Significance
The fertility of female mammals depends upon the well-orchestrated progression of oocyte meiosis, the female germ cell division that is essential for haploid oocyte formation. In preovulatory follicles, how LH induces a decrease in CNP and its encoding mRNA Nppc, a prerequisite for oocyte meiotic resumption, remains an outstanding question to understand the mechanism that controls oocyte meiosis. The present study shows that TTP, an mRNA-destabilizing protein, is one of the regulatory components responsible for the LH-induced rapid decrease in Nppc mRNA and oocyte meiotic resumption. This finding not only highlights the importance of posttranscriptional regulation in the follicle periphery to fine-tune oocyte meiosis but also provides an insight into CNP-dependent cGMP homeostasis in other physiological systems.
Keywords: TTP/Zfp36, CNP/Nppc, mRNA degradation, oocyte meiosis, mural granulosa cells
Abstract
C-natriuretic peptide (CNP) and its receptor guanylyl cyclase, natriuretic peptide receptor 2 (NPR2), are key regulators of cyclic guanosine monophosphate (cGMP) homeostasis. The CNP-NPR2-cGMP signaling cascade plays an important role in the progression of oocyte meiosis, which is essential for fertility in female mammals. In preovulatory ovarian follicles, the luteinizing hormone (LH)-induced decrease in CNP and its encoding messenger RNA (mRNA) natriuretic peptide precursor C (Nppc) are a prerequisite for oocyte meiotic resumption. However, it has never been determined how LH decreases CNP/Nppc. In the present study, we identified that tristetraprolin (TTP), also known as zinc finger protein 36 (ZFP36), a ubiquitously expressed mRNA-destabilizing protein, is the critical mechanism that underlies the LH-induced decrease in Nppc mRNA. Zfp36 mRNA was transiently up-regulated in mural granulosa cells (MGCs) in response to the LH surge. Loss- and gain-of-function analyses indicated that TTP is required for Nppc mRNA degradation in preovulatory MGCs by targeting the rare noncanonical AU-rich element harbored in the Nppc 3′ UTR. Moreover, MGC-specific knockout of Zfp36, as well as lentivirus-mediated knockdown in vivo, impaired the LH/hCG-induced Nppc mRNA decline and oocyte meiotic resumption. Furthermore, we found that LH/hCG activates Zfp36/TTP expression through the EGFR-ERK1/2–dependent pathway. Our findings reveal a functional role of TTP-induced mRNA degradation, a global posttranscriptional regulation mechanism, in orchestrating the progression of oocyte meiosis. We also provided a mechanism for understanding CNP-dependent cGMP homeostasis in diverse cellular processes.
Cyclic guanosine monophosphate (cGMP) is a ubiquitous second messenger that regulates myriads functions, such as the cell cycle, differentiation, apoptosis, and metabolism (1). During recent decades, C-natriuretic peptide (CNP), encoded by natriuretic peptide precursor C (Nppc), has emerged as a critical regulator of cGMP homeostasis by activating its transmembrane guanylyl cyclase receptor, natriuretic peptide receptor 2 (NPR2), which increases the intracellular cGMP concentration (2, 3). Increasing evidence from mouse models of genetic depletion or mutation of Nppc or Npr2 and related clinical assays have indicated that the CNP-NPR2-cGMP signaling cascade is critical for the development and function of the cardiovascular (4, 5), skeletal (6, 7), nervous (8, 9), and female reproductive systems (10). In particular, CNP-NPR2-cGMP signaling is the central regulator of the progression of oocyte meiosis (11), the female germ cell division that is essential for haploid oocyte formation.
In mammals, oocytes are arrested at the diplotene stage of meiosis until the surge of luteinizing hormone (LH) restarts meiosis and initiates the ovulatory process. In preovulatory follicles, LH-induced CNP-NPR2-cGMP signaling inhibition is the most prominent hallmark for meiotic resumption. Although LH also activates cGMP hydrolysis (12) and decreases guanylyl cyclase activity (13, 14) and NPR2 affinity for CNP in ovarian follicles (15), mouse models that prevent cGMP hydrolysis or/and NPR2 inactivation can reinitiate meiosis (12, 16), suggesting that these alterations are not indispensable for LH-induced meiotic resumption. These facts suggest a notable change upstream of the CNP-NPR2-cGMP signaling cascade; indeed, remarkable decreases in CNP and Nppc messenger RNA (mRNA) have been reported in mouse (14, 17–19), rat (13), pig (20), and human (17) follicles, and the time course of the Nppc/CNP decrease almost coincides with the resumption of meiosis (14). Previous studies have established the critical role of low-level CNP in decreasing cGMP and restarting meiotic progression. The incubation of isolated cumulus-oocyte complexes (COCs) with low-level CNP that recapitulates the follicular concentration after LH administration resumes meiosis; however, high levels of CNP maintain meiotic arrest (11, 20, 21). Thus, the LH-induced decrease in CNP levels is thought to be important for fine-tuning meiotic resumption (14, 22, 23). However, the mechanism underlying decreased CNP is unknown and thus represents an unanswered question in our understanding of the mechanism that controls oocyte meiosis (22). Given that the turnover of CNP is very rapid (24), a decrease in Nppc mRNA could rapidly decrease the amount of CNP (14). In addition, the LH-induced decrease in CNP is independent of the natriuretic peptide clearance receptor NPR3 (18). Therefore, we focused on the possible mechanism underlying the LH-induced decrease in Nppc mRNA.
By reanalyzing a previously published transcriptome that presented rapid effects of LH on gene expression in the mural granulosa cells (MGCs) of mouse preovulatory follicles (25), we noticed that zinc finger protein 36 homolog (Zfp36) that encodes tristetraprolin (TTP) showed an expression pattern that was inversely related to the LH-induced decrease in Nppc mRNA. TTP is an RNA-binding protein that regulates mRNA stability by binding to AU-rich elements (AREs) in the 3′ untranslated regions (UTRs) of target mRNAs and destabilizes mRNA via poly(A) tail removal or deadenylation (26, 27). Furthermore, we noticed that Nppc mRNA is a representative ARE-containing transcript. Based on these findings, we hypothesized that TTP might target and degrade Nppc mRNA and thus could be the key factor underlying the LH-induced decrease in Nppc mRNA.
In the present study, we identified that TTP-induced Nppc mRNA degradation is a regulatory component of meiotic progression in preovulatory follicles. Notably, we reported that a rare noncanonical ARE motif mediated the TTP-induced destabilization of Nppc mRNA. Additionally, we found that LH transiently activated Zfp36 via the epidermal growth factor receptor (EGFR)-extracellular regulated kinase (ERK)1/2-dependent pathway. Thus, focusing on the upstream part of the signaling cascade, the present study determined a mechanism by which CNP-NPR2-cGMP signaling is inactivated during meiotic resumption and ovulation processes and provides insight into CNP-dependent cGMP homeostasis in other physiological systems.
Results
Zfp36/TTP Is Transiently Up-Regulated in Response to the LH/hCG Surge and Targets the 3′ UTR of the Nppc mRNA.
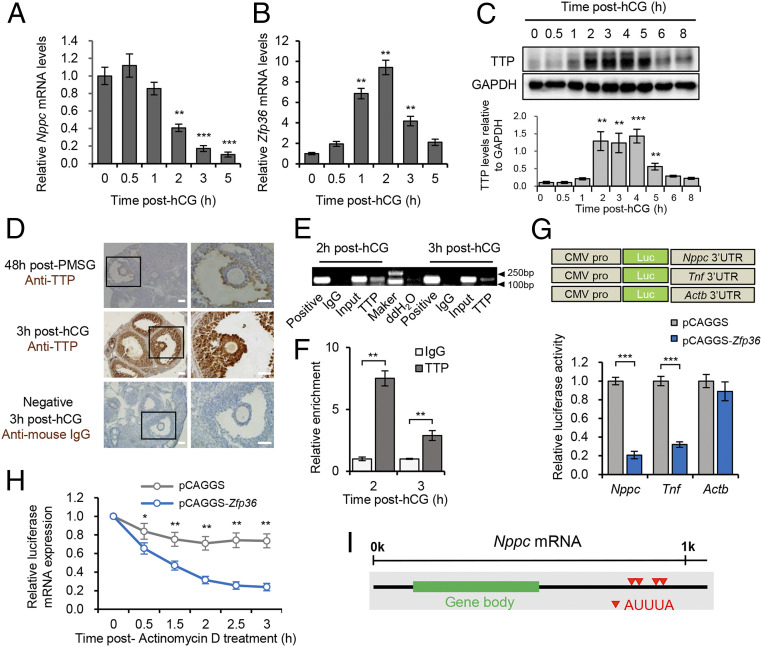
To explore the mechanism underlying the LH-induced decrease in Nppc mRNA, we tested the previous notion that the LH-induced decrease in Nppc mRNA is a universal switch for CNP-NPR2-cGMP signaling inhibition. Female Institute of Cancer Research (ICR) mice were administered with human chorionic gonadotropin (hCG) to mimic the endogenous LH surge (14, 17–19), and then we detected the Nppc mRNA levels in MGCs and ovaries at various time points. We found that the Nppc mRNA levels decreased immediately in MGCs and ovaries to less than 50% by 2 h post-hCG and further declined to 10% by 5 h post-hCG (Fig. 1A and SI Appendix, Fig. S1A). Our findings, together with previous results obtained from mice with a different genetic background (17–19) and from pigs (20), indicated that the rapid decrease in the Nppc mRNA levels is a hallmark event common to different genotypes and species.
Fig. 1.
TTP is up-regulated in response to the LH/hCG surge and targets the Nppc 3′ UTR. (A and B) RT-qPCR detection of the expression dynamics of Nppc (A) and Zfp36 (B) mRNA in preovulatory MGCs in response to hCG administration. (C) Western blotting analysis for TTP in preovulatory MGCs at different time points after hCG administration. The upper and lower bands indicate phosphorylated (inactive) and unphosphorylated (active) forms of TTP, respectively. (D) Immunohistochemical staining of TTP in preovulatory follicles before and after hCG administration. (Right) Higher magnification of boxed regions. (Scale bar, 100 μm.) (E) RIP analysis for the direct binding of TTP to the Nppc 3′ UTR. The RIP sample was subjected to RT-PCR, and the amplified products were visualized using agarose gel electrophoresis. (F) Quantitative detection of the interaction between TTP and Nppc 3′ UTR by RIP–RT-qPCR. (G) Schematic diagram of the luciferase constructs containing full-length Nppc 3′ UTR, Tnf 3′ UTR (targeted control), or Actb 3′ UTR (nontargeted control). Relative luciferase activity of different luciferase vectors, which were cotransfected with a Zfp36 overexpression vector or empty vector in HEK293 cells. (H) Measurement of the stability of the luciferase mRNA that contained the Nppc 3′ UTR. The luciferase vector was cotransfected with the Zfp36 expression vector or empty vectors in HEK293 cells, which were treated with ActD to terminate transcription. Total RNAs were prepared at the indicated time points to detect the remaining luciferase mRNA levels after ActD treatment. (I) Schematic diagram depicting the location of canonical AU-rich elements (AREs, presented as red triangles) in the Nppc 3′ UTR. All data are presented as the mean ± SEM of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. In A, B, and C, * indicates a significant difference compared with data at 0 h post-hCG.
Next, to identify the potential factor controlling Nppc expression levels, we reanalyzed previously published transcriptome data that identified early response genes in MGCs at 1 h post-hCG. We noticed that the expression of the Zfp36 gene, whose product, TTP, is responsible for degrading mRNAs that have AU-rich 3′ UTRs, was up-regulated by 3.3-fold within 1 h after LH/hCG administration (25). Given that the up-regulation of Zfp36 coincided with the decrease in Nppc levels and that the Nppc 3′ UTR is considerably AU-rich, we hypothesized that Zfp36/TTP might be implicated in the decrease in Nppc mRNA. To this end, we isolated MGCs from preovulatory follicles at various times after injection with hCG and detected the expression dynamics of Zfp36 using real-time reverse transcription quantitative PCR (RT-qPCR). In agreement with the results of previous transcriptome data (25), we found a transient and significant increase in Zfp36 mRNA, by approximately sevenfold and tenfold at 1 and 2 h post-hCG, respectively, in MGCs and ovaries, followed by a rapid decline to the basal level (Fig. 1B and SI Appendix, Fig. S1B). In addition, the transient activation of Zfp36 mRNA in MGCs was accompanied by a transiently increased abundance of TTP at 2 to 4 h in response to hCG injection (Fig. 1C). Collectively, these results suggested that Zfp36/TTP is activated in response to hCG during a specific time window in preovulatory follicles. Moreover, by analyzing the previously published transcriptome data of ovarian MGCs pre- and post-LH/hCG administration from different species (28–31), we found that activation of Zfp36/TTP in preovulatory MGCs in response to the LH/hCG surge appears to be common in these species (SI Appendix, Fig. S2).
Next, immunohistochemical detection indicated that the LH/hCG-induced TTP is mainly localized in MGCs and cumulus cells (Fig. 1D), which overlaps with the distribution of Nppc mRNA in the preovulatory follicle (11). The spatiotemporally associated patterns, together with the negatively correlated expression dynamics between Nppc and Zfp36, suggested that Zfp36-encoded TTP might be an inducible repressor of Nppc mRNA in response to the LH/hCG surge. Therefore, we determined whether TTP could target the 3′ UTR of Nppc mRNA. Given that the high level of TTP protein appeared by 2 and 3 h post-hCG and Nppc mRNA was significantly decreased at 2 h post-hCG, we isolated preovulatory MGCs at 2 and 3 h post-hCG and used RNA immunoprecipitation (RIP) to test the binding of TTP to the Nppc mRNA. In a TTP-specific pulldown, Nppc mRNA, but not nontargeted beta actin (Actb) mRNA, was significantly enriched compared with the Immunoglobulin G (IgG) controls (Fig. 1 E and F and SI Appendix, Fig. S3A). The specificity of the interaction was confirmed by sequencing the RIP-qPCR amplicon.
Having confirmed the binding of TTP to the Nppc 3′ UTR, we next examined whether the Nppc 3′ UTR can mediate the TTP-induced mRNA destabilization. We generated luciferase constructs containing the full-length Nppc 3′ UTR, the tumor necrosis factor (Tnf) 3′ UTR, or the Actb 3′ UTR. The Tnf 3′ UTR was used as the targeted control because Tnf mRNA is a well-accepted target of TTP (32), whereas the Actb 3′ UTR was used as the nontargeted control (Fig. 1G). Humanembryonic kidney (HEK293) cells were cotransfected with the psiCHECK2 luciferase vector containing Nppc 3′ UTR or the control 3′ UTRs, together with a Zfp36 overexpression vector or empty vector. Compared with cells transfected with the empty vector, overexpression of Zfp36 significantly reduced the luciferase activity of the reporter containing the full-length Nppc 3′ UTR to the level comparable to that of the reporter containing full-length Tnf 3′ UTR but did not alter the luciferase activity of the reporter containing full-length Actb 3′ UTR (Fig. 1G). To further determine the role of Nppc 3′ UTR in mediating TTP-induced destabilization of target mRNA, we measured the luciferase mRNA stability after blocking transcription using actinomycin D (ActD), which is frequently used to detect mRNA stability by inhibiting de novo mRNA synthesis, thus facilitating the measurement of the remaining mRNA (33–35). The results showed that TTP overexpression significantly accelerated the destabilization of luciferase mRNA containing the full-length Nppc 3′ UTR to approximately a quarter of that of the control vector after 3 h (Fig. 1H), which is comparable to the rate of decline of Nppc mRNA in MGCs following the LH/hCG surge. The reliability of the mRNA stability assay was supported by the fact that TTP overexpression significantly degraded luciferase mRNA containing the Tnf 3′ UTR but not that containing the Actb 3′ UTR (SI Appendix, Fig. S3 B and C). These results indicated that TTP can destabilize the Nppc mRNA via its 3′ UTR. Similar results were also obtained using the bovine NPPC 3′ UTR (SI Appendix, Fig. S3 D and E). Collectively, these experiments demonstrated that Zfp36/TTP can be activated in preovulatory follicles in response to the LH/hCG surge and then targets the Nppc 3′ UTR, which might contribute to hCG-induced destabilization of Nppc mRNA.
TTP Targets the Noncanonical ARE Motif in the Nppc 3′ UTR.
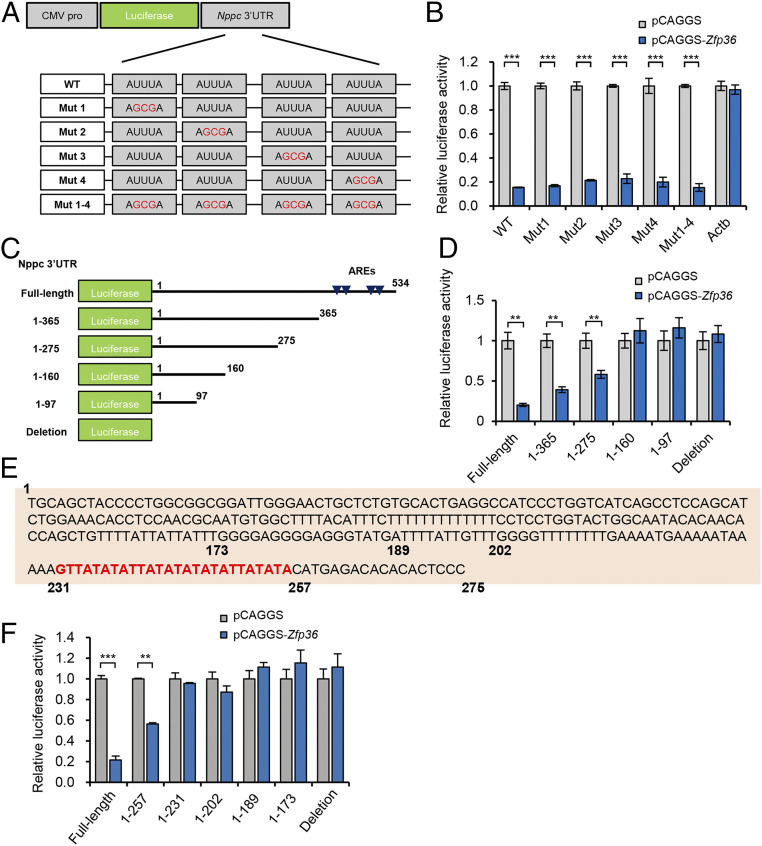
Luciferase assays demonstrated that the full-length Nppc 3′ UTR contains functional repressive motifs that respond to TTP. AREs are essential for recruiting TTP to the 3′ UTR of target mRNA; therefore, we attempted to determine the potent ARE(s) that direct TTP-induced Nppc mRNA degradation. As expected, we found four scattered AUUUA motifs, the canonical ARE sequence, located in the Nppc 3′ UTR (Fig. 1I and SI Appendix, Fig. S4A). We then constructed a range of luciferase reporters that contained different mutant ARE motifs (Fig. 2A); however, none of the detected canonical ARE motifs were essential for mediating TTP-induced repression of any reporters (Fig. 2B). To exclude possible synergistic or additive effects of these canonical ARE motifs, we further constructed a reporter containing the 3′ UTR in which all putative ARE motifs were mutated (Fig. 2A). Unexpectedly, in line with the results of each single mutation, the tetrad mutation also led to a significant decrease in luciferase activity (Fig. 2B), indicating that the reporter still retains significant AU richness in the 3′ UTR, and implying that other unidentified motif(s) might underlie the TTP-induced degradation of Nppc mRNA. This unexpected observation was reminiscent of the presence of noncanonical, but AU-rich 3′ UTR motifs, also known as non-AUUUA AREs. Previous studies have shown that canonical ARE motifs are dispensable for rapid mRNA degradation; however, noncanonical ARE motifs can functionally mediate the rapid destabilization of target mRNA (36, 37). Thus, we screened the Nppc 3′ UTR and characterized multiple non-AUUUA but AU-rich motifs. We hypothesized that the Nppc 3′ UTR contained repressive noncanonical AREs that could mediate TTP-induced destabilization of Nppc mRNA.
Fig. 2.
TTP targets the noncanonical ARE motifs in the Nppc 3′ UTR. (A) Schematic diagram of the luciferase reporters that contained single or all mutant canonical ARE motifs in the Nppc 3′ UTR. (B) Relative luciferase activity of luciferase reporters containing different mutant ARE motifs, which were cotransfected with the Zfp36 overexpression vector (pCAGGS-Zfp36) or empty vector (pCAGGS) in HEK293 cells. The reporter containing the Actb 3′ UTR was detected as a nontargeted control. (C) Schematic diagram of the luciferase reporters that contained different truncated regions from the proximal to distal portions of the Nppc 3′ UTR. Canonical ARE sites located in the Nppc 3′ UTR are indicated by blue arrows. (D) Relative luciferase activity of luciferase reporters containing different Nppc 3′ UTR truncations, which were cotransfected with the Zfp36 overexpression vector or empty vectors in HEK293 cells. The reporters that contained full-length or deleted Nppc 3′ UTRs were used as positive or negative control, respectively. (E) The sequence of the Nppc 3′ UTR containing putative noncanonical ARE motifs (highlighted in red). (F) Relative luciferase activity of luciferase reporters containing further truncated Nppc 3′ UTR fragments. All data are presented as the mean ± SEM of at least three independent experiments. **P < 0.01, ***P < 0.001.
To confirm the presence and location of repressive noncanonical ARE motifs, we next constructed a range of luciferase reporters that contained different truncated fragments from the proximal to distal portions of the Nppc 3′ UTR (Fig. 2C). Similar to the results using the full-length Nppc 3′ UTR, overexpression of TTP significantly decreased the luciferase activity of the reporter constructs containing longer proximal regions (1 to 365, 1 to 275), even though the truncated region contained canonical ARE sites (Fig. 2D). However, when shorter fragments (1 to 160, 1 to 97) were retained, the luciferase activity of these reporters were not repressed by TTP overexpression, which was comparable to the full-length Nppc 3′ UTR deletion reporter construct (Fig. 2D). This result suggested that the uncharacterized repressive motifs were located in the 160 to 275 region of the Nppc 3′ UTR. Additionally, we observed an increased trend of luciferase activity when distal fragments (downstream from 275) were deleted (Fig. 2D), implying that weak repressive AU-rich motifs were located in this region.
Next, we performed a more detailed analysis of the 160 to 275 region of the Nppc 3′ UTR and confirmed the location of the noncanonical repressive ARE, which was characterized by UU/UA dinucleotide clusters (231 to 257) (Fig. 2E). More importantly, we provided direct evidence that this non-AUUUA ARE motif could functionally mediate TTP-induced destabilization of target mRNA (Fig. 2F). Interestingly, by screening the Nppc 3′ UTR of different mammalian species, we found that the UU/UA dinucleotide clusters are highly conserved (SI Appendix, Fig. S4B). Collectively, these observations showed that TTP can bind to the Nppc 3′ UTR via the noncanonical non-AUUUA ARE motif to destabilize its target mRNA.
Nppc mRNA in Preovulatory MGCs Is Degraded by Up-Regulated TTP.
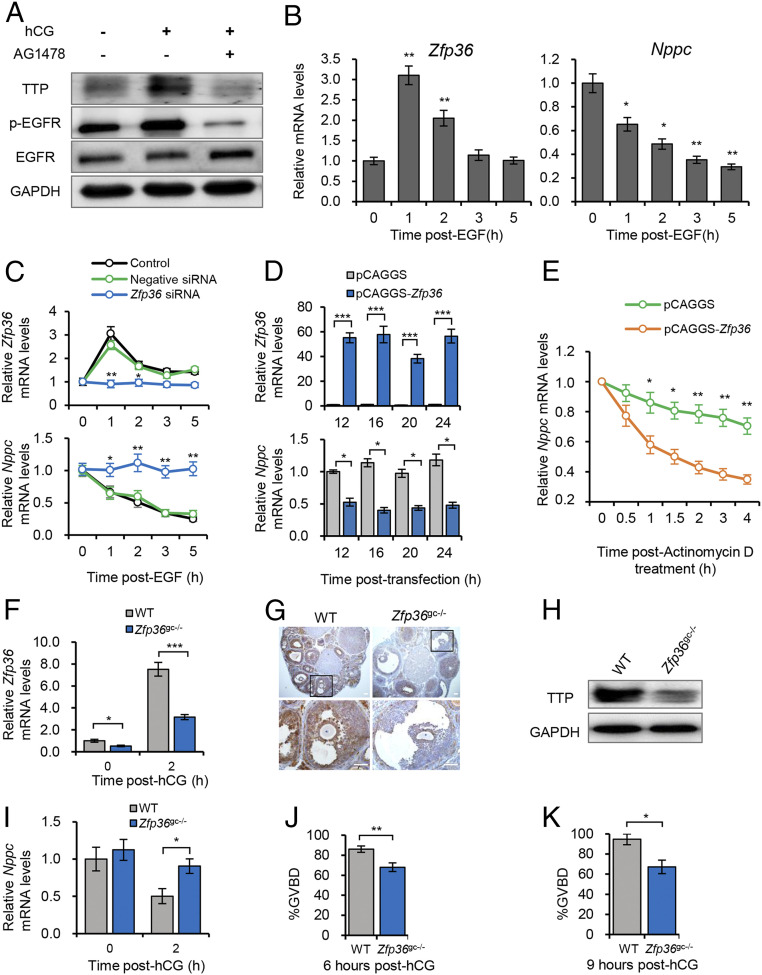
Having established the correlation between TTP and the noncanonical ARE motif in the Nppc 3′ UTR, we next aimed to test whether TTP can regulate Nppc expression in preovulatory MGCs. We used a well-established in vitro model using MGCs isolated from preovulatory follicles, which maintain high levels of Nppc mRNA via supplementation with estrogen (18, 38). Given that LH failed to decrease Nppc mRNA in vitro because of the dilution of LH-induced epidermal growth factor (EGF)-like peptides in the culture medium (18), we recapitulated the LH-induced rapid decrease of Nppc mRNA by modulating EGFR signaling, as reported in previous studies (18, 39). In addition, we found that the administration of the EGFR kinase inhibitor, AG1478, completely blocked the hCG-induced rapid up-regulation of TTP (Fig. 3A), implying that the activation and function of TTP largely depends on LH/hCG-activated EGFR signaling. Moreover, our results showed that Zfp36 was rapidly and transiently up-regulated and that Nppc mRNA was decreased in cultured mouse MGCs in response to EGF supplementation (Fig. 3B), thus recapitulating the LH/hCG-induced changes in ovaries and MGCs (Fig. 1 A and B and SI Appendix, Fig. S1 A and B). These findings support the feasibility of the model to study the role of TTP in controlling endogenous Nppc expression. Furthermore, the in vitro model has the unique advantages of MGC-specific genetic manipulation of Zfp36 and efficient transcriptional termination to assess mRNA stability.
Fig. 3.
TTP degrades endogenous Nppc mRNA in preovulatory MGCs. (A) Western blotting analysis for TTP, as well as phosphorylated and total EGFR, in preovulatory MGCs in response to administration of hCG and AG1478, a specific EGFR inhibitor. MGCs were isolated from in vitro cultured preovulatory follicles. (B) Expression dynamics of Nppc and Zfp36 in in vitro cultured MGCs in response to EGF administration. * indicates significant difference compared with data at 0 h post-hCG. (C) Effect of Zfp36 knockdown on the EGF-induced decrease in Nppc mRNA in in vitro cultured MGCs. Total RNAs were prepared at the indicated time points after EGF administration to detect Zfp36 (Upper) and Nppc (Lower) mRNA levels using RT-qPCR. (D) Effect of Zfp36 overexpression on the Nppc mRNA levels in in vitro cultured MGCs. Nppc expression was maintained at a high level before overexpressing Zfp36. Total RNAs were prepared at the indicated time points after transfection to detect Zfp36 (Upper) and Nppc (Lower) mRNA levels. (E) Measurement of the stability of Nppc mRNA in MGCs following transfection with the Zfp36 overexpression vector (pCAGGS-Zfp36) or empty vector. Total RNAs were prepared at the indicated time points to detect the remaining Nppc mRNA levels after ActD treatment. (F–H) The detection of Zfp36 knockout efficiency in MGCs at 2 h post-hCG, at which point Zfp36 should be fully activated in response to hCG administration. The expression levels of Zfp36/TTP in MGCs were detected at 0 h and 2 h post-hCG using RT-qPCR (F), immunohistochemistry (G), and Western blotting (H). (G, Lower) Higher magnification of boxed regions. (Scale bar, 100 μm.) (I) Effects of MGC-specific depletion of Zfp36 on Nppc mRNA levels at 0 h and 2 h post-hCG. (J and K) Evaluation of oocyte meiotic resumption scored as GVBD percentage at 6 h (J) and 9 h (K) post-hCG. Representative images show the morphology of oocytes isolated from preovulatory follicles of wild-type (WT) and Zfp36gc−/− mice (Right), five to eight mice were used at each time point. (Scale bar, 100 μm.) Data show the means ± SEMs of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Using the in vitro model, we next determined if TTP up-regulation was essential and sufficient to decrease Nppc mRNA levels via loss- and gain-of-function studies. As expected, when the expression of Zfp36/TTP was reduced to basal level via small interfering (siRNA)-mediated knockdown (Fig. 3C and SI Appendix, Fig. S5A), EGF supplementation failed to induce the rapid decrease in Nppc mRNA but maintained the steady-state expression levels in preovulatory MGCs, which contrasted with the groups transfected with the blank-control and negative-control siRNAs (Fig. 3C). In contrast, overexpression of TTP was sufficient to maintain Nppc mRNA at low levels in preovulatory MGCs (Fig. 3D and SI Appendix, Fig. S5B). This result was consistent with the observation from the in vivo experiment that down-regulated Zfp36 was associated with failure to decrease the Nppc mRNA level (Fig. 4C). Next, we attempted to confirm that TTP-induced rapid decrease in Nppc mRNA is dependent on mRNA destabilization. We found that Nppc mRNA maintained a relatively stable level until 4 h after ActD treatment; however, Zfp36 overexpression significantly accelerated the destabilization of Nppc mRNA, similar to that of the targeted control Tnf mRNA (Fig. 3E and SI Appendix, Fig. S5C). By contrast, accelerated destabilization of nontargeted control Actb mRNA was not observed following Zfp36 overexpression (SI Appendix, Fig. S5D). Using cultured bovine MGCs as the model, our results also suggest that the physiological role of EGF-induced TTP in degrading NPPC mRNA might be common to different species (SI Appendix, Fig. S5 E–I).
Fig. 4.
LH/hCG activates Zfp36/TTP through the EGFR-ERK1/2–dependent pathway. (A) Effect of the EGFR-ERK1/2 pathway on the expression of Zfp36 and its associated transcription factors. mRNA expression levels of Zfp36, Nppc, Elk1, and Egr1 in in vitro cultured MGCs that were treated with either EGF or AG1478 (a specific EGFR inhibitor), U0126 (a specific ERK inhibitor), or their various combinations were detected. AG1478 or U0126 was administered 1 h before EGF supplementation. (B) Western blotting analysis for TTP, EGR1, and phosphorylated and total ERK1/2 or ELK1 in in vitro cultured MGCs that were treated with either EGF, AG1478, U0126, or their various combinations for 20 min and 1 h. (C) Expression levels of Zfp36, Nppc, Elk1, and Egr1 mRNA in isolated preovulatory MGCs from mice that were intraperitoneally administrated with hCG, with or without PD0325901 (a specific ERK inhibitor). MGCs were collected at 3 h after hCG administration, and PD0325901 was injected at 4 h before hCG. (D) Western blotting analysis for TTP, EGR1, and phosphorylated and total EGFR, ERK1/2, or ELK1 in MGCs from in vitro cultured preovulatory follicles that were treated with either hCG, AG1478, U0126, or their various combinations. (E) Western blotting analysis for TTP, EGR1, and phosphorylated and total EGFR, ERK1/2, or ELK1 in isolated preovulatory MGCs from mice that were injected intraperitoneally with either PD0325901, AG1478, or their various combinations at 4 h before hCG administration. All data are presented as the mean ± SEM of at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. (F) Working model of the signaling pathways that regulate the CNP-NPR2-cGMP signaling cascade and meiotic resumption in preovulatory follicles. Pathways reported previously and those identified in the present study are indicated in black and blue, respectively.
Finally, to determine the physiological consequences of TTP deficiency in vivo, we crossed Zfp36flox/flox mice and Fshr-Cre mice to knock out Zfp36 specifically in MGCs. We detected that in the resulting Zfp36gc−/− mice, Zfp36 expression was significantly decreased in preovulatory MGCs before and after hCG administration (Fig. 3F). The reduction in TTP protein levels in MGCs was further confirmed by immunohistochemical and Western blotting detection at 2 h post-hCG (Fig. 3 G and H), at which point the high-level expression of Zfp36 should appear in preovulatory follicles (Fig. 1B and SI Appendix, Fig. S1B). Incomplete up-regulation of TTP/Zfp36, in turn, impaired the hCG-induced rapid decrease in Nppc mRNA (Fig. 3I). When oocyte meiotic resumption, scored as percent germinal vesicle breakdown (GVBD), was evaluated, Zfp36gc−/− mice showed impaired meiotic resumption compared with that in the wild-type controls (Fig. 3 J and K). These findings were also supported by results of lentivirus-mediated short hairpin RNA (shRNA) interference of Zfp36 in preovulatory follicles (SI Appendix, Fig. S6 A–F). Collectively, our results from in vitro and in vivo models demonstrated that TTP-dependent mRNA degradation underlies the rapid decrease in Nppc mRNA in preovulatory follicles, which plays a functional role in fine-tuning the progression of oocyte meiosis.
LH/hCG Activates Zfp36 through the EGFR-ERK1/2–Dependent Pathway.
Having confirmed the functional role of TTP-mediated mRNA degradation of Nppc mRNA in preovulatory MGCs, we next attempted to understand the mechanism underlying the transient up-regulation of Zfp36 in response to the LH/hCG surge. Multiple molecular and cellular evidence indicated that Zfp36 transcription is induced by E26 transformation-specific (ETS) transcription factor ELK1 (ELK-1) and early growth response 1 (EGR-1) in the EGFR-ERK1/2–dependent pathway in somatic cells (40–43). Given that LH-activated EGFR-ERK1/2 signaling in preovulatory follicles is essential to initiate meiotic resumption (22, 44), we hypothesized that the rapid induction of Zfp36/TTP in response to LH/hCG might depend on the EGFR-ERK1/2 pathway. Therefore, we examined the hypothesis using both in vitro and in vivo models. In in vitro cultured MGCs, accompanied by the rapidly increased Zfp36 and decreased Nppc mRNA levels, we observed that the transcriptional activation of Egr1, but not Elk1, responded rapidly to EGF stimulation through the EGFR-ERK1/2–dependent pathway (Fig. 4A). Furthermore, Western blotting and immunofluorescence assays showed that ERK1/2, as well as transcription factor ELK1, could be phosphorylated (activated) in response to EGF stimulation within 20 min (Fig. 4B and SI Appendix, Fig. S7 A and B). Subsequently, a marked increase in EGR1 was observed after 1 h (Fig. 4B and SI Appendix, Fig. S7C), along with significantly increased TTP levels (Fig. 4B). All these changes in response to EGFR signaling activation could be attenuated by AG1478 (an EGFR signaling-specific inhibitor) or U0126 (an ERK1/2-specific inhibitor) (Fig. 4 A and B). Moreover, to further confirm the dependence of TTP induction on the ERK1/2 pathway, mice were intraperitoneally injected with the ERK1/2 inhibitor 4 h before hCG administration. Consistent with the results from the in vitro model, in vivo inhibition of the ERK1/2 pathway significantly blocked the induction of Egr1 and Zfp36 expression, and the decrease in Nppc mRNA levels (Fig. 4C). Thus, the results of in vitro and in vivo assays indicated that the ERK1/2 pathway is essential for rapidly activating the transcriptional induction system of Zfp36.
Given that EGFR is not the only mediator of LH/hCG-induced ERK1/2 activation (45–47), we next examined if EGFR was indispensable in mediating the LH/hCG-induced activation of the ERK1/2 pathway and downstream transcriptional induction system of Zfp36. To this end, isolated mouse preovulatory follicles were cultured and treated with hCG, which resulted in the hCG-induced activation/expression of the ERK1/2 pathway, as well as TTP (Fig. 4D). Additionally, the changes in transcription factor levels could be completely reversed by blocking EGFR signaling (Fig. 4D). ERK inhibition did not attenuate EGFR phosphorylation/activation (Fig. 4D). Notably, EGFR inhibition and ERK inhibition, alone or in combination, showed a similar effect of blocking hCG-induced TTP expression (Fig. 4D), suggesting that the ERK1/2 pathway acts downstream of EGFR signaling. These findings were further confirmed using in vivo models (Fig. 4E). Collectively, these results demonstrated that the preovulatory LH surge induces transient expression of Zfp36/TTP and its transcriptional induction system via the EGFR-ERK1/2 signaling pathway.
Discussion
The progression of gonadotropin-controlled oocyte meiosis is tightly regulated by the activity of the CNP-NPR2-cGMP signaling cascade. Before the LH surge, CNP-NPR2-cGMP signaling maintains the active state. The “arrester,” CNP, is primarily synthesized by MGCs and binds to NPR2 throughout the follicle to stimulate cGMP production, thus maintaining meiotic arrest. Following the LH surge, CNP-NPR2-cGMP signaling inhibition occurs consecutively on multiple levels; however, it remains unclear what mechanism maintains the inhibition of the cascade, achieving sustained cGMP at low levels before and during meiotic resumption. Earlier studies proposed that the LH-induced rapid dephosphorylation (inactivation) of NPR2 guanylyl cyclase might contribute to CNP-NPR2-cGMP signaling inhibition, thus causing the rapid decrease in cGMP. However, further studies have indicated that the NPR2 dephosphorylation-dependent rapid decrease in cGMP (13, 14) is dispensable for LH-induced meiotic resumption because the mouse model that prevents NPR2 dephosphorylation (inactivation) only attenuated part of the cGMP decrease, which resulted in delayed, but not failed, meiotic resumption (16). Similarly, LH-induced phosphodiesterase 5 (PDE5), which directly hydrolyzes cGMP, only partially contributes to the rapid cGMP decrease and does not affect the timing of meiotic resumption. More importantly, mice carrying both active NPR2 and mutated PDE5 failed to completely block cGMP decrease (12). Therefore, these results are reminiscent of upstream changes to the signaling cascade (i.e., the LH/hCG-induced decrease in follicular ligand CNP and its encoding mRNA Nppc), which was documented as an important fail-safe mechanism that may ensure LH-induced CNP-NPR2-cGMP signaling inhibition and meiotic resumption (22).
Our data further emphasized the importance of ovarian follicular somatic cells in regulating cGMP homeostasis. To maintain cGMP at high levels and sustain meiotic arrest, somatic cells not only produce the ligand (CNP) that activates its guanylyl cyclase (NPR2) (11) but also provide guanylyl substrate for de novo synthesis of cGMP (48). After the LH surge, cGMP begins to decrease rapidly in somatic cells via the synergistic effect of decreased activity and affinity of NPR2 guanylyl cyclase (14–16), direct hydrolysis of cGMP (12), and decreased concentrations of CNP (17–19). Functional redundancy also suggests that the timing of meiotic resumption and ovulation is critical, and multiple pathways might have evolved as a fail-safe system to ensure LH-induced meiotic resumption. Compared with the rapid decrease of NPR2 activity and activated hydrolysis of cGMP that occurs within 30 min, the decrease of CNP is a relatively slow event (50% decrease within ∼2 h and further decrease until the nuclear envelope is completely broken down) but is concurrent with the progression of meiotic resumption (14). Thus, the decrease of CNP/Nppc in the preovulatory follicle was thought to potentially contribute to driving meiotic resumption, and the mechanism underlying the decrease was considered as an important and long-standing unanswered question (14, 22). Our study supported the idea that the delayed decrease in Nppc mRNA could impair the progression of meiotic resumption. These results, together with previous works that focused on NPR2 inactivation and cGMP hydrolysis (12, 14, 16), allow us to presume that NPR2 inactivation and cGMP hydrolysis may preferentially contribute to the rapid initiation of meiotic resumption, while Nppc mRNA decline is primarily responsible for the later meiotic progression.
In the present study, we revealed that the preovulatory LH surge can stimulate Zfp36 expression via activation of ELK-1 and EGR-1 transcription factors through the EGFR-ERK1/2 signaling pathway. Up-regulated TTP subsequently targets noncanonical ARE motifs in the 3′ UTR of the Nppc mRNA, which induces its rapid degradation (Fig. 4F). The turnover of CNP is rapid (24), and the LH-induced decrease in the CNP peptide is independent of the natriuretic peptide clearance receptor NPR3 (18); therefore, the rapid degradation of Nppc mRNA would lead to a decrease in CNP peptide levels, thus maintaining CNP-NPR2-cGMP signaling inhibition during meiotic resumption.
We provided the functional link between TTP-mediated mRNA degradation and oocyte meiotic progression. The involvement of mRNA degradation-mediated posttranscriptional mechanism in regulating CNP-NPR2-cGMP signaling has never been reported. Furthermore, our results highlight the role of the rare noncanonical AU-rich element in this process. Among the ARE-binding proteins that regulate posttranscriptional mechanisms, TTP has the most specific recognition and binding ARE sequences (i.e., canonical AUUUA motifs) (26, 27). However, non-AUUUA, but AU-rich motifs, can also destabilize target mRNA (36, 37, 49). The UU/UA dinucleotide clusters observed in the Nppc 3′ UTR, although generally rare in vertebrate genomes, were shown to correlate positively with TTP-induced mRNA destabilization (49). Our results, together with those of previous studies, supported the idea that noncanonical ARE motifs efficiently mediate the mRNA-destabilizing function.
The present study identified that TTP-induced mRNA degradation, a global posttranscriptional regulatory mechanism, acts as a regulatory component of orchestrating the signals for oocyte meiotic maturation. We report that Nppc mRNA is the functional target of TTP, via rare noncanonical ARE motifs. This finding might shed light on the mechanism of CNP-dependent cGMP homeostasis in regulating other physiological processes, considering the significant regulatory role of CNP-NPR2-cGMP signaling in the development and function of the cardiovascular, skeletal, and nervous systems.
Materials and Methods
Animal Studies and Ethical Approval.
All female ICR mice used in this study were from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). Fshr-Cre mice and loxP-flanked Zfp36 mice (Zfp36flox/flox) were gifts from Dr. Louis Dubeau (University of Southern California, Los Angeles, CA) and Dr. Perry J. Blackshear (National Institute of Environmental Health Sciences, NIH), respectively. All mice were maintained in a climate-controlled room on a 12 h light/darkness cycle and allowed food and water ad libitum. The China Agricultural University Institutional Animal Care and Use Committee approved this study, which was performed in accordance with the committee’s guidelines. All efforts were made to minimize animal suffering.
Cell culture, isolation, and culture of follicles, Western blotting, immunofluorescence and histologic analysis, RIP are described in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Louis Dubeau (University of Southern California) and Dr. Su Youqiang (Nanjing Medical University) for providing the Fshr-Cre mice and breeding schemes. We also thank Dr. Perry J. Blackshear (National Institute of Environmental Health Sciences, NIH) and Gu Ling (Nanjing Agricultural University) for providing the Zfp36flox/flox mice and breeding schemes. We thank Dr. Wang Chao (China Agricultural University) for his helpful suggestions and for providing the lentiviral vectors. This work was supported by grants from the National Natural Science Foundation of China (Grants 31972573 and 31672426), the National Key R&D Program (Grants 2017YFD0501901 and 2017YFD0501905), the Fundamental Research Funds for the Central Universities (Grant 2020TC004), and the Beijing Innovation Consortium of Agriculture Research System.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018345118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Beavo J. A., Brunton L. L., Cyclic nucleotide research–Still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3, 710–718 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Potter L. R., Natriuretic peptide metabolism, clearance and degradation. FEBS J. 278, 1808–1817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter L. R., Abbey-Hosch S., Dickey D. M., Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr. Rev. 27, 47–72 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Moyes A. J., et al., Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Invest. 124, 4039–4051 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ono K., et al., A single-nucleotide polymorphism in C-type natriuretic peptide gene may be associated with hypertension. Hypertens. Res. 25, 727–730 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Chusho H., et al., Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. U.S.A. 98, 4016–4021 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miura K., et al., An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS One 7, e42180 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Q., Zhang L., C-type natriuretic peptide functions as an innate neuroprotectant in neonatal hypoxic-ischemic brain injury in mouse via natriuretic peptide receptor 2. Exp. Neurol. 304, 58–66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ter-Avetisyan G., et al., Loss of axon bifurcation in mesencephalic trigeminal neurons impairs the maximal biting force in Npr2-deficient mice. Front. Cell. Neurosci. 12, 153 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt H., et al., C-type natriuretic peptide (CNP) is a bifurcation factor for sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 106, 16847–16852 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M., Su Y. Q., Sugiura K., Xia G., Eppig J. J., Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 330, 366–369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egbert J. R., Yee S. P., Jaffe L. A., Luteinizing hormone signaling phosphorylates and activates the cyclic GMP phosphodiesterase PDE5 in mouse ovarian follicles, contributing an additional component to the hormonally induced decrease in cyclic GMP that reinitiates meiosis. Dev. Biol. 435, 6–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egbert J. R., et al., Dephosphorylation and inactivation of NPR2 guanylyl cyclase in granulosa cells contributes to the LH-induced decrease in cGMP that causes resumption of meiosis in rat oocytes. Development 141, 3594–3604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson J. W., et al., Luteinizing hormone reduces the activity of the NPR2 guanylyl cyclase in mouse ovarian follicles, contributing to the cyclic GMP decrease that promotes resumption of meiosis in oocytes. Dev. Biol. 366, 308–316 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao X., et al., Epidermal growth factor-mobilized intracellular calcium of cumulus cells decreases natriuretic peptide receptor 2 affinity for natriuretic peptide type C and induces oocyte meiotic resumption in the mouse. Biol. Reprod. 95, 45 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Shuhaibar L. C., et al., Dephosphorylation of juxtamembrane serines and threonines of the NPR2 guanylyl cyclase is required for rapid resumption of oocyte meiosis in response to luteinizing hormone. Dev. Biol. 409, 194–201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamura K., et al., Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes. Hum. Reprod. 26, 3094–3101 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Lee K. B., et al., Hormonal coordination of natriuretic peptide type C and natriuretic peptide receptor 3 expression in mouse granulosa cells. Biol. Reprod. 88, 42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Xie F., Zamah A. M., Cao B., Conti M., Multiple pathways mediate luteinizing hormone regulation of cGMP signaling in the mouse ovarian follicle. Biol. Reprod. 91, 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W., et al., Brain natriuretic peptide and C-type natriuretic peptide maintain porcine oocyte meiotic arrest. J. Cell. Physiol. 230, 71–81 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Xi G., et al., Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 106, 198–209 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Jaffe L. A., Egbert J. R., Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu. Rev. Physiol. 79, 237–260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shuhaibar L. C., et al., Intercellular signaling via cyclic GMP diffusion through gap junctions restarts meiosis in mouse ovarian follicles. Proc. Natl. Acad. Sci. U.S.A. 112, 5527–5532 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt P. J., Richards A. M., Espiner E. A., Nicholls M. G., Yandle T. G., Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 78, 1428–1435 (1994). [DOI] [PubMed] [Google Scholar]
- 25.Carletti M. Z., Christenson L. K., Rapid effects of LH on gene expression in the mural granulosa cells of mouse periovulatory follicles. Reproduction 137, 843–855 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beisang D., Bohjanen P. R., Perspectives on the ARE as it turns 25 years old. Wiley Interdiscip. Rev. RNA 3, 719–731 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks S. A., Blackshear P. J., Tristetraprolin (TTP): Interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta 1829, 666–679 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christenson L. K., et al., Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 27, 1153–1171 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jo M., et al., Development and application of a rat ovarian gene expression database. Endocrinology 145, 5384–5396 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Wissing M. L., et al., Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum. Reprod. 29, 997–1010 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Xu F., et al., Dynamics of the transcriptome in the primate ovulatory follicle. Mol. Hum. Reprod. 17, 152–165 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carballo E., Lai W. S., Blackshear P. J., Feedback inhibition of macrophage tumor necrosis factor-alpha production by tristetraprolin. Science 281, 1001–1005 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Lee H. H., et al., Tristetraprolin regulates expression of VEGF and tumorigenesis in human colon cancer. Int. J. Cancer 126, 1817–1827 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Masuda K., et al., Arid5a controls IL-6 mRNA stability, which contributes to elevation of IL-6 level in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 9409–9414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Tubergen E., et al., Tristetraprolin regulates interleukin-6, which is correlated with tumor progression in patients with head and neck squamous cell carcinoma. Cancer 117, 2677–2689 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng S. S., Chen C. Y., Shyu A. B., Functional characterization of a non-AUUUA AU-rich element from the c-jun proto-oncogene mRNA: Evidence for a novel class of AU-rich elements. Mol. Cell. Biol. 16, 1490–1499 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarkar B., Xi Q., He C., Schneider R. J., Selective degradation of AU-rich mRNAs promoted by the p37 AUF1 protein isoform. Mol. Cell. Biol. 23, 6685–6693 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., et al., Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 10, 558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji T., Kiyosu C., Akiyama K., Kunieda T., CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol. Reprod. Dev. 79, 795–802 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Florkowska M., et al., EGF activates TTP expression by activation of ELK-1 and EGR-1 transcription factors. BMC Mol. Biol. 13, 8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai W. S., Thompson M. J., Taylor G. A., Liu Y., Blackshear P. J., Promoter analysis of Zfp-36, the mitogen-inducible gene encoding the zinc finger protein tristetraprolin. J. Biol. Chem. 270, 25266–25272 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Lai W. S., Thompson M. J., Blackshear P. J., Characteristics of the intron involvement in the mitogen-induced expression of Zfp-36. J. Biol. Chem. 273, 506–517 (1998). [DOI] [PubMed] [Google Scholar]
- 43.Sobolewski C., Sanduja S., Blanco F. F., Hu L., Dixon D. A., Histone deacetylase inhibitors activate tristetraprolin expression through induction of early growth response protein 1 (EGR1) in colorectal cancer cells. Biomolecules 5, 2035–2055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang M., Ouyang H., Xia G., The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 15, 399–409 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Fan H. Y., et al., MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 324, 938–941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menon B., Franzo-Romain M., Damanpour S., Menon K. M., Luteinizing hormone receptor mRNA down-regulation is mediated through ERK-dependent induction of RNA binding protein. Mol. Endocrinol. 25, 282–290 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panigone S., Hsieh M., Fu M., Persani L., Conti M., Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol. Endocrinol. 22, 924–936 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wigglesworth K., et al., Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc. Natl. Acad. Sci. U.S.A. 110, E3723–E3729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Saif M., Khabar K. S., UU/UA dinucleotide frequency reduction in coding regions results in increased mRNA stability and protein expression. Mol. Ther. 20, 954–959 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.