Introduction
In response to the SARS-CoV-2 pandemic, 2 messenger RNA (mRNA) lipid-stabilized vaccines involving new technologies have been launched with unprecedented urgency. Early postrelease data have shown these vaccines to be extremely effective and safe. Some reports have emerged suggesting that the second dose of the Moderna (mRNA-1273) vaccine can cause adverse dermatologic reactions. This article describes a series of both localized and generalized mild adverse cutaneous reactions in a community-based outpatient dermatology practice.
Case series
Local eruptions
Case 1
A 67-year-old Caucasian woman with a history of mild atopy, but no history of SARS-CoV-2 infection, developed an itchy 7-cm erythematous red patch at the vaccine injection site of the upper portion of her left arm, 1 week after receiving her first Moderna vaccine. The rash lasted for 1 week, with some improvement after topical corticosteroid use. There was no skin reaction to the second vaccine.
Case 2
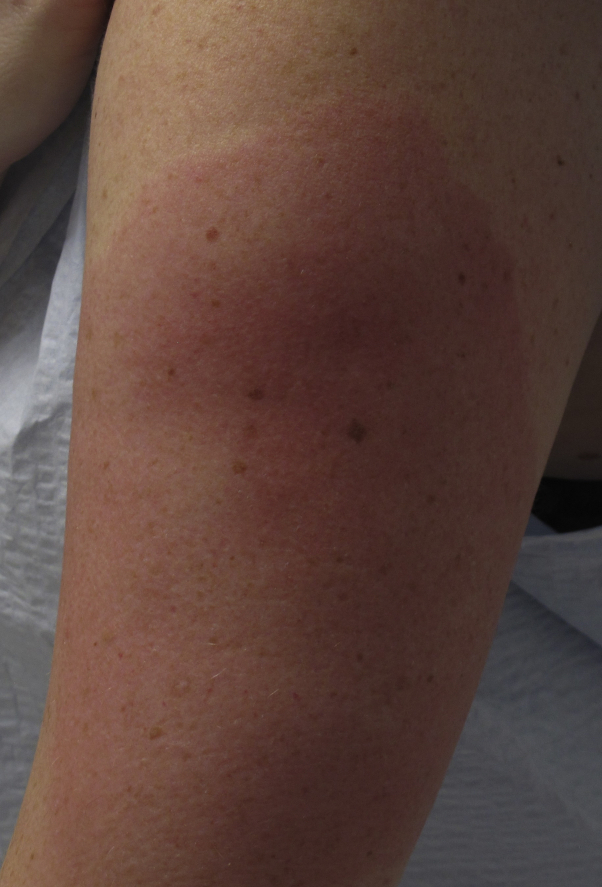
A 40-year-old Caucasian woman with an atopic family history and no history of SARS-CoV-2 disease or exposure presented with swelling and progressive erythema on the left arm 8 days after receiving the first Moderna COVID-19 vaccine. The erythema extended down her arm over the ensuing days. Her internist prescribed erythromycin for presumed erysipelas. A sharply demarcated warm urticarial oval patch was observed with erythema, extending gravitationally down her arm (Fig 1). After receiving the second inoculation, she experienced some mild swelling at the injection site; however, it was not as severe as after the first injection.
Fig 1.
Case 2 with sharply demarcated urticarial localized skin reaction after vaccination.
Case 3
A 53-year-old Caucasian woman with mild atopic background and no history of prior SARS-CoV-2 infection received the first Moderna SARS-CoV-2 vaccine with some mild sensitivity at the injection site and tenderness (but no erythema) for 3 days, which spontaneously resolved. The day after the second vaccine, she experienced flu-like symptoms with generalized weakness and diarrhea and developed a tender erythematous urticarial red ring on the injected arm. At the height of her symptoms, she could not raise her arm above 90° angle. The rash and symptoms resolved without any treatment within 3 days.
General eruptions
Case 4
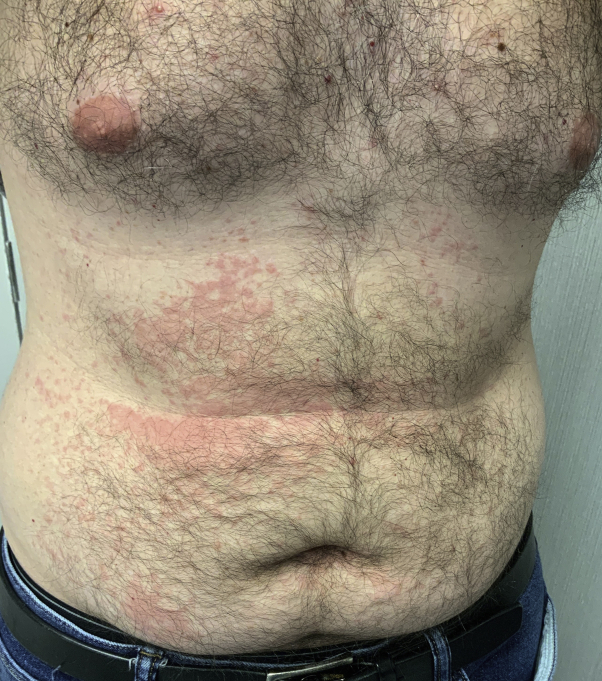
A 45-year-old Caucasian man with a history of atopy and seasonal allergies but no SARS-CoV-2 exposure developed a pruritic morbilliform rash with no shortness of breath 8 days after the first Moderna SARS-CoV-2 vaccine. The rash spread to the arms and abdomen (Fig 2). The rash resolved spontaneously over a week. He experienced no skin or other reactions after the second vaccination.
Fig 2.
Case 4 with urticarial morbilliform diffuse skin reaction.
Case 5
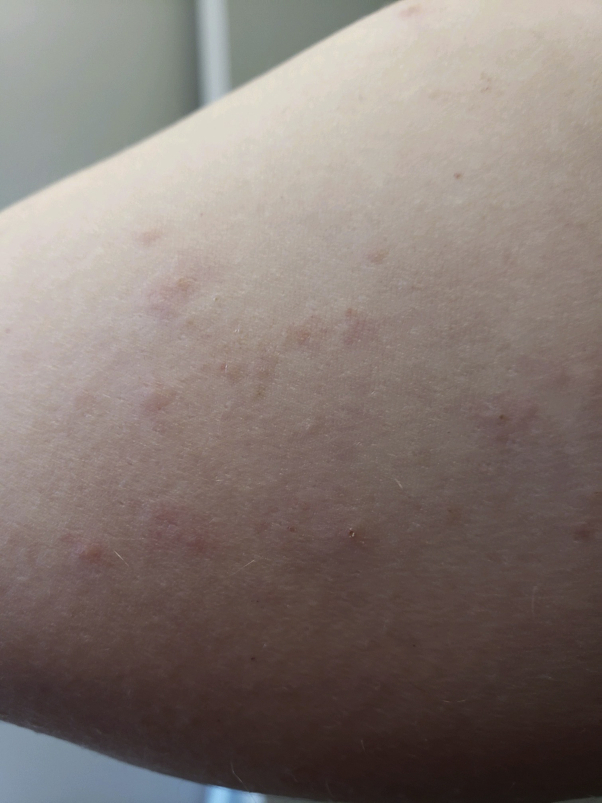
A 31-year-old Caucasian woman with a prior history of guttate psoriasis but no prior SARS-CoV-2 infection developed an urticarial papular eruption on the contralateral aspect of the right arm 3 days after receiving the second Moderna SARS-CoV-2 vaccine in the left arm (Fig 3). She had an identical reaction after the first vaccine. However, the second vaccine reaction was accompanied by low-grade fever, generalized achiness, and malaise. The rash faded over the following week without treatment.
Fig 3.
Case 5 with urticarial papular rash on the contralateral aspect of the vaccinated arm.
Case 6
An 88-year-old Caucasian woman with a history of multiple drug allergies developed generalized and progressing pruritus over 3 days after receiving the first Moderna SARS-CoV-2 vaccine. She also described a pins and needles sensation on the limbs and dysesthesia on the tongue but no difficulty in swallowing. No overt rash developed. The symptoms were partially relieved with the use of Benadryl (Johnson and Johnson) and Sarna (Stiefel) lotion. She experienced no skin reaction after receiving the second vaccine.
Histopathology
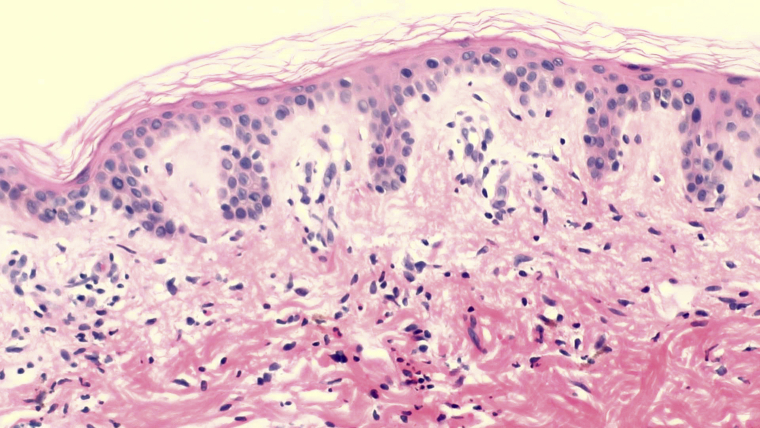
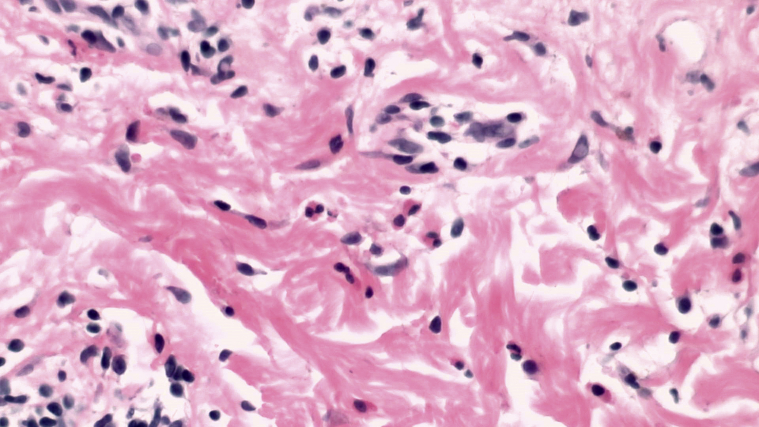
Histopathology of 6 biopsies from 4 patients demonstrated changes of urticarial allergic dermatitis. Pathology of both localized and generalized reactions demonstrated similar patterns of inflammation. There was some spongiosis within the epidermis and a superficial and deep perivascular and interstitial mixed cell infiltrate containing variable numbers of eosinophils and neutrophils (Figs 4 and 5). No vasculitis was identified. In the patient described in case 4, there were abundant eosinophils, whereas they were less common in the other patients and relatively rare in 1 patient. Neutrophils were also present in all cases in varying numbers. Typical changes of erysipelas, including subepidermal edema and extravasated erythrocytes, were not present.
Fig 4.
Specimen from the patient in case 4 at scanning power demonstrated spongiosis and a superficial and deep, perivascular, and interstitial infiltrate, mixed cell type with numerous eosinophils and occasional neutrophils. All patients demonstrated a relatively variable number of eosinophils and neutrophils within the inflammatory cell infiltrate.
Fig 5.
Specimen from the patient in case 4 at high power showed abundant interstitial eosinophils within the reticular dermis.
The conclusions that may be drawn based upon this small number of cases are limited.
Discussion
There are currently 11 approved vaccines worldwide and 86 candidate vaccines, and 20 vaccines are currently in stage 3 clinical trials.1 The 2 approved mRNA vaccines in the United States have more than 90% immunization response rates.2,3 Both mRNA vaccines are lipid nanoparticle-encapsulated mRNA-based vaccines that encode the perfusion stabilized full-length spike protein of SARS-CoV-2.4 In phase 1 through 3 trials of the Pfizer-BioNTech (BNT162b2; Pfizer, Inc) and Moderna (mRNA-1273) vaccines, minor immediate and delayed local skin reactions, including pain, redness, and swelling, were observed more often with the vaccine than with the placebo.2,3,5, 6, 7 These reactions are distinct from the anaphylaxis reported with the Pfizer vaccine, which is rare but 10 times more common with Pfizer vaccine than with all previous vaccines.8 Despite these rates being higher than those with placebo, it remains unclear whether excipients such as polyethylene glycol (PEG) and its derivatives (such as polysorbates) in the vaccine are responsible for some of these reactions.9 The predominance of women having adverse cutaneous reactions to the vaccine in this series appears to parallel women having increased systemic side effects, including anaphylaxis.
The etiology of skin responses described in this article (4 to the first shot of the vaccine and 2 to the second shot) is unclear. In 3 of these patients, the rash occurred after the first vaccination but not the second one. One patient had mild recurrence after the second vaccination, 1 had a similar reaction to the first vaccination, and 1 had a worse reaction. These differences remain difficult to understand. The responses may be immunoglobulin E- (IgE-) or non-IgE-mediated, and it is unclear which component of the vaccines is responsible (vaccine antigen, residual nonhuman protein, preservatives, or stabilizers/excipients). Historically, IgE-mediated reactions are more typically associated with the inactive components of the vaccine (ie, eggs, gelatin, and latex). The mRNA SARS-CoV-2 vaccines available in the United States have PEG 2000 (ALC-0159 = 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide) or its derivative polysorbate 80 in their excipients.8 The cross-reactivity to polysorbate 80 is thought to be due to the shared chemical moiety, –(CH2CH2O)n, which has also been described to cause reactivity.10, 11, 12 Thousands of medications, cosmetics, and foods contain either PEGs or structurally similar polysorbates. Immediate hypersensitivity to PEG and cross-reactivity to polysorbate 80 is thought to be underrecognized and increasing in frequency in recent years.10,13 Individuals who experience anaphylaxis to these vaccines are advised to avoid all PEG 2000-formulated mRNA vaccines and injectable polysorbate 80 products.10 This lipid-based nanoparticle stabilization of the vaccine is unique and novel to the mRNA vaccines and, to date, not in wide use. Therefore, impressions cannot be based on historical data.
Urticarial allergic dermatitis is used to denote the lesions that clinically resemble urticaria but have some histologic features that are not typically seen in urticaria. Clinically, the patients described here had the edematous papules and plaques prototypical of urticaria. However, the lesions lasted for more than 24 hours and did not come and go. In addition, there was slight spongiosis present within the epidermis, which is not present in acute or chronic urticaria. Although the eruptions in these patients are urticarial, they are not true urticaria just as the urticarial lesions of bullous pemphigoid, arthropod bites, leukocytoclastic vasculitis, or drug eruption are not genuine urticaria.14
The reactions described here were of 2 types: those that occurred only at the injection site and those that developed away from the injection site. The reactions that were not localized to the injection site included a generalized morbilliform eruption, 1 involved the contralateral aspect of the arm, and another resulted in generalized pruritus. All reactions described were minor and did not require stopping the completion of the vaccination series. Most of the patients in this series were women, and all patients were Caucasian. This may reflect the disparate vaccine availability and acceptance within different demographic populations. Although both mRNA vaccines are widely distributed in our geographic location, in this small series of cutaneous reactions, hypersensitivity cases were found only with the Moderna mRNA vaccine. Whether vaccine-induced skin reaction is a marker of better acquired immunity or related to prior coronavirus/SARS-CoV-2 exposure and immunity remains to be determined. Although surveillance over the next months through the Vaccine Adverse Event Reporting System (https://vaers.hhs.gov) should reveal any serious toxicities, these mild skin reactions are easily managed and of no apparent serious sequelae.
Conflicts of interest
None disclosed.
Acknowledgments
Special thanks to Ms Phyllis Strum, BA and Drs Sari Weinstein, MD, Elie B Lowenstein, MD, and Roxanne Abitbol, MD for their contribution to this article.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.COVID19 Vaccine Tracker Updated June 11, 2021. https://covid19.trackvaccines.org/
- 2.Walsh E.E., Frenck R.W., Jr., Falsey A.R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver S.E., Gargano J.W., Marin M. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson L.A., Anderson E.J., Rouphael N.G. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal K.G., Freeman E.E., Saff R.R. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N Engl J Med. 2021;384(7):643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dooling K., McClung N., Chamberland M. The Advisory Committee on Immunization Practices' interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone C.A., Jr., Liu Y., Relling M.V. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. J Allergy Clin Immunol Pract. 2019;7(5):1533–1540.e8. doi: 10.1016/j.jaip.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calogiuri G., Foti C., Nettis E., Di Leo E., Macchia L., Vacca A. Polyethylene glycols and polysorbates: two still neglected ingredients causing true IgE-mediated reactions. J Allergy Clin Immunol Pract. 2019;7(7):2509–2510. doi: 10.1016/j.jaip.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Cabanillas B., Akdis C., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of polyethylene glycol? Allergy. 2021;76(6):1617–1618. doi: 10.1111/all.14711. [DOI] [PubMed] [Google Scholar]
- 13.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9(2):670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 14.Kossard S., Hamann I., Wilkinson B. Defining urticarial dermatitis: a subset of dermal hypersensitivity reaction pattern. Arch Dermatol. 2006;142(1):29–34. doi: 10.1001/archderm.142.1.29. [DOI] [PubMed] [Google Scholar]