INTRODUCTION
Magnetic resonance (MR) imaging is an advanced modality currently reserved for supplemental breast cancer screening in high-risk individuals, with excellent sensitivity and specificity reported in recent literature.1–5 Although MR imaging–specific impact on breast cancer mortality is difficult to assess, supplemental screening with MR imaging has been associated with detection of earlier-stage disease and improved 10-year survival.6,7 Although mammography is the standard of care for population-wide screening known to decrease mortality, questions of overdiagnosis and overtreatment persist.8 With increasingly sophisticated understanding of breast cancer heterogeneity and outcomes based on cancer subtypes, there is growing impetus to parse out modality-based cancer yield both in number and in the type of cancers detected. The advantage of contrast-enhanced MR imaging as a functional imaging modality optimized to capture biologically more aggressive tumors that may be mammographically occult is the basis of a growing interest in expanding the role of MR imaging in breast cancer screening. Furthermore, there is growing evidence that MR imaging also outperforms mammography and sonography in moderate-risk women in cancer yield, prompting more recent broadening of MR imaging screening indications in certain guidelines.9 However, patient access to MR imaging remains limited, and the cost and time currently associated with the examination can be prohibitive. Efforts to improve feasibility of wider implementation have focused on streamlining examination acquisition and interpretation while preserving diagnostic accuracy. This article therefore (1) provides an evidence-based overview of current MR imaging screening indications, (2) considers the rationale for expanding its use in the population, and (3) discusses challenges and potential solutions in improving its cost-effectiveness with abbreviated approaches, notably via abbreviated and ultrafast MR imaging protocols.
CURRENT MAGNETIC RESONANCE IMAGING SCREENING INDICATIONS
High-Risk Screening
Women with an estimated lifetime risk (LTR) of greater than or equal to 20% to 25% for developing breast cancer are defined as high risk as per the American Cancer Society (ACS) guidelines.10 A woman’s LTR is usually estimated based on family history and risk modeling algorithms. There is well-established evidence supporting MR imaging screening in this group,11–13 for whom annual supplemental MR imaging in addition to mammography is currently the standard of care in breast cancer screening.
Hereditary and Familial Risks
Although there are variations in what constitutes high risk across multidisciplinary guidelines, BRCA germline mutation carriers and untested first-degree relatives are universally acknowledged as harboring risks an order of magnitude greater than that of the general population, and are thought to benefit the most from MR imaging screening9,10,14–20(see Table 2). Pooled data have shown that the average risk of developing breast cancer by age 70 years is 65% for BRCA1 mutation carriers and 45% for BRCA2 mutation carriers.21 Compared with women without mutations, BRCA1 and BRCA2 carriers have, on average, respectively 30-fold and 10-fold to 16-fold higher LTR of breast cancer.21 In women with BRCA mutations who undergo screening, mammography has low sensitivity because of high breast density and more rapidly growing tumors in younger women.6 Prospective trials have shown that annual supplemental MR imaging in conjunction with mammography typically doubles the sensitivity of mammography alone and generally achieves sensitivities greater than 90%.3,4,11,13,22,23 Further ultrasonography or clinical breast examination are not additive24(Table 1). False-positive rates are increased with addition of screening MR imaging to annual mammography, but these false-positives tend to decrease in successive incident rounds of screening. In a multicentered prospective trial from the Netherlands, the sensitivity advantage of MR imaging versus mammography was greatest at prevalent round (93% vs 20%; P = .003) but maintained in subsequent rounds (77% vs 29%; P = .02); and the false-positive rate of MR imaging decreased in subsequent rounds (from 14% to 8%).25 MR imaging–detected cancers also have a favorable stage distribution in BRCA mutation carriers, primarily composed of sub–1-cm invasive cancers and in situ cancers at incident rounds of screening with low rates of node positivity (12%–26%), associated with improved 10-year survival.24 Importantly, timing the frequency of screening has been a challenge in this population. Particularly in BRCA1 carriers less than 50 years of age, who are known to have the highest rate of interval cancers because of a high prevalence of rapidly growing tumors, annual imaging may not be adequate.26–28 The main strategy has been to shorten the screening interval to every 6 months using various examination combinations, such as staggering mammography and MR imaging, or using biannual MR imaging with annual mammography, or by supplementing annual mammography/MR imaging with biannual ultrasonography.23,29 MR imaging screening is generally deemed cost-effective for BRCA carriers assuming perfect attendance, particular for BRCA1 carriers, and adherence to the examination is high among carriers confirmed by genetic testing (80%–90%).30,31
Table 2.
Multidisciplinary recommendations for annual supplemental magnetic resonance imaging screening in higher-than-average-risk women
| Organization | BRCA Carriers/First-Degree Relativesa | Family History | Prior Radiation | Personal History | Dense Tissue | History of Atypiab |
|---|---|---|---|---|---|---|
| ACS 2007 | BRCA1/2/select mutations | If LTR ≥20% | Age 10–30 y | NR | NR | NR |
| ACR 2018 | BRCA1/2/select mutations | If LTR ≥20% | Age<30 y | If early diagnosis (before age 50) | If personal history (prior breast cancer) | If other risk factors |
| ASBrS 2019 | BRCA1/2/select mutations | If strong family history | Age 10–30 y | If early diagnosis (before age 50 y) | If personal history (prior breast cancer) | NR |
| NCCN 2020 | BRCA1/2/select mutations | If family history suggests hereditary pattern despite absence of mutation (eg, early diagnosis before age 30 y) | Age<30 y | NR | NR | If LTR ≥20% |
| EUSOBI 2015 | BRCA1/2/select mutations | Selective | Age<30 y | NR | NR | NR |
| ECIBS 2020c | NS | NR | NS | NS | NR | NR |
| ACOG 2017 | BRCA1/2/select mutations | If LTR ≥20% | Age 10–30 y | If other risks | NR | NR |
Abbreviations: ACR, American College of Radiology; ASBrS, American Society of Breast Surgeons; ECIBS, European Commission Initiative on Breast Cancer; EUSOBI, European Society of Breast Imaging; NCCN, National Comprehensive Cancer Network; NR, screening not recommended or insufficient evidence to recommend for or against; NS, not specified.
MR imaging is consistently recommended for BRCA mutation carriers and untested first-degree relatives, but more variably recommended or considered for other mutations.
Atypia refers to atypical epithelial hyperplasia, including atypical lobular hyperplasia, lobular carcinoma in situ, and atypical ductal hyperplasia.
The ECIBS guidelines primarily address average-risk women that attend organized screening programs in Europe but also include select higher-than-average-risk groups such as those with family history or high breast tissue density.
Table 1.
Comparison of diagnostic performance using magnetic resonance imaging versus mammography or sonography in multimodality breast cancer screening among high-risk women based on outcomes of prospective studies
| Sensitivity | Specificity | PPV3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Patients (n) | Rounds (n)a | Inclusion | MR Imaging (%) | MG (%) | US (%) | MR Imaging (%) | MG (%) | US (%) | MR Imaging (%) | MG (%) | US (%) | Interval CA (n) |
| 202011 | 8782 | 20,053 | BRCA+/Fam | 91 | 41 | NA | 87 | 92 | NA | 20 | 26 | NA | 12 |
| 201912,b | 674 | 2812 | Fam | 98 | 87 | NA | 84 | 91 | NA | 27 | 28 | NA | 1 |
| 201713 | 296 | 1170 | BRCA+/Fam | 68 | 37 | 32 | 95 | 98 | 95 | 25 | 34 | 10 | 3 |
| 201522 | 559 | 1506 | BRCA+/Fam | 90 | 38 | 38 | 89 | 97 | 97 | 20 | 28 | 27 | 1 |
| 201423 | 221 | 1855 | BRCA+ | 100 | 27 | 77 | 56 | 82 | 84 | NA | NA | NA | 1 |
| 201226 | 612 | 612 | Mixed/Dense | 88 | 52 | 45 | 76 | 91 | 90 | 23 | 38 | 12 | 9 |
| 20113 | 501 | 1592 | BRCA+/Fam | 91 | 50 | 52 | 97 | 99 | 98 | 56 | 71 | 62 | 3 |
| 20104 | 687 | 1679 | BRCA+/Fam | 93 | 33 | 37 | 98 | 99 | 98 | 48 | 39 | 36 | 0 |
All studies included are prospective in design.
Abbreviations: BRCA+, BRCA1 or BRCA2 mutation carriers; Interval CA, interval cancers after all-modality screening; Fam, familial risk for breast cancer as defined by a calculated lifetime risk of breast cancer greater than or equal to 20%; NA, not available; PPV3, positive predictive value indicating the rate of positive biopsies among biopsied lesions.
Number of rounds of screening indicates rounds that specifically include MR imaging.
Randomized controlled study comparing MR imaging versus mammography.
Annual breast MR imaging is also recommended in less common mutations associated with high risk of breast cancer, such as TP53 (Li-Fraumeni syndrome; 95% by age 90 years) and PTEN (Bannayan-Riley-Ruvalcaba syndrome, Cowden syndrome; 85% by age 80 years), and is increasingly considered in additional mutations associated with moderate to high risk of breast cancer, including CDH1, STK11, PALB2, CHEK2, ATM, BARD1, and NF1, with individual decisions often guided by family history because of a further increased risk.32–36 However, most women with a family history of breast cancer do not have an identified genetic mutation, and 15% of all breast cancers occur in this group.37,38 Family history in these women therefore serves as the primary basis for calculating LTR via modeling algorithms, and is a direct indication for supplemental MR imaging screening if calculated risk exceeds 20%.10 In this group, MR imaging has been found to increase detection of early-stage cancer compared with mammography (14 per 1000 vs 5 per 1000 cancers; P<.0003).12 Notably, in contrast with BRCA carriers, women at high risk without BRCA mutations have been found to have significantly lower adherence to supplemental screening MR imaging.39,40 There is also evidence that, although supplemental MR imaging is underused in high-risk women, many who undergo breast MR imaging may not be appropriately high risk.41,42 For example, for those with family history who undergo multigene panel testing in recent years, unclassified genetic variations of uncertain significance (VUS) are increasingly encountered. Although work is being done to better classify these genetic variants to provide clinically actionable information, detection of any unknown variant (including BRCA1/2 VUS) is currently not an indication for intensified breast cancer screening.43
Prior Chest Radiation
Women with prior childhood chest radiation are another group at high risk for developing breast cancer later in life, for whom annual screening MR imaging is consistently recommended (Table 2). By age 50 years of age, 1 in 3 women with prior chest radiation (risk greatest if subjected to ≥20 Gy) are diagnosed with breast cancer, a risk on par with that of BRCA1 mutation carriers.44,45 Multiple studies have shown increased sensitivity of both mammography and MR imaging in this group (94%–100%), with supplemental MR imaging yielding additional cancers.46–48 Therefore, annual screening mammography and breast MR imaging are recommended, starting at age 25 years or 8 years following chest radiation, whichever occurs last.10 However, breast cancer screening adherence among childhood cancer survivors is poor. Notably, mammography screening adherence in women who have received chest radiation is lower than among their average-risk peers in the general population. Nearly half of survivors younger than 40 years of age have never obtained a mammogram, and only 52% of women aged 40 to 50 years undergo regular mammography screening.49–51 Adherence for MR imaging screening is markedly worse, and efforts to improve adherence have had marginal effects. In a recent randomized controlled study, although educational interventions increased the rate of uptake of mammography screening (from 18% to 33%), MR imaging uptake was not significantly changed (from 13% to 16%), and overall screening adherence in this population remains poor. Primary barriers identified by women survivors to completing screening included lack of physician recommendation, deferred action by survivor, absence of symptoms, and cost, which is a concern specific to MR imaging.51
EVOLVING MAGNETIC RESONANCE IMAGING SCREENING INDICATIONS
Moderate-Risk Screening
Despite a lack of consensus among different guidelines, there is increasing evidence supporting expanding the indications of MR imaging screening to include women at intermediate risk for breast cancer, as defined by an estimated LTR of 15% to 20% as per the ACS.10 In particular, several subgroups of women with higher-than-average risk for breast cancer are considered, including those with a personal history of breast cancer, dense breast tissue, or a history of atypical epithelial hyperplasia (atypical ductal hyperplasia [ADH], atypical lobular hyperplasia [ALH], and lobular carcinoma in situ [LCIS]). Although the 2007 ACS guidelines reported insufficient evidence to recommend for or against MR imaging screening in these groups, the more recent 2018 American College of Radiology (ACR) and 2020 National Comprehensive Cancer Network (NCCN) guidelines suggest considering MR imaging in some or all of these women, particularly in conjunction with other overlapping risk factors that may increase LTR to greater than 20% (see Table 2).
Breast Cancer Survivors
Women with a personal history of breast cancer are at considerable risk of developing a second breast cancer, with cumulative risks estimated at 5.4% in 5 years and 19.3% in 10 years following initial diagnosis.52,53 In women diagnosed with breast cancer before age 50 years, the LTR for a second breast cancer is greater than 20%.54 Additional independent predictors of increased risk for a second breast cancer within 5 years also include aggressive tumor biology in the first cancer, treatment without radiation, and heterogeneously dense breasts on mammography.52 Therefore, the ACR Appropriateness Criteria currently recommend annual screening MR imaging in conjunction with mammography for women with breast cancer diagnosed before age 50 years, and for women with breast cancer history and dense breasts.9 Mammographic sensitivity is limited in the treated breast because of postsurgical parenchymal distortion, scarring, and fat necrosis. Although there are currently no prospective data, a growing body of retrospective studies have consistently shown superior sensitivities using MR imaging compared with mammography in women with a personal history of breast cancer (80%–100% vs 0%–53%),55,56 and supplemental MR imaging in this group has been shown to perform as well as, if not better than, in those with genetic and familial predispositions57(Fig. 1). The 2018 ACR recommendations aim to optimize early detection of second breast cancers and improve survival but have not been universally adopted across multidisciplinary guidelines, pending data on mortality benefits, which are currently unknown (see Table 2).
Fig. 1.
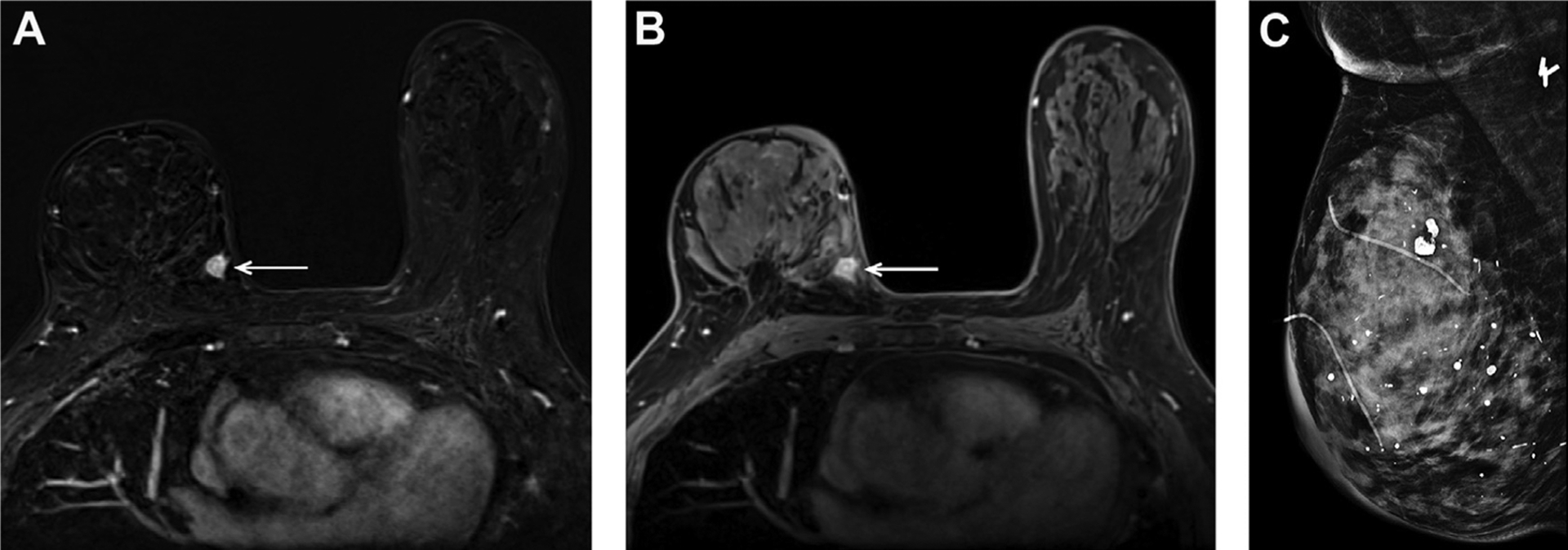
A 73-year-old woman with dense breast tissue and a prior history of right breast cancer treated with lumpectomy and radiotherapy 6 years prior, found to have a small recurrent invasive ductal carcinoma in the medial posterior right breast on MR imaging, as shown here on axial T1W postcontrast subtraction (A) and nonsubtraction (B) images (arrows). This mass was mammographically occult on the same-day surveillance mammogram (C).
Extremely Dense Tissue
Women with extremely dense breast tissue not only have poor sensitivity on mammography (on the order of 61%–65%)58,59 but also have inherently increased risk for breast cancer (approximately double the average risk)60 (Fig. 2). Clinical presentation of interval cancers following a negative mammogram is 18 times more likely in women with extremely dense breasts than in women with fatty breasts, a statistic that underscores the importance of supplemental imaging in this group.61 Although digital breast tomosynthesis (DBT) has improved both sensitivity and specificity of cancer detection, it has had minimal benefit in the extremely dense subgroup.62,63 Breast MR imaging has the highest sensitivity for cancer detection and is not limited by breast density.64 However, ultrasonography is currently more commonly used in supplemental screening because of wider availability and lower cost of implementation, although its cost-effectiveness has been questioned given the small additional cancer yield.65 In comparison, MR imaging significantly outperforms ultrasonography in incremental cancer yield (3–4 per 1000 vs 15 per 1000) and has a lower false-positive rate compared with ultrasonography,66 suggesting MR imaging may be the better supplemental screening method for women with dense breasts. Since 2019, a federal law has been introduced in the United States that mandates the US Food and Drug Administration to develop standardized breast density notification language, which is the first step in paving the way for a more standardized supplemental imaging regimen in dense women.67
Fig. 2.
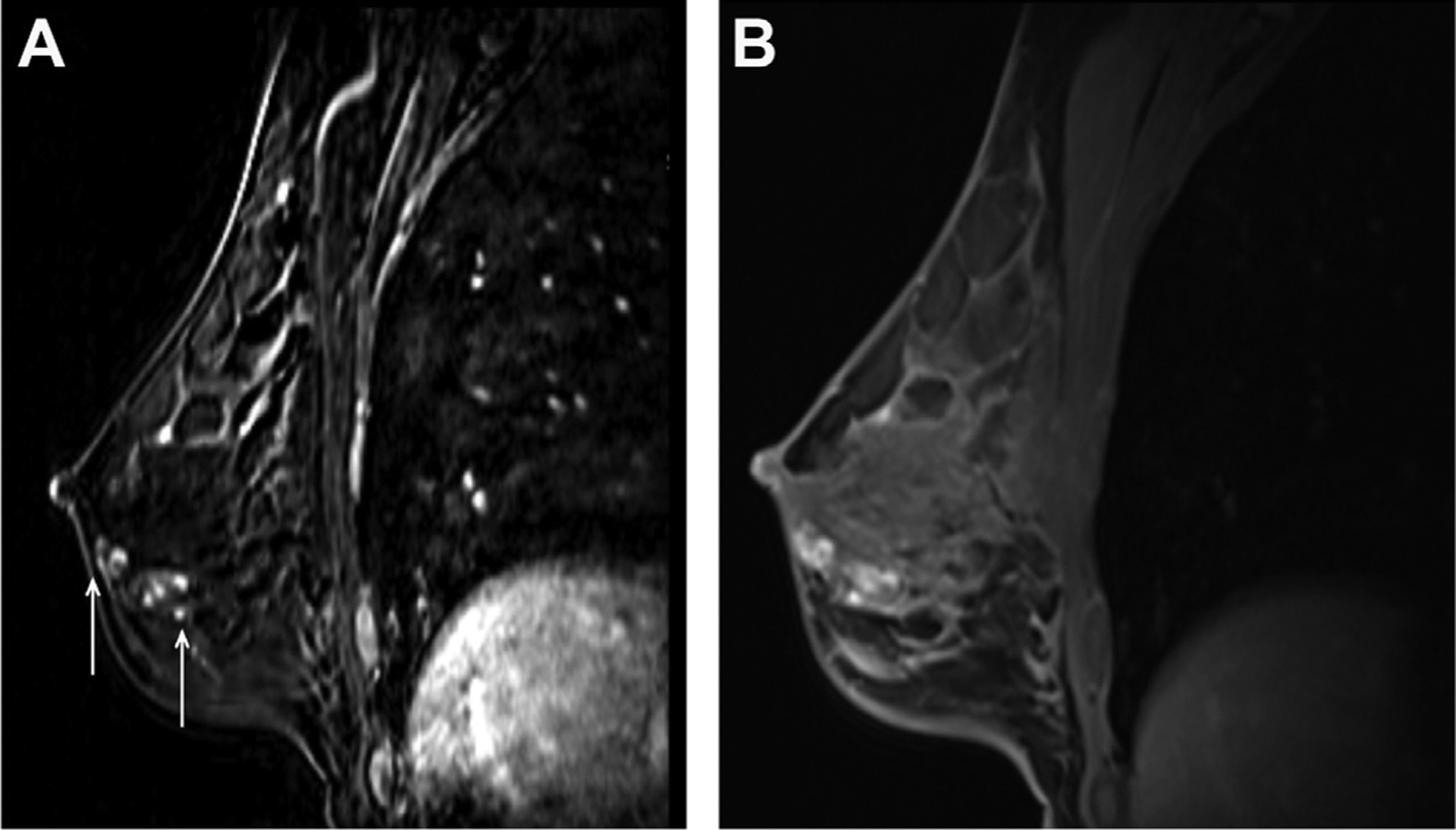
A 31-year-old woman with BRCA1 mutation and extremely dense breast tissue with segmental distribution of clumped non–mass enhancement identified on MR imaging screening without mammographic correlate, as shown on sagittal postcontrast T1W subtraction image (A) (arrows), with subsequent biopsy yielding intermediate-grade to high-grade ductal carcinoma in situ. This finding was mammographically occult and the disease blends in with dense fibroglandular tissue, as shown on the corresponding sagittal postcontrast T1W image (B).
The Dutch DENSE trial is the first and largest randomized controlled trial to date, evaluating women with extremely dense breast tissue on mammography (as per quantitative volumetric assessment), who are randomized to undergo mammography screening with or without supplemental MR imaging (n = 40,373) (age, 50–75 years). The prevalent round screening results with 2-year follow-up showed that supplemental MR imaging screening not only yielded an additional 16.5 per 1000 cancers but was also associated with a 50% reduction in interval cancer rate, suggesting a potential mortality benefit.68 The ECOG-ACRIN 1141 trial, early results were similarly positive. In this prospective study comparing performance of DBT versus abbreviated MR imaging (ABMR) among women with dense breasts (heterogeneously and extremely dense) (n = 1444; age, 40–75 years), ABMR detected significantly more invasive cancers than DBT (11.8 per 1000 vs 4.8 per 1000), with no interval cancers observed during follow-up.69 Results for incident rounds of screening are currently pending for both studies, which will help further inform how MR imaging may best be used in women with dense breast tissue.
Atypical Epithelial Hyperplasia
Atypical epithelial hyperplasia refers to a spectrum of proliferative epithelial lesions that are nonobligate precursors to malignancy and are also biological indicators for future increased risk for developing breast cancer. Women with atypical epithelial hyperplasia such as ADH, ALH, and LCIS have a 3 to 10 times higher relative risk for breast cancer than the general population.70,71 Although these women are currently classified in the intermediate-risk category as per the 2007 ACS guidelines (stated as associated with 15%–20% LTR), more recent data with long-term follow-up indicate this population has an LTR greater than 20%, more consistent with high-risk classification, for which annual MR imaging would be recommended. In longitudinal studies including nearly 1000 women from the Mayo Benign Breast Disease Cohort and the Nashville Breast Cohort, 20% to 30% developed breast cancer on average 12 to 25 years following initial detection of atypical epithelial hyperplasia.72,73 Women with ADH, ALH, and LCIS are prone to developing higher-grade invasive tumors, and those who undergo mammography screening have a significantly higher interval cancer rate compared with the general population (2.6 per 1000 vs 0.9 per 1000; P = .002).73,74 For these reasons, adjunct screening with MR imaging may add value in this population, given its potential to decrease interval cancers as well as to increase detection of more aggressive disease based on more robust data in other populations. The ACR and NCCN currently both recommend consideration of supplemental MR imaging in women with a history of atypical epithelial hyperplasia, particularly if other risk factors such as family history coexist and if cumulative LTR exceeds 20% (see Table 2). However, current evidence for supplemental MR imaging screening in this population is limited and more data are needed.
Average-Risk Screening
Breast MR imaging screening is currently not recommended in average-risk women (LTR<15% as per the ACS). Although mammography screening has been highly effective in early cancer detection in average-risk women more than 40 years of age, interval cancers persist at a rate of 13% to 38%.75,76 In contrast, more recent controversies regarding mammography have centered on detection of low-grade disease, which may contribute to overdiagnosis. Functional advantage of MR imaging in preferentially detecting the more aggressive spectrum of disease may be a potential answer to overcoming the shortcomings of mammography. Therefore, MR imaging screening is now beginning to be considered in the average-risk population.
A prospective study of supplemental MR imaging in average-risk women with negative mammography and ultrasonography examinations (n = 2120) found an overall incremental cancer detection rate (CDR) of 15.5 per 1000, which is highly comparable with incremental cancer yield by MR imaging in high-risk women (CDR of 10–20 per 1000).2,77,78 Importantly, MR imaging–detected cancers in the study were small (median, 8 mm), frequently high grade (46%), and largely node negative (93.4%), with no interval cancers observed.2 This increased sensitivity for smaller node-negative invasive cancers at MR imaging compared with mammography (average size at detection, 8 mm vs 1–2 cm) and the decreased interval cancer rate suggest a potential to downstage disease and further improve breast cancer–specific survival.4,77,79 In the study by Kuhl and colleagues,2 the zero interval cancer rate in the context of a substantial decrease in incremental CDR from prevalent round to incident round screening (22.6 per 1000 to 6.9 per 1000) supports a likely stage shift in cancer detection using MR imaging. In addition, it is noteworthy that current risk-based screening recommendations only rely on known risk factors, and that most women with breast cancer have no known risk factors before diagnosis. For example, 89% of women with breast cancer do not have a first-degree family history, and 90% to 95% of these women have no known genetic mutations.80,81 It is thus likely that there are women with underestimated risk. As previously discussed, supplemental MR imaging has been shown to be of value, for example, in the subpopulation with dense breast tissue.
MAGNETIC RESONANCE IMAGING SCREENING OUTCOMES
Multimodality Comparison
MR imaging screening has focused on women at high risk for breast cancer; therefore, screening outcomes using MR imaging are primarily based on the high-risk population, which in itself has a higher disease prevalence and lower mammographic sensitivity. Compared with mammography and sonography, MR imaging has a significantly higher sensitivity for all breast cancers. The average sensitivity of MR imaging is around 95%, compared with 40% for mammography and 45% for ultrasonography based on prospective trial data, with MR imaging yielding an additional 8 to 13 per 1000 cancers not otherwise detected by mammography and/or sonography (see Table 1).3,4,11–13,22,23,66 Additional cancer yield is greatest at prevalent round MR imaging screening and decreases at subsequent incident rounds of screening, because of initial capture of cancers that had gone undetected by routine mammography or sonography.4,12,13,22,66 MR imaging sensitivity particularly outshines that of mammography for small node-negative invasive cancers7,12,66,82(Fig. 3). For example, in a large randomized trial published in the Lancet in 2019 evaluating 4 rounds of screening in 1355 women, MR imaging not only detected more cancers than mammography (40 vs 15 cancers; P = .0017) but also detected smaller and less frequently node-positive cancers (median size, 9 mm vs 17 mm; P = .010) (11% vs 63%; P = .014), resulting in a significant shift in tumor stage in the MR imaging group and suggesting a potential for improved survival.12 Furthermore, a divergent trend of MR imaging preferentially detecting invasive and higher-grade in situ disease as opposed to mammography preferentially detecting in situ and lower-grade invasive disease has been observed,77 highlighting inherent differences between functional and anatomic depiction of breast cancer. Although direct assessment of MR imaging–specific effect on long-term breast cancer survival is not available, there are consistent data showing significant improvement of at least near-term survival (10-year survival) in high-risk women who undergo MR imaging screening compared with those who do not, because of early detection of more aggressive disease.7,12 In addition, supplemental MR imaging screening in addition to mammography over multiple rounds has also been observed to curtail interval cancer rates by up to 50%, capturing tumors with worse prognostic features and poorer survival outcomes, and is therefore expected to improve mortality.12,68,76
Fig. 3.
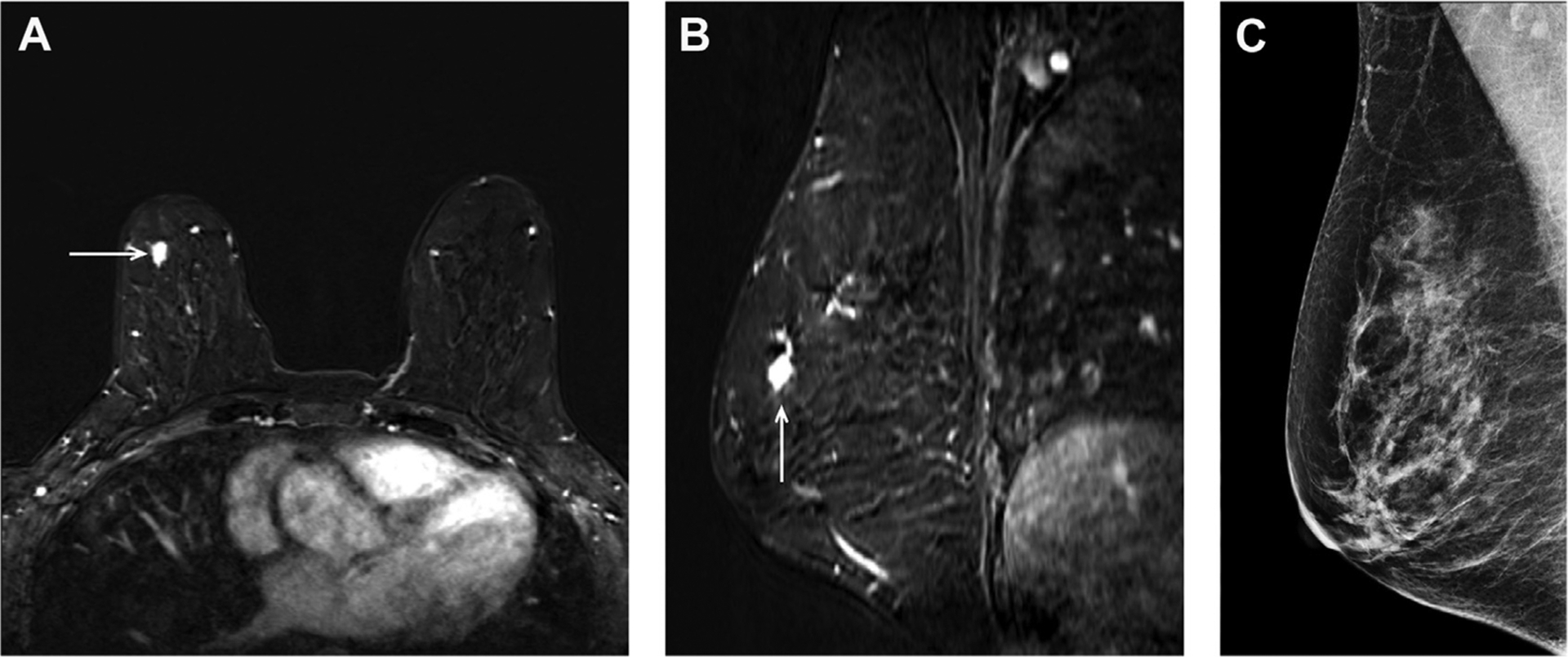
A 61-year-old woman with BRCA2 mutation and heterogeneously dense breast tissue with an MR imaging–detected small HER2+ invasive breast cancer in the right breast, as shown in axial (A) and sagittal (B) projections of postcontrast T1-weighted (T1W) subtraction images (arrows). This small invasive ductal carcinoma was identified on supplemental screening MR imaging following negative screening mammogram (C) and ultrasonography 5 months prior, and did not have axillary nodal spread at the time of diagnosis.
False-Positives
The specificity of breast MR imaging is moderate and typically less than that of mammography in early studies (wide-ranging specificities, 56%–98%23,82); however, it has improved over time as imaging technique and clinical experience have matured (higher specificities, often >95% [95%–98%], reported in recent studies1–4). In particular, there is a consistent and significant improvement of specificity from the prevalent round to the incident rounds of screening in prospective trials across multiple years (range, 82%–85% vs 92%–96%), suggesting that limited specificity may be less problematic in the setting of ongoing MR imaging screening.13,22 The positive predictive value (PPV3) of MR imaging biopsy currently ranges between 20% and 56%, which is comparable with that of mammography (26%–71%), with MR imaging biopsy positivity overrepresented by invasive rather than in situ malignancy (see Table 1).3,4,11–13,22,23,66 In addition, a recent study from 2018 evaluating false-positive findings at multimodality screening found that MR imaging lesions that underwent biopsy were twice as likely to contain atypia than mammographic or tomographic lesions at biopsy, which potentially has implications in risk-based screening regimens.83 Compared with supplemental MR imaging screening, which yields an additional 10 to 15 per 1000 cancers compared with mammography with a small loss in specificity, supplemental ultrasonography yields only 3 to 4 per 1000 additional cancers with further loss in specificity without improvement in sensitivity compared with MR imaging. For example, in a prospective multimodality trial involving 2662 patients, the number of screens needed to detect 1 cancer was 127 for mammography, 234 for supplemental ultrasonography, and 68 for MR imaging following negative mammogram and ultrasonography examinations.66
False-Negatives
MR imaging does not detect all breast cancers. Particularly in the high-risk population, most prospective studies show nonzero interval cancer rates despite annual supplemental MR imaging screening using modern technology (magnets and dedicated breast coils of 1.5–3 T), because of a highly aggressive subset of breast cancers that are rapidly growing, outpacing the frequency of screening11–13(see Table 1). In contrast, low-grade ductal carcinoma in situ, which typically manifests as microcalcifications best seen on mammography and may not enhance on MR imaging, contributes to most of a small incremental MR imaging–occult cancer yield at mammography (0%–12.5%).12,13,22,23 The relevance of these false-negative findings on MR imaging is unknown but questionable, given unlikely mortality significance and current debate of overdiagnosis and overtreatment with regard to in situ disease. Retrospective data suggest that concurrent mammography may be of little value in younger high-risk women undergoing MR imaging screening (age<50 years), who may benefit from reduced radiation dose by forgoing mammography.84,85 Furthermore, the limited incremental yield of mammography compared with MR imaging is also unlikely to improve despite technological advancement of DBT. For example, in a recent review of 4418 screening MR imaging scans performed either following a negative DBT or a negative digital mammography (DM) study, the incremental cancer detection rates did not differ significantly between the 2 groups.86 In contrast, although ultrasonography provides a higher number of incremental cancers detected in addition to DM (about 3–4 per 1000), it provides little to no incremental yield when MR imaging is performed and may reduce overall specificity by up to 5% to 6%.13,22,66 Thus, in women who undergo annual MR imaging screening, concurrent mammography primarily provides added sensitivity for in situ disease, and additional ultrasonography has limited value. However, at this time, mammography is recommended by most guidelines as the primary screening test, because it remains the only test with proven long-term mortality benefit.
BARRIERS TO MAGNETIC RESONANCE IMAGING IN SCREENING
Despite the many potential benefits of MR imaging, there are barriers to wider use, primarily caused by limited availability and cost-effectiveness. Dynamic contrast-enhanced (DCE) MR imaging currently has to meet designated technical specifications (in-plane pixel ≤1 mm, slice thickness ≤3 mm), and standard protocols include T2-weighted and T1-weighted precontrast and 3 postcontrast sequences to generate an enhancement kinetic curve. Therefore, typical examination acquisition requires, on average, 20 to 30 minutes per examination. Both long scan time and limited access to MR imaging scanners currently limit wider use of MR imaging. At the patient level, longer examination duration and the need for intravenous contrast are also less tolerated. In addition, the current cost of the examination may be prohibitive. Although MR imaging screening has been found to be cost-effective among very high-risk women, such as BRCA mutation carriers, in terms of quality-adjusted life-years gained, it may not be cost-effective in non-BRCA high-risk women in its current form, and is less likely to be cost-effective in moderate-risk or average-risk women.87–89 However, there is evidence to suggest that MR imaging screening has the potential to become more cost-effective than mammography over time if routinely used, particularly if the cost of the examination decreases.90 Even as MR imaging screening indications continue to evolve, overall adherence to MR imaging among currently eligible women is poor. In 1 study with long-term follow-up, the frequency of MR imaging screening among high-risk women (cancer free with LTR ≥20%) was less than 40%; of the group, 25% at 4 years and 40% at 8 years did not report any form of screening.91 Therefore, to optimize MR imaging adoption and enable wider use, a significantly streamlined and less costly examination is mandatory. Current efforts therefore focus on shortening the MR imaging examination in order to minimize both examination acquisition time and interpretation time while maintaining high sensitivity. A shorter and better-tolerated examination will reduce the cost by increasing capacity and throughput, thereby improving accessibility. Variations of abbreviated MR imaging protocols have emerged in recent years and early clinical studies have shown promising results, with abbreviated MR imaging usually achieving similar diagnostic accuracy to the full diagnostic MR imaging protocol.92,93 Ultrafast imaging with high temporal resolution is also being introduced into abbreviated protocols to further enhance performance.94,95 Details on early outcomes of abbreviated MR imaging are covered in another article in this issue.
Although noncontrast techniques such as diffusion-weighted imaging are also being investigated, such techniques currently have a low sensitivity, particularly for small lesions, compared with contrast-enhanced MR imaging, thus intravenous contrast remains essential at present. Beyond MR imaging, parallel efforts are also underway to investigate other promising functional imaging modalities, such as contrast-enhanced spectral mammography, which has sensitivity comparable with and specificity slightly higher than MR imaging with likely lower costs and better accessibility.92
SUMMARY
MR imaging has a clear modality-based advantage compared with mammography and sonography in early breast cancer detection, and its role in screening is evolving. There is strong evidence to support MR imaging screening in high-risk women, and growing interest and varying levels of evidence for MR imaging screening in moderate-risk women. Among those screened with MR imaging, mammography offers a small incremental cancer yield, and ultrasonography is not additive. Although MR imaging screening in the average-risk population has the potential to improve detection of more relevant disease and minimize overdiagnosis, MR imaging in its current form would not be cost-effective. Abbreviated techniques have streamlined MR imaging to improve the feasibility of wider use. However, more robust data are needed to further consolidate and refine the role of MR imaging in breast cancer screening.
KEY POINTS.
Magnetic resonance (MR) imaging has a modality-based advantage compared to mammography and sonography in early detection of invasive breast cancer, which is being leveraged to optimize screening outcomes.
Supplemental screening with MR imaging has been found to be of value in high-risk women as well as in certain subgroups of higher-than-average-risk women, but careful cost-benefit considerations are needed.
Overall adherence to MR imaging among currently eligible women is poor even as screening indications of MR imaging continue to evolve.
CLINICS CARE POINTS.
In women who undergo annual MR imaging for screening, concurrent mammography primarily provides added sensitivity for in-situ disease, but additional sonography has limited value.
BRCA1 mutation carriers less than 50 years of age who undergo screening have the highest rate of interval cancers despite annual MR imaging and may benefit from increased frequency of screening.
Unclassified genetic VUS, including BRCA1/2 VUS, without other risk factors are not indications for supplemental screening with MR imaging.
Acknowledgments
This work has not received grant funding.
Footnotes
DISCLOSURE
The authors have nothing to disclose pertaining to this work.
REFERENCES
- 1.Vreemann S, Gubern-Mérida A, Schlooz-Vries MS, et al. Influence of risk category and screening round on the performance of an MR imaging and mammography screening program in carriers of the BRCA mutation and other women at increased risk. Radiology 2018;286(2):443–51. [DOI] [PubMed] [Google Scholar]
- 2.Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology 2017; 283(2):361–70. [DOI] [PubMed] [Google Scholar]
- 3.Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): Final results. Invest Radiol 2011;(46):94–105. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010; 28(9):1450–7. [DOI] [PubMed] [Google Scholar]
- 5.Mann RM, Nariya C, Moy L. Breast MRI: state of the art. Radiology 2019;293(3):520–36. [DOI] [PubMed] [Google Scholar]
- 6.Warner E, Kimberley H, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol 2011;(29):1664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans DG, Harkness EF, Howell A, et al. Intensive breast screening in BRCA2 mutation carriers is associated with reduced breast cancer specific and all cause mortality. Heredit Cancer Clin Pract 2016;(14):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houssami N Overdiagnosis of breast cancer in population screening: does it make breast screening worthless? Cancer Biol Med 2017;14(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018;15(3 Pt A):408–14. [DOI] [PubMed] [Google Scholar]
- 10.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 11.Chiarelli AM, Blackmore KM, Muradali D, et al. Performance measures of magnetic resonance imaging plus mammography in the high risk Ontario Breast Screening Program. J Natl Cancer Inst 2020; 112(2):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saadatmand S, Amarens Geuzinge H, Rutgers EJT, et al. MRI versus mammography for breast cancer screening in women with familial risk (FaMRIsc): a multicentre, randomised, controlled trial. Lancet Oncol 2019;20(8):1136–47. [DOI] [PubMed] [Google Scholar]
- 13.van Zelst JCM, Mus RDM, Woldringh G, et al. Surveillance of women with the BRCA1 or BRCA2 mutation by using biannual automated breast US, MR imaging, and mammography. Radiology 2017; 285(2):376–88. [DOI] [PubMed] [Google Scholar]
- 14.The American Society of Breast Surgeons - Position statement on screening mammography. 2019. Available at: https://www.breastsurgeons.org/docs/statements/Position-Statement-on-Screening-Mammography.pdf. Accessed May 24, 2020.
- 15.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology - Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 1.2020 (December. 4, 2019). 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf. Accessed May 21, 2020. [DOI] [PubMed]
- 16.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology – Breast Cancer Screening and Diagnosis. Version 1.2019 (May 17, 2019). 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed May 21, 2020. [DOI] [PubMed]
- 17.Mann RM, Balleyguier C, Baltzer PA, et al. Breast MRI: EUSOBI recommendations for women’s information. Eur Radiol 2015;25(12):3669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The European Commission Initiative on breast cancer (ECIBC) guidelines for breast cancer screening. Available at: https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines. Accessed April 27, 2020.
- 19.ACOG (American College of Obstetricians and Gynecologists): Committee on practice bulletins - Gynecology, Committee on genetics, Society of Gynecologic Oncology. Practice Bulletin No 182: Hereditary Breast and Ovarian Cancer Syndrome. Obstet Gynecol 2017;130(3):e110–26. [DOI] [PubMed] [Google Scholar]
- 20.ACOG (American College of Obstetricians and Gynecologists): Committee on Gynecologic Practice. ACOG Committee Opinion no. 593: Management of women with dense breasts diagnosed by mammography. Obstet Gynecol 2014;123(4): 910–1. [DOI] [PubMed] [Google Scholar]
- 21.Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: A combined analysis of 22 studies. Am J Hum Genet 2003;72(5):1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riedl CC, Nikolaus L, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol 2015; 33(10):1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosse K, Monika G, Gossmann A, et al. Supplemental screening ultrasound increases cancer detection yield in BRCA1 and BRCA2 mutation carriers. Arch Gynecol Obstet 2014;289(3):663–70. [DOI] [PubMed] [Google Scholar]
- 24.Warner E Screening BRCA1 and BRCA2 mutation carriers for breast cancer. Cancers (Basel) 2018; 10(12):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriege M, Brekelmans CTM, Boetes C, et al. Differences between first and subsequent rounds of the MRISC breast cancer screening program for women with a familial or genetic predisposition. Cancer 2006;106(11):2318–26. [DOI] [PubMed] [Google Scholar]
- 26.Rijnsburger AJ, Inge-Marie O, Kaas R, et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: Long-term follow-up of Dutch MRISC screening study. J Clin Oncol 2010;28(36):5265–73. [DOI] [PubMed] [Google Scholar]
- 27.Chereau E, Catherine U, Balleyguider C, et al. Characteristics, treatment, and outcome of breast cancers diagnosed in BRCA1 and BRCA2 gene mutation carriers in intensive screening programs including magnetic resonance imaging. Clin Breast Cancer 2010;10(2):113–8. [DOI] [PubMed] [Google Scholar]
- 28.Shah P, Rosen M, Sopfer J, et al. Prospective study of breast MRI in BRCA1 and BRCA2 mutation carriers: effect of mutation status on cancer incidence. Breast Cancer Res Treat 2009;118(3):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guindalini RSC, Yonglan Z, Abe H, et al. Intensive surveillance with biannual dynamic contrast-enhanced magnetic resonance imaging downstages breast cancer in BRCA1 mutation carriers. Clin Cancer Res 2019;25(6):1786–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA 2006;295(20):2374–84. [DOI] [PubMed] [Google Scholar]
- 31.Ehsani S, Strigel RM, Pettke E, et al. Screening magnetic resonance imaging recommendations and outcomes in patients at high risk for breast cancer. Breast J 2015;21(3):246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Easton DF, Pharoah PDP, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med 2015;372(23):2243–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiovitz S, Korde LA. Genetics of breast cancer: a topic in evolution. Ann Oncol 2015;26(7):1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couch FJ, Hermela S, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol 2017;3(9):1190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adank MA, Senno V, Oldenburg RA, et al. Excess breast cancer risk in first degree relatives of CHEK2 *1100delC positive familial breast cancer cases. Eur J Cancer 2013;49(8):1993–9. [DOI] [PubMed] [Google Scholar]
- 36.Antoniou AC, Silvia C, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med 2014;371(6):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst 1998;90(15): 1138–45. [DOI] [PubMed] [Google Scholar]
- 38.Margolin S, Hemming J, Rutqvist LE, et al. Family history, and impact on clinical presentation and prognosis, in a population-based breast cancer cohort from the Stockholm County. Fam Cancer 2006;2006(5):4. [DOI] [PubMed] [Google Scholar]
- 39.Do WS, Weiss JB, McGregor HF, et al. Poor compliance despite equal access: Military experience with screening breast MRI in high risk women. Am J Surg 2019;217(5):843–7. [DOI] [PubMed] [Google Scholar]
- 40.Beattie MS, Blumenthal E, Creasman J, et al. Abstract P2-02-02: uptake and predictors of screening breast MRI in high risk women. Cancer Res 2010;70(24 supplement). Available at: https://cancerres.aacrjournals.org/content/70/24_Supplement/P2-02-02. [Google Scholar]
- 41.Miller JW, Sabatino SA, Thompson TD, et al. Breast MRI use uncommon among U.S. women. Cancer Epidemiol Biomarkers Prev 2013;22(1):159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miles R, Wan F, Onega TL, et al. Underutilization of supplemental magnetic resonance imaging screening among patients at high breast cancer risk. J Womens Health (Larchmt) 2018;27(6): 748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The American College of Medical Genetics and Genomics (ACMG) Joint Guidelines for Determining Disease-Causing Potential of DNA Sequence Variations. 2015. Available at: https://www.genome.gov/sites/default/files/media/files/2019-03/American%20College%20of%20Medical%20Genetics%20and%20Genomics%20Report.pdf. Accessed May 23, 2020.
- 44.Mulder RL, Kremer LCM, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 2013;14(13): e621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrhardt MJ, Howell CR, Hale K, et al. Subsequent breast cancer in female childhood cancer survivors in the St Jude Lifetime Cohort Study (SJLIFE). J Clin Oncol 2019;37(19):1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol 2013;31(18): 2282–8. [DOI] [PubMed] [Google Scholar]
- 47.Tieu MT, Cigsar C, Ahmed S, et al. Breast cancer detection among young survivors of pediatric Hodgkin lymphoma with screening magnetic resonance imaging. Cancer 2014;120(16):2507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freitas V, Scaranelo A, Menezes R, et al. Added cancer yield of breast magnetic resonance imaging screening in women with a prior history of chest radiation therapy. Cancer 2013;119(3):495–503. [DOI] [PubMed] [Google Scholar]
- 49.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 2009;301(4):404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathan PC, Kirsten Kimberlie N, Mahoney MC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med 2010;153(7):442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oeffinger KC, Ford JS, Moskowitz CS, et al. Promoting breast cancer surveillance: The EMPOWER Study, a randomized clinical trial in the Childhood Cancer Survivor Study. J Clin Oncol 2019;37(24): 2131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JM, Buist DSM, Houssami N, et al. Five-year risk of interval-invasive second breast cancer. J Natl Cancer Inst 2015;107(7):djv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804):1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Punglia RS, Hassett MJ. Using lifetime risk estimates to recommend magnetic resonance imaging screening for breast cancer survivors. J Clin Oncol 2010;28(27):4108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magn Reson Imaging 2019;50(2):377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wernli KJ, Ichikawa L, Kerlikowske K, et al. Surveillance breast MRI and mammography: Comparison in women with a personal history of breast cancer. Radiology 2019;292(2):311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehman CD, Lee JM, Demartini WB, et al. Screening MRI in women with a personal history of breast cancer. J Natl Cancer Inst 2016;108(3):djv349. [DOI] [PubMed] [Google Scholar]
- 58.Wanders JO, Holland K, Veldhuis WB, et al. Volumetric breast density affects performance of digital screening mammography. Breast Cancer Res Treat 2017;162(1):5–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol 2017;208(1):222–7. [DOI] [PubMed] [Google Scholar]
- 60.Brandt KR, Scott CG, Ma L, et al. Comparison of clinical and automated breast density measurements: Implications for risk prediction and supplemental screening. Radiology 2016;279(3): 710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyd NF, Martin LJ, Yaffe MJ, et al. Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res 2011; 13(6):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA 2016;315:1784–6. [DOI] [PubMed] [Google Scholar]
- 63.Lowry KP, Rebecca Yates C, Miglioretti DL, et al. Screening performance of digital breast tomosynthesis vs digital mammography in community practice by patient age, screening round, and breast density. JAMA Netw Open 2020;3(7):e2011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol 2019; 29(4):1762–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sprague BL, Stout NK, Schechter C, et al. Benefits, harms, and cost-effectiveness of supplemental ultrasonography screening for women with dense breasts. Ann Intern Med 2015;162(3):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berg WA ZZ, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307(13):1394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FDA (Food and Drug Administration) - Proposed MQSA updates including requirement of breast density reporting. 2019. Available at: https://www.federalregister.gov/documents/2019/03/28/2019-05803/mammography-quality-standards-act. Accessed May 24, 2020.
- 68.Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med 2019;381(22): 2091–102. [DOI] [PubMed] [Google Scholar]
- 69.Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs Digital Breast Tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA 2020;323(8):746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.King TA, Pilewskie M, Muhsen S, et al. A 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol 2015; 33(33):3945–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Page DL, Dupont WD. Premalignant conditions and markers of elevated risk in the breast and their management. Surg Clin North Am 1990;70(4): 831–51. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperplasia of the breast — risk assessment and management options. N Engl J Med 2015;372(1): 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hartmann LC, Radisky DC, Frost MH, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila) 2014;7(2): 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Houssami N, Abraham LA, Onega T, et al. Accuracy of screening mammography in women with a history of lobular carcinoma in situ or atypical hyperplasia of the breast. Breast Cancer Res Treat 2014;145(3): 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burnside ES, Daniel V, Blanks RG, et al. Association between screening mammography recall rate and interval cancers in the UK breast cancer service screening program: A cohort study. Radiology 2018;288(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houssami N, Hunter K. The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer 2017;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sung JS, Sarah S, Brooks J, et al. Breast cancer detected at screening MR imaging and mammography in patients at high risk: method of detection reflects tumor histopathologic results. Radiology 2016; 280(3):716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strigel RM, Jennifer R, Burnside ES, et al. Screening breast MRI outcomes in routine clinical practice: Comparison to BI-RADS benchmarks. Acad Radiol 2017;24(4):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Welch HG, Prorok PC, O’Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med 2016; 375(15):1438–47. [DOI] [PubMed] [Google Scholar]
- 80.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet 2001;358(9291):1389–99. [DOI] [PubMed] [Google Scholar]
- 81.Claus EB, Schildkraut JM, Thompson WD, et al. The genetic attributable risk of breast and ovarian cancer. Cancer 1996;77(11):2318–24. [DOI] [PubMed] [Google Scholar]
- 82.Warner E, Messersmith H, Causer P, et al. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 2008;148(9):671–9. [DOI] [PubMed] [Google Scholar]
- 83.Kuhl CK, Annika K, Strobel K, et al. Not all false positive diagnoses are equal: On the prognostic implications of false-positive diagnoses made in breast MRI versus in mammography/digital tomosynthesis screening. Breast Cancer Res 2018;20(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vreemann S, van Zelst JCM, Schlooz-Vries M, et al. The added value of mammography in different age-groups of women with and without BRCA mutation screened with breast MRI. Breast Cancer Res 2018;20(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo G, Scaranelo AM, Aboras H, et al. Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology 2017;285(1):36–43. [DOI] [PubMed] [Google Scholar]
- 86.Roark AA, Dang PA, Niell BL, et al. Performance of screening breast MRI after negative Full-Field Digital Mammography versus after negative Digital Breast Tomosynthesis in women at higher than average risk for breast cancer. AJR Am J Roentgenol 2019; 212(2):271–9. [DOI] [PubMed] [Google Scholar]
- 87.Moore SG, Shenoy PJ, Fanucchi L, et al. Cost-effectiveness of MRI compared to mammography for breast cancer screening in a high risk population. BMC Health Serv Res 2009;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Griebsch I, Broem J, Boggis C, et al. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs X-ray mammography of women at a high familial risk of breast cancer. Br J Cancer 2006;95(7):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pataky R, Armstrong L, Chia S, et al. Cost-effectiveness of MRI for breast cancer screening in BRCA1/2 mutation carriers. BMC Cancer 2013;13:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mango VL, Goel A, Mema E, et al. Breast MRI screening for average-risk women: A monte carlo simulation cost-benefit analysis. J Magn Reson Imaging 2019;49(7):e216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaeffer M, May BJ, Hogan BC, et al. Breast cancer screening adherence at multiple timepoints over eight years among women in a familial cohort. J Clin Oncol 2019;37(15_suppl):1557. [Google Scholar]
- 92.Kuhl CK, Simone S, Strobel K, et al. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014;32(22): 2304–10. [DOI] [PubMed] [Google Scholar]
- 93.Leithner D, May L, Morris EA, et al. Abbreviated MRI of the breast: Does it provide value? J Magn Reson Imaging 2019;49(7):e85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mann RM, Mus R, van Zelst J, et al. A novel approach to contrast-enhanced breast magnetic resonance imaging for screening: high-resolution ultrafast dynamic imaging. Invest Radiol 2014;49(9): 579–85. [DOI] [PubMed] [Google Scholar]
- 95.Abe H, Mori N, Tsuchiya K, et al. Kinetic analysis of benign and malignant breast lesions with ultrafast dynamic contrast-enhanced MRI: Comparison with standard kinetic assessment. AJR Am J Roentgenol 2016;207(5):1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]


