Abstract
As COVID-19 adversely affects patients with cancer, prophylactic strategies are critically needed. Using a validated antibody assay against SARS-CoV-2 spike protein, we determined a high seroconversion rate (94%) in 200 patients with cancer in New York City that had received full dosing with one of the FDA-approved COVID-19 vaccines. On comparison with solid tumors (98%), a significantly lower rate of seroconversion was observed in patients with hematologic malignancies (85%), particularly recipients following highly immunosuppressive therapies such as anti-CD20 therapies (70%) and stem cell transplantation (73%). Patients receiving immune checkpoint inhibitor therapy (97%) or hormonal therapies (100%) demonstrated high seroconversion post vaccination. Patients with prior COVID-19 infection demonstrated higher anti-spike IgG titers post vaccination. Relatively lower IgG titers were observed following vaccination with the adenoviral than with mRNA-based vaccines. These data demonstrate generally high immunogenicity of COVID-19 vaccination in oncology patients and identify immunosuppressed cohorts that need novel vaccination or passive immunization strategies.
Keywords: cancer, COVID-19, vaccine, hematologi malignancies
Graphical abstract
Evaluating the IgG levels against SARS-CoV-2 spike protein, Thakkar et al. demonstrate high rates of seroconversion in a diverse cohort of patients with cancer, while identifying lower immunogenicity in patients with hematologic malignancies and in patients having received immunosuppressive therapies.
Introduction
COVID-19 can result in increased morbidity and mortality in patients with cancer (Kuderer et al., 2020; Mehta et al., 2020; Bakouny et al., 2020), suggesting the need for prophylactic strategies in this immunosuppressed population. In patients with cancer who were affected by COVID-19, increased age, comorbidities, poor performance status, and thoracic and hematologic malignancies have been identified as adverse prognostic indicators for reduced survival (Grivas et al., 2021; Robilotti et al., 2020; Mehta et al., 2020). Follow-up studies on seroconversion in cancer patients with COVID-19 demonstrated that while most will develop antibody response similar to the general population, subgroups of cancer patients with hematologic malignancies, receiving anti-CD20 antibody therapies and stem cell transplantation, exhibit lower rates of seroconversion (Thakkar et al., 2021; Marra et al., 2020). These results suggested that overall high seroconversion rates might be anticipated in patients with malignancies following COVID-19 vaccinations as well, with likely reduced immunogenicity in certain subgroups of patients manifesting from different degrees and mechanisms of immune suppression. Patients with cancer can be immunocompromised due to a multitude of factors, such as the underlying malignancy itself, bone marrow suppressive effects of cytotoxic chemotherapy, and prior or ongoing treatments with a high degree of immunosuppressive effects, such as corticosteroids, B-cell depleting therapies (i.e., anti-CD20 antibodies), cell therapies (especially chimeric antigen receptor [CAR]-T cell), and stem cell transplantation.
It is critical to understand the immunogenicity of approved vaccines for assessing the need of ongoing social isolation and other strategies to mitigate the risk of contracting COVID-19 by immunosuppressed patients, and for designing and rapidly conducting clinical studies focused on passive immunization strategies and vaccine trials assessing unique schedules to enable boosting of the immune response. However, trials of the currently approved COVID-19 vaccines in general excluded patients with a diagnosis of a malignancy, therefore information on the safety and efficacy of these vaccines regarding the development of effective immunity currently is extremely sparse (Friese et al., 2021). Given the higher morbidity and mortality of patients with cancer and COVID-19, their ongoing need to be exposed to the healthcare system, and their frequent need for immunosuppressive therapies, patients with cancer have been identified as a high-priority subgroup for COVID-19 vaccinations, an effort supported by multiple key organizations (Ribas et al., 2021; Van Der Veldt et al., 2021; Desai et al., 2021).
While patients with cancer clearly represent a highly susceptible group with a strong and immediate need to be protected by available, effective vaccines, there remain many uncertainties. For example, following certain immunosuppressive therapies, such as an autologous or allogeneic stem cell transplantation, anti-CD20 therapies, or T cell-directed regimens, vaccinations have low efficacy and their best timing is unclear (Jaffe et al., 2006; Rubin et al., 2014). Such guidance is also lacking for patients undergoing cytotoxic chemotherapy. One randomized study did not suggest notable differences in influenza vaccine immunogenicity dependent on whether vaccination was given on the day of chemotherapy or during the neutropenic period of the treatment cycle (Keam et al., 2017). While many agencies have suggested administering vaccines 1–2 weeks prior to a chemotherapy dose, this recommendation has not been practical with limited vaccination slot availability, variable chemotherapy (e.g., weekly), and vaccine administration schedules (e.g., two doses of BNT162b2 are recommended to be given 21 days apart while two doses of mRNA-1273 are given 28 days apart), leading to liberal recommendations to allow the most rapid vaccination of these immunosuppressed patients (Desai et al., 2021). Vaccine safety and immunogenicity information is also generally lacking in the context of therapies that stimulate the immune system, such as immune checkpoint inhibitor (ICI) therapy, with a few studies suggesting general safety and possibly heightened immunity in this context (Waissengrin et al., 2021).
To narrow this key knowledge gap, we conducted this study to comprehensively determine the immunogenicity of vaccines in a cohort of patients with a diagnosis of a malignancy in New York City via evaluation of rates of anti-spike immunoglobulin G (IgG) antibody positivity following vaccination with one of the three Food and Drug Administration (FDA)-approved COVID-19 vaccines.
Results
Study cohort
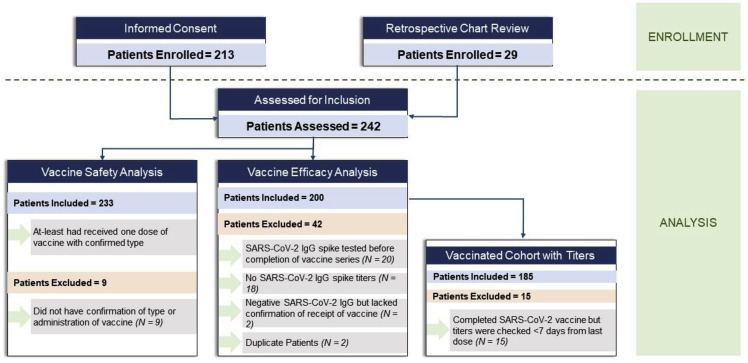
Two hundred and thirteen patients were enrolled in the study via informed-consent process. An additional 29 patients with cancer who underwent SARS-CoV-2 spike IgG testing were identified by retrospective chart review. Eighteen patients did not have a SARS-CoV-2 spike IgG test performed after consenting and were excluded. Another 20 patients were excluded as they had a SARS-CoV-2 spike IgG test before completion of a full vaccination series according to FDA guidance (6 with negative and 14 with positive results). Two more patients were excluded who had a negative SARS-CoV-2 spike IgG and no clear documentation of dates or types of vaccine, and two more patients were excluded due to duplicate medical records. Finally, 233 patients with cancer having completed the FDA-recommended two doses of the mRNA vaccines (BNT162b2 [Polack et al., 2020] or mRNA-1273 [Baden et al., 2020]) or one dose of the adenoviral vaccine (AD26.COV2.S [Sadoff et al., 2021]) were included in the safety analysis (Figure 1 ). A cohort of 200 patients underwent a SARS-CoV-2 spike IgG test and was included in the immunogenicity analysis. Serological data (positive or negative IgG test) from these 200 patients was used in association studies between cancer subtypes and treatments. We also investigated the association between the quantitative titer of SARS-CoV-2 spike IgG and cancer subtypes and treatments. One hundred and eighty-five of 200 patients had IgG titers available that were at least 7 days after the last dose of the vaccine (“vaccinated cohort with titers”). Twenty-six de-identified patients without a cancer diagnosis who had completed COVID-19 vaccination and received a SARS-CoV-2 IgG spike antibody test >7 days after their most recent vaccine dose were used as a control cohort (Table S1). This is represented in the CONSORT diagram (Figure 1).
Figure 1.
CONSORT diagram showing the patient cohort
Two hundred and thirteen patients consented to study participation and 29 were enrolled via retrospective chart review. Ultimately based on study criteria, 233 patients were evaluable for vaccine safety analysis and 200 patients were evaluable for vaccine efficacy analysis. One hundred and eighty-five of the 200 patients evaluated for vaccine efficacy analysis were then further assessed as a vaccinated cohort for antibody titer comparisons.
Baseline characteristics
A total of 200 patients who completed their full vaccination schedule according to FDA guidance were included in the efficacy study. The median age of the patient population was 67 years (range 27–90 years). Fifty-eight percent (116/200) of patients were female and 42% (84/200) were male. The ethnicity/race of the patients represented the diverse patient population of the Bronx, New York. Sixty-four patients (32%) identified their ethnicity as African American, 78 (39%) as Hispanic, 40 (22%) as Caucasian, 10 (5%) as Asian, and 5 (3%) as other ethnicities. One hundred and thirty-four patients (67%) were diagnosed with a solid tumor while 66 patients (33%) had a hematologic malignancy with a balanced representation of all common cancer types (Table 1 ). As patients were recruited from our outpatient hematology/oncology clinics, most patients had an active cancer diagnosis. One hundred and fifty patients (75%) had an active malignancy and 135 patients (67%) were in active cancer therapy at the time of their vaccination, with 112 (56%) patients on active chemotherapy. Thirty-eight (19%) patients were on active chemotherapy within 48 h of at least one of the vaccine doses. Types of cancer therapies are listed in detail in Table 2 . One hundred and fifteen patients (54%) had completed vaccination with the BNT162b2 vaccine and 62 (31%) with the mRNA-1273 mRNA vaccine, while 20 (10%) had received the single dose of Ad26.COV2.S vaccine. Three patients had received a complete mRNA vaccination series; however, the information about the type (BNT162b2 versus mRNA-1273) was not available.
Table 1.
Baseline characteristics of the cohort
| Characteristic | N | % |
|---|---|---|
| Age, median (range) | 67 (27–90) years | |
| Sex | ||
| Male | 84 | 42% |
| Female | 116 | 58% |
| Race | ||
| White | 43 | 22% |
| African American | 64 | 32% |
| Hispanic | 78 | 39% |
| Asian | 10 | 5% |
| Other | 5 | 3% |
| Type of malignancy | ||
| Solid tumor | 134 | 67% |
| Hematologic malignancy | 66 | 33% |
| Malignancy category | ||
| Solid tumor | ||
| Breast | 51 | 26% |
| Gastrointestinal | 27 | 14% |
| Genitourinary | 18 | 9% |
| Gynecologic oncology | 10 | 5% |
| Thoracic/head & neck | 25 | 13% |
| Skin/musculoskeletal | 2 | 1% |
| Carcinoma of unknown primary | 1 | 1% |
| Hematologic malignancy | ||
| Lymphoid | 26 | 13% |
| Myeloid | 18 | 9% |
| Plasma cell | 22 | 11% |
| Cancer status at the time of vaccine | ||
| Active | 110 | 55% |
| Progressive | 7 | 4% |
| Relapse/recurrent | 33 | 17% |
| Remission | 50 | 25% |
| Type of vaccine | ||
| BNT162b2 | 115 | 58% |
| mRNA-1273 | 62 | 31% |
| AD26.COV2.S | 20 | 10% |
| mRNA (type unknown) | 3 | 2% |
Table 2.
Types of cancer therapy in the cohort
| Type of cancer treatment | n | % |
|---|---|---|
| Antibody-drug conjugate | 7 | 4% |
| Anti-CD20 antibody therapy | 23 | 12% |
| Anti-CD38 antibody therapy | 10 | 5% |
| Anti-HER2 antibody therapy | 16 | 8% |
| AR-targeted therapy | 6 | 3% |
| BCL-2 inhibitor | 7 | 4% |
| BTK inhibitor | 2 | 1% |
| CAR-T cell therapy | 3 | 2% |
| CDK4/6 inhibitors | 5 | 3% |
| Chemotherapy | 112 | 56% |
| Clinical trial (experimental therapy) | 7 | 4% |
| EGFR inhibitor | 1 | 1% |
| Hormonal therapy (ADT, OFS, and AI) | 47 | 24% |
| Immune checkpoint inhibitor | 31 | 16% |
| Immunomodulator | 22 | 11% |
| mTOR inhibitors | 2 | 1% |
| No treatment | 11 | 6% |
| Protease inhibitor | 19 | 10% |
| Radiation | 55 | 28% |
| Stem cell transplant | 26 | 13% |
| Supportive care | 6 | 3% |
| Surgery | 59 | 30% |
| TGFβ inhibitor | 3 | 2% |
| Tyrosine kinase inhibitor | 9 | 5% |
| VEGF inhibitor | 7 | 4% |
AR, androgen receptor; BCL-2, B cell lymphoma 2; BTK, Bruton’s tyrosine kinase; EGFR, epidermal growth factor receptor; ADT, androgen deprivation therapy; OFS, ovarian function suppression; AI, aromatase inhibitors; mTOR, mammalian target of rapamycin; TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor.
Overall immunogenicity and safety of SARS-CoV2 vaccine
The anti-SARS-CoV-2 spike protein antibody test (Abbott) was performed, which demonstrated a high rate of seropositivity (94%) with only 13 (6%) patients with a negative value (titer below 50 arbitrary units per milliliter [AU/mL]). Percent positivity appeared similarly between the vaccine types (BNT162b2 95%, mRNA-1273 94%, and Ad26.COV2.S 85%), with a trend toward lesser positivity with the Ad26.COV2.S vaccine. We also assessed antibody titers in a subcohort of 185 patients with available IgG levels >7 days after final dose of vaccine (vaccinated cohort matching the definition of our non-cancer control cohort). The median time between spike antibody test and vaccine dose for this subcohort is 30 days (interquartile range 19–53 days). In solid malignancy patients the median was 31.5 days, and in patients with hematologic malignancies the median was 28.5 days.
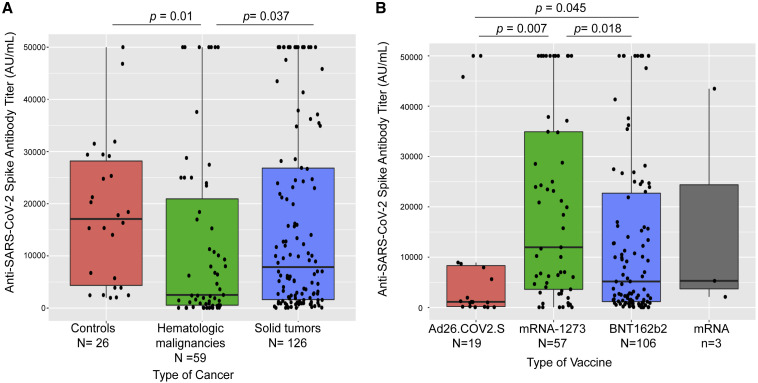
Highest IgG titers were seen with the mRNA-1273 vaccine (median 11,963 AU/mL, standard deviation [SD] 18,742), followed by the BNT162b2 vaccine (median 5,173 AU/mL, SD 16,699) and the single-dose Ad26.COV2.S vaccine (median 1,121 AU/mL, SD 17,571) (p < 0.05, Kruskall-Wallis test, Figure 2 B). Recognizing that the Ad26.COV2.S vaccine was introduced late onto the market, which might or might not account for the lower titers of spike antibodies, we assessed associations of antibody seropositivity and antibody titers with time from completion of vaccination. While there was no association with titer levels, we found a statistically significant positive association between the time from vaccination until IgG testing and antibody seropositivity (p = 0.03, Kruskal-Wallis test). We then conducted a multivariate analysis with a generalized linear model and observed that the relationship between vaccine type and titers remained significant after accounting for the effect of time from vaccine (Figure S1).
Figure 2.
Association of anti-SARS-CoV-2 spike IgG with vaccine types and cancer types
(A) Patients with hematologic malignancies had lowest titers when compared with those with solid tumors and non-cancer patient controls. No difference was seen between patients with solid tumors and controls.
(B) Anti-spike protein IgG antibody titers (AU/mL) were significantly higher in patients who received mRNA vaccines than in those who received adenoviral vaccine.
Box plots here and in subsequent figures show median (horizontal bar), the 75th and 25th quartiles, and error bars depicting the largest and smallest values (up to 1.5 times the interquartile range). Differences assessed by Kruskal-Wallis test.
Vaccinations appeared to be generally very safe in this cohort, with mostly mild and moderate anticipated adverse effects reported. In the safety analysis, 139 patients had received BNT162b2 first dose, 131 patients BNT162b2 second dose, 71 patients mRNA-1273 first dose, 64 patients mRNA-1273 second dose, and 23 patients the single-dose Ad26.COV2S vaccine. Across all the doses, 194 vaccination episodes were reported to lead to no adverse effects overall. Sore arm and muscle aches were the first and second most common reported adverse effects in 131 and 49 instances, respectively. A comprehensive analysis of adverse effect profile of each type of vaccine is presented in Figures S4 and S5.
Solid tumors versus hematologic malignancies
In the cohort of patients with solid tumors, seropositivity post vaccination was high (98%), while a significantly lower seropositivity rate was seen in patients with hematologic malignancies (85%, p = 0.001, Fisher's exact test). Analysis of the subcohort of 185 patients with available IgG titers >7 days post vaccination revealed significantly higher titer values in solid tumors (median 7,858 AU/mL, SD 18,103) than hematologic malignancies (median 2,528 AU/mL, SD 12,338, p = 0.013, Kruskal-Wallis test). Furthermore, to ensure that the difference in titers was not confounded by different time intervals from vaccination, we conducted a multivariate analysis using time from vaccination to IgG assay testing as a confounder and determined that lower titers in hematologic malignancies than in solid tumors were still significant (p = 0.0012). Comparison of titers from non-cancer controls (Table S1) revealed no significant difference when compared with solid-tumor patients but showed a statistically significant difference when compared with patients with hematologic malignancy (p = 0.01, Kruskal-Wallis test) (Figure 2A).
Association with active cancer therapies and immunosuppressive therapies
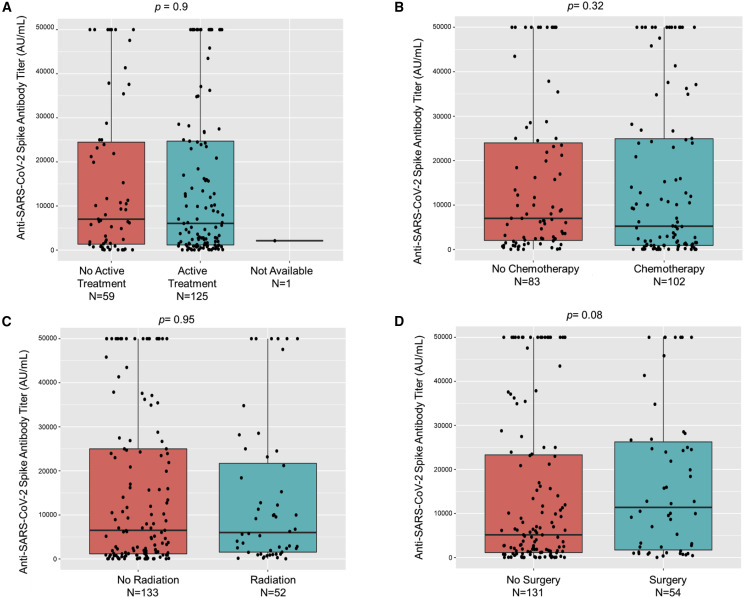
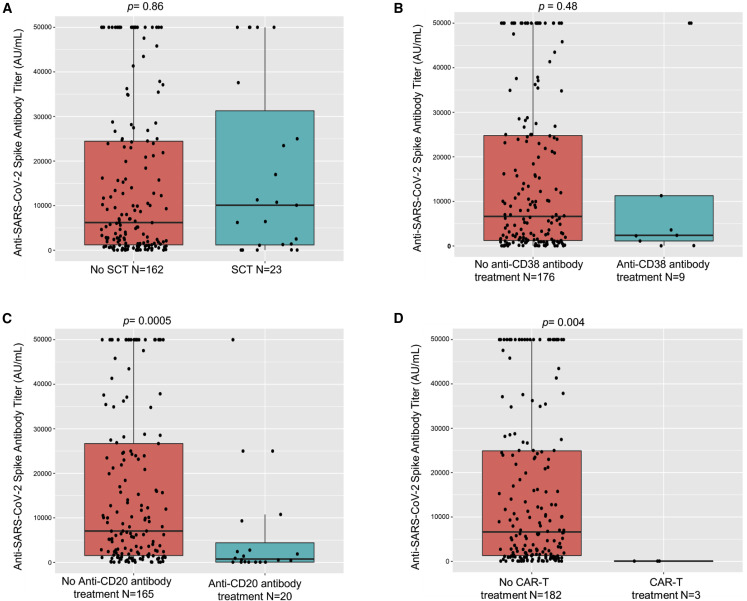
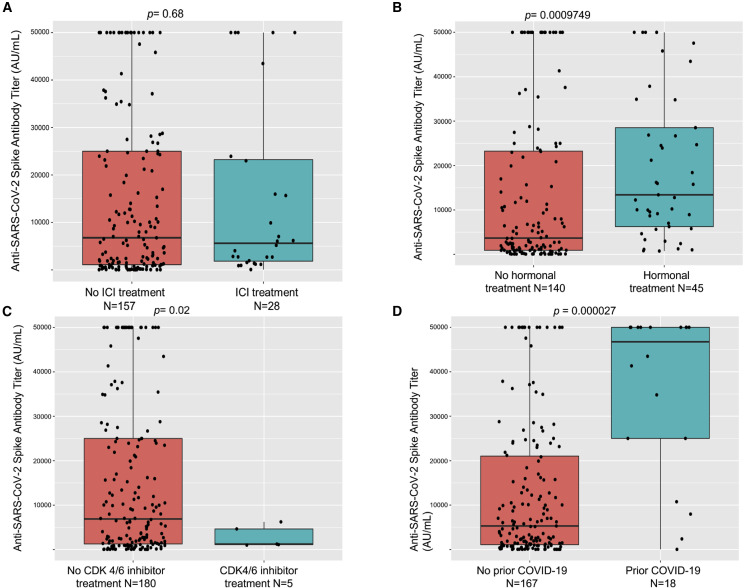
No significant differences in seroconversion were seen when comparing patients on active cancer therapy with patients who were not (96% versus 93%). However, significantly lower rates of seropositivity were seen in patients on active cytotoxic chemotherapy (92%) versus others (99%, p = 0.04) without notable differences in titer levels (Figure 3 ). Next, we focused our analysis on patients who had received specific immunosuppressive therapies, such as stem cell transplantation, anti-CD20 therapy, or CAR-T cell therapy. We observed significantly lower seroconversion rates in patients who underwent these therapies: stem cell transplant (73%, p = 0.0002, Fisher's exact test), anti-CD20 therapies (70%, p = 0.0001, Fisher's exact test) and CAR-T cell treatments (all three patients remained seronegative after vaccination, p = 0.0002, Fisher's exact test) (Table 3 ). Of the 26 stem cell transplant patients, 23 received an autologous and 3 an allogeneic transplant (2 seropositive, 1 seronegative). Accordingly, significantly lower titer levels were also seen in patients receiving anti-CD20 therapies compared with the overall group of patients (Figure 4 ). These results highlighted the continued susceptibility of patients receiving these therapies during the pandemic.
Figure 3.
Association of anti-SARS-CoV-2 spike IgG with therapy
(A–C) Anti-spike protein IgG antibody titers (AU/mL) after full vaccination did not significantly differ in patients receiving active therapy (A), chemotherapy (B), or radiation therapy (C) when compared with respective counterparts.
(D)Patients that had received surgery versus no surgery had no significant difference in titer levels (p = 0.08).
Box plots are shown with differences assessed by Kruskal-Wallis test.
Table 3.
Association of anti-spike IgG with disease characteristics
| Type of malignancy | Positive anti-SARS-CoV-2 spike IgG patients, n (%) | Negative anti-SARS-CoV-2 spike IgG patients, n (%) | p value |
|---|---|---|---|
| Solid malignancy | 131 (98%) | 3 (2%) | 0.001053∗ |
| Hematologic malignancy | 56 (85%) | 10 (15%) | |
| Type of cancer therapy | |||
| Anti-CD20 | 16 (70%) | 7 (30%) | 0.0001168∗∗ |
| Stem cell transplant | 19 (73%) | 7 (27%) | 0.0002866∗∗ |
| CAR-T cell therapy | 0 (0%) | 3 (100%) | 0.0002178∗∗ |
| Hormonal therapy | 47 (100%) | 0 (0%) | 0.04129∗∗ |
| Immune checkpoint inhibitor therapy | 30 (97%) | 1 (3%) | 0.6962 |
∗Statistically significant when compared with each other.
∗∗Statistically significant when compared with overall cohort.
Figure 4.
Association of anti-SARS-CoV-2 spike IgG with immunosuppressive therapies
(A and B) Anti-spike protein IgG antibody titers (AU/mL) after full vaccination did not significantly differ in patients having received stem cell transplantation (SCT) (A) or anti-CD38 antibody therapy (B) when compared with respective counterparts.
(C and D) Patients receiving anti-CD20 antibody treatments (C) or CAR-T cell therapy (D) had a significantly lower titer after vaccination when compared with respective counterparts.
Box plots are shown with differences assessed by Kruskal-Wallis test.
Associations with other patient demographics and treatments
These analyses are available in Table S2.
Age
Our patient population had a wide age range (27–90 years). We studied the association between age and SARS-CoV-2 IgG spike antibody seroconversion rates and observed no statistically significant association between these variables (p = 0.13, Kruskal-Wallis test).
Ethnicity
Given the ethnically diverse cohort in this study, we studied the association between seropositivity and patient ethnicity. We observed that there was no statistically significant association between ethnicity and spike antibody seroconversion rates (p = 0.4574, Fisher's exact test).
Time since immunosuppressive therapy
We also studied the association between time since specific immunosuppressive therapies and immunogenicity. We divided patients into two groups: <365 days and >365 days since anti-CD20 antibody therapy or stem cell transplant and anti-SARS-CoV-2 spike IgG testing. The comparison between seropositivity and time since immunosuppressive treatment was not statistically significant (p = 1, Fisher's exact test).
Steroid use
Our cohort included 55 patients who had used steroids (daily or occasional) at the time of vaccination. Five had a negative spike IgG result and 50 had positive results. This was not statistically different from the entire cohort (p = 0.348, Fisher's exact test).
Treatment within 48 h of a vaccine dose
We collected data to evaluate whether patients who received active cancer therapies 48 h before or after a vaccine dose had lesser seropositivity rates. Thirty-eight patients met the above criteria. We observed that three patients were seronegative, and there was no statistically significant association regarding whether patients received cancer therapies within 48 h of the vaccine or not (p = 0.7, Fisher's exact test). These patients were compared with the entire cohort. These analyses are available in Table S2.
Association with additional cancer therapies
We observed high rates of post-vaccination seroconversion in patients on hormonal therapy (100% seropositivity, p = 0.04) and ICI therapy (97%, p = 0.69, Fisher's exact test) when compared with the rest of the cohort. Interestingly, while all patients on CDK4/6 inhibitor treatment showed positive anti-spike IgG test results, notably antibody titers were very low in this small subset (n = 5, median 1,242 AU/mL SD 2,435 versus median 6,887 AU/mL, SD 17,843 for overall cohort) (Figure 5 ). Given the known involvement of the CDK4/6 pathway in immune activation (Chen-Kiang, 2003; Cingöz and Goff, 2018; Laphanuwat and Jirawatnotai, 2019), this might be biologically plausible and warrants further studies into the impact of CDK4/6 inhibitor on vaccine efficacy. We also noted trends toward lower titers among other subgroups, such as patients having received BCL2-or BTK-targeted therapy, consistent with prior observations on their negative impact on vaccine efficacy (Pleyer et al., 2021) (Figure S3).
Figure 5.
Association of anti-SARS-CoV-2 spike IgG with type of therapy and prior COVID-19 history
(A) Anti-spike protein IgG antibody titers (AU/mL) after full vaccination did not significantly differ in patients receiving immune checkpoint inhibitor (ICI) therapy when compared with those who did not.
(B) Patients receiving hormonal therapy had a significantly higher titer after vaccination.
(C) Patients receiving CDK4/6 inhibitor therapy had a significantly lower titer after vaccination.
(D) Patients with prior COVID-19 infection had significantly higher IgG titers.
Box plots are shown with differences assessed by Kruskal-Wallis test.
Association with prior COVID-19
Previous studies have reported heightened antibody responses to COVID-19 vaccinations in patients with a prior COVID-19 infection (Krammer et al., 2021). Our cohort included 22 patients with cancer who had known prior COVID-19, and a high rate of seroconversion was seen in this subset (21/22 seroconverted for a 95% seroconversion rate with one patient not seroconverting having received an autologous stem cell transplant). Antibody titers in previously infected patients were significantly higher than those who were not known to be previously infected (prior COVID-19: median 46,737 AU/mL, SD 18,681; others: median 5,296 AU/mL, SD 16,193, p < 0.001, Kruskall-Wallis test) (Figure 5D).
Discussion
COVID-19 disease has had a devastating impact worldwide and especially so among patients with a cancer diagnosis. Various factors adversely affect outcomes in cancer patients affected with COVID-19, including impact of underlying disease on performance status, age/comorbidities of affected patients, immune suppression related to disease such as in patients with hematologic malignancies, and immune-suppressive effects of disease-directed therapies (Lee et al., 2020a, 2020b; Jee et al., 2020; García-Suárez et al., 2020; Mehta et al., 2020; Westblade et al., 2020). In addition, patients with cancer requiring active therapy face frequent exposure to the healthcare system, increasing the risk of acquiring COVID-19. Lastly, treatment modifications due to the ongoing pandemic can compromise disease outcomes, amplifying the urgent need to implement widespread vaccination of patients with malignant disease—an initiative with broad support from a large swath of cancer care/advocacy organizations.
While all three FDA-approved vaccines, the mRNA-based mRNA-1273 (Moderna) and BNT162b2 (Pfizer/BioNTech) and the adenovirus-based Ad26.COV2.S (Johnson & Johnson), yield a high level of protection against the current circulating variants in the general population, limited data regarding their immunogenicity among patients with a cancer diagnosis are available. Recent studies from the United Kingdom and France have reported lower seroconversion rates following a single dose of mRNA vaccination (Monin-Aldama et al., 2021, Barrière et al., 2021). Lower seropositivity rates have also been observed in patients with chronic lymphocytic leukemia and myeloma (Herishanu et al., 2021; Terpos et al., 2021; Bird et al., 2021) and in those undergoing therapy with BTK inhibitors or venetoclax/anti-CD20 therapy, in line with our observations (Herishanu et al., 2021). These early studies clearly highlight the need to complete full vaccination schedules for optimum seroconversion and also emphasize the need for larger cohort studies to determine the immunogenicity of COVID-19 vaccines among patients receiving distinct cancer therapeutics.
Several shortcomings of our study need to be listed. These include limited representation of some patient cohorts not allowing clear conclusions regarding seroconversion rates among less common malignancy types or less frequently used treatment approaches. Our cohort also over-represented patients on active therapy, as recruitment occurred over a short period in our outpatient departments. In addition, our study relies solely on the anti-spike protein IgG levels as a surrogate for immunity to COVID-19. Admittedly, the anti-spike IgG antibody used in our study, albeit specific to the receptor binding domain of the spike protein, might still not necessarily correlate with virus-neutralizing activity. Our study also did not evaluate the level of SARS-CoV-2-specific T cell responses. Further research will be needed to directly assess virus neutralization and cellular immunity (Bange et al., 2021). Another potential limitation is underestimation of titer values for anti-spike antibodies, as evidence suggests that titers may rise over time, and the upper limit of detection of our assay is 50,000 AU/mL (Widge et al., 2020); however, a cutoff of 7 days was used to match the control cohort and eliminate bias in the analysis. Lastly, some observations are based on smaller subsets and post hoc analyses, so that larger studies are needed for validation.
Our study, along with other emerging data, strongly highlights the continued need to vaccinate patients with a cancer diagnosis urgently and broadly, as vaccinations are likely to be highly effective. On the other hand, our study highlights at-risk cohorts of patients, in particular patients with hematologic malignancies following receipt of immunosuppressive therapies such as stem cell transplantation, anti-CD20 therapies, and CAR-T cell treatments. These cohorts of patients could potentially benefit from passive immunization with anti-COVID antibodies in the face of the ongoing pandemic. In fact, monoclonal anti-COVID-19 antibodies have shown therapeutic and prophylactic potential in transplant or at-risk patient cohorts (Rizk et al., 2021; Hurt and Wheatley, 2021; Dhand et al., 2021). In addition, higher doses or booster doses of some vaccines or vaccinations of mixed vaccine types might offer stronger immunogenicity and need to be explored in immunosuppressed patients. Lastly, protective measures such as masking and social distancing will remain logical aspects of defensive management strategies for highly immune-suppressed patients during the pandemic until safe herd immunity levels of population-level vaccinations are reached.
In summary, we present a large cohort of patients with malignancy who underwent full COVID-19 vaccination according to FDA guidance. In this cohort of ethnically diverse patients with broad representation of a wide range of malignancies and therapies, very high seropositivity rates were observed, in contrast to previously published smaller cohort studies focusing on unique subsets of susceptible patients or non-standard vaccination schedules. Statistically significantly lower seropositivity rates were observed in patients with hematologic malignancies and patients having received immunosuppressive therapies. Our findings support broad and urgent COVID-19 vaccinations in patients with a cancer diagnosis to enable optimal cancer treatment delivery during the ongoing COVID-19 pandemic.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| None | Not applicable | Not applicable |
| Bacterial and virus strains | ||
| None | Not applicable | Not applicable |
| Biological samples | ||
| Serum sample | Patients recruited in this study | In this study |
| Chemicals, peptides, and recombinant proteins | ||
| None | Not applicable | Not applicable |
| Critical commercial assays | ||
| AdviseDx SARS-CoV-2 IgG II assay | Abbott | Please provide the catalog number from Abbott |
| Deposited data | ||
| Computer code | Github | https://github.com/kith-pradhan/CovidVaccineReport/blob/main/report.R |
| Experimental models: Cell lines | ||
| None | Not applicable | Not applicable |
| Experimental models: Organisms/strains | ||
| None | Not applicable | Not applicable |
| Oligonucleotides | ||
| None | Not applicable | Not applicable |
| Recombinant DNA | ||
| None | Not applicable | Not applicable |
| Software and algorithms | ||
| R 3.6.2 | https://www.r-project.org/ | https://www.r-project.org/ |
| Other | ||
| Clinical data | Electronic medical record | Medical record number |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Balazs Halmos, bahalmos@montefiore.org.
Materials availability
This study did not generate new unique reagents.
Data and code availability
The published article includes all data generated and analyzed during this study. Data will be made available freely from the corresponding authors upon request. The utilized computer code has been deposited in GitHub (https://github.com/kith-pradhan/CovidVaccineReport/blob/main/report.R). All analyses were conducted with built-in and freely available R packages.
Experimental model and subject details
Patient data collection
The study was approved by the Montefiore-Einstein Institutional Review Board. This study was designed as a cross-sectional cohort study and enrolled subjects being seen in the outpatient practices of the Montefiore/Einstein Cancer Center during April 2021. Participants were enrolled in the study after signing informed consent. Subjects underwent anti-SARS-CoV-2 spike IgG assay, completed a questionnaire focusing on details and adverse effects of COVID-19 vaccination and provided optional consent for future biobanking for research. The protocol also allowed data collection via retrospective chart review for a small number of patients who underwent anti-SARS-CoV-2 spike IgG antibody testing after vaccination as ordered at the discretion of their oncologist. Safety data for vaccines for patients recruited via informed consent was collected via questionnaires with an optional telephone call if patients did not remember dates of the vaccine. Assessment was done at the time when patients signed the informed consent. Safety data for patients recruited via retrospective chart review was collected if data was available as part of electronic medical record. Data for cancer-directed therapy was retrieved from retrospective chart review and strict criteria were used to classify them (e.g., hormonal therapy strictly included androgen deprivation, ovarian function suppression and aromatase inhibitors. Steroids were not considered a part of hormonal therapy). Active cancer means patient with an initial cancer diagnosis on treatment including surgery, radiation, neoadjuvant, adjuvant or systemic chemotherapy or maintenance therapy (ex- lenalidomide for myeloma or immunotherapy maintenance for non-small cell lung cancer) or not on treatment and under active surveillance. Remission means patient with cancer diagnosis in the past who has completed cancer-directed therapy and is now only undergoing surveillance. Relapse/recurrent means patient with cancer diagnosis who had completed cancer-directed therapy and achieved remission or was on maintenance therapy now experiencing disease that needs additional treatment. Progressive means patient with cancer diagnosis who developed disease progression while on systemic therapy.
Method details
Anti-SARS-CoV-2 spike IgG assay
The AdviseDx SARS-CoV-2 IgG II assay was used for the assessment of anti-spike IgG antibody testing. AdviseDx is an automated, two-step chemiluminescent immunoassay performed on the Abbott i2000SR instrument. The assay is designed to detect IgG antibodies directed against the receptor binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2. The RBD is a portion of the S1 subunit of the viral spike protein and has a high affinity for the angiotensin converting enzyme 2 (ACE2) receptor on the cellular membrane(Pillay, 2020; Yang et al., 2020).
The procedure, in brief, is as follows. Patient serum containing IgG antibodies directed against the RBD is bound to microparticles coated with SARS-CoV-2 antigen. The mixture is then washed of unbound IgG and anti-human IgG, acridinium-labeled, secondary antibody is added and incubated. Following another wash, sodium hydroxide is added and the acridinium undergoes an oxidative reaction which releases light energy which is detected by the instrument and expressed as relative light units (RLU). There is a direct relationship between the amount of anti-spike IgG antibody and the RLU detected by the system optics. The RLU values are fit to a logistic curve which was used to calibrate the instrument and expresses results as a concentration in AU/mL (arbitrary units/milliliter). This assay recently has shown high sensitivity (100%) and positive percent agreement with other platforms including a surrogate neutralization assay (Bradley et al., 2021) and also demonstrated high specificity both in the post COVID-19 infection and post vaccination settings. The cutoff value for this assay is 50 AU/mL with <50 AU/ml values reported as negative and the maximum value is 50000 AU/mL.
Quantification and statistical analysis
Association between two categorical variables was tested with a Fisher exact test. Association between one categorical and one ordinal variable was tested with a Kruskal-Wallis Rank Sum test. Pre-specified hypotheses to be tested included assessing correlation of seropositivity with solid and hematologic malignancies and between the overall cohort and highly immunosuppressive therapies. All analyses were done in R (version 3.6.2).
Acknowledgments
We acknowledge Albert Einstein Cancer Center grant P30 CA013330 and NCORP grant 2UG1CA189859-06 in providing funding for this project. This work was supported partly by the Jane A. and Myles P. Dempsey fund.
Author contributions
A.T., A.V., and B.H. conceived and managed the study; J.D.G.-L., N.G., R.G., L.C.S., S.R., S.Y.K., B.K., R.A.S., N.K., L.B.-R., M.M., S.G., J.S., B.G., and D.M. participated in patient recruitment and data curation; K.P. oversaw data analyses; S.P. and R.P.-S. contributed to project administration; Y.D.G. and L.W. oversaw investigations. All authors contributed to writing the manuscript.
Declaration of interests
A.V. has received research funding from GlaxoSmithKline, BMS, Janssen, Incyte, MedPacto, Celgene, Novartis, Curis, Prelude, and Eli Lilly and Company, has received compensation as a scientific advisor to Novartis, Stelexis Therapeutics, Acceleron Pharma, and Celgene, and has equity ownership in Stelexis Therapeutics. All other authors declare no competing interests.
Published: August 9, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ccell.2021.06.002.
Supplemental information
References
- Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakouny Z., Hawley J.E., Choueiri T.K., Peters S., Rini B.I., Warner J.L., Painter C.A. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38:629–646. doi: 10.1016/j.ccell.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange E.M., Han N.A., Wileyto P., Kim J.Y., Gouma S., Robinson J., Greenplate A.R., Hwee M.A., Porterfield F., Owoyemi O., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021 doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., Petit E., Leysalle A., Raimondi V., Carles M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021 doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird S., Panopoulou A., Shea R.L., Tsui M., Saso R., Sud A., West S., Smith K., Barwood J., Kaczmarek E., et al. Response to first vaccination against SARS-CoV-2 in patients with multiple myeloma. Lancet Haematol. 2021;8:e389–e392. doi: 10.1016/S2352-3026(21)00110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B.T., Bryan A., Fink S.L., Goecker E.A., Roychoudhury P., Huang M.-L., Zhu H., Chaudhary A., Madarampalli B., Lu J.Y.C., et al. Anti-SARS-CoV-2 antibody levels are concordant across multiple platforms but are not fully predictive of sterilizing immunity. medRxiv. 2021 doi: 10.1101/2021.04.26.21256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen-Kiang S. Cell-cycle control of plasma cell differentiation and tumorigenesis. Immunol. Rev. 2003;194:39–47. doi: 10.1034/j.1600-065x.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Cingöz O., Goff S.P. Cyclin-dependent kinase activity is required for type I interferon production. Proc. Natl. Acad. Sci. 2018;115:E2950–E2959. doi: 10.1073/pnas.1720431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Gainor J.F., Hegde A., Schram A.M., Curigliano G., Pal S., Liu S.V., Halmos B., Groisberg R., Grande E., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat. Rev. Clin. Oncol. 2021;18:313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhand A., Lobo S.A., Wolfe K., Feola N., Nabors C. Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: early single-center experience. Clin. Transplant. 2021;35:e14245. doi: 10.1111/ctr.14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese C.R., Choueiri T.K., Duma N., Farmakiotis D., Grivas P., Rini B.I., Shah D.P., Thompson M.A., Pergam S.A., Mishra S., Warner J.L. Care without a compass: including patients with cancer in COVID-19 studies. Cancer Cell. 2021 doi: 10.1016/j.ccell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Suárez J., De La Cruz J., Cedillo Á., Llamas P., Duarte R., Jiménez-Yuste V., Hernández-Rivas J.Á., Gil-Manso R., Kwon M., Sánchez-Godoy P., et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J. Hematol. Oncol. 2020;13:133. doi: 10.1186/s13045-020-00970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivas P., Khaki A.R., Wise-Draper T.M., French B., Hennessy C., Hsu C.Y., Shyr Y., Li X., Choueiri T.K., Painter C.A., et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer Consortium. Ann. Oncol. 2021;32:787–800. doi: 10.1016/j.annonc.2021.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herishanu Y., Avivi I., Aharon A., Shefer G., Levi S., Bronstein Y., Morales Moshiashvili M., Ziv-Baran T., Shorer Y., Scarfo L., et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021 doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt A.C., Wheatley A.K. Neutralizing antibody therapeutics for COVID-19. Viruses. 2021;13:628. doi: 10.3390/v13040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D., Papadopoulos E.B., Young J.W., O'reilly R.,J., Prockop S., Kernan N.A., Jakubowski A., Boulad F., Perales M.A., Castro-Malaspina H., Small T.N. Immunogenicity of recombinant hepatitis B vaccine (rHBV) in recipients of unrelated or related allogeneic hematopoietic cell (HC) transplants. Blood. 2006;108:2470–2475. doi: 10.1182/blood-2006-04-006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee J., Foote M.B., Lumish M., Stonestrom A.J., Wills B., Narendra V., Avutu V., Murciano-Goroff Y.R., Chan J.E., Derkach A., et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J. Clin. Oncol. 2020;38:3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keam B., Kim M.K., Choi Y., Choi S.J., Choe P.G., Lee K.H., Kim T.M., Kim T.Y., Oh D.Y., Kim D.W., et al. Optimal timing of influenza vaccination during 3-week cytotoxic chemotherapy cycles. Cancer. 2017;123:841–848. doi: 10.1002/cncr.30468. [DOI] [PubMed] [Google Scholar]
- Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., Shete S., Hsu C.-Y., Desai A., De Lima Lopes G., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. The Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laphanuwat P., Jirawatnotai S. Immunomodulatory roles of cell cycle regulators. Front. Cell. Dev. Biol. 2019;7:23. doi: 10.3389/fcell.2019.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W., Curley H.M., Fittall M.W., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L.Y.W., Cazier J.-B., Starkey T., Briggs S.E.W., Arnold R., Bisht V., Booth S., Campton N.A., Cheng V.W.T., Collins G., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A., Generali D., Zagami P., Cervoni V., Gandini S., Venturini S., Morganti S., Passerini R., Orecchia R., Curigliano G. Seroconversion in patients with cancer and oncology health care workers infected by SARS-CoV-2. Ann. Oncol. 2020;32:113–119. doi: 10.1016/j.annonc.2020.10.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Goel S., Kabarriti R., Cole D., Goldfinger M., Acuna-Villaorduna A., Pradhan K., Thota R., Reissman S., Sparano J.A., et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10:935–941. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monin-Aldama L., Laing A.G., Muñoz-Ruiz M., Mckenzie D.R., Del Molino Del Barrio I., Alaguthurai T., Domingo-Vila C., Hayday T.S., Graham C., Seow J., et al. Interim results of the safety and immune-efficacy of 1 versus 2 doses of COVID-19 vaccine BNT162b2 for cancer patients in the context of the UK vaccine priority guidelines. medRxiv. 2021 doi: 10.1101/2021.03.17.21253131. [DOI] [Google Scholar]
- Pillay T.S. Gene of the month: the 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. J. Clin. Pathol. 2020;73:366–369. doi: 10.1136/jclinpath-2020-206658. [DOI] [PubMed] [Google Scholar]
- Pleyer C., Ali M.A., Cohen J.I., Tian X., Soto S., Ahn I.E., Gaglione E.M., Nierman P., Marti G.E., Hesdorffer C., et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137:185–189. doi: 10.1182/blood.2020008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Sengupta R., Locke T., Zaidi S.K., Campbell K.M., Carethers J.M., Jaffee E.M., Wherry E.J., Soria J.C., D'souza G. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov. 2021;11:233–236. doi: 10.1158/2159-8290.CD-20-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk J.G., Forthal D.N., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Pfeiffer J.P., Lewin J.C. Expanded access programs, compassionate drug use, and emergency use authorizations during the COVID-19 pandemic. Drug Discov. Today. 2021;26:593–603. doi: 10.1016/j.drudis.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robilotti E.V., Babady N.E., Mead P.A., Rolling T., Perez-Johnston R., Bernardes M., Bogler Y., Caldararo M., Figueroa C.J., Glickman M.S., et al. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26:1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L.G., Levin M.J., Ljungman P., Davies E.G., Avery R., Tomblyn M., Bousvaros A., Dhanireddy S., Sung L., Keyserling H., Kang I. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. [DOI] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpos E., Trougakos I.P., Gavriatopoulou M., Papassotiriou I., Sklirou A.D., Ntanasis-Stathopoulos I., Papanagnou E.D., Fotiou D., Kastritis E., Dimopoulos M.A. Low neutralizing antibody responses against SARS-CoV-2 in elderly myeloma patients after the first BNT162b2 vaccine dose. Blood. 2021 doi: 10.1182/blood.2021011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar A., Pradhan K., Jindal S., Cui Z., Rockwell B., Shah A.P., Packer S., Sica R.A., Sparano J., Goldstein D.Y., et al. Patterns of seroconversion for SARS-CoV-2 IgG in patients with malignant disease and association with anticancer therapy. Nat. Cancer. 2021;2:392–399. doi: 10.1038/s43018-021-00191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Veldt A.A.M., Oosting S.F., Dingemans A.C., Fehrmann R.S.N., Geurtsvankessel C., Jalving M., Rimmelzwaan G.F., Kvistborg P., Blank C.U., Smit E.F., et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat. Med. 2021;27:568–569. doi: 10.1038/s41591-021-01240-w. [DOI] [PubMed] [Google Scholar]
- Waissengrin B., Agbarya A., Safadi E., Padova H., Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol. 2021;22:581–583. doi: 10.1016/S1470-2045(21)00155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westblade L.F., Brar G., Pinheiro L.C., Paidoussis D., Rajan M., Martin P., Goyal P., Sepulveda J.L., Zhang L., George G., et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell. 2020;38:661–671.e2. doi: 10.1016/j.ccell.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2020;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Petitjean S.J.L., Koehler M., Zhang Q., Dumitru A.C., Chen W., Derclaye S., Vincent S.P., Soumillion P., Alsteens D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020;11:4541. doi: 10.1038/s41467-020-18319-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all data generated and analyzed during this study. Data will be made available freely from the corresponding authors upon request. The utilized computer code has been deposited in GitHub (https://github.com/kith-pradhan/CovidVaccineReport/blob/main/report.R). All analyses were conducted with built-in and freely available R packages.