Dear Editor,
Delayed large local skin reactions following COVID-19 mRNA vaccine administration have been recently reported in three cases series [[1], [2], [3]]. We report here a case of delayed large local reaction which shows this phenomenon can also occur after administration of an adenovirus-vectored (ChAdOx1) vaccine.
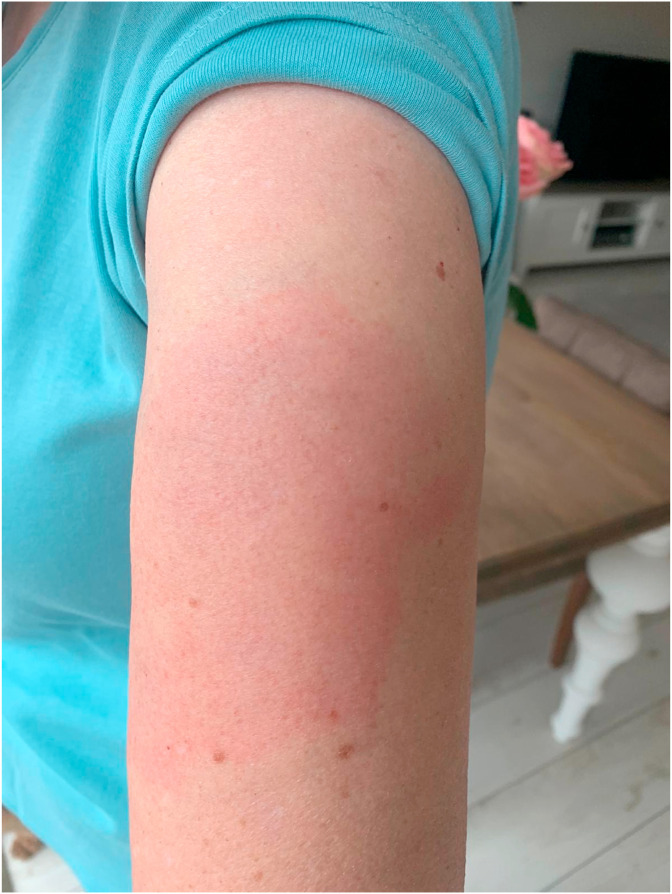
A 60-year-old female nurse, without any predisposing disease, developed a 15 × 10 cm large erythematous lesion on her left upper arm (Fig. 1 ) on D16 after receipt of the first dose of the ChAdOx1 vaccine. Two days prior, she had developed headache and chills, without other signs or symptoms. Her general physician prescribed a course of oral flucloxacillin for presumptive cellulitis. Within a day, the erythema started to fade, with complete resolution after 4 days (D20 post-vaccination). A SARS-CoV-2 PCR test initiated on presentation on D16 was negative. Our patient still has to receive her second vaccination.
Fig. 1.
Erythematous lesion around the vaccine injection site on D16 post-vaccination (the day of onset of the eruption).
Short-lived local reactions are common adverse effects of intramuscular vaccinations, and are not confined to a particular antigen or vaccine platform. Blumenthal et al. [1], Ramos and Kelso [2] and Johnston [3] et al., however, recently reported three series of delayed extensive local reactions to the two registered mRNA vaccines, comprising 39 cases following mRNA-1273 [[1], [2], [3]], and one single case following BNT162b2 vaccine administration [2].
Among the three published case series, 34/40 patients (85%) were women, predominantly white, and with a median age between 38 and 51 years old (overall range 25–89) [[1], [2], [3]]. The median onset of the skin reaction was 7–8 days (overall range 2–21 days) after the first vaccination, with a median duration of 5 days (overall range 1–21 days).
In total, 28/40 individuals (70%) developed a similar reaction, in no case aggravated reaction, after the second vaccination.
Treatment for symptom alleviation included topical steroids, oral antihistamines, and cool compresses. Two patients in total received antibiotics on the assumption of a cellulitis rather than an allergic reaction. Some patients received a premedication in anticipation of another reaction following second vaccination; however, a clear correlation between prevention of recurrence and premedication was not observed [1].
The underlying reaction – two studies found biopsy-supported evidence of a delayed-type or T-cell mediated hypersensitivity [1,3] – might, however, be less vaccine platform-, let alone vaccine-specific than it appeared from the initial reports. It has been speculated that the delayed appearance might be related to the later antigen (SARS-CoV-2 spike protein) production following mRNA application as compared to classical ‘prêt-à-porter’ antigen presentation up from the timepoint of injection [1]. However, with adenoviral vector vaccines, antigen production is also delayed, as DNA of the spike protein is delivered within the viral envelope, and host cells start producing the spike protein in a way similar to mRNA vaccines [4]. The case reported here shows that this phenomenon may be common to more COVID-19 vaccines, including vector-based ones.
Conceivably, an alternative mechanism triggering those delayed reaction might not be related to viral antigen but rather to some excipients. The mRNA-1273 vaccine contains PEG 2000 dimyristoyl glycerol (as well as tromethamine and proprietary SM-102), while the BNT162b2 vaccine contains 2 [(PEG)-2000]-N,N-ditetradecylacetamide. PEGs are known to cause immediate hypersensitivity reactions, but they can also cause delayed hypersensitivity reactions. Interestingly, the ChAdOx1 vaccine contains polysorbate 80, and cross-reactivity with PEGs may occur. Perhaps, the observed frequency of delayed cutaneous reactions (mRNA-1273≫ BNT162b2≫ ChAdOx1) might be a reflection of an underlying predisposition and reactogenicity to these vaccine ingredients, possibly in descending order [5].
Further observation of this colloquially dubbed ‘COVID Arm’ phenomenon will enhance our understanding of this self-limiting and fully reversible transient reaction.
Funding
No funding received.
Authors’ contributions
MPG conceived the paper and wrote the first draft. All authors contributed to the data analysis and interpretation and contributed to the writing of the final version of the paper.
Funding source
All sources of funding should also be acknowledged and you should declare any involvement of study sponsors in the study design; collection, analysis and interpretation of data; the writing of the manuscript; the decision to submit the manuscript for publication. If the study sponsors had no such involvement, this should be stated.
Declaration of competing interest
A conflicting interest exists when professional judgement concerning a primary interest (such as patient's welfare or the validity of research) may be influenced by a secondary interest (such as financial gain or personal rivalry). It may arise for the authors when they have financial interest that may influence their interpretation of their results or those of others. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
References
- 1.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021 Apr 1;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos C.L., Kelso J.M. COVID Arm": very delayed large injection site reactions to mRNA COVID-19 vaccines. J Allergy Clin Immunol Pract. 2021 Apr 20 doi: 10.1016/j.jaip.2021.03.055. S2213-2198(21)00443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston M.S., Galan A., Watsky K.L., Little A.J. Delayed localized hypersensitivity reactions to the moderna COVID-19 vaccine - a case series. JAMA Dermatol. 2021 May 12 doi: 10.1001/jamadermatol.2021.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21(4):195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkowski K., Mirakian R., Till S., et al. Adverse reactions to COVID-19 vaccines: a Practical approach. Clin Exp Allergy. 2021 April 4 doi: 10.1111/cea.13880. https://doi:10.1111/cea.13880 [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]