Abstract
Extracellular vesicle (EV) biology involves understanding the cellular and molecular mechanisms of cell communication. Studies conducted so far with various bacterial infection models demonstrate the release of various types of EVs that include exosomes and microvesicles. Depending upon the infection and cell type, EV cargo composition changes and ultimately might impact the host immune response and bacterial growth. The mechanisms behind the EVs release, cargo composition, and impact on the immune system have not been fully investigated. Future research needs to include in vivo models to understand the relevance of EVs in host immune function during bacterial infection, and to determine aspects that are shared or species-specific in the host. This would aid in the development of EVs as therapeutics or as markers of disease.
Keywords: Extracellular vesicles, Immune response, Bacterial infection
Extracellular vesicles (EVs) were identified around 1980, but then referred to as small membrane vesicles and originally identified as particles that are released to eject unwanted cellular components. EVs are released by most cell types and this is a phenomenon that helps cells communicate with each other. EVs are classified into exosomes and microvesicles based on size and origin. EVs that are released by the endocytic pathway and 30–100 nm in diameter are defined as exosomes. The term exosome was coined by Trams et al. [[1], [2], [3]]. EVs that bud off of the plasma membrane at 100–1000 nm in diameter are referred to as ectosomes or microvesicles [4,5]. Previous reports have demonstrated that bacterial and viral infections can impact EVs release [6] [Fig. 1]. Furthermore, immune cell-derived EVs play a role in inflammation and impact the immune system. For example, B lymphocyte produced EVs carry the MHCII antigen complex that activates CD4+T cells [7]. The biogenesis of EVs, cargo compositions, and the role of EVs on the immune system during bacterial infections will be discussed in this review.
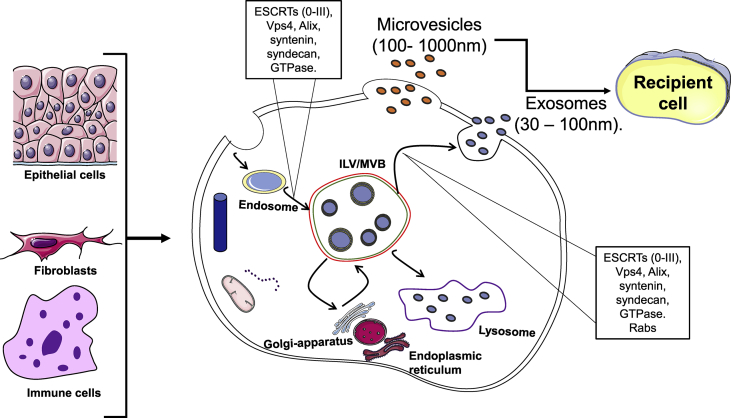
Fig. 1.
Extracellular vesicles biogenesis. Extracellular vesicle biogenesis in different cell types can release different EVs (exosomes and ecotosomes or microvesicles). Exosomes processing includes invagination of the plasma membrane to form endosomes. These endosomes will form intraluminal vesicles (ILVs) and lead to multi-vesicular bodies (MVBs) using biogenesis proteins (i.e ESCRTs, ALIX and Syntaxin and Rabs) resulting in exosomes release. Ectosomes or microvesicles are as result of the outward budding of the plasma membrane.
Biogenesis and release of extracellular vesicles (exosomes and microvesicles)
EVs isolated in different studies vary in size and originate either from the endosome or plasma membrane. Several Rabs (Ras-related protein GTPase), ESCRT (endosomal sorting complexes required for transport) 0, I, II and III proteins, Sytenin I, TSG101 (tumor susceptibility 101), ALIX (apoptosis-linked gene 2-interacting protein X), syndecan-1, phospholipids, tetraspanins, ceramides, sphingomyelinases, and SNARE [soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor] proteins play a role in the EV biogenesis and release process [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]]. Originally, the ESCRT complex's role in EV biogenesis was studied in yeast [21]. ESCRT proteins themselves are involved in restricting NF-κB constitutive signaling in a TNFR1 and LTBR receptor-dependent manner [22]. These receptors are concentrated in the absence of ESCRT components Tsg101, Vps28, UBAP1, and CHMP4B [22]. The release of EVs can be ESCRT-dependent or –independent [7]. The ESCRT complex are involved in several cellular functions; thus, determining the roles of individual ESCRT proteins independent of EVs biogenesis and release are difficult [23] [Fig. 1].
ESCRT-dependent EV biogenesis
The ESCRT dependent mechanisms produce both exosomes and microvesicles. Microvesicle formation includes ectosomal budding of the cargo with the plasma membrane from cell surface [Fig. 1]. This process results in the release of 100–1000 nm ectosomes or microvesicles. The exosome biogenesis process involves the invagination of the plasma membrane to create endosomes called multivesicular bodies (MVBs) that contain intraluminal vesicles (ILVs). These MVBs can either fuse with lysozyme to degrade the cargos or release exosomes through the plasma membrane. ESCRT complex is involved in the biogenesis of the EVs. ESCRT contains five protein complexes (ESCRT 0, I, II, III, and Vps4). The ESCRT-0 is a heterodimer in 1:1 ratio with Vps27/HRs (HGF-regulated tyrosine kinase substrate) and Hse1/STAM (signal transducing adaptor molecule) [7,10,14,16,17,[23], [24], [25]]. ESCRT-0 binds to endosomal membrane lipids and ubiquitinated proteins to execute the first sorting step in ILV cargo. In the next step, ESCRT-0 recruits ESCRT-1 that contains Vps23/TSG101, Vps28, Vps37, and Mvb12 or ubiquitin-associated protein 1 (UBAP1) and this process further enhances cargo selection. ESCRT-II (Vps36/Eap45, Vps22/Eap22, and 2 Vps25/Eap20) forms a Y-shaped structure where Vps28 of ESCRT-I binds to the GLUE domain of the ESCRT-II protein Vps36/EAp45 which controls the initial formation of the inward endosomal membrane buds and also stabilizes the growing vesicle [10,24,26]. Thus, the three ESCRT complexes interact with each other to sort ubiquitinated proteins to the multivesicular body (MVB) pathway and cargo molecules are likely transferred between ESCRT complexes. ESCRT-III contains four core subunits (Vps20/CHMP6, Snf7/CHMP4, Vps24/CHMP3 and Vps/CHMP2) with three accessory proteins Did2/CHMP1, Vps60/CHMP5 and Ist1. The two arms of ESCRT-II (Vps25/Eap20) interact with ESCRT-III subunit Vps20/CHMP6 (charged multi-vesicular body protein 6) that further recruits CHMP proteins 2, 3, 4 to induce vesicle budding until they are released by VPS4 ATPase [19,27,28]. Furthermore, Vps4 binding with ESCRT-III subunits Snf7 and Vps2 is essential for neck constriction, disassembly and recycling of the ESCRT-III subunits to the cytoplasm [19,27,28] Also, in some cell types ILVs inward budding can occur in a GTPase Arf6 and phospholipase D2 (PLD2) dependent manner with Alix-syntenin-syndecan complex in coordination with ESCRT I and III [8].
ESCRT-independent EV biogenesis
The proof for ESCRT-independent exosome formation came from cell culture studies utilizing knockdown methods to prevent expression of ESCRT proteins [29]. ESCRT-independent exosome biogenesis includes ceramide and tetraspanins. Ceramide as a cone-shaped lipid might promote membrane budding or convert into sphingosine 1-phosphate (S1P) to bind on MVBs and literature suggests that maturation of exosomes occurs by S1P [18,30,31]. Tetraspanins (CD63, CD9, and CD81) are suggestive markers of exosomes, interact with cell adhesion and signaling molecules and might be involved in inward budding of the vesicles [32,33]. For example, pre-melanosome protein and β-catenin were shown to be loaded by CD63 and CD9 [34,35].
Microvesicle biogenesis
Microvesicle biogenesis involves cargo accumulation on the cytosolic side of the plasma membrane in coordination with membrane lipids, and the production involves outward cell membrane budding with several mechanisms being implicated in this process. For example, ESCRT proteins Tsg101 and Vps4 have been suggested in microvesicle biogenesis [1,24]. Also, calcium as a co-factor activates cytoskeleton proteins to enhance microvesicle outward budding. Furthermore, Ca2+ activates enzymes such as flippase, floppase and scramblase that are involved in regulating plasma membrane phospholipid translocation which is a key process for outward membrane budding [36] [Fig. 1].
Role of Rab GTPases in extracellular vesicle biogenesis
Rab GTPases were originally identified in reticulocyte exosomes. There are 70 distinct Rab proteins that appear to be involved in different functions that include cargo transport, recycling, and endocytic pathways which are activated when bound to GTP. About 28 of them have been identified in exosomes released from various cell types. Most of these Rabs were proposed to be involved in EV biogenesis and release while so far only some of the Rabs functions have been studied. The first mammalian Rabs identified were Rab 5 and Rab 7, while Rab 4 (a, b), Rab 5 (a, b,c), Rab 7 (a, b), Rab 11 (a,b), Rab 25 and Rab 11c were most studied for their function [[37], [38], [39], [40], [41], [42], [43], [44]]. Rab 5 and Rab 7 have been shown to regulate endocytic cargo uptake and transport to endosomal and lysosomal compartments [[37], [38], [39],41]. The pervasive Rab GTPases Rab 5, Rab 4, Rab 11 are involved in early endocytic while Rab 7 and Rab 9 regulate late endocytic pathways. Furthermore, Rab 11 and Rab35 recycle endosomal membrane components to the plasma membrane while Rab 27 is involved in transporting endosomal and lysosomal molecules to plasma membrane [44]. In addition, Rab 27 along with secretory GTPase Rab3 and Rab11 regulate exocytosis. In K562 cells, overexpression of Rab 11 moderately stimulated exosome release while its inhibition decreased exosome release. Furthermore, in calcium dependent exosome release, Rab11 plays a role in promoting the fusion of MVBs. In neuronal cells, Rab 11 regulates exosome release. Rab 35 is localized at the plasma membrane which mainly regulates different proteins recycling from endosomes. In vitro studies with oligodendroglial cells indicated the role of Rab 35 in exosome release and is dependent on intracellular calcium levels. A role for Rab 27 in exosome release was demonstrated by knockdown experiments with HeLa cells and breast cancer cell lines. The exosome content was not altered with Rab27 depletion suggesting no role in protein sorting to the ILVs. Moreover, exosomal secretion by Rab 11 and Rab 35 seems to be ESCRT-independent. The downstream mechanisms involved in exosomes release and the role of Rabs in these events remains to be investigated.
Composition of extracellular vesicle cargoes
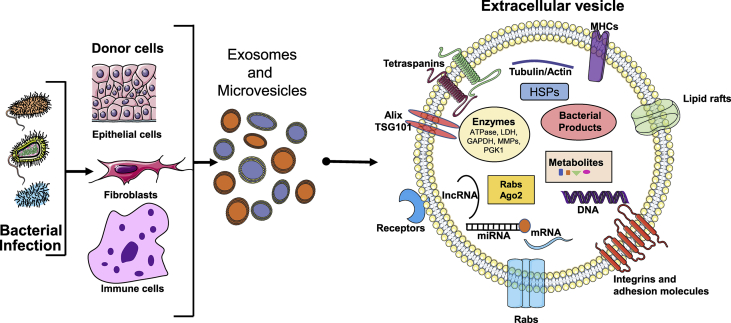
An example EV structure and cargos are shown in Fig. 2. The components of EVs differ based on the origin of the cells and the type of bacterial infection. Exosomes contain flotillin, caveolin, annexins, GTPases, tetraspanins (CD9, CD63, CD81), heat-shock proteins (HSP60, HSP70, and HSP90), phospholipases and also EV membrane proteins (Alix, TSG101), microtubule and cytoskeleton protein, antigen presentation molecules (MHC-I, MHC-II), signaling molecules (CD55, CD59, CD82, and Rabs) and mitochondrial DNA, single-strand DNA and double-strand DNA [[45], [46], [47], [48], [49], [50], [51], [52], [53]]. EVs originated from the plasma membrane (microvesicles) contain tetraspanins, integrins, and proteoglycans, Tya, C1a, and CD35. Both EVs contain DNA sequences, mRNA, microRNAs (miRNAs), long intergenic non-coding RNAs (lincRNAs) and circular RNAs (circRNAs) [[45], [46], [47], [48], [49], [50], [51], [52], [53]]. EVs appear to also transfer toxins, as well: an example includes Bacillus anthracis-produced anthrax toxin from human epithelial cells [54]. EVs RNA packaging appears to be specific and previously Y-box protein 1 was implicated in packaging some miRNA [55]. EVs can transfer these molecules to a recipient cell by direct fusion with the plasma membrane or by phagocytosis, micropinocytosis or endocytosis [56,57]. Transfer of DNA can activate stimulator of interferon genes (STING) in recipient cells [58]. Interestingly, epidermal growth factor receptor (EGFR) and topoisomerase-I inhibitor treatments have been shown to package DNA into exosomes with an unknown mechanism involved in the process [58,59] (Fig. 2).
Fig. 2.
Extracellular vesicle cargo composition during bacterial infection. Bacterial infection can lead to the release of different types of EVs. EVs are made up of major histocompatibility complex (MHCs), lipid rafts, tetraspanins, ESCRT proteins (Alix/TSG101), Rabs and integrin adhesion molecules. These surface molecules likely help with EV entry into the recipient cells. EVs cargo contains several different types of molecules that include heat shock proteins (HSPs), enzymes (ATPase, lactose dehydrogenase, GAPDH, MMPs, PGK1), small RNAs (miRNA, lncRNA), mRNA, DNA, metabolites, bacterial products).
Role of biogenesis proteins in size, morphology, and composition of the EVs
Previously, studies with cell culture models and knockdown technology demonstrated the role of EV biogenesis proteins in the size and release of EVs. For example, Colombo et al. demonstrated that knockdown of ESCRT-0/I (HRS, STAM1 or TSG101) genes in HeLa-CIITA cells that express major histocompatibility complex II (MHC II) resulted in 50% inhibition while ESCRT-III (CHMP4C, VPS4B, VTA1, ALIX) knockdown induced at least 50% increase in exosome secretion [24]. In MCF7 cells, inhibition of VPS4 did not impact CD63-specific EV release [14]. Trajkovic et al. demonstrated a similar phenomenon with VPS4 knock-down in Oli-neu cells (a mouse oligodendroglial cell) with no impact on CD63 or proteolipid protein-specific EVs release [18]. Transfection of human CD4 T cells with double negative (dn) VPS4 (VPS4dn-GFP) disrupted the function of endogenous VPS4 and resulted in a decrease in EVs by T cells [60]. Most recently, Jackson et al. reported that VPS4 inhibition in HEK293 cells decreases EV numbers as well as the protein (CD63 and CD9) and miRNA cargo [12]. Several molecules such as cytokine IL-2, granulocyte macrophage colony stimulating factor, hepatocyte growth factor, platelet-derived growth factor and epidermal growth factor induce Hrs, STAM1 and STAM2 tyrosine phosphorylation [[61], [62], [63]]. These molecules are known to be involved in vesicle transport. Hrs knockdown (hrs−/-) in a fibroblastoid cell line (derived from E9.5 hrsfloxp/floxp mouse embryos) resulted in very low levels of STAM 1 and STAM 2 expression [14]. Transfection of hrs plasmid to the hrs−/- cells resulted in ubiquitinated protein accumulation and full restoration of STAMs [14]. In human liver stem-cells (HLSCs) Alix knockdown did not impact EVs release; however, miRNA expression and in turn the transfer to endothelium was decreased [11]. Furthermore, in HeLa and Hep-2 cells knockdown of Hrs, Tsg101, Vps22, and Vps24 together resulted in enlarged MVEs with some empty and others with single membrane containing lysosomal contents [29]. The morphology of the quadruple knockdown cells appeared similar to Tsg101 single depleted cells with a more dramatic impact on the endocytic structures [29]. It is likely that smaller vesicles fused to become the enlarged structures. Interestingly, quadruple knockdown cells still have the ILVs and MVEs, suggesting the existence of ESCRT-independent mechanisms involved in MVEs. All these data suggest that depending upon the cell type used, different EV biogenesis proteins are involved in EV and cargo formation with ESCRT-dependent or -independent mechanisms playing a role in EV biogenesis and release.
EVs released during bacterial infection impact biological function
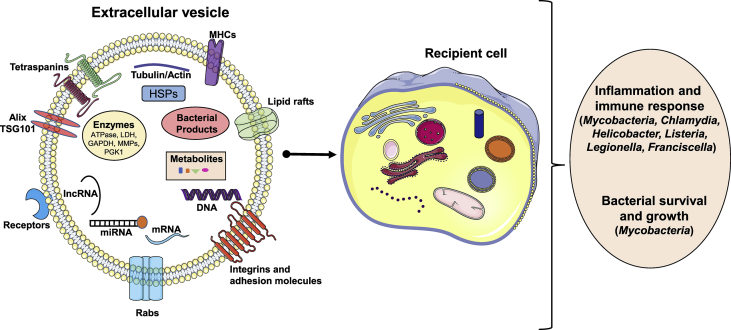
EVs released during bacterial infection impact the immune system and infection outcomes. The heterogeneity in EV size, content and cell of origin can have an impact on recipient cells resulting in cell survival or cell death or inflammation and immune response, or help the pathogen entry and survival in the recipient cells [Fig. 3]. For example, Mycobacterium tuberculosis (M. tb) or Mycobacterium avium is an intracellular bacterium that lives in the phagosome of a host macrophage. During infection with mycobacteria species, exosomes were released from monocytic leukemia (THP-1), monocyte/macrophage cells (Raw264.7) and in the serum of infected mice [45,[64], [65], [66], [67]]. These exosomes have been shown to induce TNF-α and IL-12 production, recruit macrophages and neutrophils in vivo suggesting that mycobacteria regulate host cells during infection [64]. EVs released during mycobacterium infection also contain bacterial product glycopeptidolipids (GPLs) [65]. This is made up of tripeptide amino alcohol amide linked fatty acid and methylated rhamnose and a 6-deoxytalose. Studies have indicated that GPLs interact with the host cells and mediate mycobacterial growth and survival [65]. Importantly, EVs released from mycobacteria infected cells have been shown to induce pro-inflammatory responses relative to EVs released from control cells in a TLR2, TLR4 and Myd88 dependent manner [45,65,68]. Russel et al. showed the role of other mycobacterial products such as PIM2, mycoside transfer to uninfected macrophages [69]. Also, exosomes released during mycobacterial infection or M. tb culture filtrate protein (CFP) treated macrophages also activate antigen-specific CD4+ and CD8+ T cells in vivo. When mice were treated with exosomes released from M. tb CFP macrophages, protection was observed to low-dose aerosolized M. tb infection, similar to the BCG-vaccinated mice. Moreover, mycobacterial RNA enhances phagosome maturation resulting in antibacterial immunity in recipient cells [70]. These data suggested that EVs contain mycobacterial antigens to stimulate an immune response.
Fig. 3.
Extracellular vesicles impact host immune response and bacterial survival. EVs enter into recipient cells by either endocytosis or pinocytosis. The EV cargo impacts the host immune response and in certain cases help spread pathogen infection.
Furthermore, exosomes released from Salmonella infected macrophages reported being pro-inflammatory with TNF-α production in human monocytes [71]. Interestingly, these exosomes appear to contain LPS, a cell wall component of gram-negative bacteria [71]. Exosomes released during mycoplasma infection seem to induce IFN-γ and IL-10 by B cells and this appears to inhibit T cell activation [72].
A previous study by Ettelaie et al. mentioned the release of microparticles during Chlamydia pneumoniae infection; interestingly, the method used here to obtain microparticles would enrich EVs [73]. The authors suggested the releases of C. pneumoniae into the microparticles could impact dissemination through blood, with a potential consequence to inflammation and cardiovascular conditions, in particular atherosclerosis [73]. In a separate line of investigation, Frohlich et al. indicated that EVs may contain Chlamydia proteins that might help bacteria to transfer virulence factors [74]. Most recently an in vitro study suggested a role for EVs in dendritic cell maturation during Chlamydia muridarum infection [75]. Furthermore, Chlamydia psittaci infected dendritic cells showed increased exosome release that induced IFN-γ production by natural killer cells (NK). Also, IFN- γ in co-ordination with TNF- α induces apoptosis in infected and non-infected cells to reduce chlamydial growth and spread to the neighboring cells [76]. Vromman et al. demonstrated that Chlamydia trachomatis DUF582 protein interacts with host Hrs and TSG101 proteins involved in EV biogenesis, however, inhibition of these proteins expression did not impact chlamydial growth in cell culture model (HeLa and HEK-293 cells) [77]. All the studies conducted with chlamydiae include cell culture models, it is yet to be determined if EVs play a role in bacterial infection and immune response in the in vivo models.
Additionally, even though limited studies have been published, other bacterial infections have been shown to induce EV release that can impact the host. For example, during uropathogenic E. coli infection urothelial cells released exosomes were capable of recruiting mast cells and inducing bladder urothelium barrier dysfunction in a pyroptosis dependent manner [78]. Exosomes derived from serum of patients with chronic gastritis and positive for Helicobacter pylori stimulated soluble IL-6 receptor (R) in human gastric epithelial cells (GES-1), further resulted in the release of pro-inflammatory cytokine IL1-α. Treatment with neutralizing antibodies to soluble IL-6R in the presence of serum exosomes decreased IL-1α release indicating the IL-6R role in IL-1α release and barrier dysfunction [79]. EVs containing DNA of Listeria, legionella pneumophila, franciscella tularensis could stimulate cGAS-STING innate inflammatory signaling pathway in recipient cells. Interestingly, with Listeria infection this process leads to the suppression of T cells and reduction in antibacterial defense [80] [Fig. 3]. All these reports suggest that bacterial infection induces the release of EVs and the cargos may have impact on inflammation, innate and adaptive immune systems.
Conclusions
The literature demonstrates that release of EVs during extracellular and intracellular bacterial infections is part of the normal immune response. These EVs have been shown to contain DNA, RNA, miRNA, protein, and microbial components. The majority of the EV studies were reported using cell culture models, so to determine the relevance for the in vitro observations animal models with cell-specific knockout of exosome release are needed. Determining the relevance of EVs in host–pathogen interactions is at an early stage of investigation, and more work is needed to understand the biological relevance of EVs during infection. The bigger questions that have not been answered in the field of bacterial infections include: (1) Do the EVs impact immune cell function and inflammatory response? (2) Does the release of EVs help in bacterial invasion? (3) What are the roles of EVs in disease severity and outcomes? (4) Do specific bacteria impact the EV biogenesis process, and does the composition of EVs vary with the type of pathogen? (5) What are the mechanisms involved in the transfer of microbial components to EVs, and is it the same for intracellular and extracellular bacteria? (6) Do EVs play a major role in antigen presentation and subsequent immune response? (7) Do uninfected cells also release EVs during bacterial infection, and how does that impact the host in terms of inflammation and immune response outcomes? The results from the proposed studies will determine the role of EVs in host defense and disease outcomes.
Conflicts of interest
No conflicts of interest.
Acknowledgements
The research was supported by NIH R21AI146521 (LY). In addition, LY is also supported by NIH P20GM121293 and USDA-ARS Project 6026-51000-010-06S.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Trams E.G., Lauter C.J., Salem N., Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 2.Bobrie A., Colombo M., Krumeich S., Raposo G., Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Yuan X., Shi H., Wu L., Qian H., Xu W. Exosomes in cancer: small particle, big player. J Hematol Oncol. 2015;8:83. doi: 10.1186/s13045-015-0181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor D.D., Taylor C.G., Jiang C.G., Black P.H. Characterization of plasma membrane shedding from murine melanoma cells. Int J Canc. 1988;41:629–635. doi: 10.1002/ijc.2910410425. [DOI] [PubMed] [Google Scholar]
- 5.Cocucci E., Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. doi: 10.1016/j.tcb.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues M., Fan J., Lyon C., Wan M., Hu Y. Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics. 2018;8:2709–2721. doi: 10.7150/thno.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Curr Opin Cell Biol. 2011;23:452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baietti M.F., Zhang Z., Mortier E., Melchior A., Degeest G., Geeraerts A. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 9.Henne W.M., Buchkovich N.J., Emr S.D. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Hurley J.H. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45:463–487. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iavello A., Frech V.S., Gai C., Deregibus M.C., Quesenberry P.J., Camussi G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int J Mol Med. 2016;37:958–966. doi: 10.3892/ijmm.2016.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson C.E., Scruggs B.S., Schaffer J.E., Hanson P.I. Effects of inhibiting VPS4 support a general role for ESCRTs in extracellular vesicle biogenesis. Biophys J. 2017;113:1342–1352. doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi H., Tanaka N., Asao H., Miura S., Kyuuma M., Semura K. Hrs, a mammalian master molecule in vesicular transport and protein sorting, suppresses the degradation of ESCRT proteins signal transducing adaptor molecule 1 and 2. J Biol Chem. 2005;280:10468–10477. doi: 10.1074/jbc.M409969200. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf D., Isaacs A.M. The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem Soc Trans. 2010;38:1469–1473. doi: 10.1042/BST0381469. [DOI] [PubMed] [Google Scholar]
- 16.Saksena S., Sun J., Chu T., Emr S.D. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32:561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 18.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 19.Wollert T., Wunder C., Lippincott-Schwartz J., Hurley J.H. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodman P.G., Futter C.E. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408–414. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt O., Teis D. The ESCRT machinery. Curr Biol. 2012;22:R116–R120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maminska A., Bartosik A., Banach-Orlowska M., Pilecka I., Jastrzebski K., Zdzalik-Bielecka D. ESCRT proteins restrict constitutive NF-kappaB signaling by trafficking cytokine receptors. Sci Signal. 2016;9:ra8. doi: 10.1126/scisignal.aad0848. [DOI] [PubMed] [Google Scholar]
- 23.Hurley J.H., Hanson P.I. Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat Rev Mol Cell Biol. 2010;11:556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 25.Malerod L., Stuffers S., Brech A., Stenmark H. Vps22/EAP30 in ESCRT-II mediates endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007;8:1617–1629. doi: 10.1111/j.1600-0854.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 26.Babst M., Katzmann D.J., Snyder W.B., Wendland B., Emr S.D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 27.Chiaruttini N., Redondo-Morata L., Colom A., Humbert F., Lenz M., Scheuring S. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell. 2015;163:866–879. doi: 10.1016/j.cell.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y., Kimpler L.A., Naismith T.V., Lauer J.M., Hanson P.I. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7-1 with itself, membranes, and the AAA+ ATPase SKD1. J Biol Chem. 2005;280:12799–12809. doi: 10.1074/jbc.M413968200. [DOI] [PubMed] [Google Scholar]
- 29.Stuffers S., Sem Wegner C., Stenmark H., Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10:925–937. doi: 10.1111/j.1600-0854.2009.00920.x. [DOI] [PubMed] [Google Scholar]
- 30.Kajimoto T., Okada T., Miya S., Zhang L., Nakamura S. Ongoing activation of sphingosine 1-phosphate receptors mediates maturation of exosomal multivesicular endosomes. Nat Commun. 2013;4:2712. doi: 10.1038/ncomms3712. [DOI] [PubMed] [Google Scholar]
- 31.Bari R., Guo Q., Xia B., Zhang Y.H., Giesert E.E., Levy S. Tetraspanins regulate the protrusive activities of cell membrane. Biochem Biophys Res Commun. 2011;415:619–626. doi: 10.1016/j.bbrc.2011.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charrin S., Jouannet S., Boucheix C., Rubinstein E. Tetraspanins at a glance. J Cell Sci. 2014;127:3641–3648. doi: 10.1242/jcs.154906. [DOI] [PubMed] [Google Scholar]
- 33.Pols M.S., Klumperman J. Trafficking and function of the tetraspanin CD63. Exp Cell Res. 2009;315:1584–1592. doi: 10.1016/j.yexcr.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Andreu Z., Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savina A., Furlan M., Vidal M., Colombo M.I. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 37.Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 38.Vitelli R., Santillo M., Lattero D., Chiariello M., Bifulco M., Bruni C.B. Role of the small GTPase Rab7 in the late endocytic pathway. J Biol Chem. 1997;272:4391–4397. doi: 10.1074/jbc.272.7.4391. [DOI] [PubMed] [Google Scholar]
- 39.Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 40.Yang M., Chen T., Han C., Li N., Wan T., Cao X. Rab7b, a novel lysosome-associated small GTPase, is involved in monocytic differentiation of human acute promyelocytic leukemia cells. Biochem Biophys Res Commun. 2004;318:792–799. doi: 10.1016/j.bbrc.2004.04.115. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y., Press B., Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Der Sluijs P., Hull M., Zahraoui A., Tavitian A., Goud B., Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci U S A. 1991;88:6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen P.I., Kong C., Su X., Stahl P.D. Rab5 isoforms differentially regulate the trafficking and degradation of epidermal growth factor receptors. J Biol Chem. 2009;284:30328–30338. doi: 10.1074/jbc.M109.034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh P.P., Li L., Schorey J.S. Exosomal RNA from Mycobacterium tuberculosis-infected cells is functional in recipient macrophages. Traffic. 2015;16:555–571. doi: 10.1111/tra.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo Cicero A., Stahl P.D., Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77. doi: 10.1016/j.ceb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathieu M., Martin-Jaular L., Lavieu G., Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 49.Gutierrez-Vazquez C., Villarroya-Beltri C., Mittelbrunn M., Sanchez-Madrid F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251:125–142. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Guescini M., Genedani S., Stocchi V., Agnati L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm. 2010;117:1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 52.Guescini M., Guidolin D., Vallorani L., Casadei L., Gioacchini A.M., Tibollo P. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Exp Cell Res. 2010;316:1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 53.Sansone P., Savini C., Kurelac I., Chang Q., Amato L.B., Strillacci A. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc Natl Acad Sci U S A. 2017;114:E9066–E9075. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abrami L., Brandi L., Moayeri M., Brown M.J., Krantz B.A., Leppla S.H. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5:986–996. doi: 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shurtleff M.J., Temoche-Diaz M.M., Karfilis K.V., Ri S., Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prada I., Meldolesi J. Binding and fusion of extracellular vesicles to the plasma membrane of their cell targets. Int J Mol Sci. 2016;17:1296. doi: 10.3390/ijms17081296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKelvey K.J., Powell K.L., Ashton A.W., Morris J.M., McCracken S.A. Exosomes: mechanisms of uptake. J Circ Biomark. 2015;4:7. doi: 10.5772/61186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitai Y., Kawasaki T., Sueyoshi T., Kobiyama K., Ishii K.J., Zou J. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. J Immunol. 2017;198:1649–1659. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- 59.Montermini L., Meehan B., Garnier D., Lee W.J., Lee T.H., Guha A. Inhibition of oncogenic epidermal growth factor receptor kinase triggers release of exosome-like extracellular vesicles and impacts their phosphoprotein and DNA content. J Biol Chem. 2015;290:24534–24546. doi: 10.1074/jbc.M115.679217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choudhuri K., Llodra J., Roth E.W., Tsai J., Gordo S., Wucherpfennig K.W. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asao H., Sasaki Y., Arita T., Tanaka N., Endo K., Kasai H. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J Biol Chem. 1997;272:32785–32791. doi: 10.1074/jbc.272.52.32785. [DOI] [PubMed] [Google Scholar]
- 62.Takeshita T., Arita T., Asao H., Tanaka N., Higuchi M., Kuroda H. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem Biophys Res Commun. 1996;225:1035–1039. doi: 10.1006/bbrc.1996.1290. [DOI] [PubMed] [Google Scholar]
- 63.Komada M., Kitamura N. Growth factor-induced tyrosine phosphorylation of Hrs, a novel 115-kilodalton protein with a structurally conserved putative zinc finger domain. Mol Cell Biol. 1995;15:6213–6221. doi: 10.1128/mcb.15.11.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh P.P., Smith V.L., Karakousis P.C., Schorey J.S. Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo. J Immunol. 2012;189:777–785. doi: 10.4049/jimmunol.1103638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhatnagar S., Schorey J.S. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anand P.K., Anand E., Bleck C.K., Anes E., Griffiths G. Exosomal Hsp70 induces a pro-inflammatory response to foreign particles including mycobacteria. PloS One. 2010;5 doi: 10.1371/journal.pone.0010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J.J., Chen C., Xie P.F., Pan Y., Tan Y.H., Tang L.J. Proteomic analysis and immune properties of exosomes released by macrophages infected with Mycobacterium avium. Microb Infect. 2014;16:283–291. doi: 10.1016/j.micinf.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Singh P.P., LeMaire C., Tan J.C., Zeng E., Schorey J.S. Exosomes released from M. tuberculosis infected cells can suppress IFN-gamma mediated activation of naive macrophages. PloS One. 2011;6 doi: 10.1371/journal.pone.0018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beatty W.L., Rhoades E.R., Ullrich H.J., Chatterjee D., Heuser J.E., Russell D.G. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 70.Cheng Y., Schorey J.S. Extracellular vesicles deliver Mycobacterium RNA to promote host immunity and bacterial killing. EMBO Rep. 2019;20:e46613. doi: 10.15252/embr.201846613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhatnagar S., Shinagawa K., Castellino F.J., Schorey J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang C., Chalasani G., Ng Y.H., Robbins P.D. Exosomes released from Mycoplasma infected tumor cells activate inhibitory B cells. PloS One. 2012;7 doi: 10.1371/journal.pone.0036138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ettelaie C., Collier M.E., James N.J., Li C. Induction of tissue factor expression and release as microparticles in ECV304 cell line by Chlamydia pneumoniae infection. Atherosclerosis. 2007;190:343–351. doi: 10.1016/j.atherosclerosis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 74.Frohlich K., Hua Z., Wang J., Shen L. Isolation of Chlamydia trachomatis and membrane vesicles derived from host and bacteria. J Microbiol Methods. 2012;91:222–230. doi: 10.1016/j.mimet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raedeem S., Russel K.R., Huang Ming Bo, Omosun Yusuf, Khan Mahfuz, Powell Michael D. Chlamydia infection-derived exosomes possess immunomodulatory properties capable of stimulating dendritic cell maturation. Journal oof advances in medicine and medical research. 2018;25:1–15. [Google Scholar]
- 76.Radomski N., Karger A., Franzke K., Liebler-Tenorio E., Jahnke R., Matthiesen S. Chlamydia psittaci-infected dendritic cells communicate with NK cells via exosomes to activate antibacterial immunity. Infect Immun. 2019;88:e00541-19. doi: 10.1128/IAI.00541-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vromman F., Perrinet S., Gehre L., Subtil A. The DUF582 proteins of Chlamydia trachomatis bind to components of the ESCRT machinery, which is dispensable for bacterial growth in vitro. Front Cell Infect Microbiol. 2016;6:123. doi: 10.3389/fcimb.2016.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Z., Li Y., Liu Q., Liu Y., Chen L., Zhao H. Pyroptosis engagement and bladder urothelial cell-derived exosomes recruit mast cells and induce barrier dysfunction of bladder urothelium after uropathogenic E. coli infection. Am J Physiol Cell Physiol. 2019;317:C544–C555. doi: 10.1152/ajpcell.00102.2019. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y., Wang X., Yu Y., Xiao Y., Huang J., Yao Z. Serum exosomes of chronic gastritis patients infected with Helicobacter pylori mediate IL-1alpha expression via IL-6 trans-signalling in gastric epithelial cells. Clin Exp Immunol. 2018;194:339–349. doi: 10.1111/cei.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nandakumar R., Windross S.J., Paludan S.R. Intercellular communication in the innate immune system through the cGAS-STING pathway. Methods Enzymol. 2019;625:1–11. doi: 10.1016/bs.mie.2019.07.007. [DOI] [PubMed] [Google Scholar]