Keywords: ATP, cytoskeleton, injury, neuron, pannexin 1, peripheral nerve, Ras homolog family member A, Schwann cells
Abstract
Pannexin 1 (Panx 1), as a large-pore membrane channel, is highly permeable to ATP and other signaling molecules. Previous studies have demonstrated the expression of Panx 1 in the nervous system, including astrocytes, microglia, and neurons. However, the distribution and function of Panx 1 in the peripheral nervous system are not clear. Blocking the function of Panx 1 pharmacologically (carbenoxolone and probenecid) or with small interfering RNA targeting pannexins can greatly reduce hypotonicity-induced ATP release. Treatment of Schwann cells with a Ras homolog family member (Rho) GTPase inhibitor and small interfering RNA targeting Rho or cytoskeleton disrupting agents, such as nocodazole or cytochalasin D, revealed that hypotonicity-induced ATP release depended on intracellular RhoA and the cytoskeleton. These findings suggest that Panx 1 participates in ATP release in Schwann cells by regulating RhoA and the cytoskeleton arrangement. This study was approved by the Animal Ethics Committee of Nantong University, China (No. S20180806-002) on August 5, 2018.
Chinese Library Classification No. R446; R741; Q493.8
Introduction
Schwann cells not only act as physical support cells for axons but also release multiple signaling molecules that interact with long axons. As the first response after sciatic nerve injury, these cells act by dedifferentiation, proliferation, and release of abundant signaling molecules to modulate nerve injury repair (Su et al., 2019; Wei et al., 2019). Among these signaling molecules, extracellular ATP can be released from Schwann cells as well as peripheral neurons (Fields and Stevens, 2000) and contributes to their communication (Lyons et al., 1995; Negro et al., 2016). For example, ATP release in Schwann cells is induced by glutamate (Liu and Bennett, 2003) or uridine triphosphate (Liu et al., 2005), and purinergic receptors are expressed in several peripheral nervous system cell types that mediate neuron-Schwann cell signaling transmission, which contributes to a variety of functions. ATP, as a key signaling molecule, mediates peripheral sensitization after sciatic nerve injury (Tsuda et al., 2010). Evidence for ATP release in Schwann cells is primarily focused on formation and delivery of intracellular vesicles (Shin et al., 2012). However, regulation of ATP release in Schwann cells is still incompletely understood.
Pannexins (Panx) are large-pore membrane channels that are highly permeable to ATP and other signaling molecules. The Panx family mainly consists of three members: Panx 1, Panx 2, and Panx 3 (Baranova et al., 2004). Among these, Panx 1 is the best characterized and is nearly ubiquitously expressed in neurons and glial cells (Baranova et al., 2004). In microglia (Mousseau et al., 2018), astrocytes (Iglesias et al., 2009; Suadicani et al., 2012), and the dorsal root ganglion (Zhang et al., 2015), Panx 1 has been reported to mediate ATP release and other regulated functions. Nevertheless, the distribution of Panx 1 in Schwann cells and its role in regulating ATP release remain unclear.
Cell swelling is an early event that occurs under pathophysiological conditions in response to various types of injury, such as trauma, ischemia, and stroke (Vardjan et al., 2016; Begum et al., 2018; He et al., 2019; Yang et al., 2020). Changes in cellular hydration are an important factor in regulating cellular function (Häussinger, 1996; Lang et al., 1998). Moreover, hypotonic challenge is a common stimulus that promotes ATP release in airway epithelia (Seminario-Vidal et al., 2011). Ras homolog family member A (RhoA) is an important signaling molecule in Schwann cells for processes including proliferation, migration, and myelination (Wen et al., 2017; Tan et al., 2018). Moreover, Rho GTPases are key signaling molecules for ATP release from epithelial cells (Seminario-Vidal et al., 2011). Thus, we proposed that Panx 1-mediated ATP release is regulated by Rho signaling. Panx 1 delivery to the cell surface is dependent on cytoskeletal interactions (Bhalla-Gehi et al., 2010; Boyce et al., 2015). Additionally, numerous studies have shown that organelle organization, membrane trafficking, and exocytosis are often closely associated with cytoskeletal organization (Bhalla-Gehi et al., 2010; Jaqaman and Grinstein, 2012). According to a previous study using Panx 1-green fluorescent protein to assess its trafficking and interaction with the cytoskeletal network, Panx 1 closely interacts with actin microfilaments (Bhalla-Gehi et al., 2010). In this study, we explored the distribution and function of Panx 1 in Schwann cells and determined whether RhoA and the cytoskeleton are involved in its mechanism.
Materials and Methods
Ethics statement
Postnatal day 1–3 CD-1 mice (for primary Schwann cell cultures) and adult male mice (aged 6–8 weeks, specific-pathogen-free level) (for western blot assays) were provided by the Experimental Animal Center of Nantong University (SYXK (Su) 2015-0016, SYXK (Su) 2017-0046). Animal procedures were approved by the Animal Ethics Committee of Nantong University, China (No. S20180806-002) on August 5, 2018.
Cell culture, transfection, and incubation
Primary Schwann cell cultures were produced as previously described (Su et al., 2019). Briefly, cells were isolated from the sciatic nerves of newborn mice and plated on 100 μg/mL poly-D-lysine-coated 35 cm2 dishes in Dulbecco’s modified Eagle medium (Sigma-Aldrich, St. Louis, MO, USA) containing 10% horse serum (Sigma-Aldrich). The medium was replaced with Dulbecco’s modified Eagle medium/10% horse serum supplemented with 0.5 μM forskolin (Sigma-Aldrich) and 1 ng/mL heregulin β-1 (Peprotech, Rocky Hill, NJ, USA) every 3 days. Within 10 days, Schwann cells were purified with 0.25% trypsin (Sigma-Aldrich) for approximately 10 seconds to obtain > 95% pure Schwann cells, and a majority of fibroblasts still adhered to the culture dish, as determined by immunocytochemistry (Su et al., 2016). RSC96 and Hepa1-6 cells were purchased from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China) and were maintained in Dulbecco’s modified Eagle medium/10% fetal bovine serum (Gibco, Carlsbad, CA, USA).
Small interfering RNA (siRNA) sequences were transferred into cells using RNAiMAX (Invitrogen, Carlsbad, CA, USA) at 2 or 3 days post-plating, and scrambled siRNA was used as a control. The RhoA siRNA, Panx 1 siRNA, and a universal negative control siRNA sequences were provided by Biomics Biotechnologies Co., Ltd. (Nantong, Jiangsu Province, China).
The cells were rinsed twice with isotonic solution containing (mM): 140 NaCl, 1 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES (Sigma-Aldrich), and 10 glucose (Sigma-Aldrich) (pH 7.2–7.4, 290 mOsm) and pre-incubated as indicated in the following assay solutions (Liu et al., 2008). Hypotonic solutions (pH 7.2–7.4, 200 mOsm) were composed of a HEPES buffered solution containing (mM): 1 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.2–7.4, 20 mOsm) (Liu et al., 2008). Inhibitors including carbenoxolone (CBX; Sigma-Aldrich), probenecid (Sigma-Aldrich), C3 transferase (Cytoskeleton, Denver, CO, USA), nocodazole (Sigma-Aldrich), and cytochalasin D (Sigma-Aldrich) dissolved in dimethyl sulfoxide (DMSO) were incubated in buffer immediately before use. Vehicle solutions were mixed with DMSO at the same volume in hypotonic solutions. DMSO has no effect when incubated at a low concentration (< 0.1%).
Quantitative real-time polymerase chain reaction
RNA obtained from Schwann cells was isolated using TRIzol/chloroform (Invitrogen), and complementary DNA was synthesized with a Prime-Script RT reagent Kit (TaKaRa, Dalian, Liaoning Province, China) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (RT-PCR) assays were conducted with SYBR Green and a Step-one RT-PCR system (Applied Biosystems, Foster City, CA, USA). The procedure was as follows: 95°C for 10 minutes and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The levels of target mRNA were normalized to the endogenous control glyceraldehyde 3-phosphate dehydrogenase by the 2–ΔΔCT method. Quantitative RT-PCR was used to identify the best siRNA sequence for knocking down the expression of target genes. All reactions were performed in triplicate. All primers and siRNAs used in this experiment are listed in Tables 1 and 2.
Table 1.
Quantitative real-time polymerase chain reaction primers
| Gene | Sense sequence (5'–3') | Anti-sense sequence (5'–3') |
|---|---|---|
| Panx 1 | CCT CAT TAA CCT CAT TGT GTA T | CAT TGT AGC CTT CAG ACT TG |
| Panx 2 | TGT GGT CTA TAC TCG CTA TG | CTC CTG CTG GAT GTC TAG |
| Panx 3 | CTC AGA TTA TGG ACT ATG AAC AC | TCA GAA GGT AAC TTG GAG AAT |
| RhoA | TGC TTG CTC ATA GTC TTC A | GGC GGT CAT AAT CTT CCT |
Panx: Pannexin; RhoA: Ras homolog family member A.
Table 2.
Related siRNA sequences
| siRNA | Sequence (5'–3') |
|---|---|
| Panx 1 siRNA1 | GGC UGC CUU UGU GGA UUC Att |
| Panx 1 siRNA2 | GGA ACU UGA CAA AGU CUA Ctt |
| Panx 1 siRNA3 | GGU GCU GGA GAA CAU UAA Att |
| RhoA siRNA1 | Sense: CAG GUA GAG UUG GCU UUA UdTdT |
| Anti-sense: AUA AAG CCA ACU CUA CCU GdTdT | |
| RhoA siRNA2 | Sense: CAC UGA UGU UAU ACU GAU GdTdT |
| Anti-sense: CAU CAG UAU AAC AUC AGU GdTdT | |
| RhoA siRNA3 | Sense: GUC AAG CAU UUC UGU CCA AdTdT |
| Anti-sense: UUG GAC AGA AAU GCU UGA CdTdT |
Panx 1: Pannexin 1; RhoA: Ras homolog family member A; siRNA: small interfering RNA.
Immunofluorescence
The procedure was conducted as previously described (Su et al., 2019). Briefly, after fixing with 4% paraformaldehyde for 12 minutes, Schwann cells were permeabilized with 0.1% Triton X-100 for 10 minutes and blocked in 10% bovine serum albumin for 1 hour. Cells were processed for immunofluorescence with the following primary antibodies overnight at 4°C: S100β (mouse; 1:500; Sigma) and Panx 1 (rabbit; 1:200; Santa Cruz Biotechnology, Dallas, TX, USA). After washing, the cells were incubated with the following fluorescent secondary antibodies for 1 hour at room temperature: anti-mouse Alex-488 and anti-rabbit Cy3 (1:1000; Jackson ImmunoResearch, West Grove, PA, USA). In the negative control, primary antibodies were replaced with phosphate buffered saline, and the other procedures were the same as above. Cells were imaged with a Nikon fluorescence microscope (Tokyo, Japan).
Western blot assay
Proteins were extracted from adult mouse brains and cultured Schwann cells with a radioimmunoprecipitation assay lysis buffer containing complete protease inhibitors (1:100; Roche, Basel, Switzerland) and 1 mM phenylmethylsulfonyl fluoride and measured with a bicinchoninic acid protein assay kit (Beyotime, Haimen, China). Isolated proteins (20 µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis after boiling for 10 minutes at 95°C and then transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking in 5% (w/v) nonfat instant milk in Tris-buffered saline containing 0.1% Tween-20 for 2 hours, the membranes were probed with the following antibodies overnight at 4°C: Panx 1 (1:200; rabbit; Santa Cruz Biotechnology), RhoA (1:300; mouse; Abcam, Cambridge, MA, USA), glyceraldehyde 3-phosphate dehydrogenase (1:1000; mouse, Proteintech, Chicago, IL, USA), and β-actin (1:1000; mouse; Sigma). Next, the blots were incubated with an anti-mouse/rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000; Jackson Immuno Research, West Grove, PA, USA) for 1 hour at room temperature and visualized with an enhanced chemiluminescence solution (Thermo Fisher, Waltham, MA, USA). Chemiluminescence was determined with a Bio-Rad ChemiDoc (Hercules, CA, USA) for several seconds. The intensity of bands was analyzed by ImageJ software (National Institutes of Health, Bethesda, MA, USA).
ATP and lactate dehydrogenase measurements
Extracellular ATP concentrations in Schwann cells were determined by the luciferase-luciferin test as previously reported (Zhang et al., 2007). In brief, Schwann cell medium was collected before and after treatment at different time points. The medium was incubated with an ectonucleotidase inhibitor, dipyridamole (10 M), during the entire experiment to reduce ATP hydrolysis. The sample was mixed with ATP assay mix at the same volume. Luminescence was examined using a V3.1 Sirius luminometer (Berthold Detection Systems, Baden, Germany). The luminescence of the recording medium was measured as the background. The lactate dehydrogenase (LDH) activity assay was conducted with an LDH kit (Roche) by measuring nicotinamide adenine dinucleotide oxidation with pyruvate as the substrate. The reaction product was assayed according to the absorbance at a wavelength of 490 nm using a spectrophotometer (Thermo Fisher).
Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). Statistical comparisons among groups were assessed with one-way analysis of variance and Student’s t-test using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered statistically significant.
Results
Characterization of Panx 1 expression in mouse Schwann cells
To explore the expression of Panx in Schwann cells, we examined the mRNA levels in pure cultured Schwann cells by quantitative RT-PCR. Panx 1 is highly expressed compared with other subtypes (Figure 1A). Therefore, we focused on the role of Panx 1 in our study. Western blotting and double immunofluorescence labeling detected a single band and co-localization with S100β, a specific Schwann cell marker, respectively, in pure cultured Schwann cells (Chen et al., 2012) (Figure 1B and C). Immunohistochemistry analysis showed that Panx 1 co-localized with S100β in sciatic nerves (Figure 1D). Together, these results showed that Schwann cells express Panx 1.
Figure 1.
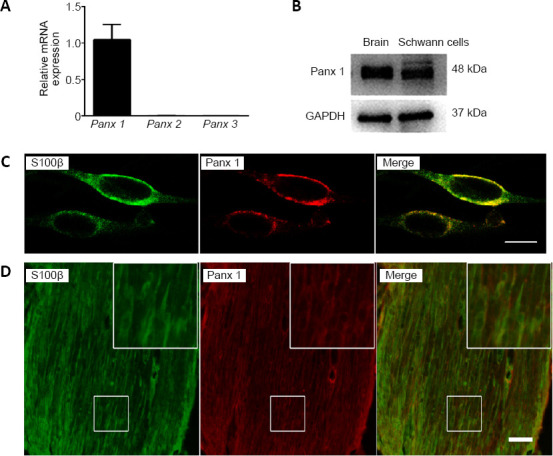
Expression of Panx 1 in Schwann cells.
(A) mRNA levels of Panx in Schwann cells. Data are expressed as the mean ± SEM (n = 4) and were analyzed by one-way analysis of variance or Student’s t-test. (B) The Panx 1 protein level in Schwann cells is shown; mouse brain tissue was used as a positive control. (C) Double immunostaining images show that Panx 1 (red, stained by Cy3) is expressed in cultured Schwann cells, as shown by the presence of S100β (green, stained by Alex-488). (D) Example images show double immunostaining with S100β (green, stained by Alex-488) and Panx 1 (red, stained by Cy3) in mouse sciatic nerves. The insets showing the boxed areas at higher magnification. Scale bars: 20 μm in C, 50 μm in D. GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; Panx: pannexin.
Hypotonic solution-induced Panx 1 activation increases ATP release
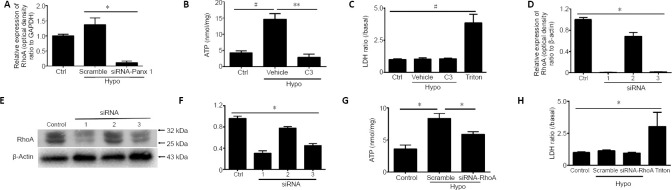
We investigated the role of Panx 1 in regulating hypotonic solution-induced ATP release. Treatment with a hypotonic solution for 2, 4, 8, or 12 minutes induced significant increases in ATP release in cultured Schwann cells (Figure 2A). After examining the increases in ATP release at different time points, we chose hypotonic solution treatment for 12 minutes for use in subsequent studies. Notably, the effect of activated Panx was abolished by pretreatment with two Panx blockers, CBX and probenecid (Karatas et al., 2013). Pretreatment with CBX (100 μM, 60 minutes) or probenecid (500 μM, 60 minutes) led to a reduction of ATP release induced by hypotonic solution in Schwann cells (Figure 2C). Because Panx 1 blockers may affect channels other than Panx 1 (Chekeni et al., 2010; Chen et al., 2014), we next used Panx 1 siRNA to knock down Panx 1 expression in Schwann cells. We initially screened and selected the most effective Panx 1 siRNAs in RSC96 cells or Schwann cells (Figure 3A–C). Surprisingly, we found that hypotonic solution could dramatically increase Panx 1 expression. However, the increase was markedly abolished after transfection with a Panx 1-specific siRNA (Figure 3D). Moreover, hypotonicity-induced ATP release was prevented with Panx 1 siRNA treatment but not with non-targeting siRNA treatment (Figure 3E).
Figure 2.
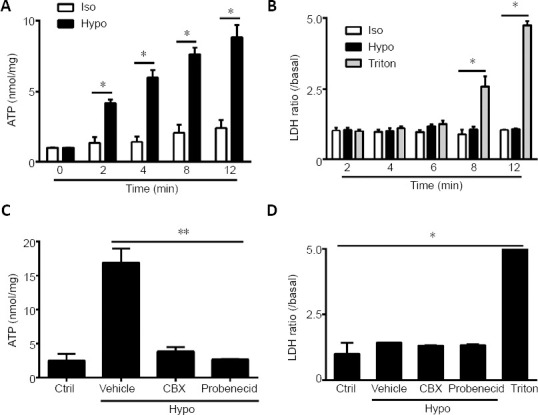
Panx 1 blockers reduced ATP release induced by hypotonic challenge in Schwann cells.
(A, B) Time course of ATP and LDH release from cultures treated in the Iso, Hypo, and Triton conditions. The Triton group was used as a positive control in the LDH assay. (C, D) ATP and LDH release were assessed under different conditions. Schwann cells were pretreated with carbenoxolone (100 μM) and probenecid (100 μM) for 60 minutes before exposure to the indicated solutions for 12 minutes. Dimethyl sulfoxide at the same volume in isotonic solution was used as a Ctrl. Data are expressed as the mean ± SEM (n = 4). *P < 0.05, **P < 0.01 (one-way analysis of variance or Student’s t-test). CBX: Carbenoxolone; Ctrl: control; Hypo: hypotonic; Iso: isotonic; LDH: lactate dehydrogenase; Vehicle: dimethyl sulfoxide.
Figure 3.
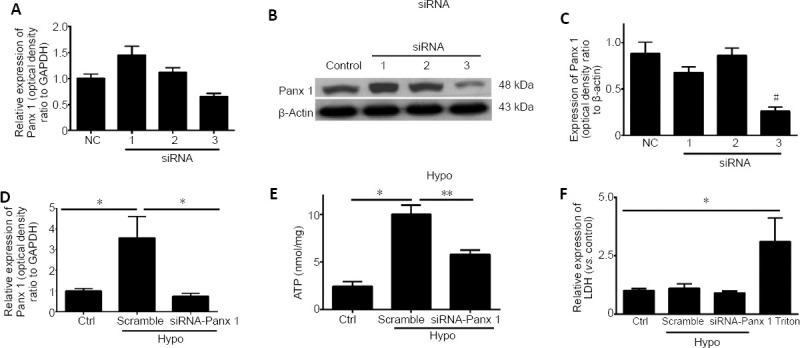
siRNA knockdown of Panx 1 attenuates hypotonic solution-induced ATP release.
(A) mRNA levels of Panx 1 in Schwann cells treated with scrambled siRNA (NC) and Panx 1-siRNA (1–3). (B, C) Panx 1 protein bands in RSC96 cells in the scrambled (control) and Panx 1-siRNA (1–3) groups. (D) The relative expression of Panx 1 in isotonic solution after transfection with scrambled siRNA (Ctrl) or in hypotonic (Hypo) solution after transfection with scrambled and siRNA-Panx 1 (#3) is shown. (E) ATP release was assessed using scrambled siRNA and Panx 1-siRNA-treated Schwann cells. (F) Lactate dehydrogenase (LDH) release was assessed under the same conditions as ATP release. Data are expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01. #P < 0.05, vs. control group (one-way analysis of variance or Student’s t-test). Panx 1: Pannexin 1; siRNA: small interfering RNA.
During the same period, we detected LDH activity in cells under different conditions. Under all conditions, except for Triton-treated cells, only low levels of LDH were detected, suggesting that ATP release was not caused by cell lysis (Chen et al., 2012) (Figures 2B, D, and 3F). Thus, the findings demonstrate that Panx 1 activation is involved in ATP release induced by hypotonic challenge.
RhoA GTPase activity regulates Panx 1-mediated ATP release
We first examined the expression of RhoA under hypotonic conditions. The results showed that hypotonicity-induced RhoA upregulation was significantly attenuated by Panx 1 knockdown via siRNA (Figure 4A), suggesting that RhoA is important in regulating Panx 1-mediated ATP release. Next, we further investigated the role of RhoA with a Rho GTPase inhibitor and Rho-siRNA. Pretreatment with C3 transferase, a potent Rho GTPase inhibitor (2 μg/mL, 1 hour) reduced ATP release induced by hypotonic solution (Figure 4B). We next utilized Hepa1-6 cells to select the most effective siRNA for knockdown of RhoA to reduce its expression in Schwann cells. The results showed that the increase in ATP release induced by hypotonic solution was abolished but not in the scrambled siRNA group (Figure 4D–G). During the same period, we also detected LDH activity in the medium of treated and untreated cells to exclude the release of ATP as a side effect resulting from cell lysis (Figure 4C and H). Taken together, we concluded that RhoA GTPase is involved in Panx 1-mediated ATP release induced by a hypotonic solution in Schwann cells.
Figure 4.
Inhibition of RhoA activation reduced hypotonic solution-induced ATP release.
(A) Relative expression of RhoA upon treatment with isotonic (Ctrl) or hypotonic (Hypo) solution after transfection with scrambled and Panx 1 siRNA (3). (B) ATP release was assessed in Schwann cells treated with C3 transferase. Dimethyl sulfoxide at the same volume in isotonic solution was used as a control (Ctrl). (C) Lactate dehydrogenase (LDH) release was assessed under the same conditions as ATP release in B. (D) RhoA mRNA levels in Schwann cells treated with scrambled siRNA (NC) and RhoA-siRNA (1–3). (E, F) Western blot images and mean analysis showing the levels of RhoA protein in Hepa 1–6 cells from the scrambled (Ctrl) and Panx 1-siRNA (1–3) groups. (G) ATP was assessed in scrambled siRNA- and RhoA-siRNA (3)-treated Schwann cells. Cells were treated with isotonic solution after transfection with scrambled siRNA as a control. (H) LDH release was assessed under the same conditions as ATP release. Data are expressed as the mean ± SEM (n = 3). *P < 0.05, **P < 0.01 (one-way analysis of variance or Student’s t-test). GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; LDH: lactate dehydrogenase; Panx 1: pannexin 1; RhoA: Ras homolog family member A; siRNA: small interfering RNA; Vehicle: dimethyl sulfoxide.
Cytoskeletal organization controls hypotonicity-induced ATP release
According to our immunostaining results in cultured Schwann cells, Panx 1 is expressed primarily on the surface of the cell membrane (Figure 1C). To evaluate the role of microtubules, cells were pretreated with the microtubule disrupting agent nocodazole (10 μM) for 2 hours before the addition of hypotonic solution for 12 minutes. The results revealed that hypotonic solution-induced ATP release was markedly attenuated in the nocodazole group (Figure 5A). Therefore, we then utilized cytochalasin D (1 μM) to disrupt actin filaments for 2 hours before hypotonic solution was added for 12 minutes. Hypotonic challenge-induced ATP release was markedly attenuated by treatment with cytochalasin D (Figure 5A). To exclude the possibility of cell lysis, an LDH assay was conducted at the same time (Figure 5B). Thus, we concluded that an intact cytoskeleton is essential for hypotonicity-induced ATP release.
Figure 5.
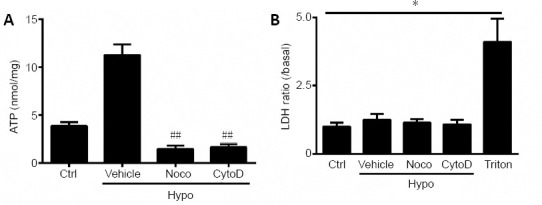
An intact cytoskeleton is required for ATP release during hypotonic challenge.
(A, B) Schwann cells were incubated with nocodazole (Noco) or cytochalasin D (CytoD) for 2 hours before treatment with hypotonic (Hypo) solutions. ATP (A) and lactate dehydrogenase (LDH) (B) release were assessed under different conditions. Dimethyl sulfoxide at the same volume in isotonic solution was used as a control (Ctrl). Data are expressed as the mean ± SEM (n = 3). *P < 0.05. ##P < 0.01 (one-way analysis of variance or Student’s t-test). LDH: Lactate dehydrogenase; Vehicle: dimethyl sulfoxide.
Discussion
ATP acts as a key regulator in the peripheral nervous system in processes such as the formation and maintenance of neuropathic pain (Burnstock, 2017; Wei et al., 2019) and the mediation of interactions of neurons and Schwann cells (Fields and Stevens, 2000). Here, our work primarily revealed the contribution of Panx 1 to the regulation of ATP release and its regulatory mechanism. We suggest that emerging evidence of mechanisms of ATP release will help to develop potential treatments for diseases.
Our study provides new information about the role of Panx 1 in regulating ATP release in Schwann cells. We first determined that Panx 1 is highly expressed in Schwann cells. Furthermore, Panx 1-mediated ATP release induced by a hypotonic stimulus is markedly reduced after treatment with Panx 1 and RhoA siRNA and cellular cytoskeleton blockers. Therefore, our findings demonstrate that Panx 1 contributes to ATP release in Schwann cells, and this process is accompanied by RhoA activation and cytoskeleton rearrangement.
It is well known that the Panx channels are highly permeable to ATP. The ATP that interacts with purinoceptors is an important inflammatory mediator released by activated cells after injury or trauma (Burnstock, 2006, 2017). Our previous study revealed that Schwann cells express several subtypes of P2X receptors (Su et al., 2019). Schwann cells can release ATP in both physiological and pathological conditions, and this released ATP further activates Schwann cells and neurons to release signaling molecules. Therefore, the role of Panx 1 in regulating ATP release is of great interest.
Membrane stretch induced by a hypotonic stimulus can result in Panx activation in several cell types and under certain conditions. In human bronchial epithelial cells, Panx 1 mediated ATP release under hypotonic conditions, which was markedly inhibited by Panx 1 RNA interference, CBX, and treatment with a Panx 1-selective blocking peptide,10 Panx 1 (Seminario-Vidal et al., 2011). Here, we showed that Panx 1 contributes to ATP release in hypotonic stress, and the effect was significantly attenuated under conditions of Panx 1 channel blockade with CBX or probenecid and siRNA knockdown of Panx 1. Connexins, such as CX43 and CX32 (De Vuyst et al., 2006), are expressed in Schwann cells and involved in ATP release (Zhao et al., 1999; Nualart-Marti et al., 2013). Moreover, we also found expression of CX43 in primary cultured Schwann cells (data not shown). Therefore, under different conditions, connexins and Panx 1 are both important for ATP release in Schwann cells.
The intercellular mechanisms that might regulate hypotonic solution-induced ATP release in Schwann cells are unknown. Hypotonic stress promotes Rho activation, as previously reported in some cells (Koyama et al., 2001; Kawamura et al., 2003; Krepinsky et al., 2003). Our study suggests that RhoA signaling mediates hypotonicity-induced ATP release. We first demonstrated that hypotonic solution induced an increase in RhoA expression, and we showed that knocking down RhoA with siRNA-RhoA or adding the potential Rho GTPase inhibitor C3 transferase markedly impaired hypotonicity-induced ATP release. These results are consistent with those of a previous study (Seminario-Vidal et al., 2011), which showed that Rho-regulated Panx 1 channel opening is involved in ATP release in airway epithelial cells.
We have not further addressed the mechanism by which RhoA regulates Panx 1-induced ATP release. However, given that RhoA is as a regulator of the cytoskeleton and facilitates membrane trafficking (Riento and Ridley, 2003; Pardo-Pastor et al., 2018), we speculate that cytoskeleton rearrangement is involved in hypotonicity-induced ATP release in Schwann cells. With pretreatment with the microtubule disrupting agent nocodazole and the potent actin polymerization inhibitor cytochalasin D, we demonstrated that the cytoskeleton is required for hypotonicity-induced ATP release. Although we did not detect any morphological change within 12 minutes after hypotonic-related solutions were applied (data not shown), the detailed mechanism of regulation in the cytoplasm still requires further investigation.
However, accumulating evidence is revealing that intracellular calcium increases when a hypotonic stimulus is applied (Boudreault and Grygorczyk, 2004; Murana et al., 2017). Channel activation and ATP release are significantly inhibited upon intracellular store depletion by thapsigargin or treatment with the calcium chelator BAPTA (Murana et al., 2017). Furthermore, ATP released by Panx 1 can increase intracellular calcium spread to the surrounding cells (Scemes and Giaume, 2006; Spray and Hanani, 2019). Overall, increased intracellular calcium may have a specific effect on hypotonicity-induced ATP release, but this still requires further investigation. In this study, we characterized the expression and function of Panx 1 in Schwann cells following hypotonic-induced ATP release. An increased Panx 1 level is an important regulator in hypotonicity-induced ATP release. Moreover, using blockers and RhoA knockdown via siRNA, we found that RhoA signaling and cytoskeletal organization regulate Panx 1-mediated ATP release. Our study provides new evidence that expression of Panx 1 in Schwann cells contributes to ATP release.
Footnotes
C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Kreiner L, Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, Nos. 31900718 (to ZYW), 31872773 (to GC); the National Key Research and Development Program of China, No. 2017YFA0104704 (to GC); Basic Research Program of the Education Department of Jiangsu Province of China, Nos. 19KJB180024 (to ZYW), 18KJB180020 (to WXS); Postdoctoral Science Foundation of China, No. 2019M651925 (to ZYW), Jiangsu Students’ Platform for Innovation and Entrepreneurship Training Program of China, No. 201810304031Z (to YJD); Six Talent Peaks Project in Jiangsu Province of China, No. WSN-007 (to WXS). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Ethics Committee of Nantong University, China (No. S20180806-002) on August 5, 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by the National Natural Science Foundation of China, Nos. 31900718 (to ZYW), 31872773 (to GC); the National Key Research and Development Program of China, No. 2017YFA0104704 (to GC); Basic Research Program of the Education Department of Jiangsu Province of China, Nos. 19KJB180024 (to ZYW), 18KJB180020 (to WXS); Postdoctoral Science Foundation of China, No. 2019M651925 (to ZYW), Jiangsu Students’ Platform for Innovation and Entrepreneurship Training Program of China, No. 201810304031Z (to YJD); Six Talent Peaks Project in Jiangsu Province of China, No. WSN-007 (to WXS).
References
- 1.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Begum G, Song S, Wang S, Zhao H, Bhuiyan MIH, Li E, Nepomuceno R, Ye Q, Sun M, Calderon MJ, Stolz DB, St Croix C, Watkins SC, Chen Y, He P, Shull GE, Sun D. Selective knockout of astrocytic Na(+) /H(+) exchanger isoform 1 reduces astrogliosis, BBB damage, infarction, and improves neurological function after ischemic stroke. Glia. 2018;66:126–144. doi: 10.1002/glia.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhalla-Gehi R, Penuela S, Churko JM, Shao Q, Laird DW. Pannexin1 and pannexin3 delivery, cell surface dynamics, and cytoskeletal interactions. J Biol Chem. 2010;285:9147–9160. doi: 10.1074/jbc.M109.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boudreault F, Grygorczyk R. Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol. 2004;561:499–513. doi: 10.1113/jphysiol.2004.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce AK, Kim MS, Wicki-Stordeur LE, Swayne LA. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem J. 2015;470:319–330. doi: 10.1042/BJ20141551. [DOI] [PubMed] [Google Scholar]
- 6.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling: therapeutic developments. Front Pharmacol. 2017;8:661. doi: 10.3389/fphar.2017.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Park CK, Xie RG, Berta T, Nedergaard M, Ji RR. Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137:2193–2209. doi: 10.1093/brain/awu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Zhang Z, Wei Z, Cheng Q, Li X, Li W, Duan S, Gu X. Lysosomal exocytosis in Schwann cells contributes to axon remyelination. Glia. 2012;60:295–305. doi: 10.1002/glia.21263. [DOI] [PubMed] [Google Scholar]
- 11.De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006;25:34–44. doi: 10.1038/sj.emboj.7600908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- 13.Häussinger D. The role of cellular hydration in the regulation of cell function. Biochem J. 1996;313:697–710. doi: 10.1042/bj3130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He HY, Ren L, Guo T, Deng YH. Neuronal autophagy aggravates microglial inflammatory injury by downregulating CX3CL1/fractalkine after ischemic stroke. Neural Regen Res. 2019;14:280–288. doi: 10.4103/1673-5374.244793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaqaman K, Grinstein S. Regulation from within: the cytoskeleton in transmembrane signaling. Trends Cell Biol. 2012;22:515–526. doi: 10.1016/j.tcb.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Koçak E, Sen ZD, Dalkara T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013;339:1092–1095. doi: 10.1126/science.1231897. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–31117. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol. 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krepinsky JC, Ingram AJ, Tang D, Wu D, Liu L, Scholey JW. Nitric oxide inhibits stretch-induced MAPK activation in mesangial cells through RhoA inactivation. J Am Soc Nephrol. 2003;14:2790–2800. doi: 10.1097/01.asn.0000094085.04161.a7. [DOI] [PubMed] [Google Scholar]
- 21.Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 22.Liu GJ, Bennett MR. ATP secretion from nerve trunks and Schwann cells mediated by glutamate. Neuroreport. 2003;14:2079–2083. doi: 10.1097/00001756-200311140-00014. [DOI] [PubMed] [Google Scholar]
- 23.Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci. 2005;21:151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- 25.Lyons SA, Morell P, McCarthy KD. Schwann cell ATP-mediated calcium increases in vitro and in situ are dependent on contact with neurons. Glia. 1995;13:27–38. doi: 10.1002/glia.440130104. [DOI] [PubMed] [Google Scholar]
- 26.Mousseau M, Burma NE, Lee KY, Leduc-Pessah H, Kwok CHT, Reid AR, O’Brien M, Sagalajev B, Stratton JA, Patrick N, Stemkowski PL, Biernaskie J, Zamponi GW, Salo P, McDougall JJ, Prescott SA, Matyas JR, Trang T. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv. 2018;4 doi: 10.1126/sciadv.aas9846. eaas9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murana E, Pagani F, Basilico B, Sundukova M, Batti L, Di Angelantonio S, Cortese B, Grimaldi A, Francioso A, Heppenstall P, Bregestovski P, Limatola C, Ragozzino D. ATP release during cell swelling activates a Ca(2+)-dependent Cl(-) current by autocrine mechanism in mouse hippocampal microglia. Sci Rep. 2017;7:4184. doi: 10.1038/s41598-017-04452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negro S, Bergamin E, Rodella U, Duregotti E, Scorzeto M, Jalink K, Montecucco C, Rigoni M. ATP released by injured neurons activates Schwann cells. Front Cell Neurosci. 2016;10:134. doi: 10.3389/fncel.2016.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nualart-Marti A, del Molino EM, Grandes X, Bahima L, Martin-Satué M, Puchal R, Fasciani I, González-Nieto D, Ziganshin B, Llobet A, Barrio LC, Solsona C. Role of connexin 32 hemichannels in the release of ATP from peripheral nerves. Glia. 2013;61:1976–1989. doi: 10.1002/glia.22568. [DOI] [PubMed] [Google Scholar]
- 30.Pardo-Pastor C, Rubio-Moscardo F, Vogel-González M, Serra SA, Afthinos A, Mrkonjic S, Destaing O, Abenza JF, Fernández-Fernández JM, Trepat X, Albiges-Rizo C, Konstantopoulos K, Valverde MA. Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci U S A. 2018;115:1925–1930. doi: 10.1073/pnas.1718177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 32.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O’Neal WK, Penuela S, Laird DW, Boucher RC, Lazarowski ER. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–26286. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin YH, Lee SJ, Jung J. Secretion of ATP from Schwann cells through lysosomal exocytosis during Wallerian degeneration. Biochem Biophys Res Commun. 2012;429:163–167. doi: 10.1016/j.bbrc.2012.10.121. [DOI] [PubMed] [Google Scholar]
- 35.Spray DC, Hanani M. Gap junctions, pannexins and pain. Neurosci Lett. 2019;695:46–52. doi: 10.1016/j.neulet.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su WF, Gu Y, Wei ZY, Shen YT, Jin ZH, Yuan Y, Gu XS, Chen G. Rab27a/Slp2-a complex is involved in Schwann cell myelination. Neural Regen Res. 2016;11:1830–1838. doi: 10.4103/1673-5374.194755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su WF, Wu F, Jin ZH, Gu Y, Chen YT, Fei Y, Chen H, Wang YX, Xing LY, Zhao YY, Yuan Y, Tang X, Chen G. Overexpression of P2X4 receptor in Schwann cells promotes motor and sensory functional recovery and remyelination via BDNF secretion after nerve injury. Glia. 2019;67:78–90. doi: 10.1002/glia.23527. [DOI] [PubMed] [Google Scholar]
- 38.Suadicani SO, Iglesias R, Wang J, Dahl G, Spray DC, Scemes E. ATP signaling is deficient in cultured Pannexin1-null mouse astrocytes. Glia. 2012;60:1106–1116. doi: 10.1002/glia.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan D, Wen J, Li L, Wang X, Qian C, Pan M, Lai M, Deng J, Hu X, Zhang H, Guo J. Inhibition of RhoA-subfamily GTPases suppresses schwann cell proliferation through regulating akt pathway rather than ROCK pathway. Front Cell Neurosci. 2018;12:437. doi: 10.3389/fncel.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuda M, Tozaki-Saitoh H, Inoue K. Pain and purinergic signaling. Brain Res Rev. 2010;63:222–232. doi: 10.1016/j.brainresrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Vardjan N, Horvat A, Anderson JE, Yu D, Croom D, Zeng X, Lužnik Z, Kreft M, Teng YD, Kirov SA, Zorec R. Adrenergic activation attenuates astrocyte swelling induced by hypotonicity and neurotrauma. Glia. 2016;64:1034–1049. doi: 10.1002/glia.22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei Z, Fei Y, Su W, Chen G. Emerging role of Schwann cells in neuropathic pain: receptors, glial mediators and myelination. Front Cell Neurosci. 2019;13:116. doi: 10.3389/fncel.2019.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen J, Qian C, Pan M, Wang X, Li Y, Lu Y, Zhou Z, Yan Q, Li L, Liu Z, Wu W, Guo J. Lentivirus-mediated RNA interference targeting RhoA slacks the migration, proliferation, and myelin formation of Schwann cells. Mol Neurobiol. 2017;54:1229–1239. doi: 10.1007/s12035-016-9733-5. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Ye G, Zhang YL, He HW, Yu BQ, Hong YM, You W, Li X. Transfer of mitochondria from mesenchymal stem cells derived from induced pluripotent stem cells attenuates hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells. Neural Regen Res. 2020;15:464–472. doi: 10.4103/1673-5374.266058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Laumet G, Chen SR, Hittelman WN, Pan HL. Pannexin-1 up-regulation in the dorsal root ganglion contributes to neuropathic pain development. J Biol Chem. 2015;290:14647–14655. doi: 10.1074/jbc.M115.650218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 47.Zhao S, Fort A, Spray DC. Characteristics of Gap junction channels in schwann cells from wild-type and connexin-null mice. Ann N Y Acad Sci. 1999;883:533–537. [PubMed] [Google Scholar]