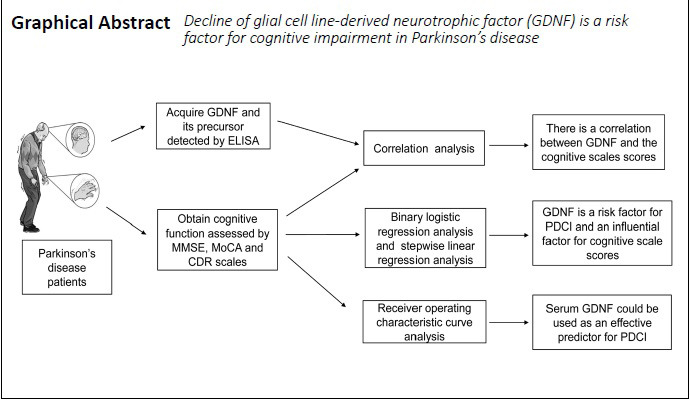
Keywords: biomarkers, cognition, factors, GDNF, neurodegenerative diseases, neurons, Parkinson's disease, risk factors
Abstract
Glial cell line-derived neurotrophic factor (GDNF) plays an important role in the protection of dopaminergic neurons, but there are few reports of the relationship between GDNF and its precursors (α-pro-GDNF and β-pro-GDNF) and cognitive impairment in Parkinson’s disease. This study aimed to investigate the relationship between the serum levels of GDNF and its precursors and cognitive impairment in Parkinson’s disease, and to assess their potential as a diagnostic marker. Fifty-three primary outpatients and hospitalized patients with Parkinson’s disease (23 men and 30 women) with an average age of 66.58 years were enrolled from the Affiliated Hospital of Xuzhou Medical University of China in this case-control study. The patients were divided into the Parkinson’s disease with cognitive impairment group (n = 27) and the Parkinson’s disease with normal cognitive function group (n = 26) based on their Mini-Mental State Examination, Montreal Cognitive Assessment, and Clinical Dementia Rating scores. In addition, 26 age- and sex-matched healthy subjects were included as the healthy control group. Results demonstrated that serum GDNF levels were significantly higher in the Parkinson’s disease with normal cognitive function group than in the other two groups. There were no significant differences in GDNF precursor levels among the three groups. Correlation analysis revealed that serum GDNF levels, GDNF/α-pro-GDNF ratios, and GDNF/β-pro-GDNF ratios were moderately or highly correlated with the Mini-Mental State Examination, Montreal Cognitive Assessment, and Clinical Dementia Rating scores. To explore the risk factors for cognitive impairment in patients with Parkinson’s disease, logistic regression analysis and stepwise linear regression analysis were performed. Both GDNF levels and Hoehn-Yahr stage were risk factors for cognitive impairment in Parkinson’s disease, and were the common influencing factors for cognitive scale scores. Neither α-pro-GDNF nor β-pro-GDNF was risk factors for cognitive impairment in Parkinson’s disease. A receiver operating characteristic curve of GDNF was generated to predict cognitive function in Parkinson’s disease (area under the curve = 0.859). This result indicates that the possibility that serum GDNF can correctly distinguish whether patients with Parkinson’s disease have cognitive impairment is 0.859. Together, these results suggest that serum GDNF may be an effective diagnostic marker for cognitive impairment in Parkinson’s disease. However, α-pro-GDNF and β-pro-GDNF are not useful for predicting cognitive impairment in this disease. This study was approved by Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, China (approval No. XYFY2017-KL047-01) on November 30, 2017.
Chinese Library Classification No. R441; R447; R741
Introduction
Many non-motor symptoms of Parkinson’s disease (PD) lead to painful experiences, which may increase overall disability rates in the patient population (Poewe et al., 2017; Goulding et al., 2019). In recent years, the non-motor symptoms of PD, and especially cognitive impairment, have received increasing attention (Calabresi et al., 2013). Approximately 30% of patients with PD have mild cognitive impairment, which can develop into dementia (Delgado-Alvarado et al., 2016). The early diagnosis of mild cognitive impairment can be difficult because of its subtle symptoms. Moreover, cognitive rating scales are based on subjective judgments and are sensitive to changes in the patient’s state. An objective and accurate marker to identify PD with cognitive impairment (PDCI) as early as possible is therefore urgently needed.
The current hypotheses regarding the pathogenesis of cognitive impairment include α-synuclein pathology (Halliday et al., 2014), tau and amyloid pathologies (Compta et al., 2011; Howlett et al., 2015), neurotransmitter disorders (Halliday et al., 2014), genetic risk (Aarsland et al., 2017), and neurotrophic factor deficiencies (Leverenz et al., 2011; Lim et al., 2016). The neurotrophic factor deficiency hypothesis suggests that neurotrophic factors play a vital role in neuronal plasticity and learning, as well as other cognitive functions. For example, it has been reported that the deterioration of cognitive function in patients with PD is related to neurotrophic factors in the cerebrospinal fluid and plasma (Leverenz et al., 2011; Lim et al., 2016).
Glial cell line-derived neurotrophic factor (GDNF) is a potent growth factor that is a member of the transforming growth factor-β superfamily. For the past few decades, our laboratory has focused on investigating the protective effects of GDNF on dopaminergic neurons. Our previous results demonstrated that GDNF can activate the PI3 kinase/Akt pathway and promote the expression of calbindin-D28K (Wang et al., 2008). GDNF can also reduce the apoptosis rate of calbindin-D28K-positive MN9D cells after treatment with 6-hydroxydopamine (Sun et al., 2011) and promote the survival of neurons in the substantia nigra pars compacta of rats after treatment with 6-hydroxydopamine (Yu et al., 2009). GDNF is also reported to be essential for maintaining the survival of dopaminergic neurons in the nigrostriatum (Cao et al., 2008). In patients with mild cognitive impairment and Alzheimer’s disease, decreased peripheral serum GDNF levels have been observed (Forlenza et al., 2015). However, it has not yet been determined whether there is a correlation between the levels of GDNF and its precursors and cognitive function in PD. Therefore, this study investigated the relationship between serum GDNF and its precursors and PDCI, to look for possible risk factors in the process of PDCI. By analyzing the relationship between serum GDNF and its precursors and PDCI, potential biomarkers for PDCI and possible intervention measures for the diagnosis of PD may be revealed.
Participants and Methods
Participants
Subject screening
In this case-control study, fifty-three primary outpatients and hospitalized patients with PD were included from the Affiliated Hospital of Xuzhou Medical University, China, from October 2017 to August 2018. The detailed demographic information, medical history, course of disease, motor symptoms, and treatment were collected and evaluated by two experienced neurologists, to ensure a diagnostic accuracy of over 90% for PD. All cognitive scales were administered when patients were in a good mental state and willing to cooperate.
Inclusion criteria for patients with PD
-
(1)
Aged ≥ 55 and ≤ 75 years old.
-
(2)
Ability to complete all neuropsychological, psychiatric, and behavioral assessments under the guidance of a physician, and to listen, speak, read and understand. This ability was estimated by the neurologist based on demographic characteristics (e.g., education level or occupation) before the test (Litvan et al., 2012).
-
(3)
A diagnosis of PD was independently made by two experienced neurologists according to the UK Brain Bank Criteria (Hughes et al., 1992), with reference to the Movement Disorder Society diagnostic criteria (Postuma et al., 2015).
Exclusion criteria for patients with PD
-
(1)
History of neurological disorders other than PD, such as craniocerebral injury, stroke, and other types of dementia, as ascertained by computed tomography or magnetic resonance imaging.
-
(2)
Drug-induced, head trauma-induced, vascular, or other secondary parkinsonism.
-
(3)
Parkinsonism-plus syndromes and other neurodegenerative diseases.
-
(4)
Severe anxiety, depression, schizophrenia, or other mental disorders.
-
(5)
Systemic diseases, including heart, liver, and renal diseases, and other diseases that may affect cognitive function.
Healthy controls
The age- and sex-matched healthy participants were recruited from the Medical Examination Center of the Affiliated Hospital of Xuzhou Medical University, and had no history of major disease and no global cognitive impairment. Subjects who failed to complete the cognitive tests or who had taken drugs that may affect cognitive function within the last month were excluded.
Ethics approval
This study was approved by Ethics Committee of the Affiliated Hospital of Xuzhou Medical University, China (approval No. XYFY2017-KL047-01) on November 30, 2017. Informed consent forms were signed by the subjects themselves.
Sample collection
Serum samples were collected on the first morning after admission. Patients fasted, including water, from 22:00 the previous day, and 5 mL of serum was collected from each patient the next morning (between 07:00 and 08:00). The samples were centrifuged at 1000 × g for 10 minutes at 4°C. Before centrifugation, the samples were allowed to remain at room temperature for up to 2 hours. To ensure that the serum components were not destroyed, they were dispensed into 500 μL Eppendorf tubes (BD Biosciences, Beijing, China) immediately after centrifugation and stored at –80°C for later assays. When serum had been collected from all subjects, GDNF and GDNF precursor (α-pro-GDNF and β-pro-GDNF) levels were measured in patients with PD and controls using enzyme-linked immunosorbent assay kits (GDNF: R&D Systems, Minneapolis, MN, USA; GDNF precursors: Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) in strict accordance with the manufacturers’ instructions.
Neuropsychological assessments
The cognitive function of each patient was evaluated in the morning on day 2 of admission, after the patient’s venous blood had been collected. A comprehensive assessment of cognitive domains was performed when patients were in a good mental state and willing to cooperate. Demographic and clinical data of PD and healthy control participants were also collected, including sex, age, education (years), and anamnesis, as well as the disease duration and Hoehn-Yahr stage of all patients with PD. The MMSE, MoCA, and CDR were used to evaluate patients’ cognitive function. The primary outcome was the scores of the cognitive function scales.
Cognitive impairment was defined as a total Mini-Mental State Examination (MMSE) score of < 26 out of 30 (Dubois et al., 2007); a Montreal Cognitive Assessment (MoCA) score of < 26 out of 30 (Kandiah et al., 2014), with 1 point added to the score if the number of years of education was ≤ 12 years; and a Clinical Dementia Rating (CDR) score of ≥ 0.5 (Maiti et al., 2020). Based on the cognitive scale scores, patients with PD were divided into the PDCI group and the PD with normal cognitive function (PDN) group. In the PDCI group, patients with PD had global cognitive impairment without a detailed distinction of their cognitive domains; subjects with an MMSE score of < 26, a MoCA score of < 26, and a CDR score of ≥ 0.5 were included. In the PDN group, patients with PD did not have global cognitive impairment; subjects with an MMSE score of ≥ 26, a MoCA score of ≥ 26, and a CDR score of < 0.5 were included. In the healthy control group, the age-and sex-matched healthy participants presented no history of major disease, no global cognitive impairment, and had an MMSE score of ≥ 26, a MoCA score of ≥ 26, and a CDR score of < 0.5. Three cognitive scales were used to evaluate the cognitive function of the subjects, and the intersection was taken to avoid false-negative or false-positive values caused by the use of a single cognitive scale.
Quality assessment
All subjects evaluated using the cognitive function scales were subjected to a random selection process for evaluation by a third experienced neurologist. If the score was quite different from that given by the first two doctors, the scores of the cognitive function scales were discussed and determined by the three doctors together. To minimize bias in the experiment, we conducted a large, detailed literature search before the study to determine the appropriate cognitive function scales. Prior to the assessment of cognitive function, the two experienced neurologists systematically studied the use of the cognitive function scales. When a patient’s mental state was good and they were willing to cooperate, a comprehensive assessment of cognitive function was conducted.
Statistical analysis
Professional biostatisticians provide guidance for the statistical analysis of this study. We performed the statistical analysis using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The indexes, such as age and serum GDNF and GDNF precursor concentrations, which were normally distributed, were expressed as the mean ± standard deviation (SD). Education, disease duration, and the cognitive scale scores were represented as the median (interquartile range) [M(QR)] because they were not normally distributed. The Mann-Whitney U test was used to compare nonparametric data, such as disease duration, between two groups. For comparisons among multiple groups, one-way analysis of variance was used to analyze parametric data, and the least significant difference post hoc test (such as for the comparison of serum GDNF precursor concentration and the GDNF/α-pro-GDNF and GDNF/β-pro-GDNF ratios among the three groups) or the Dunnett’s T3 test (such as for the comparison of age and α-pro-GDNF/β-pro-GDNF ratios among the three groups) was used for further pairwise comparison, depending on the homogeneity of variance. Education and the cognitive scale scores, which were nonparametric, were analyzed among the three groups using the Kruskal-Wallis test, and the Bonferroni test was used for further pairwise comparisons to correct the P-value, to control the total probability of type I errors. Statistical significance was defined as two-sided P < 0.05/n (n represents the number of groups; Bland and Altman, 1995). Numerical data were compared using the chi-squared test. The correlations between serum GDNF and its precursors and the cognitive scale scores were measured using Spearman’s rank correlation test, with P < 0.05 considered statistically significant. Binary logistic regression analysis was used to test the risk factors of PDCI, and stepwise linear regression analysis was used to examine the factors that influence MMSE, MoCA, and CDR scores.
Results
Participant demographics
A total of 79 participants were included in the study: 26 in the healthy control group, 26 in the PDN group, and 27 in the PDCI group (Figure 1). Demographic and clinical data analysis and a comparison of serum GDNF and its precursor levels were performed among the three groups (n = 79). Correlation, regression, and receiver operating characteristic (ROC) curve analyses were performed in all patients with PD (n = 53).
Figure 1.
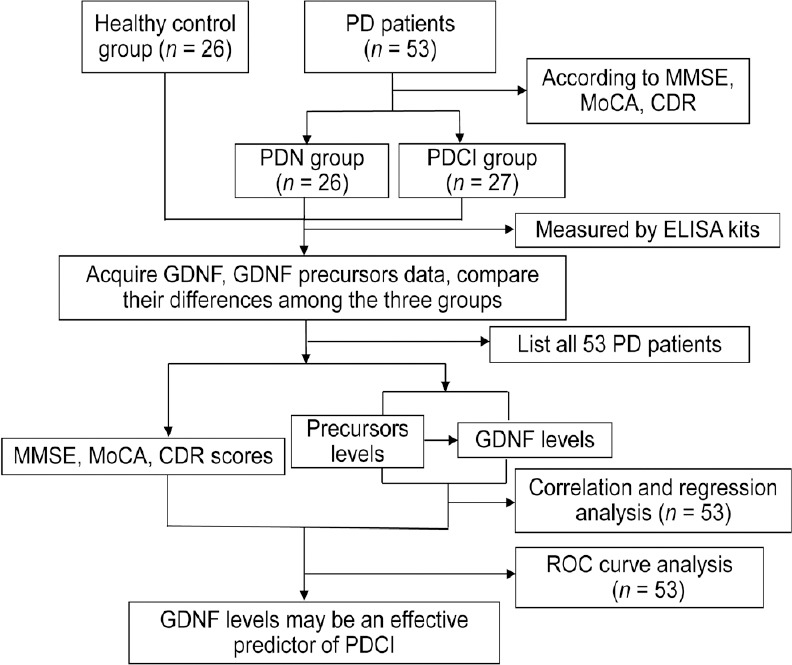
Summary diagram of the study.
CDR: Clinical Dementia Rating; ELISA: enzyme-linked immunosorbent assay; GDNF: glial cell line-derived neurotrophic factor; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; PD: Parkinson’s disease; PDCI: Parkinson’s disease with cognitive impairment; PDN: Parkinson’s disease with normal cognitive function; ROC: receiver operating characteristic.
General characteristics of the three groups of subjects
A total of 26, 27, and 26 subjects were included in the PDN, PDCI, and healthy control groups, respectively. Demographics, clinical characteristics, and disease status are shown in Table 1. There were no significant differences in sex, age, smoking, alcohol consumption, hypertension, or the proportion of subjects with high school education and above among the three groups. There were significant differences in years of education and the proportion of subjects with diabetes among the three groups, and there were also differences in Hoehn-Yahr stage and disease duration between the PDN and PDCI groups (P < 0.05).
Table 1.
Demographic and clinical data of Parkinson's disease and healthy control participants
| Items | Healthy control | PDN | PDCI | P |
|---|---|---|---|---|
| n | 26 | 26 | 27 | |
| Gender [male, n(%)]a | 14 (53.8) | 13 (50.0) | 10 (37.0) | 0.494 |
| Age (mean±SD, years)b | 64.73±3.75 | 65.04±10.55 | 68.07±6.81 | 0.210 |
| Hoehn-Yahr stage[n(stage)] | ||||
| (1/1.5/2/2.5/3/4)c | 1 (1) | 2 (1.5) | 0.002* | |
| Education [senior high school and up, n(%)]a | 8 (30.8) | 4 (15.4) | 3 (11.1) | 0.161 |
| Education [M(QR), years]d | 9 (3) | 9 (3.75) | 0 (6) | 0.000* |
| Disease duration [M(QR), months] | ||||
| High blood pressure [n(%)]a | 11 (42.3) | 4 (15.4) | 8 (29.6) | 0.102 |
| Diabetes [n(%)]a | 8 (30.8) | 2 (7.7) | 2 (7.4) | 0.026* |
| Smoking [n(%)]a | 2 (7.7%) | 5 (19.2) | 2 (7.4) | 0.307 |
| Drinking [n(%)]a | 5 (19.2) | 5 (19.2) | 3 (11.1) | 0.653 |
Hoehn-Yahr stage: 1, unilateral involvement only; 1.5, unilateral and axial involvement; 2, bilateral involvement without impairment of balance; 2.5, mild bilateral disease with recovery on pull test; 3, mild to moderate bilateral disease, some postural instability, physically independent; 4, severe disability, still able to walk or stand unassisted. a: chi-squared test; b: one-way analysis of variance, post hoc testing: Dunnett's T3 test; c: Mann-Whitney U test; d: Kruskal-Wallis rank sum test. *P < 0.05. PDCI: Parkinson's disease with cognitive impairment; PDN: Parkinson's disease with normal cognitive function.
Comparison of GDNF and its precursor levels among the three groups
There were significant differences in serum GDNF levels among the PDN, healthy control, and PDCI groups (P < 0.001; Figure 2 and Additional Table 1). Furthermore, the levels of GDNF in the PDN group were significantly higher than those in the healthy control group (P < 0.001) and PDCI group (P < 0.001; Figure 2 and Additional Table 1). Although the concentration of GDNF appeared to be slightly higher in the healthy control group, it was not significantly different between the healthy control and PDCI groups (Figure 2 and Additional Table 1).
Figure 2.
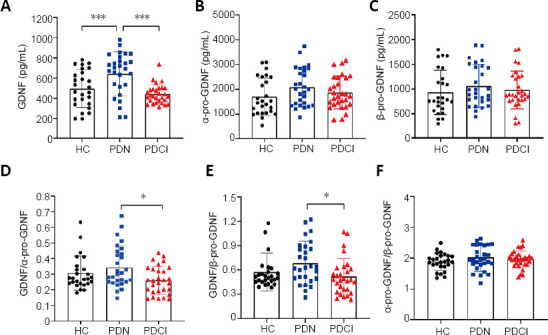
Comparison of levels of GDNF and its precursors between the three groups.
(A–C) Levels of GDNF (A), α-pro-GDNF (B), and β-pro-GDNF (C) among the three groups. (D–F) Ratios of GDNF/α-pro-GDNF (D), GDNF/β-pro-GDNF (E), and α-pro-GDNF/β-pro-GDNF (F) among the three groups. n = 26 (HC), 26 (PDN), and 27 (PDCI). The three groups were assessed using one-way analysis of variance followed by post hoc testing: Dunnett’s T3 test (GDNF, α-pro-GDNF/β-pro-GDNF) or the least significant difference method (α-pro-GDNF, β-pro-GDNF, GDNF/α-pro-GDNF, GDNF/β-pro-GDNF). *P < 0.01, ***P < 0.001. GDNF: Glial cell line-derived neurotrophic factor; HC: healthy control; PDCI: Parkinson’s disease with cognitive impairment; PDN: Parkinson’s disease with normal cognitive function.
Additional Table 1.
Comparison of levels of GDNF and its precursors between the three groups
| Group | HC | PDN | PDCI | F | P |
|---|---|---|---|---|---|
| GDNF (pg/mL)1 | 494.80±188.92* | 679.43±175.58 | 444.15±96.11* | 16.101 | <0.001 |
| α-pro-GDNF (pg/mL)2 | 1704.55±747.69 | 2135.10±761.04 | 1824.16±654.44 | 2.469 | 0.091 |
| β-pro-GDNF (pg/mL)2 | 933.30±445.35 | 1081.74±412.65 | 944.67±357.38 | 1.081 | 0.344 |
| GDNF/α-pro-GDNF2 | 0.31±0.11 | 0.34±0.11 | 0.27±0.09* | 3.297 | 0.042 |
| GDNF/β-pro-GDNF2 | 0.57±0.23 | 0.68±0.23 | 0.54±0.22 | 2.991 | 0.056 |
| α-pro-GDNF/β-pro-GDNF1 | 1.88±0.28 | 2.04±0.47 | 1.97±0.27 | 1.402 | 0.253 |
Three groups were assessed with one-way analysis of variance. Pairwise comparison between groups. 1: Using Dunnett's T3 method; 2: Using LSD method. *P < 0.05, vs. PDN group. CDR: Clinical dementia rating, GDNF: Glial cell line-derived neurotrophic factor; MMSE: Mini-mental state examination, MoCA: Montreal cognitive assessment
Moreover, the GDNF precursor levels and the GDNF/α-pro-GDNF, GDNF/β-pro-GDNF, and α-pro-GDNF/β-pro-GDNF ratios were also compared among all groups. There were significant differences in the GDNF/α-pro-GDNF ratio among the PDN, healthy control, and PDCI groups (P = 0.042; Figure 2) and Additional Table 1). Furthermore, the GDNF/α-pro-GDNF ratio in the PDN group was significantly higher than that in the PDCI group (P = 0.012); whereas there were no significant differences between the PDN and healthy control groups or between the PDCI and healthy control groups ((Figure 2) and Additional Table 1). Furthermore, there were no significant differences in GDNF precursor level, GDNF/β-pro-GDNF ratio, or α-pro-GDNF/β-pro-GDNF ratio among the three groups ((Figure 2) and Additional Table 1).
Cognitive scale scores in the three groups
The MMSE scores were significantly different among the three groups (P < 0.001; Table 2). Furthermore, the MMSE scores were significantly different between the healthy control and PDCI groups, and between the PDN and PDCI groups, but were not significantly different between the healthy control and PDN groups. The unadjusted P-values were P < 0.001, P < 0.001, and P = 0.951, respectively, and the Bonferroni-adjusted values were P < 0.001, P < 0.001, and P = 1.000, respectively. The MoCA results were similar to those of the MMSE scores (P < 0.001; Table 2). More details are shown in (Table 2).
Table 2.
Comparisons of cognitive scale scores of the three groups
| Healthy control group (n = 26) | PDN group (n = 26) | PDCI group(n = 27) | P | |
|---|---|---|---|---|
| MMSE | 29(2)# | 29(2)# | 19(10) | < 0.001 |
| MoCA | 27(2.25)# | 26.5(1)# | 10(8) | < 0.001 |
| CDR | 0(0)# | 0(0)# | 1(1) | < 0.001 |
Data are expressed as the M(QR). The results among the three groups were compared with the Kruskal-Wallis test and corrected by Bonferroni method (P < 0.001). #P < 0.05, vs. PDCI group. CDR: Clinical Dementia Rating; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; PDCI: Parkinson's disease with cognitive impairment; PDN: Parkinson's disease with normal cognitive function.
Correlation between GDNF and its precursor levels and cognitive scores
To verify the value of GDNF and its precursors in clinical practice, the correlations between the serum levels of GDNF and its precursors and cognitive scores were analyzed (Figure 3) and Additional Table 2). There were positive correlations between GDNF levels and the MMSE and MoCA scores (r = 0.610, P < 0.001 and r = 0.579, P < 0.001, respectively), and a negative correlation between GDNF levels and CDR scores (r = –0.573, P < 0.001; Figure 3 and Additional Table 2). The correlations between GDNF precursor levels and cognitive scores were also analyzed (Figure 3 and Additional Table 2).
Figure 3.
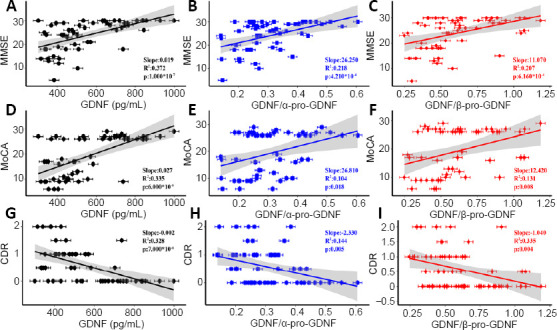
Spearman’s correlations between GDNF and its precursors and cognitive scores.
(A–C) Spearman’s correlations between MMSE scores and GDNF (r = 0.610, P < 0.001) (A), GDNF/α-pro-GDNF (r = 0.467, P < 0.001) (B), and GDNF/β-pro-GDNF (r = 0.455, P < 0.001) (C). (D–F) Spearman correlations between MoCA scores and GDNF (r = 0.579, P < 0.001) (D), GDNF/α-pro-GDNF (r = 0.323, P = 0.018) (E), and GDNF/β-pro-GDNF (r = 0.362, P = 0.008) (F). (G–I) Spearman’s correlations between CDR scores and GDNF (r = –0.573, P < 0.001) (G), GDNF/α-pro-GDNF (r = –0.379, P = 0.005) (H), and GDNF/β-pro-GDNF (r = –0.390, P = 0.004) (I). The correlation coefficients and P-values are indicated, and the shadows depict 95% confidence intervals. n = 53. CDR: Clinical Dementia Rating; GDNF: glial cell line-derived neurotrophic factor; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment.
Additional Table 2.
Spearman correlations between GDNF and its precursors and cognitive scores in Parkinson's disease patients
| MMSE | MoCA | CDR | ||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| GDNF | 0.610 | <0.001††† | 0.579 | <0.001††† | -0.573 | <0.001††† |
| α-pro-GDNF | 0.034 | 0.810 | 0.175 | 0.210 | -0.103 | 0.463 |
| β-pro-GDNF | 0.023 | 0.870 | 0.098 | 0.484 | -0.049 | 0.729 |
| GDNF/α-pro-GDNF | 0.467 | <0.001††† | 0.323 | 0.018†† | -0.379 | 0.005†† |
| GDNF/β-pro-GDNF | 0.455 | <0.001††† | 0.362 | 0.008†† | -0.390 | 0.004†† |
| α-pro-GDNF/β-pro-GDNF | -0.001 | 0.997 | 0.141 | 0.313 | -0.091 | 0.515 |
The correlation between serum GDNF and its precursors and the cognitive scales scores was measured by the Spearman's rank correlation test. †† P < 0.01, ††† P < 0.001. CDR: Clinical dementia rating, GDNF: Glial cell line-derived neurotrophic factor; MMSE: Mini-mental state examination, MoCA: Montreal cognitive assessment.
Regression analyses of risk factors for PDCI
To determine risk factors for PDCI, a binary logistic regression analysis was first conducted, which included the following variables: sex, age, education (years), Hoehn-Yahr stage, disease duration, GDNF (pg/mL), GDNF precursors (pg/mL), GDNF/α-pro-GDNF ratio, GDNF/β-pro-GDNF ratio, and α-pro-GDNF/β-pro-GDNF ratio. A likelihood ratio test was used (Table 3). GDNF level and Hoehn-Yahr stage had a significant effect on cognition. Next, a stepwise linear regression analysis (backward linear regression) was performed (Table 4). The variables affecting the MMSE score were GDNF level, Hoehn-Yahr stage, and α-pro-GDNF level (Table 4). The variables affecting the MoCA score were GDNF level, Hoehn-Yahr stage, and education (Table 4), and those affecting the CDR score were GDNF level and Hoehn-Yahr stage (Table 4). Education only affected the MoCA score among the three cognitive scales.
Table 3.
Binary logistic regression analysis of cognition in patients with Parkinson's disease
| B | SE | WALD | P | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Hoehn-Yahr stage | 1.447 | 0.580 | 6.232 | 0.013 | 4.250 | 1.365–13.233 |
| GDNF | 0.012 | 0.003 | 12.601 | < 0.001 | 0.988 | 0.981–0.995 |
| Constant | 4.004 | 1.655 | 5.855 | 0.016 | 54.799 |
A likelihood ratio test was used to evaluate the factors that influence cognition in patients with Parkinson's disease. To exclude confounding factors at baseline, we included all variables with differences between the groups in the demographic and clinical data (Table 1) and the variables to be analyzed in the study. Thus, sex, age, education, Hoehn-Yahr stage, disease duration, GDNF (pg/mL), a-pro-GDNF (pg/mL), β-pro-GDNF (pg/mL), GDNF/a-pro- GDNF ratio, GDNF/β-pro-GDNF ratio, and a-pro-GDNF/β-pro-GDNF ratio were included in the equation. Hoehn-Yahr stage and GDNF had a significant effect on cognitive function in patients with Parkinson's disease. n = 53. GDNF: Glial cell line-derived neurotrophic factor; SE: standard error; B: unstandardized coefficients
Table 4.
Stepwise linear regression analysis of MMSE, MoCA, and CDR scores in patients with Parkinson's disease
| Model | Variable | R2 | B | SE | β | t | P |
|---|---|---|---|---|---|---|---|
| Constant | 22.541 | 2.724 | 8.274 | < 0.001 | |||
| MMSE | GDNF | 0.561 | 0.022 | 0.004 | 0.633 | 5.742 | < 0.001 |
| Hoehn-Yahr stage | –2.779 | 0.635 | –0.424 | –4.374 | < 0.001 | ||
| a-pro-GDNF | –0.003 | 0.001 | –0.315 | –2.893 | 0.006 | ||
| Constant | 9.383 | 3.312 | 2.833 | 0.007 | |||
| MoCA | GDNF | 0.551 | 0.021 | 0.005 | 0.483 | 4.670 | < 0.001 |
| Hoehn-Yahr stage | –2.204 | 0.843 | –0.271 | –2.616 | 0.012 | ||
| education | 0.398 | 0.182 | 0.238 | 2.186 | 0.034 | ||
| Constant | 0.993 | 0.291 | 3.419 | 0.001 | |||
| CDR | GDNF | 0.465 | –0.002 | 0 | –0.470 | –4.436 | < 0.001 |
| Hoehn-Yahr stage | 0.276 | 0.072 | 0.404 | 3.818 | < 0.001 |
Stepwise linear regression analysis was used to evaluate the factors that influence MMSE, MoCA, and CDR scores in patients with Parkinson's disease. To exclude confounding factors at baseline, we included all variables with differences between the groups in the demographic and clinical data (Table 1) and the variables to be analyzed in the study. Thus, sex, age, education, Hoehn-Yahr stage, disease duration, GDNF (pg/mL), a-pro-GDNF (pg/mL), β-pro-GDNF (pg/mL), GDNF/a-pro-GDNF ratio, GDNF/β-pro-GDNF ratio, and a-pro-GDNF/β-pro-GDNF ratio were included in the equation. n = 53. Model MMSE: Constant, GDNF (pg/mL), Hoehn-Yahr stage, a-pro-GDNF; Model MoCA: Constant, GDNF (pg/mL), Hoehn-Yahr stage, education; Model CDR: Constant, GDNF (pg/mL), Hoehn- Yahr stage. CDR: Clinical Dementia Rating; GDNF: glial cell line-derived neurotrophic factor; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; SE: standard error; B: unstandardized coefficients; β: standardized coefficients.
ROC curve of GDNF levels predicting cognitive function in PD
The PDN and PDCI groups were analyzed to evaluate the accuracy of GDNF levels in the diagnosis of PDCI (Figure 4). The distinction between PDN and PDCI was based on MMSE, MoCA, and CDR scores. The diagnostic accuracy was determined using ROC curve analysis (AUC = 0.859, P < 0.001, 95% confidence interval: 0.736–0.939). The best cut-off value of serum GDNF levels for PDCI diagnosis was 508.991 pg/mL, with a sensitivity and specificity of 85.19% and 84.62%, respectively. Further analysis was performed to determine whether the combination of GDNF, GDNF/α-pro-GDNF ratio, and GDNF/β-pro-GDNF ratio had a higher diagnostic accuracy for PDCI. Logistic regression was performed to fit the data, and ROC analysis was used for comparison (AUC = 0.862, P < 0.001; Figure 4). The results revealed that the combination was not significantly better than GDNF alone.
Figure 4.
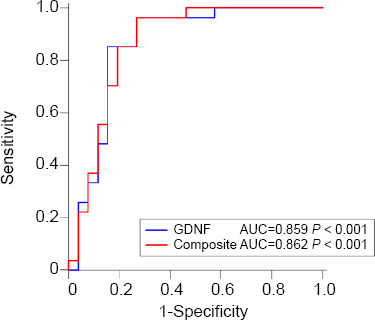
ROC curves for GDNF and composite biomarkers as related to PDCI.
ROC analyses of GDNF and composite biomarkers in the serum of PD patients. Composite = (GDNF vs. GDNF/a-pro-GDNF vs. GDNF/β-pro-GDNF). The GDNF cutoff point of PDCI diagnosis based on the ROC curve: *508.99 pg/mL. AUC: Area under curve; GDNF: glial cell line-derived neurotrophic factor; PDCI: Parkinson’s disease with cognitive impairment; ROC: receiver operating characteristic.
Discussion
We aimed to investigate the relationship between serum levels of GDNF and its precursors and PDCI, and to find a potential biomarker for the early diagnosis of PDCI. Our results indicate that GDNF levels are significantly different between patients with PD with different cognitive functions, and are correlated with a variety of cognitive scales that objectively evaluate and predict cognitive function in patients with PD.
Gui et al. (2017) demonstrated that neuroinflammation results in decreased GDNF, accompanied by learning and memory impairments, and that GDNF reduces these impairments when injected into the lateral ventricle. In the present study, reduced GDNF levels were also found in patients with PDCI, whose global cognition was significantly worse than that of the healthy control and PDN groups. These findings support the previous experimental results. In another animal experiment, Pertusa et al. (2008) reported that GDNF secreted by astrocytes enhances neuronal function, characterized by the increased local synthesis of neurotransmitters, such as acetylcholine, dopamine, and 5-hydroxytryptamine. Moreover, GDNF improves neuronal atrophy in aged rats, thereby further improving their spatial learning and memory ability. These findings all indicate the importance of GDNF for cognitive function.
Analysis of the cognitive scale scores and serum levels after eliminating confounding factors revealed differences in GDNF levels and related parameters between the PDN and PDCI groups. Correlation and regression analyses demonstrated that serum GDNF levels, as well as Hoehn-Yahr stage and education, had a significant relationship with cognition in patients with PD. However, education had a significant effect on MoCA scores only (Table 4). The reason for this finding may be the large proportion of visuospatial and executive functions and the difficulty of delayed recall in the MoCA scale, which may require higher education. Moreover, most subjects had low MoCA scores. The assessment of PDCI can therefore not rely solely on the MMSE or MoCA scale, but requires the combination of multiple cognitive scales. Thus, our findings also provide guidance for clinical practice. Because GDNF levels were correlated with the scores of various neuropsychological scales, including the MMSE, MoCA, and CDR, it is expected that preliminary screening for cognitive function can be achieved by collecting data on GDNF and its precursors in patients with PD, and vice versa. Because cerebrospinal fluid is strongly associated with the central nervous system, previous studies of peripheral markers of neurodegenerative diseases have mostly focused on cerebrospinal fluid markers. However, a lumbar puncture is an invasive operation that is difficult to carry out widely, and a substitute is therefore necessary. Straten et al. (2009) demonstrated a correlation between peripheral blood GDNF levels and cerebrospinal fluid GDNF levels in healthy people, indicating that the levels of GDNF in peripheral blood may be used to indirectly reflect the cerebrospinal fluid levels of GDNF. Moreover, a recent study on Alzheimer’s disease diagnostic markers by Jia et al. (2019) reported biomarkers in the peripheral blood that had equivalent diagnostic efficacy to those in cerebrospinal fluid. Furthermore, Lonka-Nevalaita et al. (2010) revealed that both GDNF and pro-GDNF are secretory proteins, thus providing a theoretical basis for the study of serum GDNF and its precursors.
To date, alterations in GDNF levels in neurodegenerative diseases remain inconclusive (Straten et al., 2009; Forlenza et al., 2015; Rocha et al., 2018; Virachit et al., 2019). In view of this, we compared the serum levels of GDNF and its precursors in patients with PD based on our research and the previous knowledge in this field. In the present study, there were no significant differences in GDNF or GDNF precursor levels between the healthy control and PD groups. In contrast, GDNF levels were significantly higher in the PDN group than in the PDCI group, which is consistent with results from a previous study (Wang et al., 2011). There were also significant differences in GDNF/α-pro-GDNF ratios between these two groups. This study provides a preliminary description of both GDNF and GDNF precursor levels in these three different populations. However, a larger sample size is needed to verify whether this finding applies to the wider population.
In the current study, the highest serum GDNF levels occurred in the PDN group, rather than in the healthy control group. GDNF levels were significantly lower in the healthy control and PDCI groups than in the PDN group; this finding was inconsistent with previously reported changes (Liu et al., 2020). Because of the recognized neuroprotective properties of GDNF, we hypothesized that elevated GDNF levels in the PDN group may indicate that the system is activated to combat an increasing loss of dopaminergic neurons. Based on this hypothesis, the decrease in GDNF levels in the PDCI group, indicating a failure of the system to compensate this, may be one of the reasons for the decline in cognitive function in these patents. It has been reported that PDN patients are generally in the early stages of the disease, while PDCI tends to occur later (Poewe et al., 2017). This effect can possibly be attributed to the protective effects of GDNF on dopaminergic neurons, which may lead to high expression of GDNF. A large number of studies can confirm this hypothesis; some studies have demonstrated that GDNF expression is increased in the substantia nigra and striatum of rats with MPTP-induced injury in the early days after injury occurs (Grunblatt et al., 2001; Mandel et al., 2002). It has also been reported that GDNF is highly expressed in dopaminergic neurons with 6-hydroxydopamine-induced injury in the early days following injury, demonstrating a protective effect of GDNF in injured dopaminergic neurons (Gao et al., 2016). It is speculated that increased levels of GDNF reduce the activity of tyrosine hydroxylase enzymes in dopaminergic neurons to compensate for the synthesis and release of dopamine, thereby protecting the injured dopaminergic neurons (Lonka-Nevalaita et al., 2010). The protective mechanisms of GDNF on dopaminergic neurons may also occur through the following molecular pathways. GDNF can activate the PI3 kinase/Akt pathway and promote the expression of calbindin-D28K, which protects neurons from degeneration (Wang et al., 2008). Moreover, GDNF regulates adhesion molecules, efficiently repairs damaged cells, and increases phosphorylation of neural cell adhesion molecules related to the Fyn pathway, thus promoting axonal growth in damaged dopaminergic neurons (Cao et al., 2008). It also mediates neural cell adhesion molecule-140 translocation into lipid rafts, to increase the viability of dopaminergic neurons damaged by 6-hydroxydopamine (Uhlen et al., 2017).
The serum levels of GDNF in the PDCI group were lower than those in the PDN group in the present study, which might be because long-term GDNF overexpression may lead to compensatory changes in excess of nutritional demands (Georgievska et al., 2002; Barroso-Chinea et al., 2016). In contrast, moderate GDNF overexpression can compensate for a damaged neurotrophic environment (Chauhan et al., 2001) and provides the possibility of restoring the dynamic balance of physiological dopamine (Kramer and Liss, 2015). It is also possible that, with the development of the disease, extensive apoptosis of nigrostriatal dopaminergic neurons occurs. This is accompanied by apoptosis of the glial cells around dopaminergic neurons, leading to a decrease in GDNF expression, and further accelerating dopaminergic neuron apoptosis, thus creating a vicious cycle.
However, it is somewhat puzzling that GDNF levels appeared lower in the PDCI group compared with the healthy control group, although this was not significant. Three possible reasons are suggested. First, it may be related to the cognitive levels of the included patients with PD. In our study, there were few patients with PD with very severe cognitive impairment and the overall sample size was small, which may affect the results. Further research should include more patients with PD with severe cognitive impairment. Second, a local reduction of GDNF in the brain can lead to the long-distance transfer of GDNF from the body to compensate, thus preventing accurate detection of local reductions in GDNF. Third, because high GDNF levels can cause cerebellar toxicity (Lang et al., 2006) and are closely related to glioma development and progression (Miao et al., 2000), the protective mechanisms of the body may limit continuous increases, leading to a decrease in GDNF.
We speculated that the altered GDNF levels were caused by changes in GDNF precursors. Gu et al. (2020) demonstrated that, at the gene level, the expression of β-pro-GDNF is significantly higher than that of α-pro-GDNF in the mouse brain, whereas it is opposite at the protein level. The aforementioned higher protein β-pro-GDNF levels compared with α-pro-GDNF levels are consistent with the concentrations of GDNF precursors that we detected in the serum in the present study. Both the change trends of the precursors and the sum of the precursors were consistent with those of GDNF; however, there were no statistical differences among the three groups.
Although the present study preliminarily suggests that GDNF may be an effective predictor of PDCI, it does not clarify whether GDNF is a main factor in PDCI pathogenesis, or if it is an upstream or downstream change (or if it is an additional phenomenon of PD). We hypothesize that changes in GDNF precursors levels lead to changes in GDNF levels, although the current data on GDNF precursors cannot reasonably explain this phenomenon. Our research was limited, the sample size was small, and GDNF and its precursors were measured by enzyme-linked immunosorbent assay kits, all of which may affect the reliability of the results. Moreover, this was a cross-sectional study from just a single time point. Thus, although our GDNF precursor data were inconclusive, we hope to stimulate further research by reporting them here. Our research group is also investigating the production of GDNF precursor antibodies. A final limitation of our study is that not all follow-up data were collected from the patients with PD. In the future, longitudinal studies will therefore be conducted with the follow-up data to increase the credibility of this research. In summary, our research results indicate that serum GDNF may be an effective diagnostic marker for cognitive impairment in PD.
Additional files: Open peer review report 1 (85.2KB, pdf) .
Additional Table 1: Comparison of levels of GDNF and its precursors between the three groups.
Additional Table 2: Spearman correlations between GDNF and its precursors and cognitive scores in Parkinson’s disease patients.
Acknowledgments
We would like to acknowledge the Department of Anatomy and Neurobiology of Xuzhou Medical University and Department of Neurology of the Affiliated Hospital of Xuzhou Medical University of China for providing the experimental platform. We would particularly like to acknowledge Ke Wang from Department of Epidemiology and Biostatistics, Xuzhou Medical University, for his assistance in statistical analysis. We would like to acknowledge Guowei Wu from Department of Language Science and Art, Jiangsu Normal University, for his assistance in drawing pictures. We would also like to thank all contributors for their support and effort.
Footnotes
P-Reviewer: Mitrofanis J; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Haase R, Qiu Y, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare that they have no conflict of interest.
Financial support: This work was funded by the National Natural Science Foundation of China, No. 81971006 (to DSG); the Postgraduate Research and Practice Innovation Program of Jiangsu Province of China, Nos. KYCX18_2193 (to MYS), KYCX18_2171 (to CXT). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: The procedures were performed in accordance with ethical standards of the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University of China (approval No. XYFY2017-KL047-01) on November 30, 2017. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Declaration of participant consent: The authors certify that they have obtained all appropriate participant consent forms. In the form the participants have given their consent for their images and other clinical information to be reported in the journal. The participants understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatistician of Xuzhou Medical University of China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: John Mitrofanis, University of Sydney Anatomy, Australia.
Funding: This work was funded by the National Natural Science Foundation of China, No. 81971006 (to DSG); the Postgraduate Research and Practice Innovation Program of Jiangsu Province of China, Nos. KYCX18_2193 (to MYS), KYCX18_2171 (to CXT).
References
- 1.Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, Ballard C. Cognitive decline in Parkinson disease. Nat Rev Neurol. 2017;13:217–231. doi: 10.1038/nrneurol.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barroso-Chinea P, Cruz-Muros I, Afonso-Oramas D, Castro-Hernandez J, Salas-Hernandez J, Chtarto A, Luis-Ravelo D, Humbert-Claude M, Tenenbaum L, Gonzalez-Hernandez T. Long-term controlled GDNF over-expression reduces dopamine transporter activity without affecting tyrosine hydroxylase expression in the rat mesostriatal system. Neurobiol Dis. 2016;88:44–54. doi: 10.1016/j.nbd.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabresi P, Castrioto A, Di Filippo M, Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson’s disease. Lancet Neurol. 2013;12:811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- 5.Cao JP, Wang HJ, Yu JK, Yang H, Xiao CH, Gao DS. Involvement of NCAM in the effects of GDNF on the neurite outgrowth in the dopamine neurons. Neurosci Res. 2008;61:390–397. doi: 10.1016/j.neures.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson’s disease brain. J Chem Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 7.Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, de Silva R, Lees AJ, Revesz T. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important. Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delgado-Alvarado M, Gago B, Navalpotro-Gomez I, Jimenez-Urbieta H, Rodriguez-Oroz MC. Biomarkers for dementia and mild cognitive impairment in Parkinson’s disease. Mov Disord. 2016;31:861–881. doi: 10.1002/mds.26662. [DOI] [PubMed] [Google Scholar]
- 9.Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 10.Forlenza OV, Miranda AS, Guimar I, Talib LL, Diniz BS, Gattaz WF, Teixeira AL. Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J Alzheimers Dis. 2015;46:423–429. doi: 10.3233/JAD-150172. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Kang XY, Sun S, Li L, Zhang BL, Li YQ, Gao DS. Transcription factor Six2 mediates the protection of GDNF on 6-OHDA lesioned dopaminergic neurons by regulating Smurf1 expression. Cell Death Dis. 2016;7:e2217. doi: 10.1038/cddis.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- 13.Goulding SR, Sullivan AM, O’Keeffe GW, Collins LM. The potential of bone morphogenetic protein 2 as a neurotrophic factor for Parkinson’s disease. Neural Regen Res. 2020;15:1432–1436. doi: 10.4103/1673-5374.274327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunblatt E, Mandel S, Maor G, Youdim MB. Gene expression analysis in N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mice model of Parkinson’s disease using cDNA microarray: effect of R-apomorphine. J Neurochem. 2001;78:1–12. doi: 10.1046/j.1471-4159.2001.00397.x. [DOI] [PubMed] [Google Scholar]
- 15.Gu XH, Li H, Zhang L, He T, Chai X, Wei H, Gao DS. Differential expression of glial cell line-derived neurotrophic factor splice variants in the mouse brain. Neural Regen Res. 2020;15:270–276. doi: 10.4103/1673-5374.265561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gui L, Lei X, Zuo Z. Decrease of glial cell-derived neurotrophic factor contributes to anesthesia- and surgery-induced learning and memory dysfunction in neonatal rats. J Mol Med (Berl) 2017;95:369–379. doi: 10.1007/s00109-017-1521-9. [DOI] [PubMed] [Google Scholar]
- 17.Halliday GM, Leverenz JB, Schneider JS, Adler CH. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlett DR, Whitfield D, Johnson M, Attems J, O’Brien JT, Aarsland D, Lai MK, Lee JH, Chen C, Ballard C, Hortobagyi T, Francis PT. Regional multiple pathology scores are associated with cognitive decline in lewy body dementias. Brain Pathol. 2015;25:401–408. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, Zhou C, Liang F, Shi S, Wang S, Qin W, Wang Q, Li F, Wang Q, Li Y, Shen L, Wei Y, Jia J. Concordance between the assessment of Abeta42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15:1071–1080. doi: 10.1016/j.jalz.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Kandiah N, Zhang A, Cenina AR, Au WL, Nadkarni N, Tan LC. Montreal cognitive assessment for the screening and prediction of cognitive decline in early Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:1145–1148. doi: 10.1016/j.parkreldis.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Kramer ER, Liss B. GDNF-Ret signaling in midbrain dopaminergic neurons and its implication for Parkinson disease. FEBS Lett. 2015;589:3760–3772. doi: 10.1016/j.febslet.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 24.Leverenz JB, Watson GS, Shofer J, Zabetian CP, Zhang J, Montine TJ. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim NS, Swanson CR, Cherng HR, Unger TL, Xie SX, Weintraub D, Marek K, Stern MB, Siderowf A, Investigators P, Alzheimer’s Disease Neuroimaging I. Trojanowski JQ, Chen-Plotkin AS. Plasma EGF and cognitive decline in Parkinson’s disease and Alzheimer’s disease. Ann Clin Transl Neurol. 2016;3:346–355. doi: 10.1002/acn3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litvan I, Goldman JG, Troster AI, Schmand BA, Weintraub D, Petersen RC, Mollenhauer B, Adler CH, Marder K, Williams-Gray CH, Aarsland D, Kulisevsky J, Rodriguez-Oroz MC, Burn DJ, Barker RA, Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: movement disorder society task force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Tong S, Ding L, Liu N, Gao D. Serum levels of glial cell line-derived neurotrophic factor and multiple neurotransmitters: In relation to cognitive performance in Parkinson’s disease with mild cognitive impairment. Int J Geriatr Psychiatry. 2020;35:153–162. doi: 10.1002/gps.5222. [DOI] [PubMed] [Google Scholar]
- 28.Lonka-Nevalaita L, Lume M, Leppanen S, Jokitalo E, Peranen J, Saarma M. Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci. 2010;30:11403–11413. doi: 10.1523/JNEUROSCI.5888-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiti B, Koller JM, Snyder AZ, Tanenbaum AB, Norris SA, Campbell MC, Perlmutter JS. Cognitive correlates of cerebellar resting-state functional connectivity in Parkinson disease. Neurology. 2020;94:e384–e396. doi: 10.1212/WNL.0000000000008754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandel S, Grunblatt E, Maor G, Youdim MB. Early and late gene changes in MPTP mice model of Parkinson’s disease employing cDNA microarray. Neurochem Res. 2002;27:1231–1243. doi: 10.1023/a:1020989812576. [DOI] [PubMed] [Google Scholar]
- 31.Miao HQ, Lee P, Lin H, Soker S, Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 32.Pertusa M, Garcia-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J, Cristofol R, Sarkis C, Sanfeliu C. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging. 2008;29:1366–1379. doi: 10.1016/j.neurobiolaging.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 33.Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 34.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 35.Rocha NP, Ferreira JPS, Scalzo PL, Barbosa IG, Souza MS, Christo PP, Reis HJ, Teixeira AL. Circulating levels of neurotrophic factors are unchanged in patients with Parkinson’s disease. Arq Neuropsiquiatr. 2018;76:310–315. doi: 10.1590/0004-282X20180035. [DOI] [PubMed] [Google Scholar]
- 36.Straten G, Eschweiler GW, Maetzler W, Laske C, Leyhe T. Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer’s disease and normal controls. J Alzheimers Dis. 2009;18:331–337. doi: 10.3233/JAD-2009-1146. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Li F, Gao X, Zhu Y, Chen J, Zhu X, Yuan H, Gao D. Calbindin-D28K inhibits apoptosis in dopaminergic neurons by activation of the PI3-kinase-Akt signaling pathway. Neuroscience. 2011;199:359–367. doi: 10.1016/j.neuroscience.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 38.Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnström H, Glimelius B, et al. A pathology atlas of the human cancer transcriptome. Science. 2017 doi: 10.1126/science.aan2507. doi: 101126/scienceaan2507. [DOI] [PubMed] [Google Scholar]
- 39.Virachit S, Mathews KJ, Cottam V, Werry E, Galli E, Rappou E, Lindholm P, Saarma M, Halliday GM, Shannon Weickert C, Double KL. Levels of glial cell line-derived neurotrophic factor are decreased, but fibroblast growth factor 2 and cerebral dopamine neurotrophic factor are increased in the hippocampus in Parkinson’s disease. Brain Pathol. 2019;29:813–825. doi: 10.1111/bpa.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang HJ, Cao JP, Yu JK, Zhang LC, Jiang ZJ, Gao DS. Calbindin-D28K expression induced by glial cell line-derived neurotrophic factor in substantia nigra neurons dependent on PI3K/Akt/NF-kappaB signaling pathway. Eur J Pharmacol. 2008;595:7–12. doi: 10.1016/j.ejphar.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Hou Z, Yuan Y, Hou G, Liu Y, Li H, Zhang Z. Association study between plasma GDNF and cognitive function in late-onset depression. J Affect Disord. 2011;132:418–421. doi: 10.1016/j.jad.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Yu ZQ, Zha JH, Liu HM, Ding YX, Wang YQ, Wang HJ, Gao DS. Effect of intranigral injection of GDNF and EGF on the survival and possible differentiation fate of progenitors and immature neurons in 6-OHDA-lesioned rats. Neurochem Res. 2009;34:2089–2101. doi: 10.1007/s11064-009-9995-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


