Abstract
This special issue contains four review articles that analyze the development and biology of innate lymphoid cells (ILCs), which are the most recently-discovered group of innate immune cells. This unique group of lymphoid cells lacks the RAG gene and consequently does not express B cell nor T cell antigen-specific receptors. They are abundant at mucosal surfaces, where they play a role in immunity and homeostasis. The ILCs are the focus of intensive research efforts to understand their development and function.
This special issue contains four review articles by experts in the emerging field of innate lymphoid cell (ILC) development and biology.
The first review, by Dr. Tsukasa Nabekura and Dr. Akira Shibuya [1], describes common features of Nature Killer (NK) cells and a subset of ILCs, ILC1, such as their requirement for T-bet transcription factor for maturation, secretion of high quantities of IFN-γ, dependence on IL-15 for development, and expression of specific cytokine receptors. They also summarize the differences between NK cells and ILC1s. NK cells exhibit powerful cytotoxic activity and migrate in lymphatic and blood vessels, while ILC1s are not cytotoxic and are mainly tissue-resident. In the second part of their review, the authors analyze the role of ILC1s in liver, intestines, adipose tissues and several other tissues such as salivary glands, kidney, skin and lung. In the liver, after infection with mouse cytomegalovirus or during drug-induced acute liver injury, conventional type 1 dendritic cells release large amounts of IL-12, which stimulates ILC1s to produce IFN-γ. This limits the replication of the virus in the liver and confers resistance to viral infection. In drug-induced acute liver injury, ILC1s are the first cells to be activated among several lymphocyte subsets and have a protective effect on injured hepatocytes by producing INF-γ, increasing expression of the anti-apoptotic protein Bcl-xL. The authors also summarize studies that established the major role of intestinal ILC1s against bacteria and parasites through the rapid production of IFN-γ. Interestingly, ILC1s have a complex role during the development of obesity and contribute to the establishment of an inflammatory environment in adipose tissues, resulting in glucose intolerance and insulin resistance. Finally, the authors discuss studies that show that ILC1s can proliferate and differentiate into long-lived cells that produce increased IFN-γ in response to specific antigens such as mouse cytomegalovirus. Overall, this interesting review shows that tissue-resident ILC1s can protect the host against bacteria or parasites by rapidly producing large amounts of IFN-γ; but they also control local inflammation, modulate tissue lesions, and maintain homeostasis.
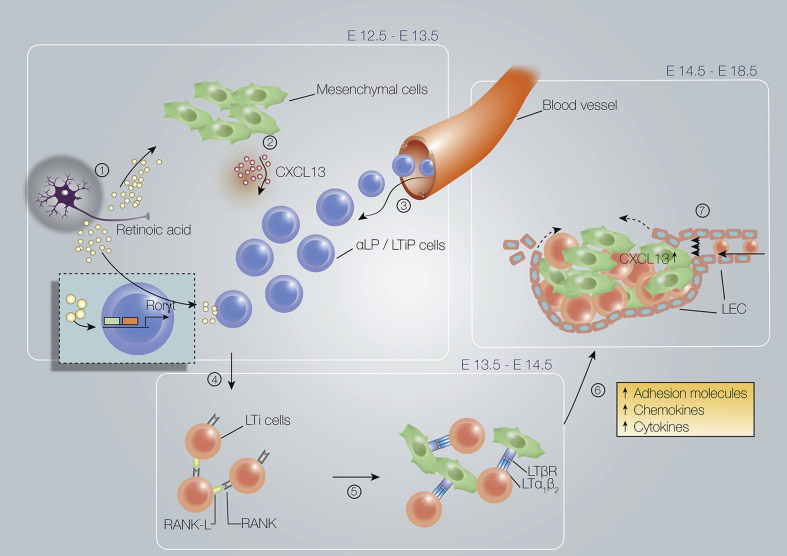
The second article, by Dr. Serge van de Pavert [2], analyzes the role of Lymphoid Tissue Inducer (LTI) cells, which are members of the ILC family, in the formation of lymph nodes (LN). In the first part of this review, the ontogeny of LTi is described. The author presents evidence that mouse LTi progenitors appear around embryonic day 8 (E8.5) and derive from embryonic hemogenic sources: the embryonic vitelline artery or the aorta-gonad-mesonephros. Embryonic LTi cells implicated in LN formation are replaced by bone-marrow hematopoietic stem cells derived LTi cells in adult mice. In fetal liver (FL), the common lymphoid precursors differentiate and generate LTi precursor cells that have left the FL to colonize the LN anlagen. In this tissue, the expression of the retinoic acid-receptor-related orphan nuclear receptor gamma (RORγt) triggers LTi commitment and differentiation of LTi precursors into LTi0 and mature LTi4 cells. Two sections of this review describe and analyze the complex role of LTi cells in embryonic LN [Fig. 1] and of ILC3 and LTi-like cells in adult LNs and gut. This comprehensive review, written by an expert in the field, should help readers understand how LTi cells contribute to the formation of LNs in embryos and adults.
Fig. 1.
Earliest phases in lymph node formation. (1) Retinoic acid induces expression of (2) CXCL13 by mesenchymal cells, which (3) attracts LTiP and LTi cells from the blood. (4) RANK-L expressed on LTi cells and mesenchymal cells interacts with RANK on the LTi cells, leading to (5) lymphotoxin-α1β2 (LTα1β2) expression on LTi4 cells. (6) Interaction of LTi4 cells with lymphotoxin-β receptor (LTβR)-expressing stromal cells results in differentiation into stromal organizer cells. (7) After lymphotoxin signaling, lymphatic endothelial cells migrate to the lymph node anlagen. Source: van de Pavert [2].
The third review, by Dr. Rachel Golub [3], summarizes the current state of knowledge about the role of Notch signaling pathways in fetal and adult ILC development and differentiation. The Notch signaling pathway and the methods used to study this pathway are presented in the first section, followed by a description of the role of Notch in ILC development. While Notch1 is involved in T lymphocyte development, only Notch2 transcripts are expressed in lymphoid precursors of ILC, with expression of Id2 and CXCR6 correlating with ILC commitment. Interestingly, Notch2 signaling is not required for early stages of fetal ILC lineage commitment. In addition, Notch signaling is dispensable for fetal LTi development, whereas it is required for ILC3 differentiation. Finally, Notch is required for normal ILC3 differentiation but not for LTi. A separate section of the review shows how Notch signaling maintains an equilibrium among the heterogeneous subsets of ILC3 in the adult small intestine. The last part of this review is devoted to the complex topic of Notch signaling pathways in regulation of ILC function. It has been established that Notch signaling is involved in the acquisition of Th17 inflammatory function by ILC2s. These KLRG2hi ILC2s, defined as inflammatory ILC2s (iILC2s), express GATA3 (a key transcription factor of ILC2) but also high levels of Dtx1 and low levels of Rorγt (a key transcription factor of ILC3), allowing the same cell to share biological functions of ILC2 and ILC3. Blocking Notch1 and/or Notch2 in vivo demonstrated that iILC2 plasticity is dependent on both Notch1 and Notch2. This section also analyzes the complex mechanisms potentially controlled by Notch signaling to modulate the abundance of ILCs in different tissues and to regulate their function.
The last article, by Dr. Jean-Charles Guéry and colleagues [4], analyzes the role of sex steroid hormones on group 2 ILC development and tissue-specific immunity. In the first part of this review, the authors describe the development of ILCs and NK cells from common lymphoid precursors. They then briefly discuss ILC function in homeostatic and pathophysiological situations. In the second section of this article, the authors introduce the basics of estrogen and androgen receptor signaling pathways; they analyze studies showing that ILC2 express high levels of androgen receptor; and they discuss the expression of co-regulators of androgen receptor like chaperone proteins or pioneer factors, which are known to enhance androgen receptor action. In the third section of this review, they discuss evidence that ILC2s are differentially regulated in males and females and that androgen-receptor signaling in ILC2s inhibits IL-33-driven ILC2 expansion in males. They also describe experiments from their own laboratory demonstrating that 5α-dihydrotestosterone or selective androgen receptor modulators (SARMs) trigger the development and differentiation of CD90dull ILC2s in vitro, of which 40% express the KLRG1 molecule. In contrast, the androgen receptor antagonist Flutamide promotes the development of CD90hi KLRGneg ILC2s, which are similar to the tissue-resident lung ILC2s found in female mice. Experimental evidence suggests that male ILC2s produce less IL-5 and IL-13 after stimulation by IL-2 and IL-33. Finally, ILC2s can increase airway inflammation through IL-5 and IL-13 production, and these cells can promote lung homeostasis because IL-13 triggers epithelial cell survival. In addition, ILC2s release amphiregulin, which contributes to the regeneration of the bronchial epithelium.
These results suggest that androgen signaling decreases the development of ILC2s in lungs, explaining the weaker airway inflammation observed in male mice when compared to females, and they correlate with the observations that asthma is more frequent in women than in men. In addition, the number of circulating ILC2s in asthmatic men is significantly lower than in women patients. Thus, SARMs, which stimulate androgen receptors of ILC2s and decrease ILC2 abundance in lungs, should be considered as targets for treatment of allergic asthma.
Taken together, these four articles provide the reader with an overview of the rapidly expanding literature on ILC development, and suggest clinical applications that can already be envisioned based on our understanding of ILC biology.
Conflicts of interest
The authors are editors with Biomedical Journal.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Nabekura T., Shibuya A. Type 1 innate lymphoid cells: soldiers at the front line of immunity. Biomed J. 2021;44:115–122. doi: 10.1016/j.bj.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van de Pavert S.A. Lymphoid tissue inducer (LTi) cell ontogeny and functioning in embryo and adult. Biomed J. 2021;44:123–132. doi: 10.1016/j.bj.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golub R. The Notch signaling pathway involvement in innate lymphoid cell biology. Biomed J. 2021;44:133–143. doi: 10.1016/j.bj.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanquart S., Laffont S., Guéry J.C. Sex hormone regulation of innate lymphoid cells. Biomed J. 2021;44:144–156. doi: 10.1016/j.bj.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]