Significance
Army ants form some of the largest insect societies on the planet and famously forage in mass raids, in which many thousands of ants stream out of the nest in search of live prey. Here we show that this complex collective behavior has evolved from group raiding, which is practiced by relatives of army ants with smaller colonies. Through laboratory experiments, we discovered that group raids and mass raids follow similar organizational principles and that mass raids emerge from group raids when colony size is artificially increased. This suggests that ancient expansions in colony size, rather than changes in individual behavioral rules, led to the evolution of mass raids in the first army ants.
Keywords: collective behavior, social behavior, communication, complex systems, Formicidae
Abstract
The mass raids of army ants are an iconic collective phenomenon, in which many thousands of ants spontaneously leave their nest to hunt for food, mostly other arthropods. While the structure and ecology of these raids have been relatively well studied, how army ants evolved such complex cooperative behavior is not understood. Here, we show that army ant mass raiding has evolved from a different form of cooperative hunting called group raiding, in which a scout directs a small group of ants to a specific target through chemical communication. We describe the structure of group raids in the clonal raider ant, a close relative of army ants in the subfamily Dorylinae. We find evidence that the coarse structure of group raids and mass raids is highly conserved and that all doryline ants likely follow similar behavioral rules for raiding. We also find that the evolution of army ant mass raiding occurred concurrently with expansions in colony size. By experimentally increasing colony size in the clonal raider ant, we show that mass raiding gradually emerges from group raiding without altering individual behavioral rules. This suggests that increasing colony size can explain the evolution of army ant mass raids and supports the idea that complex social behaviors may evolve via mechanisms that need not alter the behavioral interaction rules that immediately underlie the collective behavior of interest.
The foraging behavior of army ants in the subfamily Dorylinae is an iconic collective spectacle that has captivated people for hundreds of years (1–4). Army ants live in huge colonies that contain 104 to 107 workers. Unlike most other ants, they are often subterranean and hunt live arthropods—often other social insects—in a striking form of foraging called mass raiding (1–3, 5, 6). While many ant species forage collectively, few raid insect colonies, making army ants unusual both in their diet and in the specific form of foraging they employ. At least tens of thousands of army ants participate in a mass raid, streaming spontaneously out of the nest in a column—or, in the species with the largest colonies, a swarm (3, 7, 8)—in search of prey. At the outset, the ants have no information about prey location. However, a few scouts search slightly ahead of the raid, and when they encounter prey they lay a pheromone trail back to the raid front and recruit nestmates for a collective attack (9). Army ants have been studied extensively (e.g., refs. 1–3, 5, 7), but how their mass raids evolved is unknown. Indeed, how complex social behaviors generally evolve is not well understood—especially over deep time—and few cases have been studied in mechanistic detail.
To understand how the mass raids of army ants evolved we must identify their ancestral form, understand how this other form of foraging was organized, and explain how it was modified over evolutionary time. Army ants do not constitute a biological clade; instead, army ants are functionally defined by a life history that combines mass raiding, nomadism (frequent colony relocations), and reproduction by colony fission (new colonies arise from large colonies splitting in two). Within the subfamily Dorylinae army ants have evolved twice (10), and the life histories and the structure of the mass raids are remarkably similar between these two independent lineages (3). The other members of the Dorylinae are also often subterranean and prey on live arthropods but live in much smaller colonies of only hundreds of workers. They are rarely encountered in the field, and little is known about their behavior. Sporadic and usually partial observations suggest that many non-army ant dorylines display a different form of foraging called group raiding (SI Appendix, Supplementary Note 1 and Table S1), in which scouts find prey before recruiting a raiding party from the nest (4, 11). W. M. Wheeler, who first observed these group raids in 1918, noted similarities in diet and foraging behavior with army ants and suggested that this had evolutionary significance (12). In 1958, E. O. Wilson hypothesized more explicitly that army ant mass raiding may have evolved from group raids (13, 14). However, because non-army ant dorylines have hardly been studied, no quantitative description of group raids is available. Thus, a formal evolutionary analysis of foraging behavior in dorylines is lacking, and the functional evolutionary relationship between group raiding and mass raiding remains unknown.
Group and mass raiding, like other forms of collective foraging, are not typically defined in a mutually exclusive manner, which hinders our ability to compare them (see SI Appendix, Supplementary Note 1 for discussion of ant foraging terminology). Drawing from the literature, we recognize them as representing two distinct behavioral syndromes, differentiated by the manner in which the raiding column leaves the nest (spontaneously in army ants; induced by a scout in other dorylines), where they recruit to the food (at the raid front in army ants; within the nest in other dorylines), how many ants participate in the raid (at least tens of thousands in army ants; dozens or hundreds in other dorylines), and how many food sources they raid simultaneously (several for army ants; only one for other dorylines) (see SI Appendix, Table S1 for full definition). All doryline raids observed in the wild conform to this strict dichotomy; no species has been observed performing both forms of raiding or displaying raids with both group- and mass-raiding features (SI Appendix, Table S2). Furthermore, alternative types of collective foraging common to other ant lineages (SI Appendix, Supplementary Note 1) have never been observed in the Dorylinae. Together, this lends promise to the hypothesis that group raids may be ancestral to mass raids and more generally suggests that understanding how non-army ant dorylines forage will reveal the evolutionary origins of mass raids. Here we combine phylogenetic reconstructions, automated behavioral quantifications of group raiding behavior, and experimental manipulations of colony compositions in a non-army ant doryline to show that mass raiding has likely evolved from group raiding via simple scaling effects related to increasing colony sizes.
Results and Discussion
Group Raids Have Stereotyped Structure.
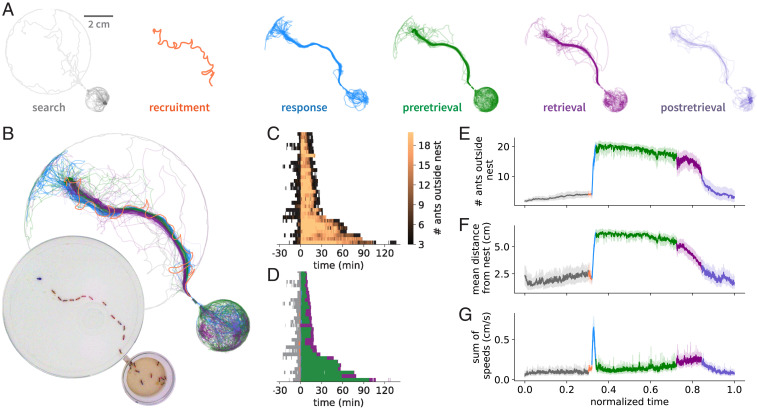
To furnish a detailed understanding of group raiding we systematically studied foraging behavior in the clonal raider ant, Ooceraea biroi, the only non-army ant doryline that has been propagated in the laboratory. In our efforts to establish this species as an experimental model we have developed high-throughput, automated tracking approaches to monitor individual and collective behavior (15, 16), allowing us to study doryline foraging behavior quantitatively and under controlled laboratory conditions. In a first experiment, we set up nine colonies each of 25 individually tagged ants and filmed and tracked their foraging behavior while offering them a single small fire ant pupa once every 12 h (for experimental details see Methods). Overall, we analyzed tracking data for 31 raids (Methods) (17). We found that O. biroi, like other non-army ant dorylines (11, 18), forages in scout-initiated group raids (Movies S1–S6; for ant foraging terminology see SI Appendix, Table S1). We decompose group raids into six distinct phases (Fig. 1 A and B and SI Appendix, Fig. S1). First, in the “search” phase, one or a few scouts explore the arena. Once a scout has discovered food, she examines it briefly before becoming highly excited. In the “recruitment” phase, she runs homeward, and as she enters the nest the ants inside become active. In the “response” phase, a large proportion of ants inside the nest run toward the scout, exit the nest in single file, and move toward the food, retracing the scout’s homeward trajectory (Fig. 1 A–C). Most ants then stay on or near the food for a few minutes, while some run back and forth between the food and the nest, which we call the “preretrieval” phase. Variation in the length of this phase explains most variation in raid length, but its function is currently unknown (Fig. 1D and SI Appendix, Fig. S2). Next, during the “retrieval” phase, one to three ants begin to independently drag or carry the food back home, with no apparent help from their nestmates (Fig. 1 and Movie S2). Finally, in the “postretrieval” phase, the last ants outside gradually return to the nest. To visualize the temporal structure of these raids we aligned and rescaled each phase of each raid and quantified three informative features: the number of ants outside the nest, the mean distance from the nest, and the sum of the speeds of all ants (Fig. 1 E–G). Our analyses show that group raids are highly stereotyped and mostly vary in the duration of the phases. Moreover, these raids are remarkably similar to published descriptions of group raids in other non-army ant dorylines (11, 18), suggesting that group raids are stereotyped not just within O. biroi but across the Dorylinae generally.
Fig. 1.
The anatomy of a group raid. (A) Trajectories of ants at each phase of a representative group raid (Movies S1 and S2), separated into six sequential phases (see Methods). The orange track in the recruitment phase depicts the path taken by the recruiting ant, whereas tracks in all other phases depict the paths of all ants in the colony. (B) Overlay of trajectories from all six phases. (Inset) Snapshot of the colony at the peak of the response phase. A short tunnel separates the nest (small circle) from the foraging arena (large circle), and the food (blue spot) is at the top left. (C) Heat map showing the number of ants outside the nest over time. Thirty-one raids are sorted vertically by their duration and are aligned to the start of recruitment. (D) Representing each phase of each raid by the same color code as in B shows that variation in raid length is primarily determined by the length of the preretrieval phase (SI Appendix, Fig. S2). We do not show the postretrieval phase here, because it has constant length by definition (see Methods). (E–G) Aligning and rescaling each phase of each raid (see Methods) and plotting the time course of the mean number of ants outside the nest (E), their mean distance from the nest (F), and the sum of the speeds of all ants (a measure of collective activity) (G) shows that the temporal structure of group raids is highly stereotyped. The error bands in E–G represent the 95% CI of the mean.
Army Ant Mass Raids Evolved from Group Raids.
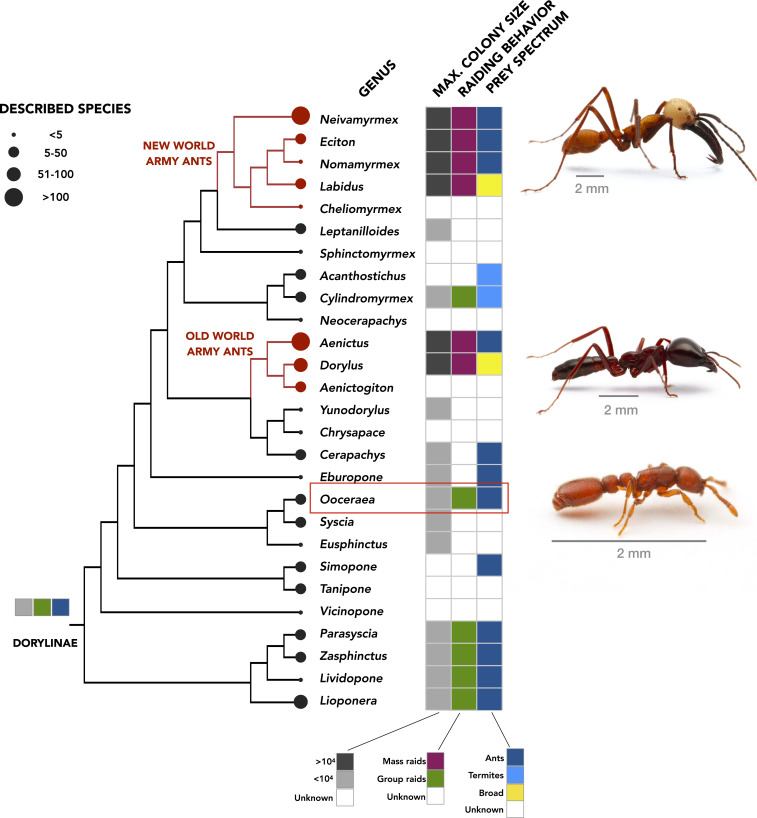
Next, to infer the evolutionary relationship between group raiding and mass raiding, we combined our data on O. biroi with published descriptions of doryline biology and mapped relevant life history traits (taken from the literature; see SI Appendix, Table S2) onto a consensus genus-level phylogeny of the Dorylinae (10). Given the cryptic lifestyle of non-army ant dorylines, information on their biology is unfortunately sparse, which resulted in gaps in our character matrix. However, as our goal here is a coarse-grained inference of the lifestyle of the most recent common ancestor of the Dorylinae, these gaps do not constitute a prohibitive constraint. First, we reconstructed the colony size of the most recent common ancestor of extant doryline ants. We classified each extant doryline genus as having either small or large colonies, separated by a threshold of 104 workers (see SI Appendix, Table S2 for detailed sizes). Our reconstructions (see Methods) found that ancestral dorylines lived in small colonies and that the two origins of army ants were each associated with massive expansions in colony size (Fig. 2 and SI Appendix, Fig. S3 and Table S3). Next, we reconstructed the diet of the ancestral dorylines by classifying the food spectra of extant genera (SI Appendix, Table S2). We found that ancestral dorylines were specialist predators of ants (Fig. 2 and SI Appendix, Fig. S4 and Table S3). Such predators are a priori unlikely to be solitary foragers, because ant colonies are well-defended and able to kill solitary intruders. In addition, there are no recorded observations of doryline ants foraging (i.e., retrieving food) solitarily, or through any form of collective behavior other than group and mass raiding. Together, this lends support to the notion that the ancestral doryline ants foraged collectively through a form of raiding behavior. Indeed, as noted earlier, all observed doryline foraging can be classified as either group or mass raiding, according to the criteria we set out earlier (SI Appendix, Tables S1 and S2 and Supplementary Note 1; see Methods for the classification method). Our formal reconstructions found that ancestral dorylines indeed foraged in group raids. A recent phylogeny of the Dorylinae estimated that their most recent common ancestor lived roughly 74 Ma (10). Thus, our analyses suggest that group raiding is an ancient form of foraging and that non-army ant dorylines have employed this strategy for tens of millions of years. Moreover, our reconstructions show that the origins of army ants were associated with transitions from group raiding to mass raiding, likely independently in the New World and Old World army ants (Fig. 2 and SI Appendix, Fig. S5 and Table S3) (4, 10, 13, 14). In other words, these analyses show that mass raids evolved from ancestral group raids and, by extension, that studying O. biroi might provide mechanistic insight into how a group raid might be transformed into a mass raid.
Fig. 2.
Phylogeny of the Dorylinae, showing all extant genera, along with maximum colony size, type of raiding behavior, and prey spectrum, where known. Ancestral reconstructions on a consensus cladogram (10) are shown at the base of the tree (SI Appendix, Figs. S3–S5 and Methods). Photographs from top to bottom show workers of the army ants Eciton burchellii and Dorylus molestus as well as the clonal raider ant O. biroi (highlighted by a red box). E. burchellii and D. molestus images credit: D.K. O. biroi image credit: Alexander Wild (photographer).
Recruitment and Response Are Homologous across Group and Mass Raids.
To understand this transformation, we must be able to compare group and mass raids explicitly. This requires the use of a common vocabulary to describe the elements of group and mass raids. Our phylogenetic analyses demonstrate that group raids are homologous to mass raids, which suggests we may apply our quantitative description of group raids to mass raids as well. Specifically, we may consider mass raids to entail the same set of phases in the same sequence, beginning with search and ending after prey retrieval. This would allow us to identify homologous phases, and ask in which phase(s) the evolutionary modifications occurred.
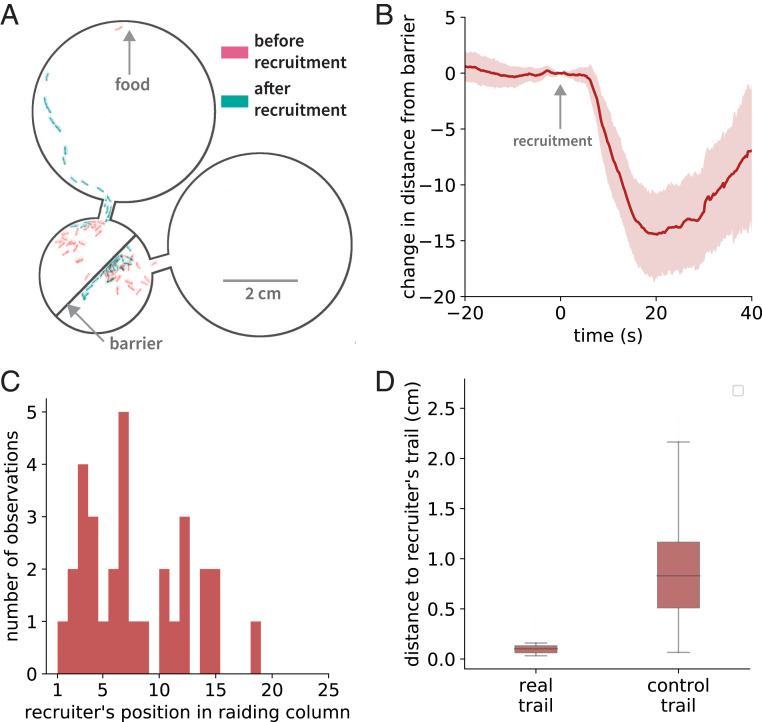
Intuitively, one might expect the response phase of a group raid to be homologous to the onset of a mass raid, because these are superficially similar: They both represent columns of ants streaming out of the nest. However, homology is better established by identifying the behavioral rules involved in each case. Based on our own observations, as well as previous work on army ants and two distantly related non-army ant dorylines (3, 9, 11, 18), we hypothesized that at least two distinct, scout-derived signals determine the spatial and temporal structure of group raids. First, we asked how the scout activates nestmates during recruitment. We conducted an experiment in a modified arena that had a porous wall in the middle of the nest chamber and separate foraging arenas connected to each nest half (Fig. 3A). In each trial, food was placed in one foraging arena, and when a scout with access to that arena located the food she recruited the ants in her nest half, which formed a column that traveled to the food. Shortly after the scout entered the nest, the ants in the other nest half moved toward the wall separating the two halves (Fig. 3 A and B, SI Appendix, Fig. S6, and Movie S7) (17). This suggests that the scout releases an attractive recruitment pheromone as she enters the nest (SI Appendix, Supplementary Note 2). Second, we asked whether the scout lays a pheromone trail back to the nest during recruitment and whether that trail is sufficient to guide the responding ants. Scout-initiated raiding has evolved independently on a few occasions in distantly related ant subfamilies, and in several cases the scout must lead the raiding party to the target. In other words, in these other species information about target location resides primarily in the scout, rather than in a pheromone trail (e.g., refs. 19–23). In contrast, we found that in O. biroi the scout usually (in 30/31 raids) does not lead the raiding column (Fig. 3C). However, the trajectories of the responding ants closely recapitulate the homebound trajectory of the scout, suggesting that the scout indeed deposits trail pheromone on her way to the nest (Fig. 3D). Information about prey location therefore resides exclusively in the scout’s trail. This use of pheromones is highly reminiscent of recruitment at the raid front in army ant mass raids, where scouts lay pheromone trail from prey to the raid front where they elicit recruitment (9). Together, this suggests that group- and mass-raiding dorylines use chemical information in the same way and that the recruitment and response phases of a group raid are homologous to recruitment and response at the raid front in mass raids.
Fig. 3.
A trail and a recruitment pheromone determine the spatial and temporal structure of group raids, respectively. (A) The recruitment pheromone is attractive and acts at a distance. The image shows a modified nest with a porous barrier down the middle. On the left side, a scout releases recruitment pheromone, causing the ants to leave the nest. The ants on the right side, meanwhile, run toward the barrier instead of leaving the nest. (B) The distance between the barrier and the center of mass of ants on the side opposite to that of the scout as a function of time since recruitment. The center of mass travels toward the barrier after recruitment, which shows that the recruitment pheromone is attractive (n = 31 raids, error band shows 95% CI of the mean). (C) A histogram of the scouts’ position in the raiding column shows that scouts do not typically lead raids. (D) The outbound trajectories of responding ants are significantly closer to their scout’s inbound trajectory than they are to trajectories of scouts in other group raids (see Methods), showing that the responding ants indeed follow their scout’s trail to the food (n = 31 raids, two-sided Welch’s t test t = 22.77, P < 7 × 10−29).
Behavioral Rules for Search Are Conserved across Group and Mass Raids.
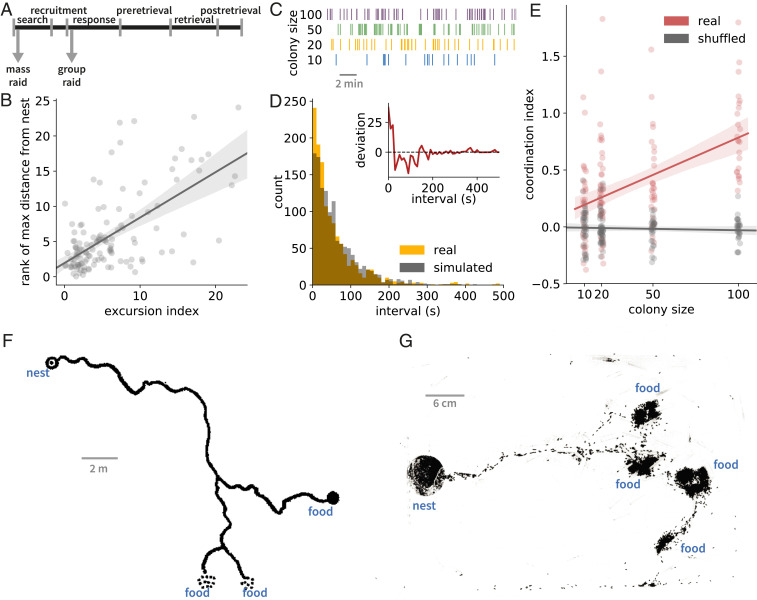
Considering a mass raid to have the same sequence of phases as a group raid, it follows that, as with the other phases, the search phases of group and mass raids are also homologous (Fig. 4A). In other words, despite their apparent differences, the onset of a mass raid is actually homologous to the search phase of a group raid (Fig. 4A). Mass raids begin when workers spontaneously and synchronously leave the nest in “pushing parties” (3, 24, 25). At first, small groups of workers hesitantly leave the nest to explore its immediate vicinity. They lay trail pheromone as they walk, returning after only a few steps out. Ants continue to leave the nest, walking further and further out, confidently following their predecessors’ trail. When they reach untrodden ground, they also hesitate and turn, spreading outward along the raid front. Over time, this leads to a dynamic fan of ants traveling outward, leaving a strong, elongating trail back to the nest in its wake (3, 24, 25). In the species with the largest colonies the ants at the raid front can be so numerous that the raid advances as a swarm (3). In our initial O. biroi experiments, however, the search phase seemed less coordinated (Fig. 1 E–G).
Fig. 4.
Group raids turn into mass raids with increasing colony size. (A) The onset of a mass raid is homologous to the search phase of a group raid, despite the superficial resemblance to its response phase. Arrows indicate when a column of ants leaves the nest in each type of raid. (B) On average, early excursions (low excursion index) terminate closer to the nest than later excursions (high excursion index) (n = 127 excursions, colony size 25, linear regression r = 0.65, P = 2 × 10−16). (C) Four example sequences of nest exit times, sorted by colony size. (D) An example distribution of interexit intervals in a colony of size 20. This distribution (in amber) deviates significantly from a simulated exponential distribution (in gray) (Anderson–Darling k-sample test P = 0.001). (Inset) Difference between this distribution and 1,000 simulated exponential distributions, as a function of the interval, showing an increased coefficient of variation (red line is mean difference, with 95% CI). Nest exits in close succession (i.e., short intervals) are overrepresented in the empirical distribution compared to simulated distributions. (E) The coordination index (see Methods) of real interexit intervals (red data points) increases as a function of colony size (n = 131 exit sequences, linear regression r = 0.48, P = 4.9 × 10−9), but the coordination index of shuffled interval sequences (gray data points) does not (n = 131 exit sequences, linear regression r = 0.79, P = 0.89). (F) Schematic of a mass raid of the army ant Aenictus laeviceps, reformatted with modifications from ref. 42. (G) Snapshot (background-subtracted and contrast-enhanced; see Methods) of an O. biroi raid in a colony with ca. 5,000 workers. The raid shows all the major features of the army ant mass raid depicted in F. Error bands in B and E depict the 95% CI of the regression line.
We thus asked whether O. biroi scouts follow the same basic behavioral rules for search that translate into pushing parties in mass-raiding army ants. First, we analyzed our tracking data from colonies of 25 workers to see whether ants incrementally increase their foraging distance by extending previously traveled paths. We found that O. biroi often (in 21/31 raids) search an arena that is initially void of trail pheromone in a series of excursions (see Methods). Further analysis of these excursions revealed that, on average, early excursions terminate close to the nest, while later excursions terminate farther away (Fig. 4B). Additionally, ants walk faster (SI Appendix, Fig. S7A) and spend longer outside (SI Appendix, Fig. S7B) in later excursions and are more likely to follow trail at the beginning, rather than the end, of the outbound leg of each excursion (SI Appendix, Fig. S7C) (17). Thus, it appears that, as in army ants, O. biroi workers lay pheromone trail as they leave the nest in the search phase, and indeed, appear to follow the same rules during search behavior. Taken together, our results suggest that the basic behavioral rules underlying search behavior are conserved between army ants and their non-army ant relatives.
Despite the similarities in individual behavior, the emergent collective search patterns in a mass raid and a group raid are strikingly different. Understanding how mass raids evolved thus requires us to understand how the search phase of the ancestral group raid was modified without changes to individual worker behavior. In other words, we must explain how pushing parties emerge during search in mass raids. Unlike in army ants, where workers leave the nest en masse to go on a raid, O. biroi workers typically leave the nest during the search phase in a seemingly sporadic manner (Fig. 1E). To study the temporal structure of search in O. biroi and to quantify the synchronicity in the search phase we conducted an experiment with four colonies of size 20. To control for the possibility that ants behave differently when food is in the arena we specifically selected periods when the arena was empty (i.e., the ca. 20 h after each raid each day, resulting in a total of 43 search events). We then recorded each time an ant exited the nest in each event. We analyzed the resulting sequences of interexit intervals by comparing them to the uncorrelated, exponentially distributed expectation from a random Poisson process (Fig. 4 C and D and Methods). We found that nearly all distributions deviated significantly from the random expectation (SI Appendix, Fig. S8A), exhibiting increased coefficients of variation (SI Appendix, Fig. S8B), overrepresentation of short intervals (SI Appendix, Fig. S8C), and positive correlations between consecutive intervals (SI Appendix, Fig. S9A), implying that workers leave the nest in quick succession more often than expected by chance (Fig. 4D and SI Appendix, Fig. S8C). This suggests that, while the apparent synchronicity is weak, a significant positive feedback—which characterizes the onset of army ant raids—also underlies the search activity of O. biroi.
Increasing Colony Size Transforms Stereotyped Group Raids into Mass Raids.
Army ants live in much larger colonies than non-army ant dorylines, and expansions in colony size within the Dorylinae align perfectly with the evolutionary transition to mass-raiding behavior (Fig. 2). We hypothesized that this increase in colony size could explain the origins of mass raiding.
To understand the effect of colony size on the emergent search and raiding behavior in dorylines we established O. biroi colonies with 10, 50, or 100 workers, alongside the colonies of 20 workers described above. Although these colony sizes do not approach those of army ants, this experiment is nonetheless informative regarding the general scaling effects of colony size. Moreover, as the nest chambers of our experimental arenas are much larger than even the largest colonies, this experiment manipulates colony size but not their effective density. Across all colony sizes, colonies mostly exhibited the same stereotypical raid dynamics (Movie S8), with the number of ants participating in the raids increasing proportionally to colony size (SI Appendix, Fig. S9B). Analyzing their interexit interval distributions during search (Fig. 4C), we observed the same increase in their coefficient of variation compared to the random expectation as before (SI Appendix, Fig. S8B). Moreover, the correlation between consecutive intervals, as measured by the autocorrelation function of the sequences, markedly increased with colony size (SI Appendix, Fig. S9A), as did a “coordination index” that we computed from the autocorrelation function (Fig. 4E and Methods) (17). Thus, as colony size increases, search behavior in O. biroi begins to resemble the onset of highly bursty, coordinated army ant mass raids. However, unlike in a full-blown mass raid, these bursts typically attenuate quickly. Nevertheless, we observed multiple events in colonies of ≥50 ants in which positive feedback among the ants spontaneously produced a column that traveled away from the nest, headed by an obvious pushing party that formed without recruitment, in what resembled the onset of a mass raid (Movie S9).
To test whether these scaling effects persist at colony sizes that approach those of army ants, we established two O. biroi colonies of roughly 5,000 workers each, an order of magnitude larger than naturally occurring colonies (26), and filmed their raids in large arenas (see Methods). The resulting raids involved thousands of ants and displayed trail bifurcations, simultaneously targeting multiple food sources (Fig. 4 F and G, Movie S10, and SI Appendix, Table S4). Most initial recruitment events now occurred outside the nest and usually at the raid front (43 out of 47). Thus, increasing colony size eventually transforms stereotyped group raids into raids that display all the defining features of army ant mass raids (SI Appendix, Table S1).
Together, our results suggest that all doryline ants share fundamental rules of search and recruitment behavior. At small colony sizes, these rules manifest as scout-initiated group raids. However, as colony size increases, either experimentally within species or naturally between species across evolutionary time, these rules gradually give rise to spontaneously initiated mass raids in which many ants leave the nest in quick succession, advance in pushing parties, and recruit at the raid front rather than at the nest. The difference between search behavior in group raiders and mass raiders may thus be largely driven by the effects of increasing colony size. In other words, expansions in colony size in the ancestors of army ants are sufficient to have caused the transition from group raiding to mass raiding behavior.
Conclusion
Typically, the mechanism for behavioral evolution is thought to be the modification of neural circuits for that behavior. For instance, courtship decisions in fruit flies evolve through modifications to the internal physiology and/or synaptic strength of courtship neurons (27, 28). Vocal learning circuits in birds are thought to have evolved via the duplication and modification of ancestral motor circuits (29). Animals typically acquire the ability to perceive new stimuli by duplicating or modifying old receptor genes or evolving new ones and/or enhancing their sensitivity to old stimuli through expansions in existing sensory processing systems (30, 31). Similar modifications have been observed or proposed to explain a variety of evolutionary changes in motor systems (32). In all these cases, the circuits immediately involved in that behavior are modified, altering the computations they perform on the timescale of that behavior.
Our data suggest a slightly less direct mechanism for the evolution of foraging behavior in army ants. We propose that all doryline ants share similar neural circuits for raiding behavior and that instead the evolution of mass raiding from group raiding depends on a change elsewhere—likely in the circuits that ultimately regulate colony size. Changes in group size are known to induce qualitative changes in collective behavior in other cases as well. For instance, golden shiners form polarized swarms or milling schools depending on their group size (33). For the last few decades, it has been understood that changes in the size of an ant colony can also have dramatic effects on the organization of its foraging behavior. Theoretical work proposes that ant colonies may transition from individual to collective foraging with increasing colony size (34) and suggests that this may be driven by positive feedback within the nest (35). Empirical work demonstrates that Pharaoh’s ants (Monomorium pharaonis) undergo a phase transition from disordered to ordered foraging as their colony size increases (36). Moreover, colony size has been generally associated with foraging mode across the ant phylogeny. Species with small colonies typically rely on individual foragers, species with moderately sized colonies tend to use a mixture of individual and collective foraging strategies, and species with large colonies tend to rely primarily on cooperative, collective foraging (37). Here we provide a concrete example in which such changes in colony size have been instrumental in shaping the evolution of collective behavior over tens of millions of years.
Methods
Colony Maintenance.
O. biroi colonies were maintained in the laboratory at 25 °C in boxes with a water-saturated plaster of Paris floor. Like many other doryline ants, colonies of this species undergo stereotypical cycles, alternating between reproductive phases, during which the ants lay eggs and do not forage, and brood care phases, during which colonies contain larvae and workers forage for food. During the brood care phase, experimental colonies were fed with frozen Solenopsis invicta brood. All experiments were performed using ants from clonal line B (38). For all experiments other than the one with very large colonies, all ants were 1 mo old, were from the same source colony, and had been reared under the same conditions.
Behavioral Tracking Setup.
Behavioral experiments were conducted in artificial arenas constructed from layers of cast acrylic, with a plaster of Paris floor. Each arena was a square of side 10 cm, in which we laser-cut a nest chamber and a foraging arena, connected to each other by a narrow tunnel (Fig. 1). The nest chamber had a diameter of 2 cm, the tunnel was ∼2 mm wide and ∼6 mm long, and the foraging arena had a diameter of 6.5 cm. The floor of the foraging arena was covered with vapor-permeable Tyvek paper to make it less attractive as a nesting site and discourage colonies from emigrating there, while keeping it suitable as a foraging arena. For all experiments in these artificial arenas ants were introduced to the nest chamber at the start of the reproductive phase. During this period, the tunnel was sealed to prevent ants from entering the foraging arena; 2 to 4 d after introduction, the ants laid eggs in the nest chamber. Ten days later, the eggs hatched into larvae; 4 to 6 d after this, when the larvae were in their third or fourth instar, we placed food (i.e., a single frozen S. invicta pupa) in the foraging arena, unsealed the tunnel, and filmed the ants foraging.
We filmed colonies at 5 or 10 Hz and 2,592 × 1,944 pixel resolution, using webcams (Logitech C910) in enclosed containers with controlled light-emitting diode lighting at ∼27 °C and ∼60% humidity.
Tagged-Ant Experiment.
Nine colonies of ants were established from a single cohort of 1-mo-old ants that were entering the reproductive phase. Each colony consisted of 25 ants, and each ant was tagged with an ordered pair of color dots that was unique within the colony. Specifically, each ant was painted on her thorax and gaster with one of five colors of oil-paint markers (Uni Paint Markers PX-20 and PX-21), a previously used technique (15, 16, 39). At the end of the experiment, we counted all larvae and found that each colony had between 20 and 25 larvae. In other words, the larvae:adults ratio [a known source of variation in colony foraging (40)] was close to 1:1 in all colonies.
We define foraging events as events in which the ants were provided food, discovered it, and in which the food subsequently entered the nest. For the 8 d of the tracking period (i.e., when the larvae were between ∼5 and 13 d old), every 12 h, we cleaned each foraging arena with water (to remove trail pheromone from the previous foraging event) and placed a single S. invicta pupa (infused with 0.05% bromophenol blue to aid visualization; Movie S1 and Fig. 1B) at its far end. We then unsealed the tunnel and allowed the ants to explore the arena. We filmed the arena for roughly 4 h thereafter, at 10 frames per second, after which we resealed the arena. For the first 5 d (i.e., the first 10 foraging events), each colony was given a small (worker-destined) S. invicta pupa. For the next 3 d, we presented colonies with large (queen-destined) or small (worker-destined) pupae in alternation. The difference in feeding did not affect the coarse structure of the colonies’ foraging behavior. Here, we do not differentiate between these foraging events, and we will analyze the fine differences between them in a subsequent publication. In some cases, colonies emigrated to the foraging arena. For the next event in such colonies, if the ants had not moved back to the nest chamber we presented them with a S. invicta pupa but did not record foraging. All chambers had their plaster floor watered periodically to saturation.
In sum, we recorded 90 foraging events across nine colonies. All foraging events resulted in raids, with scouts locating the food and recruiting their nestmates to it. Twenty-two foraging events ended in emigration, where the ants moved their nest (i.e., their larvae and all adults) to the food. In 18 events, the ants appeared to eat the S. invicta pupa in situ (although we cannot exclude the possibility that they tore it into small pieces before carrying it home, and we cannot be certain that only adults ate the food). The 50 remaining events ended in retrieval, i.e., with the ants transporting the pupa into the nest. We never observed emigration again in subsequent experiments and only observed a single further instance of eating in situ, possibly due to subtle differences in experimental design. Thus, we excluded these events from our analysis here. Of the 50 events that ended in retrieval, 19 were excluded from analysis due to failures in data acquisition or cases where the colony was unsettled at the time of food presentation. Our final dataset thus consisted of 31 foraging events from seven colonies.
Annotation of Group Raid Phases.
Based on our manual observations of the raids, we identified six discrete, sequential phases of each raid. We defined the search phase as the period beginning at the start of the video and ending at the time at which the next phase (i.e., recruitment) begins. For the group raids that we analyze here, scout ants necessarily located the food during the search phase. The recruitment phase begins when a scout leaves the food and runs homeward, and it ends when the scout recruits her nestmates, which commences the response phase. The recruitment phase only includes successful recruitment. In some cases, scout ants may run homeward from the food without initiating a response; however, as we cannot judge whether these instances constitute attempted recruitment, we do not use them to define the beginning and end of the recruitment phase. We define the beginning of the response phase as the first ant of a column leaving the nest, and the end as the moment when the tail of the column reaches the food. This commences the preretrieval phase, which ends when ants begin to move the food back home. We define the retrieval phase as beginning when the position of the food has noticeably changed and ending when the food enters the nest. We define the final phase, postretrieval, as beginning when the food has entered the nest and arbitrarily end it 500 s later.
For all raids, we manually annotated the corresponding videos, specifically recording five time points that allow us to define these six phases. These time points are the time at which a scout leaves the food on her recruitment run, the time at which the leader of the column of ants responding to recruitment enters the foraging arena, the time at which the last ant in the column arrives at the food, the time at which the position of the food begins to change, and the time at which the food enters the nest. In colonies of 25 ants these time points may be recorded with minimal subjectivity, as assessed by repeated annotations of the same raids, and by comparisons of recorded time points between observers. For all raids analyzed here, a single observer (V.C.) annotated all videos. We also recorded the identities of the scouts that successfully initiated raids and all ants that contributed to retrieving food.
Tracking Tagged Ants.
Videos from this experiment were processed using anTraX (16) to produce the spatial (x, y) coordinates of each ant in the colony during each of the events. Tracking quality was quantified using the assignment error (see ref. 16 for definition) for each colony and each event separately. As in the context of this paper we have used individual trajectories for the analysis of the recruitment and response phases, we have quantified the assignment error in the time period starting just before start of recruitment phase and ending just after the end of the response phase. The average tracking error was estimated to be 1.8%, with the largest error across all events being 5%.
Visualization of the Average Raid Structure.
While the temporal ordering of the phases is identical across raids, the duration of each of the phases varies considerably between events (Fig. 1D and SI Appendix, Fig. S2). In order to analyze the average time course of colony activity during the raid we computed the mean duration of each of the phases across all 31 raiding events. We then rescaled each phase of each raid so that it equaled the mean phase duration. Rescaling was done by dividing the time points of each phase in each event by the ratio between the average phase duration and the current phase duration. We then interpolated and resampled each of the computed measures (number of ants outside the nest, their average distance from the nest, and the sum of their absolute velocities), so that all events had the same time axes, and applied a moving average filter with a window size of 1 s to smooth out tracking noise. The average of these rescaled time-dependent measures together with their 95% CIs is shown in Fig. 1 E–G.
Analysis of the Scout’s Position in the Raiding Column.
To ask whether the scout led the raid, we ranked her position in the raiding column in each raid. To do this, we took advantage of the fact that in all analyzed raids the responding ants walked in a single file. We ranked all ants by the time they crossed the halfway mark between the nest and the food (Fig. 3C). Observations of the videos suggested that changes in the ants’ ranks were minimal (i.e., they did not often overtake each other), and selecting alternative points at which to rank the ants did not noticeably alter the distribution of the scout’s rank across raiding events.
Analysis of Trail following during the Response Phase.
To ask whether the ants in the response phase follow the specific trail laid by the scout in the recruitment phase, we asked whether the x, y coordinates during their outbound journey were closer to the x, y coordinates of the recruiting scout during her inbound journey than expected by chance.
For each raid, let the set of the recruiter’s coordinate vector be
| [1] |
where represents the coordinate vector of the recruiter at time t, is the time at the start of the recruitment phase, and is the time at the end of the recruitment phase.
Similarly, the set of all coordinate vectors of all responding ants is
| [2] |
where represents the coordinate vector of ant a at time t, is the time at the start of the response phase, is the time at the end of the response phase, and A is the set of ants that participate in the response to recruitment.
For each time point in the response, and for each ant participating in the response, we define as its minimum distance to the recruiter’s track:
| [3] |
For each raid event we then computed a measure of trail following, defined as
| [4] |
If the ants are not following the recruiter’s trail, we might still expect to have a relatively low value, because the positions of the nest and the food remain constant across each raid (and thus substantially constrain the initial and final coordinates of each ant’s trajectory). To account for this inherent spatial structure in our null expectation, we compared the set of response coordinates to the coordinates of scouts from all raids other than their own. For each set of response coordinates we thus generated 30 minimum-distance values. We then compared the distribution of 31*30 “control” values to the distribution of 31 true values using a Welch’s t test.
Detection and Analysis of Excursions in the Search Phase.
We define an excursion as the trajectory of a scout from the moment she is leaving the nest to the moment she enters back. To identify excursions in our data, for each ant we identified all pairs of transitions across the nest threshold and extracted the trajectory segments between them. For each excursion we then calculated a number of summary features: its duration, its maximum distance from the nest, and the ant’s mean speed. We excluded excursions in which ants traveled ≥3.5 times their maximum distance from the nest, as these represent cases where the scout travels along the arena’s wall or is walking in circles in the arena, events that are artifacts of our experimental setup, that are unlikely to occur in natural contexts, and that have different dynamics that obscure the pattern visible in other excursions. We then ranked these values within each event and plotted the excursion rank versus its index in the event across all events (Fig. 4C and SI Appendix, Fig. S7 A and B).
To ask how ants follow trails during these excursions, we also selected the outbound leg of each excursion by truncating the excursion at the time at which the ant reached its maximum distance (in that excursion) from the nest. For each coordinate vector in each outbound leg we classified it as either on- or off-trail, depending on whether it mapped to a previously occupied pixel on a 100- × 100-pixel binary map (where each pixel represents a square of side 1 mm) of all previous ant locations, excluding the focal excursion, in that search phase. We then rescaled all such binary sequences to be the same length so that we could align the beginning and end of the outbound legs of each excursion (SI Appendix, Fig. S7C).
Barrier Experiment.
To study the nature of recruitment, we modified our artificial arenas as depicted in Fig. 3. We laser-cut cast acrylic porous barriers of 0.8-mm thickness, with multiple holes with a diameter of ∼50 µm, so that ants could not contact each other from across such barriers but could communicate via volatile pheromones. Each barrier was placed in the middle of a nest that had two foraging arenas, essentially creating two nests separated by this porous barrier. We established colonies of 20 1-mo-old, phase- and genotype-matched ants in each nest half in each of eight replicate nests. The ants laid eggs in each nest half 2 d later. In the subsequent brood care phase, each day (except for a handful of days interspersed through the experiment when we fed and watered all colonies while preventing them from leaving their nest halves) we placed a single S. invicta pupa in the foraging chamber of one nest half of each artificial arena, alternating which half received food each day. In this experiment, a number of colonies often failed to detect the food (because the ants never left their nest). Nonetheless, we recorded 35 instances of foraging in five artificial nests across a 2-wk period. Of our 35 replicate events, we excluded four events from a single colony from further analysis, because the scout in these events did not enter the nest or because (in one case) the colony was too active in the search phase for effective recruitment.
As tracking the ants in the dense chamber is impractical, we used an alternative approach to understand the recruitment dynamics in the nest. We subtracted each frame in the video from the background image (an image which includes all image features, but without the ants; see ref. 16 for the procedure used to generate these images) and converted it to gray scale. As ants are darker than the background, the value of each pixel, , in this image was taken as the probability that it contains an ant. We then compute the center of mass coordinates for each “half-colony” by summing over all pixels that belong to it:
| [5] |
where C refers to the centroid’s coordinates, and refer to the coordinates of each pixel, and refers to the pixel gray value.
For each frame we found the position of the centroid and recorded its distance to the barrier separating the two nest halves as the length of the perpendicular from the centroid to the barrier. We then aligned the time series of centroid distance (from the barrier) to the time of recruitment (which we define as the time at which a majority of ants on the scout’s nest half are activated and begin to move) and averaged across events (Fig. 3B and SI Appendix, Fig. S6).
For the statistical analysis comparing distances before and after the scout releases recruitment pheromone (SI Appendix, Fig. S6A), we manually selected a frame from each video roughly 1 to 2 s before release and compared the distance of the centroid from the barrier at this timepoint to its distance 20 s later. To ensure that our manual selection of the initial frame was accurately identifying a time shortly before recruitment, we also defined the “ant mass” in each nest half by
| [6] |
We then plotted the ant mass in the scout’s nest half over time from the time recruitment and found that shortly after the initial frame, this “ant mass” decreased sharply—an indication that the ants in the scout’s nest half actually left the nest in response to recruitment (SI Appendix, Fig. S6).
Colony Size Experiment.
To ask how increasing colony size altered the structure of search behavior we established three or four colonies each of 10, 20, 50, or 100 untagged workers. As before, all workers were 1 mo old and were selected from a single cohort from a large source colony. They were placed in artificial arenas identical to those used in the tagged-ants experiment when they were entering the reproductive phase and laid eggs simultaneously in their new nests shortly thereafter. In the subsequent brood care phase, when their larvae were ∼5 d old, we began tracking. Here, every day for 10 d, we gently transferred ants in the foraging arena into the nest, sealed the connecting tunnel, cleaned the foraging arena with water, saturated the plaster base of each colony, and placed food (a single small S. invicta pupa) in the foraging arena before reopening the tunnel and starting tracking. Roughly 4 h later, we then fed each colony in proportion to their colony size (to control their nutritional states). Specifically, we placed S. invicta pupae inside each nest, maintaining a constant 1:10 food items:ants ratio. On rare occasions when a colony did not locate the food in the arena within 4 h we placed it inside the nest. We then continued filming the colony for the next ∼20 h. We repeated this process through the brood care phase, until the larvae had pupated. This experimental design allowed us to study how varying colony size alters the structure of the raid (SI Appendix, Fig. S9B), and more importantly, how it alters the behavior of ants searching for food when there is no food in the arena—the primary focus of our statistical analyses (Fig. 4).
Exit Counting Analysis and Controls.
To analyze the temporal structure of search behavior we recorded the time at which each ant exited the nest (and entered the foraging arena). We used anTraX to track ant movement while in the foraging arena. Since the ants were not individually tagged in this experiment we did not obtain complete trajectories, but rather a collection of short tracklets, some of which were single-ant and some were multi-ant (16). We marked the entrance to the tunnel and filtered all tracklets that originated with an ant emerging from the tunnel (all tracklets that have their first blob overlapping with the entrance mark and have no parent tracklets, or multi-ant tracklets with only one single-ant tracklet parent that start at the tunnel entrance). For each of these tracklets we recorded the first frame as an “exit time” of one ant. While the false-positive rate of this detection process is minimal the false-negative (unrecorded exits) is more substantial, as some cases where ants leave the nest in close proximity, which prevents their segmentation, are recorded as single exits. However, for all the analyses described below, these errors work to decrease the reported effect.
Overall, across all colony sizes, we had 150 time series of intervals between subsequent nest exits. We excluded samples (i.e., time series) that had fewer than 200 total exits from subsequent analysis. As our analysis was focused on short-term activity fluctuations, we detrended each time series with a third-degree polynomial to account for slow modulations of activity that might correspond to effects such as buildup of colony hunger, circadian cycles, etc. For each time series we then assessed the first 10 lags of the autocorrelation function (SI Appendix, Fig. S9A). The mean autocorrelation was higher for larger colony sizes at most initial lags. To quantify a “coordination index” for ants leaving the nest together, we summed the unbiased autocorrelation over the first 10 lags and compared this value across samples:
| [7] |
where refers to the i-th interexit interval in the detrended sequence, k refers to the lag, refers to the empirical mean, refers to the empirical SD, and N refers to the size of the interexit sequence.
Quantifying the Number of Ants That Participate in the Raid.
As a proxy for the true number of ants involved in raids we used the maximum number of detected blobs outside the nest in any single frame throughout the raid, whether these blobs corresponded to individual or several ants. While this is an underestimation of the real number of ants participating in the raid, it provides an estimate that is sufficient for the purpose of testing whether larger colonies have more total participation (SI Appendix, Fig. S9B). Moreover, the negative bias in the estimation increases as a function of colony size.
Enlarged O. biroi Colony Experiment.
We established two O. biroi colonies in the brood-care phase with roughly 5,000 workers each. All workers in both colonies were of clonal line B and included multiple age classes, representative of natural colonies. Preliminary experiments suggested that colonies of this size settle relatively rapidly, and we found that after 12 h in a new nest the colonies behaved qualitatively indistinguishably from “settled” colonies that had lived in a nest for a long period. For each foraging event we anesthetized each colony with CO2 and transferred it into a new arena (roughly 60 cm × 34 cm) with a fresh plaster of Paris base and a circular nest chamber (radius 6 cm) with a single sealed exit.
O. biroi workers have a strong thigmotactic tendency, and in large, featureless arenas they spend substantial proportions of time following the outer walls. To ameliorate this effect, we scattered a number of small, transparent acrylic bricks (3 × 0.3 × 0.3 cm) throughout the arena. Pilot experiments suggested that introducing these bricks inside the arena would enable the workers to follow the short local edges, diminishing the amount of time they take to locate the food and creating more naturalistic conditions. Additional pilot experiments showed that adding such edges or changing arena size and geometry did not qualitatively affect the ants’ ability to raid and were not sufficient to induce bifurcations in raiding trails.
Roughly 12 to 16 h after introducing each large colony to its new nest we placed three to seven piles of fire ant brood far from the nest and then unsealed the nest exit and allowed each colony to explore the arena. We filmed each colony’s foraging behavior for the next ∼24 h. We repeated this process seven times for one colony and four times for the other, with 1 to 3 d between subsequent foraging events. Together, we filmed 11 foraging events in the brood-care phase in these large arenas, of which we excluded one because the ants were alarmed at the start of filming. We manually annotated the remaining foraging events to assess whether recruitment occurred inside or outside the nest, whether or not recruitment events resulted in bifurcation of the trail, and to estimate approximately how many ants participated in the raid (SI Appendix, Table S4).
To create the image shown in Fig. 4G we selected a representative snapshot from the middle of one of these raids, performed background subtraction, and then uniformly increased the contrast of the image to make the ants more visible.
Ancestral-State Reconstructions.
We used the phylogenetic consensus topology of the Dorylinae from ref. 10. We searched the natural history literature on doryline ants to find information on character states for a number of characters: colony size, prey spectrum, and various features of foraging behavior (raid initiation, recruitment, number of ants in the raid, and trail bifurcation) that are characteristic of either group or mass-raiding behavior (SI Appendix, Tables S1 and S2). Since there is very little evidence from multiple species within each genus (and little quantitative data anywhere in the Dorylinae), we chose to collapse character states for each trait into a genus-level categorical assessment. There were no major ambiguities within any genus. This within-genus concordance may in principle be partly an artifact of insufficient species-level data or of insufficient sampling within each species. However, in some genera (e.g., Eciton, Dorylus, Neivamyrmex, Aenictus, Lioponera, and Zasphinctus) information about several species is available, and incongruity is never observed. Some species within these genera are well-studied, and it is unlikely that they contain substantial hitherto unknown variation in their colony size, prey spectrum, or raiding behavior (see references in SI Appendix, Table S2 for more detail). Together, this suggests that substantial incongruity within genera is unlikely.
For colony size, we first recorded the maximum reported colony size for any colony in the genus to the nearest order of magnitude. We then classified genera with maximum colony sizes above a threshold of 104 workers as genera with “large” colonies and genera with fewer than 104 workers as possessing “small” colonies (SI Appendix, Table S2). As is clear from the distribution of colony sizes in SI Appendix, Table S2, varying the exact value of this threshold does not affect the classification of any genera. We then used this binary classification of maximum colony size to reconstruct the ancestor of all dorylines (SI Appendix, Fig. S3).
To infer the ancestral states of foraging behavior (SI Appendix, Fig. S5), we classified each genus as either a group raider, a mass raider, or as “unknown,” based on their four foraging characters’ states (SI Appendix, Table S2). There were no inconsistencies across the four characters for any genus, i.e., any species with one character state typical of group raiding had other character states also typical of group raiding or had no information regarding other character states. Thus, if a genus had at least two known character states we classified it as either a group or mass raider. We classified genera with information for one or no characters as “unknown.”
We then reconstructed ancestral states for maximum colony size (SI Appendix, Fig. S3), prey spectrum (SI Appendix, Fig. S4), and raiding behavior (SI Appendix, Fig. S5) using maximum parsimony and maximum likelihood with a one-parameter Markov k-state model, both implemented in Mesquite (41) (SI Appendix, Table S3). Given the paucity of character data, we interpret this reconstruction largely qualitatively, ignoring inferred character states for all intermediate nodes except the doryline most recent common ancestor.
Supplementary Material
Acknowledgments
We thank Leonora Olivos Cisneros and Stephany Valdés Rodríguez for assistance with ant maintenance, Jim Petrillo and The Rockefeller University Precision Instrumentation Technologies facility for assistance with arena construction, the Rockefeller University High Performance Computing Core for computing infrastructure, and members of the Laboratory of Social Evolution and Behavior for helpful discussion. This work was supported by a Faculty Scholars Award from the Howard Hughes Medical Institute and the National Institute of General Medical Sciences of the NIH under Award R35GM127007, both to D.J.C.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. A.G. was supported by the Human Frontier Science Program (LT001049/2015). This is Clonal Raider Ant Project paper 16.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2026534118/-/DCSupplemental.
Data Availability
All behavioral data and code for the analyses presented in this paper are available at Zenodo, DOI: 10.5281/zenodo.4708446.
References
- 1.Kronauer D. J. C., Army Ants: Nature’s Ultimate Social Hunters (Harvard University Press, 2020). [Google Scholar]
- 2.Gotwald W. H., Army Ants: The Biology of Social Predation (Cornell University Press, 1995). [Google Scholar]
- 3.Schneirla T. C., Army Ants: A Study in Social Organization (W. H. Freeman & Co. Ltd., 1971). [Google Scholar]
- 4.Hölldobler B., Wilson E. O., The Ants (Belknap Press, 1990). [Google Scholar]
- 5.Kronauer D. J. C., Recent advances in army ant biology (Hymenoptera: Formicidae). Myrmecol. News 12, 51–65 (2009). [Google Scholar]
- 6.Borowiec M. L., Generic revision of the ant subfamily Dorylinae (Hymenoptera, Formicidae). ZooKeys 608, 1–280 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks N. R., Gomez N., Goss S., Deneubourg J. L., The blind leading the blind in army ant raid patterns: Testing a model of self-organization (Hymenoptera: Formicidae). J. Insect Behav. 4, 583–607 (1991). [Google Scholar]
- 8.Deneubourg J. L., Goss S., Franks N., Pasteels J. M., The blind leading the blind: Modeling chemically mediated army ant raid patterns. J. Insect Behav. 2, 719–725 (1989). [Google Scholar]
- 9.Chadab R., Rettenmeyer C. W., Mass recruitment by army ants. Science 188, 1124–1125 (1975). [DOI] [PubMed] [Google Scholar]
- 10.Borowiec M. L., Convergent evolution of the army ant syndrome and congruence in big-data phylogenetics. Syst. Biol. 68, 642–656 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Hölldobler B., Communication, raiding behavior and prey storage in Cerapachys (Hymenoptera; Formicidae). Psyche (Stuttg.) 89, 3–23 (1982). [Google Scholar]
- 12.Wheeler W. M., The Australian ants of the ponerine tribe Cerapachyini. Proc. Am. Acad. Arts Sci. 53, 215–265 (1918). [Google Scholar]
- 13.Wilson E. O., Observations on the behavior of the cerapachyine ants. Insectes Soc. 5, 129–140 (1958). [Google Scholar]
- 14.Wilson E. O., The beginnings of nomadic and group-predatory behavior in the ponerine ants. Evolution 12, 24–36 (1958). [Google Scholar]
- 15.Ulrich Y., Saragosti J., Tokita C. K., Tarnita C. E., Kronauer D. J. C., Fitness benefits and emergent division of labour at the onset of group living. Nature 560, 635–638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gal A., Saragosti J., Kronauer D. J. C., anTraX, a software package for high-throughput video tracking of color-tagged insects. eLife 9, e58145 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra V., Gal A., Kronauer D. J. C., Data and code: Colony expansions underlie the evolution of army ant mass raiding. Zenodo. 10.5281/zenodo.4708446. Deposited 14 May 2021. [DOI] [PMC free article] [PubMed]
- 18.Gobin B., et al., A new type of exocrine gland and its function in mass recruitment in the ant Cylindromyrmex whymperi (Formicidae, Cerapachyinae). Naturwissenschaften 88, 395–399 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Mill A. E., Predation by the ponerine ant Pachycondyla commutata on termites of the genus Syntermes in Amazonian rain forest. J. Nat. Hist. 18, 405–410 (1984). [Google Scholar]
- 20.Longhurst C., Baker R., Howse P. E., Termite predation by Megaponera foetens (FAB.) (Hymenoptera: Formicidae)—Coordination of raids by glandular secretions. J. Chem. Ecol. 5, 703–719 (1979). [Google Scholar]
- 21.Bayliss J., Fielding A., Termitophagous foraging by Pachycondyla analis (Formicidae, Ponerinae) in a Tanzanian coastal dry forest. Sociobiology 39, 103–122 (2002). [Google Scholar]
- 22.Grasso D. A., Ugolini A., Visicchio R., Le Moli F., Orientation of Polyergus rufescens (Hymenoptera, Formicidae) during slave-making raids. Anim. Behav. 54, 1425–1438 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Topoff H., LaMon B., Goodloe L., Goldstein M., Social and orientation behavior of Polyergus breviceps during slave-making raids. Behav. Ecol. Sociobiol. 15, 273–279 (1984). [Google Scholar]
- 24.Schneirla T. C., Studies on army ants in Panama. J. Comp. Psychol. 15, 267–299 (1933). [Google Scholar]
- 25.Leroux J. R., Densité des colonies et observations sur les nids des dorylines Anomma nigricans Illiger (Hym. Formicidae) dans la région de Lamto (Côte d’Ivoire). Bull. Soc. Zool. Fr. 102, 51–62 (1977). [Google Scholar]
- 26.Tsuji K., Yamauchi K., Production of females by parthenogenesis in the ant, Cerapachys biroi. Insectes Soc. 42, 333–336 (1995). [Google Scholar]
- 27.Seeholzer L. F., Seppo M., Stern D. L., Ruta V., Evolution of a central neural circuit underlies Drosophila mate preferences. Nature 559, 564–569 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Y., et al., Neural evolution of context-dependent fly song. Curr. Biol. 29, 1089–1099.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Chakraborty M., Jarvis E. D., Brain evolution by brain pathway duplication. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370, 20150056 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auer T. O., et al., Olfactory receptor and circuit evolution promote host specialization. Nature 579, 402–408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krubitzer L., The magnificent compromise: Cortical field evolution in mammals. Neuron 56, 201–208 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Katz P. S., Hale M. E., “Evolution of motor systems” in Neurobiology of Motor Control: Fundamental Concepts and New Directions, Hooper S. L., Büschges A., Eds. (John Wiley & Sons, 2017), pp. 135–176. [Google Scholar]
- 33.Tunstrøm K., et al., Collective states, multistability and transitional behavior in schooling fish. PLOS Comput. Biol. 9, e1002915 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collignon B., Deneubourg J. L., Detrain C., Leader-based and self-organized communication: Modelling group-mass recruitment in ants. J. Theor. Biol. 313, 79–86 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Bonabeau E., Theraulaz G., Deneubourg J. L., Group and mass recruitment in ant colonies: The influence of contact rates. J. Theor. Biol. 195, 157–166 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Beekman M., Sumpter D. J. T., Ratnieks F. L. W., Phase transition between disordered and ordered foraging in Pharaoh’s ants. Proc. Natl. Acad. Sci. U.S.A. 98, 9703–9706 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckers R., Goss S., Deneubourg J. L., Pasteels J. M., Colony Size, communication and ant foraging strategy. Psyche (Stuttg.) 96, 239–256 (1989). [Google Scholar]
- 38.Kronauer D. J. C., Pierce N. E., Keller L., Asexual reproduction in introduced and native populations of the ant Cerapachys biroi. Mol. Ecol. 21, 5221–5235 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Trible W., et al., orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170, 727–735.e10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulrich Y., Burns D., Libbrecht R., Kronauer D. J. C., Ant larvae regulate worker foraging behavior and ovarian activity in a dose-dependent manner. Behav. Ecol. Sociobiol. 70, 1011–1018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddison W. P., Maddison D. R., Mesquite: A modular system for evolutionary analysis, Version 3.61. http://www.mesquiteproject.org/. Accessed 20 May 2021.
- 42.Schneirla T. C., Reyes A. Y., Raiding and related behaviour in two surface-adapted species of the old world doryline ant, Aenictus. Anim. Behav. 14, 132–148 (1966). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All behavioral data and code for the analyses presented in this paper are available at Zenodo, DOI: 10.5281/zenodo.4708446.