Abstract
Encouraging results have been reported for the use of transcranial magnetic stimulation-based nerve stimulation in studies of the mechanisms of neurological regulation, nerve injury repair, and nerve localization. However, to date, there are only a few reviews on the use of transcranial magnetic stimulation for diabetic neuropathy. Patients with diabetic neuropathy vary in disease progression and show neuropathy in the early stage of the disease with mild symptoms, making it difficult to screen and identify. In the later stage of the disease, irreversible neurological damage occurs, resulting in treatment difficulties. In this review, we summarize the current state of diabetic neuropathy research and the prospects for the application of transcranial magnetic stimulation in diabetic neuropathy. We review significant studies on the beneficial effects of transcranial magnetic stimulation in diabetic neuropathy treatment, based on the outcomes of its use to treat neurodegeneration, pain, blood flow change, autonomic nervous disorders, vascular endothelial injury, and depression. Collectively, the studies suggest that transcranial magnetic stimulation can produce excitatory/inhibitory stimulation of the cerebral cortex or local areas, promote the remodeling of the nervous system, and that it has good application prospects for the localization of the injury, neuroprotection, and the promotion of nerve regeneration. Therefore, transcranial magnetic stimulation is useful for the screening and early treatment of diabetic neuropathy. Transcranial magnetic stimulation can also alleviate pain symptoms by changing the cortical threshold and inhibiting the conduction of sensory information in the thalamo-spinal pathway, and therefore it has therapeutic potential for the treatment of pain and pain-related depressive symptoms in patients with diabetic neuropathy. Additionally, based on the effect of transcranial magnetic stimulation on local blood flow and its ability to change heart rate and urine protein content, transcranial magnetic stimulation has potential in the treatment of autonomic nerve dysfunction and vascular injury in diabetic neuropathy. Furthermore, oxidative stress and the inflammatory response are involved in the process of diabetic neuropathy, and transcranial magnetic stimulation can reduce oxidative damage. The pathological mechanisms of diabetic neuropathy should be further studied in combination with transcranial magnetic stimulation technology.
Keywords: autonomic neuropathy, central nervous system, depression, inflammation, oxidative stress, pain, peripheral nerve, plasticity, recovery, regeneration, vascular
Introduction
Overview of transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) is a focused, non-invasive form of cerebral cortical and local stimulation, and its application was first reported in the functional localization of the central nervous system (Barker et al., 1985; Xu and Sun, 2020; Yang et al., 2020). By choosing different stimulation patterns and setting different parameters, TMS can be used in central and peripheral nervous system lesions for evaluating the excitability and integrity of the corticospinal tract and determining the degree of damage to motor function and the motor conduction pathway (Takahashi et al., 2015). Furthermore, TMS can affect local cerebral blood flow, change oxidative stress levels, and promote limb functional recovery after nerve injury (Beaulieu et al., 2013). Currently, TMS research has focused primarily on neurological regulatory mechanisms, as well as combined diagnostic and clinical treatment techniques.
Based on Faraday’s law of induction, TMS generates an induced electric field in the conductor by varying the magnetic field (Barker et al., 1985). When the induced current reaches a critical level of intensity, the neuronal axon hillock or interneurons can be depolarized, and neurological function can be regulated by activating or changing the excitability of neurons (Takahashi et al., 2015). By selecting the appropriate stimulating coil (circular, “8-shaped”, biconical, arc-shaped, H-shaped or quatrefoil-shaped), specific cerebral cortical (or deeper) regions can be targeted by varying the stimulation intensity, effective depth, and actuating range.
There are basically three stimulation patterns: single-pulse TMS, double-pulse TMS (paired-pulse TMS), and repetitive-pulse TMS (rTMS). Single-pulse TMS is targeted to the motor cortex with the help of neuronavigation and by measuring motor-evoked potential amplitudes from the peripheral muscles to assess cortical reactivity. Paired-pulse TMS uses paired-pulse conditioning stimuli to probe local facilitatory and inhibitory function at specific interstimulus intervals in the motor cortex, including short-interval intracortical inhibition and long-interval intracortical inhibition. Patterned trains of pulses (i.e. rTMS) induce changes in cortical excitability and metabolism that last beyond the stimulation itself. On-off patterns of high frequency subthreshold stimulation (> 1 Hz, commonly > 3 or 5 Hz) can increase the motor-evoked potential amplitude and excitability of neurons, while continuous low-frequency subthreshold stimulation (≤ 1 Hz) can reduce excitability. A new form of rTMS, called theta burst stimulation (TBS), has been developed and has been applied as intermittent TBS and continuous TBS protocols (Fried et al., 2017; Lanza et al., 2020).
Synaptic transmission can be strengthened or weakened over a long period of time by external factors. Based on the plasticity of synapses, central and peripheral TMS is mainly applied as high-frequency stimulation for long-term potentiation or as low-frequency stimulation for long-term depression, to achieve two-way adjustment of neural excitability (George et al., 1996). The different modes of nerve stimulation by TMS include stimulation of the morphological unit to achieve local stimulation, stimulation of functional units to achieve interactions between different brain functional areas, and stimulation of the neural network to achieve stimulation at a distal site. To date, TMS has mainly been conducted for central and peripheral nervous system injury and nerve diagnostics, with favorable results. However, research on extensive polyneuropathy, such as diabetic neuropathy (DN), remains limited.
Research state of diabetic neuropathy
DN mainly involves somatic and/or autonomic nerve damage to the peripheral nervous system caused by high glucose concentration. Clinically, DN is divided into two types: (1) typical DN, including distal symmetric polyneuropathy (DSPN) and autonomic neuropathy; and (2) atypical DN, including single peripheral neuropathy, radiculopathy, and polyradiculopathy. DSPN is the most common clinical form of DN, characterized by pain, paresthesia, and late-stage complications, such as sleep deprivation, depression, foot ulcers, hemorrhage, motor function decline, and even amputation (Zakin et al., 2019). A high incidence of these complications increases the risk of death and takes a heavy toll on the quality of life of the patient. At this point, the goals of DN treatment include maintaining normal blood glucose levels, relieving pain, and expectant treatment of complications at various organ system levels. Although priority treatment remains maintaining normal or near-normal levels of blood glucose, intensified hypoglycemic therapy does not necessarily control the progression of neuropathy in type 1 or type 2 diabetes (Pop-Busui et al., 2017; Johann et al., 2018). Clinically, symptomatic control of DN typically focuses on relieving pain. Nevertheless, data suggest that roughly 50% of diabetic peripheral neuropathy cases may not involve pain symptoms (Jensen et al., 2011). Therefore, in early diagnosis or among patients with mild complications, it is rather difficult to introduce intervention owing to the presence of risk factors, resulting in irreversible neuropathy among a substantial number of patients. Therefore, improvement of differential diagnosis and effective intervention, based on rigorous research, are required to diagnose and treat DN (Zakin et al., 2019). Basic and clinical studies of TMS technology have demonstrated the advantages of the technology for diagnostics and neuromodulation. Thus, TMS is considered a highly promising approach for the diagnosis and treatment of DN involving neurodegeneration, pain, blood flow change, autonomic nervous disorders, vascular endothelial injury, and depression. In the following section, based on a review of 111 references, we evaluate and discuss the research findings and clinical application potential of TMS for DN.
In this review, we aimed to collect and assess significant papers on the beneficial effects of TMS for DN treatment. We discuss the research value of TMS in various treatments for neurodegeneration, pain, blood flow change, autonomic nervous disorders, vascular endothelial injury, and depression (Figure 1).
Figure 1.
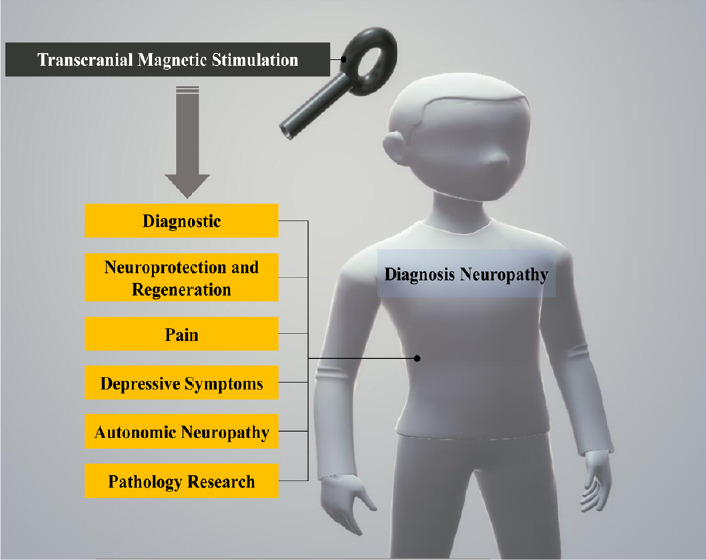
Application of transcranial magnetic stimulation in diabetic neuropathy.
Search Strategy
An electronic search of the MEDLINE database for literature describing TMS and DN of SCI from 1985 to 2020 was performed using the following search conditions: SCI (MeSH Terms) AND (TMS, DN (MeSH Terms) OR Diabetic, TMS (MeSH Terms) OR Blood sugar (MeSH Terms). The results were further screened by title and abstract to only acquire papers related to Diabetic and/or TMS. Unrelated diseases were excluded.
In addition, an electronic search of the Medline database for magnetic stimulation in various research studies of DN was completed. This included publications prior to March 2020, with the following search criteria: inducing DN diagnosis, DN clinical symptom, and DN pathological mechanism. Subsequent searches were completed that were specifically relevant to the application of TMS technology with the following terms: diagnosis, peripheral nerve, pain, depression, vascular, autonomic neuropathy, oxidative stress, inflammation. Articles that did not correspond to pharmaceutical research were excluded.
Application of Transcranial Magnetic Stimulation in Diabetic Neuropathy
Diagnostic value of transcranial magnetic stimulation for diabetic neuropathy
Neuropathy starts at the early stage of diabetes, and free glucose in the blood after food consumption causes DSPN and subsequent disease progression (Pafili et al., 2018). Thus, subjects with impaired glucose tolerance are generally more likely to develop neuropathy than subjects with impaired fasting glucose (Bongaerts et al., 2012). Experts from the American Diabetes Association recently pointed out that DSPN screening should be conducted among patients with prediabetic and neuropathic symptoms (American Diabetes Association, 2018). Generally, pre-diabetic DSPN is less serious than dominant diabetes and mainly affects small nerve fibers, possibly involving pain. However, because small and giant nerve fibers are functionally complementary, damage to these nerves results in insignificant symptoms. Thus, the screening examination needs to cover both types of fibers to better assess the damage to small nerve fibers (Pafili et al., 2018). According to the American Diabetes Association 2018 Clinical Practice Guidelines (American Diabetes Association, 2018), the recommended protective sensory test method can only detect severe sensory loss (Orosz et al., 2017). Thus, more accurate early screening is needed to improve evaluation. Magnetic resonance and ultrasound-based neuroimaging have been useful for several focal and inflammatory neuropathies, and TMS technology may help address the space and time limits associated with these methods.
By itself, TMS may not be sensitive enough to assess DN-associated neuropathy. Thus, multi-segmental surface electrodes can be applied to detect motor-evoked potential and accurately control the irradiation/excitation and dose-effect relationship. Other assessment methods, including functional magnetic resonance imaging (fMRI), electroencephalogram (EEG) and ultrasound blood flow monitoring, can also be employed to establish a possible link between various functional parameters (Du et al., 2018; Che et al., 2019; Selvarajah ett al., 2019; Derosiere et al., 2020). Combined with these methods, TMS can be of great worth in the early detection and diagnosis of neuropathy or angiopathy in patients with DN. In asymptomatic neurodegenerative disorder patients, changes in short-interval intracortical inhibition and intracortical facilitation produced by TMS might precede the onset of neurodegenerative changes (Lanza et al., 2020).
Neurotransmission is highly dependent on the availability of glucose for energy. Corticospinal excitability is not affected directly by glucose, suggesting that acute changes in glucose levels do not alter TMS measures of corticospinal or intracortical excitability (Toepp et al., 2019). DN studies on the relationship between neurotransmitters, ion channels and motor cortical excitability need to be based on an understanding of the pharmacological mechanisms of various drugs. For example, the single patch-clamp technique cannot adequately evaluate overall drug efficacy. Thus, it is necessary to advance nerve electrophysiological research in clinical neurology and psychopharmacology. Huang et al. (2017) used high-frequency intermittent TMS and continuous TBS to precisely target and stimulate the left primary motor cortex, and found that patients with drug addiction had reduced cortical plasticity. Thus, TMS may be used as an evaluation method for diabetes and DN-related pharmacological research for medication testing and treatment development.
Application of transcranial magnetic stimulation for neuroprotection and neuroregeneration
Diabetic axonal injury is characterized by slowly progressing peripheral neuropathy. In patients with DN, the most distal part of the lower limbs is usually the first to succumb to neuropathy, with sensory disturbance being the most common symptom. Motor symptoms gradually manifest as the disease progresses (Tesfaye et al., 2010). Recently, neuroplasticity involving the central nervous system and the interaction between axons, glial cells, and the microenvironment have become the focus of study. Early in the course of type 2 diabetes, giant and small fibers are affected simultaneously to varying degrees (Ziegler et al., 2014). When blood glucose levels are high, the major pathological pathways of DN include polyol, pentose phosphate, hexosamine and protein kinase C pathways, as well as the activation of oxidative stress and inflammatory responses that act on neurons and Schwann cells, resulting in diabetic neurological dysfunction (Goncalves et al., 2017). C57BL/6N mice show a significant hippocampal neuroinflammatory response after 8–12 weeks on a high fructose diet. Microglial and astrocytic activation lead to gliosis and a significant drop in the total number of hippocampal neurons and newborn neurons (Li et al., 2019). The extracellular matrix supports peripheral cells and regulates the maintenance and repair mechanisms, so as to accelerate the regeneration and recovery of the nerve fibers remaining in the proximal nerve ends (Yasuda et al., 2003; Sango et al., 2017). Matrix metalloproteinases (MMPs), which are a component of the extracellular matrix regulatory system, are activated by hyperglycemia, oxidative stress and inflammatory cytokines. In the dorsal root ganglia of diabetic rats, MMP-2 is involved in axonal degeneration (Yasuda et al., 2003), and up-regulated expression of MMP-2 and MMP-9 after axotomy inhibits abnormal pain (Kuhad et al., 2015). Thus, while MMPs promote neural regeneration and increase neuropathic pain, they can also inhibit pain and block neural regeneration. These findings stress the difficulty of target nerve regeneration after injury and the role of neural plasticity in the development of neuropathic pain.
In 2004, the effects of oscillating magnetic fields on nerve regeneration were studied for the first time. Using coils to generate oscillating magnetic fields and stimulate the head of rats with substantia nigra injuries, researchers found that nerve regeneration in the subventricular zone was enhanced (Arias-Carrion et al., 2004). Based on this study, rats with white matter demyelination were subjected to an oscillating magnetic field with similar parameters. This treatment promoted the proliferation and migration of neural stem cells in the injured area and enhanced myelin sheath repair (Sherafat et al., 2012). Moreover, another study found that neural regeneration was enhanced in the hippocampus of magnetically-stimulated C57BL/6 mice (Cuecurazzu et al., 2010). Further studies showed that rTMS could enhance the effect of brain-derived neurotrophic factor in promoting motor learning and nerve regeneration (Gersner et al., 2011; Deveci et al., 2020). Wang and colleagues showed that rTMS activates the brain-derived neurotrophic factor–tyrosine receptor kinase B–N-methyl-D-aspartate receptor signaling pathway in rat cortical neurons (Wang et al., 2011). Furthermore, Villamar et al. showed that dopamine release was increased in the caudate nucleus and corpus striatum in healthy volunteers given rTMS to stimulate the prefrontal cortex (Villamar et al., 2012). These findings indirectly suggest that TMS neuroregulation techniques may promote neural regeneration.
Therefore, the application of TMS for targeted central and peripheral intervention may be a new way to treat neuropathy in patients with DN. Further research is needed to clarify the mechanisms by which TMS affects central neuromodulation, pain relief and neural regeneration.
Application of transcranial magnetic stimulation for relieving diabetic neuropathy-associated pain
Neuropathic pain is defined as pain caused by somatosensory system damage or disease (Pop-Busui et al., 2016), including burning, stinging or tingling (like an electric shock), and exhibits spontaneous or induced characteristics. In the past decade, researchers have made considerable progress in understanding the mechanisms of pain, and have elucidated the mechanisms by which inflammatory mediators cause peripheral sensitization (Basbaum et al., 2009) and central sensitization (Kuner et al., 2010) to induce inflammatory and neuropathic pain (Selvarajah et al., 2018). However, gaps remain in our knowledge of pain relief.
Treatment for pain caused by DN primarily involves preventing or delaying the progression of DN and interfering with pathways that perceive the noxious stimulus or transmit pain. At present, the clinical treatment for pain is still limited to drug therapy (Papanas et al., 2016; Pop-Busui et al., 2016; American Diabetes Association, 2018). However, the efficacy of these compounds is limited, with a relief rate for pain of no more than 50%. Additionally, treatment with different compounds has significant side effects (Papanas et al., 2016; Yekkirala et al., 2017). As a result, the direction of treatment has evolved toward multimodal management, including physical therapy and psychological intervention.
rTMS can excite multiple primary and secondary motor regions of the brain. The thalamus is the most important pain integration center and the main intermediate structure relaying sensory information to the cortex. The adjustment of pain in the advanced center involves mutual inhibition between the posterolateral nucleus and the posteromedial nucleus of the thalamus. rTMS directly excites the thalamus through the cortico-thalamic projection system, thereby inhibiting the transmission of sensory information through the spino-thalamic pathway (Gustin et al., 2014). rTMS stimulation of the primary motor cortical M1 region of patients with spinal cord injury has been reported to inhibit excessive excitation of thalamic and spinal neurons, thereby effectively alleviating pain (Gustin et al., 2014). The electrophysiological mechanisms by which rTMS alleviates neuropathic pain involves altering sensory thresholds in the cerebral cortex (Johnson et al., 2006). Chronic neuralgia involves secondary central sensitization, causing patients to suffer from temperature sensory regulatory dysfunction, and rTMS of the motor cortex can relieve pain and improve the associated temperature sensory function (Lefaucheur et al., 2008). rTMS has been shown to have a good therapeutic effect in peripheral neuralgia (Leung et al., 2009). The mechanisms by which rTMS exerts this therapeutic effect may include regulation of hemispheric inhibition, inhibition or facilitation of cortical excitability, and modulating cerebral blood flow, metabolism and pain signaling in the pain transmission pathway (Antonino et al., 2016). It has been shown that muscle pain exerts modulatory effects on sensorimotor cortical excitability, and that left dorsolateral prefrontal cortical rTMS has analgesic effects and modulates pain-induced sensorimotor cortical adaptations. These findings suggest an important role of prefrontal-basal ganglia communication in sensorimotor cortical excitability and pain processing (De et al., 2019). H-coil rTMS can act on the deep and extensive cerebral cortex. The application of high frequency (20 Hz) H-coil rTMS to the motor cortex can reduce lower extremity pain within 3 weeks in patients (Onesti et al., 2013). Other researchers used high-frequency (10 Hz) 8-coil rTMS to stimulate the motor cortex corresponding to the lower limbs on the scalp at Cz (hot point of the tibialis anterior muscle) in DN patients. Lower extremity pain relief was observed to last up to 5 weeks (Abdelkader et al., 2019). Therefore, the pain relief effect of rTMS in DN patients is clear.
The analgesic effect of TMS has been widely used in a variety of neuropathic pain treatments. However, the reported effects of rTMS on pain have been inconsistent, because of small numbers of patients, differences in TMS parameter settings, and lack of maintenance protocols. Therefore, specific brain region localization and comparable coil placement sites require further technical exploration through a brain navigation system. In addition, electrophysiological and imaging techniques (such as EEG and MRI) need to be used to assess the effects of TMS on other regions of the brain.
Application of transcranial magnetic stimulation for treating diabetic neuropathy-associated depressive symptoms
Current treatments for diabetes mellitus-associated depression include conventional hypoglycemic and antidepressant medications or drug combinations. It has been shown that the development of diabetes mellitus-associated depression may involve hypothalamic-pituitary-adrenal axis hyperactivity, insulin resistance, hippocampal neuronal regeneration disorder, intracerebral inflammation, and brain-derived neurotrophic factor deficiency (Ma et al., 2015). The United States Food and Drug Administration has approved TMS as a treatment for severe depressive disorder in adults without psychotic symptoms (Chaudhary et al., 2012). Diabetic peripheral neuropathic pain is a greater determinant of depression than other diabetes-related complications. Patients with diabetes-associated depression have a change in blood flow in the left dorsolateral prefrontal cortex. Stimulation of the dorsolateral prefrontal cortex with high-frequency rTMS can increase frontal lobe activity, and depressive symptoms can be alleviated after the metabolism in the left prefrontal cortex is improved (Fox et al., 2013). Depressive symptoms are associated with both painless and painful DN. Some painful symptoms (i.e., painful colds and electrical shocks) are significantly higher predictors of depression than tingling and numbness (Amato et al., 2016). Application of TMS for depression related to fibromyalgia may involve inhibition of motor cortical and descending pain systems (Cardinal et al., 2019). Therefore, the early screening and treatment of pain with TMS can be of clinical value in the prognosis and therapy of depressive symptoms in patients with DN. A recent study showed that changes in intestinal microbes can affect the gene expression profile of the entire prefrontal cortex (Hoban et al., 2016). Intestinal microbes are associated with a common mental disorder-depression. Based on previous animal experiments, the lack or alteration of intestinal microbes affects neurogenesis, blood–brain barrier function, and microglial maturation (Erny et al., 2015; Möhle et al., 2016).
At present, the pathogenesis by which depression arises as a complication of diabetes mellitus is unclear. The application of TMS has practical significance for the improvement of symptoms and related regulatory mechanisms of depression in the high-glucose state. Based on cortical plasticity, TMS technology could be applied to relieve depressive symptoms and assess the effect of antidepressants. Studying the relationship between pain and depressive symptoms with TMS may be helpful for the early screening and treatment of depressive symptoms in patients with DN. Further exploration of the impact of TMS on intestinal flora changes may provide new insight into the relationship between neuropathy and depression.
Application of transcranial magnetic stimulation in diabetic autonomic neuropathy
Diabetic autonomic neuropathy mainly affects gastrointestinal, cardiovascular, urinary, and genital functions (Vinik et al., 2003). Its clinical manifestations include orthostatic hypotension, gastroparesis, and erectile dysfunction. Clinically, anticholinergics and tricyclic antidepressants are used to improve diabetic autonomic neuropathy symptoms. At present, little is known about the control of autonomic function in the cerebral cortex. There is increasing interest in the potential therapeutic uses of magnetic stimulation. For example, some researchers have investigated the effects of magnetic stimulation on the human sympathetic nervous system, and found that TMS can cause transient changes in the cardiovascular system (Kaur et al., 2020). Researchers have also used rTMS to stimulate the primary motor cortical M1 region with the aim of elucidating the neural connection between the brain and the kidneys. The data show that the urine protein content in both healthy and diabetic patients is significantly increased. It has been speculated that there may be a functional connection between the brain and the kidneys via autonomic nerves (Rosaria et al., 2014). The short-term effects of TMS on autonomic nerves indirectly suggest that there is a potential risk of autonomic hyperreflexia-related side effects from TMS-targeted stimulation of other therapeutic targets. Further investigation may be needed to determine whether the short-term effects of TMS on the autonomic nervous system are beneficial in patients with DN.
Application of transcranial magnetic stimulation in vascular injury-related diabetic neuropathy
Patients with diabetes or pre-diabetes have increased morbidity and mortality owing to cardiovascular diseases (American Diabetes Association, 2018; Andersson et al., 2018; Pasquel et al., 2018) and microvascular complications (Vas et al., 2016). Changes in the vascular structure of the DN endothelium, that is, endothelial cell proliferation and basement membrane thickening, have been reported to lead to a narrowing of the lumen of the blood vessels and neurofibrillary ischemia, along with other vascular changes, such as increased blood viscosity (Nukada et al., 2014). Because dorsal root ganglia have the ability to regulate blood flow, their oxygen tension is lower and their neurovascular barrier is weaker than peripheral nerve trunks, and therefore, sensory neurons in dorsal root ganglia may be more susceptible to microvascular changes (Kobayashi et al., 2018). Given that endothelial dysfunction is an important event in the etiology of DN, endothelial dysfunction alone is sufficient to cause neuropathy. In the context of DN, the term “microvascular endothelial dysfunction” is often used to describe microvascular endothelium-dependent vasodilation due to reduced nitric oxide secretion, while endothelial cell adhesion and steady-state changes in thrombotic factors also contribute to microvascular endothelial dysfunction. In diabetes and even pre-diabetes, hyperglycemia affects neurovascular endothelial function in a variety of ways, resulting in decreased bioavailability of nitric oxide and prostacyclin, which affects neurovascular flow and leads to ischemia-related neurofibrillary damage (Chapouly et al., 2016). At present, microvascular blood flow changes in peripheral nerve injury is a hot topic, and TMS can improve cerebral blood flow. However, the effects of TMS on microvascular blood flow and vascular endothelial protective mechanisms in patients with DN have not been reported.
Application of Transcranial Magnetic Stimulation in Pathological Research on Diabetic Neuropathy
The pathophysiology of DN involves complex interactions between metabolism, the immune system, lifestyle, and genetic factors. Recent preclinical and clinical observations as well as epidemiological studies have shown that oxidative stress and inflammatory processes are important contributors to the pathogenesis of DN (Fernyhough et al., 2015; O’Brien et al., 2017; Feldman et al., 2019).
Oxidative stress
Previous studies have shown that oxidative stress in rat models of diabetes leads to a decrease in nerve conduction and neurovascular flow velocities (Stevens et al., 2000; Cameron et al., 2001), impairing nerve conduction (Li et al., 2004; Obrosova et al., 2004) and causing small fiber neuropathy (Ilnytska et al., 2006). This is associated with the activation of polyadenosine diphosphate-ribose polymerase 1 and the loss of nicotinamide adenine dinucleotide (NAD+)/adenosine triphosphate. The inhibitory effect of polyadenosine diphosphate-ribose polymerase 1 has been shown to counteract diabetes-induced oxidative stress and improve nerve fiber function in rats and mice (Lupachyk et al., 2011). Mitochondrial dysfunction caused by hyperglycemia has been reported to promote cytochrome c release from the mitochondria into the cytoplasm and activate the apoptosis-related protein, caspase-3, thereby inducing neuronal apoptosis (Li et al., 2001). Researchers have also found that hyperglycemia significantly increases the activity of aldose reductase, leading to the accumulation of excessive sorbitol in the body that, in turn, subjects cells to hypertonic conditions and edema, and results in nerve cell injury (Schmidt et al., 2001). It has been further demonstrated that DN can be aggravated by a decline in the antioxidant defense capacity and an increase in the generation of reactive oxygen species. The aggravation of DN is mainly associated with impaired blood flow and hypoxia of the endoneurium, reduced nerve conduction velocity, small fiber neuropathy, and axonal atrophy (Fernyhough et al., 2015).
Studies have shown that certain TMS application protocols can alleviate oxidative stress. For example, high-frequency rTMS reduced cell loss and oxidative damage in an oxidative stress model (Tunez et al., 2006). Low-frequency rTMS can reduce oxidative stress markers in the cerebrospinal fluid of patients with spinocerebellar degeneration (Sandrini et al., 2011). Furthermore, another study found that low-frequency TMS downregulates stress genes, fos and dusp1 in blood leukocytes (Tasset et al., 2013). In an experimental model of autoimmune encephalomyelitis, high-frequency rTMS application decreased oxidative stress and cell damage (Medina et al., 2017). However, the effects of TMS on oxidative stress and vascular endothelial injury in DN have not yet been reported.
Inflammatory response
In the DN animal models of type 1 diabetes and type 2 diabetes, it was found that the inflammatory response may lead to the degeneration of myelinated and unmyelinated nerve fibers, and may also cause damage to the blood-nerve barrier and the microvascular system (Jolivalt et al., 2016; O’Brien et al., 2017). Gene expression analysis of the sciatic nerve in the DN mouse model revealed a severe dysregulation of inflammation and immune regulatory pathways (O’Brien et al., 2015). Further studies showed that pro-inflammatory cytokines—interleukin (IL)-6, IL-1β and tumor necrosis factor-α—reduce nerve conduction velocity and cause neuropathic pain in DN animal models (Zhou et al., 2014; Pop-Busui et al., 2016). Immune mediators and other cytokines are also involved in the transition from painless DN to painful DN (Spallone et al., 2013; Cohen et al., 2014). Inhibition of IL-1β, IL-6 and tumor necrosis factor-α using drugs or neutralizing antibody restores nerve conduction velocity and relieves pain (Gabay et al., 2011; Urabe et al., 2015; Zhou et al., 2016). Furthermore, studies have shown that IL-4 and IL-10 have protective effects in DN (Clark et al., 2013). Injection of IL-6 causes neuropathic pain in patients with DSPN (Zhou et al., 2016), while IL-6 treatment is beneficial to the recovery of nerve structure and function in DN animals (Cox et al., 2017). Thus, it is speculated that inflammation is involved not only in the neurodegenerative disorder but also in the regeneration process (Lang et al., 2014). The underlying mechanisms remain to be elucidated (Cox et al., 2017). Further study is needed to clarify the mechanisms underpinning the analgesic effects of TMS and its role in improving motor function and blood flow.
Prospects and Problems
As discussed above, only a few studies have focused on the application of TMS in patients with DN. However, other studies show that TMS can be applied in various clinical diseases that share characteristics with DN (Figure 2 and Table 1).
Figure 2.
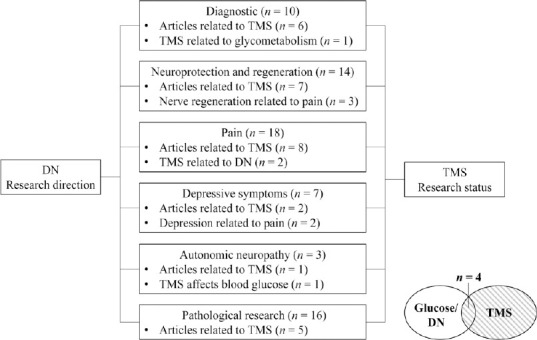
Summary chart of references.
n represents the number of cited references. DN: Diabetic neuropathy; TMS: transcranial magnetic stimulation.
Table 1.
Study on the correlation of TMS in DN branch fields
| DN related clinical problems and research direction | Research type | TMS | TMS-DN | ||
|---|---|---|---|---|---|
| Core content | Reference | Core content | Reference | ||
| Diagnostic techniques | Multiple combination technique | EEG/fMRI | Huang et al. (2017); Du et al. (2018); Che et al. (2019); Gerard et al. (2020) | – | |
| Application | Addictive drugs-synaptic plasticity | Corticospinal/intracortical | Toepp et al. (2019) | ||
| Nerve injury | Application | Hippocampal | Arisa et al. (2004) | – | – |
| Subventriculsar zone | Cuccurazzu et al. (2010) | ||||
| White matter | Sherafat et al. (2012) | ||||
| Mechanism | BDNF GluR1 | Gersner et al. (2011) | |||
| BDNF-TrkB | Wang et al. (2011) | ||||
| Dopamine | Villaecimar et al. (2012) | ||||
| BDNF | Deveci et al. (2020) | ||||
| Pain | Application | Centrally/peripherally originated neuropathic pain | Johnson et al. (2006); | Motor cortex (H-coil) | Onesti et al. (2013) |
| Lefaucheur et al. (2008); | |||||
| Leung et al. (2009); | Motor cortex (8-coil) | Abdelkader et al. (2019) | |||
| Gustin et al. (2014) | |||||
| Mechanism | Synaptic plasticity | Antonino et al. (2016) | – | ||
| Depressive symptom | Application | Relief symptom | Fox et al. (2013) | – | |
| Pain related | Cardinalet al. (2019) | ||||
| DAN | Application | Neuro-cardiac-guided | Kaur et al. (2020) | Brain-kidneys | Rosaria et al. (2014) |
| Pathomechanism (oxidative stress) | Application | High-frequency | Tunez et al. (2006); | – | |
| Medina et al. (2017) | |||||
| Mechanism | Low-frequemcy | Sandrini et al. (2011); | |||
| Tasseta et al. (2013) | |||||
BDNF: Brain-derived neurotrophic factor; DAN: diabetic autonomic neuropathy; DN: diabetic neuropathy; EEG: electroencephalography; fMRI: functional magnetic resonance imaging; TMS: transcranial magnetic stimulation; TMS-DN: application of TMS in DN; TrkB: tyrosine receptor kinase B.
In the field of magnetic genetics, considerable attention has been given to non-invasive magnetic field neuromodulation (Pang et al., 2017). Recently, a study showed that magnetic stimulation of hippocampal cells that express magnetic field-induced proteins does not induce generation of current. Thus, there are great challenges in the field of ferritin-based magnetic genetics. Furthermore, this result supports the therapeutic effectiveness of TMS. Thus, TMS research may provide inspiration for future neuromodulation and magnetic genetics research (Xu et al., 2019).
DN is a complicated, progressive disease that involves multiple organ systems in the whole body and is difficult to treat. Thus, researchers need to deepen our understanding of DN and seek innovative solutions for DN diagnosis and treatment by focusing on multidisciplinary connections. In the screening for prediabetes, it is difficult to use a single examination technique to obtain dynamic comparison results in a short time. Furthermore, there is currently no reliable brain–spinal medical diagnostic method for patients with DN (Segerdahl et al., 2018). Based on the corticospinal regulatory effect of TMS (Gerard et al., 2020), it is reasonable to speculate that the application of TMS technology for hemodynamic and nerve conduction deficits in DN may help identify early pathological changes, which may be helpful for the early screening of peripheral nerve degeneration and microvascular dysfunction, assessment of prognosis, and timely intervention of targeted treatment. A limited number of clinical studies have shown that DN starts with microvascular injury and may further cause axonal degeneration as a consequence of ischemia and/or hypoxia (Gongalves et al., 2017). Collectively, the studies to date suggest that TMS has therapeutic value in neuroprotection, regrowth, remyelination and microvascular flow improvement. Thus, TMS technology is helpful to clarify the progress of DN and explore the optimal treatment parameters. Recent studies have revealed that sensory excitability could induce axonal dysfunction earlier than motor dysfunction, which is associated with later onset of pain (Sung et al., 2017; Fried et al., 2019). Hehl and colleagues applied TMS over the dominant primary motor cortex and observed changes in sensorimotor function with a significant decline commencing in the mid-thirties (Hehl et al., 2020). Therefore, early TMS intervention may have clinical potential for prognostic evaluation and injury control. In patients with DN with pain-related depressive symptoms, TMS has practical application value in mechanism research and symptom control. Owing to its complex mechanism and lack of research measures, diabetic autonomic nerve dysfunction has not received much attention. Because of cortical plasticity, TMS affects the autonomic nervous system in the short term, resulting in changes in heart rate and kidney function. Therefore, the effects on diabetic autonomic neuropathy should be considered before applying TMS to patients with DN.
In conclusion, previous application of TMS technology in pathological research as well as in the diagnosis and treatment of neuropathies has provided the theoretical basis for the use of TMS technology to diagnose and treat DN. Based on the studies to date, promising results may be obtained in future research. However, the following problems need to be resolved: (1) The intervention methods for patients with diabetes vary, and thus the TMS application results are easily affected by a variety of symptomatic drugs. Therefore, it is difficult to set the admission standard and follow up on cases, resulting in the need for more animal experiments for TMS applications. (2) There is no uniform standard for setting TMS parameters and for the evaluation of treatment duration and effect for different targets. Thus, it is necessary to compare the dose–effect relationships of large samples in the early stages of an investigation. (3) TMS technology requires more investment in manpower and equipment using fMRI, EEG and/or ultrasonography to monitor TMS and study the dynamic changes in nerve injury and repair.
Footnotes
P-Reviewer: Ruiz ML; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Qiu Y, Song LP; T-Editor: Jia Y
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the Science and Technology Project of Nantong City of China, No. JC2018060 (to XX).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Moisés León Ruiz, Servicio de Neurología, Clínica San Vicente, Spain.
Funding: This work was financially supported by the Science and Technology Project of Nantong City of China, No. JC2018060 (to XX).
References
- 1.Abdelkader AA, El Gohary AM, Mourad HS, El Salmawy DA. Repetitive TMS in treatment of resistant diabetic neuropathic pain. Egypt J Neurol Psychiatry Neurosurg. 2019;55:30. [Google Scholar]
- 2.Amato CD, Morganti R, Greco C, Gennaro FD, Cacciotti L, Longo S, Mataluni G, Lauro D, Marfia GA, Spallone V. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Res Clin Pract. 2016;13:418–428. doi: 10.1177/1479164116653240. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association (2018)10. Microvascular complications and foot care: standards of medical care in diabetes-2018. Diabetes Care. 41:S105–118. doi: 10.2337/dc18-S010. [DOI] [PubMed] [Google Scholar]
- 4.Andersson T, Hjerpe P, Carlsson AC, Pivodic A, Wandell P, Manhem K, Bengtsson Bostrom K. Mortality trends and cause of death in patients with new-onset type 2 diabetes and controls: a 24-year follow-up prospective cohort study. Diabetes Res Clin Pract. 2018;138:81–89. doi: 10.1016/j.diabres.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 5.Antonino N, Demetrio M, Margherita R, Carmen T, Vincenzo R, Alberto C, Silvia M, Calabro RS, Angelo Q. Non-invasive brain stimulation, a tool to revert maladaptive plasticity in neuropathic pain. Front Hum Neurosci. 2016;10:376. doi: 10.3389/fnhum.2016.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias-Carrión O, Verdugo-Díaz L, Feria-Velasco A, Millán-Aldaco D, Gutiérrez AA, Hernández-Cruz A, Drucker-Colín R. Neurogenesis in the subventricular zone following transcranial magnetic field stimulation and nigrostriatal lesions. J Neurosci Res. 2004;78:16–28. doi: 10.1002/jnr.20235. [DOI] [PubMed] [Google Scholar]
- 7.Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 8.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaulieu LD, Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Clin Neurophysiol. 2013;43:251–260. doi: 10.1016/j.neucli.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Bongaerts BW, Rathmann W, Kowall B, Herder C, Stockl D, Meisinger C, Ziegler D. Postchallenge hyperglycemia is positively associated with diabetic polyneuropathy: the KORA F4 study. Diabetes Care. 2012;35:1891–1893. doi: 10.2337/dc11-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardinal TM, Antunes LC, Brietzke AP, Parizotti CS, Carvalho F, De SA, da-Silva Torres IL, Fregni F, Caumo W. Differential neuroplastic changes in fibromyalgia and depression indexed by up-regulation of motor cortex inhibition and disinhibition of the descending pain system: an exploratory study. Front Hum Neurosci. 2019;13:138. doi: 10.3389/fnhum.2019.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron NE, Tuck Z, McCabe L, Cotter MA. Effect of the hydroxyl radical scavenger, dimethyl thiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia. 2001;4:1161–1169. doi: 10.1007/s001250100626. [DOI] [PubMed] [Google Scholar]
- 13.Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau AP, Renault MA. Impaired Hedgehog signaling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res. 2016;109:217–227. doi: 10.1093/cvr/cvv263. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary K, Rana AC, Bala R, Seth N. Review: depression as a common disorder in teenagers and its treatment. Int J Pharm Bio Sci. 2012;2:269–279. [Google Scholar]
- 15.Che XW, Cash B, Chung SW, Bailey N, Fitzgerald PB, Fitzgibbon BM. The dorsomedial prefrontal cortex as a flexible hub mediating behavioral as well as local and distributed neural effects of social support context on pain: a theta burst stimulation and TMS-EEG study. Neuroimage. 2019;201:116053. doi: 10.1016/j.neuroimage.2019.116053. [DOI] [PubMed] [Google Scholar]
- 16.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:7656. doi: 10.1136/bmj.f7656. [DOI] [PubMed] [Google Scholar]
- 18.Cox AA, Sagot Y, Hedou G, Grek C, Wilkes T, Vinik AI, Ghatnekar G. Low-dose pulsatile interleukin-6 as a treatment option for diabetic peripheral neuropathy. Front Endocrinol (Lausanne) 2017;8:89. doi: 10.3389/fendo.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuccurazzu B, Leone L, Podda MV, Piacentini R, Riccardi E, Ripoli C, Azzena GB, Grassi C. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp Neurol. 2010;226:173–182. doi: 10.1016/j.expneurol.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 20.De Martino E, Seminowicz DA, Schabrun SM, Petrini L, Graven-Nielsen T. High frequency repetitive transcranial magnetic stimulation to the left dorsolateral prefrontal cortex modulates sensorimotor cortex function in the transition to sustained muscle pain. Neuroimage. 2019;186:93–102. doi: 10.1016/j.neuroimage.2018.10.076. [DOI] [PubMed] [Google Scholar]
- 21.De-Ridder D, Vanneste S, Van Laere K, Menovsky T. Chasing map plasticity in neuropathic pain. World Neurosurg. 2013;80:901. doi: 10.1016/j.wneu.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Derosiere G, Vassiliadis P, Duque J. Advanced TMS approaches to probe corticospinal excitability during action preparation. Neuroimage. 2020;213:116746. doi: 10.1016/j.neuroimage.2020.116746. [DOI] [PubMed] [Google Scholar]
- 23.Deveci S, Matur Z, Kesim Y, Senturk G, Sargın-Kurt G, Ugur SA, Oge AE. Effect of the brain-derived neurotrophic factor gene Val66Met polymorphism on sensory-motor integration during a complex motor learning exercise. Brain Res. 2020;1732:146652. doi: 10.1016/j.brainres.2020.146652. [DOI] [PubMed] [Google Scholar]
- 24.Du J, Hu J, Hu J, Xu Q, Zhang Q, Liu L, Ma M, Xu G, Zhang Y, Liu X, Lu G, Zhang Z, Yang F. Aberrances of cortex excitability and connectivity underlying motor deficit in acute stroke. Neural Plast. 2018;2018:1318093. doi: 10.1155/2018/1318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermohlen O, Chun E, Garrett WS, McCoy KD, Diefenvach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the cns. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne D W, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Primer. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 27.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15:89. doi: 10.1007/s11892-015-0671-9. [DOI] [PubMed] [Google Scholar]
- 29.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with tms based on intrinsic connectivity. Neuroimage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried PJ, Ali J, Davila-Pérez P, Pascual-Leone A. Reproducibility of single-pulse, paired-pulse, and intermittent theta-burst TMS measures in healthy aging , type-2 diabetes, and alzheimer’s disease. Front Aging Neurosci. 2017;9:263. doi: 10.3389/fnagi.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fried PJ, Pascual-Leone A, Bolo NR. Diabetes and the link between neuroplasticity and glutamate in the aging human motor cortex. Clin Neurophysiol. 2019;130:1502–1510. doi: 10.1016/j.clinph.2019.04.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabay E, Wolf G, Shavit Y, Yirmiya R, Tal M. Chronic blockade of interleukin-1 (IL-1) prevents and attenuates neuropathic pain behavior and spontaneous ectopic neuronal activity following nerve injury. Eur J Pain. 2011;15:242–248. doi: 10.1016/j.ejpain.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 33.George MS, Wassermann EM, Post RM. Transcranial magnetic stimulation: a neuropsychiatric tool for the 21st century. J Neuropsychiatry Clin Neurosci. 1996;8:373–382. doi: 10.1176/jnp.8.4.373. [DOI] [PubMed] [Google Scholar]
- 34.Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13:135–147. doi: 10.1038/nrneurol.2016.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gustin SM, Wrigley PJ, Youssef AM, McIndoe L, WiLcox SL, Rae CD, Siddall PJ, Henderson LA. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain. 2014;155:1027–1036. doi: 10.1016/j.pain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hehl M, Swinnen SP, Cuypers K. Alterations of hand sensorimotor function and cortical motor representations over the adult lifespan. Aging. 2020;12:4617–4640. doi: 10.18632/aging.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoban AE, Stilling RM, Ryan FJ, Shanahan F, Dinan TG, Claesson MJ. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang X, Chen YY, Shen Y, Cao X, Li A, Liu Q, Li Z, Zhang LB, Dai W, Tan T, Arias-Carrion O, YX Cue, H Su, Yuan TF. Methamphetamine abuse impairs motor cortical plasticity and function. Mol Psychiatry. 2017;22:1274–1281. doi: 10.1038/mp.2017.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly (ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Johann, Jende, Groener J B, Oikonomou D, Heiland S, Kopf S, Pham M, Nawroth P, Bendszus M, Kurz FT. Diabetic neuropathy differs between type 1 and type 2 diabetes: insights from magnetic resonance neurography. Ann Neurol. 2018;83:588–598. doi: 10.1002/ana.25182. [DOI] [PubMed] [Google Scholar]
- 43.Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain. 2006;123:187–192. doi: 10.1016/j.pain.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 44.Jolivalt CG, Frizzi KE, Guernsey L, Marquez A, Ochoa J, Rodriguez M, Calcutt NA. Peripheral neuropathy in mouse models of diabetes. Curr Protoc Mouse Biol. 2016;6:223–255. doi: 10.1002/cpmo.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaur M, Michael JA, Hoy KE, Fitzgibbon BM, Ross MS, Iseger TA, Arns M, Hudaib AR, Fitzgerald PB. Investigating high- and low-frequency neuro-cardiac-guided TMS for probing the frontal vagal pathway. Brain Stimul. 2020;13:931–938. doi: 10.1016/j.brs.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi M, Zochodne DW. Diabetic neuropathy and the sensory neuron: new aspects of pathogenesis and their treatment implications. J Diabetes Investig. 2018;9:1239–1254. doi: 10.1111/jdi.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuhad A, Singh P, Chopra K. Matrix metalloproteinases: potential therapeutic target for diabetic neuropathic pain. Expert Opin Ther Targets. 2015;19:177–185. doi: 10.1517/14728222.2014.960844. [DOI] [PubMed] [Google Scholar]
- 48.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 49.Lang BT, Wang J, Filous AR, Au NP, Ma CH, Shen Y. Pleiotropic molecules in axon regeneration and neuroinflammation. Exp Neurol. 2014;258:17–23. doi: 10.1016/j.expneurol.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Lanza G, Aricò D, Lanuzza B, Cosentino FII, Tripodi M, Giardina F, Bella R, Puligheddu M, Pennisi G, Ferri R, Pennisi M. Facilitatory/inhibitory intracortical imbalance in REM sleep behavior disorder: early electrophysiological marker of neurodegeneration. Sleep. 2020;43:zsz242. doi: 10.1093/sleep/zsz242. [DOI] [PubMed] [Google Scholar]
- 51.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. J Neurol Neurosurg Psychiatry. 2008;79:1044–1049. doi: 10.1136/jnnp.2007.135327. [DOI] [PubMed] [Google Scholar]
- 52.Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, Saitoh Y, Andre-Obadia N, Rollnik J, Wallace M, Chen R. rTMS for suppressing neuropathic pain: a meta- analysis. Pain. 2009;10:1205–1216. doi: 10.1016/j.jpain.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Li F, Szabo C, Pacher P, Southan GJ, Abatan OI, Charniauskaya T, Stevens MJ, Obrosova IG. Evaluation of orally active poly (ADP-ribose) polymerase inhibitor in streptozotocin-diabetic rat model of early peripheral neuropathy. Diabetologia. 2004;47:710–717. doi: 10.1007/s00125-004-1356-0. [DOI] [PubMed] [Google Scholar]
- 54.Li JM, Yu R, Zhang LP, When SY, Wang SJ, Zang XY, Xu Q, Kong LD. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: a benefit of short-chain fatty acids. Microbiome. 2019;7:98. doi: 10.1186/s40168-019-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li P, He QP, Quyang YB, Liu CL, Hu BR, Siesjo BK. Early release of cytochromes C and activation of caspase 3 in hyperglycemic rats subjected to transient forebrain ischemia. Brain Res. 2001;30:69–76. doi: 10.1016/s0006-8993(01)01997-7. [DOI] [PubMed] [Google Scholar]
- 56.Lupachyk S, Shevalye H, Maksimchyk Y, Drel VR, Obrosova IG. PARP inhibition alleviates diabetes-induced systemic oxidative stress and neural tissue 4-hydroxynonenal adduct accumulation: correlation with peripheral nerve function. Free Radic Biol Med. 2011;50:1400–1409. doi: 10.1016/j.freeradbiomed.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma B, Yu J, Xie C, Sun L, Lin S, Ding J, Luo J, Cai H. Toll-like receptors promote mitochondrial translocation of nuclear transcription factor nuclear factor of activated T-cells in prolonged microglial activation. J Neurosci. 2015;35:10799–10814. doi: 10.1523/JNEUROSCI.2455-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Medina-Fernandez FJ, Escribano BM, Agüera E, Aguilar-Luque M, Feijoo M, Luque E, Garcia-Maceira FI, Pascual-Leone A, Drucker-Colin R, Tunez I. Effects of transcranial magnetic stimulation on oxidative stress in experimental autoimmune encephalomyelitis. Free Radic Res. 2017;51:460–469. doi: 10.1080/10715762.2017.1324955. [DOI] [PubMed] [Google Scholar]
- 59.Möhle L, Mattei D, Heimesaat MM, Bereswill S, Fischer A, Alutis M, French T, Hambardzumyan D, Matzinger P, Dunay IR, Wolf SA. Ly6Chi monocytes provide a link between antibiotic-induced changes in gut microbiota and adult hippocampal neurogenesis. Cell Rep. 2016;15:1945–1956. doi: 10.1016/j.celrep.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 60.Nukada H. Ischemia and diabetic neuropathy. Handb Clin Neurol. 2014;126:469–487. doi: 10.1016/B978-0-444-53480-4.00023-0. [DOI] [PubMed] [Google Scholar]
- 61.O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16:465–477. doi: 10.1016/S1474-4422(17)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Brien PD, Hur J, Hayes JM, Backus C, Sakowski SA, Feldman EL. BTBR ob/ob mice as a novel diabetic neuropathy model: Neurological characterization and gene expression analyses. Neurobiol Dis. 2015;73:348–355. doi: 10.1016/j.nbd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly (ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 64.Onesti E, Gabriele M, Cambieri C, Ceccanti M, Raccah R, Di Stefano G, Biasiotta A, Truini A, Zangen A, Inghilleri M. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain. 2013;17:1347–1356. doi: 10.1002/j.1532-2149.2013.00320.x. [DOI] [PubMed] [Google Scholar]
- 65.Orosz A, Baczko I, Nyiraty S, Korei AE, Putz Z, Takacs R, Nemes A, Varkonyi TT, Balogh L, Abraham G, Kempler P, Papp JG, Varro A, Lengyel C. Increased short-term beat-to-beat QT interval variability in patients with impaired glucose tolerance. Front Endocrinol. 2017;8:129. doi: 10.3389/fendo.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pafili K, Papanas N, Ziegler D. Neuropathy in diabetes: “one cannot begin it too soon”. Angiology. 2018;69:752–754. doi: 10.1177/0003319717751759. [DOI] [PubMed] [Google Scholar]
- 67.Pang K, You H, Chen Y, Chu P, Hu M, Shen J, Guo W, Xie C, Lu B. MagR alone is insufficient to confer cellular calcium responses to magnetic stimulation. Front Neural Circ. 2017;11:1–13. doi: 10.3389/fncir.2017.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papanas N, Ziegler D. Emerging drugs for diabetic peripheral neuropathy and neuropathic pain. Expert Opin Emerg Drugs. 2016;21:393–407. doi: 10.1080/14728214.2016.1257605. [DOI] [PubMed] [Google Scholar]
- 69.Pasquel FJ, Gregg EW, Ali MK. The evolving epidemiology of atherosclerotic cardiovascular disease in people with diabetes. Endocrinol Metab Clin North Am. 2018;47:1–32. doi: 10.1016/j.ecl.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL. Inflammation as a therapeutic target for diabetic neuropathies. Curr Diab Rep. 2016;16:29. doi: 10.1007/s11892-016-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rosaria L, Valentina D, Antonio L. Proteinuric effect of transcranial magnetic stimulation in healthy subjects and diabetic patients with Stage 3-4 CKD. Nephrol Dial Transplant. 2014;29:573–579. doi: 10.1093/ndt/gft454. [DOI] [PubMed] [Google Scholar]
- 73.Sandrini M, Umiltà C, Rusconi E. The use of transcranial magnetic stimulation in cognitive neuroscience: a new synthesis of methodological issues. Neurosci Biobehav Rev. 2011;35:516–536. doi: 10.1016/j.neubiorev.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Sango K, Mizukami H, Horie H, Yagihashi S. Impaired axonal regeneration in diabetes, perspective on the underlying mechanism from in vivo and in vitro experimental studies. Front Endocrinol. 2017;8:12. doi: 10.3389/fendo.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–171. doi: 10.2337/diabetes.52.1.165. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt RE, Dorsey DA, Beaudet LN, Plurad SB, Parvin CA, Yarasheski KE, Smith SR, Lang HJ, Williamson JR, Ido Y. Inhibition of sorbitol dehydrogenase exacerbates autonomic neuropathy in rats with streptozotocin induced diabetes. J Neuropathol Exp Neurol. 2001;60:1153–1169. doi: 10.1093/jnen/60.12.1153. [DOI] [PubMed] [Google Scholar]
- 77.Segerdahl AR, Themistocleous AC, Fido D, Bennett DL, Tracey I. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain. 2018;141:357–364. doi: 10.1093/brain/awx337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Selvarajah D, Wilkinson I D, Fang F, Sankar A, Davies J, Boland E, Harding J, Rao G, Gandhi R, Tracey I, Tesfaye S. Structural and functional abnormalities of the primary somatosensory cortex in diabetic peripheral neuropathy: a multimodal mri study. Diabetes. 2019;68:796–806. doi: 10.2337/db18-0509. [DOI] [PubMed] [Google Scholar]
- 79.Sherafat MA, Heibatollahi M, Mongabadi S, Moradi F, Javan M, Ahmadiani A. Electromagnetic field stimulation potentiates endogenous myelin repair by recruiting subventricular neural stem cells in an experimental model of white matter demyelination. J Mol Neurosci. 2012;48:144–153. doi: 10.1007/s12031-012-9791-8. [DOI] [PubMed] [Google Scholar]
- 80.Spallone V, Greco C. Painful and painless diabetic neuropathy: one disease or two. Curr Diab Rep. 2013;13:533–549. doi: 10.1007/s11892-013-0387-7. [DOI] [PubMed] [Google Scholar]
- 81.Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- 82.Sung J Y, Tani J, Chang TS, Lin CSY. Uncovering sensory axonal dysfunction in asymptomatic type 2 diabetic neuropathy. PLoS One. 2017;12:e0171223. doi: 10.1371/journal.pone.0171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takahashi S, Ukai S, Tsuji T, Ueyama T, Kono M, Yamanaka N, Shinosaki K. Reduction of cortical excitability and increase of thalamic activity in a low-frequency rTMS treatment for chronic tinnitus. Neurocase. 2015;21:339–344. doi: 10.1080/13554794.2014.893000. [DOI] [PubMed] [Google Scholar]
- 84.Tasseta I, Herrerab AP, Medina FJ, Carrión ÓA, Colínd RD, Túnez I. Extremely low-frequency electromagnetic fields activate the antioxidant pathway Nrf2 in a Huntington’s disease-like rat model. Brain Stimul. 2013;6:84–86. doi: 10.1016/j.brs.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 85.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Túnez I, Montilla P, del Carmen MM, Medina FJ, Drucker-Colín R. Effect of transcranial magnetic stimulation on oxidative stress induced by 3-nitropropionic acid in cortical synaptosomes. Neurosci Res. 2006;56:91–95. doi: 10.1016/j.neures.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Urabe H, Terashima T, Lin F, Kojima H, Chan L. Bone marrow-derived TNF-alpha causes diabetic neuropathy in mice. Diabetologia. 2015;58:402–410. doi: 10.1007/s00125-014-3440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vas PRJ, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol. 2016;4:723–725. doi: 10.1016/S2213-8587(16)30063-8. [DOI] [PubMed] [Google Scholar]
- 89.Villamar MF, Santos PA, Fregni F, Zafonte R. Noninvasive brain stimulation to modulate neuroplasticity in traumatic brain injury. Neuromodulation. 2012;15:326–338. doi: 10.1111/j.1525-1403.2012.00474.x. [DOI] [PubMed] [Google Scholar]
- 90.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 91.Wang HY, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A, Friedman E, Quartarone A, Ghilardi MF. Repetitive transcranial magnetic stimulation enhances bdnf-trkb signaling in both brain and lymphocyte. J Neurosci. 2011;31:11044–11054. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu AH, Sun YX. Research hotspots and effectiveness of repetitive transcranial magnetic stimulation in stroke rehabilitation. Neural Regen Res. 2020;15:2089–2097. doi: 10.4103/1673-5374.282269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu FX, Zhou L, Wang XT, Jia F, Ma KY, Wang N, Lin Li, Xu FQ, Shen Y. Magneto is ineffective in controlling electrical properties of cerebellar Purkinje cells. Nat Neurosci. 2019;9:1–9. doi: 10.1038/s41593-019-0475-3. [DOI] [PubMed] [Google Scholar]
- 94.Yang YW, Pan WX, Xie Q. Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity. Neural Regen Res. 2020;15:1986–1994. doi: 10.4103/1673-5374.282239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol. 2003;69:229–285. doi: 10.1016/s0301-0082(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 96.Yekkirala AS, Roberson DP, Bean BP, Woolf CJ. Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov. 2017;16:545–564. doi: 10.1038/nrd.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zakin E, Abrams R, Simpson DM. Diabetic neuropathy. Semin Neurol. 2019;39:560–569. doi: 10.1055/s-0039-1688978. [DOI] [PubMed] [Google Scholar]
- 98.Zhou J, Zhou S. Inflammation: therapeutic targets for diabetic neuropathy. Mol Neurobiol. 2014;49:536–546. doi: 10.1007/s12035-013-8537-0. [DOI] [PubMed] [Google Scholar]
- 99.Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016;13:141. doi: 10.1186/s12974-016-0607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ziegler D, Papanas N, Zhivov A, Allgeier S, Winter K, Ziegler I, Brüggemann J, Strom A, Peschel S, Kohler B, Stachs O, Guthoff RF, Roden M. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63:2454–2463. doi: 10.2337/db13-1819. [DOI] [PubMed] [Google Scholar]
- 101.Toepp SL, Turco CV, Locke MB, Nicolini C, Ravi R, Nelson AJ. The impact of glucose on corticospinal and intracortical excitability. Brain Sci. 2019;9:339. doi: 10.3390/brainsci9120339. [DOI] [PMC free article] [PubMed] [Google Scholar]


