Significance
Ocean warming has caused catastrophic losses of corals on reefs worldwide and is intensifying faster than the adaptive rate of most coral populations that remain. Human interventions, such as propagation of heat-resistant corals, may help maintain reef function and delay further devastation of these valuable ecosystems as society confronts the climate crisis. However, exposing adult corals to a complex suite of new environmental conditions could lead to tradeoffs that alter their heat stress responses, and empirical data are needed to test the utility of this approach. Here, we show that corals transplanted to novel reef conditions did not exhibit changes in their heat stress response or negative fitness tradeoffs, supporting the inclusion of this approach in our management arsenal.
Keywords: climate change, coral bleaching, ocean warming, adaptive management, restoration
Abstract
Urgent action is needed to prevent the demise of coral reefs as the climate crisis leads to an increasingly warmer and more acidic ocean. Propagating climate change–resistant corals to restore degraded reefs is one promising strategy; however, empirical evidence is needed to determine whether stress resistance is affected by transplantation beyond a coral’s native reef. Here, we assessed the performance of bleaching-resistant individuals of two coral species following reciprocal transplantation between reefs with distinct pH, salinity, dissolved oxygen, sedimentation, and flow dynamics to determine whether heat stress response is altered following coral exposure to novel physicochemical conditions in situ. Critically, transplantation had no influence on coral heat stress responses, indicating that this trait was relatively fixed. In contrast, growth was highly plastic, and native performance was not predictive of performance in the novel environment. Coral metabolic rates and overall fitness were higher at the reef with higher flow, salinity, sedimentation, and diel fluctuations of pH and dissolved oxygen, and did not differ between native and cross-transplanted corals, indicating acclimatization via plasticity within just 3 mo. Conversely, cross-transplants at the second reef had higher fitness than native corals, thus increasing the fitness potential of the recipient population. This experiment was conducted during a nonbleaching year, so the potential benefits to recipient population fitness are likely enhanced during bleaching years. In summary, this study demonstrates that outplanting bleaching-resistant corals is a promising tool for elevating the resistance of coral populations to ocean warming.
The global climate crisis is threatening the survival of coral reef ecosystems around the world. As climate change increases the temperature of the world’s oceans (1), marine heatwaves are becoming increasingly frequent (2), leading to widespread coral bleaching (3, 4), a heat stress response in which the coral–algal symbiosis breaks down and the algae (dinoflagellates in the family Symbiodiniaceae) are expelled from the host (5, 6). This dysbiosis has myriad negative consequences, ranging from declines in coral calcification and reproduction to extensive coral mortality (7–11). These bleaching-associated outcomes affect the function of the entire reef ecosystem, as coral biomineralization is necessary to build and maintain the physical framework that is required to support the immense biodiversity typical of a healthy coral reef (12–14). Deterioration of the reef structure is also being exacerbated by ocean acidification (15), another climate change stressor that has also led to declines in net ecosystem calcification (16–18). An important ongoing question is whether coral populations have the capacity to acclimatize or adapt to climate change stressors fast enough to avoid further catastrophic losses (19) and whether human intervention can enhance this process to help corals keep pace with a rapidly changing environment (20). Encouragingly, there is evidence that coral populations are becoming more resistant to bleaching during heat stress (21, 22); however, this nominal improvement may be coming at the expense of certain species, as only the more tolerant taxa remain following the selective sieve of major bleaching mortality events (11, 23–25).
Action is urgently needed to protect and promote coral reef resilience in the face of climate change (26), as even the most optimistic emissions reductions (e.g., carbon neutral by 2050) will be accompanied by decades of continued ocean warming (2). Adaptive management strategies, such as selective propagation of climate change–resistant corals (e.g., via assisted gene flow, selective breeding), have recently gained attention as a potential intervention to prevent the extinction of reefs and species (20, 26–29). Propagation of individuals with desired phenotypes (e.g., rapid growth, bleaching resistance) for coral reef recovery and restoration is a promising approach; however, the utility of these trait-guided efforts depends on the survival of coral transplants, which requires strong local measures to promote reef health (e.g., limiting pollution and overfishing), as well as the retention of beneficial traits following transplantation to novel physicochemical and ecological conditions and their integration into the population; otherwise, fitness gains due to increased survival during heatwaves may be cancelled or even outweighed by factors such as compensatory declines in growth and/or reproductive output. Determining the feasibility of these approaches therefore requires improved knowledge of the fundamental mechanisms of coral acclimatization, since we do not know whether or for how long these phenotypes are retained following exposure to novel environmental regimes within or across generations. Rigorous experimental evaluation that incorporates the complexity of the natural reef environment is therefore needed to address this question, the results of which are important not only for restoration but also for understanding the capacity for coral populations to withstand rapid environmental change resulting from anthropogenic activities.
A first step in testing whether bleaching resistance is the result of local adaptation or acclimatization is to identify individuals with higher temperature thresholds for bleaching within a population. Bleaching-resistant corals are often found in locations with higher mean temperatures [e.g., shallow inshore reefs with restricted water flow (30–32)] or those with larger magnitude or higher frequency fluctuations in temperature than surrounding reefs (33–36), though not always (37). Reefs with conditions that promote these local threshold maxima are likely excellent resources for selecting the most bleaching-resistant genets of the various species found in a region but only if elevated heat tolerance is retained when environmental conditions change. There is evidence of local adaptation to different thermal regimes between populations (38–41) and that heat tolerance can be heritable (42–45), yet much remains to be learned about mechanisms determining bleaching tolerance within populations. In particular, acclimatization can contribute to gains in heat tolerance (34), yet adult corals can also maintain their relative (if not absolute) bleaching performance following acclimatization in common garden settings (41, 46). It is therefore critical to understand the relationship between acclimatization and genotype-specific fixed effects in determining coral bleaching thresholds, as both these mechanisms influence the persistence of adaptive traits through time and space (47).
Coral bleaching events provide an opportunity to identify bleaching-resistant individuals within populations already exhibiting higher mean bleaching thresholds and have the advantage of allowing assessment of relative performance in a natural context. Here, we identified bleaching-resistant individuals of two important reef-building species, Montipora capitata and Porites compressa, from a site with higher bleaching thresholds relative to nearby reefs (48). Bleaching-resistant coral genets were identified here as those that remained fully pigmented while as much as 79% of live coral bleached during the peak of a coral bleaching event that occurred in a Kāne‘ohe Bay, Hawai‘i in 2015 (49), the second of two consecutive annual bleaching events in the region (50). After allowing for one year of recovery from the heat stress event, the effects of acclimatization to a novel physicochemical environment on coral acute heat stress response and fitness were tested by reciprocally transplanting ramets of each genet between two patch reefs with distinct environmental conditions. In addition, the physiological plasticity of each species was examined by measuring coral survival, growth, metabolism, tissue energetics, and feeding rates in their native versus cross-transplanted environments at 3 and 6 mo posttransplantation. These experiments are a critical step toward understanding the biological basis and utility of selecting and propagating climate change–resistant corals for enhancing coral reef resilience to climate change.
Results
Distinct Physicochemical Dynamics Characterized Each Reef.
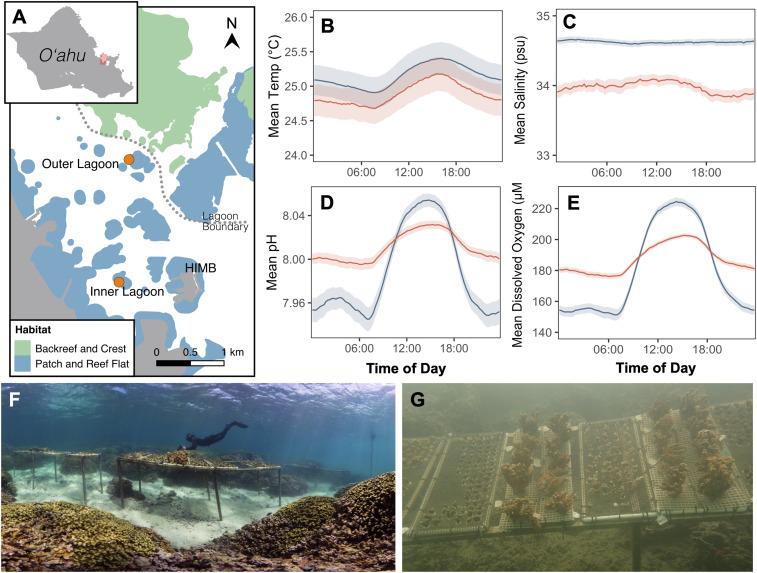
Temperature and light dynamics were similar between the two patch reefs (Fig. 1 A and B and SI Appendix, Table S1 and Figs. S1 and S2), as were mean pH, dissolved oxygen (DO), and light (SI Appendix, Table S1). In contrast, salinity differed between the two sites (Fig. 1C and SI Appendix, Fig. S1), and diel fluctuations in pH and DO were 2.92-fold and 2.68-fold greater at the Outer Lagoon than the Inner Lagoon reef, respectively (Fig. 1 D and E and SI Appendix, Fig. S1 and Table S1). Sedimentation rates were 8.27-fold higher at the Outer Lagoon (0.324 ± 0.066 g · day−1) than the Inner Lagoon reef (0.039 ± 0.003 g · day−1; SI Appendix, Table S1), and relative flow rates were approximately twofold higher at the Outer Lagoon reef (SI Appendix, Table S1).
Fig. 1.
(A) Map of the southern region of Kāneʻohe Bay on the island of Oʻahu (Inset) indicating locations of the Inner and Outer Lagoon reefs. Daily physicochemical dynamics of the seawater above the reef benthos at the Inner Lagoon (orange) versus Outer Lagoon (blue) reefs show the mean diel cycles of (B) seawater temperature, (C) salinity, (D) pH, and (E) DO. Diel cycles are shown as means ± 95% CI. Experimental setup at (F) the Outer Lagoon reef (image credit: The Ocean Agency) and (G) the Inner Lagoon reef.
Corals Exhibited High Phenotypic Plasticity in Response to Novel Reef Environments.
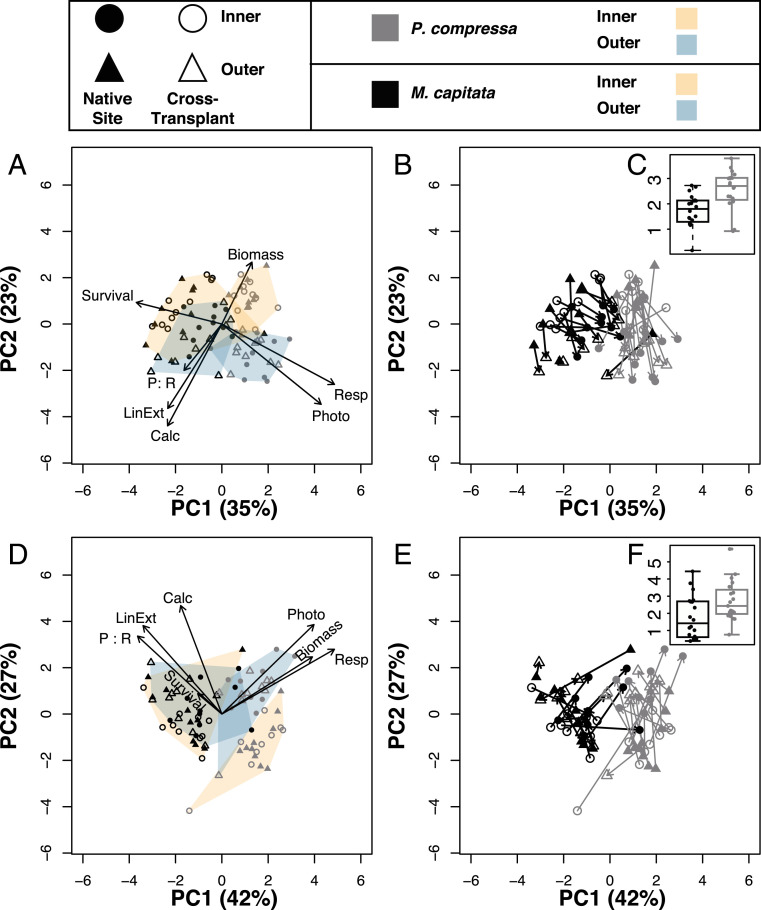
Significant differences in coral phenotypes were observed between the two destinations and species at 3 and 6 mo following transplantation (P = 0.001; PERMANOVA; Fig. 2 A and D). There was also a significant interaction between species and destination at both time points (P = 0.003 for T3 and P = 0.001 for T6; permutational multivariate analysis of variance [PERMANOVA]). In contrast, origin was not a significant factor at either time point, indicating that both species had acclimatized to their destination reef as early as 3 mo following transplantation. Phenotypic plasticity, quantified as the principal component (PC) distance between each genet’s native versus cross-transplanted phenotype, did not differ between the two origin populations for either species. Plasticity did differ between species, with P. compressa exhibiting higher phenotypic plasticity than M. capitata at both T3 (P < 0.0004) and T6 (P = 0.017) (Fig. 2 B, C, E, and F). The traits contributing most strongly to differences between destinations included metabolic rates and growth, which were higher at the Outer Lagoon reef after 3 mo, whereas biomass and survival were higher at the Inner Lagoon reef (Fig. 2A). All traits were higher at the Outer Lagoon reef 6 mo posttransplantation (Fig. 2D). Univariate analyses of each response metric indicated that both species exhibited variation between genets in the magnitude and in some cases direction of their response to each environment, indicating the presence of genotype–environment interactions (SI Appendix, Figs. S3–S8 and Tables S2–S19).
Fig. 2.
Principal component analysis of coral performance following 3 (A–C) and 6 mo (D–F) of transplantation. Polygons outline the ordination groups, with Inner Lagoon in orange and Outer Lagoon in blue, whereas vectors in A and D indicate the loadings of the phenotypic variables to the PCs, with length of arrow signifying strength of loading. Plasticity, calculated as the distance in principal component space between each genet’s native phenotype (filled symbols) versus cross-transplanted phenotype (open symbols), are indicated by lines in B and E. The boxplots and data points for plasticity values of each species are shown in C and F.
Coral Fitness Differed between Reefs but No Evidence of Site Specialization or Tradeoffs.
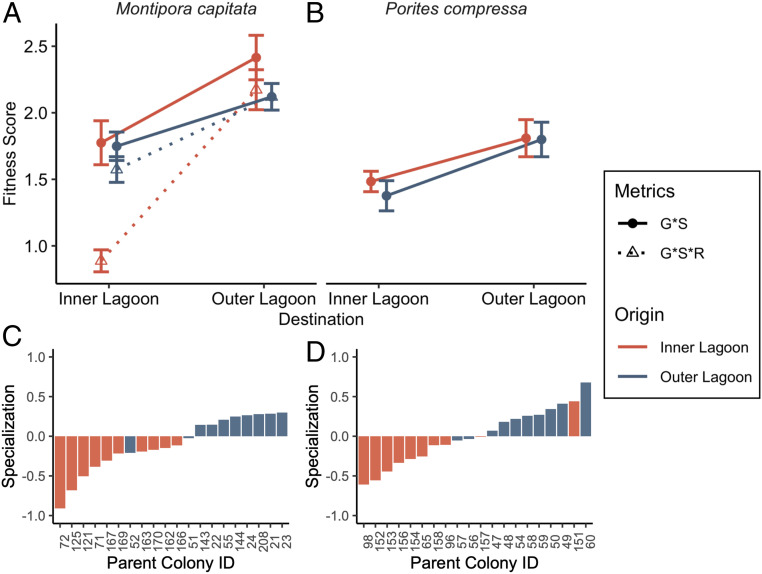
Improvements in performance of M. capitata cross-transplanted to the Outer Lagoon reef did not incur a tradeoff with reproduction, as there was no relationship between growth and fecundity (SI Appendix, Fig. S9). In the reverse direction, cross-transplants of M. capitata at the Inner Lagoon reef that had a decline in overall performance showed a strong positive relationship between growth and reproduction (P < 0.005, r2 = 0.700; SI Appendix, Fig. S9), whereas native corals at both reefs showed no relationship between growth and reproduction, indicating that there were no negative tradeoffs between growth and reproduction for any of the four transplant histories. Because the fitness of coral transplants depends on their ability to survive and reproduce in their new environment and because reproductive output of colonial organisms like corals is positively correlated with size (51), an integrative metric of coral fitness was calculated as the product of survival, growth, and (for M. capitata only) reproductive success of each genet. Corals at the Outer Lagoon reef showed significantly higher fitness scores than corals at the Inner Lagoon reef, but there were no differences in fitness between native and cross-transplanted corals at the Outer Lagoon reef (Fig. 3 A and B and SI Appendix, Tables S20 and S21). In contrast, at the Inner Lagoon reef, cross-transplants of M. capitata displayed higher fitness than native corals but only when accounting for differences in reproductive success (Fig. 3A and SI Appendix, Tables S20 and S21). Local specialization of each genet was calculated by considering its relative fitness in its native versus cross-transplanted environment. In general, corals exhibited positive specialization values at the Outer Lagoon reef, whereas corals native to the Inner Lagoon reef showed negative specialization values (Fig. 3 C and D), indicating corals performed better at the Outer Lagoon reef even when it was not their native environment. One genet of P. compressa native to the Inner Lagoon had a positive local specialization score and was the only genet of either species native to the Inner Lagoon that had higher fitness at its native reef (Fig. 3D).
Fig. 3.
Fitness score for (A) M. capitata and (B) P. compressa. Fitness score is a product of net growth and survival (G*S; solid lines) and for M. capitata was also calculated as the product of net growth, survival, and reproductive success (G*S*R; dashed lines). n = 10; error bars indicate SEM. Magnitude of local specialization for each genet of (C) M. capitata and (D) P. compressa. Local specialization values are defined as the difference in fitness score (G*S only) of a genet at its origin and destination reef, divided by the mean fitness score of all conspecifics at the destination reef. Positive values indicate local site specialization; negative values indicate destination reef favorable.
Coral Acute Heat Stress Response Was Unaffected by Transplantation.
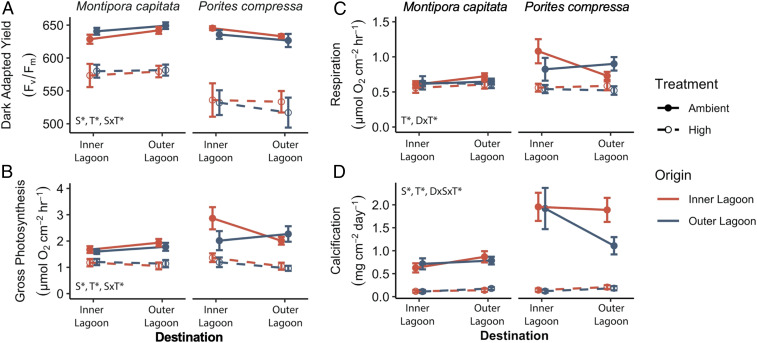
At the initiation of the acute heat stress aquarium experiment (maximum daily temperature of 27 °C across all treatments; SI Appendix, Fig. S10), there were no significant differences in performance metrics (i.e., light-enhanced dark respiration [LEDR], Fv/Fm, and gross photosynthesis) between species, treatments, origins, or destinations (SI Appendix, Fig. S11). At the end of the 10-d heat stress, the heat treatment reached a daily maximum of 32 °C (maximum monthly mean 4 °C), while the ambient treatment reached a daily maximum of 28 °C (SI Appendix, Fig. S10). Linear mixed models (SI Appendix, Table S22) indicated that temperature was a significant factor across all parameters examined, with corals in the heat treatment exhibiting declines in photochemical yield (Fig. 4A and SI Appendix, Table S23), metabolic rates (Fig. 4 B and C and SI Appendix, Table S24), and calcification rates (Fig. 4D and SI Appendix, Table S25). Origin was not a significant factor for any of the metrics examined (Fig. 4 and SI Appendix, Tables S23–S25), indicating that there was no decline in the heat stress response capacity of cross-transplanted corals relative to ramets that remained at their native reef, and thus, exposure to a novel environment did not alter heat stress responses of these bleaching-resistant corals. Overall, P. compressa showed the greatest declines in performance metrics in response to heat stress, with declines in photochemical yield, photosynthesis, and calcification exceeding those of M. capitata (Fig. 4 and SI Appendix, Tables S23–S25).
Fig. 4.
Coral performance following acute heat stress (high temperature; 32 °C) versus controls (ambient temperature; 27 to 28 °C). (A) Photosynthetic efficiency (dark-adapted yield; Fv/Fm), (B) gross photosynthesis rates, (C) LEDR rates, and (D) calcification rates. n = 8 to 10; error bars indicate SEM. Inset indicates statistically significant fixed effects (S, species; T, temperature; D, destination).
Discussion
Coral Heat Stress Responses Unaffected by Transplantation.
Transplantation of bleaching-resistant corals to a novel environment in situ did not alter their heat stress response, despite transplants exhibiting high levels of phenotypic plasticity for other traits. Because bleaching-resistant corals tend to have lower mortality (49) and higher reproductive success (7, 52, 53) than bleaching-sensitive conspecifics following a bleaching event, they have a clear selective advantage during and in the years following these events. Harnessing these natural advantages by propagating bleaching-resistant individuals is a promising approach to maintain reef function increasing the bleaching resistance of a population using native (i.e., endemic, local) coral stocks. Furthermore, relative bleaching resistance of M. capitata and P. compressa has persisted through multiple in situ bleaching events (54, 55), indicating that bleaching resistance is retained and will likely continue during future heatwaves of similar magnitude. This, in combination with heat stress response being unaffected by both transplantation and acclimatization to a complex in situ environment, makes bleaching resistance a promising trait for selecting individuals to enhance resistance of coral populations to climate change. Finally, because M. capitata and P. compressa represent divergent lineages of two globally distributed coral genera, these patterns may be shared with species on reefs around the world.
Fitness Consequences of Coral Acclimatization to Novel Environments.
The identification of negative tradeoffs during acclimatization is important for informing trait-guided restoration. Indeed, corals acclimatizing to new thermal regimes can exhibit declines in growth and/or reproduction (38, 40), reducing the potential benefits of their introduction. Here, despite corals exhibiting high levels of phenotypic plasticity across a range of traits including metabolism, feeding, growth, and reproduction following transplantation to reefs with distinct physicochemical conditions, negative tradeoffs were not observed for either species. In general, corals at the Outer Lagoon performed better overall, and improvements in any one trait did not come at the cost of another. These results are consistent with data from other reef systems that demonstrate an absence of tradeoffs between bleaching and reproduction (56) and between resistance traits against multiple stressors (57) and holds promise that these bleaching-resistant genets may also withstand additional stressors. Critically, bleaching-resistant cross-transplants maintained fitness equal to or higher than that of native corals, despite having acclimatized to substantially different environmental regimes. This indicates that these corals would have a neutral or positive effect on the fitness of recipient populations, even during a nonbleaching year, and would likely elevate the recipient population’s fitness during future heatwaves due to their greater bleaching resistance. The duration of elevated fitness in cross-transplants, which lasted at least 11 mo in this study, remains unknown and could be the result of a temporary carryover of the energetic benefits of having originated from a more favorable reef environment. This potential lag effect, as well as seasonal cycles, have been shown to affect corals on an annual cycle (e.g., refs. 58 and 59), and future work is needed to assess multiyear influences on coral fitness. However, even if this carryover were transient, the long-term fitness effects for recipient populations are likely net positive due to the transplants’ higher expected relative performance during increasingly common marine heatwaves. These results are a necessary first step to validate trait-guided approaches in reef restoration and adaptive management. Additional work is needed to determine the persistence of these traits in the population, which requires they be both heritable, as has been shown for several species (42–45), and introduced in sufficient abundance. Initial studies indicate that stress-resistant corals must be introduced in numbers equivalent to at least 2 to 5% of the population per year for several decades in order to achieve adaptive gains in heat tolerance that can keep pace with climate change (60). As such, work is needed to scale up these approaches if they are to have a meaningful impact on coral reef resistance to ocean warming. However, this approach cannot work in isolation, and it is imperative that investments in adaptive management are supported by strong local measures to maintain water quality and limit overfishing (61, 62). In addition, these measures cannot replace global measures urgently needed to reduce carbon emissions and slow the intensification of climate change.
Genotype–Environment Effects.
Despite consistently higher mean coral performance at the Outer Lagoon reef, in many cases, these differences in performance between the two reefs for an individual metric were not significant due to a strong genotype–environment (GxE) effect. Growth in particular showed a strong GxE effect, aligning with recent work cautioning against using growth alone as a predictive trait for future coral performance as it can vary across time (63, 64). Furthermore, heat tolerance in a stressful environment does not ensure rapid growth in a less stressful environment (65). Our results do support the need for a genetically diverse “planting stock” to account for the wide range of expressed phenotypes in different reef environments (66). In summary, this study indicates that heat stress response was not plastic in M. capitata and P. compressa, and past bleaching resistance is thus likely indicative of future coral performance.
Biologically Guided Strategies for Coral Reef Restoration.
There is mounting evidence that the current rate of ocean warming is outpacing the “natural” dispersal rate of heat-tolerant genets and the generation times required for adaptation to increase heat tolerance of coral populations (67). This reality underscores the need for scientifically informed human interventions in management and restoration. Here, we show that the heat stress response of bleaching-resistant corals was unaltered following transplantation into novel environments, and this was accomplished without incurring fitness costs. While more work is needed to determine how well bleaching resistance persists across generations, these results support the use of active restoration for promoting climate resilient reefs. Additional traits are also important when selecting individuals for restoration [e.g., ocean acidification tolerance, disease resistance, and genetic diversity (68)], although the plasticity of many of these traits are not well described. Encouragingly, relative growth during acidification stress is consistent in several coral species (69) and thus, along with bleaching resistance, may be a useful selection marker for promoting climate change resilient reefs via active management.
Site selection for nurseries and outplanting is also an important consideration to maximize restoration success, as water quality is critical for outplant survival (62) and can be managed at the local level. Here, we found that the reef with the greatest water flow, diel physicochemical variation, and distance from land resulted in higher coral growth and fitness. Sufficient water flow is generally beneficial for coral performance across reef systems (70), and both flow and temperature variability can mitigate bleaching responses (21, 36, 70), indicating that these may be generalizable environmental characteristics of reefs that promote coral fitness and bleaching resistance (although, for exception, see ref. 37). Our results highlight the importance of local management of water quality and ecosystem health (e.g., limiting fishing pressure) and the need to select sites for nurseries and outplanting that promote high coral fitness, as this could accelerate the successful establishment of corals. Furthermore, in situ nursery sites that promote faster growth would provide obvious logistical benefits, leading to shorter residence times for individuals and greater yields for outplanting. Assisted gene flow using climate change–resistant genets could complement traditional conservation measures such as marine protected areas, which could provide favorable habitat for stress-resistant outplants, and in coordination with less directed approaches [e.g., adaptation networks (71)] to preserve genetic diversity. Restoration targets may include reefs damaged directly by human activity (e.g., ship groundings, dredging, etc.) or indirectly via bleaching-related mortality. While the former may not always be “high-fitness” sites, this study indicates that using corals from favorable sites or nurseries may still benefit the recipient population at a “low-fitness” reef because: 1) corals from a “high-fitness” reef had higher reproductive success than native corals, likely boosting the fitness of the recipient population, and 2) introduction of bleaching-resistant individuals would likely improve the fitness of that population during increasingly frequent marine heatwaves. Another strategy is to introduce bleaching-resistant genotypes into populations with lower bleaching thresholds (e.g., cooler or more stable mean temperatures), and evidence is accumulating that relative bleaching performance is maintained following acclimatization to both aquarium (40) and distinct in situ conditions [this study (46)]. However, caution must be taken before moving heat-tolerant corals beyond the thermal regime to which they are adapted, as corals introduced to cooler climates can suffer significant cold stress in winter, exhibiting reductions in growth, reproduction, and survival (38, 40). Promisingly, this study showed that bleaching-resistant corals exhibited increased fitness (growth, reproduction, and survival) following transplantation despite the energetic demands of acclimatizing to a complex suite of environmental conditions, a necessary prerequisite for assisted gene flow to successfully introduce heat-resistant alleles into recipient coral populations. Future work is needed to determine whether elevated fitness and bleaching resistance persist across generations and to increase the scalability of such efforts. Coral outplanting efforts are already occurring at a scale of tens of thousands of outplants each year in some regions (72), and initial analyses of these types of coral restoration efforts indicate positive returns on that investment for a diversity of coral species, indicating that this approach is both scalable and economically feasible (73). When accompanied by strong local measures to mitigate nonclimate related stressors, adaptive reef management could preserve species diversity and promote reef resilience to climate change, temporarily buying these invaluable ecosystems time as society struggles with reigning in the current climate catastrophe.
Materials and Methods
Site Selection and Characterization.
The coral-dominated patch reefs in the Kāneʻohe Bay lagoon (Fig. 1A) are exposed to distinct seawater conditions that result from spatial gradients within the lagoon driven by differences in seawater residence times (74), freshwater and nutrient input (75), and human influence (76). Here, we characterized the physicochemical conditions of two patch reefs with contrasting seawater residence times and terrestrial influence: 1) a nearshore Inner Lagoon reef (21.4343°N, 157.7991°W) with long seawater residence times (30+ days) located 0.75 km from shore and 2) an offshore Outer Lagoon reef (21.4516°N, 157.7966°W) with short seawater residence times (<1 d) located 1.6 km from shore (Fig. 1A). Seawater temperature, salinity, pH, DO, and photosynthetically active radiation were measured every 15 min above the reef benthos at each site (2 m depth). Sedimentation rates were measured every 2 wk, and relative water flow was measured at least monthly at each reef using the clod card dissolution technique (77).
Reciprocal Transplant Setup.
During the peak of the 2015 coral bleaching event in Kāneʻohe Bay, bleaching prevalence for each of the two dominant reef-building corals, M. capitata and P. compressa, was up to 69 to 87% of the population, respectively (SI Appendix, Fig. S12). At that time, 10 bleaching-resistant (i.e., fully pigmented) colonies of each species were visually identified and tagged at both the Inner and Outer Lagoon reefs, and their health was monitored for the following year (SI Appendix, Fig. S12; (49)). One year later, a portion of each colony was collected from the reef and fragmented into ramets, and a reciprocal transplant was initiated where half of the ramets from each colony remained at their origin reef while the other half were cross-transplanted to the other reef.
Coral Performance.
A total of 3 mo following transplantation, coral survival was quantified for all fragments, and skeletal accretion, linear extension, and dark-adapted photochemical efficiency (Fv/Fm) were quantified for 10 fragments per parent per transplant treatment (n = 800). Half of these remained in the field, and the other half were assessed for photosynthesis and LEDR rates, tissue biomass and lipid content, and skeletal surface area. A subset of these (one per parent per history; n = 80) were used to quantify heterotrophic feeding rates. All of the above measures were repeated for the remaining 400 fragments at 6 mo posttransplantation. Reproductive output of M. capitata (one fragment per parent per history; n = 40) was quantified at 9 to 11 mo posttransplantation by measuring the volume of egg–sperm bundles released from each individual across all nights in the months of June, July, and August and was normalized to planar surface area of live tissue.
Fitness and Local Specialization.
A cumulative metric of coral fitness (i.e., fitness score) was calculated as the product of survival, growth (skeletal mass), and, for M. capitata only, reproductive success. The proportion of individual fragments from each genet and history that survived at 6 mo was multiplied by their respective growth (represented as the total increase in skeletal mass across the 6 mo), and, for M. capitata only, by the proportion of genets from each history that successfully reproduced following transplantation. This fitness score (W) was used to calculate local specialization (S) with the difference in fitness (W) between the home and transplanted environment for each genet divided by the mean fitness of all corals of that species at that transplant site, regardless of origin (as in refs. 78 and 79).
Acute Heat Stress Challenge.
A subset of coral fragments (two per genet per transplant history; 160 fragments total) were used for an acute heat stress experiment following 6 mo of acclimatization. The high-temperature treatment was ramped 1 °C per day for 6 d, reaching a maximum of 32.0 °C (maximum monthly mean [MMM] + 4 °C) for 5 d (SI Appendix, Fig. S10). Maximum temperatures in the ambient treatment ranged from 26 to 28.6 °C over the course of the experiment. Coral skeletal accretion, photochemical efficiency (Fv/Fm), photosynthesis, and respiration rates for each fragment were determined at the beginning and end of the experiment as described above.
Statistical Analyses.
Statistical analyses were conducted in R Statistical Programming (80). Principal component analysis (PCA) was used to determine the percent variance explained by seven physiological variables (biomass, calcification, linear extension, gross photosynthetic rate, LEDR, P:R, and survival) in the separation of the transplant groups. PCA was conducted on the scaled and centered data using the prcomp function in the Vegan package (81). Phenotypic plasticity of each genet was calculated as the PCA distance between that genet’s native versus cross-transplanted phenotype in two-dimensional trait space (i.e., PC1 versus PC2), which accounts for correlations among traits (as in ref. 82). Differences in plasticity were tested using a two-way ANOVA. Univariate analyses were performed using linear mixed-effect models and are described in detail in SI Appendix, Supplementary Methods and Table S1.
Supplementary Material
Acknowledgments
This work would not have been possible without the support of volunteers in the field, and we would particularly like to thank Yanitza Grantcharska, Aileen Maldonado, Dyson Chee, and the Hawaii Institute of Marine Biology boating and diving team led by Jason Jones. We also recognize technical help from Brian Glazer and Stanley Lio with sensors. This work was supported by the Paul G. Allen Family Foundation (to R.D.G.), the University of Pennsylvania (K.L.B.), and NSF awards OCE-1923743 to K.L.B., OCE-PRF 1323822 to H.M.P., and NSF Graduate Research Fellowships to A.S.H. and E.A.L.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025435118/-/DCSupplemental.
Data Availability
All raw data and scripts have been deposited in Zenodo and are publicly available (DOI: 10.5281/zenodo.4315627).
References
- 1.Johnson G. C., Lyman J. M., Warming trends increasingly dominate global ocean. Nat. Clim. Chang. 10, 757–761 (2020). [Google Scholar]
- 2.Frölicher T. L., Fischer E. M., Gruber N., Marine heatwaves under global warming. Nature 560, 360–364 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Hughes T. P., et al., Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Hoegh-Guldberg O., et al., Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Jokiel P. L., “Temperature stress and coral bleaching” in Coral Health and Disease, Rosenberg E., Loya Y., Eds. (Springer Berlin Heidelberg, 2004), pp. 401–425. [Google Scholar]
- 6.Oakley C. A., Davy S. K., “Cell biology of coral bleaching” in Coral Bleaching: Patterns, Processes, Causes and Consequences, van Oppen M. J. H., Lough J. M., Eds. (Ecological Studies, Springer International Publishing, 2018), pp. 189–211. [Google Scholar]
- 7.Ward S., Harrison P., Hoegh-Guldberg O., Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. Proceedings of the 9th International Coral Reef Symposium 2, 1123-1128 (2000).
- 8.Baird A. H., Marshall P. A., Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 237, 133–141 (2002). [Google Scholar]
- 9.Baker A. C., Glynn P. W., Riegl B., Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008). [Google Scholar]
- 10.Hughes T. P., et al., Global warming impairs stock-recruitment dynamics of corals. Nature 568, 387–390 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Hughes T. P., et al., Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Fordyce A. J., Ainsworth T. D., Heron S. F., Leggat W., Marine heatwave hotspots in coral reef environments: Physical drivers, ecophysiological outcomes, and impact upon structural complexity. Front. Mar. Sci. 6, 498 (2019). [Google Scholar]
- 13.Leggat W. P., et al., Rapid coral decay is associated with marine heatwave mortality events on reefs. Curr. Biol. 29, 2723–2730.e4 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Hughes T. P., et al., Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 9, 40–43 (2019). [Google Scholar]
- 15.Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A., Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Eyre B. D., et al., Coral reefs will transition to net dissolving before end of century. Science 359, 908–911 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Andersson A. J., Gledhill D., Ocean acidification and coral reefs: Effects on breakdown, dissolution, and net ecosystem calcification. Annu. Rev. Mar. Sci. 5, 321–348 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Albright R., et al., Reversal of ocean acidification enhances net coral reef calcification. Nature 531, 362–365 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Edmunds P. J., Gates R. D., Acclimatization in tropical reef corals. Mar. Ecol. Prog. Ser. 361, 307–310 (2008). [Google Scholar]
- 20.van Oppen M. J. H., Oliver J. K., Putnam H. M., Gates R. D., Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. U.S.A. 112, 2307–2313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sully S., Burkepile D. E., Donovan M. K., Hodgson G., van Woesik R., A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coles S. L., et al., Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 6, e5347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loya Y., et al., Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001). [Google Scholar]
- 24.McClanahan T. R., The relationship between bleaching and mortality of common corals. Mar. Biol. 144, 1239–1245 (2004). [Google Scholar]
- 25.Edmunds P. J., Implications of high rates of sexual recruitment in driving rapid reef recovery in Mo’orea, French Polynesia. Sci. Rep. 8, 16615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medicine National Academies of Sciences Engineering , A Decision Framework for Interventions to Increase the Persistence and Resilience of Coral Reefs (National Academies Press, 2019). [Google Scholar]
- 27.van Oppen M. J. H., et al., Shifting paradigms in restoration of the world’s coral reefs. Glob. Change Biol. 23, 3437–3448 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Anthony K. R. N., et al., Operationalizing resilience for adaptive coral reef management under global environmental change. Glob. Change Biol. 21, 48–61 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anthony K. R. N., et al., Interventions to help coral reefs under global change-A complex decision challenge. PLoS One 15, e0236399 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jokiel P. L., Brown E. K., Global warming, regional trends and inshore environmental conditions influence coral bleaching in Hawaii. Glob. Change Biol. 10, 1627–1641 (2004). [Google Scholar]
- 31.Woesik R., et al., Climate-change refugia in the sheltered bays of Palau: Analogs of future reefs. Ecol. Evol. 2, 2474–2484 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castillo K. D., Helmuth B. S. T., Influence of thermal history on the response of Montastraea annularis to short-term temperature exposure. Mar. Biol. 148, 261–270 (2005). [Google Scholar]
- 33.Oliver T. A., Palumbi S. R., Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30, 429–440 (2011). [Google Scholar]
- 34.Palumbi S. R., Barshis D. J., Traylor-Knowles N., Bay R. A., Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Schoepf V., Stat M., Falter J. L., McCulloch M. T., Limits to the thermal tolerance of corals adapted to a highly fluctuating, naturally extreme temperature environment. Sci. Rep. 5, 17639 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Safaie A., et al., High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 9, 1671 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klepac C. N., Barshis D. J., Reduced thermal tolerance of massive coral species in a highly variable environment. Proc. Biol. Sci. 287, 20201379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howells E. J., Berkelmans R., van Oppen M. J. H., Willis B. L., Bay L. K., Historical thermal regimes define limits to coral acclimatization. Ecology 94, 1078–1088 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Kenkel C. D., et al., Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol. Ecol. 22, 4335–4348 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Schoepf V., et al., Stress-resistant corals may not acclimatize to ocean warming but maintain heat tolerance under cooler temperatures. Nat. Commun. 10, 4031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barshis D. J., Birkeland C., Toonen R. J., Gates R. D., Stillman J. H., High-frequency temperature variability mirrors fixed differences in thermal limits of the massive coral Porites lobata. J. Exp. Biol. 221, jeb188581 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Kenkel C. D., Setta S. P., Matz M. V., Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity 115, 509–516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dixon G. B., et al., CORAL REEFS. Genomic determinants of coral heat tolerance across latitudes. Science 348, 1460–1462 (2015). [DOI] [PubMed] [Google Scholar]
- 44.Quigley K. M., Bay L. K., van Oppen M. J. H., Genome-wide SNP analysis reveals an increase in adaptive genetic variation through selective breeding of coral. Mol. Ecol. 29, 2176–2188 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Yetsko K., et al., Genetic differences in thermal tolerance among colonies of threatened coral Acropora cervicornis: Potential for adaptation to increasing temperature. Mar. Ecol. Prog. Ser. 646, 45–68 (2020). [Google Scholar]
- 46.Morikawa M. K., Palumbi S. R., Using naturally occurring climate resilient corals to construct bleaching-resistant nurseries. Proc. Natl. Acad. Sci. U.S.A. 116, 10586–10591 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drury C., Resilience in reef-building corals: The ecological and evolutionary importance of the host response to thermal stress. Mol. Ecol. 29, 448–465 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Schoepf V., Jury C. P., Toonen R. J., McCulloch M. T., Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proc. Biol. Sci. 284, 20172117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuda S., et al., Coral bleaching susceptibility is predictive of subsequent mortality within but not between coral species. Front. Ecol. Evol. 8, 1–14 (2020). [Google Scholar]
- 50.Bahr K. D., Rodgers K. S., Jokiel P. L., Impact of three bleaching events on the reef resiliency of Kāne‘ohe Bay, Hawai’i. Front. Mar. Sci. 4, 398 (2017). [Google Scholar]
- 51.Hall V. R., Hughes T. P., Reproductive strategies of modular organisms: Comparative studies of reef- building corals. Ecology 77, 950–963 (1996). [Google Scholar]
- 52.Fisch J., Drury C., Towle E. K., Winter R. N., Miller M. W., Physiological and reproductive repercussions of consecutive summer bleaching events of the threatened Caribbean coral Orbicella faveolata. Coral Reefs 38, 863–876 (2019). [Google Scholar]
- 53.Howells E. J., et al., Species-specific trends in the reproductive output of corals across environmental gradients and bleaching histories. Mar. Pollut. Bull. 105, 532–539 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Ritson-Williams R., Gates R. D., Coral community resilience to successive years of bleaching in Kane‘ohe Bay, Hawai’i. Coral Reefs 39, 757–769 (2020). [Google Scholar]
- 55.Innis T., et al., Marine heatwaves depress metabolic activity and impair cellular acid-base homeostasis in reef-building corals regardless of bleaching susceptibility. Glob. Change Biol. 10.1111/gcb.15622 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Lenz E. A., “Identifying opportunities for resilience in reef-building corals as ocean warming continues,” PhD dissertation, University of Hawai’i at Manoa, Honolulu, Hawaii (2020) pp. 1–199.
- 57.Wright R. M., et al., Positive genetic associations among fitness traits support evolvability of a reef-building coral under multiple stressors. Glob. Change Biol. 25, 3294–3304 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Fitt W. K., McFarland F. K., Warner M. E., Chilcoat G. C., Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685 (2000). [Google Scholar]
- 59.Jurriaans S., Hoogenboom M. O., Seasonal acclimation of thermal performance in two species of reef-building corals. Mar. Ecol. Prog. Ser. 635, 55–70 (2020). [Google Scholar]
- 60.Bay R. A., Rose N. H., Logan C. A., Palumbi S. R., Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 3, e1701413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ware M., et al., Survivorship and growth in staghorn coral (Acropora cervicornis) outplanting projects in the Florida Keys National Marine Sanctuary. PLoS One 15, e0231817 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Foo S. A., Asner G. P., Impacts of remotely sensed environmental drivers on coral outplant survival. Restor. Ecol. 29, e13309 (2021). [Google Scholar]
- 63.Edmunds P. J., Putnam H. M., Science-based approach to using growth rate to assess coral performance and restoration outcomes. Biol. Lett. 16, 20200227 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edmunds P. J., Intraspecific variation in growth rate is a poor predictor of fitness for reef corals. Ecology 98, 2191–2200 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Bay R. A., Palumbi S. R., Transcriptome predictors of coral survival and growth in a highly variable environment. Ecol. Evol. 7, 4794–4803 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drury C., Manzello D., Lirman D., Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis. PLoS One 12, e0174000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quigley K. M., Bay L. K., van Oppen M. J. H., The active spread of adaptive variation for reef resilience. Ecol. Evol. 9, 11122–11135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller E. M., Bartels E., Baums I. B., Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. Elife 7, e35066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jury C. P., Delano M. N., Toonen R. J., High heritability of coral calcification rates and evolutionary potential under ocean acidification. Sci. Rep. 9, 20419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Page C. E., et al., Seeking resistance in coral reef Ecosystems: The interplay of biophysical factors and bleaching resistance under a changing climate: The interplay of a reef’s biophysical factors can mitigate the coral bleaching response. BioEssays 41, e1800226 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Webster M. S., et al., Who should pick the winners of climate change? Trends Ecol. Evol. 32, 167–173 (2017). [DOI] [PubMed] [Google Scholar]
- 72.Lirman D., Schopmeyer S., Ecological solutions to reef degradation: Optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ 4, e2597 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suggett D. J., et al., Optimizing return-on-effort for coral nursery and outplanting practices to aid restoration of the Great Barrier Reef: Optimizing coral restoration return-on-effort. Restor. Ecol. 27, 683–693 (2019). [Google Scholar]
- 74.Lowe R. J., Falter J. L., Monismith S. G., Atkinson M. J., Wave-driven circulation of a coastal reef–lagoon system. J. Phys. Oceanogr. 39, 873–893 (2009). [Google Scholar]
- 75.Drupp P., De Carlo E. H., Mackenzie F. T., Bienfang P., Sabine C. L., Nutrient inputs, phytoplankton response, and CO2 variations in a semi-enclosed subtropical embayment, Kaneohe Bay, Hawaii. Aquat. Geochem. 17, 473–498 (2011). [Google Scholar]
- 76.Bahr K. D., Jokiel P. L., Toonen R. J., The unnatural history of Kāne’ohe Bay: Coral reef resilience in the face of centuries of anthropogenic impacts. PeerJ 3, e950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jokiel P. L., Morrissey J., Water motion on coral reefs: Evaluation of the “clod card” technique. Mar. Ecol. Prog. Ser. 93, 175–181 (1993). [Google Scholar]
- 78.Hereford J., A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588 (2009). [DOI] [PubMed] [Google Scholar]
- 79.Kenkel C. D., Almanza A. T., Matz M. V., Fine-scale environmental specialization of reef-building corals might be limiting reef recovery in the Florida Keys. Ecology 96, 3197–3212 (2015). [DOI] [PubMed] [Google Scholar]
- 80.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2017). [Google Scholar]
- 81.Oksanen J., et al., The vegan package: community ecology package. R package version 1, 1–190 (2007). [Google Scholar]
- 82.Abbott J. M., DuBois K., Grosberg R. K., Williams S. L., Stachowicz J. J., Genetic distance predicts trait differentiation at the subpopulation but not the individual level in eelgrass, Zostera marina. Ecol. Evol. 8, 7476–7489 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw data and scripts have been deposited in Zenodo and are publicly available (DOI: 10.5281/zenodo.4315627).