Significance
Auditory neuropathy spectrum disorder (ANSD) is a confounding auditory disease in which the subjects respond to sound but have difficulties in speech discrimination. Herein, we examined two Asian families with hereditary late-age–onset ANSD. By linkage analysis and exome sequencing, we identified the TMEM43-p.(Arg372Ter) variant as the etiology of the disease. To examine the mechanism of TMEM43 on ANSD, we generated a knock-in mouse with the p.(Arg372Ter) variant that recapitulated the progressive hearing loss phenotype of the two families. We discovered that variation in TMEM43 impairs the connexin-linked function of mediating passive conductance current in cochlear glial cells. Based on the pathology involving cochlear glial cells, we performed cochlear implant on the human patients, and their speech discrimination ability was restored.
Keywords: auditory neuropathy spectrum disorder, cochlea, glia-like supporting cells, connexins
Abstract
Genes that are primarily expressed in cochlear glia-like supporting cells (GLSs) have not been clearly associated with progressive deafness. Herein, we present a deafness locus mapped to chromosome 3p25.1 and an auditory neuropathy spectrum disorder (ANSD) gene, TMEM43, mainly expressed in GLSs. We identify p.(Arg372Ter) of TMEM43 by linkage analysis and exome sequencing in two large Asian families segregating ANSD, which is characterized by inability to discriminate speech despite preserved sensitivity to sound. The knock-in mouse with the p.(Arg372Ter) variant recapitulates a progressive hearing loss with histological abnormalities in GLSs. Mechanistically, TMEM43 interacts with the Connexin26 and Connexin30 gap junction channels, disrupting the passive conductance current in GLSs in a dominant-negative fashion when the p.(Arg372Ter) variant is introduced. Based on these mechanistic insights, cochlear implant was performed on three subjects, and speech discrimination was successfully restored. Our study highlights a pathological role of cochlear GLSs by identifying a deafness gene and its causal relationship with ANSD.
Auditory neuropathy spectrum disorder (ANSD) is defined as an inability in speech discrimination despite preserved sensitivity to sound (1). Clinically, ANSD has been characterized by the presence of otoacoustic emission (OAE) and/or cochlear microphonics (CM) and the concurrent absence of averaged auditory brainstem responses (ABR) or presence of abnormal ABR (2, 3). The presence of OAE and/or CM is indicative of normal cochlear outer hair cell (OHC) activity, whereas the abnormal ABR is indicative of disrupted auditory nerve (AN) activity (2). Up to now, research has suggested that ANSD is caused by a malfunctioning of the inner hair cells (IHC), the synapse between the IHCs and AN, or AN itself such as demyelination or desynchronization (4–6). However, in the organ of Corti of inner ear, glia-like supporting cells (GLSs) comprise major cell types in addition to OHCs and IHCs. GLSs are defined as glia-like cells due to a presence of typical glia markers such as GFAP and GLAST (7). While hair cells play critical roles in mechanoreception and synaptic transmission by converting the acoustic energy into electrochemical signals that are relayed to the brainstem (7), GLSs reside adjacent to hair cells and play critical roles in development and maintenance of auditory system (7–10). Developing mammalian cochlea can be categorized in two anatomical regions as greater epithelial ridge (GER) and lesser epithelial ridge (LER) (11). The GER refers to the area medial to pillar cells including IHCs and inner GLSs of Kolliker’s organ such as inner phalangeal cells and border cells (12, 13). The LER spans the area radial to pillar cells, consisting of OHCs and outer GLSs such as Hensen’s cells and Deiters’ cells (12). The GLSs in Kolliker’s organ have been studied to generate ATP- and TMEM16A-dependent spontaneous activity that depolarizes IHCs (10, 11, 13), generate spontaneous Ca2+ signaling for OHC refinement (14), and spontaneously regenerate hair cell in the neonatal mouse cochlea (9, 15, 16). Despite their essential function at prehearing stages, the precise role of GLSs in hearing or speech discrimination, especially their contribution to late-onset ANSD, remains unknown.
The GLSs are physically coupled to each other by gap junctions (7). Gap junctions consist of two hemi channels, which are encoded by connexin genes, that meet at the plasma membrane of adjacent cells to achieve cell coupling. The connexins provide a pathway for rapid removal of ions from the region of the hair cells during sound transduction (17) by recycling and regulating intracellular K+ and maintaining pH homeostasis (18–20). Connexin26 (Cx26, GJB2) and Connexin30 (Cx30, GJB6) are the two predominantly expressed connexins in the GLSs of mammalian cochlea (21) and also the major deafness genes known to induce high incidence of nonsyndromic hearing loss (21–24). Variants in connexins break the endocochlear potential of the inner ear and lead to hearing loss (21). Although a pathological role of connexin variants on ANSD has been suggested (25), a combination of heterozygous phenotypes, diverse Cx26 and Cx30 variants (21), and various interacting proteins (26, 27) make it difficult to determine the molecular and cellular mechanism of connexin-related ANSD. Moreover, none of the known interacting proteins for connexins has been associated with ANSD.
In this study, we identified two Asian families with hereditary late-onset ANSD with progressive hearing loss. By linkage analysis and exome sequencing, we determined TMEM43-p.(Arg372Ter) variant as the origin of the disease. In order to examine the role of TMEM43, we generated a knock-in (KI) mouse with p.(Arg372Ter) variant which recapitulated a progressive hearing loss with ANSD phenotypes. Ex vivo and in vitro studies further demonstrated that TMEM43 physically interacts with Cx26 and Cx30 in the GLSs. When the p.(Arg372Ter) variant was introduced, passive conductance current in GLSs was disturbed with histological abnormalities in GLSs. Based on the mechanistic insights, cochlear implant (CI) was performed on patients with p.(Arg372Ter) variant, and their speech discrimination was successfully restored. Our study identifies an interacting protein of connexins in cochlea and introduces a role of GLSs in late-onset ANSD.
Results
TMEM43 Is a Novel Deafness Gene.
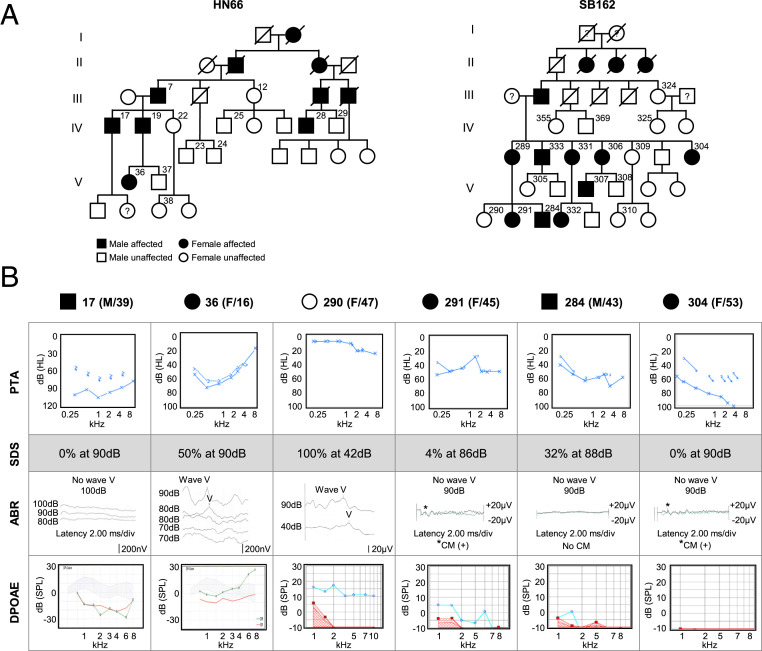
We identified two large five-generation pedigrees of Chinese Han family (HN66) and Korean family (SB162), nonconsanguineously segregating the late-onset progressive ANSD in an autosomal dominant fashion (Fig. 1A and SI Appendix, Fig. S1A). Unlike the normal subject (#290) in SB162, the affected subjects (#17, #291, #284, and #304) from families HN66 and SB162 displayed elevated pure-tone audiogram (PTA) thresholds and disproportionately lower speech discrimination score (SDS) for the PTA thresholds. Importantly, complete absence of ABR was noted from these affected subjects, despite the presence of either distortion-product OAE (DPOAE) or CM (Fig. 1B), which are classic signs of ANSD. CM measurement was not performed from subjects #17 and #36. In subjects #374 and #376 from SB162, elevation of low-frequency pure-tone hearing thresholds, which was not subjectively detectable and not accompanied by decrease in speech discrimination, began slowly from the age of 10 (SI Appendix, Fig. S1A), and the typical ASND symptoms appeared from the middle of second decade as shown in subject #36 from HN66 (a 16-y-old female) (Fig. 1B). She showed a significant elevation of ABR threshold as high as 80 dB, while DPOAE response was completely preserved (Fig. 1B). This suggests that phenotype for #36 can be interpreted as the stage of transition to canonical ANSD. In addition, we measured PTA in time series from three individuals (#284, #374, and #376) and found a time-dependent progression of hearing loss (SI Appendix, Fig. S1A). Given significant aggravation of hearing over 15 y in #284 from SB162, we concluded that the progression of ANSD appears to be particularly pronounced in the 30s (SI Appendix, Fig. S1A).
Fig. 1.
Pedigree and audiological assessment of family HN66 and SB162. (A) Pedigree of Chinese family HN66 and a Korean family SB162 family segregates ANSD in an autosomal dominant fashion. Open and filled symbols indicate unaffected and affected statuses, respectively. Square and circle symbols indicate male and female, respectively. Subject numbers are superscripted. (B) PTA, SDS, ABR, and DPOAE of six subjects from HN66 and SB162. The behavioral hearing threshold of each subject can be identified in the PTA results, and ABR tests identify the electrophysiological hearing threshold where wave V disappears. In DPOAE, red line indicates level of noise, and blue or green line indicates level of response. The gray shading in #17 and #36 indicates a normative region. The control subject #290 shows 40 dB or lower ABR threshold. #36, #291, and #304 show no response to 90 dB stimulus (#291 and #304) or 80 dB of hearing threshold as shown by the overlapping wave V to the threshold stimulus of the same magnitude in the two repetitive measurements (#36) in ABR, while displaying either positive DPOAE responses (#36 and #291) or presence of CM (asterisk) (#304). The subject #36 showed disordered waveform differentiation in ABR responses and significant elevation of ABR threshold up to 80 dB, with preserved DPOAE response. #17 and #284 who do not elicit any ABR response show none or very subtle DPOAE response which does not suffice for diagnosis for ANSD. Subjects #17 and #36 from Chinese family HN66 share the same ABR scale, and subjects #290, #291, #284, and #304 from Korean family SB162 share the same ABR scale.
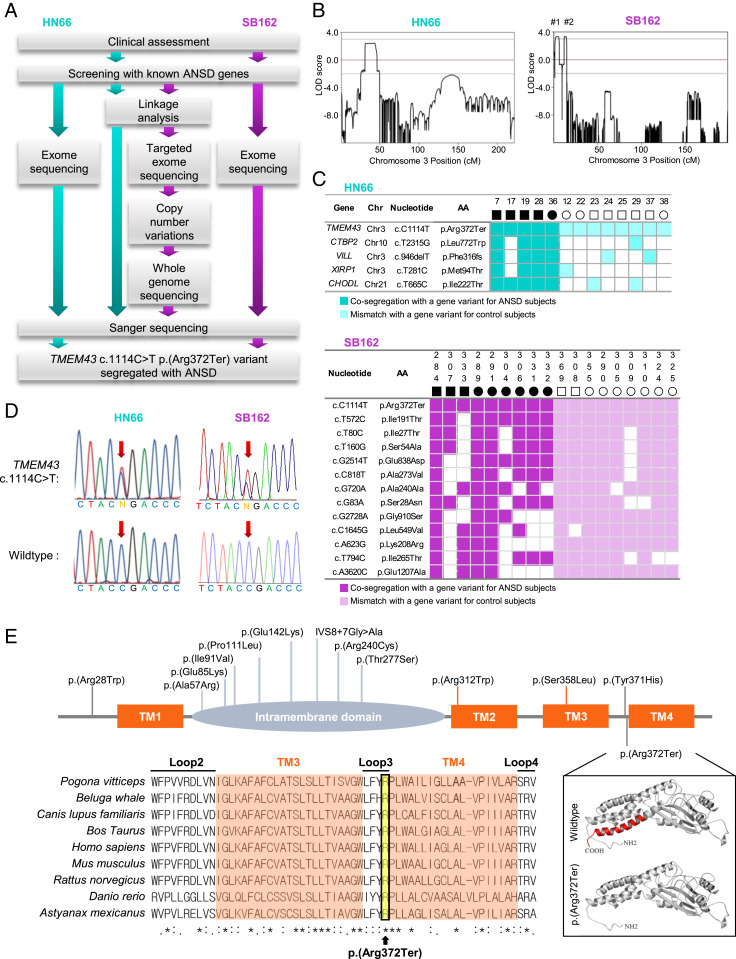
A stepwise genetic analysis was carried out to identify the pathogenic variants of families HN66 and SB162, separately (Fig. 2A). To identify the chromosomal locus of ANSD in the two families, we conducted a whole-genome linkage-scan on nine subjects (five affected and four unaffected) in HN66 and 18 subjects (10 affected and 8 unaffected) in SB162 (SI Appendix, Fig. S1B). As a result, we identified a candidate region on chromosome (Chr) 3: 13,165,401 to 22,769,511 with the highest parametric (LOD) value in both families. We calculated LOD of 2.4 across the entire chromosomes in HN66 (Fig. 2 B, Left) and LOD of greater 3.0 in SB162 (Fig. 2 B, Right). In particular, we found two regions (Region #1: Chr 3: 1,946,000 to 5,956,000; Region #2: Chr 3: 11,883,000 to 14,502,000) in the chromosome 3p25.1 with a genetic length of 6.7 and 3 cM, in SB162 (Fig. 2 B, Right and SI Appendix, Fig. S2 A, Inset). Given the failure to show any link with the previously reported ANSD genes such as OTOF (NM_194248, 2p23.3) (28), DIAPH3 (NM_001042517, 13q21.2) (29), SLC17A8 (NM_139319, 12q23.1) (30), AIFM1 (NM_004208, Xq26.1) (31), OPA1 (NM_130837, 3q29) (32), and an ANSD gene reported by us, ATP1A3 (NM_152296.4, 19q13.2) (33), we suspected an involvement of another gene.
Fig. 2.
A p.(Arg372Ter) variant of TMEM43 is the causative variant of ANSD in family HN66 and SB162. (A) Overall workflow of variant detection in HN66 and SB162. (B) Merlin multipoint linkage analysis demonstrates LOD scores greater than 3.0 on chromosome 3p25.1. Region #1 (Chr. 3: 1,946,000 to 5,956,000), region #2 (Chr. 3:11,883,000 to 14,502,000). (C) Genetic candidates of the ANSD after exome sequencing of genomic DNA from three subjects (#22, #28, and #36) in HN66 and four subjects (#289, #290, #291, and #284) in SB162. Subsequent segregation study with additional subjects to identify TMEM43 variant in HN66 and SB162 with full-match was performed. Cosegregation with a gene variant for ANSD subjects in HN66 and SB162 are indicated as cyan and purple box, respectively. Mismatch with a gene variant for control subjects are lightly colored. Chr; chromosome, AA; amino acid. (D) Representative DNA sequence chromatograms of c.1114C > T variant in TMEM43. Red arrow signifies allele c.1114C. (E) The variant spectrum of TMEM43 associated with ARVC and Emery–Dreifuss muscular dystrophy 7 (EDMD7) reported in human gene mutation database (HGMD) (Top) and the variants identified in the present study [Bottom, p.(Arg372Ter)]. The conservative amino acid sequences of TMEM43 are shown below. Asterisks, identical amino acids; orange box, TM domains; yellow box, p.(Arg372Ter). A protein structure modeling of WT human TMEM43 and the variant TMEM43 lacking TM4 (red) is shown. TM, transmembrane domain.
To narrow down another gene, we employed various advanced genomic sequencing techniques to evaluate the affected families. Although the identification of a causative gene for ANSD from these two families was done independently and blindly in China and South Korea, the linkage interval shared by the two families narrowed down to Chr 3: 13,165,401 to 14,502,000. Exome sequencing was first performed from two affected subjects (#36 and #28) and one unaffected subject (#22), yielding five candidate variants cosegregating with the phenotype (XIRP1, CTBP2, CHODL, TMEM43, and VILL) (SI Appendix, Table S1). Next, we performed detailed Sanger sequencing of these variants from 10 additional subjects in HN66 and zeroed in on only one variant [p.(Arg372Ter) from TMEM43] which cosegregated with the ANSD phenotype (Fig. 2 C and D). This variant fell within the linkage interval of HN66 (Chr 3: 13,165,401 to 22,769,511). For SB162, we employed two diagnostic pipelines in a parallel fashion (Fig. 2A). First, we performed targeted exome sequencing of the two regions obtained from the linkage analysis among four subjects (#284, #289, #290, and #291), leading to identification of two nonsynonymous variants [TMEM43-p.(Arg372Ter) and FBLN2-p.(Asp851His)] in Region #2 that cosegregated with the ANSD phenotype (SI Appendix, Table S2, Top). Of the two variants, we excluded FBLN2-p.(Asp851His), based on its high occurrence (0.4%) among the healthy Korean population in two independent databases, Korean Reference Genome Database (KRGDB) (n = 1722) and in-house Samsung Genome Institute control subjects database (n = 400), zeroing in on p.(Arg372Ter) from TMEM43 (NM_024334) as the only candidate. No convincing copy number variations, such as a large genomic deletion and duplication, were detected within the locus (SI Appendix, Fig. S2B). To exclude noncoding causative variants, we also performed whole genome sequencing of the linkage interval from eight subjects (#284, #289, #290, #304, #307, #309, #324, and #332). The filtering step left us with one nonsynonymous variant [TMEM43-p.(Arg372Ter)] and eighteen noncoding region variants that cosegregated with the deaf phenotype within the linkage interval (SI Appendix, Table S3). We excluded all of the 18 variants, based on their high occurrence (>0.4%) among the two aforementioned Korean databases from normal controls and detection of these variants among unaffected subjects or normal controls (n = 65) by Sanger sequencing, leaving p.(Arg372Ter) from TMEM43 (NM_024334) as the only candidate (SI Appendix, Table S3). Separately from the first pipeline and independently of linkage analysis, we performed exome sequencing from the same four subjects (#284, #289, #290, and #291), identifying 20 possible candidate variants through an extensive filtering process in SB162 (SI Appendix, Table S2, Bottom). We performed detailed Sanger sequencing of these variants from 14 additional subjects in the SB162 and excluded 19 variants from the potential candidates again leaving only one variant p.(Arg372Ter) from TMEM43 as a candidate (Fig. 2 C and D and SI Appendix, Fig. S1C). Taken together, we narrowed down to TMEM43- p.(Arg372Ter) as the potential causative gene for ANSD in two families, HN66 and SB162.
Next, we closely examined the amino acid sequence of TMEM43 for potential pathogenicity. Previously, p.(Ser358Leu) in TMEM43 has been shown to cause familial arrhythmogenic right ventricular cardiomyopathy (ARVC) (34, 35). Along with the variant spectrum of TMEM43 associated with the heart diseases reported in the human gene mutation database (Fig. 2 E, Top), the arginine at position 372 as well as amino acids in the transmembrane domain (TM) and loop regions of TMEM43 are highly conserved across organisms (Fig. 2 E, Bottom and SI Appendix, Fig. S3). A protein structure modeling of human TMEM43 showed that p.(Arg372Ter), which was expected to introduce a premature stop codon, caused a truncation of the last 29 amino acids of the TMEM43 protein. (Fig. 2 E, Inset). This variant was predicted to be pathogenic, according to the guideline for the interpretation of classifying pathogenic variants (PS3, PM2, PP1_Strong, and PS4_Supporting) (36, 37) and high Combined Annotation-Dependent Depletion score of 45 (38). However, the ANSD subject of HN66 and SB162 displayed symptoms of neither arrhythmia nor any other heart abnormalities. Conversely, hearing loss has not been reported in TMEM43-p.(Ser358Leu) ARVC patients (34, 35). This suggests that p.(Ser358Leu) and p.(Arg372Ter) of TMEM43 exert a completely different pathophysiological mechanism, leading to pleiotropy of this gene.
TMEM43 Is Expressed in GLSs.
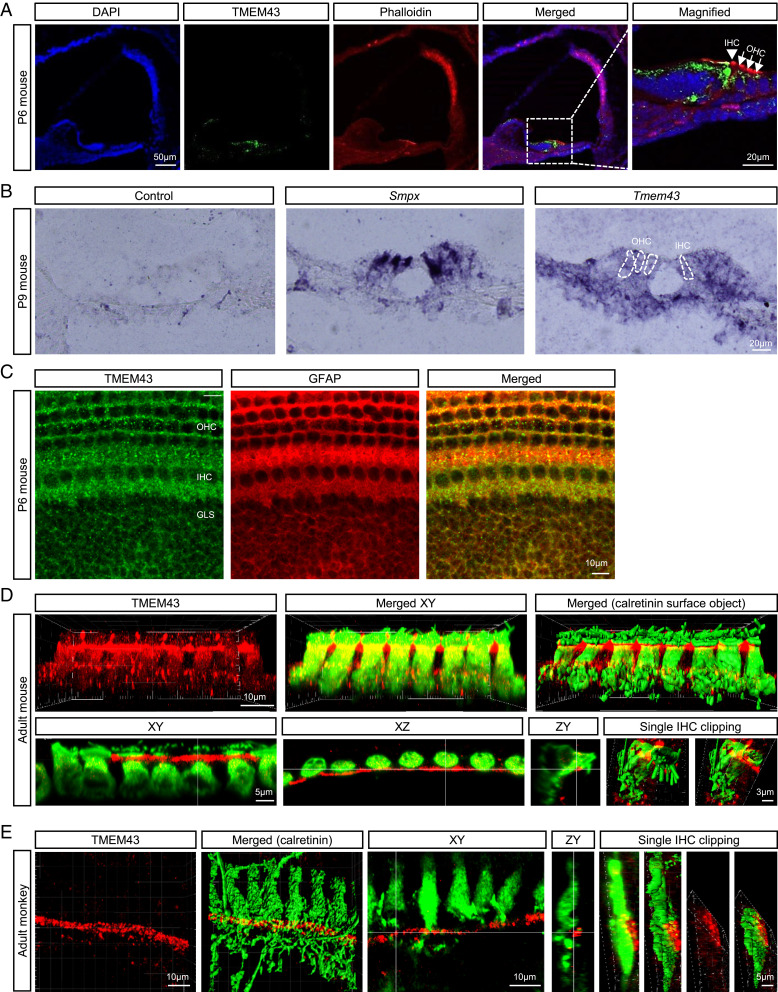
To investigate the molecular and cellular mechanisms of TMEM43-associated ANSD, we took a reverse translational approach to phenocopy the genetic disposition in mouse model. The cochlear expression of TMEM43 was firstly confirmed by RT-PCR (SI Appendix, Fig. S4A). TMEM43 was expressed throughout the mouse body including cochlea, heart, eye, brain, and kidney (SI Appendix, Fig. S4A). To specify TMEM43 expression in the cochlea tissue, we performed immunostaining using anti-TMEM43 antibody. The expression of TMEM43 protein was evaluated at various time points along the postnatal development of hearing in the mouse cochlea (SI Appendix, Fig. S4 B and C). The previous studies have reported that TMEM43 is localized predominantly to the inner nuclear membrane in multiple noncardiac cell types and also shows some expression outside the nucleus, including the endoplasmic reticulum (35, 39). In the case of ex vivo cochlea, TMEM43 was mainly expressed in the organ of Corti, along the entire cochlear length, at postnatal day 4 (P4) through P20 (Fig. 3A and SI Appendix, Fig. S4 B and C). At P20, the expression became more restricted to the apical membrane of the inner border cells and the cell junctions of the inner sulcus cells (SI Appendix, Fig. S4B). Throughout the early developmental period, up to P20, the TMEM43 expression was found mainly at inner GLSs of Kolliker’s organ, while hair cell expression is comparably sparse, at both protein and messenger RNA (mRNA) levels (Fig. 3 A and B, SI Appendix, Fig. S4 B and C, and Movies S1 and S2). TMEM43 expression in cochlear glial cell was confirmed again by coexpression with glia-specific GFAP signal (Fig. 3C). Adult mice at 1, 2, and 4 mo still maintained TMEM43 protein expression in GLSs (Fig. 3D and SI Appendix, Fig. S6C). This glia-specific TMEM43 expression was recapitulated in adult primates corresponding to age 40's in humans, showing expression in the junctional area between the inner supporting cells and IHCs, without overlapping with calretinin staining (Fig. 3E). The specificity of the antibody was confirmed via a significant reduction of immunoreactivity from the cultured mouse organ of Corti when infected with lentivirus carrying Tmem43-short hairpin RNA (shRNA) (SI Appendix, Fig. S5 A and B). These results strongly suggest that TMEM43 may play a critical role in cochlear GLSs.
Fig. 3.
TMEM43 is expressed in the GLSs of mammalian cochlea. (A) Frozen-section images of the mouse cochlea at P6 immunolabeled by anti-TMEM43 (green) and counterstained by phalloidin (red). TMEM43 immunoreactivity is detected mainly at the organ of Corti, and the magnified image shows TMEM43 immunoreactivity at the supporting cells of the organ of Corti. Arrow head, IHCs; arrows, OHCs. (B) In situ hybridization with a control probe, hair cell marker Smpx probe, and Tmem43 probe showing Tmem43 mRNA expression in the Organ of Corti. The dotted lines indicate OHCs and IHCs. (C) Immunostained cochlea tissue with TMEM43 and glial marker GFAP. (D and E) Imaris-reconstructed images of cochlea display TMEM43 expression (red) in GLSs that does not colocalize with hair cells (green) in adult mouse (D) nor adult monkey (E). XY, XZ, and ZY section views and surface object clipping images of a single IHC show that TMEM43 immunoreactivity is found primarily in the apical potion of inner border cell, juxtaposing the upper lateral membrane of calretinin-positive IHC.
Localization of TMEM43 in the Plasma Membrane and Loss of Protein Stability in TMEM43-p.(Arg372Ter).
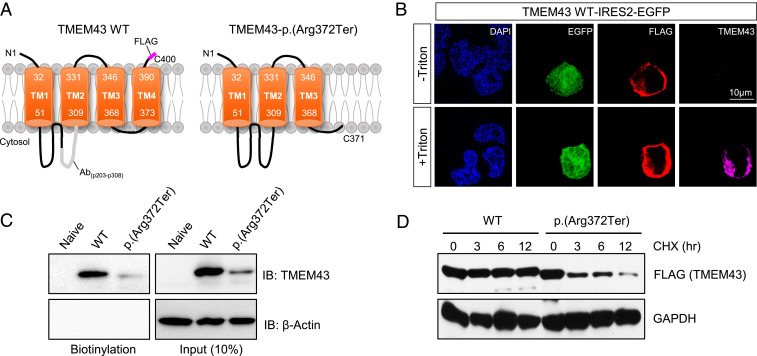
Based on hydrophobicity analysis, the secondary structure of TMEM43 was predicted to have four TMs and one intramembrane domain with its N- and C-terminal domains located at extracellular space (SI Appendix, Fig. S3A and Fig. 4A) (34). Bioinformatics revealed that TMEM43 was phylogenetically close among many species (SI Appendix, Fig. S3B), and the four TMs were highly conserved among different species (SI Appendix, Fig. S3C). We verified the predicted TMEM43 topology through immunocytochemistry with or without cell permeabilization. FLAG-tagged TMEM43 was transfected to human embryonic kidney (HEK) 293T cells and double stained with anti-FLAG and anti-TMEM43 with epitope targeting p203-p308 at intracellular loop1 (Fig. 4A). We found that TMEM43 immunoreactivity was positive only with cell permeabilization, indicating that the loop1 of TMEM43 resides at the intracellular space (Fig. 4B). In addition, a positive FLAG signal without cell permeabilization was detected (Fig. 4B), indicative of membrane expression of TMEM43 and confirming the topology. To verify the cell surface localization of TMEM43, we performed cell surface biotinylation assay. We found that TMEM43 wild-type (WT) protein trafficked to the plasma membrane, whereas TMEM43-p.(Arg372Ter) showed almost complete disappearance of the biotinylated protein along with a significant reduction in total protein (Fig. 4C). Analysis of protein stability by the cycloheximide chase assay implies that this reduction might have been due to a decreased protein stability of TMEM43-p.(Arg372Ter) (Fig. 4D). Together, these results demonstrate the putative protein topology of TMEM43 and its localization to plasma membrane.
Fig. 4.
TMEM43-p.(Arg372Ter) variant shows decreased cell surface expression with reduced protein stability. (A) Membrane topology of TMEM43 WT and p.(Arg372Ter). Antibody epitope of TMEM43 is indicated as Ab, and FLAG is tagged at C-terminal. (B) Immunostaining result of TMEM43 expressing HEK293T cells with and without cell permeabilization. Cells were double stained with FLAG and TMEM43 antibody. (C) Cell surface biotinylation assay in TMEM43 WT and p.(Arg372Ter)-transfected HEK293T cells blotted with TMEM43 antibody. (D) Cycloheximide chase (CHX) assay with FLAG antibody. Band sizes for TMEM43 WT and TMEM43-p.(Arg372Ter) are 44 kD and 41 kD each.
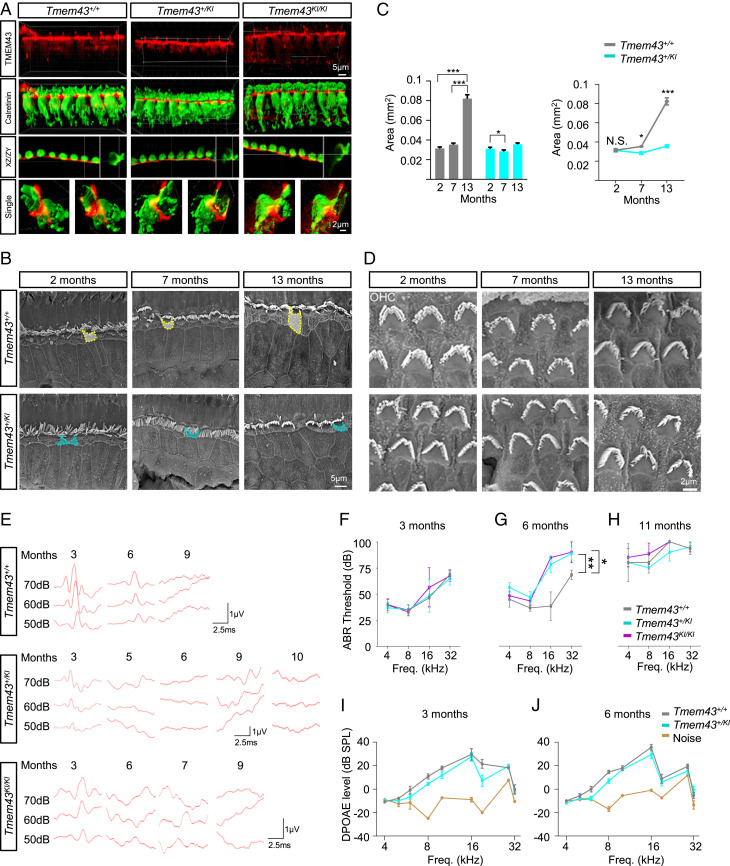
Generation of p.(Arg372Ter) Knock-In Mice Displaying Morphological Change in GLSs with Progressive Hearing Loss.
To examine the pathogenic effect of mutant TMEM43 on the in vivo function of cochlea, a KI mouse (Tmem43KI) harboring the human variant, p.(Arg372Ter) of TMEM43 in the corresponding mouse Tmem43 sequence (C57BL/6J; 129S-Tmem43tm1Cby), was successfully established (SI Appendix, Fig. S6 A and B). The mutant TMEM43 protein was present in the GLSs till the adult period, as shown by a positive immunostaining from Tmem43KI mouse models (Fig. 5A and SI Appendix, Fig. S6C). Scanning electron microscopic (sEM) examinations showed that the apical surface area of GLSs, specifically the inner border cells of the organ of Corti from Tmem43+/KI, became significantly narrower than that of Tmem43+/+ as early as 7-mo-old (Fig. 5 B and C). This reduced size of GLSs became more prominent at 13 mo of age in Tmem43+/KI (Fig. 5 B and C). In contrast, the shape and the arrangement of hair cell stereocilia as well as the number of synaptic ribbons were not different between the two genotypes (Fig. 5D and SI Appendix, Fig. S7). The sEM images of OHCs showed no gross differences between stereocilia of Tmem43+/+ and Tmem43+/KI mice at 2-, 7-, and 13-mo of age (Fig. 5D). When number of hair cells were quantified and compared in Tmem43+/+, Tmem43+/KI, and Tmem43KI/KI mice, neither IHC nor OHC number showed significant difference in 3-mo-old mice (SI Appendix, Fig. S8). A slight decrease in the number of IHC (∼15 to 45% segment) was observed only in 7-mo-old Tmem4KI/KI (SI Appendix, Fig. S8). In addition, the spiral ganglion neuron (SGN) density of Tmem43+/KI was not significantly different from Tmem43+/+ at all cochlear regions examined (SI Appendix, Fig. S9). These results together indicate that Tmem43KI mice display morphological dysfunction mostly in GLSs and possibly IHCs.
Fig. 5.
Tmem43-p.(Arg372Ter) KI mice display morphological and physiological dysfunction. (A) Confocal micrographs of the organs of Corti from 1-mo-old littermates of Tmem43+/+, Tmem43+/KI, and Tmem43KI/KI. Three-dimensional rendered images were obtained from the apical turn of cochlea immunolabeled with anti-TMEM43 (red, first row) and anti-calretinin (green, second row). XZ and ZY section views are shown (third row). Surface object clipping images of a single IHC (fourth row, Left) and cut-away object images of the same IHC (fourth row, Right) are shown. Cut-away plane is parallel to the cuticular plate. (B) sEM findings at the level of reticular lamina of hair cells. Yellow and cyan dotted lines each indicate apical surface area of inner border cells of Tmem43+/+ and Tmem43+/KI, respectively. (C) Summary graphs of apical surface area of inner border cells (n = 50) from B. (D) Representative sEM images of whole mounts from apical turns of cochlea showing intact OHC bundles in Tmem43+/KI mice that are comparable to Tmem43+/+. (E) Representative ABR waveforms in response to 16 kHz tone burst sound that are recorded longitudinally at time intervals from the same mice in each genotype. y-axis, sound pressure level (SPL). (F) Mean ± SEM. ABR thresholds from littermate Tmem43+/+ (n = 10), Tmem43+/KI (n = 10), and Tmem43KI/KI (n = 10) at 3 mo. (G) Mean ± SEM. ABR thresholds from littermate Tmem43+/+ (n = 3), Tmem43+/KI (n = 12), and Tmem43KI/KI (n = 3) measured at 6 mo. (H) Mean ± SEM. ABR thresholds from littermate Tmem43+/+ (n = 3), Tmem43+/KI (n = 3), and Tmem43KI/KI (n = 3) measured at 11 mo. (I) Mean ± SEM growth functions of DPOAE thresholds from 4 to 32 kHz for littermate Tmem43+/+ (n = 6) and Tmem43+/KI (n = 6) measured at 3 mo. (J) Mean ± SEM growth functions of DPOAE thresholds from 4 to 32 kHz for littermate Tmem43+/+ (n = 8) and Tmem43+/KI (n = 8) measured at 6 mo. The DPOAE responses from Tmem43+/KI are not significantly different from Tmem43+/+ (ANOVA, P > 0.05) at whole kHz and significantly higher than noise level (yellow) at the frequencies from 8 to 19 kHz.
To investigate the age-related temporal progression of hearing loss from the Tmem43KI mice, ABR thresholds at 16 kHz from Tmem43+/KI and Tmem43 KI/KI mice were evaluated (Fig. 5E). At 3 mo of age, neither significant elevation of ABR thresholds nor reduction of suprathreshold amplitude of ABR wave I from Tmem43+/KI or Tmem43 KI/KI mice compared with littermate Tmem43+/+ control mice were observed (Fig. 5 E and F and SI Appendix, Fig. S10A). However, we were able to observe a significant distortion of ABR waveforms from Tmem43+/KI at 5 mo of age at 16 kHz (Fig. 5E) and finally noticed a significant elevation of ABR thresholds starting at frequencies of 16 and 32 kHz from both Tmem43+/KI and Tmem43KI/KI mice compared with littermate Tmem43+/+ control mice at 6 mo of age (Fig. 5 E and G). With response to 8 kHz tone burst sounds in which there was no ABR threshold difference among genotypes at 6 mo of age (Fig. 5G), significantly reduced suprathreshold amplitude of ABR wave I from Tmem43+/KI and Tmem43 KI/KI compared with that from Tmem43+/+ was also noted (SI Appendix, Fig. S10B). At 9 mo or thereafter, significantly elevated ABR thresholds were observed throughout all frequencies irrespective of genotypes, indicative of presbycusis (Fig. 5 E and H). These results indicate that the Tmem43KI mice experience progressive hearing loss, just as the ANSD subjects of families HN66 and SB162.
To examine whether the progressive hearing loss of Tmem43KI mice originates from OHC dysfunction, we measured DPOAE responses from 4 to 32 kHz in both Tmem43+/+ and Tmem43+/KI mice at 3 and 6 mo. The DPOAE responses from the two genotypes were not significantly different but higher than the noise level at the frequencies from 8 to 19 kHz at both 3 and 6 mo (Fig. 5 I and J). Given that the ABR threshold of Tmem43+/KI was significantly higher than that of Tmem43+/+ at 16 kHz (Fig. 5G), the DPOAE results indicate a preserved OHC function in these frequency ranges and reflect the characteristics of nonhair cell–related ANSD rather than OHC-related hearing loss, in the Tmem43KI mice. In other words, the progressive hearing loss in Tmem43KI may not originate from OHC. Furthermore, the electrocardiography of the Tmem43+/KI mice did not show any sign of ARVC (SI Appendix, Fig. S11), again confirming a profoundly distinct pathogenic effect of p.(Arg372Ter) compared to p.(Ser358Leu).
TMEM43-p.(Arg372Ter) Variant Disrupts Prehearing Supporting Cell Conductance, Mediated Primarily by Gap Junctions.
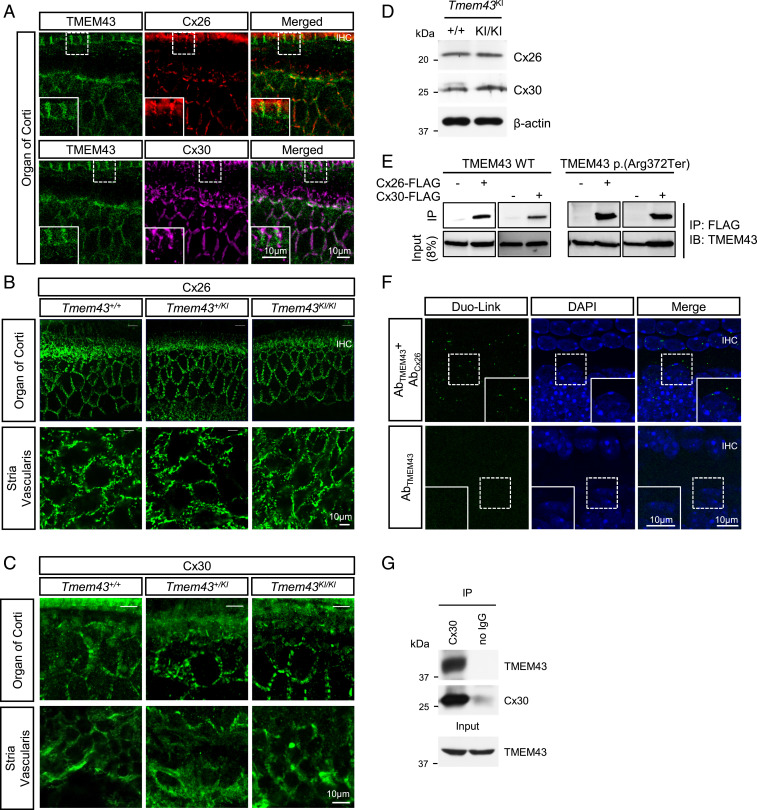
TMEM43 has been previously shown to be critical for gap junction channel function in cardiac muscles (35), raising a possibility that pathogenic effect of TMEM43-p.(Arg372Ter) involves gap junction channels in cochlea such as Cx26 or Cx30 (40). Therefore, we examined the localization of TMEM43 with connexin channels. Immunostaining data showed that TMEM43 was expressed in GLSs along with Cx26 and Cx30 (Fig. 6A). In addition, the cochlea of Tmem43+/+, Tmem43+/KI, and Tmem43KI/KI all showed a similar Cx26 and Cx30 expression level (Fig. 6 B and C and SI Appendix, Fig. S12). Cx26 and Cx30 protein expression level in Tmem43KI/KI was confirmed again with Western blot, which showed similar protein expression level when compared to Tmem43+/+ (Fig. 6D). In order to check the protein interaction of TMEM43 with these connexin channels, we next performed coimmunoprecipitation (co-IP) assay in heterologous expression system. Both TMEM43 WT and TMEM43-p.(Arg372Ter) protein were immunoprecipitated with either Cx26 or Cx30 protein when coexpressed in HEK293T cells (Fig. 6E). TMEM43 and connexin interaction was further examined in cochlea tissue by Duolink proximity ligation assay (PLA). A positive PLA signal was observed in cochlear GLSs with TMEM43 and Cx26 antibodies but not with TMEM43 antibody alone (Fig. 6F), indicating a close proximity of the two proteins (<40 nm). Consistent with the co-IP results in in vitro system, TMEM43 was pulled down with anti-Cx30 antibody in the endogenous cochlea tissue (Fig. 6G). These results together indicate that TMEM43 interacts with the connexin channels.
Fig. 6.
TMEM43 interacts with Cx26 and Cx30 gap junction channels. (A) Immunohistochemistry data showing TMEM43 expression with Cx26 and Cx30 in GLSs (P20). Merged images are shown in Bottom. TMEM43, green; Cx26, red; Cx30, magenta; DAPI, blue. (B) Confocal micrographs of Cx26 expression in the organ of Corti (Top) and Stria Vascularis (Bottom) of Tmem43+/+, Tmem43+/KI, and Tmem43KI/KI (5 mo). (C) Confocal micrographs of Cx30 expression in the organ of Corti (Top) and Stria Vascularis (Bottom) of Tmem43+/+, Tmem43+/KI, and Tmem43KI/KI (7 mo). There was no difference among the genotypes. (D) Western blot result from cochlea tissue lysates of Tmem43+/+ and Tmem43KI/KI mice (p6), blotted with anti-Cx26 and anti-Cx30. (E) Co-IP data showing that both TMEM43 WT and TMEM43-p.(Arg372Ter) are immuno-pulled down with Cx26 and Cx30 in in vitro. (F) Duolink PLA with anti-TMEM43 and anti-Cx26 (Top). Duolink PLA signal was amplified as a red fluorescent, indicative of close proximity of TMEM43 and Cx-26. Red signal was pseudo colored as green for better data display. Lower is a negative control data without Cx26 antibody. (G) Co-IP data using cochlea tissue (p6). Protein lysate was pulled down with Cx30 antibody and blotted with anti-TMEM43.
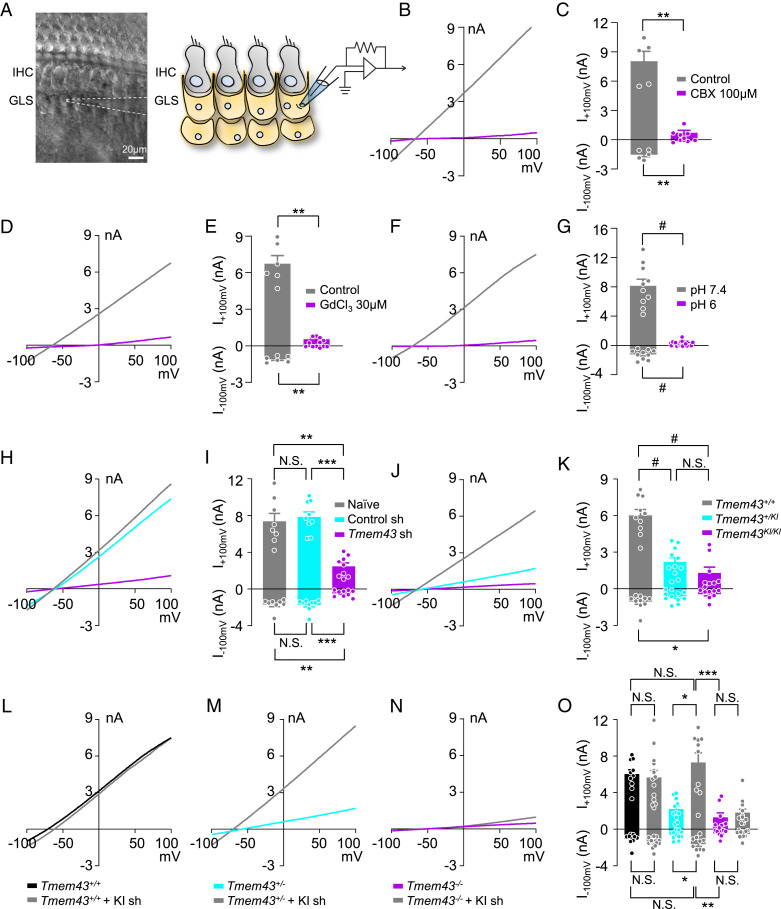
It has been reported that K+ channel expression is low in GLSs, so most of their resting membrane conductance is mediated by gap junctions, and gap junction blockers or isolation of GLSs increase their membrane resistance dramatically (10), implying that gap junction channels mediate passive conductance current in GLSs. To test a possible involvement of TMEM43 in connexin channel function, we performed whole-cell patch clamp in GLSs from acutely dissected cochlea tissue (Fig. 7A). Indeed, the large passive current with a linear current–voltage relationship was recorded from GLSs. The large passive current was abolished when gap junction channel blocker carbenoxolone (CBX) was treated (Fig. 7 B and C). As the GLS gap junction networks are known to take part in recirculation of cochlear K+ ions (8), we treated a broad-spectrum nonselective cation channel blocker GdCl3 (41). Treatment of GdCl3 (30 μM) also abolished the passive current of GLSs (Fig. 7 D and E). We next examined whether pH change can gate the passive conductance of GLS as some connexin channels are reported to be pH sensitive (8). When extracellular pH was lowered to 6, the passive conductance current was completely abolished (Fig. 7 F and G). These results indicate that GLSs display the large passive conductance current which is cationic and pH sensitive. The similar elimination of the passive conductance current was observed when cultured cochlea tissue was infected with a virus carrying Tmem43 shRNA (Fig. 7 H and I). We subsequently measured the passive conductance current from Tmem43KI mouse. The passive conductance current from GLSs of Tmem43+/+ was significantly reduced by 89% in Tmem43KI/KI mouse (Fig. 7 J and K), indicating that TMEM43 contributes majorly to the passive conductance of the GLSs. The heterozygote mouse, Tmem43+/KI, which mimics the counterpart human ANSD subjects, showed a significant reduction of 63% in the passive conductance current in GLSs (Fig. 7 J and K), confirming the significant dominant-negative pathogenic effect of the TMEM43 variant on the function of GLSs. To rescue the impaired TMEM43-p.(Arg372Ter)–mediated current, we generated an shRNA specifically targeting the KI sequence of Tmem43KI mouse. This Tmem43 KI shRNA targets only KI allele but not WT allele (SI Appendix, Fig. S5C and Fig. 7 L and O). When Tmem43+/KI heterozygote cochlea culture was infected with the virus carrying Tmem43 KI shRNA, the reduced passive conductance current was rescued to the level comparable to Tmem43+/+, due to an unmasking of the remaining WT allele (Fig. 7 M and O). However, this Tmem43 KI shRNA did not rescue the passive current from Tmem43KI/KI cochlea, because these homozygote mice carried no WT allele to be unmasked (Fig. 7 N and O). These results clearly explain the autosomal dominant effect of TMEM43-p.(Arg372Ter) and emphasize the role of TMEM43 in connexin-linked function in mediating the passive conductance current in GLSs.
Fig. 7.
TMEM43-p.(Arg372Ter) variant disrupts prehearing supporting cell conductance, mediated primarily by gap junctions. (A) Differential interference contrast (DIC) image of freshly isolated mouse cochlea with glass pipette (white dotted line) whole-cell patch-clamped to a GLS (Left). Illustration depicting cochlea tissue with patch-clamped GLS (Right). (B, D, and F) Representative current–voltage (I-V) curve measured from GLSs of control (gray) and CBX-treated (B), GdCl3-treated (D) and pH6-treated (F) (purple) cochlea. (C, E, and G) Summary bar graph of B (C), D (E), and F (G). (H) Representative I-V curve measured from naïve (gray), control shRNA-treated (cyan), and Tmem43 shRNA-treated (purple) cochlea. (I) Summary bar graph of (H). (J) Representative current–voltage relationship of Tmem43+/+ (gray), Tmem43+/KI (cyan) and Tmem43KI/KI (purple). (K) Summary bar graph of GLS currents from (J). (L–N) GLS current measured from Tmem43 KI shRNA uninfected and infected cochlea of Tmem43+/+ (L), Tmem43+/KI (M), and Tmem43KI/KI (N) mice. Note that the Tmem43 KI shRNA rescues impaired passive current of Tmem43+/KI. (O) Summary bar graph of L–N.
Cochlear Implant Restores the Impaired Speech Discrimination in the ANSD Subjects.
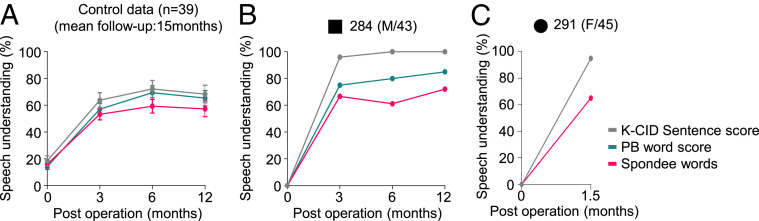
Clinically, determining the exact lesion site of hearing loss is critical for choosing a proper treatment. For example, subjects with conductive hearing loss or moderate sensorineural hearing loss due to limited damages to OHCs would benefit from hearing aids, whereas subjects with severe to profound sensorineural hearing loss, as a result of significant damages to OHCs or IHCs, would require a CI. A subset of sensorineural hearing loss mainly affecting more central structures such as SGN or cochlear nerve or central hearing loss originating from the brainstem or brain may benefit from brainstem implant but not from either hearing aids nor CI. Because the main damage in Tmem43+/KI mouse was restricted to GLSs, but not to the hair cells or SGNs at minimum 6∼7 mo of age, we could predict that subjects from SB162 could benefit from CI. Consequently, CI was performed on #284 and #291, even though their PTA results did not meet a conventional criterion of CI (PTA equal to or exceeding 70 dB) (42). Postoperatively, the open-set speech understanding of #284 and #291 with a deaf duration of about 15 y restored much more rapidly than that of control adult cochlear implantees (n = 39), as measured by speech evaluation test tools (Fig. 8 A–C and SI Appendix, Fig. S13A) (43, 44). However, the restoration of speech discrimination ability in #304 with a longer deaf duration (about 25 y) was not as rapid (SI Appendix, Fig. S13 A and B). Notably, the responsiveness of cochlear nerve to stimuli of #304, measured by intracochlear electrically evoked ABR, was restored immediately after cochlear implantation (SI Appendix, Fig. S13C). These results suggest that limited improvement of speech discrimination in #304 is presumably due to cortical damage related with longer deaf duration but not due to CI-related issues. Based on our results, early cochlear implantation may be recommended for restoration of speech discrimination in the case of TMEM43-p.(Arg372Ter) among late-onset, progressive ANSD subjects.
Fig. 8.
Timely cochlea implantations in family SB162 rescue impaired speech discrimination. (A) Longitudinal change of postoperative auditory performance from control subjects (n = 39) with adult-onset progressive hearing loss. (B) Speech discrimination performance scores of subject #284 measured at 3, 6, and 12 mo following cochlear implantation. (C) Speech discrimination performance scores of subject #291 measured at 1.5 mo following cochlear implantation. K-CID Sentence score, Korean Central Institute for the Deaf score; PB word score, Phonetically Balanced word score; Spondee words, a word with two syllables—disyllabic, that is, pronounced with equal emphasis on first and second syllables.
Discussion
In this study, we have identified a deafness locus, Chr 3: 13,165,401 to 14,502,000 (3p25.1), and a deafness gene, TMEM43, whose variation can cause ANSD post lingually. The unique ANSD phenotype shared by two unrelated families significantly strengthens the causal link between this variant and ANSD. Unbiased exome sequencing screening was performed for both HN66 and SB162 in a parallel fashion with linkage analysis to minimize errors arising from spurious linkages. Significant overlap of linkage interval between HN66 and SB162 in Chr 3: 13,165,401 to 14,502,000 also made it unlikely for our linkage data to be reflection of spurious linkage. Further, we have identified and characterized the role of TMEM43 in connexin-linked function contributing to the passive conductance current in GLSs which is necessary for hearing and speech discrimination (SI Appendix, Fig. S14). Until now, only the pathological functions of TMEM43 have been implicated in arrhythmia (34, 35) and tumor progression (45). The variant p.(Ser358Leu) of TMEM43 has been reported to cause ARVC by decreasing the expression and localization of tight junctions, redistributing the gap junction proteins from the surface to the cytoplasm and decreasing the conduction velocity (35). On the other hand, Tmem43+/KI mice did not show any sign of ARVC (SI Appendix, Fig. S11), implying that the pathogenic mechanism of ARVC and ANSD would be different. However, we cannot rule out the possibility that underlying mechanism of ARVC and ANSD is independent. This is because even Tmem43-p.(Ser358-Leu) variant alone did not show any sign of ARVC in Tmem43+/Ser358Leu mice, and Tmem43Δ/Δ mice also did not show any sign of ARVC (46). Thus, absence of ARVC from the Tmem43+/KI mice does not necessarily support the hypothesis that the underlying mechanism of ARVC and ANSD would be unrelated.
Previous studies have reported the presence of high conductance, octanol-, and flufenamic acid–sensitive GLS currents (47, 48). In this study, we have established a more detailed characterization of the CBX-, GdCl3-, and low pH–sensitive and TMEM43-dependent passive conductance current in the GLSs of intact cochlea. In the cochlea, the endocochlear potential of 80 mV is generated by maintaining high K+ concentration in the endolymph, and this potential is critical for activation of hair cells (49). The presence of passive conductance current implicates an important role of GLSs in homeostasis of K+ and volume regulation. We have observed a significantly smaller inner border cell size of Tmem43KI mice compared to control, with loss of passive conductance current. We hypothesize that the smaller inner border cells have smaller capacity for K+ uptake. The loss of the passive conductance current indicates impaired recycling of K+. Such breakdown of K+ homeostasis would make hair cells difficult to depolarize, resulting in a disruption of speech discrimination ability. Consistent with this hypothesis, we observed the increasing apical surface area in inner border cell at the age between 7 mo and 13 mo. As one of the major functions of cochlear supporting cells, including inner border cells, is to regulate cochlear K+ homeostasis via leak K+ channels and gap junction channels, we hypothesized that the increase in inner border cell is to maintain its homeostasis during aging process. The detailed mechanisms await future investigation.
Our study demonstrates that hearing impairment of Tmem43+/KI and Tmem43KI/KI begins at some time between 3 and 6 mo (equivalent to twenties in humans). This raises a fundamental question of why canonical ANSD does not occur in younger age. The passive conductance current recordings from the cochlea of P5 to 7 pups already revealed a 63% reduction in passive conductance current in heterozygous Tmem43KI mice. We also showed that lowering the pH to 6 reduced the passive current of GLS. Thus, the late-onset hearing loss could be explained by reduction of cochlear pH in aging mouse and human, similar to what happens in aging brain (50). Therefore, the normal hearing in heterozygote Tmem43KI mice and the ANSD subjects at younger age could be due to the remaining passive conductance current that might be enough to maintain homeostatic functions of GLSs. In contrast, the hearing loss in the aging Tmem43KI mice and ANSD subjects can be explained with further reduction of the remaining current during aging, in which gap junction channels are disturbed. Unfortunately, due to the technical limitation of recording currents from adult cochlea tissue, we could only record the passive conductance current from the cochlea of P5 to 7 pups. The possibility of the remaining passive conductance current in aged WT and Tmem43KI mice needs to be tested in the future.
Our study provides a potential prognostic genetic marker TMEM43-p.(Arg372Ter) for ANSD patients. More importantly, our study suggests that if a causative gene of ANSD is expressed mainly at GLSs, it could also be used as a favorable prognostic marker for CI. Although CI successfully rehabilitates severe to profound sensorineural hearing loss in many cases, adult ANSD patients usually hesitate to receive the surgery due to uncertainty of surgery outcome regarding speech discrimination, side effects including loss of residual hearing and high cost. Given this, an ANSD-causative variant of GLS-specific gene such as p.(Arg372Ter) of TMEM43 could serve as a useful indicator of positive CI surgery outcome if the surgery is timely done. #284 and #291 who received CI at the age 43 and 50, respectively, recovered the sentence understanding ability to 95 to 100% much more rapidly than did control adult cochlear implantees (n = 39). Thus, we may have to recommend the ANSD patients carrying TMEM43-p.(Arg372Ter) to receive a CI surgery as soon as they start to experience significantly diminished speech discrimination even though they retain significant residual sound detection ability. Our study provides an important step toward identifying more TMEM43-related ANSD patients, calling for further study with larger number of subjects carrying this allele.
In conclusion, we have characterized the role of TMEM43 in connexin-linked function and have delineated that an alteration of this protein leads to functional and morphological abnormalities in GLSs, resulting in the failure to maintain speech discrimination in aged human. Through these mechanistic insights, we have elucidated a link between abnormality in cochlear GLSs and impaired human speech discrimination. We further provide a model platform in which the personalized timing and mode of auditory rehabilitation can be determined, highlighting the importance of a precision medicine–based approach.
Materials and Methods
Family Data.
Clinical examination was performed on 20 subjects from Korean family SB162 and 16 subjects from Chinese family HN66. Informed consent was obtained from all participants. This study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB-B-1007-105-402) and the ethics committee of Xiangya Hospital of Central South University (ID: 201603518).
Animals and Housing.
Tmem43-p.(Arg372Ter) KI (C57BL/6J; 129S-Tmem43tm1Cby) mice, 129Sv/Ev mice, C57BL/6J mice, and crab-eating macaque were used. All animals were kept on a 12 h light–dark cycle in a specific-pathogen–free facility with controlled temperature and humidity and had free access to food and water. All experimental procedures were conducted according to protocols approved by the directives of the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital and the Institutional Animal Care and Use Committee of the Institute for Basic Science.
Immunocytofluorescence on Cochlear Tissue.
Mouse inner ears (C57BL/6) at various time points postnatally and the cochlea from three monkeys were fixed in ice cold 4% paraformaldehyde for 1 h. Cochlear turns were excised and incubated in blocking buffer and incubated overnight at 4 °C with primary antibodies diluted in the blocking buffer. After three washes, the cochlear turns were reacted with secondary antibodies diluted in blocking buffer for 1 h at room temperature. Samples were then rinsed once with blocking buffer and twice with phosphate-buffered saline. Using Fluorsave reagent, the tissues were mounted on glass slides and covered with coverslip. High-resolution images were obtained using a confocal laser scanning microscope.
Electrophysiological Recording in Cochlear Supporting Cells.
Cochlea of P5-P7 Tmem43-p.(Arg372Ter) KI mouse were isolated in Hepes buffer containing: 144 NaCl, 5.8 KCl, 1.3 CaCl2, 2 MgCl2, 10 Hepes, 0.7 NaH2PO4 and 5.6 D-glucose in millimolar (pH 7.4). All recordings were done with same Hepes buffer. Stria vascularis and tectorial membrane were carefully peeled off, and the remaining cellular organization of the organ of Corti was left intact. Acutely dissected cochlea turn was used within 2 h of dissection. Recording electrodes (7 to 11MΩ) supplemented with 126 K-Gluconate, 5 Hepes, 0.5 MgCl2, and 10 BAPTA in millimolar (pH 7.3) were advanced through tissue under positive pressure.
Data Analysis and Statistical Analysis.
Off-line analysis was carried out using Clampfit version 10.4.1.10 and GraphPad Prism version 7.02 software. Significance levels were given as: N.S., P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and #P < 0.0001.
For full, detailed materials and methods, refer to SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Young-Woo Seo at Korea Basic Science Institute Gwang-Ju Center for his assistance with image analysis with Imaris software. This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (Grant HI15C1632 and HI17C0952 to B.Y.C.); the Creative Research Initiative Program, National Research Foundation of Korea (NRF) (Grant 2015R1A3A2066619 to C.J.L.); Grant IBS-R001-D2 from the Institute for Basic Science from the Ministry of Science (to C.J.L.); the Basic Science Research Program through the NRF, funded by the Ministry of Education (Grant 2019R1I1A3A01063625 to E.Y.); the National Natural Science Foundation of China (Grants 82071065 and 81771023 to Y.F.); and the Major State Basic Research Development Program of China (973 Program) (Grant 2014CB541702 to Y.F.). Xuezhong Liu is supported by NIH Grants R01DC005575, R01DC012115, and A.N. is supported by NIH Grant T32 DC015995.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. U.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2019681118/-/DCSupplemental.
Data Availability
All study data related to this work are available in the main text and supplementary materials.
References
- 1.Elzouki A. Y., Harfi H. A., Nazer H. M., Textbook of Clinical Pediatrics (Lippincott Williams & Wilkins, Philadelphia, 2001), pp. 1800. [Google Scholar]
- 2.Zeng F. G., Kong Y. Y., Michalewski H. J., Starr A., Perceptual consequences of disrupted auditory nerve activity. J. Neurophysiol. 93, 3050–3063 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Norrix L. W., Velenovsky D. S., Auditory neuropathy spectrum disorder: A review. J. Speech Lang. Hear. Res. 57, 1564–1576 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Starr A., et al., Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser). Brain 126, 1604–1619 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Harrison R. V., An animal model of auditory neuropathy. Ear Hear. 19, 355–361 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Starr A., Picton T. W., Sininger Y., Hood L. J., Berlin C. I., Auditory neuropathy. Brain 119, 741–753 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Wan G., Corfas G., Stone J. S., Inner ear supporting cells: Rethinking the silent majority. Semin. Cell Dev. Biol. 24, 448–459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao H. B., Kikuchi T., Ngezahayo A., White T. W., Gap junctions and cochlear homeostasis. J. Membr. Biol. 209, 177–186 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellado Lagarde M. M., et al., Spontaneous regeneration of cochlear supporting cells after neonatal ablation ensures hearing in the adult mouse. Proc. Natl. Acad. Sci. U.S.A. 111, 16919–16924 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H. C., et al., Spontaneous activity of cochlear hair cells triggered by fluid secretion mechanism in adjacent support cells. Cell 163, 1348–1359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eunyoung Yi J. L. C. J. L., Developmental role of Anoctamin-1/TMEM16A in Ca2+ dpendent volume change in supporting cells of the mouse cochlea. Exp. Neurobiol. 22, 322–329 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley M. W., Cellular commitment and differentiation in the organ of Corti. Int. J. Dev. Biol. 51, 571–583 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Tritsch N. X., Yi E., Gale J. E., Glowatzki E., Bergles D. W., The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Ceriani F., et al., Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. EMBO J. 38, e99839 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGovern M. M., Randle M. R., Cuppini C. L., Graves K. A., Cox B. C., Multiple supporting cell subtypes are capable of spontaneous hair cell regeneration in the neonatal mouse cochlea. Development 146, dev171009 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., et al., Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J. Neurosci. Methods 164, 271–279 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Forge A., et al., Gap junctions and connexin expression in the inner ear. Novartis Found. Symp. 219, 134–150, discussion 151–156(1999). [DOI] [PubMed] [Google Scholar]
- 18.Kammen-Jolly K., et al., Connexin 26 in human fetal development of the inner ear. Hear. Res. 160, 15–21 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Li-dong Z., Jun L., Yin-yan H., Jian-he S., Shi-ming Y., Supporting cells-a new area in cochlear physiology study. J. Otol. 3, 9–17 (2008). [Google Scholar]
- 20.Kikuchi T., Kimura R. S., Paul D. L., Takasaka T., Adams J. C., Gap junction systems in the mammalian cochlea. Brain Res. Brain Res. Rev. 32, 163–166 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Mei L., et al., A deafness mechanism of digenic Cx26 (GJB2) and Cx30 (GJB6) mutations: Reduction of endocochlear potential by impairment of heterogeneous gap junctional function in the cochlear lateral wall. Neurobiol. Dis. 108, 195–203 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen-Salmon M., et al., Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr. Biol. 12, 1106–1111 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbe C. B., Harris K. C., Runge-Samuelson C. L., Flanary V. A., Wackym P. A., Connexin 26 and connexin 30 mutations in children with nonsyndromic hearing loss. Laryngoscope 114, 607–611 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Teubner B., et al., Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum. Mol. Genet. 12, 13–21 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Manchaiah V. K., Zhao F., Danesh A. A., Duprey R., The genetic basis of auditory neuropathy spectrum disorder (ANSD). Int. J. Pediatr. Otorhinolaryngol. 75, 151–158 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Giepmans B. N., Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 62, 233–245 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Batissoco A. C., et al., A cell junctional protein network associated with connexin-26. Int. J. Mol. Sci. 19, 2535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasunaga S., et al., A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat. Genet. 21, 363–369 (1999). [DOI] [PubMed] [Google Scholar]
- 29.Schoen C. J., et al., Increased activity of diaphanous homolog 3 (DIAPH3)/diaphanous causes hearing defects in humans with auditory neuropathy and in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 107, 13396–13401 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruel J., et al., Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am. J. Hum. Genet. 83, 278–292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong L., et al., Mutations in apoptosis-inducing factor cause X-linked recessive auditory neuropathy spectrum disorder. J. Med. Genet. 52, 523–531 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarelli R., et al., OPA1-related auditory neuropathy: Site of lesion and outcome of cochlear implantation. Brain 138, 563–576 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han K. H., et al., ATP1A3 mutations can cause progressive auditory neuropathy: A new gene of auditory synaptopathy. Sci. Rep. 7, 16504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merner N. D., et al., Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 82, 809–821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siragam V., et al., TMEM43 mutation p.S358L alters intercalated disc protein expression and reduces conduction velocity in arrhythmogenic right ventricular cardiomyopathy. PLoS One 9, e109128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oza A. M.et al.; ClinGen Hearing Loss Clinical Domain Working Group , Expert specification of the ACMG/AMP variant interpretation guidelines for genetic hearing loss. Hum. Mutat. 39, 1593–1613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards S., et al., Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rentzsch P., Witten D., Cooper G. M., Shendure J., Kircher M., CADD: Predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bengtsson L., Otto H., LUMA interacts with emerin and influences its distribution at the inner nuclear membrane. J. Cell Sci. 121, 536–548 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Johnson S. L., et al., Connexin-mediated signaling in nonsensory cells is crucial for the development of sensory inner hair cells in the mouse cochlea. J. Neurosci. 37, 258–268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sackin H., Mechanosensitive channels. Annu. Rev. Physiol. 57, 333–353 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Entwisle L. K., Warren S. E., Messersmith J. J., Cochlear implantation for children and adults with severe-to-profound hearing loss. Semin. Hear. 39, 390–404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee D. S., et al., Cross-modal plasticity and cochlear implants. Nature 409, 149–150 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Lee J. S., et al., PET evidence of neuroplasticity in adult auditory cortex of postlingual deafness. J. Nucl. Med. 44, 1435–1439 (2003). [PubMed] [Google Scholar]
- 45.Jiang C., et al., TMEM43/LUMA is a key signaling component mediating EGFR-induced NF-κB activation and tumor progression. Oncogene 36, 2813–2823 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Stroud M. J., et al., Luma is not essential for murine cardiac development and function. Cardiovasc. Res. 114, 378–388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirko P., Gale J. E., Ashmore J. F., Intercellular Ca2+ signalling in the adult mouse cochlea. J. Physiol. 597, 303–317 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jagger D. J., Forge A., Compartmentalized and signal-selective gap junctional coupling in the hearing cochlea. J. Neurosci. 26, 1260–1268 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nin F., et al., The endocochlear potential depends on two K+ diffusion potentials and an electrical barrier in the stria vascularis of the inner ear. Proc. Natl. Acad. Sci. U.S.A. 105, 1751–1756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forester B. P., et al., Age-related changes in brain energetics and phospholipid metabolism. NMR Biomed. 23, 242–250 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data related to this work are available in the main text and supplementary materials.