Keywords: autografts, axon growth, drug delivery, glial cell-derived neurotrophic factor, growth factors, mineral coatings, nerve grafting, nerve growth factor
Abstract
The gold standard for treating peripheral nerve injuries that have large nerve gaps where the nerves cannot be directly sutured back together because it creates tension on the nerve, is to incorporate an autologous nerve graft. However, even with the incorporation of a nerve graft, generally patients only regain a small portion of function in limbs affected by the injury. Although, there has been some promising results using growth factors to induce more axon growth through the nerve graft, many of these previous therapies are limited in their ability to release growth factors in a sustained manner and tailor them to a desired time frame. The ideal drug delivery platform would deliver growth factors at therapeutic levels for enough time to grow axons the entire length of the nerve graft. We hypothesized that mineral coated microparticles (MCMs) would bind, stabilize and release biologically active glial cell-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) in a sustained manner. Therefore, the objective of this study was to test the ability of MCMs releasing growth factors at the distal end of a 10 mm sciatic nerve graft, to induce axon growth through the nerve graft and restore hind limb function. After sciatic nerve grafting in Lewis rats, the hind limb function was tested weekly by measuring the angle of the ankle at toe lift-off while walking down a track. Twelve weeks after grafting, the grafts were harvested and myelinated axons were analyzed proximal to the graft, in the center of the graft, and distal to the graft. Under physiological conditions in vitro, the MCMs delivered a burst release of NGF and GDNF for 3 days followed by a sustained release for at least 22 days. In vivo, MCMs releasing NGF and GDNF at the distal end of sciatic nerve grafts resulted in significantly more myelinated axons extending distal to the graft when compared to rats that received nerve grafts without growth factor treatment. The rats with nerve grafts incorporated with MCMs releasing NGF and GDNF also showed significant improvement in hind limb function starting at 7 weeks postoperatively and continuing through 12 weeks postoperatively when compared to rats that received nerve grafts without growth factor treatment. In conclusion, MCMs released biologically active NGF and GDNF in a sustained manner, which significantly enhanced axon growth resulting in a significant improvement of hind limb function in rats. The animal experiments were approved by University of Wisconsin-Madison Animal Care and Use Committee (ACUC, protocol# M5958) on January 3, 2018.
Chinese Library Classification No. R456; R364; R605
Introduction
Peripheral nerve injuries have an incidence of roughly 13 to 23 per 100,000 people per year, resulting in difficult outcomes related to function and sensation (Evans, 2001; Taylor et al., 2008; Sullivan et al., 2016). Although there are quality microsurgical techniques for nerve repair and nerve grafts are commonly used, recovery of function is inadequate, especially those injuries that are not amenable to direct suturing, larger gaps, and lesions that are a long distance from target muscle (Deumens et al., 2010). Moreover, development of neuropathic pain is frequently observed following nerve injury. This greatly compromises the quality of life of affected individuals (Deumens et al., 2010). In order to improve the quality of life for those suffering from large gap peripheral nerve injuries, novel methods are needed to increase the amount of function regained when repairing these damaged nerves.
Autologous nerve grafts are widely regarded as the gold standard for treating large nerve gaps in patients (Siemionow and Brzezicki, 2009; Geissler and Stevanovic, 2019). However, this remains an imperfect solution, as many patients only regain a small portion of function. Thus, although autografts have shown the best results for treating nerve gaps, the functional recovery is inadequate. In an analysis of the reconstruction of 132 median nerves, only 49.2% had good recovery (anti-gravity) and none of the 13 patients with grafts longer than 7 cm achieved any useful recovery (Kallio and Vastamaki, 1993). Since nerve grafting requires that the regenerating fibers cross two coaptation sites, it increases the risk of axonal loss and misdirection. Recent research has been aimed at improving axonal growth through the nerve graft using growth factor therapy, with some promising results (Hoyng et al., 2014).
Growth factors play an important role during nerve regeneration in cell survival, axonal sprouting and growth, cell migration, cell proliferation, and cell differentiation. It has been well-demonstrated that overexpression of two specific growth factors in autografts, glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF), increases neuronal survival and leads to a significant increase in motor axon and sensory axon growth, respectively (Hoyng et al., 2014; Rosich et al., 2017; Ortmann and Hellenbrand, 2018). To have the desired impact, the growth factors must stay active at therapeutic levels for a time period that allows the axons to grow through the autograft. However, if they stay at a therapeutic level or higher for longer than desired, this can cause axon entrapment and impair functional recovery (Hoyng et al., 2014). Hoyng et al. (2014) used lentiviral vectors to upregulate growth factors in a nerve graft, and although this resulted in large grafts full of axons, there were actually fewer axons distal to the grafts and diminished functional recovery. These results, in addition with the short half-lives, high biological activity, and pleiotropic effects of GDNF and NGF, highlight a need for a delivery strategy that provides control of dosage, location, and timing of growth factor delivery (Tria et al., 1994).
In recent research, mineral coatings have been used to achieve a sustained delivery of multiple therapeutic proteins, including vascular endothelial growth factor, transforming growth factor beta 1, bone morphogenetic protein 2, fibroblast growth factor-2, insulin-like growth factor-1, neurotrophin-3, and interleukin-10 (Yu et al., 2014, 2017; Chamberlain et al., 2015; Hanna et al., 2016; Hellenbrand and Hanna, 2016; Clements et al., 2018; Hellenbrand et al., 2019). These studies showed that the mineral coatings are able to incorporate therapeutic proteins, deliver the protein in a sustained manner for an extended timeframe, and retain the biological activity of the proteins adsorbed onto the mineral coating. Growth factor incorporation into mineral coatings grown in simulated body fluids is particularly advantageous, as it allows for a high level of control over coating properties and resultant protein release kinetics (Yu et al., 2017). This control over growth factor delivery time frame would make it achievable to tailor the growth factor delivery to the length of graft used, thus maximizing axon growth without causing axon entrapment. The objective of this study was to test the efficacy of one variation of mineral coating (4.2 mM bicarbonate) on one length of nerve graft (10 mm), to induce more axon growth through a nerve graft and promote significantly more functional recovery. Thus, although in this current study we are not tailoring the growth factor release to the length of graft, this study serves as an important first step in developing mineral coated microparticles (MCMs) capable of tailoring the growth factor delivery profile to the specific length of nerve graft.
Materials and Methods
Animals
The University of Wisconsin-Madison Animal Care and Use Committee (ACUC, protocol# M5958) approved all procedures on January 3, 2018, which followed the NIH Guide for animal care. Seventy-two inbred male Lewis rats weighing ~250 g were used (Envigo, Huntingdon, UK). The inbred Lewis rat strain enabled the use of a nerve graft from a donor rat without the need of immunosuppression (Hellenbrand et al., 2016). For all surgical procedures, an intraperitoneal injection of 90 mg/kg ketamine (Akorn, Inc., Lake Forest, IL, USA) and 9 mg/kg xylazine (Bimeda, Oakbrook Terrace, IL, USA)was given for the anesthetic agent. Following surgical procedures, the rats received subcutaneous injections of Carprofen (Rimadyl) 5 mg/kg to control pain. They also received an antibiotic enriched diet of Uniprim (Envigo, Huntingdon, UK; Cat# TD.06596), for 7 days following surgery. All rats were given food and water ad libitum, housed at room temperature with two rats per cage and standard light/dark cycle.
Development of MCMs
Absorbable β-tricalcium phosphate microparticles were obtained from Plasma Biotal, Ltd. (Derbyshire, UK) (Figure 1A). The mineral coating was formed as previously reported (Hellenbrand et al., 2019). Briefly, microparticles were incubated at 37°C (pH 6.80) in modified simulated body fluid (mSBF) for 1 week. The mSBF has the same ionic composition as compared to human plasma except double the calcium and phosphate ions, and was created by adding the following reagents (Thermo Fisher Scientific, Waltham, MA, USA) in the order shown into deionized water: 141 mM NaCl, 4.0 mM KCl, 0.5 mM MgSO4, 1.0 mM MgCl2, 4.2 mM NaHCO3, 20.0 mM N-(2-hydroxyethyl) piperazine-N-(2-ethanesulfonic acid) (HEPES), 5.0 mM CaCl2, and 2.0 mM KH2 PO4. The coated microparticles were strained through a 40 μm screen and a scanning electron microscope (EDS; LEO 1530 field emission scanning microscope; Zeiss, Oberkochen, Germany) was used to analyze the composition of the mineral coating.
Figure 1.
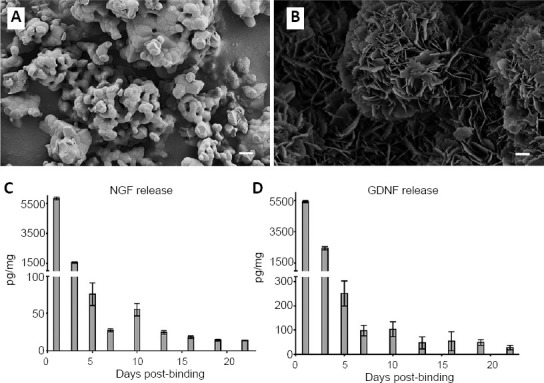
Mineral coating on β-TCP microparticles and cytokine delivery in vitro.
Scanning electron microscopy images of β-TCP microparticles (A) and β-TCP microparticles after incubation in modified simulated body fluid (B), revealing a continuous mineral coating that covered the entire surface. Over the first 3 days, there was a burst release of NGF and GDNF followed by a sustained release for at least 22 days (C, D). Scale bars: 1 µm in A and B. Error bars represent ± SEM in C and D. GDNF: Glial cell-derived neurotrophic factor; NGF: nerve growth factor; β-TCP: β-tricalcium phosphate.
Characterizing growth factor delivery profile in vitro
The dosages of GDNF and NGF were roughly based on previous studies (Vejsada et al., 1998; Boyd and Gordon, 2003; Lin et al., 2016; Tajdaran et al., 2016). Although these previous studies were not a sustained release, they did show NGF having an effect at much lower doses than GDNF. Thus, we used 2.5 times more GDNF than NGF. For growth factor binding, 5 mg MCMs were incubated in 500 μL of 1× PBS (0.1% BSA) containing 2 μg recombinant rat NGF and 5 μg recombinant rat GDNF (R & D Systems¸ Minneapolis, MN, USA; Cat# 556-NG and Cat# 512-GF) for 1 hour at 37°C.
To determine the delivery profile of NGF and GDNF from MCMs in vitro, the MCMs loaded with NGF and GDNF were incubated at physiological conditions (pH 7.4, 37°C) in simulated body fluid consisting of the same ionic constituents as blood plasma. Five mg MCMs loaded with NGF and GDNF were incubated in 500 µL simulated body fluid. Every other day the MCMs were centrifuged 1000 × g for 5 minutes, the solution without the MCM pellet was removed and stored at –80°C, then the MCM pellet was resuspended in 500 µL of fresh simulated body faluid. An enzyme-linked immuno-sorbent assay kit (Abcam, Cambridge, UK; Cat# ab100757 and Cat# ab213901) was used to quantify the NGF and GDNF released into the simulated body fluid.
To view MCMs on the distal end of sciatic nerve grafts (SNGs), 10 mg/mL MCMs were incubated in 10 µg/mL bovine serum albumin (BSA) conjugated to a fluorescein isothiocyanate (FITC) fluorophore (Thermofisher scientific, Waltham, MA, USA; Cat# A23015). A layer of fluorescently tagged MCMs was placed in a petri dish and then the cut end of the nerve was placed against the layer of MCMs, which created a thin layer of MCMs adsorbed to the nerve graft (Figure 2A and B). To determine the amount of MCMs that were adsorbed onto each nerve graft, the petri dish was weighed before and after the MCMs were adsorbed on the nerve graft. This process was repeated on 12 nerve ends and the amount of MCMs adsorbed was 143 ± 0.025 µg on each nerve.
Figure 2.
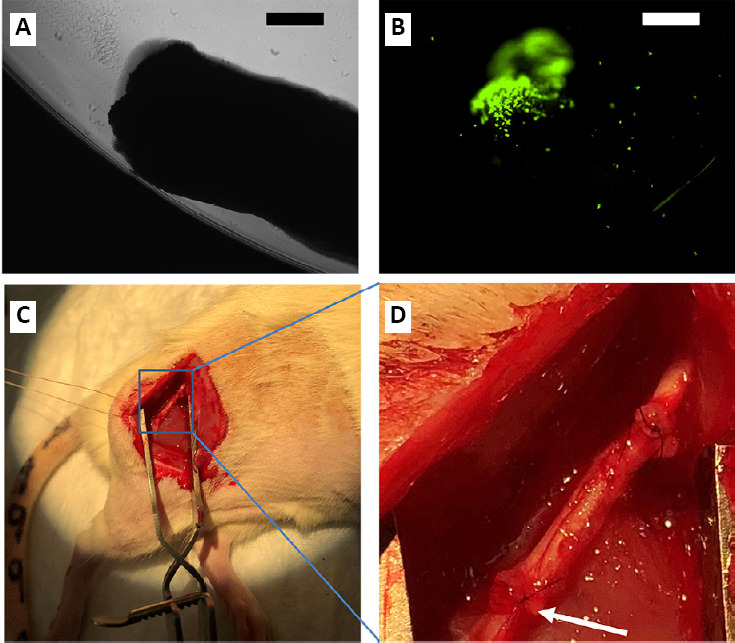
The distal end of a 10 mm SNG was dipped into MCMs that were labeled with a FITC fluorophore.
The nerve was then imaged in bright field (A), and with the FITC filter on (B), showing the MCMs localized to the distal end of the graft. For testing in vivo, male Lewis rats had 6 mm of the right sciatic nerve removed and a 10 mm-long isograft from a donor rat was micro-sutured end-to-end (C, D). The arrow points to the distal end of the SNG where the MCMs are incorporated. Scale bars equal 500 µm in A and B. FITC: Fluorescein isothiocyanate; MCM: mineral coated microparticle; SNG: sciatic nerve graft.
Testing growth factor delivery in situ
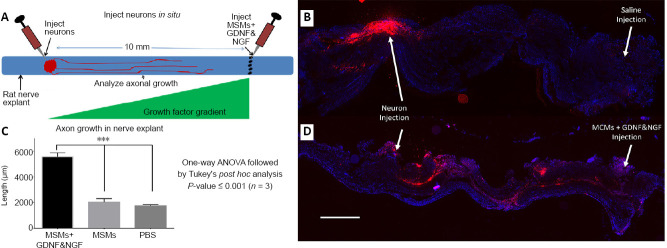
An in situ model was developed, to test the efficacy of MCMs releasing NGF and GDNF to induce axonal growth from a 10 mm distance in a nerve (Figure 3A). Dorsal root ganglion (DRG) neurons were purchased (SIGMA, St. Louis, MO, USA; Cat# R8820N), and their cell membrane was labeled with Vybrant Dil per manufacturer’s instructions (Molecular Probes, Eugene, OR, Cat# V22885). Male Lewis rats were anesthetized and their sciatic nerves were harvested and placed in Hank’s balanced salt solution on ice. Immediately after harvesting the nerves we injected a 20 µL volume of either PBS only, or PBS containing 150 µg MCMs, or PBS containing 150 µg MCMs loaded with NGF and/or GDNF, 3 mm proximal to the tibial nerve bifurcation. Then 20,000 Vybrant Dil labeled DRG neurons in 20 µL were injected 10 mm proximal to the MCMs or saline injection. To allow time for axons to grow, the nerves were cultured for 7 days in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 1% N2 supplement, 2% fetal bovine serum and Pen/Strep. After 7 days, the nerves were fixed in 4% paraformaldehyde (PFA) overnight, cryopreserved in 30 % sucrose for 48 hours, and 20 µm thick frozen longitudinal sections were taken. A Keyence BZ-9000 microscope (Keyence, Osaka, Japan) was used to image the sections. The distance that Vybrant Dil labeled membranes were observed was measured using ImageJ (NIH, Bethesda, MD, USA).
Figure 3.
Testing growth factor delivery from MCMs in situ.
Sciatic nerves were harvested from Lewis rats, dorsal root ganglion neurons labeled with Vybrant Dil were injected into the nerve and either saline, MCMs, or MCMs + NGF & GDNF were injected 10 mm distal to the injection of neurons (A). After injection, the nerves were cultured for 7 days, then fixed and sectioned longitudinally. When saline was injected there was minimal axon growth toward the injection (B). However when MCMs + NGF & GDNF was injected, the Vybrant red was observed significantly further distances toward the growth factor injection (C, D). n = 3; ***P < 0.001 (one-way analysis of variance followed by Tukey’s post hoc test); Red = Vybrant Dil labeled neurons; blue = 2-(4-amidinophenyl)-1H -indole-6-carboxamidine (DAPI). Scale bar: 500 µm in B and D. Error bars represent ± SEM (C). GDNF: Glial cell-derived neurotrophic factor; MCM: mineral coated microparticle; NGF: nerve growth factor.
Sciatic nerve grafting
To determine the number of rats needed in each group, a power analysis was performed with 80% power and a significance level (alpha) of 0.05 (two-tailed Student’s t-test, GraphPad StatMate 2.0 (GraphPad Software, San Diego, CA, USA). Nine rats were randomly assigned to each test group as shown in Table 1. The rats were assessed to ensure there were no hind limb motor deficits before inclusion in the study. Male Lewis rats were anesthetized as aforementioned, an incision approximately 2 cm long was made on the right leg along the femur, the muscles were separated to expose the sciatic nerve, and 6 mm of sciatic nerve was removed. Then, 10 mm of sciatic nerve from a donor rat was micro-sutured end-to-end on both the proximal end and distal end with two standard 9-0 Nylon sutures in the no treatment (NT) group (Figure 2C and D). For the groups treated with MCMs, the distal end of the SNG was dipped in the MCMs as described in the in vitro characterization section, before being micro-sutured into the recipient rat (Table 1). In order to allow for retraction of nerve endings and suturing without tension, a longer nerve graft was used. The nerve grafts were harvested 12 weeks postoperatively, which allowed time for grafts to incorporate with the nerves and axonal growth to occur.
Table 1.
Descriptions of treatments tested
| Group | Treatments |
|---|---|
| NT | 10 mm isograft with no treatment |
| MCMs | 10 mm isograft with MCMs |
| MCMs + NGF | 10 mm isograft with MCMs loaded with NGF |
| MCMs + GDNF | 10 mm isograft with MCMs loaded with GDNF |
| MCMs + NGF&GDNF | 10 mm isograft with MCMs loaded with NGF and GDNF |
MCMs were only incorporated on the distal end of the isograft in the treatment groups (n = 9 rats in each group). GDNF: Glial cell-derived neurotrophic factor; MCM: mineral coated microparticle; NGF: nerve growth factor.
Functional testing
For each rat, the right, experimental leg was subjected to sciatic nerve grafting, while the left sciatic nerve remained intact. All rats began functional testing at 5 weeks postoperatively, which consisted of a gait analysis of the angle of the ankle at toe lift off to assess functional recovery after sciatic nerve graft placement (Lee et al., 2013; Rui et al., 2014; Wang et al., 2018). The gait analysis was performed weekly for 12 weeks. Before the gait analysis each week, the lower limbs were shaved, and toenails were trimmed. Each rat was marked with a black marker dot at 3 locations along the lower leg: mid-way up the shaft of the leg, the back edge of the heel, and the joint at the fifth metatarsal head.
The video gait analysis was performed by having each rat walk along the full length of a Plexiglas walkway (100 cm × 10 cm × 30 cm), starting from the far left-side entrance until the exit on the right-most side of the walkway. Footage of the rats’ gait was captured using an iPhone 7, with a 12-megapixel wide-angle camera with f/1.8 aperture and optical image stabilization. The footage was then uploaded to a computer, where Adobe Premiere Pro CC Software (Adobe, San Jose, CA, USA) was utilized to obtain specific frame shots of the toe-off phase from the gait analysis for each rat. Then, the angle of ankle at toe lift off was measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA). Three frames of the toe lift-off stage were used for analysis and angle measurement every week for each rat. Each video was assessed by two different raters, who were blinded to the treatment groups, and the final result was the average of the two raters. A larger ankle angle observed is associated with a larger stride length in the rat steps, which correlates with an increase of functional recovery.
Tissue harvest and myelin staining
Twelve weeks postoperatively, the rats were given a lethal dose of isoflurane, perfused transcardially with 0.9% saline to flush the blood followed by 4% PFA in 0.1 M PBS, pH 7.4. The sciatic nerves were harvested and placed in 4% PFA for 24 hours and then parsed into three segments: a 3 mm-long segment taken 3 mm proximal to the SNG, a 3 mm-long segment taken in the center of the SNG, and a 3 mm-long segment taken 3 mm distal to the SNG. To view myelinated axons, the 3 mm-long segments were rinsed twice in 1× PBS, then placed in 2% osmium tetroxide in 1× PBS for 2 hours, before being dehydrated in ethanol and paraffin embedded (Di Scipio et al., 2008). The paraffin embedded segments were then sectioned transversally 5 µm thick, placed on slides and cover slipped with Permount. All sections were imaged under the same parameters at 20× on a Keyence BZ-9000 (Keyence Corporation, Itasca, IL, USA). An assessment of the myelinated axons was conducted using the Keyence BZ-II Analyzer software (Keyence Corporation) using the same threshold for all images. Only axons larger than 1 µm in diameter were analyzed.
Statistical analysis
Prism 6 (GraphPad Software, San Diego, CA, USA) was used for statistical analyses. One-way analysis of variance (ANOVA) was used to analyze axon growth in situ, myelinated axon count, and myelinated axon size. Two-way ANOVA with days as the repeated measures was used to analyze functional scores. If ANOVA results were significant, Dunnett’s test was used to compare all treatments to the controls (NT group), or Tukey’s test was used to find means that are significantly different from each other. Differences were deemed significant at P < 0.05. Data are presented as mean ± standard error of the mean (SEM).
Results
MCMs and growth factor delivery in vitro
After incubation in a 4.2 mM bicarbonate mSBF solution, scanning electron microscopy images revealed a continuous mineral coating that covered the entire surface of the MCMs (Figure 1B). An in vitro release profile of growth factors was quantified in SBF for 22 days. NGF expressed an initial burst release for the first 3 days (day 1: 5.48 ± 0.09 ng, day 3: 1.03 ± 0.05 ng), followed by a linear continuous release and the MCMs were still releasing 13.6 ± 0.18 pg of NGF per mg MCMs on day 22 (Figure 1C). GDNF also expressed an initial burst release for the first 3 days (day 1: 5.42 ± 0.07 ng, day 3: 2.44 ± 0.1 ng), followed by a linear continuous release and the MCMs were still releasing 28.3 ± 9.46 pg of GDNF per mg MCMs on day 22 (Figure 1D).
MCMs delivering growth factors in situ
After culturing the nerve explants containing the labeled DRG neurons for 7 days, the nerves were fixed in PFA and sectioned longitudinally. The DRG neurons were labeled extensively with Vybrant Dil and easily visible (Figure 3B and D). The DRG neurons in both the nerves injected with saline or MCMs alone, exhibited limited axon growth and the Vybrant Dil labeling was only visible within ~2 mm of the DRG injection site (Saline injection 1.73 ± 0.09 mm; MCM injection 2.00 ± 0.29 mm). In the group injected with MCMs + NGF & GDNF, the Vybrant Dil was observed at significantly further distances extending toward the growth factor injection (MCMs + NGF & GDNF injection 5.59 ± 0.31 mm; F(2, 6) = 73.47, P < 0.0001, one-way ANOVA; Figure 3C), than the nerves injected with PBS (P < 0.0001) and the nerves injected with MCMs alone (P = 0.0001). There was no significant difference between the nerves injected with PBS and the nerves injected with MCMs alone (P = 0.739).
Functional recovery
Due to abnormal walking gaits, one rat from the NT group, one rat from the MCM group, one rat from the MCM + NGF group, and one rat from the MCM + NGF & GDNF group, were removed from analysis of ankle angle at toe lift-off. When walking these four rats would drag their feet slightly and swing their foot sideways during stepping, making it unfeasible to measure the ankle angle.
As previously shown, measuring the ankle angle at the toe lift-off phase is a reliable measure of functional recovery after nerve injury, where uninjured rats have an ankle angle of approximately 100° on toe lift-off (Lee et al., 2013). We observed significant differences in ankle angle at toe lift-off among our groups (F(4,40) = 5.312, P = 0.0016, two-way ANOVA). When compared to the NT group, there were no significant differences in ankle angle for the MCM group (Pweek 12 = 0.2342, Figure 4A). The MCM+NGF group had significantly larger angles than the NT group on weeks 5, 6 and 8 (Pweek 5 = 0.0235, Pweek 6 = 0.0121, Pweek 8 = 0.0316), but leveled off and was not significantly different on week 12 (Pweek 12 = 0.9700, Figure 4B). The MCM+GDNF group had significantly larger angles than the NT group on weeks 8, 9 and 11 (Pweek 8 = 0.0065 Pweek 9 = 0.0087, Pweek 11 = 0.0151), but also leveled off and was not significantly different on week 12 (Pweek 12 = 0.1160, Figure 4C). The MCM + NGF & GDNF group had significantly larger ankle angles on toe lift-off than the NT group starting on week 7 (Pweek 7 = 0.0239) and continuing through week 12 (Pweek 12 = 0.0012; Figure 4D).
Figure 4.
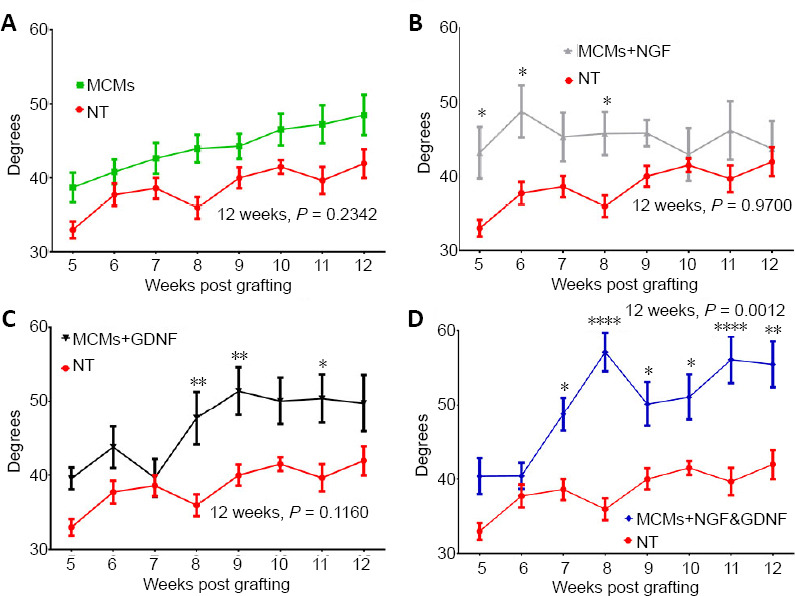
Functional recovery was assessed by measuring the angle of the ankle angle at toe liftoff.
There was no significant difference in ankle angle in the group treated with MCMs compared to the no treatment (NT) controls (A). The rats treated with MCMs + NGF had significantly larger angles on weeks 5 and 6 but planed off and were not significantly different on week 12 (B). The rats treated with MCMs + GDNF had significantly larger angles on week 9, but they also leveled off and were not significantly different from the NT group on week 12 (C). The rats treated with MCMs + NGF & GDNF were significantly higher than the rats receiving no treatment starting on week 7 and continuing through week 12 (D). *P < 0.05, **P < 0.01, ***P < 0.001 (two-way analysis of variance followed by Dunnett’s test). Error bars represent ± SEM; n = 9. GDNF: Glial cell-derived neurotrophic factor; MCM: mineral coated microparticle; NGF: nerve growth factor.
Myelinated axons
At 12 weeks postoperatively, the sciatic nerves were harvested and viewed under the operating microscope. All isografts anatomically incorporated with the sciatic nerve both proximally and distally with no signs of infection or graft rejection.
Myelinated axons were assessed in transverse sections 3 mm proximal to the graft, in the center of the graft, and 3 mm distal to the graft. When observing across all groups, the axons proximal to the graft (12.88 ± 0.65 µm2) were significantly larger with thick myelination (F(2,72) = 87.40, P < 0.0001, one-way ANOVA) compared to the axons in the graft (4.67 ± 0.38 µm2, P < 0.0001) and axons distal to the graft (6.08 ± 0.30 µm2, P < 0.0001).
Proximal to the graft, there were no significant differences between groups in the total number of axons (F(4,20) = 0.6259, P = 0.6495, one-way ANOVA; (Figure 5F), and in the size of the axons counted (F(4,20) = 0.6697, P = 0.6206, one-way ANOVA; (Figure 5G). However, there were significant differences between groups in the total number of axons counted in the center of the graft (F(4,20) = 11.13, P < 0.0001, one-way ANOVA; Figure 5F). The rats treated with MCM + GDNF had significantly more axons in their grafts than the NT group (P = 0.0035), and the MCMs group (P = 0.0040). The rats treated with MCMs + NGF & GDNF also had significantly more axons than the NT group (P = 0.0004), and the MCMs group (P = 0.0004). There were no significant differences between groups in the size of the axons present in the center of the graft (F(4,20) = 0.9718, P = 0.4448, one-way ANOVA; (Figure 5G). Distal to the graft, there were significant differences between groups in the total number of axons counted (F(4,20) = 8.012, P = 0.0005, One-way ANOVA; Figure 5F). The rats treated with MCMs + NGF & GDNF had significantly more axons distal to the graft than the NT group (P = 0.0056), the MCMs group (P = 0.0004), and the MCMs + NGF group (P = 0.0025). There were no significant differences between groups in the size of the axons present distal to the graft (F(4,20) = 0.0601, P = 0.9928, one-way ANOVA; Figure 5G).
Figure 5.
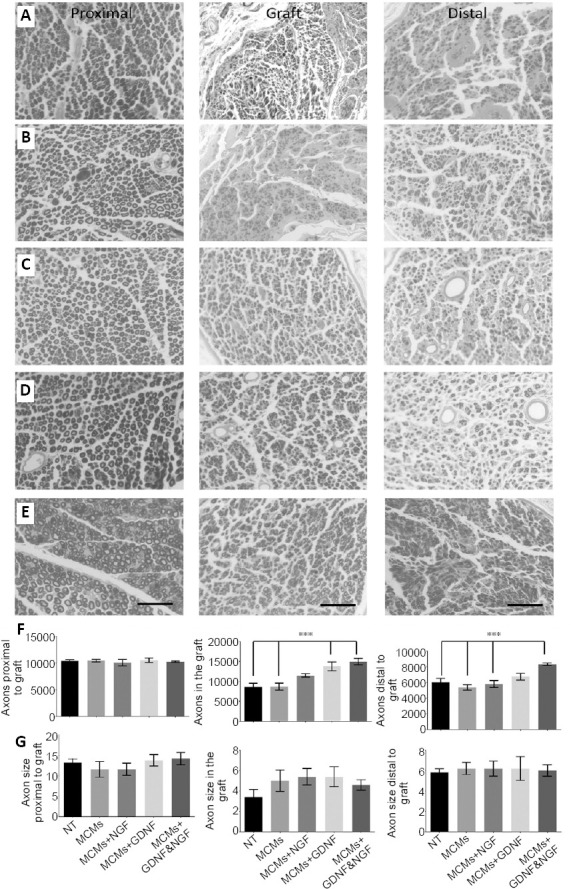
Twelve weeks after grafting, the rats were harvested and myelinated axons were labeled with osmium tetroxide.
Transverse sections were taken 3 mm proximal to the graft (first column), in the center of the 10 mm graft (second column), and 3 mm distal to the graft (third column). Micrographs were taken with a Keyence BZ9000 microscope of rats with an isograft and no treatment (NT group) (A), rats treated with MCMs only (B), rats treated with MCMs + NGF (C), rats treated with MCMs + GDNF (D), and rats treated with MCMs + NGF & GDNF (E). Scale bars: 50 µm. For all groups, the axons appeared larger with thick myelination in the proximal sections, compared to both in the graft and distal to the graft where the axons were smaller with a thin layer of myelination. Although, there were no significant differences among the groups in total numbers of axons proximal to the graft (P = 0.6495, one-way ANOVA), in the grafts of rats treated with MCMs + GDNF or the combination of MCMs + NGF & GDNF had significantly more myelinated axons (P < 0.0001, one-way ANOVA) and distal to the graft, only the rats treated with the combination of MCMs + NGF & GDNF had significantly more myelinated axons (P = 0.0005, one-way ANOVA) (F). There were no significant differences between groups in terms of axon size (µm2) proximal to the graft, in the graft, or distal to the graft (G). ***P < 0.001 (one-way ANOVA followed by Tukey’s post hoc test); error bars represent ± SEM; n = 4 per group. ANOVA: Analysis of variance; GDNF: glial cell-derived neurotrophic factor; MCM: mineral coated microparticle; NGF: nerve growth factor.
Discussion
Similar to what has been previously shown (Khalil et al., 2017; Orth et al., 2017; Yu et al., 2017; Clements et al., 2018; McMillan et al., 2018; Clements and Murphy, 2019; Fontana et al., 2019; Hellenbrand et al., 2019), the mineral coatings grown in this study bind proteins with a high affinity and release biologically active proteins over a controllable time frame. Here, we hypothesized that mineral coatings generating a sustained release of NGF and GDNF would induce significantly more axons to grow through a nerve autograft, resulting in a significant improvement in functional recovery. The in vitro release profile shows a burst release of NGF and GDNF for the first 3 days followed by a sustained release for at least 22 days. It is important to note, the enzyme-linked immuno-sorbent assay measure the amount of growth factor that is immunoreactive, however, this does not confirm that the growth factor is biologically active. Even though our in vivo data demonstrates that the growth factor released from MCMs is bioactive, future studies should include in vitro neurite outgrowth assays to confirm growth factor biological activity before using the MCMs for delivery in vivo. Although the growth factor delivery profile in vivo may vary from the in vitro data, this in vitro data provides an approximate timeline of the growth factor delivery. Importantly this approximate growth factor delivery timeline is evidence that MCMs have the capability to release growth factors for a timeline needed to grow axons through a 10 mm graft. Previous research, using the sensory pinch test on 10 mm grafts, shows that axons regenerating into nerve grafts will have an initial delay period of approximately 3.6 days, and then the axons grow at a rate of approximately 1.5 mm per day (Holmquist et al., 1993; Danielsen et al., 1995).
To determine if MCMs releasing NGF and GDNF could create a natural diffusion growth factor gradient in nerve tissue and promote axon growth from a distance of 10 mm, an in situ model was used. After 7 days of culturing the nerves containing DRG neurons, there was very little growth from the DRG neurons when saline or unloaded MCMs were injected. However, when MCMs loaded with NGF and GDNF were injected, there was robust growth from the DRG neurons, with Vybrant Dil labeling extending the full 10 mm and reaching the MCMs in some of the nerves. Although this model resulted in axon growth approximations from DRG neurons, future in situ experiments should also include motor neurons to detect the effects on both sensory neurons and motor neurons. When treating in vivo, the MCMs releasing GDNF and NGF are incorporated on the distal end of a 10 mm graft. This differs from the in situ model because in vivo the axons must first grow across the proximal nerve graft interface to be within 10 mm of the MCMs. Regardless, the in situ model provides a growth factor gradient approximation, demonstrating that the MCMs releasing growth factors are expected to promote more axon growth once the axons cross the proximal nerve graft interface in vivo.
When testing in vivo, the axons proximal to the graft were much larger than the axons observed in the graft or distal to the graft for all groups, which is reminiscent of the arborization that occurs when nerves are cut (Witzel et al., 2005). Even with growth factor treatment, the axons were smaller both in the graft and distal to the graft for all groups tested. We did not observe any significant differences between the groups in terms of axon size. In terms of axons counted, both the MCMs + GDNF and the MCMs + NGF & GDNF significantly increased the number of myelinated axons counted in the graft, but only the combination growth factor delivery of MCMs + NGF & GDNF significantly increased the number of myelinated axons distal to the graft. In contrast, the MCMs + NGF did not significantly increase the number of axons in the graft or distal to the graft. A couple possible reasons for the lack of myelinated axon growth when delivering NGF are 1) NGF acts predominantly on tyrosine kinase A receptors, which are expressed predominantly on nonmyelinated axons (Benito-Gutierrez et al., 2006), and 2) NGF was given at a lower concentration than GDNF. The concentration of NGF and GDNF used was based on previous studies where NGF had effects at lower concentration than GDNF (Vejsada et al., 1998; Boyd and Gordon, 2003; Lin et al., 2016; Tajdaran et al., 2016). Importantly, this study was aimed at developing a new local sustained drug delivery platform, which differed from the previous studies. Thus, determining the concentration of growth factor to be delivered from MCMs based on previous literature was not ideal. Although, this study was an important first step in developing and testing the MCMs and growth factor release, there was only one concentration of growth factor tested. It would be beneficial for a future study to be conducted testing multiple concentrations of growth factors released from MCMs, to determine an optimal dose of growth factor delivery from MCMs.
The overall objective of this study was to increase the number of axons growing through a SNG and thus improve the amount of hind limb function innervated from the newly grown axons. As shown previously, measuring the ankle angle at toe lift-off was a more precise and a better prognostic indicator of functional recovery over time as compared to other methods such as measuring sciatic functional index (Lee et al., 2013; Rui et al., 2014; Wang et al., 2018). In this study, the NT group that received grafts with no other treatment improved from an ankle angle of 34° ± 1.12° at 5 weeks of to an ankle angle of 41.92° ± 1.94° at 12 weeks. The group that received unloaded MCMs also improved over time (MCMsweek 5 = 38.69° ± 2°, MCMsweek 12 = 48.47° ± 2.73°) and appeared to do slightly better than the NT group, however there was no significant difference between these groups at any time point tested. At the time of harvest (12 weeks) only the MCMs + NGF & GDNF group had significantly larger ankle angles at toe lift-off compared to rats with a graft only. This correlated with our histological data showing that the MCMs + NG & GDNF was the only group with significantly more axons distal to the graft. Interestingly, there were earlier points that reached functional significance for both the MCMs + NGF group (weeks 5 & 6) and the MCMs + GDNF group (week 9). Although speculative, it is possible that axons within these groups grew through the SNG, but then the axons were pruned at a later time point due to inadequate survival signals. Regardless, the ankle angle measurements clearly show that MCMs releasing both NGF and GDNF have the capability to significantly increase axon growth through a nerve graft resulting in a significant improvement of functional recovery. A limitation of this study is the lack of testing sensory function and neuropathic pain. NGF effects sensory axons, which could explain why the GDNF group had better ankle angle measurements. Future studies should include tests for motor function, sensory function, and neuropathic pain.
Although this study was a necessary first step in testing growth factor delivery from MCMs to induce axon growth through a nerve graft, the end goal would be to tailor the growth factor release profile for the time needed to grow the axons through the graft. In clinic, many of the nerves repaired require a longer graft than the 10 mm grafts used here in rats, which highlights the need to tailor the growth factor release profile to the length of graft used. Ideally, the growth factors should remain at a localized therapeutic level for a time period long enough to grow robust myelinated axons the entire length of the graft and growth factor release should be finished when the axons reach the distal end, thus not causing axon entrapment. This highlights the advantages of mineral coatings, because coating properties can be adjusted to fine-tune the growth factor release profile (Yu et al., 2014, 2017). In this study, we observed some axon entrapment both in situ and in vivo. In some of the nerves in situ, axons reached the MCMs and lingered around them instead of advancing. Also in vivo there were almost twice as many axons in the graft compared to distal to the graft, which may be caused by the extended growth factor release. It may be necessary to have a depot of sustained growth factor or release in the distal nerve stump, to entice the axons to grow out of the graft and into the distal stump. In conclusion, the MCMs bound, stabilized and released growth factors in a sustained manner, which enhanced axon growth through a 10 mm nerve graft and improved functional recovery in rats. However, future work should consist of testing different growth factor release profiles from MCMs, on multiple lengths of nerve graft, and MCMs in the distal nerve stump.
Footnotes
C-Editors: Zhao M, Li CH; T-Editor: Jia Y
Conflicts of interest: WLM is a co-founder and stockholder of Dianomi Therapeutics. The other authors declare that they have no competing interests.
Financial support: None.
Institutional review board statement: The animal experiments were approved by University of Wisconsin-Madison Animal Care and Use Committee (ACUC, protocol# M5958) on January 3, 2018.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Benito-Gutierrez E, Garcia-Fernandez J, Comella JX. Origin and evolution of the Trk family of neurotrophic receptors. Mol Cell Neurosci. 2006;31:179–192. doi: 10.1016/j.mcn.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain CS, Lee JS, Leiferman EM, Maassen NX, Baer GS, Vanderby R, Murphy WL. Effects of BMP-12-releasing sutures on Achilles tendon healing. Tissue Eng Part A. 2015;21(5-6):916–927. doi: 10.1089/ten.tea.2014.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clements AEB, Murphy WL. Injectable biomaterials for delivery of interleukin-1 receptor antagonist: Toward improving its therapeutic effect. Acta Biomater. 2019;93:123–134. doi: 10.1016/j.actbio.2019.04.051. [DOI] [PubMed] [Google Scholar]
- 5.Clements AEB, Groves ER, Chamberlain CS, Vanderby R, Murphy WL. Microparticles locally deliver active interleukin-1 receptor antagonist in vivo. Adv Healthc Mater. 2018;7:e1800263. doi: 10.1002/adhm.201800263. [DOI] [PubMed] [Google Scholar]
- 6.Danielsen N, Kerns JM, Holmquist B, Zhao Q, Lundborg G, Kanje M. Predegeneration enhances regeneration into acellular nerve grafts. Brain Res. 1995;681:105–108. doi: 10.1016/0006-8993(95)00300-f. [DOI] [PubMed] [Google Scholar]
- 7.Deumens R, Bozkurt A, Meek MF, Marcus MA, Joosten EA, Weis J, Brook GA. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92:245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Di Scipio F, Raimondo S, Tos P, Geuna S. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microsc Res Tech. 2008;71:497–502. doi: 10.1002/jemt.20577. [DOI] [PubMed] [Google Scholar]
- 9.Evans GR. Peripheral nerve injury: a review and approach to tissue engineered constructs. Anat Rec. 263:396–404. doi: 10.1002/ar.1120. [DOI] [PubMed] [Google Scholar]
- 10.Fontana G, Martin HL, Lee JS, Schill K, Hematti P, Murphy WL. Mineral-coated microparticles enhance mRNA-based transfection of human bone marrow cells. Mol Ther Nucleic Acids. 2019;18:455–464. doi: 10.1016/j.omtn.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geissler J, Stevanovic M. Management of large peripheral nerve defects with autografting. Injury 50 Suppl. 2019;5:S64–67. doi: 10.1016/j.injury.2019.10.051. [DOI] [PubMed] [Google Scholar]
- 12.Hanna A, Thompson DL, Hellenbrand DJ, Lee JS, Madura CJ, Wesley MG, Dillon NJ, Sharma T, Enright CJ, Murphy WL. Sustained release of neurotrophin-3 via calcium phosphate-coated sutures promotes axonal regeneration after spinal cord injury. J Neurosci Res. 2016;94:645–652. doi: 10.1002/jnr.23730. [DOI] [PubMed] [Google Scholar]
- 13.Hellenbrand DJ, Hanna A. Treating spinal cord injury via sustained drug delivery from calcium phosphate coatings. Neural Regen Res. 2016;11:1236–1237. doi: 10.4103/1673-5374.189176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hellenbrand DJ, Kaeppler KE, Ehlers ME, Thompson CD, Zurko JC, Buchholz MM, Springer AR, Thompson DL, Ibrahim RK, Hanna A. Immunohistochemical assessment of rat nerve isografts and immunosuppressed allografts. Neurol Res. 2016;38:1094–1101. doi: 10.1080/01616412.2016.1248626. [DOI] [PubMed] [Google Scholar]
- 15.Hellenbrand DJ, Reichl KA, Travis BJ, Filipp ME, Khalil AS, Pulito DJ, Gavigan AV, Maginot ER, Arnold MT, Adler AG, Murphy WL, Hanna AS. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. J Neuroinflammation. 2019;16:93. doi: 10.1186/s12974-019-1479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmquist B, Kanje M, Kerns JM, Danielsen N. A mathematical model for regeneration rate and initial delay following surgical repair of peripheral nerves. J Neurosci Methods. 1993;48(1-2):27–33. doi: 10.1016/s0165-0270(05)80004-4. [DOI] [PubMed] [Google Scholar]
- 17.Hoyng SA, De Winter F, Gnavi S, de Boer R, Boon LI, Korvers LM, Tannemaat MR, Malessy MJ, Verhaagen J. A comparative morphological, electrophysiological and functional analysis of axon regeneration through peripheral nerve autografts genetically modified to overexpress BDNF, CNTF, GDNF, NGF, NT3 or VEGF. Exp Neurol. 2014;261:578–593. doi: 10.1016/j.expneurol.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Kallio PK, Vastamäki M. An analysis of the results of late reconstruction of 132 median nerves. J Hand Surg Br. 1993;18:97–105. doi: 10.1016/0266-7681(93)90205-t. [DOI] [PubMed] [Google Scholar]
- 19.Khalil AS, Yu X, Xie AW, Fontana G, Umhoefer JM, Johnson HJ, Hookway TA, McDevitt TC, Murphy WL. Functionalization of microparticles with mineral coatings enhances non-viral transfection of primary human cells. Sci Rep. 2017;7:14211. doi: 10.1038/s41598-017-14153-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JY, Giusti G, Wang H, Friedrich PF, Bishop AT, Shin AY. Functional evaluation in the rat sciatic nerve defect model: a comparison of the sciatic functional index, ankle angles, and isometric tetanic force. Plast Reconstr Surg. 2013;132:1173–1180. doi: 10.1097/PRS.0b013e3182a3bfeb. [DOI] [PubMed] [Google Scholar]
- 21.Lin KM, Shea J, Gale BK, Sant H, Larrabee P, Agarwal J. Nerve growth factor released from a novel PLGA nerve conduit can improve axon growth. J Micromech Microeng. 2016;26:045016. [Google Scholar]
- 22.McMillan A, Nguyen MK, Gonzalez-Fernandez T, Ge P, Yu X, Murphy WL, Kelly DJ, Alsberg E. Dual non-viral gene delivery from microparticles within 3D high-density stem cell constructs for enhanced bone tissue engineering. Biomaterials. 2018;161:240–255. doi: 10.1016/j.biomaterials.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orth M, Kruse NJ, Braun BJ, Scheuer C, Holstein JH, Khalil A, Yu X, Murphy WL, Pohlemann T, Laschke MW, Menger MD. BMP-2-coated mineral coated microparticles improve bone repair in atrophic non-unions. Eur Cell Mater. 2017;33:1–12. doi: 10.22203/eCM.v033a01. [DOI] [PubMed] [Google Scholar]
- 24.Ortmann SD, Hellenbrand DJ. Glial cell line-derived neurotrophic factor as a treatment after spinal cord injury. Neural Regen Res. 2018;13:1733–1734. doi: 10.4103/1673-5374.238610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosich K, Hanna BF, Ibrahim RK, Hellenbrand DJ, Hanna A. The effects of glial cell line-derived neurotrophic factor after spinal cord injury. J Neurotrauma. 2017;34:3311–3325. doi: 10.1089/neu.2017.5175. [DOI] [PubMed] [Google Scholar]
- 26.Rui J, Runge MB, Spinner RJ, Yaszemski MJ, Windebank AJ, Wang H. Gait cycle analysis: parameters sensitive for functional evaluation of peripheral nerve recovery in rat hind limbs. Ann Plast Surg. 2014;73:405–411. doi: 10.1097/SAP.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 27.Siemionow M, Brzezicki G. Chapter 8: Current techniques and concepts in peripheral nerve repair. Int Rev Neurobiol. 2009;87:141–172. doi: 10.1016/S0074-7742(09)87008-6. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. 2016;17:2101. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tajdaran K, Gordon T, Wood MD, Shoichet MS, Borschel GH. A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts. Acta Biomater. 2016;29:62–70. doi: 10.1016/j.actbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 31.Tria MA, Fusco M, Vantini G, Mariot R. Pharmacokinetics of nerve growth factor (NGF) following different routes of administration to adult rats. Exp Neurol. 1994;127:178–183. doi: 10.1006/exnr.1994.1093. [DOI] [PubMed] [Google Scholar]
- 32.Vejsada R, Tseng JL, Lindsay RM, Acheson A, Aebischer P, Kato AC. Synergistic but transient rescue effects of BDNF and GDNF on axotomized neonatal motoneurons. Neuroscience. 1998;84:129–139. doi: 10.1016/s0306-4522(97)00497-1. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: A comparison between sciatic functional index and kinematic analysis. PLoS One. 2018;13:e0208985. doi: 10.1371/journal.pone.0208985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witzel C, Rohde C, Brushart TM. Pathway sampling by regenerating peripheral axons. J Comp Neurol. 2005;485:183–190. doi: 10.1002/cne.20436. [DOI] [PubMed] [Google Scholar]
- 35.Yu X, Khalil A, Dang PN, Alsberg E, Murphy WL. Multilayered inorganic microparticles for tunable dual growth factor delivery. Adv Funct Mater. 2014;24:3082–3093. doi: 10.1002/adfm.201302859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Biedrzycki AH, Khalil AS, Hess D, Umhoefer JM, Markel MD, Murphy WL. Nanostructured mineral coatings stabilize proteins for therapeutic delivery. Adv Mater. 2017;29 doi: 10.1002/adma.201701255. 10.1002/adma.201701255. [DOI] [PMC free article] [PubMed] [Google Scholar]