Abstract
Glutamate excitotoxicity is considered one of the major causes of retinal ganglion cell death in many retinal diseases. Retinal ganglion cell degeneration causes severe blindness since visual signals from the eye to the brain are conducted only through retinal ganglion cells. Objective: We aimed to explore the potential ameliorative effects of L. sativum against glutamate excitotoxicity-induced retinal ganglion cell damage. Methods: Pure retinal ganglion cells were divided into a control group (untreated); L. sativum-treated groups in which retinal ganglion cells were treated with 5, 10, 50, or 100 µg/mL L. sativum seed extract for 2 h; glutamate-treated groups in which cells were treated with 5, 10, 50, or 100 µM glutamate for 48 h; and L. sativum/glutamate groups [pretreatment with L. sativum for 2 h (50 or 100 µg/mL) before glutamate treatment at 100 µM for 48 h]. Cell damage was assessed by comet assay and cell viability was by MTT test. Results: Tailed DNA, tail length, and tail moment of the 50 and 100 mM glutamate-treated groups were significantly greater than those of the blank control group, while the L. sativum-treated groups demonstrated nonsignificantly different tailed DNA, tail length, and tail moment compared with the blank control group, but significantly lower values compared with the glutamate-treated groups. Conclusion: L. sativum ameliorated the cell viability in retinal ganglion cells after high-concentration glutamate exposure. L. sativum seed extracts were efficient anti-excitotoxic and antioxidant agent that might improve the clinical presentation of many neurological disorders.
Keywords: Lepidium sativum, glutamate excitotoxicity, retinal ganglion cell, cell viability, COMET assay
1. Introduction
Retinal ganglion cells (RGCs) are the sole efferent nerve cells that transmit visual impulses from the retinal bipolar and amacrine cells to the cortical visual centers (the lateral geniculate nucleus and the superior colliculus). They are categorized into 40 subtypes according to their morphology and functions. The visual information is chemical messages received by transmembrane receptors on RGCs and transformed into electrical signals that travel along the optic nerve to the visual cortex [1,2].
Many retinal pathologies result in RGC degeneration with subsequent vision loss. Increasing evidence has indicated that RGCs are vulnerable to various insults in a type-specific manner [3,4]
Glutamate excitotoxicity is considered a pathological process in which nerve cells are destroyed by extreme excitation of glutamate receptors [N-methyl-d-aspartate (NMDA) receptors]. It plays a significant role in the necrosis of neurons in many neurological disorders [5]. Glutamate receptors are expressed by all types of retinal RGCs [6] and NMDA excitotoxicity is believed to result in RGC death in many retinal pathologies [7].
In various neurodegenerative diseases, optic nerve degeneration has been confirmed as part of the pathological process of central nervous system degeneration. Retinal optical coherence tomography revealed thinning of RGCs and the optic nerve-forming axons in neurodevelopmental and neurodegenerative diseases such as autism spectrum disorders, Alzheimer’s disease [8], Parkinson’s disease [9], Huntington’s disease [10], and multiple system atrophy [11].
Medicinal plants have played a significant role in providing humans with formulations to cure and prevent various diseases and ailments. Phytotherapy is now recognized as a promising field in healthcare and serves as a supportive approach for the treatment of various common ailments [12].
Different neurological disorders such as trauma, ischemia, stroke, and epilepsy as well as neurodevelopmental disorders such as autism, despite having diverse primary causes of onset, share a common final damaging pathway known as excitotoxicity [13].
The concentration of glutamate in the synaptic cleft is a critical factor of endogenous excitotoxicity because the synaptic cleft is the site where glutamate interacts with postsynaptic receptors. Excitotoxic neuronal death is thought to be a consequence of intracellular Ca2+ influx. Under normal physiological conditions, the concentration of glutamate within the synaptic cleft increases very briefly to 1–2 mM as it is released from presynaptic vesicles by exocytosis. Synaptic transmission is terminated by the combination of reuptake by glutamate transporters, glutamate diffusion out of the synaptic cleft, and receptor di-sensitization [14,15]. Increasing evidence indicates that glutamate excitotoxicity and/or oxidative stress is associated with mitochondrial DNA (mtDNA) damage-related mitochondrial dysfunction in retinal neurodegeneration [16,17,18,19].
The currently available drug therapies for neurodegenerative diseases are palliative, with limited effectiveness and adverse side effects [20,21,22]. The major challenge for researchers is to develop a therapy that addresses the underlying cause/mechanism of degeneration with improved effectiveness and the least side effects. Therapeutic interventions that modify the progression of neurodegeneration may prove useful and plant-based interventions offer various possibilities to modify disease progression and symptoms [22].
Against this background, Lepidium sativum Linn. from the family Brassicaceae was selected on the basis of its excellent chemical, pharmacological, and nutritional profiles [23,24]
Several studies have focused on the nutritional and medicinal potential of this plant species, but our study is first of its kind to evaluate the anti-excitotoxicity of L. sativum. This species (with the common name garden cress or garden pepperwort) is an annual, edible, fast-growing herb grown throughout India, the United States, Europe, and the Middle East. The seeds and leaves of this plant are rich in numerous bioactive phytochemicals [25,26]. The seed extract of L. sativum is used as a medicine in Ayurveda (Indian system of medicine) [25] as well as having multiple applications in Saudi folk medicine, mainly in fracture healing, anti-inflammation, and at the postpartum stage [27]. It also exhibits valuable pharmacological effects, including antioxidant, anticancer, cardiovascular, diuretic [28,29,30,31] anti-inflammatory, analgesic, antipyretic [32,33], antidiabetic, antimicrobial, hypocholesterolemic, antispasmodic, antidiarrheal, and laxative effects [34].
Although a number of medicinal plants are well-known to be a potent storehouse of polyphenolic and flavonoid phytoconstituents, there have been few studies evaluating their anti-excitotoxicity properties and their ability to prevent reactive oxygen species (ROS)-mediated cellular and DNA damage. The Food and Agriculture Organization (FAO) has classified L. sativum as an underutilized or neglected crop among ancient crops.
In this context, the aim of the present study was to evaluate the comprehensive ameliorative effects of L. sativum as an edible plant herb against glutamate excitotoxicity-induced RGCs as an etiological mechanism of many neurological disorders.
2. Materials and methods
2.1. Botanical material
Lepidium sativum L. (1.0 kg) seeds were purchased from a local market in Riyadh, Saudi Arabia. The collected seeds were washed with tap water, disinfected, rinsed with distilled water, and finally dried under shaded conditions. These dried seeds were ground into a fine powder. The plant species were taxonomically identified and authenticated by Prof. Mohamed Yousef of the Department of Pharmacognosy, College of Pharmacy, King Saud University, Saudi Arabia. Voucher specimens (LP/8619 and MO/8719) were deposited at the herbarium of the department.
2.2. Preparation of extracts
The dried, powdered seeds (1.0 kg) of L. sativum were extracted rigorously with methanol in a Soxhlet apparatus for 2 days. The combined extract was filtered through double-layered muslin cloth, centrifuged at 10,000 rpm for 5 min, and finally filtered through Whatman No. 1 filter paper to obtain a clear filtrate. The obtained filtrates were separated from the solvent on a wiped film evaporator at 50 ± 5°C under reduced pressure by using a vacuum evaporator. The yield obtained for the extract was 54.24 g. The extract was stored in capped bottles in a refrigerator at 5°C prior to use.
2.3. Phytochemical screening of L. sativum seeds
Phytochemical analysis of the crude seed extract of L. sativum was performed qualitatively to determine the presence of various phytoconstituents such as tannins, flavonoids, triterpenoids, alkaloids, saponins, and anthraquinones as plant constituents using standard phytochemical methods following the work of Evans [35]. Briefly, the presence of tannins in the plant extracts was detected by the formation of green or black precipitate upon treatment with aqueous FeCl3. The plant samples were considered positive for flavonoids when a yellow color was precipitated out with 10% lead acetate solution. Triterpenoids were screened by treating plant extracts with acetic anhydride and a few drops of conc. H2SO4. The formation of a bluish green ring or violet or blue color indicated their presence. The alkaloids were tested by treating the plant extracts with Dragendoff’s reagent, resulting in the formation of turbid precipitate at the bottom of the test tube indicating their presence. The saponins were detected in the plant material when diluted samples upon vigorous shaking resulted in stable froth formation. The plant samples were deemed positive for cardiac glycosides when a reddish-brown color appeared with conc. H2SO4. Finally, anthraquinones were screened in plant samples by dissolving plant extracts in 1% HCl followed by the addition of NH4OH reagent and purified with benzene. The formation of a pink, violet, or red color indicated their presence.
2.4. High‐performance liquid chromatography (HPLC) analysis of L. stavium seed extract
The HPLC analysis of crude methanolic L. stavium seed extract was performed on a Shimadu i-series of liquid chromatograph comprising of a solvent delivery pump Model LC-20AT, controller Model LC-2030, UV visible detector with variable wavelengths Model UV-2600, and autosampler equipped with a photodiode array detector (SPD-M30A, Shimadzu). Separation of flavonoids was performed on Inertsil ODS III analytical column with 250 mm × 4.6 mm dimensions and 5 μm particle size. The analytical column was externally protected by C18 Guard‐Pak cartridge (Waters, Milford). Injection volume was used to be 10 µL. The mobile used were: 0.2% of acetic acid in 45% of Millipore water and 0.2% of acetic acid in methanol and an isocratic program was applied. All the separations were carried out at 20°C temperatures with a 0.8 mL/min flow rate. The peaks were identified on the chromatogram by comparing their retention times and spectra with those of commercial and authentic standard solution. Identification of flavonoid compounds was based on the appearance of the absorption maxima between 275 and 320 nm in the spectrum of the individual peaks.
2.5. Preparation of retinal cell suspensions
The retinal tissues were separated from the enucleated eyeballs of newborn Sprague-Dawley rats on postnatal days 1 to 4 and incubated in precooled calcium-free and magnesium-free Earle’s Balanced Salt Solution (EBSS; Gibco, Grand Island, NY) and Hank’s Balanced Salt Solution (Life Technologies, Grand Island, NY) containing 5 mg/mL papain, 0.24 mg/mL l-cysteine, and 10 U/mL DNase І for 30 min. Then, an ovomucoid solution containing 0.1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), 0.1% ovomucoid (Sigma-Aldrich), and 1% DNase І (4 mg/mL, Sigma-Aldrich) in minimum essential medium (MEM; Gibco) was subsequently used to fully quench any residual papain activity. After centrifugation at 200×g for 10 min, the cells were resuspended in MEM containing 0.5 mg/mL BSA and then the cell suspension was filtered through the mesh filter (pore size 40 μm; BD Falcon, Franklin Lakes, NJ) to yield a single cell suspension. The procedures were conducted at room temperature in a laminar flow hood.
Ethical approval: The research related to animals’ use has been complied with all the relevant national regulations and institutional policies for the care and use of animals.
2.6. RGC purification: preparation of panning dishes and cell culture dishes/plates
Antibody-coated 10-cm Petri dishes (one dish per eight rats) were prepared for negative or positive selection by adding 15 μL of a rabbit anti-rat macrophage/Thy-1 antibody and 7 mL of 50 mM Tris-HCl (pH 9.5) per dish. The plates were swirled until the surfaces were evenly coated with the antibody-Tris solution. The panning plates were incubated overnight at 4°C. Immediately before use, the plates were rinsed three times with Dulbecco’s PBS (1×; 0.9 mM CaCl2, 0.49 mM MgCl2·6H2O, 137.9 mM NaCl, 2.67 mM KCl, 8.06 mM Na2HPO4·7H2O, 1.47 mM KH2PO4, pH 7.4; D-PBS; Gibco). Then, 1× poly-d-lysine stock (PDL; Sigma-Aldrich) was added to the cell culture plates (50 μL for the 96-well plates, 100 μL for the 24-well plates, and 500 μL for the six-well plates), and the plates were incubated overnight at room temperature. The plates were rinsed three to four times with sterile H2O and aspirated to dryness. Mouse laminin (1 mg/mL) was diluted to a final concentration of 50 μg/mL by adding 10 μL of the laminin stock to 5 mL of Neurobasal medium (Gibco, Grand Island, NY). The diluted laminin solution was mixed well, added to the dried cell culture plates, and incubated in a 37°C incubator for 2 h. The plates were rinsed with D-PBS three times before use. To isolate RGCs with immunopanning (IP), we adapted a protocol from Cold Spring Harbor Protocols.
2.7. Cell culture
The purified RGCs were seeded at the desired density on the PDL- and laminin-coated coverslips in 24-well or six-well plates in prewarmed RGC growth medium and maintained in a 37°C cell culture incubator with a humidified atmosphere containing 5% CO2 and 95% air. The RGC growth medium was improved from the formulation described in the Cold Spring Harbor Protocols, based on our repeated experiments, and contained Neurobasal medium, BSA (0.1 mg/mL; Sigma), transferrin (0.1 mg/mL; Sigma), progesterone (60 ng/mL; Sigma), putrescine (16 µg/mL; Sigma), selenium (40 ng/mL; Sigma), 3,5,3-triiodothyronine T3 (40 ng/mL; Sigma), thyroxine T4 (40 ng/mL; Sigma), B27 (20 µl/mL; Invitrogen), sodium pyruvate (1 mM; Gibco), glutamine (2 mM; Gibco), N-acetyl-l-cysteine (NAC, 5 µg/mL; Sigma), insulin (5 µg/mL; Sigma), forskolin (5 µM; Sigma), brain-derived neurotrophic factor (BDNF, 50 ng/mL; PeproTech, Rocky Hill, NJ), ciliary neurotrophic factor (CNTF, 10 ng/mL; PeproTech), basic fibroblast growth factor (bFGF, 10 ng/mL; PeproTech), and penicillin-streptomycin (100 U/mL; Gibco). Half of the medium was replenished every 3 days. However, although a high level of purity was obtained with FACS, the level of cell survival was low. The causes of this low survival are unclear.
2.8. COMET assay
The comet assay was performed in accordance with the procedure established by Singh et al. [36], with some modifications. Briefly, after 24 h of exposure of cells to the tested substance in different Petri dishes (60 × 15 mm; Greiner), the cells were trypsinized (0.1% for 4 min), suspended, homogenized in 1 mL of medium, and centrifuged (10 min at 800 rpm). Subsequently, 600 μL of low-melting agarose (0.8% in PBS) was added to the cell suspension (100 μL). Then, 100 μL of this mixture was transferred to agarose pre-coated slides. The coated slides were immersed in lysis buffer (0.045 M TBE, pH 8.4, containing 2.5% SDS) for 15 min. The slides were placed in an electrophoresis chamber containing the same TBE buffer, but devoid of SDS. The electrophoresis conditions were 2 V/cm for 2 min and 100 mA. Staining was performed using ethidium bromide (20 μg/mL at 4°C; Sigma). Observation was performed when the samples were still humid; the DNA fragment migration patterns of 100 cells for each dose level were evaluated with a fluorescence microscope [with excitation filter 420–490 nm (issue 510 nm)]. The comet tail lengths were measured from the middle of the nucleus to the end of the tail with a 40× increase for the count and measure the size of the comet. For the visualization of DNA damage, observations of ethidium bromide-stained DNA were performed using a fluorescent microscope using public domain software for image analysis based on Comet 5 image analysis software developed by Kinetic Imaging, Ltd. (Liverpool, UK), linked to a CCD camera. DNA damage was measured as tail length (TL = distance of DNA migration from the center of the body of the nuclear core) and tail intensity of DNA (TI = % of genomic DNA that migrated during the electrophoresis from the nuclear core to the tail). By presenting all three parameters together, more information on the extent of DNA damage could be obtained.
2.9. L. sativum neuroprotective effects on glutamate-induced excitotoxicity
Pure RGCs were used for the current study. They were divided into four (sets of) groups according to the various treatments: control group (untreated); L. sativum-treated groups in which RGCs were treated independently with 5, 10, 50, or 100 µg/mL L. sativum seed extract for 2 h to test the cytotoxic effects of the extract compared with control-untreated RGCs; glutamate-treated groups (glutamate treatment for 48 h using 5, 10, 50, or 100 µM glutamate); and L. sativum/glutamate groups [pretreatment with L. sativum for 2 h (50 and 100 µg/mL) before glutamate treatment at 100 µM for 48 h].
2.10. Determination of cell viability
All cultured RGCs were cultured in a 96-well plate. After treatment, the cell viability was measured using the MTT test. MTT solution (5 mg/mL) was added to each well (20 µL/well) at a final concentration of 0.5 mg/mL and incubated at 37°C for 4 h. Subsequently, the reaction was terminated by adding 200 µL of DMSO to each well for 15 min. The absorbance of each sample was measured at 490 nm using a microplate reader (Bio-Rad). The results are presented as a percentage of control (untreated cells) or glutamate excitotoxic independently.
2.11. In vitro antioxidant activity of L. stavium
The free radical-scavenging potential of methanolic seed extract of L. stavium was measured by applying two in vitro assays such as ABTS˙+ assay and DPPH˙ assay.
2.11.1. ABTS radical-scavenging activity
ABTS radical-scavenging activity of the L. stavium seed extract was performed by following the procedure described by Re et al. [37]. Equal amounts of ABST stocks (5 mL) and 2.45 mM potassium persulfate (5 mL) were mixed and allowed to stand in dark for 16 h at ambient temperature to produce ABTS radical cation (ABTS˙+). Prior to use, the solution was diluted with distilled water to obtain 0.700 ± 0.020 absorbance at 734 nm and equilibrated at 30°C. Different concentrations of plant extract were diluted with dimethyl sulfoxide (DMSO) to prepare the sample solution. 5 µL of each sample solution was merged with 195 µL ABTS˙+ solution and the reaction mixture was incubated for 6 min at room temperature. Afterwards, the absorbance of the reaction mixture was measured at 734 nm. Butylated hydroxytoluene (BHT) was used as a control and the experiments were performed in triplicates. The ABTS scavenging capacity of plant extract was expressed as IC50 (µg/mL) and the inhibition percentage was determined by applying following equation:
| (1) |
where A 0 and A 1 are the absorbance of control and sample, respectively.
2.11.2. DPPH radical-scavenging activity
DPPH free radical-scavenging activity of the extract was measured by obeying the method described by Blois [38]. DPPH solution was prepared by dissolving 0.025 g of DPPH in 1 L of methanol. Various concentrations of plant extract were diluted DMSO to obtain the sample solution. 5 µL of sample solution was added in a 96-well plate followed by the addition of 195 µL of DPPH working solution in each well and the mixture was incubated for 20 min at ambient temperature under dark conditions. After 20 min incubation, the absorbance of the reaction mixture was recorded at 515 nm. BHT was applied as a standard. All the tests were carried out in triplicates and inhibition percentage was determined by comparing absorbance values of the test sample with that of control. The DPPH scavenging ability of test sample was expressed as IC50 (µg/mL) and the inhibition percentage was calculated by applying equation (1).
2.12. Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA). The results are presented as mean ± standard deviation (S.D). Comparing between all groups using one-way ANOVA test with multiple comparisons (Dunnett s test) to compare each group with the control group for (Parametric data), and Comparing between all groups using Kruskal–Wallis test, and using Mann–Whitney test to compare each group with the control group for (Nonparametric data). Significance was assigned at the level of p < 0.05.
3. Results
3.1. HPLC-DAD detection of methanolic extract of L. stavium seed
The HPLC-DAD chromatograph of extract displayed adjacent peaks of the main flavonoids identified in the methanolic extract of L. stavium seed within shorter analysis time. Due to the close similarity in the structures, the separation of the flavonoid constituents was difficult. The separation depends on the percentage of flavonoid constituents in the extract and selection of appropriate mobile phase (methanol, water, acetic acid).
As shown in Figure 1, six major peaks for flavonoids were detected, including naringin, quercetin, naringenin, luteolin, kaempferol, and apigenin in the chromatogram of the seed extract of L.stavium. The amount of these components was calculated and presented in Table 1 and shown in Figure 1. Phytochemical screening of crude methanol extracts of L. sativum is shown in Table 2.
Figure 1.
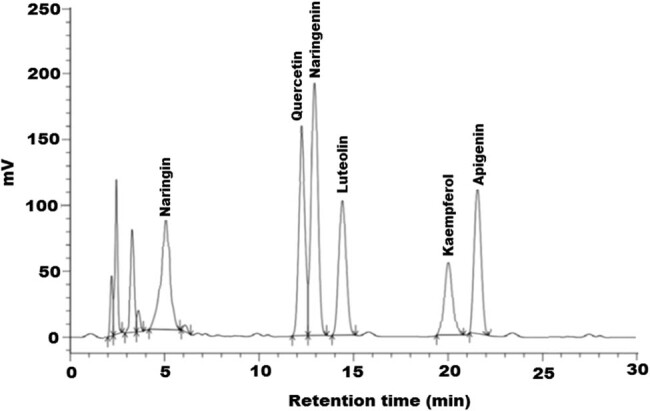
HPLC profile of flavonoids from methanolic L. sativum seed extract at 275 nm.
Table 1.
Concentration of the identified flavonoids components in the L. sativum seed extract
| L. sativum seed extract | Standard | |||||
|---|---|---|---|---|---|---|
| Peak number | Identified compounds | Retention time (t R) | Peak area | Concentration in extract (mg/g) | Retention time (t R) | Peak area |
| 1 | Naringin | 5.114 | 2,781,628 | 2.62 | 5.048 | 8,721,813 |
| 2 | Quercetin | 12.248 | 4,882,629 | 10.50 | 12.241 | 12,681,712 |
| 3 | Naringenin | 12.816 | 5,878,472 | 21.67 | 12.764 | 23,456,321 |
| 4 | Luteolin | 14.287 | 3,183,742 | 2.82 | 14.642 | 12,681,722 |
| 5 | Kaempferol | 20.211 | 24,516,535 | 1.67 | 20.212 | 17,148,150 |
| 6 | Apigenin | 21.401 | 528,396 | 4.35 | 22.116 | 2,329,872 |
Table 2.
Phytochemical screening of crude methanol extracts of L. sativum
| Identified phytoconstituents | L. sativum |
|---|---|
| Tannins | +++ |
| Flavonoids | ++ |
| Triterpenoids | ++ |
| Alkaloids | +++ |
| Saponins | ++ |
| Cardiac glycosides | − |
| Anthraquinones | +++ |
−: absent, +: low intensity, ++: medium intensity, and +++: strong intensity.
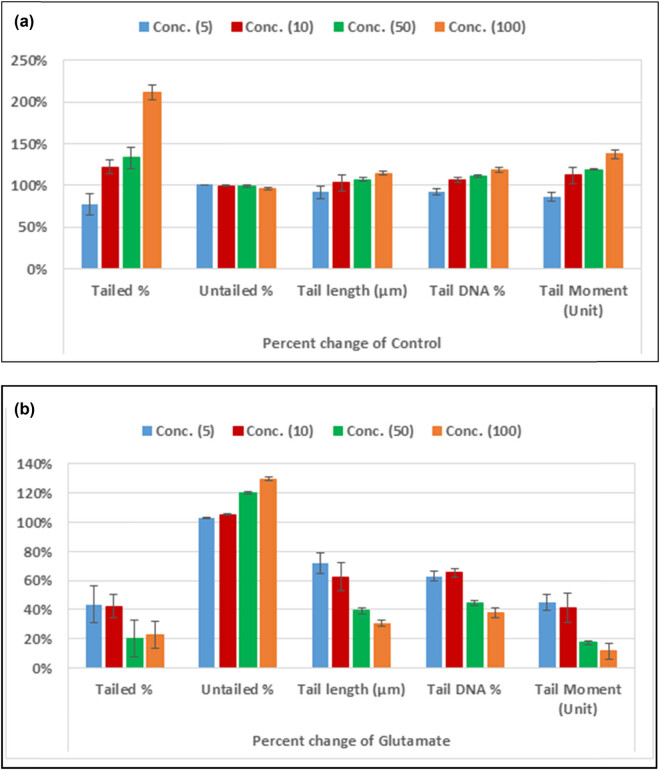
Table 3 together with Figures 2 and 3 demonstrates that the tailed DNA (TDNA), tail length (TL), and tail moment (TM) of the 50 and 100 mM glutamate-treated groups were significantly greater than those of the blank control group (p < 0.05). In addition, the L. sativum-treated groups demonstrated nonsignificantly different tailed DNA (TDNA), tail length (TL), and tail moment (TM) when compared with the blank control group, but significantly lower values when compared with the glutamate-treated groups (p < 0.05 for 5 and 10 mM and p < 0.01 for 50 and 100 mM).
Table 3.
Comparison COMET assay measured variables in glutamate intoxicated and plant extract-treated retinal cells
| Parameters | Extracts | Concentration | |||
|---|---|---|---|---|---|
| 5 µg | 10 µg | 50 µg | 100 µg | ||
| Tailed (%) | Control | 3.00 ± 1.00 | 3.00 ± 1.00 | 3.00 ± 1.00 | 3.00 ± 1.00 |
| Glutamate (5–100 µM) | 5.33 ± 1.15 | 8.67 ± 0.58 | 20.00 ± 1.73 | 27.67 ± 2.52 | |
| L. sativum | 2.33 ± 0.58b | 3.67 ± 0.58b | 4.00 ± 1.00b | 6.33 ± 1.15a,b | |
| Untailed (%) | Control | 97.00 ± 1.00 | 97.00 ± 1.00 | 97.00 ± 1.00 | 97.00 ± 1.00 |
| Glutamate (5–100 µM) | 94.67 ± 1.15 | 91.33 ± 0.58 | 80.00 ± 1.73 | 72.33 ± 2.52 | |
| L. sativum | 97.67 ± 0.58b | 96.33 ± 0.58b | 96.00 ± 1.00b | 93.67 ± 1.15a,b | |
| Tail length (µm) | Control | 1.23 ± 0.09 | 1.23 ± 0.09 | 1.23 ± 0.09 | 1.23 ± 0.09 |
| Glutamate (5–100 µM) | 1.57 ± 0.13 | 2.03 ± 0.22 | 3.36 ± 0.32 | 4.62 ± 0.41 | |
| L. sativum | 1.13 ± 0.08b | 1.27 ± 0.12b | 1.32 ± 0.03b | 1.41 ± 0.03b | |
| Tail DNA (%) | Control | 1.24 ± 0.16 | 1.24 ± 0.16 | 1.24 ± 0.16 | 1.24 ± 0.16 |
| Glutamate(5–100 µM) | 1.83 ± 0.12 | 2.03 ± 0.11 | 3.06 ± 0.15a | 3.85 ± 0.58a | |
| L. sativum | 1.15 ± 0.04b | 1.33 ± 0.04b | 1.37 ± 0.02b | 1.47 ± 0.05a,b | |
| Tail moment (unit) | Control | 1.51 ± 0.10 | 1.51 ± 0.10 | 1.51 ± 0.10 | 1.51 ± 0.10 |
| Glutamate (5–100 µM | 2.88 ± 0.41 | 4.12 ± 0.44 | 10.32 ± 1.49 | 17.96 ± 4.09 | |
| L. sativum | 1.30 ± 0.07b | 1.69 ± 0.17b | 1.80 ± 0.02b | 2.08 ± 0.11a,b | |
There is sig. diff. with the control group at 0.05 level.
There is sig. diff. with the glutamate group at 0.05 level and 0.01 level.
Figure 2.
Percentage change in the five measured comet assay variables in RGCs treated with L. sativum compared to control healthy untreated cells (a) OR compared to glutamate excitotoxic RGCs (b).
Figure 3.
Measure of glutamate-induced DNA damage through single – cell alkaline gel electrophoresis (comet) assay in control, glutamate excitotoxic RGCs (5–100 µM glutamate), and L. sativum-treated RGCs (5–100 µg L. sativum extract).
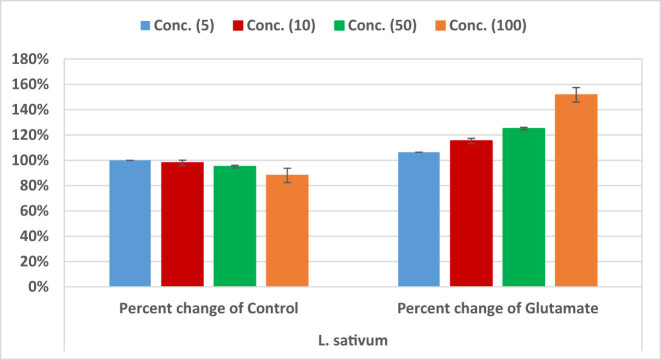
Table 4 and Figure 4 show cell viability of RGCs treated with different concentrations of the excitotoxic glutamate (5–100 µM), or L. sativum (5–100 µg) compared to healthy untreated control cells. It can be easily noticed that while cell viability of 50 and 100 µM glutamate-treated cells recorded significantly lower cell viability of 0.76 ± 0.01 and 0.58 ± 0.03, respectively, compared to control cells, 100 µg L. sativum-treated cells recorded much higher cell viability 0.88 ± 0.05. The three lower concentrations of L. sativum did not show any cytotoxic effects on RGCs. The neuroprotective effects of L. sativum can be observed in Table 5 and Figure 5. Combined treatment of cells with L. sativum (100 µg) + Glutamate (100 µM) induces remarkable increase in cell viability. Table 6 demonstrates the remarkable antioxidant and scavenging effects of L. sativum extracts. IC50 of the extract was found to be concentration-dependent.
Table 4.
Cell viability (MTT assay) of RGCs treated with different concentrations of the excitotoxic glutamate (5–100 µM), or L. sativum (5–100 µg) compared to healthy untreated control cells
| Parameters | Extracts | Concentrations | |||
|---|---|---|---|---|---|
| 5 | 10 | 50 | 100 | ||
| Control | Control | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Glutamate (µM) | 0.94 ± 0.02 | 0.85 ± 0.02 | 0.76 ± 0.01a | 0.58 ± 0.03a | |
| L. sativum (µg) | 1.00 ± 0.00b | 0.98 ± 0.02b | 0.95 ± 0.01b | 0.88 ± 0.05a,b | |
There is sig. diff. with the control group at 0.05 level.
There is sig. diff. with the glutamate group at 0.05 level and 0.01 level.
Figure 4.
Percentage change in cell viability of RGCs – treated with different concentrations of L. sativum (5–100 µg) compared to healthy untreated control cells, or glutamate excitotoxic cells.
Table 5.
Neuroprotective effects of L. sativum (50 and 100 µg) on glutamate (100 µM) – induced loss of RGCs’ viability (death)
| Treatment | Mean ± S.D |
|---|---|
| Glutamate (100 µM) | 0.76 ± 0.01 |
| L. sativum (50 µg) + Glutamate (100 µM) | 0.77 ± 0.02 |
| Glutamate (100 µM) | 0.58 ± 0.03 |
| L. sativum (100 µg) + Glutamate (100 µM) | 0.73 ± 0.02a |
There is sig. diff. with the glutamate group at 0.01 level.
Figure 5.
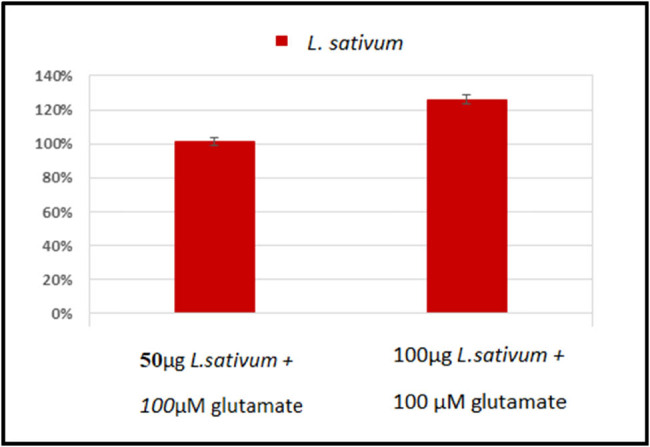
Percentage change of the neuroprotective effects of L. sativum (50 and 100 µg) on glutamate (100 µM) – induced loss of RGCs’ viability (Death).
Table 6.
Antioxidant activity of L. sativum extract
| Sample | Concentration (μg/mL) | ABTS (IC50 μg/mL) | DPPH (IC50 μg/mL) |
|---|---|---|---|
| L. stavium seed extract | 50 | 38.21 ± 0.37 | 30.24 ± 0.67 |
| 100 | 26.52 ± 0.65 | 16.39 ± 0.82 | |
| 200 | 12.45 ± 3.22 | 6.74 ± 0.01 | |
| Butylated hydroxytoluene (BHT) | 200 | 8.67 ± 0.98 | 1.26 ± 0.27 |
Comparing between all groups using One-Way ANOVA test with Multiple Comparisons (Dunnett test) to compare each group with the glutamate group.
4. Discussion
Our understanding of the etiological and biochemical mechanisms of numerous neurological disorders has immensely increased. Parallel with this, growing knowledge has been obtained that might help in the understanding of the mechanisms through which a number of naturally occurring plant extracts can affect these pathological pathways so as to offer protection against damage and support healing of neurological tissues [39].
As shown in Table 1, the results revealed that quercetin and nariginin were found in high quantity, whereas the kaempferol was recorded in low amounts. This could be easily related to the ameliorative effects of L. sativum extract on glutamate-induced DNA damage, cell viability, and the antioxidant effects or scavenging activity. This is in good agreement with two recent studies which demonstrated that quercetin and nariginin were shown to induce mitochondrial activity, significant membrane stabilization, and prevention of lipid peroxidation of treated cells [40,41]. Table 2 demonstrates the richness of L. sativum with tannins, flavonoids, alkaloids, and anthraquinones as phytochemical with well-documented therapeutic potency.
The comet assay or single-cell gel electrophoresis (SCGE) helps to define whether DNA damage-induced single-cell death (apoptosis) or cytotoxicity has occurred and to measure and compare the extent of this damage [42]. Cells embedded in agarose on a microscopic slide are lysed with detergent and high salt to form nucleoids holding supercoiled loops of DNA linked to the nuclear matrix. Electrophoresis results in structures that look like comets at alkaline pH and the intensity of the comet tail or tail length relative to the head usually reflects the number of DNA breaks [42].
Neurodegenerative diseases are a mixture of disorders of the nervous system, including the brain, spinal cord, and peripheral nerves, which have diverse etiologies [43]. Owing to the prevalence, morbidity, and mortality of different neurological disorders, they represent major social, medical, and economic issues [44]. Many environmental factors are connected to neurological disorders. Among these are food, heavy metals, chemicals, and lifestyle [44]. Food additives such as monosodium glutamate (MSG) are recognized as brain toxicants closely linked to neuronal death through glutamate-induced excitotoxicity as a major etiological mechanism in neurological disorders.
Table 3 and Figures 2 and 3 demonstrate the excitotoxic effects of glutamate on pure RGCs in culture. Dose-dependent increases of tailed%, tail length, and tail moment in glutamate-treated RGCs were observed. While low concentrations of glutamate (5 and 10 mM) did not show significant differences compared with the control, higher concentrations (50 and 100 mM) showed significant variation in the four comet assay variables as measures of DNA fragmentation. This is supported by the work of Ankarcrona et al. [45] and Culmsee et al. [46] who proved that high concentrations of glutamate (≥100 μM) can induce neuronal death, which characteristically involves DNA damage and the initiation of apoptosis in rat primary cerebral cortical cultures. Moreover, the nonsignificant findings of DNA damage with 5 and 10 mM glutamate are in good agreement with the previous work by Yang et al. [47] who reported that the exposure of neurons to a low concentration of glutamate (20 μM) for 10 min was effective to induce oxidative nuclear DNA damage, but did not cause significant cell death [48]. Based on the contribution of glutamate excitotoxicity as an etiological mechanism of many neurological disorders, the obtained data can prove that DNA damage and apoptosis are neurological signals of glutamate toxicity, which can be targeted to treat major neurodegenerative diseases such as Alzheimer’s disease [48], Parkinson’s disease [49], and retinal neurodegeneration [50].
There is no cure for many neurological disorders, but treatment of the symptoms may improve the clinical presentation of many disorders such as dementia in Alzheimer’s disease; tremor, slowness, and stiffness in Parkinson’s disease; impaired social interaction in autism spectrum disorders (ASD); and other brain-related problems. Traditional medicine has long been practiced globally in the form of using memory enhancers and medicinal plants in the treatment of dementia, amnesia, as well as Alzheimer’s disease. Most applied herbs and plants have been chemically evaluated and their efficacy has also been proven in clinical trials. However, the mechanisms underlying the activities of medicinal plants as suggested treatments for neurological disorders are still under investigation [51].
Table 3 and Figures 2 and 3 also show the ameliorative effects of L. sativum in glutamate-induced DNA damage measured as % tailing, tail length, and tail moment. Much lower values of these variables were observed with significant variation compared with glutamate-treated cells, but there were nonsignificant differences when compared with the blank control group of RGCs.
The reported protective effect of L. sativum against glutamate-induced DNA damage is supported by the recent study by Zamzami et al. [52], in which almost complete repair of DNA damage occurred posttreatment of rabbits with 200 mg and 400 mg/kg body weight L. sativum seeds. The protective effect of L. sativum seeds on hepatic DNA damage can be attributed to the antioxidant activity of L. sativum seeds through direct scavenging of free radicals or interfering with free radicals production.
Based on the fact that oxidative stress and detoxification mechanisms are linked to glutamate excitotoxicity as three etiological mechanisms in neurological disorders, the ameliorative effects of L. sativum reported in the present study can be attributed to its antioxidant and detoxification mechanisms. Through the use of multiple regression, El-Ansary [53] proved the relationship between these three mechanisms and the etiology of ASD as a neurodevelopmental disorder of remarkably increasing prevalence. They also recently suggested L. sativum as a complex nutritional supplement to treat ASD [54].
The glutamate NMDARs on membranes can be roughly divided into two groups: synaptic and extrasynaptic receptors. Some studies proved that extrasynaptic NMDARs are more responsible for glutamate excitotoxicity and DNA damage-induced cell death than synaptic ones [55,56,57,58]. It is widely believed that synaptic NMDAR activation stimulates cell survival, while extrasynaptic activation triggers cell death; in addition, it is thought that imbalance between the two contributes to neuronal dysfunction as a major cause of most neurological disorders [59]. For example, in Alzheimer’s disease, excess glutamate released from astrocytes activates extrasynaptic NMDARs and triggers proapoptotic signaling, which overcomes synaptic NMDAR-mediated survival signaling that is already weakened by other mechanisms such as the endocytosis of NMDARs. This leads to further synaptic damage and eventual neuronal death. Based on this, the anti-excitotoxic effect of L. sativum reported in the current study can help to suggest this plant as an option for treating Alzheimer’s disease [48], Parkinson’s disease [49], and retinal neurodegeneration [50].
As the retina is considered an extension of the brain, it could provide a stage for studying neurological disorders, understanding the contributing etiological mechanisms, and subsequently developing treatment strategies. Table 4 and Figure 4 demonstrate the independent effect of treating RGCs with glutamate, as an excitotoxic agent, and L. sativum as a promising source of a plant extract that might help in treating excitotoxicity. This table clearly shows that glutamate significantly reduced RGC viability at high concentrations (50 and 100 mM) when compared with healthy control cells. This is supported by the recent work by Christensen et al. [60], who proved the susceptibility of RGCs to glutamatergic excitotoxicity. On the other hand, the viability of L. sativum-treated RGCs was significantly different from that of glutamate-treated cells, but not that of control cells, especially at the concentrations of 5, 10, and 50 µg/mL. Regarding L. sativum, 100 µg/mL was the only concentration at which significantly lower cell viability was recorded compared with that of the control. Table 5 and Figure 5 show the ameliorative effects of L. sativum on glutamate-induced cell death, presenting a remarkable increase of cell viability. This is in good agreement with the previous work of Al-Sheddi et al. [61] who ascertained the cytoprotective effects of L. sativum extract against H2O2-induced cell death. The antioxidant effects of L. sativum are shown in Table 6. This could be attributed to the multiple antioxidant compounds recorded in this plant (Tables 1 and 2) which demonstrate high scavenging power of lipid peroxides and ROS [40,41].
This might be attributed to the multiple antioxidant and anti-inflammatory components of this plant, as shown in Tables 1 and 2. This is supported by the recent work of Chatoui et al. [62] who proved that the antioxidant activity of different extracts of L. sativum correlated significantly with their total flavonoids and other phenolic compounds. They suggested that L. sativum seeds could be used in food supplement preparations or as a food additive protecting against oxidative stress as a major signaling pathway related to glutamate excitotoxicity, a major etiological mechanism of almost all neurological disorders.
5. Conclusion
In conclusion, our study indicates the effectiveness of methanol extract of L. sativum in ameliorating changes in comet assay-related variables as markers of DNA damage and reduced cell viability induced in RGCs after exposure to high concentrations of glutamate. This work suggests the value of using L. sativum seed extracts as anti-excitotoxic agents, which might improve the clinical presentation of many neurological disorders.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, for supporting this work through research group number (RG-1441-540). The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Funding information: The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, for supporting this work through research group number (RG-1441-540).
Conflict of interest: Authors state no conflict of interest.
Data availability statement: The datasets of the current study are available from the corresponding author on reasonable request.
References
- [1].Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016 Jan;529(7586):345–50. [DOI] [PMC free article] [PubMed]; Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T.. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016 Jan;529(7586):345–50. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K, et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018 Jul;9(1):2759. Erratum in: Nat Commun. 2018 Aug;9(1):3203. [DOI] [PMC free article] [PubMed]; Rheaume BA, Jereen A, Bolisetty M, Sajid MS, Yang Y, Renna K. et al. Single cell transcriptome profiling of retinal ganglion cells identifies cellular subtypes. Nat Commun. 2018 Jul;9(1):2759. doi: 10.1038/s41467-018-05134-3. . Erratum in: Nat Commun. 2018 Aug;9(1):3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].El-Danaf RN, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015 Feb;35(6):2329–43. [DOI] [PMC free article] [PubMed]; El-Danaf RN, Huberman AD.. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015 Feb;35(6):2329–43.. doi: 10.1523/JNEUROSCI.1419-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ou Y, Jo RE, Ullian EM, Wong RO, Della Santina L. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J Neurosci. 2016 Aug;36(35):9240–52. [DOI] [PMC free article] [PubMed]; Ou Y, Jo RE, Ullian EM, Wong RO, Della Santina L.. Selective vulnerability of specific retinal ganglion cell types and synapses after transient ocular hypertension. J Neurosci. 2016 Aug;36(35):9240–52. doi: 10.1523/JNEUROSCI.0940-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Brain Res Rev. 2009 Apr;60(1):202–13. [DOI] [PMC free article] [PubMed]; Hulsebosch CE, Hains BC, Crown ED, Carlton SM.. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Brain Res Rev. 2009 Apr;60(1):202–13. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Evangelho K., Mogilevskaya M, Losada-Barragan M, Vargas-Sanchez JK. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmol. 2017 Dec;39(1): 259–71. [DOI] [PubMed]; Evangelho K., Mogilevskaya M, Losada-Barragan M, Vargas-Sanchez JK.. Pathophysiology of primary open-angle glaucoma from a neuroinflammatory and neurotoxicity perspective: a review of the literature. Int Ophthalmol. 2017 Dec;39(1):259–71.. doi: 10.1007/s10792-017-0795-9. [DOI] [PubMed] [Google Scholar]
- [7].den Haan J, Verbraak FD, Visser PJ, Bouwman FH. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst). 2017 Jan;6(1):162–70. [DOI] [PMC free article] [PubMed]; den Haan J, Verbraak FD, Visser PJ, Bouwman FH.. Retinal thickness in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimers Dement (Amst) 2017 Jan;6(1):162–70.. doi: 10.1016/j.dadm.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, Parisi V, et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014 Jan;9(1):e85718. [DOI] [PMC free article] [PubMed]; Yu JG, Feng YF, Xiang Y, Huang JH, Savini G, Parisi V. et al. Retinal nerve fiber layer thickness changes in Parkinson disease: a meta-analysis. PLoS One. 2014 Jan;9(1):e85718. doi: 10.1371/journal.pone.0085718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Andrade C, Beato J, Monteiro A, Costa A, Penas S, Guimarães J, et al. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s disease. Mov Disord. 2016 Mar;31(3):377–83. [DOI] [PubMed]; Andrade C, Beato J, Monteiro A, Costa A, Penas S, Guimarães J. et al. Spectral-domain optical coherence tomography as a potential biomarker in Huntington’s disease. Mov Disord. 2016 Mar;31(3):377–83.. doi: 10.1002/mds.26486. [DOI] [PubMed] [Google Scholar]
- [10].Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR 3rd, Kaufmann H. Progressive retinal structure abnormalities in multiple system atrophy. Mov Disord. 2015 Dec;30(14):1944–53. [DOI] [PMC free article] [PubMed]; Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR 3rd, Kaufmann H.. Progressive retinal structure abnormalities in multiple system atrophy. Mov Disord. 2015 Dec;30(14):1944–53. doi: 10.1002/mds.26360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shakya AK. Medicinal plants: future source of new drugs. Int J Herb Med. 2016;4(4):59–64.; Shakya AK.. Medicinal plants: future source of new drugs. Int J Herb Med. 2016;4(4):59–64. [Google Scholar]
- [12].Blandini F. An update on the potential role of excitotoxicity in the pathogenesis of Parkinson’s disease. Funct Neurol. 2010 Apr-Jun;25(2):65–71. [PubMed]; Blandini F.. An update on the potential role of excitotoxicity in the pathogenesis of Parkinson’s disease. Funct Neurol. 2010 Apr-Jun;25(2):65–71. [PubMed] [Google Scholar]
- [13].Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science 1992 Nov;258(5087):1498–501. [DOI] [PubMed]; Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL.. The time course of glutamate in the synaptic cleft. Science. 1992 Nov;258(5087):1498–501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- [14].Glavinovíc MI, Rabie HR. Monte Carlo simulation of spontaneous miniature excitatory postsynaptic currents in rat hippocampal synapse in the presence and absence of desensitization. Pflugers Arch. 1998 Jan;435(2):193–202. [DOI] [PubMed]; Glavinovíc MI, Rabie HR.. Monte Carlo simulation of spontaneous miniature excitatory postsynaptic currents in rat hippocampal synapse in the presence and absence of desensitization. Pflugers Arch. 1998 Jan;435(2):193–202. doi: 10.1007/s004240050501. [DOI] [PubMed] [Google Scholar]
- [15].Jarrett SG, Lin H, Godley BF, Boulton ME. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008 Nov;27(6):596–607. [DOI] [PubMed]; Jarrett SG, Lin H, Godley BF, Boulton ME.. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog Retin Eye Res. 2008 Nov;27(6):596–607. doi: 10.1016/j.preteyeres.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [16].Chan AS, Saraswathy S, Rehak M, Ueki M, Rao NA. Neuroglobin protection in retinal ischemia. Invest Ophthalmol Vis Sci. 2012 Feb;53(2):704–11. [DOI] [PMC free article] [PubMed]; Chan AS, Saraswathy S, Rehak M, Ueki M, Rao NA.. Neuroglobin protection in retinal ischemia. Invest Ophthalmol Vis Sci. 2012 Feb;53(2):704–11. doi: 10.1167/iovs.11-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC, et al. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011 Aug;93(2):204–12. [DOI] [PubMed]; Lee S, Van Bergen NJ, Kong GY, Chrysostomou V, Waugh HS, O’Neill EC. et al. Mitochondrial dysfunction in glaucoma and emerging bioenergetic therapies. Exp Eye Res. 2011 Aug;93(2):204–12. doi: 10.1016/j.exer.2010.07.015. [DOI] [PubMed] [Google Scholar]
- [18].Kong YX, Van Bergen N, Trounce IA, Bui BV, Chrysostomou V, Waugh H, et al. Increase in mitochondrial DNA mutations impairs retinal function and renders the retina vulnerable to injury. Aging Cell. 2011 Aug;10(4):572–83. [DOI] [PubMed]; Kong YX, Van Bergen N, Trounce IA, Bui BV, Chrysostomou V, Waugh H. et al. Increase in mitochondrial DNA mutations impairs retinal function and renders the retina vulnerable to injury. Aging Cell. 2011 Aug;10(4):572–83. doi: 10.1111/j.1474-9726.2011.00690.x. [DOI] [PubMed] [Google Scholar]
- [19].Nowacek A, Kosloski LM, Gendelman HE. Neurodegenerative disorders and nanoformulated drug development. Nanomedicine (Lond). 2009 Jul;4(5):541–55. [DOI] [PMC free article] [PubMed]; Nowacek A, Kosloski LM, Gendelman HE.. Neurodegenerative disorders and nanoformulated drug development. Nanomedicine (Lond) 2009 Jul;4(5):541–55. doi: 10.2217/nnm.09.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rao RV, Descamps O, John V, Bredesen DE. Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimers Res Ther. 2012 Jun;4(3):22. [DOI] [PMC free article] [PubMed]; Rao RV, Descamps O, John V, Bredesen DE.. Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimers Res Ther. 2012 Jun;4(3):22. doi: 10.1186/alzrt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mezeiova E, Korabecny J, Sepsova V, Hrabinova M, Jost P, Muckova L, et al. Development of 2-methoxyhuprine as novel lead for Alzheimer’s disease therapy. Molecules 2017 Jul;22(8):1265. [DOI] [PMC free article] [PubMed]; Mezeiova E, Korabecny J, Sepsova V, Hrabinova M, Jost P, Muckova L. et al. Development of 2-methoxyhuprine as novel lead for Alzheimer’s disease therapy. Molecules. 2017 Jul;22(8):1265. doi: 10.3390/molecules22081265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ahmad R, Mujeeb M, Anwar F, Husain A, Ahmad A, Sharma S. Pharmacognostical and phytochemical analysis of Lepidium sativum L. seeds. Int Curr Pharm J. 2015;4(10):442–6.; Ahmad R, Mujeeb M, Anwar F, Husain A, Ahmad A, Sharma S.. Pharmacognostical and phytochemical analysis of Lepidium sativum L. seeds. Int Curr. Pharm J. 2015;4(10):442–6. [Google Scholar]
- [23].Paikra BK, Dhongade HK, Gidwani B. Phytochemistry and pharmacology of Moringa oleifera Lam. J Pharmacopuncture. 2017 Sep;20(3):194–200. [DOI] [PMC free article] [PubMed]; Paikra BK, Dhongade HK, Gidwani B.. Phytochemistry and pharmacology of Moringa oleifera Lam. J Pharmacopuncture. 2017 Sep;20(3):194–200. doi: 10.3831/KPI.2017.20.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Al-Snafi AE. chemical constituents and pharmacological effects of Lepidium sativum – a review. Int J Curr Pharm Res. 2019;11(6):1–10.; Al-Snafi AE.. chemical constituents and pharmacological effects of Lepidium sativum – a review. Int J Curr Pharm Res. 2019;11(6):1–10. [Google Scholar]
- [25].Mali R, Mahajan S, Mehta A. Studies on bronchodilatory effect of Lepidium sativum against allergen induced bronchospasm in guinea pigs. Pharmacogn Mag. 2008;4(15):189.; Mali R, Mahajan S, Mehta A.. Studies on bronchodilatory effect of Lepidium sativum against allergen induced bronchospasm in guinea pigs. Pharmacogn Mag. 2008;4(15):189. [Google Scholar]
- [26].Ahsan SK, Tariq M, Ageel M, Alyahya MA, Shah AH. Studies on some herbal drugs used in fracture healing. Int J Crude Drug Res. 1989;27(4):235–9.; Ahsan SK, Tariq M, Ageel M, Alyahya MA, Shah AH.. Studies on some herbal drugs used in fracture healing. Int J Crude Drug Res. 1989;27(4):235–9. [Google Scholar]
- [27].Malar J, Chairman K, Singh AR, Vanmathi JS, Balasubramanian A, Vasanthi K. Antioxidative activity of different parts of the plant Lepidium sativum Linn. Biotechnol Rep (Amst). 2014 Jun;3:95–8. [DOI] [PMC free article] [PubMed]; Malar J, Chairman K, Singh AR, Vanmathi JS, Balasubramanian A, Vasanthi K.. Antioxidative activity of different parts of the plant Lepidium sativum Linn. Biotechnol Rep (Amst) 2014 Jun;3:95–8. doi: 10.1016/j.btre.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Indumathy R, Aruna A. Cytotoxic potential of various extracts of Lepidium sativum (Linn.). An in-vitro evaluation. Int J Pharm Pharm Sci. 2015;2(5):1–5.; Indumathy R, Aruna A.. Cytotoxic potential of various extracts of Lepidium sativum (Linn.). An in-vitro evaluation. Int J Pharm Pharm Sci. 2015;2(5):1–5. [Google Scholar]
- [29].Maghrani M, Zeggwagh NA, Michel JB, Eddouks M. Antihypertensive effect of Lepidium sativum L. in spontaneously hypertensive rats. J Ethnopharmacol. 2005 Aug;100(1–2):193–7. [DOI] [PubMed]; Maghrani M, Zeggwagh NA, Michel JB, Eddouks M.. Antihypertensive effect of Lepidium sativum L. in spontaneously hypertensive rats. J Ethnopharmacol. 2005 Aug;100(1–2):193–7. doi: 10.1016/j.jep.2005.02.024. [DOI] [PubMed] [Google Scholar]
- [30].Patel U, Kulkarni M, Undale V, Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Trop J Pharm Res. 2009;8(3):215.; Patel U, Kulkarni M, Undale V, Bhosale A.. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Trop J Pharm Res. 2009;8(3):215. [Google Scholar]
- [31].Sowjanya KR, Vishweswar RV, Bushra F, Kapila M, Srilakshmi M. In vitro anti-inflammatory activity of ultra soni assisted, methanol extract of Lepidium sativum linn seeds. Int J Pharm Dev Technol 2013;3:63–5.; Sowjanya KR, Vishweswar RV, Bushra F, Kapila M, Srilakshmi M.. In vitro anti-inflammatory activity of ultra soni assisted, methanol extract of Lepidium sativum linn seeds. Int J Pharm Dev Technol. 2013;3:63–5. [Google Scholar]
- [32].Al-Yahya MA, Mossa JS, Ageel AM, Rafatullah S. Pharmacological and safety evaluation studies on Lepidium sativum L., Seeds. Phytomedicine 1994 Sep;1(2):155–9. [DOI] [PubMed]; Al-Yahya MA, Mossa JS, Ageel AM, Rafatullah S.. Pharmacological and safety evaluation studies on Lepidium sativum L., Seeds. Phytomedicine. 1994 Sep;1(2):155–9. doi: 10.1016/S0944-7113(11)80035-8. [DOI] [PubMed] [Google Scholar]
- [33].Attia ES, Amer AH, Hasanein MA. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat Prod Res. 2019 Mar;33(6):901–5. [DOI] [PubMed]; Attia ES, Amer AH, Hasanein MA.. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat Prod Res. 2019 Mar;33(6):901–5. doi: 10.1080/14786419.2017.1413564. [DOI] [PubMed] [Google Scholar]
- [34].Alqahtani FY, Aleanizy FS, Mahmoud AZ, Farshori NN, Alfaraj R, Al-Sheddi ES, et al. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2019 Jul;26(5):1089–92. [DOI] [PMC free article] [PubMed]; Alqahtani FY, Aleanizy FS, Mahmoud AZ, Farshori NN, Alfaraj R, Al-Sheddi ES. et al. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J Biol Sci. 2019 Jul;26(5):1089–92.. doi: 10.1016/j.sjbs.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Evans WC. Trease and Evans’ pharmacognosy. 16th ed. Arnold: Saunders Ltd; 2009.; Evans WC. Trease and Evans’ pharmacognosy. 16th ed. Arnold: Saunders Ltd; 2009. [Google Scholar]
- [36].Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. [DOI] [PubMed]; Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- [37].Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999 May;26(9–10):1231–7. [DOI] [PubMed]; Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C.. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999 May;26(9–10):1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- [38].Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958;181(4617):1199–200.; Blois MS.. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–200.. [Google Scholar]
- [39].Blaylock RL, Maroon J. Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. Surg Neurol Int. 2012;3(1):19. [DOI] [PMC free article] [PubMed]; Blaylock RL, Maroon J.. Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. Surg Neurol Int. 2012;3(1):19. doi: 10.4103/2152-7806.92935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W, et al. Multi-therapeutic potential of naringenin (4′,5,7-trihydroxyflavonone): experimental evidence and mechanisms. Plants 2020 Dec;9(12):1784. [DOI] [PMC free article] [PubMed]; Arafah A, Rehman MU, Mir TM, Wali AF, Ali R, Qamar W. et al. Multi-therapeutic potential of naringenin (4′,5,7-trihydroxyflavonone): experimental evidence and mechanisms. Plants. 2020 Dec;9(12):1784. doi: 10.3390/plants9121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tvrdá E, Debacker M, Ďuračka M, Kováč J, Bučko O. Quercetin and naringenin provide functional and antioxidant protection to stored boar semen. Animals (Basel). 2020 Oct;10(10):1930. [DOI] [PMC free article] [PubMed]; Tvrdá E, Debacker M, Ďuračka M, Kováč J, Bučko O. Quercetin and naringenin provide functional and antioxidant protection to stored boar semen. Animals (Basel) 2020 Oct;10(10):1930. doi: 10.3390/ani10101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Afanasieva K, Sivolob A. Physical principles and new applications of comet assay. Biophys Chem. 2018 Jul;238:1–7. [DOI] [PubMed]; Afanasieva K, Sivolob A.. Physical principles and new applications of comet assay. Biophys Chem. 2018 Jul;238:1–7. doi: 10.1016/j.bpc.2018.04.003. [DOI] [PubMed] [Google Scholar]
- [43].Przedborski S, Vila M, Jackson-Lewis V. Neurodegeneration: what is it and where are we? J Clin Invest. 2003 Jan;111(1):3–10. [DOI] [PMC free article] [PubMed]; Przedborski S, Vila M, Jackson-Lewis V.. Neurodegeneration: what is it and where are we? J Clin Invest. 2003 Jan;111(1):3–10. doi: 10.1172/JCI17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Modgil S, Lahiri DK, Sharma VL, Anand A. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl Neurodegener. 2014 Apr;3(1):9. [DOI] [PMC free article] [PubMed]; Modgil S, Lahiri DK, Sharma VL, Anand A.. Role of early life exposure and environment on neurodegeneration: implications on brain disorders. Transl Neurodegener. 2014 Apr;3(1):9. doi: 10.1186/2047-9158-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995 Oct; 15(4):961–73. [DOI] [PubMed]; Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA. et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995 Oct;15(4):961–73. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- [46].Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M, et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005 Nov;25(44):10262–72. [DOI] [PMC free article] [PubMed]; Culmsee C, Zhu C, Landshamer S, Becattini B, Wagner E, Pellecchia M. et al. Apoptosis-inducing factor triggered by poly(ADP-ribose) polymerase and Bid mediates neuronal cell death after oxygen-glucose deprivation and focal cerebral ischemia. J Neurosci. 2005 Nov;25(44):10262–72.. doi: 10.1523/JNEUROSCI.2818-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang JL, Takahashi T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamateinduced oxidative DNA damage by a process involving CREB-mediated up-regulation of APE1. J Biol Chem. 2010 Sep;285(36):28191–9. [DOI] [PMC free article] [PubMed]; Yang JL, Takahashi T, Keijzers G, Mattson MP, Bohr VA.. Neurons efficiently repair glutamateinduced oxidative DNA damage by a process involving CREB-mediated up-regulation of APE1. J Biol Chem. 2010 Sep;285(36):28191–9. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1041–8. [DOI] [PMC free article] [PubMed]; Wang R, Reddy PH.. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1041–8. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wild AR, Bollands M, Morris PG, Jones S. Mechanisms regulating spill-over of synaptic glutamate to extrasynaptic NMDA receptors in mouse substantia nigra dopaminergic neurons. Eur J Neurosci. 2015 Nov;42(9):2633–43. [DOI] [PMC free article] [PubMed]; Wild AR, Bollands M, Morris PG, Jones S.. Mechanisms regulating spill-over of synaptic glutamate to extrasynaptic NMDA receptors in mouse substantia nigra dopaminergic neurons. Eur J Neurosci. 2015 Nov;42(9):2633–43. doi: 10.1111/ejn.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH, et al. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7(10):e47098. [DOI] [PMC free article] [PubMed]; Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH. et al. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7(10):e47098. doi: 10.1371/journal.pone.0047098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Akram M, Nawaz A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017 Apr;12(4):660–70. [DOI] [PMC free article] [PubMed]; Akram M, Nawaz A.. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017 Apr;12(4):660–70. doi: 10.4103/1673-5374.205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zamzami MA, Baothman OAS, Samy F, Abo-Golayel MK. Amelioration of CCl4-induced hepatotoxicity in rabbits by Lepidium sativum seeds. Evid Based Complement Alternat Med. 2019 Mar;2019:5947234. [DOI] [PMC free article] [PubMed]; Zamzami MA, Baothman OAS, Samy F, Abo-Golayel MK.. Amelioration of CCl4-induced hepatotoxicity in rabbits by Lepidium sativum seeds. Evid Based Complement Alternat Med. 2019 Mar;2019:5947234. doi: 10.1155/2019/5947234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].El-Ansary A. Data of multiple regressions analysis between selected biomarkers related to glutamate excitotoxicity and oxidative stress in Saudi autistic patients. Data Brief. 2016 Feb;7:111–6. [DOI] [PMC free article] [PubMed]; El-Ansary A.. Data of multiple regressions analysis between selected biomarkers related to glutamate excitotoxicity and oxidative stress in Saudi autistic patients. Data Brief. 2016 Feb;7:111–6. doi: 10.1016/j.dib.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].El-Ansary A, Ibrahim EM, Shafi Bhat R. Lepidium sativum seeds as a suggested complex nutritional supplement to treat biomarkers related deficits in autism. Nov Tech Nutr Food Sci. 2019;3(3):258–62.; El-Ansary A, Ibrahim EM, Shafi Bhat R.. Lepidium sativum seeds as a suggested complex nutritional supplement to treat biomarkers related deficits in autism. Nov Tech Nutr Food Sci. 2019;3(3):258–62. [Google Scholar]
- [55].Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000 Jan;20(1):22–33. [DOI] [PMC free article] [PubMed]; Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M.. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000 Jan;20(1):22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways [PubMed: 11953750]. Nat Neurosci. 2002 May;5(5):405–14. [DOI] [PubMed]; Hardingham GE, Fukunaga Y, Bading H.. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways [PubMed: 11953750] Nat Neurosci. 2002 May;5(5):405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- [57].Léveillé F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O, et al. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors [PubMed: 18711223]. FASEB J. 2008 Dec;22(12):4258–71. [DOI] [PubMed]; Léveillé F, El Gaamouch F, Gouix E, Lecocq M, Lobner D, Nicole O. et al. Neuronal viability is controlled by a functional relation between synaptic and extrasynaptic NMDA receptors [PubMed: 18711223] FASEB J. 2008 Dec;22(12):4258–71. doi: 10.1096/fj.08-107268. [DOI] [PubMed] [Google Scholar]
- [58].Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity [PubMed: 19482936]. Proc Natl Acad Sci USA. 2009 Jun;106(24):9854–9. [DOI] [PMC free article] [PubMed]; Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB.. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity [PubMed: 19482936] Proc Natl Acad Sci USA. 2009 Jun;106(24):9854–9. doi: 10.1073/pnas.0903546106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders [PubMed: 20842175]. Nat Rev Neurosci. 2010 Oct;11(10):682–96. [DOI] [PMC free article] [PubMed]; Hardingham GE, Bading H.. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders [PubMed: 20842175] Nat Rev Neurosci. 2010 Oct;11(10):682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Christensen I, Lu B, Yang N, Huang K, Wang P, Tian N. The susceptibility of retinal ganglion cells to glutamatergic excitotoxicity is type-specific. Front Neurosci. 2019 Mar;13:219. [DOI] [PMC free article] [PubMed]; Christensen I, Lu B, Yang N, Huang K, Wang P, Tian N.. The susceptibility of retinal ganglion cells to glutamatergic excitotoxicity is type-specific. Front Neurosci. 2019 Mar;13:219. doi: 10.3389/fnins.2019.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Al-Sheddi ES, Farshori NN, Al-Oqail MM, Musarrat J, Al-Khedhairy AA, Siddiqui MA. Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2). Pharm Biol. 2016;54(2):314–21. [DOI] [PubMed]; Al-Sheddi ES, Farshori NN, Al-Oqail MM, Musarrat J, Al-Khedhairy AA, Siddiqui MA.. Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2) Pharm Biol. 2016;54(2):314–21. doi: 10.3109/13880209.2015.1035795. [DOI] [PubMed] [Google Scholar]
- [62].Chatoui K, Harhar H, El Kamli T, Tabyaoui M. Chemical composition and antioxidant capacity of Lepidium sativum seeds from four regions of Morocco. Evid Based Complement Alternat Med. 2020 Jun;2020:7302727. [DOI] [PMC free article] [PubMed]; Chatoui K, Harhar H, El Kamli T, Tabyaoui M.. Chemical composition and antioxidant capacity of Lepidium sativum seeds from four regions of Morocco. Evid Based Complement Alternat Med. 2020 Jun;2020:7302727. doi: 10.1155/2020/7302727. [DOI] [PMC free article] [PubMed] [Google Scholar]