Significance
Inducible Treg (iTreg) cells are crucial for the maintenance of intestinal immune homeostasis. iTreg differentiation has been recently linked with metabolic reprogramming in fatty acid oxidation (FAO). Butyrate, a specific type of short-chain fatty acid readily produced from fiber-rich diets through microbial fermentation, was previously found to promote iTreg generation as an HDAC inhibitor and critical for intestinal homeostasis. Here, we revealed that butyrate could also facilitate iTreg differentiation by enhancing FAO. This process relies on butyrate conversion to butyryl-CoA, which directly targets CPT1A, the rate-limiting enzyme in FAO, to unleash CPT1A activity for iTreg differentiation. Together, our findings depict a previously unappreciated mechanism for the regulation of iTreg differentiation and fine-tuning of the intestinal immune homeostasis by butyrate.
Keywords: iTreg, butyrate, fatty acid oxidation, CPT1A, inflammatory bowel disease
Abstract
Inducible regulatory T (iTreg) cells play a crucial role in immune suppression and are important for the maintenance of immune homeostasis. Mounting evidence has demonstrated connections between iTreg differentiation and metabolic reprogramming, especially rewiring in fatty acid oxidation (FAO). Previous work showed that butyrate, a specific type of short-chain fatty acid (SCFA) readily produced from fiber-rich diets through microbial fermentation, was critical for the maintenance of intestinal homeostasis and capable of promoting iTreg generation by up-regulating histone acetylation for gene expression as an HDAC inhibitor. Here, we revealed that butyrate could also accelerate FAO to facilitate iTreg differentiation. Moreover, butyrate was converted, by acyl-CoA synthetase short-chain family member 2 (ACSS2), into butyryl-CoA (BCoA), which up-regulated CPT1A activity through antagonizing the association of malonyl-CoA (MCoA), the best known metabolic intermediate inhibiting CPT1A, to promote FAO and thereby iTreg differentiation. Mutation of CPT1A at Arg243, a reported amino acid required for MCoA association, impaired both MCoA and BCoA binding, indicating that Arg243 is probably the responsible site for MCoA and BCoA association. Furthermore, blocking BCoA formation by ACSS2 inhibitor compromised butyrate-mediated iTreg generation and mitigation of mouse colitis. Together, we unveil a previously unappreciated role for butyrate in iTreg differentiation and illustrate butyrate–BCoA–CPT1A axis for the regulation of immune homeostasis.
Regulatory T (Treg) cells are CD4+ T cells expressing Foxp3 that play a key role in immune suppression (1–3). They can be divided into natural Treg (nTreg) and inducible Treg (iTreg) cells (1–3). nTreg cells, which are often referred to as thymic Treg (tTreg), arise during CD4+ T cell differentiation in the thymus under the influence of relatively high-avidity interactions of the T cell receptor (TCR) with self-antigens (1–3). iTreg cells, also called peripherally induced Treg (pTreg), develop in secondary lymphoid tissues. In the presence of TGFβ1, naive CD4+ T are induced into iTreg cells upon TCR ligation and costimulation by antigen-presenting cells (APCs) in response to non-self antigens, such as allergens, food, and the commensal microbiota (1–3).
It has been demonstrated that iTreg cells are enriched in gut-associated lymphoid tissues (GALTs) and are important for the maintenance of intestinal immune homeostasis (3–5). Intestinal iTreg cells were found to be important for the regulation of inflammatory bowel diseases (IBDs), such as Crohn’s disease (CD) and ulcerative colitis (UC), which can potentially affect any portion of the gastrointestinal tract and induce many further complications such as tissue fibrosis, stenosis, fistulas, and colon cancer over time (6). Enhancement of intestinal iTreg function or adoptive transfer of iTreg could significantly alleviate IBDs in mice (7–9).
Different types of T cells are featured by distinct metabolic characteristics. Unlike effector CD4+ T cells (Teffs), including Th1, Th2, Th9, and Th17 cells, that are mainly reliant on aerobic glycolysis, iTreg cells largely rely on fatty acid oxidation (FAO) (10–12). Accumulating evidence has demonstrated that T cell differentiation is always coupled with metabolic reprogramming (13, 14). For instance, FAO needs to be established in the process of iTreg differentiation. Up-regulation of FAO improved iTreg generation, whereas impairment in FAO compromised iTreg differentiation (12, 13, 15, 16).
FAO, comprised of a cyclical series of reactions, demands different fatty acids (FAs), which can be divided into long-, medium-, and short-chain fatty acids (LCFAs, SCFAs, and MCFAs). It dominantly occurs in mitochondria and results in acetyl-CoA (AcCoA), which could be consumed in tricarboxylic acid (TCA) cycle. For the oxidation of LCFA, it initiates from LCFA activation in cytoplasm, resulting in long-chain acyl-CoA. Subsequently, these resulting molecules are converted into long-chain acyl-carnitine by carnitine palmitoyltransferase 1 (CPT1), which is anchored on the mitochondrial outer membrane. Following its shuttling into mitochondria, long-chain acyl-carnitine experiences a chain of reactions to support FAO. Apparently, the transportation of LCFA from cytoplasm into mitochondria is a prerequisite for FAO. CPT1, the rate-limiting enzyme controlling this key step, is thus recognized as a determinant for FAO. In contrast, SCFAs and MCFAs can diffuse across mitochondrial membrane and drive FAO in a CPT1-independent manner (17). Nevertheless, extensive investigations have suggested an important role for CPT1 in iTreg differentiation (12, 13, 15, 16).
In recent years, butyrate, a specific type of SCFA produced from fiber-rich diets through microbial fermentation, was shown to play a critical role in the maintenance of intestinal homeostasis and was therefore recognized as an effective ingredient from food (18–24). By modulating distinct types of immune cells, including dendritic cells (18, 19), macrophages (20), and B and T cells (21–24), butyrate contributes to the orchestration of the delicate balance in intestinal immune system. Elegant investigations have elucidated that butyrate is able to facilitate iTreg differentiation by up-regulating Foxp3 expression as a histone deacetylase (HDAC) inhibitor (22, 23). Meanwhile, butyrate, as metabolic fuel and energy source, could also support FAO in colonic epithelial cells (25). However, whether butyrate could regulate FAO to promote iTreg differentiation is unclear.
In this study, we found that increased FAO contributed to enhanced iTreg cell differentiation in response to butyrate. Butyrate was processed, by acyl-CoA synthetase short-chain family member 2 (ACSS2), into butyryl-CoA (BCoA), which played a critical role in the control of FAO by targeting CPT1A. We found that BCoA competed with malonyl-CoA (MCoA), the best-known metabolic intermediate inhibiting CPT1A, to unleash CPT1A activity for FAO and thereby iTreg differentiation. Inhibition of ACSS2 to block BCoA generation compromised butyrate-mediated iTreg generation as well as mitigation of mouse colitis. Collectively, we depicted a previously unappreciated mechanism, namely, the butyrate–BCoA–CPT1A regulatory axis, for iTreg differentiation.
Results
Butyrate Enhances CPT1-Dependent Fatty Acid Oxidation during iTreg Differentiation.
Consistent with the previous finding that butyrate functioned as an HDAC inhibitor to promote iTreg differentiation (22, 23), we observed that the presence of butyrate enhanced histone acetylation (SI Appendix, Fig. S1A) and up-regulated iTreg generation in vitro (Fig. 1A). Interestingly, the most widely used HDAC inhibitor, Trichostatin A (TSA), failed to improve iTreg differentiation as efficiently as butyrate did (Fig. 1A), despite a stronger effect from TSA on histone acetylation than that from butyrate (SI Appendix, Fig. S1A). This suggests an additional role, other than HDAC inhibition, for butyrate in iTreg differentiation.
Fig. 1.
Butyrate (But) enhances CPT1-dependent fatty acid oxidation during iTreg differentiation. (A) Impact from But and trichostatin A (TSA) on iTreg generation. For iTreg induction in vitro, naive CD4+ T cells isolated from mice were cultured with Dynabeads Mouse T-Activator CD3/CD28, rmIL-2, and rhTGFβ1 for 72 h. But or TSA at different concentrations were added at the onset of the incubation. Percentages of iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Upper) and quantified accordingly (Lower) (n = 3). (B) Evaluation of OCR contributed from different types of FAs. Naive CD4+ T cells were induced under iTreg-polarization condition as described in A for 24 h and subsequently transferred to XF Base Medium for OCR detection. Cells were incubated with acetate (Ace), But, hexanoate (Hex), or octanate (Oct), respectively, for 15 to 20 min prior to the assessment of OCR. Diagram (Left) illustrating OCR at various conditions and associated quantifications (Right) were shown (n = 4). (C) Functional comparison between different types of FAs for iTreg differentiation. Naive CD4+ T cells from mice were cultured under iTreg-polarization condition as described in A and supplemented with Ace, But, Hex, or Oct at the same concentration (125 μM). Percentages of iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right) (n = 3). (D and E) Palmitate (PA)-OCR assay in the presence of different types of FAs. Naive CD4+ T cells induced under iTreg-polarization condition as described in B were kept in XF Base Medium containing different types of FAs, including Ace, But, Hex, or Oct prior to the incubation with PA. Rotenone plus antimycin A (Rot/Ant A) in D was replaced by etomoxir (Eto) in E. Diagram (Left) illustrating PA-OCR at various conditions and associated quantifications (Right) were shown (n = 3). Data represent mean ± SD of three independent experiments, with significance determined by one-way ANOVA test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, nonsignificant.
Given that FAs can be directly oxidized through FAO, which is important for iTreg generation (12, 25), we hypothesized that butyrate might be able to accelerate FAO for iTreg differentiation. Despite relatively mild and comparable up-regulation in oxygen consumption rate (OCR) in response to different tested FAs (Fig. 1B), butyrate exhibited a more dramatic effect on iTreg differentiation as compared to acetate, hexanoate, and octanate (Fig. 1C). When LCFA, palmitate (PA), was supplemented into medium as substrates, PA-associated OCR was more significantly up-regulated by butyrate than acetate, hexanoate, and octanate (Fig. 1D). Importantly, in response to the inhibition of LCFA transportation into mitochondria by CPT1 inhibitor etomoxir (Eto) (26), this butyrate-induced increase of PA-associated OCR was abrogated (Fig. 1E), suggesting an important role for CPT1 in butyrate-induced up-regulation in OCR and LCFA oxidation. We then analyzed long-chain acyl-carnitines, which are intermediate metabolites directly produced by CPT1. Remarkable elevations in different long-chain acyl-carnitines (fivefold to eightfold) were observed after butyrate treatment (SI Appendix, Fig. S1B), further supporting a critical role of butyrate in CPT1-dependent FAO. It is also worth mentioning that none of the tested FAs affected cell survival (SI Appendix, Fig. S1C), arguing that iTreg differentiation and PA-associated OCR induced by butyrate are not due to altered cell viability. Together, butyrate has a significant impact on CPT1-dependent LCFA oxidation, but its role as a direct substrate does not seem to contribute to the increase in iTreg induction.
In addition to enhanced CPT1-dependent LCFA oxidation, the up-regulation in long-chain acyl-carnitines in response to butyrate could also benefit from improved LCFA synthesis via futile fatty acid synthesis (FAS)/FAO cycle. As illustrated previously (14, 27, 28), the so-called “futile cycle” begins the accumulation of AcCoA in mitochondria, which condenses with oxaloacetate (OAA) and results in citrate to support TCA cycle. Meanwhile, citrate resulted from the TCA cycle is exported to cytosol to fuel LCFA generation (14, 27, 28). In turn, the accumulation of newly synthesized FAs can contribute to the enhancement in FAO (14, 27, 28). Because butyrate can be processed into AcCoA in mitochondria, which has a tight connection with futile FAS/FAO cycle, we wanted to assess whether butyrate-induced long-chain acyl-carnitine accumulation is via the “futile cycle” and performed [U-13C]butyrate-tracing experiment. Interestingly, we found that only ∼0.048% acyl-carnitine molecules (mainly C16 and C18) in iTreg cells were labeled with 13C (SI Appendix, Fig. S1 D and E). This argues a rather minor contribution from butyrate to acyl-carnitine formation via futile FAS/FAO cycle. Taken together, butyrate, other than a substrate supporting FAO, plays an important role in CPT1-dependent FAO during iTreg differentiation.
Regulation of CPT1A Activity Contributes to Enhanced iTreg Cell Differentiation by Butyrate.
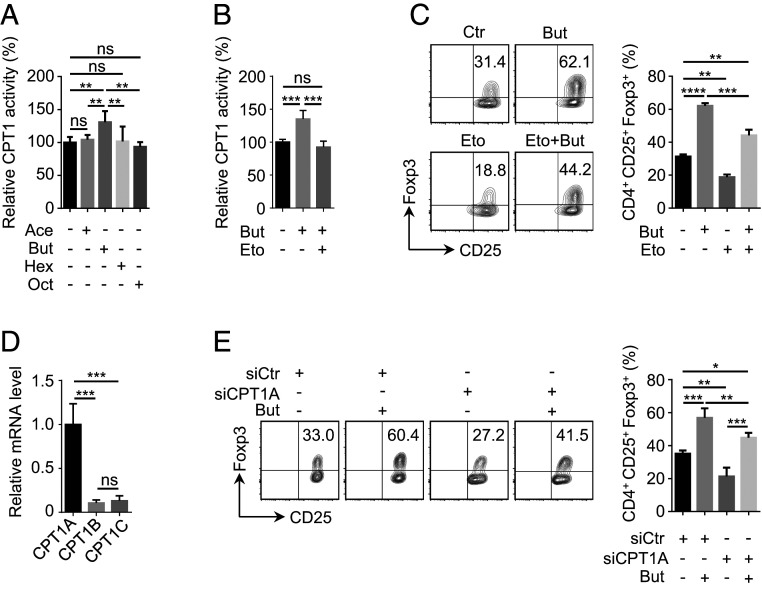
Acute abrogation of FAO was shown to impair iTreg cell differentiation (12, 13, 16). Interestingly, different types of long-chain acyl-carnitine were elevated in response to butyrate treatment (SI Appendix, Fig. S1B). Because they are direct products from CPT1, the rate-limiting enzyme in FAO, we asked whether the regulation of FAO as well as iTreg differentiation by butyrate was via CPT1. Interestingly, only butyrate, but not the other three types of FAs tested in our assay, significantly elevated CPT1 activity (Fig. 2A). Importantly, butyrate-induced up-regulation in CPT1 activity and iTreg generation was abrogated by CPT1 inhibitor Eto (Fig. 2 B and C), indicating that butyrate promoted iTreg generation through CPT1. In mammals, three isoforms of CPT1, including CPT1A, CPT1B, and CPT1C, have been identified in different tissues (29). In our analysis, we found that CPT1A was exclusively expressed in CD4+ T cells (Fig. 2D) and knockdown of CPT1A compromised butyrate-induced iTreg generation (SI Appendix, Fig. S2 and Fig. 2E). It is worth mentioning that CPT1A remained unchanged at both mRNA and protein level upon butyrate treatment (SI Appendix, Fig. S3 A and B), arguing that the regulation of CPT1A by butyrate was not at the transcriptional level. Together, these data suggest that CPT1A plays a critical role in butyrate-induced iTreg differentiation.
Fig. 2.
Regulation of CPT1A activity contributes to enhanced iTreg cell differentiation by butyrate. (A and B) Examination of impact from different types of fatty acids (FAs) on CPT1 activity. In the presence of FAs at the same concentration (125 μM), including acetate (Ace), butyrate (But), hexanoate (Hex), or octanate (Oct), naive CD4+ T cells were cultured under iTreg-polarization condition as described in Fig. 1A for 24 h and lysed for the assessment of CPT1 activity (A). Etomoxir (Eto) (5 μM), a well-documented CPT1 inhibitor (26), was added together with But for the evaluation of But-dependent up-regulation in CPT1 activity (B). (C) Analysis of iTreg generation upon the inhibition of CPT1. In the presence of But or/and Eto, naive CD4+ T cells were cultured under iTreg-polarization condition as described in Fig. 1A for 72 h. iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right). (D) Assay for mRNA levels of distinct CPT1 isoforms. RT-qPCR was performed using iTreg cells cultured as described in Fig. 1A for 24 h. (E) Evaluation of iTreg differentiation for CPT1A-depleted cells. Naive CD4+ T cells isolated from mice were transfected with siRNA against CPT1A. Following 4-h incubation, cells were replaced into fresh medium supplemented with or without But and kept under iTreg-polarization condition as described in Fig. 1A for 72 h. iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right). Data represent mean ± SD (n = 3) of three independent experiments, with significance determined by one-way ANOVA test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, nonsignificant.
Butyryl-CoA Associates with CPT1A and Antagonizes Malonyl-CoA–Mediated CPT1A Repression.
To understand the regulation of CPT1A activity by butyrate during iTreg differentiation, we started with evaluating the levels of l-carnitine and malonyl-CoA (MCoA), which are the two well-characterized metabolites directly regulating CPT1A activity in opposite ways (29). In brief, l-carnitine is CPT1A substrate, whereas MCoA is the best-known physiological inhibitor of CPT1A. In the process of de novo FAS, MCoA resulted from the carboxylation of cytosolic AcCoA by acetyl-CoA carboxylase 1 (ACC1). Subsequently, it condenses with AcCoA in a reaction catalyzed by fatty acid synthase (FASN) for the production of FAs, in particular PA (30). In our assay where levels of l-carnitine and MCoA were measured, a FASN inhibitor, cerulenin (Cer), which is able to induce MCoA accumulation, was included as a positive control (31) (SI Appendix, Fig. S4A). In response to butyrate treatment, no changes in levels of MCoA and l-carnitine were detected (SI Appendix, Fig. S4 A and B). Next, we continued to explore whether butyrate could directly regulate CPT1A activity. In our in vitro assay, CPT1A activity was down-regulated by MCoA as expected (Fig. 3A). Surprisingly, butyrate treatment did not influence CPT1A activity regardless of whether isolated mitochondria or cell lysates were used (Fig. 3A). Considering that butyrate can also be directly catalyzed into butyryl-CoA (BCoA) in cells, we decided to examine BCoA in the same enzymatic activity analysis. Interestingly, we observed that the addition of BCoA significantly increased CPT1A activity in vitro (Fig. 3A), while the presence of Eto blocked BCoA-mediated up-regulation of CPT1A activity (Fig. 3B). These data implied a direct role for BCoA in the control of CPT1A activity.
Fig. 3.
Butyryl-CoA binds to CPT1A to antagonize malonyl-CoA mediated repression. (A) Functional comparison between butyrate (But) and butyryl-CoA (BCoA) for the regulation of CPT1A activity. (Left) Mitochondria or (Right) cell lysates from iTreg cells cultured as described in Fig. 1A for 24 h were incubated with CPT1 detection buffer containing EDTA, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and palmitoyl-CoA in the presence of malnoyl-CoA (MCoA) (80 μM), But (125 μM), or BCoA (125 μM) for 5 min prior to CPT1A activity analysis. (B) Etomoxir (Eto) blocked BCoA-mediated up-regulation in CPT1A activity. In the presence of BCoA (125 μM) or/and Eto (5 μM), naive CD4+ T cells were cultured under iTreg-polarization condition as described in Fig. 1A for 24 h prior to the analysis of CPT1A activity. (C) Effect of BCoA on MCoA-mediated CPT1A inhibition. Recombinant CPT1A protein was incubated with MCoA (80 μM) and indicated concentrations of BCoA at 30 °C for 5 min. CPT1A activity was tested using CPT1 Activity Assay Kit. (D) Effect of cerulenin (Cer) on iTreg differentiation. In the presence of Cer (4 μM) or/and BCoA (125 μM), naive CD4+ T cells were cultured under iTreg-polarization condition as described in Fig. 1A for 72 h. iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right). (E–G) Assay for BCoA- and MCoA-binding to CPT1A protein. Recombinant (E and F) CPT1AWT or (G) CPT1AR243A were loaded onto the Ni-NTA biosensors in the biolayer interferometry analysis and incubated with BCoA or MCoA to detect their association. Data represent mean ± SD (n = 3) of three independent experiments, with significance determined by one-way ANOVA test. **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, nonsignificant.
We noticed that BCoA was structurally similar to the best-known physiological inhibitor of CPT1A, MCoA (SI Appendix, Fig. S5). An attractive possibility is that BCoA competes with MCoA binding on CPT1A and subsequently antagonizes MCoA-mediated CPT1A repression. To test this assumption, we established an in vitro assay with purified catalytic domain of CPT1A from Escherichia coli and found that MCoA-mediated inhibition of CPT1A was actually dampened by BCoA in a dose-dependent manner (Fig. 3C). Importantly, supplementing cells with BCoA rescued compromised iTreg generation due to MCoA accumulation induced by Cer (FASN inhibitor) (Fig. 3D). Further interaction assay revealed that BCoA could directly bind to CPT1A with even higher binding affinity than MCoA (Fig. 3 E and F). Arg243, which is completely conserved in all CPT1 members, was predicted to be critical for MCoA binding in a computation- and modeling-based analysis, which indicated that Arg243 was likely to establish polar contacts with the CoA moiety through the hydroxyl group of the pantothenic fragment in MCoA (29). In line with previous finding (29), mutating Arg243 in CPT1A to Ala (CPT1AR243A) resulted in a drastic reduction of MCoA association on CPT1A (Fig. 3G), confirming that Arg243 was required for MCoA binding. Interestingly, BCoA association with CPT1A was also significantly disrupted upon Arg243 mutation (Fig. 3G), suggesting that BCoA may interact with the same site in CPT1A as MCoA does. In addition, we noticed that CPT1A activity, which was up-regulated by BCoA in both isolated mitochondria and cell lysates (Fig. 3A), did not seem to be affected by BCoA significantly in our in vitro assay containing only BCoA and CPT1A (SI Appendix, Fig. S6). This argues that the regulation of CPT1A by BCoA is probably through interfering with MCoA association. Taken together, these results suggest that BCoA competes with MCoA to regulate CPT1A activity.
iTreg Cell Induction by Butyrate Depends on the Generation of Butyryl-CoA by ACSS2.
BCoA appeared to stimulate iTreg generation in a dose-dependent manner (Fig. 4A). To identify the enzyme responsible for catalyzing the conversion of butyrate into BCoA in iTreg, we started to focus on acyl-CoA synthetases (ACSs), including short- (ACSS), medium- (ACSM), and long-chain (ACSL) families, which have been reported to convert free fatty acid into acyl-CoA in mammalian cells (32). Considering that the butyrate molecule contains four carbon atoms, we screened all members of ACSS and ACSM families, and found that iTreg cells exclusively expressed ACSS2 and no other short and medium carbon acyl-CoA synthetases (Fig. 4B). In addition, ACSS2 appeared to be mainly present in cytoplasm (SI Appendix, Fig. S7A). To verify whether ACSS2 is capable of converting butyrate into BCoA, we first expressed recombinant ACSS2 in E. coli. Recombinant protein was functional and converted acetate into AcCoA as previously described (33) (SI Appendix, Fig. S7B). When recombinant ACSS2 was incubated with butyrate, BCoA was readily detected (Fig. 4C). Moreover, inhibiting ACSS2 by its specific inhibitor, 1-(2,3-di(thiophen-2-yl)quinoxalin-6-yl)-3-(2-methoxyethyl)urea (34), completely abrogated of AcCoA and BCoA generation (SI Appendix, Fig. S7B and Fig. 4C), confirming that ACSS2 was responsible for the conversion of butyrate into BCoA. Importantly, blocking ACSS2 also impaired butyrate-mediated up-regulation in CPT1A activity and iTreg generation, while add-back of BCoA significantly rescued ACSS2 inhibition-associated defects (Fig. 4 D and E). Collectively, ACSS2 is the metabolic enzyme catalyzing butyrate into BCoA, and it is required for butyrate-induced iTreg differentiation.
Fig. 4.
iTreg cell induction by butyrate depends on the generation of butyryl-CoA by ACSS2. (A) Functional assay of butyryl-CoA (BCoA) for iTreg differentiation. Naive CD4+ T cells from mice were cultured in the presence of BCoA and polarized as described in Fig. 1A for 72 h. Percentages of iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right). (B) mRNA levels of ACSS and ACSM members in CD4+ T cells. iTreg cells were cultured as described in Fig. 1A for 24 h and total RNA was extracted for the assessment of mRNA levels of ACSS1, ACSS2, ACSS3, ACSM1, ACSM2, ACSM3, ACSM4, and ACSM5. (C) Analysis of the transformation from butyrate (But) to BCoA by ACSS2. The production of BCoA catalyzed by recombinant ACSS2 in vitro was evaluated by HPLC. A specific ACSS2 inhibitor (ACSS2i), 1-(2,3-di(thiophen-2-yl)quinoxalin-6-yl)-3-(2-methoxyethyl)urea, was used to block ACSS2 activity. (D) ACSS2 inhibition disrupted But-induced up-regulation in CPT1A activity. In the presence of ACSS2i (5 μM), But (125 μM), and/or BCoA (125 μM), naive CD4+ T cells were cultured under iTreg-polarization condition as described in Fig. 1A for 24 h and subject to assessment of CPT1A activity. (E) ACSS2 inhibition impaired But-induced up-regulation in iTreg differentiation. Naive CD4+ T cells were cultured and stimulated as described in D for 72 h. Percentages of iTreg cells positive for CD4, CD25, and Foxp3 were analyzed by flow cytometry (Left) and quantified accordingly (Right). Data represent mean ± SD (n = 3) of three independent experiments, with significance determined by one-way ANOVA test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, nonsignificant. ND, not detectable.
ACSS2 Is Required for Butyrate-Mediated Alleviation of Colitis in Mouse.
Because butyrate has been linked with boost in colonic Treg cells and colitis alleviation (22, 23), we speculated that BCoA as well as ACSS2 might play an important role in butyrate-dependent regulation of colitis. To this end, we took advantage of a mouse model (6) for the investigation of ACSS2-BCoA function in colitis. Following dextran sodium sulfate (DSS) administration, a series of colitis-associated changes in mice were observed as expected: 1) disease activity index (DAI) score based on weight loss, liquid stool, and fecal occult blood increased drastically (Fig. 5A); 2) the length of colon was shortened (Fig. 5B); 3) inflammatory cell infiltration, mucosal edema, and injury as well as crypt damage were increased (Fig. 5C) (6). In agreement with previous observations (22), butyrate treatment significantly alleviated pathological alterations associated with colitis in mice and enhanced Treg cell frequencies in both mesenteric lymph nodes (MLNs) and colonic lamina propria (cLP) (Fig. 5 A–E). Importantly, inhibition of ACSS2 diminished butyrate-mediated effects in these mice (Fig. 5 A–E). Again, these in vivo data suggest an indispensable role for ACSS2 in butyrate-mediated iTreg differentiation in mice with colitis.
Fig. 5.
ACSS2 is required for butyrate-mediated alleviation of colitis in mouse. (A–C) Systematic evaluation of manifestation of colitis in mice administrated with ACSS2 inhibitor. To establish colitis model, 6- to 8-wk-old female C57BL/6J mice were supplied with DSS (2% [wt/vol]) in drinking water. Meanwhile, butyrate (But) (40 mg⋅kg−1⋅d−1) and/or ACSS2 inhibitor (ACSS2i) (15 mg⋅kg−1⋅d−1) was administered by oral gavage or intraperitoneal injection, respectively. (A, Left) Body weight and (Right) disease activity index (DAI) score, based on body weight loss, stool consistency, and blood in the stool, were daily recorded as described in the literature (6). Ten days later, entire colons from mice treated in different ways were removed for length assessment (B) and H&E staining for histopathological changes (C). (Scale bar, 200 μm.) (D and E) Assay for CD4+ Foxp3+ Treg in mesenteric lymph nodes (MLNs) and colonic lamina propria (cLP). Cells from (D) MLNs and (E) cLP were examined by flow cytometry according to the published method (23). Data represent mean ± SD (n = 4) with significance determined by two-way ANOVA test (A) and one-way ANOVA test (B, D, and E). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, nonsignificant.
Besides iTreg, other types of intestinal cells, such as colonic epithelial cells, gut-resident dendritic cells (DCs), and macrophages, have also been shown to be regulated by butyrate for the alleviation of colitis (18–20, 25, 35, 36). We thus continued to examine whether these cells express any of the ACSS and (or) ACSM members. Interestingly, relatively high expression of ACSS2 was detected in colonic epithelial cells, gut-resident DCs, and macrophages (SI Appendix, Fig. S8 A–C). These results imply that, in addition to iTreg cells, other intestinal cells might also be regulated by butyrate–ACSS2–BCoA axis and thus contribute to butyrate-mediated colitis alleviation.
Discussion
In this work, we unveiled a previously unappreciated role for butyrate in iTreg differentiation and identified ACSS2 as the responsible enzyme catalyzing butyrate into BCoA. By competing with MCoA, BCoA regulated CPT1A activity to support FAO for iTreg generation. Other than HDAC inhibitor, butyrate can also function as a metabolic regulator driving alterations in FAO that are required for iTreg differentiation. On one hand, butyrate regulates FAO in a CPT1A-dependent manner; on the other hand, it supports FAO with a direct but limited contribution as a carbon source.
As a SCFA, butyrate is generated from dietary fibers by anaerobic fermentation and is important for the regulation and maintenance of immune homeostasis in the gut (22–24). Colonic environment is obviously complex and composed of various cell types (37). Besides iTreg, other types of cells can be also involved in butyrate-dependent regulation (18–20, 25, 35, 36). Our observation with respect to the presence of ACSS2 in colonic epithelial cells, gut-resident DCs, and macrophages, implies that the butyrate–ACSS2–BCoA axis may exist and function in multiple types of intestinal cells. It is noteworthy that ACSM3 was also readily detected in those intestinal cells in the meantime (SI Appendix, Fig. S8 A–C), hinting a potential involvement in the functional regulation of tested intestinal cells. This obviously requires a thorough investigation in the future to get a clear view on the underlying mechanism. Moreover, butyrate was found to promote the memory potential of activated CD8+ T cells by enhancing fatty acid uptake and oxidation (24). There is also evidence demonstrating an indispensable role of CPT1A in CD8+ T memory cells development (14, 38, 39). An attractive possibility that the regulation of CD8+ T cells functions by butyrate is also via BCoA-CPT1A remains to be tested.
Although the essential role of CPT1A in iTreg differentiation has been recognized (12, 13, 15, 16), a more recent report challenged previous findings and proposed that CPT1A is dispensable for iTreg differentiation (40). Raud et al. (40) found that naive CD4+ T cells isolated from mice with a specific deletion of CPT1A in T cells (TCPT1A mice) did not seem to have any defect in iTreg differentiation and the phenotypes induced by high dose Eto (>100 μM) were due to off-target effect. However, our results demonstrated that both small interfering RNA (siRNA)-mediated CPT1A knockdown and low-dose Eto (5 μM) treatment resulted in impairment in iTreg differentiation (Fig. 2 C and E). Notably, low-dose Eto (5 μM) has been shown to inhibit CPT1A without introducing off-target effect (26). Apparently, our data support the previous conclusion that CPT1A plays an important role in iTreg differentiation (12, 13, 15, 16). TCPT1A mice are reported to be viable and can tolerate the loss of CPT1A. To reachieve metabolic homeostasis in these mice lacking CPT1A, various metabolic genes/regulators have to be readjusted to compensate for CPT1A lost as well as perturbed FAO. In contrast, blocking CPT1A function by specific inhibitor or siRNA mimics an acute disruption in FAO and might be useful for minimizing long-term metabolic adaption and directly evaluating CPT1A function in iTreg differentiation.
CPT1A activity is repressed by MCoA, which shields CPT1A at His473 from the binding of substrate metabolite l-carnitine to block acyl-carnitine formation (29). Interestingly, the free carboxyl group in MCoA, which is critical for contacting His473 and blocking l-carnitine binding in CPT1A (29), is replaced by an ethyl group in BCoA (SI Appendix, Fig. S5). Due to the lack of the free carboxyl group, BCoA is probably unable to contact with His473 in CPT1A to block l-carnitine binding as MCoA does. This delicate difference in molecular structure could also be relevant for understanding the unperturbed enzymatic activity of CPT1A in the presence of BCoA and l-carnitine in vitro (SI Appendix, Fig. S6). Apparently, MCoA is key to the control of CPT1A activity as well as FAO under variable physiological conditions (29, 41). Of note, Arg243 in CPT1A has been identified as a critical site for MCoA binding (29). Our data revealed that mutation of Arg243 abolished both MCoA and BCoA association with CPT1A, indicating that these two regulatory molecules sharing similar structure might recognize and bind to the same site in CPT1A. By competing with MCoA for the same site, BCoA abrogated MCoA-mediated CPT1A inhibition to promote FAO and iTreg differentiation.
Among all members in ACSS and ACSM family, ACSS2 was exclusively presented in iTreg (Fig. 4B). It has been shown that ACSS2 catalyzes acetate into AcCoA and is involved in the regulation of histone acetylation for gene transcription and expression (42). ACSS2 was also found to convert crotonate into crotonyl-CoA for the crotonylation on proteins (43). In this study, we revealed that ACSS2 catalyzed butyrate into BCoA. Using different SCFAs with structural similarity as substrates, ACSS2 may simultaneously impact multiple posttranslational modifications by regulating levels of their donor molecules. In addition, the functional characterization of BCoA catalyzed by ACSS2 in CPT1A regulation and iTreg differentiation depicts a pivotal regulatory axis for butyrate-induced iTreg generation.
Accumulating evidence has underscored the important role of butyrate in the control of T cell functions and immune homeostasis (22–24). Other types of FAs, such as hexanoate and octanate, were also shown to be correlated with immune disorders (44). Functional characterization of butyrate and BCoA in FAO for iTreg differentiation can aid us understand the coupling between metabolic network and T cell differentiation. Depicting the mechanism by which BCoA regulates CPT1A activity will also bring insights into the maintenance of immune homeostasis by T cells.
Materials and Methods
Animals.
C57BL/6J mice were purchased from Beijing HFK Bioscience Company. Mice were bred and cohoused four to six mice per cage, in a specific pathogen-free facility with a standard 12-h alternate light/dark cycle at an ambient temperature of 22 ± 2 °C and 30 to 70% humidity at the Animal Research Center of Northeast Normal University (Changchun, China). Health status of mice was determined via daily observation by technicians supported by veterinary care. All mouse experiments were conducted in accordance with the protocols for animal use, treatment, and killing approved by the Animal Care Committee of Northeast Normal University.
Induction and Analysis of iTreg Cells.
Primary naive CD4+ T cells were isolated from spleen of mice at the age of 6 to 8 wk with MojoSort Mouse CD4 Naive T Cell Isolation Kit (Biolegend) according to the manufacturer’s instructions. A total of 2–3 × 105 naive CD4+ T cells was cultured in RPMI 1640 medium (Sigma) supplemented with 10% FBS (Biological Industries), penicillin–streptomycin (500 U; PAN Biotech), and β-mercaptoethanol (50 μM; Sigma-Aldrich), and stimulated with Dynabeads Mouse T-Activator CD3/CD28 (Gibco) at a cell:bead ratio of 1:1 in the presence of rmIL-2 (200 U/mL; Peprotech) and rhTGFβ1 (0.5 ng/mL; Peprotech). Cells were kept in medium containing butyrate (Sigma-Aldrich), acetate (Sigma-Aldrich), hexanoate (Sigma-Aldrich), octanate (Sigma-Aldrich), [U-13C]butyrate (Sigma-Aldrich), trichostatin A (Selleck), butyryl-CoA (Sigma-Aldrich), etomoxir (Selleck), cerulenin (BioVision), or ACSS2 inhibitor, 1-(2,3-di(thiophen-2-yl)quinoxalin-6-yl)-3-(2-methoxyethyl)urea (Selleck), in the incubator with 5% CO2 for 24 or 72 h.
To analyze iTreg generation, cells were collected in PBS containing 1% FBS (vol/vol) and fixed/permeabilized using the Transcription Factor Buffer Set (BD Biosciences) according to manufacturer’s instructions. Selected protein markers were stained with FITC rat anti-mouse CD4 (clone RM4-5), PE rat anti-mouse CD25 (clone PC61), and Alexa Fluor 647 rat anti-mouse Foxp3 (clone MF23) monoclonal antibodies (BD Biosciences). Flow cytometry analysis was carried out with BD FACS Canto II flow cytometer (BD Biosciences), and acquired data were analyzed using FlowJo, version 10, software (FlowJo). For iTreg analysis, cells positive for CD4, CD25, and Foxp3 were selected and further evaluated.
Cell Viability Assay.
To analyze cell viability, cells were collected and stained with YF647-Annexin V and propidium iodide (PI) according to the manufacturer’s instruction (US Everbright). Cells negative for both YF647-Annexin V and PI were defined as viable ones. Flow cytometry analysis was carried out with BD FACS Canto II flow cytometer (BD Biosciences), and acquired data were analyzed using FlowJo, version 10, software (FlowJo).
Isolation of Different Types of Intestinal Cells.
Colonic epithelial cells, gut-resident DCs, and macrophages were isolated from C57BL/6J mice as described previously with minor modifications (45, 46). In brief, mice were killed, and the colon, with the Peyer’s patches removed, was washed with Ca2+/Mg2+-free PBS (CMF-PBS) after being cut open longitudinally. Colon was cut into small pieces and transferred into prewarmed CMF–Hank’s balanced salt solution (HBSS) with 5% FBS and 2 mM EDTA, followed by shaking at 250 rpm for 20 min at 37 °C. Supernatant containing colonic epithelial cells was then filtered (100 µm), incubated on ice for 10 min to allow sedimentation, and filtered (75 µm) again. Cell suspension was stained with PE anti-mouse CD326 (Ep-CAM) antibody (clone G8.8) (Biolegend) for the isolation of CD326+ colonic epithelial cells by BD ARIA II Cell Sorter (BD Biosciences).
For the isolation of gut-resident DCs and macrophages, residual colon tissues (after epithelium removal) were collected and prepared according to the previous procedure with minor modifications (46). Briefly, residual colon tissues were shaken in prewarmed CMF-HBSS containing 5% FBS, 1.5 mg/mL type VIII collagenase (Sigma-Aldrich), and 40 μg/mL DNase I (Sigma-Aldrich) at 250 rpm for 20 min at 37 °C. After filtering through a 100-μm cell strainer and centrifuging at 1,500 rpm for 5 min at 4 °C, the cell pellet was resuspended with ice-cold CMF-HBSS containing 5% FBS. Cell suspension was stained with APC anti-mouse CD11c (clone N418) and PE anti-mouse CD103 (clone 2E7) (Biolegend) monoclonal antibodies for the isolation of CD11c+ CD103+ gut-resident DCs by BD ARIA II Cell Sorter (BD Biosciences). Alternatively, it was stained with Brilliant Violet 421 anti-mouse/human CD11b (clone M1/70) and PE/Cyanine7 anti-mouse F4/80 (clone BM8) (Biolegend) monoclonal antibodies for isolation of CD11b+ F4/80+ gut-resident macrophages.
Extracellular Flux Analysis.
For the FAO assay, the Seahorse XFp Analyzer (Agilent), Palmitate-BSA reagent (Agilent), Mito Stress Test Kits (Agilent), and etomoxir (Eto) (Selleck) were used to assess the cell’s ability to oxidize exogenous fatty acid as per manufacturers’ instructions with minor modifications.
In brief, cells were collected and adhered to poly-d-lysine–coated XFp plates (4–6 × 105/well) via centrifugation in serum-free XF Base Medium (Agilent). For evaluation of mitochondrial oxidation contributed by different types of FAs and/or PA, cells were cultured with XF Base Medium (Agilent) in the presence of 125 μM acetate (Sigma-Aldrich), butyrate (Sigma-Aldrich), hexanoate (Sigma-Aldrich), or octanate (Sigma-Aldrich), respectively, in a non-CO2 incubator for 15 to 20 min at 37 °C. Subsequently, OCR was assessed in the presence or absence of XF Palmitate-BSA FAO Substrates (Agilent), palmitate-BSA (167 μM palmitate conjugated with 28 μM BSA), and 0.5 mM l-carnitine (Sigma-Aldrich).
OCR was measured under basal state and in response to 1 mM oligomycin (Oligo) (Agilent), 1.5 mM fluorocarbonyl cyanide phenylhydrazone (FCCP) (Agilent), and 100 nM rotenone plus 1 mM antimycin A (Rot/Ant A) (Agilent) or 5 μM Eto (Selleck) employing the Seahorse XFp Analyzer (Agilent) using Wave software, version 2.3.0 (Agilent). To compare OCR associated with different types of FAs, three to four sets of combinations that include control, acetate, butyrate, hexanoate, and octanoate were organized in separated XFp plate and detected sequentially. Data yielded from separate assessment were normalized based on “control” and analyzed with proper statistical methods using GraphPad Prism, version 6 (GraphPad Software).
Mass Spectrometry-Based Metabolomics for Acyl-Carnitine.
Metabolite extraction was performed as previously described (47) with 2-chloro-l-phenylalanine as internal standard. Liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS) analysis was performed using ultra-high-performance liquid chromatography system (1290 InfinityII; Agilent) coupled to a quadrupole time-of-flight mass spectrometry detector (6545; Agilent) outfitted with an electrospray ionization (ESI) source. LC-QTOF-MS method, performance evaluation, data processing, and acyl-carnitine identification and analysis were performed as a published report with minor modifications (48). In brief, a sample (2 μL) was injected into Waters XSelect HSS T3 chromatographic column (2.5 μm; 100 × 2 mm), maintained at 25 °C. The mobile phases were as follows: aqueous solution with 0.1% formic acid (A) and acetonitrile solution with 0.1% formic acid (B). The elution was carried out at 25 °C and the flow rate was 0.35 mL/min. The gradient was as follows: 0–2 min, 5% B; 2–10 min, 5–95% B; 10–15 min, 95% B; 15–18 min, 95–5% B. Post time was set as 3 min for system balance. Mass spectrometry was operated in both positive and negative ion modes. The parameters for both ion modes were positive mode voltage (4 kV), negative mode voltage (3.5 kV), drying gas flow (10 L/min), gas temperature (325 °C), nebulizer pressure (20 psig), fragmentor voltage (120 V), and skimmer voltage (45 V). The mass range was 50 to 1,700 m/z. Reference ions were used during MS acquisition process to ensure mass accuracy. The reference ions were 121.0509 m/z and 922.0098 m/z (ESI+), and 112.9856 m/z and 1,033.9981 m/z (ESI−). Data were acquired by Agilent Masshunter Qualitative Analysis B.07.00 software (Agilent Technologies). In the R software platform, the XCMS program was used in peak identification, retention time correction, and automatic integration pretreatment. Then the data were subjected to internal standard normalization and weight normalization, and the flesh data were subjected to internal standard normalization. The acyl-carnitine identification was based on their measured accurate m/z values (10 ppm mass error window) and on the comparison of their acquired LC-QTOF-MS/MS spectra with those available on different databases, such as Metlin (https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage), Kyoto Encyclopedia of Genes and Genomes (https://www.kegg.jp/kegg), and Personal Compound Database and Library (Agilent Technologies).
13C-Tracing Assessment.
Acyl-carnitine extraction was carried out as previously described (49) with 19:0-CoA as an internal standard for correction of ion response. Samples were then incubated on a thermomixer at 450 rpm for 20 min at 25 °C, followed by centrifugation at 12,000 rpm for 5 min at 4 °C. The clean supernatant was transferred to fresh tube and subsequently dried in the SpeedVac (Genevac) under OH mode. Dry extracts were resuspended in appropriate volume of methanol: water (9:1 [vol/vol]) prior to LC-MS analysis on a dual-gradient high-performance liquid chromatography system (UltiMate 3000; Thermo Fisher) coupled to mass spectrometer (QTRAP 6500 plus; Sciex). LC-MS performance and data processing and analysis were accomplished as reported (49). Areas corresponding to each metabolite containing 13C isotope were normalized to the area of internal standard for correction of intersample ion response.
Plasmid Construction and Protein Expression.
DNA sequences coding mouse ACSS2 gene (National Center for Biotechnology Information accession no. NM019811.3) and the catalytic domain of mouse CPT1A gene (41) from CD4+ T cells were amplified by PCR and subsequently cloned into pET28a(+) vector to construct pET28a(+)/ACSS2 and pET28a(+)/CPT1AWT, respectively. CPT1AR243A point mutations were introduced by whole-plasmid PCR followed by DpnI digest to obtain pET28a(+)/CPT1AR243A. To obtain desired recombinant proteins, DNA constructs, including pET28a(+)/ACSS2, pET28a(+)/CPT1AWT, and pET28a(+)/CPT1AR243A, were transformed into E. coli BL21 (DE3) strains, which were induced with 1 mM IPTG at 16 °C overnight. Following published procedures (50), bacterial lysates were prepared for protein purification by His affinity column with Ni-NTA agarose (Qiagen).
Assessment of Metabolic Molecules.
The level of l-carnitine was measured using l-Carnitine Assay Kit (Sigma-Aldrich) according to the kit instructions and was normalized with the concentration of total proteins from each sample. For the analysis of malonyl-CoA, cells were cultured under iTreg polarization condition for 24 h and lysed in ice-cold 5% sulfosalicylic acid (Sigma-Aldrich) containing 50 μM dithioerythritol (DTE) (Sigma-Aldrich) to obtain cell homogenates. After 600 × g centrifugation for 10 min at 4 °C, the supernatants were collected for the detection of malonyl-CoA. To assay butyryl-CoA and acetyl-CoA, an in vitro reaction system (33) with minor modifications was established. In brief, a mixture (final volume of 250 μL) contains the following: 60 mM potassium phosphate (pH 7.5), 3 mM ATP, 0.1 mM CoA-SH, 4 mM MgCl2, 1 mM DTE, 0.6 μg of recombinant ACSS2, and indicated amount of butyrate (Sigma-Aldrich) or acetate (Sigma-Aldrich). Following 30-min incubation at 30 °C, the reaction was stopped with glacial acetic acid (50 μL). To inhibit ACSS2, the reaction mixture was preincubated with specific ACSS2 inhibitor (5 μM; Selleck) for 5 min at 30 °C.
Ten-microliter aliquots of the above samples were directly injected while a pump system supplied eluent A (50 mM sodium phosphate and 37.5 mM sodium acetate, pH 4.6) and eluent B (70% eluent A in methanol). Absorbance measurements were made at 254 nm on a UV detector (SPD-10A; Shimadzu). The elution was carried out at ambient temperature and the flow rate was 1 mL/min. The profile of the gradient elution was as follows: 0 min, 30% B; 10 min, 60% B; 17.6 min, 100% B. Short-chain acyl-CoA standards (Sigma-Aldrich) were prepared at appropriate series concentrations. The level of short-chain acyl-CoA was calculated based on the peak area referenced to standards.
CPT1 Activity Assay.
Whole-cell lysates in PBS with 0.1% Triton X-100 (Sigma-Aldrich) and mitochondrial isolated with Mitochondria Isolation Kit (Beyotime Biotechnology) were prepared. CPT1 activity was assessed with CPT1 Activity Assay Kit (Suzhou Comin Biotechnology Company) according to the manufacturer’s instructions. Briefly, samples were separately mixed with reaction buffer containing 1 to 2 mM EDTA, 200 μM 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), and 80 μM palmitoyl-CoA. After 5-min preincubation at 30 °C, the reaction was initiated with the supplement of l-carnitine, followed by an immediate photometric measurement at 412 nm. As blank control, the l-carnitine was substituted for sterilized distilled water. The calculation of CPT1 activity was based on absorbance and normalized with protein concentrations.
siRNA Knockdown.
Naive CD4+ T cells were transfected with siRNAs (SI Appendix, Table S1) using the Amaxa cell electroporation device (Nucleofector II; Lonza) according to the protocol recommended by the manufacturer. Briefly, cells were resuspended in the nucleofector solution (Lonza) and mixed with indicated siRNAs (300 nM). After the transfer to cuvettes, the cells were electroporated using the X-001 pulsing parameter and replaced into wells containing 37 °C prewarmed Mouse T Cell Nucleofector Medium (Lonza) plus MEM NEAA (1%; Gibco). For functional assays, transfected cells were cultured under iTreg-polarization condition post incubation at 37 °C for 4 h.
Real-Time PCR.
Total RNA was isolated using the TRIzol reagent (Invitrogen). cDNA was synthesized with the PrimeScript RT reagent Kit plus gDNA Eraser (Takara) according to manufacturer’s instructions. Real-time PCR was performed on the QuantStudio 3 Real-Time PCR Instrument (Applied Biosystems) with a TB Green Premix Ex Taq (Tli RNaseH Plus) regent (Takara). The mRNA expression of genes was normalized to the expression of β-actin gene. Data were analyzed using the comparative cycling threshold method. Primer sequences are listed in SI Appendix, Table S1.
Western Blot.
Cell lysate preparation, SDS-PAGE, and electrophoretic transference were accomplished as previously described (42). Membranes were blocked with 5% (wt/vol) nonfat dried milk in TBS (pH 7.4) containing 0.1% Tween 20 (TBST) and incubated with the appropriate antibodies in 5% (wt/vol) BSA in TBST overnight at 4 °C. The following primary antibodies were used: acetylated-lysine antibody (CST), anti-acetyl-histone H3 (Millipore), anti-histone H3 (CST), anti-acetyl-histone H4 (Millipore), anti-histone H4 (Millipore), anti-CPT1 (Santa), or anti-β-actin (Proteintech). All primary antibody incubations were followed by incubation with secondary HRP-conjugated antibody in 5% (wt/vol) nonfat dried milk in TBST and visualized using Chemiluminescent HRP Substrate (Millipore) on Chemiluminescence Image System (Tanon Science and Technology Company).
Biolayer Interferometry Analysis.
The recombinant His-CPT1AWT (50 μg/mL) or -CPT1AR243A (50 μg/mL) were used to evaluate binding of malonyl-CoA and butyryl-CoA with CPT1A. The recombinant proteins were prepared in assay buffer (0.02% Tween 20 and 0.1% BSA in PBS, pH 7.4) and were loaded onto Ni-NTA biosensors (Pall ForteBio). After washing with assay buffer to remove unspecific bound proteins, biosensors were inserted into wells contained malnoyl-CoA (Sigma-Aldrich) or butyryl-CoA (Sigma-Aldrich) diluted with the assay buffer. To examine the disassociation of tested metabolites, biosensors were inserted into wells filled with only assay buffer. Eventually, data were analyzed using Octet System Data Analysis software 8.11 (Pall ForteBio).
Colitis Model.
DSS (MP Biomedicals) dissolved in drinking water (2% [wt/vol]) was given ad libitum to 6- to 8-wk-old female C57BL/6J mice for 10 d. Mice supplied with regular drinking water were included as negative control. Butyrate (Sigma-Aldrich) was dissolved in sterilized distilled water and given by gavage (40 mg⋅kg−1⋅d−1). ACSS2-specific inhibitor (15 mg⋅kg−1⋅d−1) (Selleck) dissolved in the vehicle containing 5% DMSO, 40% PEG300, 5% Tween 80, and 50% sterilized distilled water was administered by intraperitoneal injection every 2 d.
Evaluation of Colitis in Mice.
To assess the severity of DSS colitis, DAI was daily recorded by scoring the body weight loss, stool consistency, and blood in the stool as described in the literature (6). Afterward, mice were killed for the examination of histopathological changes in colon tissues. Entire colons were removed, and colon length was determined. Following cleaning with saline and fixation with formalin, colon samples were embedded in paraffin. Sections (3 μm) were stained with hematoxylin and eosin (H&E), and slides were blindly analyzed for intestinal inflammation according to the previous description (6). In addition, cells from MLNs and the cLP were prepared as described previously (23) for the analysis of Treg cells by flow cytometry.
Data Analysis and Statistics.
Statistical analysis was performed using GraphPad Prism, version 6 (GraphPad Software). Data are displayed as mean ± SD. One-way or two-way ANOVAs were performed in the indicated figures when more than two groups were compared. Values of P < 0.05 were considered significant. P values are indicated on graphs as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Supplementary Material
Acknowledgments
We thank Weiwei Yang (Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, China) and Xueqing Ba (Northeast Normal University, China) for critical comments. This work was supported by grants from the National Natural Science Foundation of China (32070758 and 32070896).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2014681118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Josefowicz S. Z., Lu L. F., Rudensky A. Y., Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 30, 531–564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage P. A., Malchow S., Leventhal D. S., Basic principles of tumor-associated regulatory T cell biology. Trends Immunol. 34, 33–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veenbergen S., Samsom J. N., Maintenance of small intestinal and colonic tolerance by IL-10-producing regulatory T cell subsets. Curr. Opin. Immunol. 24, 269–276 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Round J. L., Mazmanian S. K., Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. U.S.A. 107, 12204–12209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josefowicz S. Z., et al., Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482, 395–399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wirtz S., et al., Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 12, 1295–1309 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Luo Y., et al., Negligible effect of sodium chloride on the development and function of TGF-β-induced CD4+ Foxp3+ regulatory T cells. Cell Rep. 26, 1869–1879.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson F., et al., Therapeutic evaluation of ex vivo-generated versus natural regulatory T-cells in a mouse model of chronic gut inflammation. Inflamm. Bowel Dis. 19, 2282–2294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H. L., et al., Regulatory T-cell depletion in the gut caused by integrin β7 deficiency exacerbates DSS colitis by evoking aberrant innate immunity. Mucosal Immunol. 9, 391–400 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Macintyre A. N., et al., The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., et al., Histone deacetylase SIRT1 negatively regulates the differentiation of interleukin-9-producing CD4+ T cells. Immunity 44, 1337–1349 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Michalek R. D., et al., Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacIver N. J., Michalek R. D., Rathmell J. C., Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Sullivan D., et al., Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berod L., et al., De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327–1333 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Gualdoni G. A., et al., The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. FASEB J. 30, 3800–3809 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Schönfeld P., Wojtczak L., Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 57, 943–954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurav A., et al., Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions. Biochem. J. 469, 267–278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh N., et al., Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40, 128–139 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulthess J., et al., The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez H. N., et al., B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 11, 60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furusawa Y., et al., Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Arpaia N., et al., Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachem A., et al., Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Donohoe D. R., et al., The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connor R. S., et al., The CPT1a inhibitor, etomoxir induces severe oxidative stress at commonly used concentrations. Sci. Rep. 8, 6289 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulloo A. G., Gubler M., Montani J. P., Seydoux J., Solinas G., Substrate cycling between de novo lipogenesis and lipid oxidation: A thermogenic mechanism against skeletal muscle lipotoxicity and glucolipotoxicity. Int. J. Obes. Relat. Metab. Disord. 28, S29–S37 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Liu L., et al., Paradoxical coupling of triglyceride synthesis and fatty acid oxidation in skeletal muscle overexpressing DGAT1. Diabetes 58, 2516–2524 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.López-Viñas E., et al., Definition by functional and structural analysis of two malonyl-CoA sites in carnitine palmitoyltransferase 1A. J. Biol. Chem. 282, 18212–18224 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Lochner M., Berod L., Sparwasser T., Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36, 81–91 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Thupari J. N., Pinn M. L., Kuhajda F. P., Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 285, 217–223 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Watkins P. A., Maiguel D., Jia Z., Pevsner J., Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J. Lipid Res. 48, 2736–2750 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Luong A., Hannah V. C., Brown M. S., Goldstein J. L., Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J. Biol. Chem. 275, 26458–26466 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Comerford S. A., et al., Acetate dependence of tumors. Cell 159, 1591–1602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiminez J. A., Uwiera T. C., Abbott D. W., Uwiera R. R. E., Inglis G. D., Butyrate supplementation at high concentrations alters enteric bacterial communities and reduces intestinal inflammation in mice infected with Citrobacter rodentium. MSphere 2, e00243-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaudier E., et al., Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1168–G1174 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Maloy K. J., Powrie F., Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Pearce E. L., et al., Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Windt G. J. W., et al., Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raud B., et al., Etomoxir actions on regulatory and memory T cells are independent of Cpt1a-mediated fatty acid oxidation. Cell Metab. 28, 504–515.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao J. N., Warren G. Z. L., Estolt-Povedano S., Zammit V. A., Ulmer T. S., An environment-dependent structural switch underlies the regulation of carnitine palmitoyltransferase 1A. J. Biol. Chem. 286, 42545–42554 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu J., et al., Acetate promotes T cell effector function during glucose restriction. Cell Rep. 27, 2063–2074.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabari B. R., et al., Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 58, 203–215 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Preter V., et al., Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut 64, 447–458 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Mathewson N. D., et al., Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atarashi K., et al., Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angelin A., et al., Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25, 1282–1293.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Minno A., et al., Untargeted metabolomics to go beyond the canonical effect of acetylsalicylic acid. J. Clin. Med. 9, 51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam S. M., et al., A robust, integrated platform for comprehensive analyses of acyl-coenzyme as and acyl-carnitines revealed chain length-dependent disparity in fatty acyl metabolic fates across Drosophila development. Sci. Bull. (Beijing) 65, 1840–1848 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Ueki Y., et al., A consensus binding motif for the PP4 protein phosphatase. Mol. Cell 76, 953–964.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.