Abstract
Background
Increasing concern has been expressed about the declining sperm count of humans and the potential fertility effects of clomiphene citrate, a synthetic oestrogen-antagonist on human reproductive health in the last few decades. This study aims to investigate the influence of cashew nut supplemented diet on fertility activity of clomiphene citrate in male rats.
Methods
The rats were divided into six groups n = 6: rats fed basal diet; rats fed basal diet and then given clomiphene citrate (cc) orally; rats fed diet supplemented with 10% processed cashew nut and given cc orally; and rat fed diet supplemented with 20% processed cashew nut and given cc orally; rats fed diet supplemented with 10% processed cashew nut and rat fed diet supplemented with 20% processed cashew nut for fourteen days.
Results
The results revealed that there was a significant (p < 0.05) improvement of total testosterone level and epididymal sperm count, viability and progressive motility in all the groups, in comparison to control, with more significance in combination therapy groups. Similarly, increased testicular and epididymal total antioxidant capacity (TAC), nitric oxide (NO), total thiol (T-SH), non protein thiol (NPSH) levels, Glutathione-S-transferase (GST) and steroidogenic enzymes activities with concomitant decrease in reactive oxygen species (ROS) level, thiobarbituric acid reactive substances (TBARS) production and arginase activity, as well as non-observable histopathologic changes in the testes were observed in all the groups when compared with control, with more significance in combination therapy groups.
Conclusion
Therefore, this present study shows that the combination of cashew nut supplemented diet and clomiphene citrate could modulate vital biomolecules associated with male reproductive function. Thus, this finding supports the concept that the combination therapy of cashew nut and clomiphene citrate may be used to treat male partners suffering from infertility.
Keywords: Fertility, Testosterone, Clomiphene citrate, Sperm count
Graphical abstract
At a glance commentary.
Scientific background on the subject
Clomiphene citrate is a known synthetic oestrogen-antagonist, reported to have a potential fertility effects on human reproductive health in the last few decades. However, study, have shown that dietary pattern plays a pivotal role in the protection of testicular damage, and till date there is no report on the influence of cashew nut supplemented diet on fertility activity of clomiphene citrate in male infertility and reproductive dysfunction.
What this study adds to the field
The present study shows that the combination of cashew nut supplemented diet and clomiphene citrate could modulate vital biomolecules associated with male reproductive function. Thus, this finding supports the concept that the combination therapy of cashew nut and clomiphene citrate may be used to treat male partners suffering from infertility.
Infertility is defined by the World Health Organization (WHO) as the inability of a couple to achieve conception or bring pregnancy to term after one year or more of regular, unprotected sexual intercourse [1,2]. A variety of medications have been developed in an attempt to improve the sperm quality and in turn modify the male fertility potential [3]. Commonly used medications include follicle stimulating hormone (FSH), anti-estrogens, l-carnitine and antioxidants.
Clomiphene citrate, 1-[p-(beta-diethyl aminoethoxy) phenyl]-1,2-diphenyl chloroethylene, is an orally active non-steroidal agent distantly related to diethylstilbestrol. It is thought to stimulate pituitary gonadotropin release by excluding estradiol from hypothalamic receptor sites [4]. This interaction neutralizes the normal negative feedback control of estrogen and results in enhanced secretion of LH-RH, FSH-RH and gonadotropins [4]. Testosterone is produced by the Leydig cells in response to LH secretion. The concentration of testosterone in the tubular environment is believed to maintain the gametogenic function of the testis. Clomiphene citrate has also been found to increase serum level of LH and FSH in male rats which stimulate testosterone and sperm production by the testes [5]. In infertile male rats, Clomiphene citrate treatment together with testosterone administration has maintained their fertility [6].
Nuts are recommended as an important constituent of a healthy diet, of which cashew nut (Anacardium occidentale L.) occupies a central position in the diets of the human population throughout the world. In folk medicine, it is consumed orally to cure impotency and as aphrodisiac [7,8]. It has been reported that cashew nut oil improved sexual libido and potency in male albino rats [9]. Cashew nuts are also believed to increase testosterone levels [10]. Cashew nuts have various health advantages as they are significant sources of iron (essential for red blood cell function and enzyme activity), magnesium (promotes energy release and bone growth), phosphorus (builds bones and teeth) and zinc (essential for proper sperm motility and production) [9]. It has been reported that zinc, which is very abundant in cashew nut is required for a robust body as well as to boost fertility in both men and women [10]. Some studies have shown that even a short term deficiency of zinc can affect testosterone levels and decrease sperm volume [[10], [11], [12]]. There are no reports in the literature on the interaction of food with clomiphene citrate, a known fertility drug. The present study therefore was undertaken to investigate the influence of cashew nut supplemented diet on fertility activity of clomiphene citrate in male rats.
Materials and methods
Chemicals and equipments
The substrate l-arginine, as well as urea, N-(l-naphthyl)ethylene-diaminedihydrochloride, Tris–HCl buffer, bovine serum albumin, nitrate and vanadium chloride (VCl3) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The standard fertility drug (clomiphene citrate), was procured from CIPRA-MEDPRO (PTY) LTD, Rosen Heights, Pasita Street, Rosen Park, Bellville, 7530, Johannesburg, South Africa. All the other chemicals used in this experiment were of the analytical grade, while the water was glass distilled. All the kits used for the bioassay were sourced from Randox Laboratories Ltd. (Crumlin, Northern Ireland, United Kingdom).
Plant material and sample preparation
Cashew nut (A. occidentale L.) seeds were obtained from the local market in Ado - Ekiti, Nigeria. The nuts were cracked with manual cashew kernel cutter to separate the nuts from the shells. The nuts were roasted in oven at 100 °C for 2 h. The covering testa was removed by squeezing and then winnowed to obtain cream colour nuts. The nuts were milled using Kenwood blender into flour and kept in an airtight container prior to analysis. Furthermore, the proximate analysis of the sample was carried out (unpublished data) to determine the nutritional content of the nut which was used in the diet formulation. Voucher specimen of A. occidentale L. has been deposited in the Herbarium.
Animals
Thirty-six (36) adult male rats (200–250 g) were procured for the study from the Central Animal House, College of Medicine, Ekiti State University, Ado Ekiti. Nigeria. The animals were handled according to the guidelines of the National Council for Animal Experiments Control (CONCEA) and in accordance with institutional ethical committee. The animals were housed in stainless steel cages and kept in a room where 12 h light/dark cycle was maintained and they were allowed free access to water and diet throughout the period of the experiment. The ambient temperature was kept at 23 ± 1 °C.
Experimental protocol and diet formulation
The rats were acclimatized for two weeks and were randomly divided into six groups of six animals n = 6. Group I: (Control) normal control rats fed basal diet; Group II: normal rats placed on basal diet + clomiphene citrate; Group III: normal rats fed diet supplemented with 10% processed cashew nut + clomiphene citrate; Group IV: normal rats fed diet supplemented with 20% processed cashew nut + clomiphene citrate; Group V: normal rats fed diet supplemented with 10% processed cashew nut; Group VI: normal rats fed diet supplemented with 20% processed cashew nut as presented in [Table 1]. Treatment lasted for 14 days, after which the rats fasted overnight after the last treatment and were sacrificed under ketamine and xylazine anesthesia. The male rats were given diet freshly prepared according to a modified method of Akinyemi et al. (2014). Standard drug was given daily at 1.04 mg/kg body weight by gavage using a metal oropharyngeal cannula as described in the protocols used by Ref. [12].
Table 1.
Diet formulation for basal and supplemented diets for control and test groups.
| Treatment | Group I | Group II | Group III | Group IV | Group V | Group VI |
|---|---|---|---|---|---|---|
| Skimmed milk | 40.0 | 40.0 | 31.3 | 22.6 | 31.3 | 22.6 |
| Oil | 10.0 | 10.0 | 5.71 | 1.42 | 5.71 | 1.42 |
| Vitamin premix | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Corn starch | 46.0 | 46.0 | 48.99 | 51.98 | 48.99 | 51.98 |
| Cashew nut | – | – | 10.0 | 20.0 | 10.0 | 20.0 |
| Total | 100 g | 100 g | 100 g | 100 g | 100 g | 100 g |
Note: Skimmed milk = 32% protein; The vitamin premix (mg or IU/g) h was the following composition; 3200 IU vitamin A, 600 IU vitamin D3, 2.8 mg vitamin E, 0.6 mg vitamin K3, 0.8 mg vitamin B1, 1 mg vitamin B2, 6 mg niacin, 2.2 mg pantothenic acid, 0.8 mg vitamin B6, 0.004 mg vitamin B12, 0.2 mg folic acid, 0.1 mg biotin H2, 70 mg choline chloride, 0.08 mg cobalt, 1.2 mg copper, 0.4 mg iodine, 8.4 mg iron, 16 mg manganese, 0.08 mg selenium, 12.4 mg zinc, 0.5 mg antioxidant. Group I: (Control) normal control rats fed basal diet; Group II: normal rats placed on basal diet + clomiphene citrate; Group III: normal rats fed diet supplemented with 10% processed cashew nut + clomiphene citrate; Group IV: normal rats fed diet supplemented with 20% processed cashew nut + clomiphene citrate; Group V: normal rats fed diet supplemented with 10% processed cashew nut; Group VI: normal rats fed diet supplemented with 20% processed cashew nut.
Sperm count
The sperm was collected immediately after a rat was sacrificed. Briefly, epididymal sperm count was obtained by mincing the pairs of caudal epididymis in distilled water and filtering through a nylon mesh. The spermatozoa were counted by hemocytometer using the Improved Neubauer (Deep 1/10 mm. LABART, Germany) chamber as described by Pant and Srivastava [13]. A total of 400 spermatozoa from each rat were examined for morphological abnormalities.
Morphological abnormalities assay
A portion of the sperm suspension placed on a slide glass was smeared out with another slide and stained with Wells and Awa stain (0.2 g of eosin and 0.6 g of fast green dissolved in 10 and 20 ml of distilled water, respectively, and 18 ml of ethanol was added to the mixture) for morphological examination [12,14].
Sperm motility and viability assays
The sperm was collected immediately after a rat was sacrificed. Briefly, epididymal sperm was obtained by cutting the epididymis with surgical blades and released onto a sterile clean glass slide. The sperm was subsequently diluted with 2.9% sodium citrate dehydrate solution, mixed thoroughly and covered with a 24 × 24 mm coverslip before examination under a phase contrast microscopeat 200× magnification to evaluate the sperm progressive motility. The data were expressed as percentage of sperm progressive motility. Sperm viability was determined by staining with 1% eosin and 5% nigrosine in 3% sodium citrate dehydrate solution according to established procedures [12,14].
Serum testosterone
The serum total testosterone level was measured by ELISA method using DRG Elisa testosterone kit (ELISA EIA-1559, 96 Wells kit, DRG Instruments, GmbH, Marburg, Germany) according to the standard protocol supplied by the kit manufacturer.
Measurement total thiol levels
Total thiol (T-SH) content was determined according to the method previously described by Ellman [15]. Briefly, the reaction mixture consisted of 40 mL of tissue homogenate, 10 mL of 10 mM 5, 5-dithiobis 2-nitro benzoic acid and 0.1 M potassium phosphate buffer (pH 7.4) in a final volume of 200 mL. The mixture was incubated for 30 min at ambient temperature and the absorbance measured at 412 nm. A standard curve was plotted for each measurement using cysteine as a standard and the results were expressed as μmol/mg protein.
Measurement of non protein thiols levels
Non-protein thiols (NPSH) levels were determined by the method of Ellman [15]. Briefly, an aliquot of tissue homogenate was mixed (1:1) with 10% trichloroacetic acid. Subsequent to precipitation of protein, the resulting solution was centrifuged at 10,000 3 g for 5 min at 48 °C and the free SH groups were determined in the supernatant. The reaction mixture consisting of 50 mL sample, 450 mL phosphate buffer, and 1.5 mL of 0.1 mM 5, 5-dithiobis 2-nitrobenzoic acid was incubated for 10 min at 378 °C. The absorbance was measured at 412 nm. NPSH levels were expressed as μmol/mg of protein.
Measurement of Total Antioxidant Capacity (TAC)
The TAC was determined by the method of Kambayashi et al. [16]. Briefly, 90 L of phosphate-buffered saline (10 mM, pH 7.2), 50 L of myoglobin solution (18 M), 20 L of 3mMABTS solution, and 20 L of diluted tissue homogenate were added to a 96-well microplate and mixed well at 26 °C for 3min. Then, 20 L of H2O2 (250 M) was added to each well and the plate was incubated for 5min.The absorbance at 600 nm was measured using a spectrophotometer (Molecular Devices). TAC was expressed as μmol/mg of protein.
Lipid peroxidation assay
Lipid peroxidation was determined as the formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction according to previously published study [17]. Briefly, the reaction mixture consisting of 200 mL of tissue homogenate or standard (0.03 mM MDA), 200 mL of 8.1% sodium dodecyl sulfate, 500 mL of 0.8% TBA and 500 mL of acetic acid solution (2.5 M HCl, pH 3.4) was heated at 958 °C for 1 h. The absorbance was measured at 532 nm and the TBARS tissue levels were expressed as μmol MDA produced/mg of protein.
Measurement of reactive oxygen species (ROS) level
The ROS assay was performed by the method of Hayashi et al. [18]. In brief, 50 L of tissue homogenate and 1400 L sodium acetate buffer were transferred to a cuvette. After then, 1000 L of reagent mixture (N,N-diethyl-para-phenylenediamine 6 mg/mL with 4.37 M of ferrous sulfate dissolved in 0.1 M sodium acetate buffer, pH 4.8) was added at 37 °C for 5min. The absorbance was measured at 505 nm using a spectrophotometer (Molecular Devices). ROS levels from the tissue were calculated from a calibration curve of H2O2 and expressed as U/mg of protein (1 unit = 1.0 mg H2O2/L).
Measurement of nitric oxide level
The nitric oxide (NO) content in heart homogenates was estimated in a medium containing 400 mL of 2% vanadium chloride (VCl3) in 5% HCl, 200 mL of 0.1% N-(l-naphthyl) ethylenediaminedihydrochloride, 200 mL of 2% sulfanilamide (in 5% HCl). After incubating at 378 °C for 60 min, nitrite levels, which correspond to levels of NO, were determined spectrophotometrically at 540 nm [19]. Nitrite and nitrate levels were expressed as nmol of NO/mg of protein.
Arginase activity assay
The arginase activity of the tissue homogenate was assayed as described by Zhang et al. [20]. Briefly, arginase activity was determined by the measurement of urea produced by the reaction of Ehrlich's reagent. The reaction mixture contained in final concentration 1.0 mMTris-HCl buffer (pH 9.5) containing 1.0 mM MnCl2, 0.1 M arginine solution, and 50 mM of the tissue lysate in a final volume of 1.0 mL. The mixture was incubated for 10 min at 37 °C. The reaction was terminated by the addition of 2.5 mL Ehrlich reagent (2.0 g of p-dimethylaminobenzaldehyde in 20 mL of concentrated hydrochloric acid and made up to 100 mL with distilled water). The optical density reading was taken after 20 min at 450 nm. The amount of urea produced, after normalization with protein, was used as an index for arginase activity.
Determination of glutathione-S-transferase activity
Glutathione-S-transferase activity was determined according to the method of Habig et al. [21] using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The assay reaction mixture consisted of 270 μL of a solution containing (20 mL of 0.25 M potassium phosphate buffer, pH7.0, 10.5 mL of distilled water, and 500 μL of 0.1 M GSH at 25 °C), 20 μL of sample (1:50 dilution), and 10 μL of 25 mM CDNB. The reaction was monitored for 5 min (30 s intervals) at 340 nm in a Spectrophotometer (CA, USA) and the data were expressed as μmol/min/mg protein using the molar extinction coefficient of 9.6 mM−1cm−1 for CDNB conjugate.
3β-HSD and 17β-HSD determination
For the determination of testicular steriodogenic enzymes (3β and 17β -hydroxysteroid dehydrogenase (HSD)) activities, testicular tissue (50 mg/ml) was homogenized in 20% spectroscopic grade glycerol containing 5 mM potassium phosphate and 1 mM EDTA at 4 °C, centrifuged at 10,000 ×g for 30 min. Briefly, 3β-HSD was measured by mixing 250 μl of the supernatant with 250 μl of 100 μM sodium pyrophosphate buffer, pH 8.9, 10 μl ethanol containing 30 μg dihydroxylepiandrosterone (DHEA) (Sigma St Louis, USA) and 240 μl of 25 mg% BSA (Bangalore Genei, India). Enzymatic activity was measured after addition of 50 μl of 0.5 μM NAD to the mixture in a spectrophotometer at 340 nm against a blank (without NAD) [22]. 17β-HSD was measured by mixing 250 μl of the supernatant with 250 μl of 440 μM sodium pyrophosphate buffer (pH 10.2), 10 μl ethanol containing 0.3 μM testosterone (Sigma, St. Louis, USA) and 240 μl of 25 mg% BSA. Enzyme activity was measured after addition of 50 μl of 0.5 μM NAD to the mixture in a spectrophotometer at 340 nm against a blank (without NAD) [23]. One unit of enzyme activity was the amount causing a change in absorbance of 0.001/minute at 340 nm.
Data analysis
All data were tested for normality and analyzed using Prism (Graph pad, San Diego, CA, USA). One way analysis of variance (ANOVA) was used to analyze the mean while appropriate post-hoc treatment was applied. Significant difference was taken at p < 0.05 while the data was expressed as mean ± SD or SEM.
Results
Sperm analysis
There was significant (p < 0.05) increase in epididymal sperm count, viability and motility as well as decrease in total sperm abnormality in all the treated groups, in comparison to control, with more significance in combination therapy group [Table 2].
Table 2.
Sperm count, sperm progressive motility, sperm viability, sperm abnormality and testosterone level in normal rats fed with diet supplemented with processed cashew nut plus clomiphene citrate.
| Treatment groups | Sperm count (number of sperm/rat x106) | Sperm viability (%) | Sperm motility (%) | Sperm abnormality (%) | Testosterone (ng/dl) |
|---|---|---|---|---|---|
| I | 15.50 ± 1.33 | 34.40 ± 2.45 | 20.50 ± 2.06 | 16.82 ± 2.44 | 16.26 ± 2.11 |
| II | 20.30 ± 1.24a | 40.00 ± 2.64a | 30.50 ± 2.22a | 8.40 ± 1.65a | 30.63 ± 2.92a |
| III | 32.20 ± 1.25a | 46.00 ± 2.62b | 38.10 ± 2.49b | 5.40 ± 1.45b | 34.79 ± 2.69b |
| IV | 25.50 ± 1.55a | 49.00 ± 2.25b | 39.80 ± 2.94b | 5.20 ± 1.66b | 42.99 ± 3.81b |
| V | 17.30 ± 1.61 | 38.10 ± 2.55a | 28.40 ± 2.50a | 11.72 ± 1.54a | 18.35 ± 2.37 |
| VI | 19.50 ± 1.44a | 39.80 ± 2.54a | 29.90 ± 2.51a | 10.81 ± 1.45a | 28.51 ± 2.51a |
The results are presented as mean ± SEM (n = 6). aValues significantly (p < 0.05) different from control.bValues significantly (p < 0.05) different from clomiphene citrate group. For details see the legend of [Table 1].
Testosterone level, testicular 3β-HSD and 17β-HSD activities
The control rats had a decrease in serum testosterone level when compared to treated rats, which was found at increased levels in clomiphene-treated and clomiphene-treated rats pre-treated with diet containing cashew nuts [Table 2]. In a similar manner, testicular 3β-HSD and 17β-HSD activities increased significantly in treated rats compared to the control rats [Fig. 1A and B], but the increase was found to be greater in the clomiphene rats pre-treated with cashew nut (10% and 20%) supplemented diet.
Fig. 1.
Effects of dietary supplementation of processed cashew nut plus clomiphene citrate on the testicular (A) 3β- (B) 17β-hydroxyl steroid dehydrogenase (3β-HSD and 17β-HSD) activities(C) testicular nitric oxide, (D) epididymal nitric oxide (NO) level, (E) testicular arginase, (F) epididymal arginase activity in normal rats. Data are presented as mean ± SEM (n = 6). *Values significantly (p < 0.05) different from control. #Values significantly (p < 0.05) different from clomiphene citrate group.
Nitric oxide (NO) level and arginase activity
Nitric oxide (NO) level in the testes and epididymis increased significantly in non-pre-treated clomiphene-treated group compared to control rats. In the clomiphene-treated rats previously fed with cashew nut inclusive diet, the level of NO was clearly elevated compared with non-pre-treated clomiphene-treated [Fig. 1C and D]. Testicular and epididymal arginase activity of clomiphene-treated rats decreased significantly when compared to the control rats [Fig. 1E and F]. However, pre-treatment with dietary supplementation of cashew nut further caused a synergistic decrease in the arginase activity when compared with clomiphene-treated group.
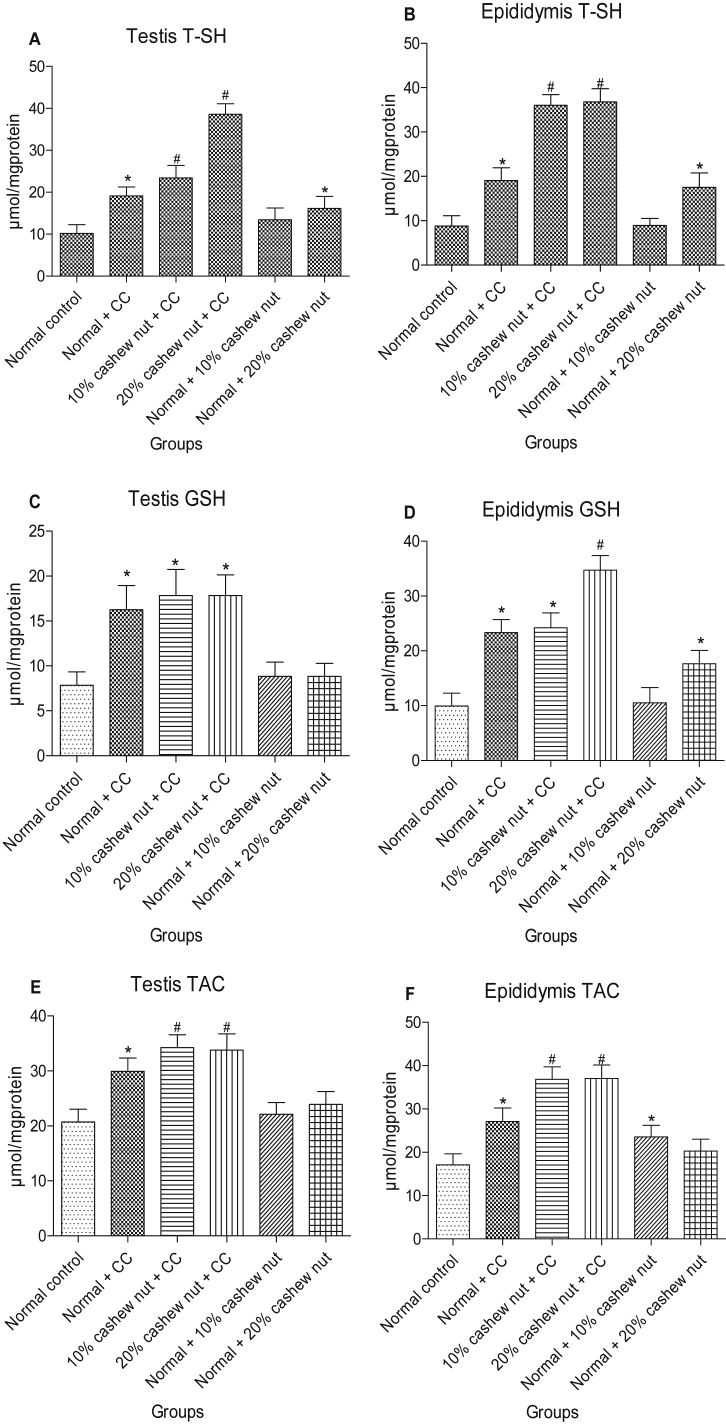
Testicular and epididymal antioxidant status
The antioxidant status of the tested organs of the experimental rats is presented in [Fig. 2, Fig. 3]. The administration of clomiphene citrate and feeding with cashew nut inclusive diet resulted in a significant increase in the CAT, SOD, GPx, GST activities as well as in GSH, TAC and T-SHs levels, but decreased ROS and TBARS levels in the testes and epididymis of treated rats, compared to control rats. Interestingly, the combination therapy with the cashew nut caused more significance on the tested antioxidant parameters when compared with non-pre-treated clomiphene citrate-treated rats.
Fig. 2.
Effects of dietary supplementation of processed cashew nut plus clomiphene citrate on the testicular and epididymal total thiol (T-SHs) level, non-protein thiol (NPSH) or reduced glutathione (GSH) level and total antioxidant capacity (TAC) in normal rats. Data are presented as mean ± SEM (n = 6). *Values significantly (p < 0.05) different from control. #Values significantly (p < 0.05) different from clomiphene citrate group.
Fig. 3.
Effects of dietary supplementation of processed cashew nut plus clomiphene citrate on the testicular and epididymal glutathione-S-transferase (GST) activity, reactive oxygen species (ROS) and thiobarbituric acid reactive substances (TBARS) levels in normal rats. Data are presented as mean ± SEM (n = 6). *Values significantly (p < 0.05) different from control. #Values significantly (p < 0.05) different from clomiphene citrate group.
Histologic examination
The histology result of the testis from negative control rat showing normal histological structure of mature functioning seminiferous tubules associated with complete spermatogenic series. Sections of the rat's testis belonging to the groups pre-treated with 10 and 20% cashew nuts inclusive diets and clomiphene citrate were not significantly different from that of the control group (H&E, X 400) [Fig. 4(I–VI)].
Fig. 4.
(A) Testis of a negative control rat showing normal histological structure of mature functioning seminiferous tubules associated with complete spermatogenic series; (B) Testis of normal rats placed on basal diet + clomiphene citrate showing similar pattern with negative control; (C) Testis of normal rats fed diet supplemented with 10% processed cashew nut + clomiphene citrate showing similar pattern with negative control; (D) Testis of normal rats fed diet supplemented with 20% processed cashew nut + clomiphene citrate showing similar pattern with negative control; (E) Testis of normal rats fed diet supplemented with 10% processed cashew nut showing similar pattern with negative control; (F) Testis of normal rats fed diet supplemented with 20% processed cashew nut showing similar pattern with negative control.
Discussion
In this study, the administration of clomiphene citrate (1.04 mg/kg) influenced fertility, characterized by the decrease in sperm abnormality, increased sperm count, viability and motility. Clomiphene citrate has been reported to enhance fertility potentials, including increase in weights and dimensions of testes, epididymis, sperm concentration and motility in most men, rat and boars [4,24].
Testicular 3β-HSD and 17β-HSD play a noteworthy impact in the biosynthesis of testosterone from cholesterol [12]. Thus, determining the activity levels of these enzymes provides essential information regarding the biosynthesis of testosterone. In the present study, administration of clomiphene citrate increased the activity levels of 3β-HSD and 17β-HSD in rats, indicating increased steroidogenesis and function of Leydig cells. Accordingly, the circulating levels of testosterone also increased in clomiphene treated rat [Table 2]. On the other hand, the combination therapy of clomiphene citrate with cashew nut supplemented diet synergistically potentiates more favourable effect in the testis. Previous studies have reported the use of clomiphene citrate in the treatment of male infertility [[25], [26], [27]] and the enhanced activity of steriodogenic enzymes may be one of its mechanisms in stimulating spermatogenesis.
The presence of normal NO level is a vital mediator in the male reproductive function, as chronic inhibition of NO affect sperm function [12,28]. NO also involve in the management of free radicals and production of superoxide anions [28], and ultimately prevent lipid peroxidation and impairment of spermatozoa function [29]. In this study, administration of clomiphene citrate drastically increase NO level in the isolated tissues (p < 0.05) when compared to control rats [Fig. 1C and D]. However, the synergistic effect of nut supplemented diets on testicular and epididymal NO levels suggest the possible improvement of male reproductive function.
The nitric oxide-cyclic guanidine monophosphate (NO-cGMP) pathway plays a major role in the male sexual function [28]. l-arginine serves as substrate for nitric oxide synthase (NOS) to produce nitric oxide (NO). However, the activity of arginase, a metalloenzyme competes with NOS in the utilization of l-arginine, hence reduced NO production by NOS, and alters NO/cGMP pathway. The key role of arginine as a substrate for both nitric oxide synthase and arginase serves as a potential point of regulation for the NO/cGMP pathway such that an up-regulation of one enzyme leads to the down-regulation of the other. This study further revealed that there was a decrease in the arginase activity in the testes and epididymides of clomiphene-treated rats and pre-treated nut's inclusive diets groups when compared with control rats [Fig. 1E and F]. The decrease in arginase activity in this study could be due to the stimulation of eNOS activity, thereby favouring increased production of nitric oxide in the testes and epididymides of the treated rats as observed in this study [30]. Hence, the implication of the pharmacological benefits of cashew nut in the prevention of male infertility.
The increase in ROS production in the male reproductive system causes damage to spermatozoa and in many cases of oligospermia [31]. In this, we observed a significant increment in the testicular and epididymal TSH, GSH levels, CAT, SOD, and GPx activities in the clomiphene-treated rats and pre-treated nut's inclusive diets groups with a concomitant reduction in ROS and TBARS production when compared with control rats. However, dietary supplementation with roasted cashew nut efficiently caused a synergistic effect on these parameters, which may be due to the phenolic compounds present in the cashew nut.
The histological sections of the current study showed that spermatogenesis was significantly preserved in the rats administered with clomiphene citrate and pre-treated with cashew nut supplemented diet. The morphological characteristics of these testes were comparable to those in control groups, with more significance in combination therapy groups.
Conclusion
Therefore, this present study shows that the combination of cashew nut supplemented diet and clomiphene citrate could modulate vital biomolecules associated with male reproductive function. Thus, this finding supports the concept that the combination therapy of cashew nut and clomiphene citrate may be used to treat male partners suffering from infertility.
Ethical statement
All procedures performed in studies involving animals were in accordance with the Institutional Animal Ethical Committee. All efforts were made to minimize the number of animals and their suffering.
Conflicts of interest
There is no financial or non-financial conflicts of interest.
Acknowledgments
The authors appreciate the effort of Dr. Fowotade, the Head of Histology Department, University of Ilorin Teaching Hospital, Ilorin, Nigeria who helped in carrying out the histopathological studies.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Akomolafe S.F., Oboh G., Akindahunsi A.A., Afolayan A.J. Tetracarpidium conophorum (Mull.Arg) Hutch &Dalziel inhibits FeSO4 -induced lipid peroxidation in rat's genitals. BMC Complement Altern Med. 2015;15:57. doi: 10.1186/s12906-015-0547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ElSheikh M.G., Hosny M.B., Elshenoufy A., Elghamrawi H., Fayad A., Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: a prospective, randomized trial. Andrology. 2015;3:864–867. doi: 10.1111/andr.12086. [DOI] [PubMed] [Google Scholar]
- 3.Comhaire F.H., Mahmoud A. The role of food supplements in the treatment of the infertile man. Reprod Biomed Online. 2003;7:385–391. doi: 10.1016/s1472-6483(10)61881-0. [DOI] [PubMed] [Google Scholar]
- 4.Patankar S.S., Kaore S.B., Sawane M.V., Mishra N.V., Deshkar A.M. Effect of clomiphene citrate on sperm density in male partners of infertile couples. Indian J Physiol Pharmacol. 2007;51:195–198. [PubMed] [Google Scholar]
- 5.Weissenberg R., Dar Y., Lunenfeld B. The effect of clomiphene citrate and its Zu or En isomers on the reproductive system of the immature male rat. Andrologia. 1992;24:161–165. doi: 10.1111/j.1439-0272.1992.tb02631.x. [DOI] [PubMed] [Google Scholar]
- 6.Rej S.K., Chatterjee R., Chatterjee A. Clomiphene and the fertility in rats. Proc Zool SOC. 1988;37:1–4. [Google Scholar]
- 7.Saxena A., Prakash P., Porwal M., Sissodia N., Sharma P. Erectile dysfunction: a review and herbs used for its treatment. Int J Green Pharm. 2012;6:109–117. [Google Scholar]
- 8.Onilude A.A., Igbinadolor R.O., Wakil S.M. Effect of time and relative humidity on the microbial load and physical quality of cashew nuts (Anacardiumoccidentale L) under storage. Afr J Microbiol Res. 2010;4:1939–1944. [Google Scholar]
- 9.Mbatchou V.C., Kosoono I. Aphrodisiac activity of oils from AnacardiumoccidentaleL. seeds and seed shells. Phytopharmacology. 2012;2:81–91. [Google Scholar]
- 10.Sinclair S. Male infertility: nutritional and environmental considerations. Altern Med Rev. 2000;5:28–38. [PubMed] [Google Scholar]
- 11.Zhao J., Dong X., Hu X., Long Z., Wang L., Liu Q. Zinc levels in seminal plasma and their correlation with male infertility: a systematic review and meta-analysis. Sci Rep. 2016;6:22386. doi: 10.1038/srep22386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akomolafe S.F., Oboh G., Akindahunsi A.A., Afolayan A.J. Ethanol-induced male infertility: effects of aqueous leaf extract of Tetracarpidium conophorum. Andrologia. 2017;49 doi: 10.1111/and.12759. [DOI] [PubMed] [Google Scholar]
- 13.Pant N., Srivastava S.P. Testicular and spermatotoxic effect of quinaphos in rats. J Appl Toxicol. 2003;23:271–274. doi: 10.1002/jat.919. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida S., Hiyoshi K., Ichinose T., Takano H., Oshio S., Sugawara I. Effect of nanoparticles on the male reproductive system of mice. Int J Androl. 2009;32:337–342. doi: 10.1111/j.1365-2605.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 16.Kambayashi Y., Binh T., Asakura H.W. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J Clin Biochem Nutr. 2009;44:46–51. doi: 10.3164/jcbn.08-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jentzsch A.M., Bachmann H., Fürst P., Biesalski H.K. Improved analysis of malondialdehyde in human body fluids. Free Radic Biol Med. 1996;20:251–256. doi: 10.1016/0891-5849(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi I., Morishita Y., Imai K., Nakamura M., Nakachi K., Hayashi T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Res Genet Toxicol Environ Mutagen. 2007;631:55–61. doi: 10.1016/j.mrgentox.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Miranda K.M., Espay M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Hein T.W., Wang W., Chang C.I., Kuo L. Constitutive expression of Arginase in microvascular endothelial cells counteracts nitric oxide mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- 21.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 22.Jarabak J., Adams J.A., Williams-Ashman H.G., Talalay P. Purification of 17β-hydroxysteroid dehydrogenase of human placenta and studies on its transdehydrogenase function. J Biol Chem. 1962;237:345–347. [PubMed] [Google Scholar]
- 23.Talalay P. Hydroxysteriod dehydrogenase. In: Colowick S.P., Kaplan N.O., editors. Methods in enzymology. Academic Press; New York: 1962. pp. 512–516. [Google Scholar]
- 24.Umesiobi D.O. Effect of oral administration of clomiphene citrate on sperm viability and fertility of boar semen. J Appl him Res. 2006;30:167–170. [Google Scholar]
- 25.Bharti S., Misro M.M., Rai U. Clomiphene citrate potentiates the adverse effects of estrogen on rat testis and down-regulates the expression of steroidogenic enzyme genes. Fertil Steril. 2013;99:140–148. doi: 10.1016/j.fertnstert.2012.08.050. e5. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility. Int J Androl. 1992;15:299–307. doi: 10.1111/j.1365-2605.1992.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 27.Sorbie P.J., Perez-Marrero R. The use of clomiphene citrate in male infertility. J Urol. 1984;131:425–426. doi: 10.1016/s0022-5347(17)50437-1. [DOI] [PubMed] [Google Scholar]
- 28.Akinyemi A.J., Adedara I.S., Thome G.R., Morsch V.R., Rovani M.T., Mujica L.K.S. Dietary supplementation of ginger and turmeric improves reproductive function in hypertensive male rats. Toxicol Rep. 2015;2:1357–1366. doi: 10.1016/j.toxrep.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal A., Hamada A., Esteves S.C. Insight into oxidative stress in varicocele-associated male infertility. Nat Rev Urol. 2012;9:678–680. doi: 10.1038/nrurol.2012.197. [DOI] [PubMed] [Google Scholar]
- 30.Durak I., Elgun S., Özbek H., Karaayvaz H., Göçmen E.H.S. Effect of cisplatin treatment on renal nitric oxide synthase activity in Guinea pigs. Acta Anaesthesiol Scand. 2002;45:119–122. [Google Scholar]
- 31.Zini A., de Lamirande E., Gagnon C. Reactive oxygen species in semen of infertile patients: levels of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl. 1993;16:183–188. doi: 10.1111/j.1365-2605.1993.tb01177.x. [DOI] [PubMed] [Google Scholar]