG1-deficiency was associated with long-term control of Mycobacterium tuberculosis infection [63]. Whether KLRG1 expression affects the competitive fitness of individual pulmonary ILC2s and controls the sex bias in ILC2s upon allergen or pathogen insult to the respiratory tract warrant further investigations.
Sex bias in ILC2 tissue repair function and plasticity
Besides their role in allergic lung inflammation, lung ILC2 promote homeostasis following viral infection [64]. Sex differences in disease control have been reported in respiratory virus infection like influenza virus [1] and M. tuberculosis [65]. Although, the role of ILC2 in lung inflammation and tissue repair in M. tuberculosis infection is still unclear, ILC2 have been thoroughly investigated in the context of influenza A virus (IAV) infection with somehow controversial conclusions [64,66]. In one study, during infection with IAV, ILC2s were necessary and sufficient to mediate the development of airway hyperreactivity through their capacity to produce IL-5 and IL-13 which resulted in more secretion of mucus from the airways [66]. However, ILC2s are also important for tissue repair and promote barrier integrity [64]. ILC2-derived IL-13 may also promote lung homeostasis by inducing epithelial cell proliferation and survival. ILC2 also secrete amphiregulin (Areg) which promotes regeneration of the bronchial epithelium [64,67], whereas IL-5 by recruiting eosinophils could have a beneficial role during the resolution phase of viral infection [68]. Thus, it is likely that pulmonary ILC2s have both pro-inflammatory and tissue repair which may depend on the timing of infection and environmental factors such as the presence of sex steroid hormones [4] or polarizing cytokines, which may limit their function or promote their plasticity [69]. By limiting ILC2 functions, IFN-γ has a detrimental role in IAV, suggesting that ILC2 activity could represent a potential target for post-infection therapy of influenza [69]. ILC2 appear well-adapted to respond to different environmental stimuli, and ILC2 plasticity is increasingly being recognized. ILC2 to ILC1 transition in response to IL-12 and IL-18 produced during influenza infection or in lung inflammation associated with chronic obstructive pulmonary disease result in ILC1/2 co-expressing type 2 genes and genes associated with ILC1s such IFN-γ [70,71]. These observations strongly suggest that ILC2-ILC1 plasticity is an important player during respiratory virus infection, whether such plasticity can be influenced by sex-hormone signaling warrant further investigation.
Of interest, the sex-bias reported in M. tuberculosis is limited to the pulmonary forms of tuberculosis (TB) [72]. Given the selective abundance of ILC2 in the lungs, these cells may represent a target of prime interest for sex hormones action. Although ILC2 may be expected to be detrimental in controlling M. tuberculosis replication through their capacity to produce type 2 cytokines thereby promoting alternative macrophage activation (a population of cells permissive to M. tuberculosis), several lines of evidence suggest that ILC2 could exert beneficial actions in the context of TB infection. ILC2 have been reported to produce GM-CSF, a cytokine known to control the growth of M. tuberculosis through its action on macrophages [73]. Indeed, mice lacking GM-CSF are highly susceptible to M. tuberculosis [74]. The higher numbers of ILC2 in the lung of female mice could therefore contribute to protect female from TB infection.
Sex bias in ILC2 biology through sex differences in IL33 production?
The neonatal period is critical for the development and seeding of ILC2 population in the lung, and only a minor fraction of ILC2s is slowly replaced by newly developing ILC2s in adulthood [75,76]. Lung epithelial and stromal cells expressed IL-33 around 2 weeks after birth causing transient ILC2 activation and proliferation of neonatal lung ILC2 and eosinophil infiltration [77,78]. Although, ILC2 develop normally in IL-33-deficient mice, they are not activated by endogenous IL-33 during the neonatal period, and present reduced effector functions in adulthood [79]. This suggests that endogenous IL-33 may serve to “train” ILC2s in the perinatal period, a process which has also been described in allergen-experienced ILC2s in adult which acquire memory-like properties [80]. Thus, sex differences in the regulation of IL-33 production during the neonatal period could also contribute to the sex-bias in ILC2 biology observed in adults. This hypothesis is however challenged by the observation that lung ILC2 numbers are similar between pre-pubescent male and female mice [51,54].
One can also hypothesize that cell-intrinsic AR signaling may control ILC2 homeostatic numbers in tissue, whereas endogenous IL-33 might exert “trained immunity-like effects” on ILC2s [79]. In mice, IL-33 is a nuclear protein expressed in epithelial cells fibroblast-like cells and myofibroblasts, rather than CD45+ hematopoietic cells, both during steady state and inflammation [81]. Age-dependent sex differences in the expression of IL-33, IL-7 and TSLP have been recently reported, with enhanced expression of lung epithelial cytokines in lung homogenates of adult female mice compared to male [82]. However, these differences were unlikely to contribute to the higher response of female ILC2s to IL-33 stimulation than male ILC2s as similar response were observed between WT and IL-33-deficient mice [82]. Of note, differentially expressed gene signatures were identified between naïve male and female lung ILC2. Female lung ILC2s were enriched for a gene signature of memory T cells and an over-representation of cellular metabolism-related genes, which could contribute to the enhanced responsiveness of female ILC2s. However, differences in individual genes involved in ILC2 development and function were not observed [82].
The mechanisms resulting in enhanced IL-33 expression in female lung tissue is currently unknown. It has been suggested that ER-signaling could positively control IL-33 protein and mRNA production in human epithelial cell lines upon challenge with Alternaria Extract or HDM [50]. However, a comprehensive in vivo evaluation of the role of ER-signaling on IL-33 up-regulation in lung epithelial cells is still awaiting. We previously reported that ERα-signaling within the hematopoietic compartment in female mice, as well as ovariectomy, had no measurable effect on ILC2s in the lung in female mice [4]. Here, we further show that E2-supplementation is neither not associated with changes in pulmonary ILC2 numbers and phenotype at steady state (Fig. 5). KLRG1 expression was identical between castrated and E2-supplemented female, and was markedly reduced as compared to lung ILC2s from male (Fig. 5). It is therefore unlikely that the enhanced numbers of lung ILC2s observed in female mice are due to estrogen-mediated regulatory mechanism at steady states. Thus, further studies regarding the potential role of estrogens in regulating indirectly ILC2 biology should use appropriate models of tissue-specific ablation of Esr1 gene.
Concluding remarks
ILC subsets at barrier surfaces maintain tissue homeostasis and respond to infection or to challenge with aeroallergens. ILCs adapt to organ milieu through their capacity to respond to environmental factors produced by non-hematopoietic cells in tissues, including neurons, epithelial cells, parenchymal and stromal cells [83]. Among these extrinsic signals, evidence is emerging that sex steroid hormones may act in a cell-intrinsic manner to regulate ILCs development, maintenance in tissues and effector functions. This has been well illustrated in particular for ILC2s for which compelling evidence support the conclusion that androgen signaling inhibits the generation and/or tissue maintenance of lung ILC2, which accounts for the reduced airway inflammation in male mice (see Fig. 6). The sexual dimorphism in ILC2 function could explain the increased prevalence of asthma in women compared to men [84]. Indeed, in a small cohort of asthmatic patients, it was reported that circulating ILC2 were less abundant in men compared to women [54]. Therefore, modulating AR activity in ILC2, or its down-stream molecular targets, might represent a novel therapeutic strategy in allergic asthma. Indeed, DHT treatment in vivo decreases pulmonary ILC2 numbers at steady state as well as their capacity to produce IL-5 and IL-13 upon ex vivo stimulation with IL-2/IL-33 [54,85]. Moreover, we recently provided evidence for the direct contribution of AR-signaling in this effect, independently of KLRG1 [85]. We showed here that AR-mediated regulation of ILC2 using SARMs is possible and further studies are needed to harness the inhibitory action of AR in ILC2 using non-steroidal SARMs, which may represent alternative approaches for the treatment of asthma in both sexes. Studies are now needed to determine whether androgens are also key inhibitors of human ILC2 in order to consider the modulation of AR as future therapeutics in asthma. In addition, understanding the contribution of estrogen signaling on NK cells functions could contribute to the development of therapeutic strategies aimed at better exploiting the anticancer activity of NK. It is therefore important to understand how their functions are regulated at the molecular level. Indeed, the available pharmacological tools to potentiate or inhibit the action of estrogen receptors are abundant and could be used for these purposes.
Fundings
Research works in our laboratory were supported by grants from Fondation pour la Recherche Médicale (DEQ20180339187), Fonds de Dotation CSL Behring, Sidaction, Société Française d'Allergologie, Agence Nationale de la Recherche contre le SIDA, Fondation Arthritis, Ligue Nationale contre le Cancer, Fondation Bristol-Myers Squibb and Fondation ARC.
Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Kadel S., Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front Immunol. 2018;9:1653. doi: 10.3389/fimmu.2018.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacquelot N., Luong K., Seillet C. Physiological regulation of innate lymphoid cells. Front Immunol. 2019;10:405. doi: 10.3389/fimmu.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartemes K., Chen C.C., Iijima K., Drake L., Kita H. IL-33-Responsive group 2 innate lymphoid cells are regulated by female sex hormones in the uterus. J Immunol. 2018;200:229–236. doi: 10.4049/jimmunol.1602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laffont S., Blanquart E., Savignac M., Cenac C., Laverny G., Metzger D. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med. 2017;214:1581–1592. doi: 10.1084/jem.20161807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laffont S., Seillet C., Guery J.C. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol. 2017;8:108. doi: 10.3389/fimmu.2017.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seillet C., Laffont S., Tremollieres F., Rouquie N., Ribot C., Arnal J.F. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- 7.Lelu K., Laffont S., Delpy L., Paulet P.E., Perinat T., Tschanz S.A. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2386–2393. doi: 10.4049/jimmunol.1101578. [DOI] [PubMed] [Google Scholar]
- 8.Calippe B., Douin-Echinard V., Delpy L., Laffargue M., Lelu K., Krust A. 17 Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 9.Griesbeck M., Ziegler S., Laffont S., Smith N., Chauveau L., Tomezsko P. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J Immunol. 2015;195:5327–5336. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim R.Y., Mangu D., Hoffman A.S., Kavosh R., Jung E., Itoh N. Oestrogen receptor β ligand acts on CD11c+ cells to mediate protection in experimental autoimmune encephalomyelitis. Brain. 2018;141:132–147. doi: 10.1093/brain/awx315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammad I., Starskaia I., Nagy T., Guo J., Yatkin E., Vaananen K. Estrogen receptor alpha contributes to T cell-mediated autoimmune inflammation by promoting T cell activation and proliferation. Sci Signal. 2018;11 doi: 10.1126/scisignal.aap9415. [DOI] [PubMed] [Google Scholar]
- 12.Zeng Z., Surewaard B.G.J., Wong C.H.Y., Guettler C., Petri B., Burkhard R. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol. 2018;19:1100–1111. doi: 10.1038/s41590-018-0211-2. [DOI] [PubMed] [Google Scholar]
- 13.Garnier L., Laffont S., Lelu K., Yogev N., Waisman A., Guery J.C. Estrogen signaling in bystander Foxp3(neg) CD4(+) T cells suppresses cognate Th17 differentiation in trans and protects from central nervous system Autoimmunity. J Immunol. 2018;201:3218–3228. doi: 10.4049/jimmunol.1800417. [DOI] [PubMed] [Google Scholar]
- 14.Zhao R., Chen X., Ma W., Zhang J., Guo J., Zhong X. A GPR174-CCL21 module imparts sexual dimorphism to humoral immunity. Nature. 2020;577:416–420. doi: 10.1038/s41586-019-1873-0. [DOI] [PubMed] [Google Scholar]
- 15.Sellau J., Groneberg M., Fehling H., Thye T., Hoenow S., Marggraff C. Androgens predispose males to monocyte-mediated immunopathology by inducing the expression of leukocyte recruitment factor CXCL1. Nat Commun. 2020;11:3459. doi: 10.1038/s41467-020-17260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zook E.C., Kee B.L. Development of innate lymphoid cells. Nat Immunol. 2016;17:775–782. doi: 10.1038/ni.3481. [DOI] [PubMed] [Google Scholar]
- 17.Cherrier D.E., Serafini N., Di Santo J.P. Innate lymphoid cell development: a T cell perspective. Immunity. 2018;48:1091–1103. doi: 10.1016/j.immuni.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Klose C.S.N., Flach M., Mohle L., Rogell L., Hoyler T., Ebert K. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Constantinides M.G., McDonald B.D., Verhoef P.A., Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishizuka I.E., Chea S., Gudjonson H., Constantinides M.G., Dinner A.R., Bendelac A. Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol. 2016;17:269–276. doi: 10.1038/ni.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyler T., Klose C.S., Souabni A., Turqueti-Neves A., Pfeifer D., Rawlins E.L. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37:634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S., Iizuka K., Kang H.S., Dokun A., French A.R., Greco S. In vivo developmental stages in murine natural killer cell maturation. Nat Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 23.Constantinides M.G., Gudjonson H., McDonald B.D., Ishizuka I.E., Verhoef P.A., Dinner A.R. PLZF expression maps the early stages of ILC1 lineage development. Proc Natl Acad Sci U S A. 2015;112:5123–5128. doi: 10.1073/pnas.1423244112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orange J.S. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs A. ILC1s in tissue inflammation and infection. Front Immunol. 2016;7:104. doi: 10.3389/fimmu.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G. Innate lymphoid cells: 10 Years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Lambrecht B.N., Hammad H., Fahy J.V. The cytokines of asthma. Immunity. 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Zeng B., Shi S., Ashworth G., Dong C., Liu J., Xing F. ILC3 function as a double-edged sword in inflammatory bowel diseases. Cell Death Dis. 2019;10:315. doi: 10.1038/s41419-019-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seehus C.R., Kadavallore A., Torre B., Yeckes A.R., Wang Y., Tang J. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun. 2017;8:1900. doi: 10.1038/s41467-017-02023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyamoto C., Kojo S., Yamashita M., Moro K., Lacaud G., Shiroguchi K. Runx/Cbfbeta complexes protect group 2 innate lymphoid cells from exhausted-like hyporesponsiveness during allergic airway inflammation. Nat Commun. 2019;10:447. doi: 10.1038/s41467-019-08365-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita H., Kubo T., Ruckert B., Ravindran A., Soyka M.B., Rinaldi A.O. Induction of human regulatory innate lymphoid cells from group 2 innate lymphoid cells by retinoic acid. J Allergy Clin Immunol. 2019;143:2190–2201. doi: 10.1016/j.jaci.2018.12.1018. e9. [DOI] [PubMed] [Google Scholar]
- 33.Wang S., Xia P., Chen Y., Qu Y., Xiong Z., Ye B. Regulatory innate lymphoid cells control innate intestinal inflammation. Cell. 2017;171:201–216. doi: 10.1016/j.cell.2017.07.027. e18. [DOI] [PubMed] [Google Scholar]
- 34.Arnal J.F., Lenfant F., Metivier R., Flouriot G., Henrion D., Adlanmerini M. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 35.Dai C., Heemers H., Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harb Perspect Med. 2017;7 doi: 10.1101/cshperspect.a030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas P. Membrane androgen receptors unrelated to nuclear steroid receptors. Endocrinology. 2019;160:772–781. doi: 10.1210/en.2018-00987. [DOI] [PubMed] [Google Scholar]
- 37.Bennett N.C., Gardiner R.A., Hooper J.D., Johnson D.W., Gobe G.C. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–827. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Pihlajamaa P., Sahu B., Janne O.A. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015;36:357–384. doi: 10.1210/er.2015-1034. [DOI] [PubMed] [Google Scholar]
- 39.Robinette M.L., Fuchs A., Cortez V.S., Lee J.S., Wang Y., Durum S.K. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ricardo-Gonzalez R.R., Van Dyken S.J., Schneider C., Lee J., Nussbaum J.C., Liang H.E. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol. 2018;19:1093–1099. doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabata H., Moro K., Fukunaga K., Suzuki Y., Miyata J., Masaki K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. doi: 10.1038/ncomms3675. [DOI] [PubMed] [Google Scholar]
- 42.Zook E.C., Ramirez K., Guo X., van der Voort G., Sigvardsson M., Svensson E.C. The ETS1 transcription factor is required for the development and cytokine-induced expansion of ILC2. J Exp Med. 2016;213:687–696. doi: 10.1084/jem.20150851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laffont S., Rouquie N., Azar P., Seillet C., Plumas J., Aspord C. X-Chromosome complement and estrogen receptor signaling independently contribute to the enhanced TLR7-mediated IFN-alpha production of plasmacytoid dendritic cells from women. J Immunol. 2014;193:5444–5452. doi: 10.4049/jimmunol.1303400. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson N., Carlsten H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. 1994;158:131–139. doi: 10.1006/cimm.1994.1262. [DOI] [PubMed] [Google Scholar]
- 45.Albrecht A.E., Hartmann B.W., Scholten C., Huber J.C., Kalinowska W., Zielinski C.C. Effect of estrogen replacement therapy on natural killer cell activity in postmenopausal women. Maturitas. 1996;25:217–222. doi: 10.1016/s0378-5122(96)01063-8. [DOI] [PubMed] [Google Scholar]
- 46.Hrushesky W.J., Gruber S.A., Sothern R.B., Hoffman R.A., Lakatua D., Carlson A. Natural killer cell activity: age, estrous- and circadian-stage dependence and inverse correlation with metastatic potential. J Natl Cancer Inst. 1988;80:1232–1237. doi: 10.1093/jnci/80.15.1232. [DOI] [PubMed] [Google Scholar]
- 47.Gruber S.A., Hoffman R.A., Sothern R.B., Lakatua D., Carlson A., Simmons R.L. Splenocyte natural killer cell activity and metastatic potential are inversely dependent on estrous stage. Surgery. 1988;104:398–403. [PubMed] [Google Scholar]
- 48.Sorachi K., Kumagai S., Sugita M., Yodoi J., Imura H. Enhancing effect of 17 beta-estradiol on human NK cell activity. Immunol Lett. 1993;36:31–35. doi: 10.1016/0165-2478(93)90065-a. [DOI] [PubMed] [Google Scholar]
- 49.Gibson D.A., Greaves E., Critchley H.O., Saunders P.T. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum Reprod. 2015;30:1290–1301. doi: 10.1093/humrep/dev067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cephus J.Y., Gandhi V.D., Shah R., Brooke Davis J., Fuseini H., Yung J.A. Estrogen receptor-alpha signaling increases allergen-induced IL-33 release and airway inflammation. Allergy. 2021;76:255–268. doi: 10.1111/all.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadel S., Ainsua-Enrich E., Hatipoglu I., Turner S., Singh S., Khan S. A major population of functional KLRG1(-) ILC2s in female lungs contributes to a sex bias in ILC2 numbers. Immunohorizons. 2018;2:74–86. doi: 10.4049/immunohorizons.1800008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couse J.F., Yates M.M., Walker V.R., Korach K.S. Characterization of the hypothalamic-pituitary-gonadal Axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 53.Entwistle L.J., Gregory L.G., Oliver R.A., Branchett W.J., Puttur F., Lloyd C.M. Pulmonary group 2 innate lymphoid cell phenotype is context specific: determining the effect of strain, location, and stimuli. Front Immunol. 2019;10:3114. doi: 10.3389/fimmu.2019.03114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cephus J.Y., Stier M.T., Fuseini H., Yung J.A., Toki S., Bloodworth M.H. Testosterone attenuates group 2 innate lymphoid cell-mediated airway inflammation. Cell Rep. 2017;21:2487–2499. doi: 10.1016/j.celrep.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmidt A., Harada S., Kimmel D.B., Bai C., Chen F., Rutledge S.J. Identification of anabolic selective androgen receptor modulators with reduced activities in reproductive tissues and sebaceous glands. J Biol Chem. 2009;284:36367–36376. doi: 10.1074/jbc.M109.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narayanan R., Coss C.C., Dalton J.T. Development of selective androgen receptor modulators (SARMs) Mol Cell Endocrinol. 2018;465:134–142. doi: 10.1016/j.mce.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto N., Yamamoto K., Koganei S., Saito N., Maruyama T., Ito M. Killer cell lectin-like receptor G1 binds three members of the classical cadherin family to inhibit NK cell cytotoxicity. J Exp Med. 2006;203:289–295. doi: 10.1084/jem.20051986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Hofmann M., Wang Q., Teng L., Chlewicki L.K., Pircher H. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura S., Kuroki K., Ohki I., Sasaki K., Kajikawa M., Maruyama T. Molecular basis for E-cadherin recognition by killer cell lectin-like receptor G1 (KLRG1) J Biol Chem. 2009;284:27327–27335. doi: 10.1074/jbc.M109.038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salimi M., Barlow J.L., Saunders S.P., Xue L., Gutowska-Owsiak D., Wang X. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grundemann C., Schwartzkopff S., Koschella M., Schweier O., Peters C., Voehringer D. The NK receptor KLRG1 is dispensable for virus-induced NK and CD8+ T-cell differentiation and function in vivo. Eur J Immunol. 2010;40:1303–1314. doi: 10.1002/eji.200939771. [DOI] [PubMed] [Google Scholar]
- 62.Meinicke H., Bremser A., Brack M., Schrenk K., Pircher H., Izcue A. KLRG1 impairs regulatory T-cell competitive fitness in the gut. Immunology. 2017;152:65–73. doi: 10.1111/imm.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cyktor J.C., Carruthers B., Stromberg P., Flano E., Pircher H., Turner J. Killer cell lectin-like receptor G1 deficiency significantly enhances survival after Mycobacterium tuberculosis infection. Infect Immun. 2013;81:1090–1099. doi: 10.1128/IAI.01199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monticelli L.A., Sonnenberg G.F., Abt M.C., Alenghat T., Ziegler C.G., Doering T.A. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neyrolles O., Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang Y.J., Kim H.Y., Albacker L.A., Baumgarth N., McKenzie A.N., Smith D.E. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaiss D.M.W., Gause W.C., Osborne L.C., Artis D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity. 2015;42:216–226. doi: 10.1016/j.immuni.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phipps S., Lam C.E., Mahalingam S., Newhouse M., Ramirez R., Rosenberg H.F. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood. 2007;110:1578–1586. doi: 10.1182/blood-2007-01-071340. [DOI] [PubMed] [Google Scholar]
- 69.Califano D., Furuya Y., Roberts S., Avram D., McKenzie A.N.J., Metzger D.W. IFN-gamma increases susceptibility to influenza A infection through suppression of group II innate lymphoid cells. Mucosal Immunol. 2018;11:209–219. doi: 10.1038/mi.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohne Y., Silver J.S., Thompson-Snipes L., Collet M.A., Blanck J.P., Cantarel B.L. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17:646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- 71.Silver J.S., Kearley J., Copenhaver A.M., Sanden C., Mori M., Yu L. Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol. 2016;17:626–635. doi: 10.1038/ni.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katsnelson A. Beyond the breath: exploring sex differences in tuberculosis outside the lungs. Nat Med. 2017;23:398–401. doi: 10.1038/nm0417-398. [DOI] [PubMed] [Google Scholar]
- 73.Nunes-Alves C., Booty M.G., Carpenter S.M., Jayaraman P., Rothchild A.C., Behar S.M. In search of a new paradigm for protective immunity to TB. Nat Rev Microbiol. 2014;12:289–299. doi: 10.1038/nrmicro3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez-Juarrero M., Hattle J.M., Izzo A., Junqueira-Kipnis A.P., Shim T.S., Trapnell B.C. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J Leukoc Biol. 2005;77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 75.Gasteiger G., Fan X., Dikiy S., Lee S.Y., Rudensky A.Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science. 2015;350:981–985. doi: 10.1126/science.aac9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schneider C., Lee J., Koga S., Ricardo-Gonzalez R.R., Nussbaum J.C., Smith L.K. Tissue-resident group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity. 2019;50:1425–1438. doi: 10.1016/j.immuni.2019.04.019. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steer C.A., Martinez-Gonzalez I., Ghaedi M., Allinger P., Matha L., Takei F. Group 2 innate lymphoid cell activation in the neonatal lung drives type 2 immunity and allergen sensitization. J Allergy Clin Immunol. 2017;140:593–595. doi: 10.1016/j.jaci.2016.12.984. e3. [DOI] [PubMed] [Google Scholar]
- 78.de Kleer I.M., Kool M., de Bruijn M.J., Willart M., van Moorleghem J., Schuijs M.J. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity. 2016;45:1285–1298. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 79.Steer C.A., Matha L., Shim H., Takei F. Lung group 2 innate lymphoid cells are trained by endogenous IL-33 in the neonatal period. JCI Insight. 2020;5 doi: 10.1172/jci.insight.135961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martinez-Gonzalez I., Matha L., Steer C.A., Ghaedi M., Poon G.F., Takei F. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity. 2016;45:198–208. doi: 10.1016/j.immuni.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 81.Cayrol C., Girard J.P. Interleukin-33 (IL-33): a nuclear cytokine from the IL-1 family. Immunol Rev. 2018;281:154–168. doi: 10.1111/imr.12619. [DOI] [PubMed] [Google Scholar]
- 82.Matha L., Shim H., Steer C.A., Yin Y.H., Martinez-Gonzalez I., Takei F. Female and male mouse lung group 2 innate lymphoid cells differ in gene expression profiles and cytokine production. PloS One. 2019;14 doi: 10.1371/journal.pone.0214286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klose C.S.N., Artis D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020;30:475–491. doi: 10.1038/s41422-020-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Laffont S., Guery J.C. Deconstructing the sex bias in allergy and autoimmunity: from sex hormones and beyond. Adv Immunol. 2019;142:35–64. doi: 10.1016/bs.ai.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Blanquart E., Mandonnet A., Cenac C., Anesi N., Mercier P., Audouard C. Targeting androgen-signaling in ILC2s protects from IL-33-driven lung inflammation, independently of KLRG1. J Allergy Clin Immunol Forthcoming. 2021 doi: 10.1016/j.jaci.2021.04.029. [DOI] [PubMed] [Google Scholar]