Keywords: Alzheimer's disease, behavioral variant frontotemporal dementia, cognitive profile, frontotemporal dementia, genetics, memory, neuropsychology, progranulin, visuoconstructive ability
Abstract
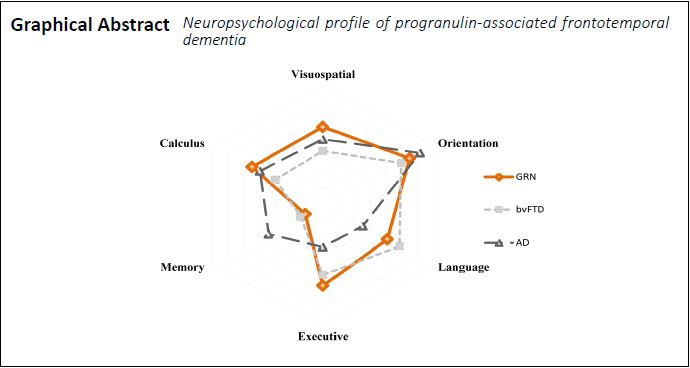
The distinction between sporadic and genetic behavioural-variant frontotemporal dementia (bvFTD) regarding some neuropsychological (NP) features remains challenging. Specifically, progranulin (GRN)-associated bvFTD frequently presents with early episodic memory impairment and some degree of parietal dysfunction which are supporters of Alzheimer’s disease (AD) diagnosis. In this context, we aimed to characterize the NP profile of GRN-bvFTD as compared to sporadic-bvFTD and AD in patients with mild dementia (Mini-Mental State Examination score ≥ 17 and Clinical Dementia Rating Scale score ≤ 1. We identified 21 patients at Centro Hospitalar e Universitário de Coimbra, Portugal with GRN mutations belonging to fifteen different families. As our focus was bvFTD variants, FTD-related aphasic forms (3 patients) were excluded. The remaining 18 GRN-bvFTD were further matched with 18 sporadic-bvFTD and 18 AD patients according to disease staging, age and education. All patients completed the Mini-Mental State Examination, Montreal Cognitive Assessment (MoCA) and a comprehensive NP assessment battery. Results were converted into z-scores. Differences between groups in individual NP measures and NP domains were assessed through non-parametric tests (Kruskal-Wallis test analysis) and eta squared (ŋ2) was calculated as a measure of effect size. Group comparisons show that GRN patients have worse performances on verbal retrieval processes (P = 0.039, ŋ2 = 0.110) and visuoconstructive abilities (P = 0.039, ŋ2 = 0.190) than sporadic bvFTD forms. When compared to AD, GRN patients present a higher impairment in frontal (P = 0.001, ŋ2 = 0.211) and parietal (P = 0.041, ŋ2 = 0.129) measures and a better performance in memory tasks (P = 0.020, ŋ2 = 0.120). Sporadic-bvFTD forms are worse than AD in frontal measures (P = 0.032, ŋ2 = 0.200), being better in both memory (P = 0.010, ŋ2 = 0.131) and visuospatial skills (P = 0.023, ŋ2 = 0.231). Considering these results, we conclude that GRN-bvFTD patients present a NP profile that associates the typical patterns of FTD and AD deficits. This is particularly expressive in visuoconstructive abilities, which was the more discriminative feature between groups, followed by episodic verbal memory. This study was approved by the Institutional Ethics Committee of Centro Hospitalar e Universitário de Coimbra, Portugal (CE-029/2019) on June 24, 2019.
Chinese Library Classification No. R441; R741
Introduction
Frontotemporal dementia (FTD) is the second most frequent early-onset degenerative dementia. It has an estimated prevalence between 1–461/100,000 worldwide (Hogan et al., 2016) and is characterized by different subtypes: (i) behavioural variant (bvFTD), responsible for 60% of all cases and characterized by a progressive decline in personality/behaviour, language and executive dysfunction; (ii) non-fluent primary progressive aphasia; and (iii) semantic dementia, which present impaired speech production or agrammatism and impaired semantic memory, respectively (Gorno-Tempini et al., 2011; Rascovski et al., 2011). Each of these clinical phenotypes reflect different topographical distributions of similar underlying pathologies. Besides being clinically heterogeneous, FTD is also variable in its genetic substrate, with three major groups of mutations: the microtubule associated protein tau gene (MAPT), the progranulin (GRN) gene and the expanded hexanucleotide repeat in a noncoding region of the chromosome 9 open reading frame 72 (C9orf72) gene. An increasing list of genes has been linked with FTD, namely: TBK1, VCP, SQSTM1, CHMP2B, CHCHD10, TARDBP, FUS, UBQLN2, CCNF, OPTN, DCTN1, TUBA4A, TREM2, HNRNP2B1 and HNRNPA1 (Guerreiro et al., 2020).
Most of the patients are considered to be sporadic forms, but a family history is present in 20 to 40% of the cases, and 10% of them are inherited in an autosomal dominant pattern (Hosokawa and Tetsuaki, 2019). GRN gene mutations are responsible for up to 20% of all familial FTD case reports (Rohlfing and Tu, 2017), however, at Centro Hospitalar e Universitário de Coimbra, Portugal, they are responsible for around 50% of genetic cases. These patients have a significantly reduced expression (up to 50%) level of GRN in cerebrospinal fluid and plasma/serum (Almeida et al., 2014) with some variations between cases. Despite having heterogeneous manifestations (even among members of the same family), the most prominent clinical phenotypes associated with GRN mutations are bvFTD and non-fluent primary progressive aphasia, with bvFTD being more frequent and more elusive (Tatton, 2014). Descriptions of the cognitive profile at the initial stages are very heterogeneous, including expressive language, executive dysfunction and also parietal lobe deficits which are uncommon in other FTD forms. Specifically, GRN patients can also present with early episodic memory impairment and parietal dysfunction that resemble AD or mild cognitive impairment due to AD, making it challenging to establish a differential diagnosis between AD and FTD (Kelley et al., 2009). Nonetheless, it is of utmost relevance that the genetic cause of the disease is identified as early as possible as innovative mutation-target treatments are already available and also to allow genetic counselling. We aimed to characterize the neuropsychological (NP) profile of GRN-bvFTD compared to sporadic-bvFTD forms and AD patients.
Subjects and Methods
Patient selection and evaluation
In this nested case-control study, the recruitment was made at the memory clinic of Centro Hospitalar e Universitário de Coimbra, Portugal, in the last 5 years (2015–2019). The initial sample was composed by a convenience sample of 21 GRN patients with at least 2 years of formal education and mild dementia staging – for these criteria we considered a Mini-Mental State Examination (MMSE; Freitas et al., 2015) ≥ 17 and a score of ≤ 1 according to the Clinical Dementia Rating scale (CDR; Hughes et al., 1982). As we were interested in bvFTD patients, all FTD-related aphasic variants (3 patients) were excluded from further analyses. The remaining 18 bvFTD patients were matched with 18 sporadic-bvFTD forms and 18 AD according to disease staging (based on CDR global score and medical consulting), age and education (Figure 1). Patients were retrospectively selected from our dementia cohort and evaluated by the same NP assessment battery. They were matched by CDR, MMSE, sex, age at evaluation and age of onset, whenever possible. In the cases where an exact control was not available, the one with the most clinical and demographic features was selected. Matching was revised by an author not involved in the selection process. All patients were thoroughly studied, with most of them being evaluated with MRI and either cerebrospinal fluid biomarkers or amyloid-PET imaging. GRN serum levels were determined by a validated commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). Values below the laboratory reference value (23.6 ng/mL) underwent mutation analysis (Almeida et al., 2014). The diagnosis of bvFTD was established by a multidisciplinary team according to the most recent international criteria (Rascovski et al., 2011). AD patients were diagnosed according to international criteria (McKhann et al., 2011). The following exclusion criteria were established at the beginning of the study: unstable clinical syndrome, with significant comorbidities; moderate to severe dementia (assessed by CDR global score); recent changes in medication; psychiatric comorbidities; and significant motor, visual, or auditory deficits, all of which may affect the NP assessment.
Figure 1.
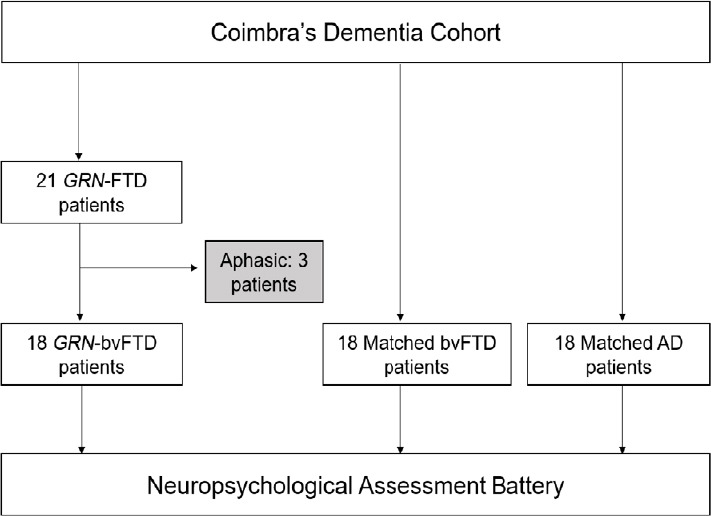
Patient inclusion and evaluation flowchart.
AD: Alzheimer’s disease; bvFTD: behavioural-variant frontotemporal dementia; GRN: progranulin.
All study participants completed the following assessment protocol: MMSE, Montreal Cognitive Assessment (MoCA; Freitas et al., 2011); and a comprehensive neuropsychological battery with normative data for the Portuguese population, the Battery of Lisbon for the Assessment of Dementia (BLAD; Guerreiro, 1998). This battery includes some tests of the Wechsler Memory Scale (WMS; Wechsler, 1945) and encompasses the following cognitive abilities: attention (Cancellation Task); verbal initiative (Semantic Fluency), motor and graphomotor initiatives; verbal comprehension (a modified version of the Token Test); verbal and non-verbal reasoning (Interpretation of Proverbs and the Raven’s Coloured Progressive Matrices – Ab series); orientation (spatial, temporal and social orientation); visuoconstructive abilities (cube copy); basic written calculus; immediate memory (Digit Span Forward); visual memory (WMS Visual Reproduction Test); working memory (Digit Span Backward); learning and verbal memory (WMS Verbal Paired-Associate Learning, Logical Memory and Word Recall). All tests were administered and scored according to standardized procedures. Individual test scores were converted into z-scores. The presence of impairment is considered when z-score < –1. The study was approved by the Institutional Ethics Committee of Centro Hospitalar e Universitário de Coimbra, Portugal (CE-029/2019) on June 24, 2019 (Additional file 1 (194KB, pdf) ) and was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. Informed consent was given by all study participants or their legal next of kin (Additional file 2 (271.1KB, pdf) ).
Statistical analysis
Normal data distribution was assessed through Kolmogorov-Smirnov normality test. One-way analysis of variance or Kruskal-Wallis was applied depending on the result of normality testing. For baseline comparison of demographic data between groups, analysis of variance and Pearson’s chi-square test were used, for numerical and nominal data, respectively. All tests were two-tailed and a P-value < 0.05 was assumed to be statistically significant. The NP assessment results were standardized according to age and education normative data for the Portuguese population (Guerreiro, 1998; Freitas et al., 2011, 2015), and z-scores were calculated. Non-parametric Kruskal-Wallis test analysis was performed for group comparisons of both NP measures and NP domains. The score of each domain was calculated by the sum of all z-scores belonging to the NP tests that integrate each domain and further divided by the total number of tests that composes it. Eta squared (ŋ2) was calculated as a measure of effect size. Post hoc Bonferroni corrections were used to adjust for multiple pair wise comparisons. All statistical analyses were performed using IBM SPSS Statistics 25 for Windows (IBM Corp, Armonk, NY, USA) package.
Results
Sample description and overview
General clinical and demographic characteristics of the study groups are presented in Tables 1 and 2. GRN patients came from fifteen different families, exhibiting four different GRN mutations.
Table 1.
General clinical and demographic characteristics of the 21 GRN patients
| Case | Sex | Age at onset (yr) | Family history | Primary diagnosis | Co-features | Disease duration (yr) | GRN serum levels (ng/mL) | GRN Mutations (NM_002087.3) |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 59 | + | bvFTD | – | 4 | 19.12 | c.900_901dupGT p.Ser301Cysfs*61 |
| 2 | M | 43 | – | bvFTD | CBS | 3 | – | c.900_901dupGT p.Ser301Cysfs*61 |
| 3 | F | 52 | – | bvFTD | – | 7 | 18 | c.768_769dupC p.Gln257Profs*27 |
| 4 | F | 65 | + | PNFA# | – | 6 | 19 | c.900_901dupGT p.Ser301Cysfs*61 |
| 5 | M | 62 | – | bvFTD | – | 5 | 14 | c.900_901dupGT p.Ser301Cysfs*61 |
| 6 | M | 61 | – | bvFTD | – | 1 | 11.7 | c.900_901dupGT p.Ser301Cysfs*61 |
| 7 | F | 65 | + | PNFA# | – | 5 | 14.35 | c.900_901dupGT p.Ser301Cysfs*61 |
| 8 | F | 57 | + | bvFTD | – | 1 | 15.2 | c.900_901dupGT p.Ser301Cysfs*61 |
| 9 | M | 52 | – | bvFTD | – | 5 | 13.9 | c.900_901dupGT p.Ser301Cysfs*61 |
| 10 | M | 58 | – | bvFTD | CBS | 2 | 18.51 | c.900_901dupGT p.Ser301Cysfs*61 |
| 11 | F | 62 | – | bvFTD | – | 5 | – | c.900_901dupGT p.Ser301Cysfs*61 |
| 12 | M | 48 | + | bvFTD | – | 5 | 15.26 | c.768_769dupCC p.Gln257Profs*27 |
| 13 | F | 57 | + | bvFTD | – | 4 | 19.7 | c.909delC p.Trp304Glyfs*57 |
| 14 | F | 59 | – | bvFTD | – | 3 | 13.52 | c.900_901dupGT p.Ser301Cysfs*61 |
| 15 | F | 55 | – | bvFTD | – | 5 | 20 | c.775_778delAAGT p.Lys259Alafs*23 |
| 16 | M | 52 | + | PNFA# | – | 5 | – | c.909delC p.Trp304Glyfs*57 |
| 17 | M | 54 | – | bvFTD | – | 2 | 19.1 | c.768_769dupCC p.Gln257Profs*27 |
| 18 | F | 54 | – | bvFTD | – | 2 | 14.8 | c.900_901dupGT p.Ser301Cysfs*61 |
| 19 | F | 50 | + | bvFTD | – | 1 | 16 | c.900_901dupGT p.Ser301Cysfs*61 |
| 20 | F | 58 | + | bvFTD | – | 2 | 12.3 | c.768_769dupCC p.Gln257Profs*27 |
| 21 | F | 55 | UKN | bvFTD | – | 5 | 17 | c.775_778delAAGT p.Lys259Alafs*23 |
Patients 5, 8, 9, 14 and 19 are relatives; patients 4, 15 and 16 are relatives. bvFTD: Behavioral variant frontotemporal dementia; CBS: corticobasal syndrome; F: female; M: Male; GRN: progranulin; PNFA: non-fluent primary progressive aphasia (%excluded patients); UKN: unknown.
Table 2.
Demographic characteristics of GRN-bvFTD, bvFTD and AD groups
| GRN-bvFTD (n=18) | bvFTD (n=18) | AD (n=18) | P-value | |
|---|---|---|---|---|
| Age at onset (yr) | 55.13±5.29 | 59.67±8.65 | 53.14±6.62 | 0.069 |
| Age at baseline (yr) | 57.25±7.09 | 62.56±8.26 | 56.29±6.41 | 0.288 |
| Education (yr) | 6.81±3.93 | 6.69±3.89 | 6.89±2.95 | 0.746 |
| Sex (M/F, n) | 7/11 | 8/10 | 7/11 | 0.765 |
Data are presented as the mean ± SD with the exception of sex. AD: Alzheimer's disease; bvFTD: behavioral variant frontotemporal dementia; F: female; GRN-bvFTD: progranulin associated bvFTD; M: male. Group comparisons were performed through one-way analysis of variance and Pearson's chi-square test.
The first clinical syndrome diagnosed was bvFTD in 18 patients (86%); two patients later developed clinical symptoms that lead to a secondary diagnosis of corticobasal syndrome. Table 2 summarizes the demographic characteristics of all the three groups (GRN-bvFTD, bvFTD, and AD). Results from one-way analysis of variance between the three groups showed no differences between groups regarding age at onset, age at baseline assessment, education or gender.
Neuropscyhological evaluation and comparison of GRN-bvFTD, sporadic bvFTD and AD groups
As a first analysis, we observed that there were no differences between the three groups regarding cognitive screening measures (MMSE: P = 0.729, ŋ2 = 0.002; and MoCA: P = 0.098, ŋ2 = 0.129; Table 3). Bonferroni post hoc test analysis showed differences between GRN-bvFTD patients and sporadic-bvFTD in the comprehensive NP assessment battery in delayed recall of Logical Memory test (P = 0.032, ŋ2 = 0.200) and in Cube copy (P = 0.011, ŋ2 = 0.451), indicating that GRN patients have a higher impairment on verbal retrieval processes (episodic memory) and visuoconstructive abilities than the sporadic FTD forms.
Table 3.
Standardized neuropsychological test scores (z-scores) for the three groups
| GRN-bvFTD (n = 18) | bvFTD (n = 18) | AD (n = 18) | P-value | Effect size (ŋ2) | |
|---|---|---|---|---|---|
| MMSE | –4.72±1.96 | –4.61±1.38 | –4.43±1.70 | 0.729 | 0.002 |
| MoCA | –3.15±1.44 | –3.55±1.12 | –3.07±1.22 | 0.098 | 0.129 |
| MoCA (EF) | –0.60±0.87 | –0.47±0.92 | –0.35±1.05 | 0.327 | 0.089 |
| MoCA (MEM) | –0.15±0.87 | –0.14±0.86 | –0.33±1.09 | 0.700 | 0.059 |
| MoCA (LANG) | –0.28±1.10 | –0.18±0.88 | –0.32±1.13 | 0.117 | 0.17 |
| MoCA (VSP) | –0.25±0.81 | –0.20±1.11 | –0.28±1.05 | 0.068 | 0.333 |
| MoCA (AT) | 0.30±1.35 | 0.20±0.73 | 0.41±1.06 | 0.501 | 0.136 |
| MoCA (OR) | 0.24±1.20 | 0.41±0.43 | 0.18±1.16 | 0.087 | 0.349 |
| Cancellation task | –0.21±0.87 | –0.64±0.17 | –0.28±0.68 | 0.105 | 0.019 |
| Digit Span | –2.22±1.00 | –1.63±1.07 | –0.44±0.10 | 0.035 | 0.227 |
| Semantic fluency | –2.55±1.00 | –2.47±1.63 | –2.53±1.05 | 0.556 | 0.001 |
| Motor initiative | –1.27±0.87 | –1.33±0.56 | 0.15±0.80 | 0.022 | 0.171 |
| Graphomotor initiative | –1.57±0.79 | –2.38±0.46 | 0.10±0.66 | 0.019 | 0.208 |
| Proverbs | –3.53±1.51 | –2.87±1.66 | –0.14±0.50 | < 0.001 | 0.302 |
| RPCM ab series | –2.52±1.43 | –2.87±1.54 | –1.86±1.01 | 0.324 | 0.062 |
| Naming | –0.87±0.45 | –0.81±0.34 | –0.99±0.22 | 0.112 | 0.043 |
| Repetition | 0.23±1.00 | 0.35±0.87 | 0.36±0.56 | 0.666 | 0.018 |
| Token Test | –1.53±0.98 | –0.66±1.44 | –0.81±0.45 | 0.099 | 0.073 |
| Word recall | –3.46±1.50 | –1.96±0.23 | –2.99±1.57 | 0.096 | 0.088 |
| Semantic memory | –3.19±1.23 | –3.47±1.68 | –2.08±1.45 | 0.677 | 0.037 |
| Verbal Paired-Associate Learning (IR) | –2.40±1.30 | –2.35±3.25 | –3.01±1.00 | 0.398 | 0.047 |
| Logical Memory (IR) | –2.07±1.15 | –2.13±0.54 | –2.66±1.38 | 0.024 | 0.281 |
| Visual Memory (IR) | –1.26±0.52 | 0.64±0.92 | –1.17±0.98 | 0.611 | 0.081 |
| Logical Memory (DR) | –1.75±0.50 | –1.01±1.15 | –3.68±0.44 | 0.006 | 0.240 |
| Basic Written Calculus | –0.95±0.22 | –0.81±0.44 | –0.90±0.45 | 0.687 | 0.001 |
| Cube copy | –1.47±0.98 | –1.02±0.87 | –1.24±0.67 | < 0.001 | 0.444 |
| Orientation | –1.10±0.22 | –0.99±0.37 | –1.23±0.67 | 0.976 | 0.054 |
Data of variables is presented as the mean ± SD. Statistically significant differences between the groups are presented in bold. AD: Alzheimer’s disease; AT: attention; bvFTD: behavioral variant frontotemporal dementia; DR: delayed recall; EF: executive functions; GRN-bvFTD: progranulin associated bvFTD; IR: Immediate Recall; LANG: Language; MEM: Memory; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; OR: orientation; RCPM: Raven’s Coloured Progressive Matrices; VSP: Visuospatial. Group comparisons were performed through non-parametric Kruskal-Wallis analysis.
A similar post hoc analysis was performed between GRN-bvFTD and AD patients, with statistically significant differences found for frontal measures and drawing: GRN patients are worse than AD in Digit Span (working memory/attention) (P = 0.003, ŋ2 = 0.227), Proverbs (verbal abstract reasoning) (P < 0.001, ŋ2 = 0.342) and in Cube copy (P = 0.020, ŋ2 = 0.329). AD patients presented a significantly lower performance in memory tests, specifically Logical Memory immediate recall (P = 0.003, ŋ2 = 0.281) and delayed recall (P = 0.002, ŋ2 = 0.240).
Regarding the differences between sporadic-bvFTD and AD patients in post hoc Bonferroni analysis, the first group presented a worst performance in frontal measures such as graphomotor initiative (flexibility and psychomotor control) (P = 0.005, ŋ2 = 0.109) and Proverbs (abstract verbal reasoning) (P = 0.001, ŋ2 = 0.284). On the other hand, AD patients performed significantly worst in Logical Memory delayed recall (P = 0.031, ŋ2 = 0.220) and Cube Copy (P = 0.027, ŋ2 = 0.330).
Next, we analysed the existence of differences between the groups in NP domains through Kruskal-Wallis non-parametric analysis, by averaging individual test z-scores within that domain. Results are presented in Table 4. In this analysis a higher score indicates a greater deviation from the norm and consequently a larger impairment.
Table 4.
Differences between groups on standardized neuropsychological domain scores (domain composite z-scores)
| Domains | GRN-bvFTD (n = 18) | bvFTD (n = 18) | AD (n = 18) | P-value | Effect size (ŋ2) |
|---|---|---|---|---|---|
| Attention/Executive Functions/ Initiative/Psychomotor Control | –1.89±0.70 | –1.64±1.01 | –0.99±0.45 | 0.035 | 0.119 |
| Language | –0.81±0.23 | –0.97±0.15 | –0.50±0.34 | 0.107 | 0.015 |
| Memory | –2.22±1.31 | –2.28±1.02 | –2.69±1.00 | 0.021 | 0.16 |
| Calculus | –0.95±0.22 | –0.81±0.44 | –0.90±0.45 | 0.533 | 0.001 |
| Visuoconstructive/Perceptive abilities | –1.47±0.98 | –1.02±0.87 | –1.24±0.67 | 0.032 | 0.444 |
| Orientation | –1.10±0.22 | –0.99±0.37 | –1.23±0.67 | 0.437 | 0.054 |
Data are presented as the mean ± SD. Statistically significant differences between the groups are presented in bold. AD: Alzheimer’s disease; bvFTD: behavioral variant frontotemporal dementia; GRN: progranulin mutation carriers. Group comparisons were performed through non-parametric Kruskal-Wallis analysis.
Bonferroni post hoc analysis showed statistically significant differences between GRN-bvFTD patients and sporadic-bvFTD forms in Memory (P = 0.039, ŋ2 = 0.110) and Visuoconstructive/Perceptive abilities (P = 0.039, ŋ2 = 0.190), with GRN-bvFTD patients presenting higher impairment. The same analysis was performed between GRN-bvFTD and AD, where differences between groups were found in Attention/Executive Functions/Initiative/Psychomotor Control (P = 0.001, ŋ2 = 0.211), Memory (P = 0.020, ŋ2 = 0.120) and Visuoconstructive/Perceptive abilities (P = 0.041, ŋ2 = 0.129) domains, with GRN-bvFTD patients being worse in EF and VSP domains, and better in memory tasks. Regarding differences between groups between sporadic-bvFTD and AD, post hoc analysis showed statistically significant differences in Attention/Executive Functions/Initiative/Psychomotor Control (P = 0.032, ŋ2 = 0.200), Memory (P = 0.010, ŋ2 = 0.131) and Visuoconstructive/Perceptive abilities domain (P = 0.023, ŋ2 = 0.231). We observed that FTD sporadic forms are worse than AD in EF and better in Memory and VSP tasks.
The main differences were between GRN-bvFTD versus AD as well as sporadic-bvFTD vs. AD patients with both FTD-groups being worse in EF and AD-patients in memory tasks. The constructive task was the more discriminative with the larger effect size (ŋ2 = 0.444), showing a profile where sporadic-bvFTD, AD and GRN present an increasing level of impairment, respectively.
Discussion
This study describes the NP profile of 18 GRN-bvFTD patients from fifteen different families, exhibiting four different GRN mutations. It reveals measurable group differences between GRN-bvFTD, sporadic-bvFTD and AD patients on standardized NP measures, underscoring the utmost importance of a comprehensive NP assessment on the identification of early stages of FTD due to GRN mutations. Prior studies have already looked for the cognitive profile of GRN mutation carriers (Le Ber et al., 2008; Moreno et al., 2009; Hallam et al., 2014), where some degree of frontal, temporal and parietal dysfunction was found on the majority of patients. Here we focused on milder forms of dementia (MMSE ≥ 17 and CDR ≤ 1).
Our results show a more severe pattern of executive dysfunction mainly due to impaired performances in attention/working memory, flexibility, abstract reasoning and psychomotor control in GRN patients when compared to sporadic-bvFTD forms and AD. But in contrast to what was previously described (Mackenzie et al., 2006; Heuer et al., 2020), in our sample the Digit Span (forward task) was not able to distinguish GRN patients from sporadic-bvFTD forms as GRN-bvFTD patients obtained lower scores not reaching statistical significance. Regarding the comparisons between sporadic-bvFTD and AD, the first ones also presented a higher level of executive dysfunction, mainly due to an impaired pattern in abstract reasoning, psychomotor control and flexibility. Similar results were found on a previous study from Stopford, Thompson, Neary, Richardson and Snowden (2012). Focusing on the memory profile, it was recently reported that sporadic-bvFTD patients perform in an intermediate level between controls and AD patients. Together with this, was purposed that sporadic-bvFTD and AD patients do not differ in free recall measures, but those with FTD would significantly benefit from cueing or recognition tasks (Poos et al., 2018). When we compared sporadic-bvFTD with AD patients, we found a pattern similar to the above-described. Both groups had impaired learning performances but AD patients presented with more severe retrieval deficits (not benefiting from cues or recognition tasks). One possible explanation for the presence of encoding deficits in sporadic-bvFTD patients is the probable development of disrupted attentional and executive control processes leading to difficulties in learning mechanisms (Glosser et al., 2002). On the other hand, GRN-bvFTD patients presented with a transitional performance between sporadic-bvFTD and AD. They are better than AD on measures of both immediate and delayed recall but worse than sporadic-bvFTD. Le Ber et al. (2008) and Snowden et al. (2006) have already found that at initial stages, GRN patients can present with episodic memory impairment that resembles AD. One possible reason is the high expression of GRN in hippocampus in which marked atrophy is frequently observed. Our results suggested this same pattern, with the majority of GRN patients revealing some degree of hippocampal dysfunction (worse performances on delayed recall when compared to immediate recall), being, however, less severe than in AD. Nevertheless, five GRN patients presented with a frontal type memory dysfunction with equal and/or better performances in delayed recall (retrieval) as compared with those from immediate recall (learning).
Regarding visuospatial abilities, AD patients showed a pattern of primary visuospatial deficits presenting with lack of elements, closing in and gestalt changes. In sporadic-bvFTD patients, a pattern suggesting frontal dysfunction was dominant, with perseveration, lack of planning and inattention being the more frequent features. In GRN-bvFTD patients, it is important to highlight that these patients exhibit a visuospatial pattern of performance that includes features of both sporadic-bvFTD and AD patients. In this specific task (cube copy), GRN-bvFTD patients presented with lack of elements and gestalt changes (features from AD) but also with perseveration and lack of planning (sporadic-bvFTD features).
Concerning language skills, no pattern of dysfunction was found in any of the three groups, consequently not allowing the distinguishing between them. This was not a surprising result since FTD-related aphasic variants as well as AD-logopenic patients were excluded from our study sample.
Spatial, temporal and social orientation were also assessed, however, no differences between groups were found. Previous studies showed contrasting results: while some authors found a clear distinct pattern between AD and sporadic-bvFTD, with AD patients presenting with more impaired results (Yew et al., 2013), other found with no differences between these groups (Ramanan et al., 2017). Similarly, the capacity of performing basic written calculus was also evaluated but no differences were found between the three groups.
Our study has some limitations. First, our sample size is relatively small and from only one center, despite being one of the largest samples where GRN-bvFTD NP profile have been evaluated. Second, we did not include non-fluent primary progressive aphasia related GRN patients since we had a very small number of patients in this specific FTD-variant. Although these limitations may reduce generalizability, the highly homogeneous constitution of the groups reduces the effect of confounding variables and allows the study of peculiar characteristics to each group. In addition, as the evaluation is standardized and done before the genetic studies, both examiners and participants were always blinded for genetic status, preventing possible interpretation biases.
Conclusion
We focused on the cognitive profile of milder forms of GRN-bvFTD associated dementia between GRN-bvFTD vs. sporadic-bvFTD and AD patients, helping to clarify the natural history of the disease and providing some important NP markers which may help to distinguish between groups. Regarding the overall cognitive profile of GRN-bvFTD patients, we found a global pattern of moderate-to-severe frontotemporoparietal deficits, corroborating Moreno et al. (2009), who purposed the term “frontotemporoparietal” dementia for characterizing GRN patients, due to parietal lobe deficits which are uncommon in other FTD forms. In contrast, in sporadic-bvFTD patients the main deficits are related to frontotemporal areas, while in AD the impaired performances are mainly associated with temporoparietal areas. Visuoconstructive task was the more discriminant feature between the groups, followed by episodic verbal memory - particularly delayed recall. The reported findings have major theoretical and clinical implications for the distinguishing between sporadic-bvFTD and AD from GRN-bvFTD patients, helping to prevent misdiagnosed cases. Future research includes the combination of these findings with behavioral assessment data aiming a full characterization of the disease course since innovative mutation-target treatments are already available.
Additional files:
Additional file 1 (194KB, pdf) : Ethical Approval Documentation (Portuguese).
Additional file 2 (271.1KB, pdf) : Model consent form (Portuguese).
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: The authors declares no conflict of interest.
Financial support: ML was supported by Portuguese Foundation for Science and Technology (FCT), No. SFRH/BD/144001/2019.
Institutional review board statement: This study was reviewed and approved by the Institutional Ethics Committee of Centro Hospitalar e Universitário de Coimbra, Portugal (CE-029/2019) on June 24, 2019.
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Coimbra’s University and Hospital Center in Portugal.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Data is only available by request to the corresponding author and depending on the idea behind the project and the aims and methods of the requirement.
Plagiarism check: Checked twice by iThenticate.
Funding: ML was supported by Portuguese Foundation for Science and Technology (FCT), No. SFRH/BD/144001/2019.
Peer review: Externally peer reviewed.
References
- 1.Almeida MR, Baldeiras I, Ribeiro MH, Santiago B, Machado C, Massano J, Guimarães J, Oliveira CR, Santana I. Progranulin peripheral levels as a screening tool for the identification of subjects with progranulin mutations in a Portuguese cohort. Neurodegener Dis. 2014;13:214–223. doi: 10.1159/000352022. [DOI] [PubMed] [Google Scholar]
- 2.Freitas S, Simões MR, Alves L, Santana I. Montreal Cognitive Assessment (MoCA): normative study for the Portuguese population. J Clin Exp Neuropsychol. 2011;33:989–996. doi: 10.1080/13803395.2011.589374. [DOI] [PubMed] [Google Scholar]
- 3.Freitas S, Simões MR, Alves L, Santana I. The relevance of sociodemographic and health variables on MMSE normative data. Appl Neuropsychol Adult. 2015;22:311–319. doi: 10.1080/23279095.2014.926455. [DOI] [PubMed] [Google Scholar]
- 4.Glosser G, Gallo JL, Clark CM, Grossman M. Memory encoding and retrieval in frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2002;16:190–196. [PubMed] [Google Scholar]
- 5.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerreiro M. Unpublished doctoral dissertation. Lisbon, Portugal: Faculty of Medicine; 1998. Contribution of Neuropsychology for the study of dementia. [Google Scholar]
- 7.Guerreiro R, Gibbons E, Tábuas-Pereira M, Kun-Rodrigues C, Santo GC, Bras J. Genetic architecture of common non-Alzheimer’s disease dementias. Neurobiol Dis. 2020 doi: 10.1016/j.nbd.2020.104946. doi: 101016/jnbd2020104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallam BJ, Jacova C, Hsiung GYR, Wittenberg D, Sengdy P, Bouchard-Kerr P, Slack P, Rademakers R, Baker M, Chow TW, Levine B, Feldman HH, Mackenzie IR. Early neuropsychological characteristics of progranulin mutation carriers. J Int Neuropsychol Soc. 2014;20:694–703. doi: 10.1017/S1355617714000551. [DOI] [PubMed] [Google Scholar]
- 9.Heuer HW, Wang P, Rascovsky K, Wolf A, Appleby B, Bove J, Bordelon Y, Brannelly P, Brushaber DE, Caso C, Coppola G, Dickerson B, Dickinson S, Domoto-Reilly K, Faber K, Ferrall J, Fields J, Fishman A, Fong J, Foroud T, et al. Comparison of sporadic and familial behavioral variant frontotemporal dementia (FTD) in a North American cohort. Alzheimers Dement. 2020;16:60–70. doi: 10.1002/alz.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogan DB, Jetté N, Fiest KM, Roberts JI, Pearson D, Smith EE, Roach P, Kirk A, Pringsheim T, Maxwell CJ. The Prevalence and Incidence of Frontotemporal Dementia: a Systematic Review. Can J Neurol Sci 43 Suppl. 2016;1:S96–109. doi: 10.1017/cjn.2016.25. [DOI] [PubMed] [Google Scholar]
- 11.Hosokawa M, Tetsuaki A. Progranulin and Frontotemporal Lobar Degeneration. In: Hara H, Hosokawa M, Nakamura S, Shimohata T, Nishihara M, editors. Progranulin and central nervous system disorders. Singapore: Springer; 2019. pp. 35–69. [Google Scholar]
- 12.Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 13.Kelley BJ, Haidar W, Boeve BF, Baker M, Graff-Radford NR, Krefft T, Frank AR, Jack CR, Jr, Shiung M, Knopman DS, Josephs KA, Parashos SA, Rademakers R, Hutton M, Pickering-Brown S, Adamson J, Kuntz KM, Dickson DW, Parisi JE, Smith GE, et al. Prominent phenotypic variability associated with mutations in Progranulin. Neurobiol Aging. 2009;30:739–751. doi: 10.1016/j.neurobiolaging.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Ber I, Camuzat A, Hannequin D, Pasquier F, Guedj E, Rovelet-Lecrux A, Hahn-Barma V, van der Zee J, Clot F, Bakchine S, Puel M, Ghanim M, Lacomblez L, Mikol J, Deramecourt V, Lejeune P, de la Sayette V, Belliard S, Vercelletto M, Meyrignac C, et al. (2008) Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 131(;t 3):732–746. doi: 10.1093/brain/awn012. [DOI] [PubMed] [Google Scholar]
- 15.Mackenzie IR, Baker M, West G, Woulfe J, Qadi N, Gass J, Cannon A, Adamson J, Feldman H, Lindholm C, Melquist S, Pettman R, Sadovnick AD, Dwosh E, Whiteheart SW, Hutton M, Pickering-Brown SM. A family with tau-negative frontotemporal dementia and neuronal intranuclear inclusions linked to chromosome 17. Brain. 2006;129:853–867. doi: 10.1093/brain/awh724. [DOI] [PubMed] [Google Scholar]
- 16.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno F, Indakoetxea B, Barandiaran M, Alzualde A, Gabilondo A, Estanga A, Ruiz J, Ruibal M, Bergareche A, Martí-Massó JF, de Munain AL. “Frontotemporoparietal” dementia: clinical phenotype associated with the c. 709-1G> A PGRN mutation. Neurology. 2009;73:1367–1374. doi: 10.1212/WNL.0b013e3181bd82a7. [DOI] [PubMed] [Google Scholar]
- 18.Poos JM, Jiskoot LC, Papma JM, van Swieten JC, van den Berg E. Meta-analytic review of memory impairment in behavioral variant frontotemporal dementia. J Int Neuropsychol Soc. 2018;24:593–605. doi: 10.1017/S1355617718000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanan S, Bertoux M, Flanagan E, Irish M, Piguet O, Hodges JR, Hornberger M. Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer’s disease. J Int Neuropsychol Soc. 2017;23:34–43. doi: 10.1017/S1355617716000837. [DOI] [PubMed] [Google Scholar]
- 20.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, et al. (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 134(;t 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohlfing FW, Tu RK. Genetics of frontotemporal dementia. AJNR Am J Neuroradiol. 2017;38:10–11. doi: 10.3174/ajnr.A4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowden JS, Pickering-Brown SM, Mackenzie IR, Richardson AM, Varma A, Neary D, Mann DM. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129(pt 11):3091–3102. doi: 10.1093/brain/awl267. [DOI] [PubMed] [Google Scholar]
- 23.Stopford CL, Thompson JC, Neary D, Richardson AM, Snowden JS. Working memory, attention, and executive function in Alzheimer’s disease and frontotemporal dementia. Cortex. 2012;48:429–446. doi: 10.1016/j.cortex.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Tatton N. FTD Research and Drug Development Landscape. The Association for Frontotemporal Degeneration. 2014. [Accessed February 1, 2020]. https://wwwtheaftdorg/wpcontent/uploads/2014/05/FTD-Research-and-Drug-Development-Landscapepdf .
- 25.Wechsler D. San Antonio, USA: Psychological Corporation; 1945. Wechsler Memory Scale. [Google Scholar]
- 26.Yew B, Alladi S, Shailaja M, Hodges JR, Hornberger M. Lost and forgotten. Orientation versus memory in Alzheimer’s disease and frontotemporal dementia. J Alzheimers Dis. 2013;33:473–481. doi: 10.3233/JAD-2012-120769. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


